Abstract
Vaccines are the most effective and sustainable intervention to control ticks and tick-borne diseases (TBD). Using a personalized vaccine design based on regional tick genotypes, a Rhipicephalus appendiculatus Subolesin protective antigen was used in a field trial evaluating tick vaccine efficacy, effectiveness, and safety in cattle infested with multiple tick species in different Ugandan agro-ecological zones. Vaccination with SUB was safe with a protective capacity against anemia and infection, and reduced the number of infested cattle, tick fitness (feeding and reproduction) with vaccine effectiveness against multiple tick species between 93.2% at 167-196 days post-vaccination (dpv) and 61.4% at 251–327 dpv. Total integrated vaccine efficacy/effectiveness was estimated as 98.8%. The Subolesin-based vaccine is protective against multiple cattle tick infestations under field conditions in Uganda. These results support registration and commercialization of the vaccine to reduce tick populations and associated risks for human and animal TBD and chemical acaracides in Uganda.
Subject terms: Recombinant vaccine, Protein vaccines
Introduction
The growing incidence of ticks and tick-borne diseases (TBD) affects human and animal health worldwide (reviewed by de la Fuente et al.1). In Uganda as in other tropical countries, cattle ticks increase risks associated with TBD transmitted to humans (e.g., Crimean-Congo Hemorrhagic Fever, CCHF2–4) and affect cattle welfare and production with increasing costs of acaricide misuse, emergency of resistant ticks and environmental pollution5. In Uganda, studies have estimated that losses of over US$ 1.1 billion occur annually due to ticks and TBD such as East Coast fever, babesiosis, anaplasmosis, and cowdriosis. At farm level, 80% of the total annual expenses incurred in the management all cattle diseases are associated with controlling TBD and the most economically relevant tick species, R. appendiculatus, R. decoloratus and A. variegatum5. To approach these challenges, vaccines are the most effective and sustainable intervention for the control of tick populations and the incidence of TBD6,7.
Tick vaccines evolved from strain-specific autovaccines with organ protein extracts8 to recombinant Rhipicephalus microplus-derived Bm86/Bm95 antigens registered and commercialized as TickGARD and Gavac (still commercially available)6,9,10 to vaccinomics11 and quantum vaccinomics12, a new research approach to identify protective epitopes (immunological quantum) for vaccine chimeric antigen design. Since Bm86/Bm95 proteins were identified, multiple tick antigens have been discovered and characterized with proven efficacy under experimental controlled conditions6,13,14. One of these antigens is Subolesin (SUB; also known as 4D8 or Akirin), which has proven efficacy in multiple hosts for the control of different tick species and other ectoparasite vectors and vector-borne pathogens15. Subolesin-based vaccines have been also evaluated under field conditions for the control of tick infestations and transmitted pathogens in Sicily16 and for the control of multi-species cattle tick infestations under pen-trial controlled conditions in Uganda17.
However, main limitations to advance in tick vaccinology include the limited collaborations with African and Asian countries with high incidence of tick infestations and TBD18 and facing challenges such as vaccine efficacy against multiple tick species, impact of tick genetic diversity on vaccine efficacy, antigen combinations, effectiveness and safety of vaccine formulations and vaccine production and administration19.
These limitations support the initiative to evaluate under field conditions a Rhipicephalus appendiculatus SUB vaccine for registration and commercialization in Uganda20. This is the first field trial globally to evaluate in different locations vaccine efficacy E (based on the effect in the reduction of tick infestations, oviposition, and fertility), vaccine effectiveness Ee (as evaluation of efficacy under field conditions considering locations with different characteristics and infestations of the same host by multiple tick species), safety and prevalence of tick-borne pathogens in cattle. The results of the study demonstrated the safety, efficacy, and effectiveness of SUB vaccine for the control of multi-species cattle tick infestations in Uganda.
Results
Vaccine efficacy E
The vaccine efficacy E with recombinant R. appendiculatus SUB antigen was evaluated under pen-controlled conditions in a previous study with values between 47% and 90% for different tick species (R. appendiculatus, R. decoloratus, A. variegatum) in B. indicus and crossbred cattle17. To extend these data for evaluation under field conditions, a field trial was designed and implemented in cattle farms at multiple Ugandan locations (Fig. 1). The results were compared between vaccinated and control groups for different tick species and at different study locations (Figs. 2–4, Table 1).
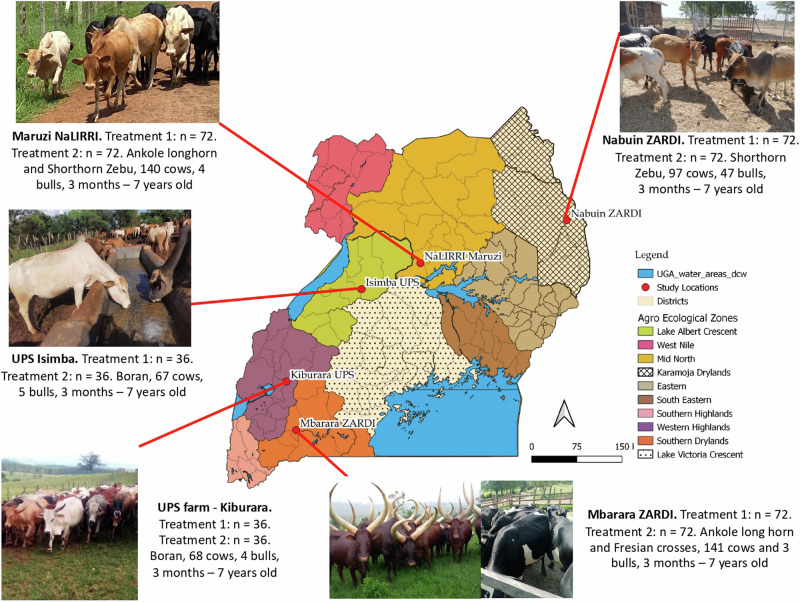
Fig. 1. Characteristics of the Ugandan locations included in the field trial.
Data includes geographical locations Mbarara ZARDI (Mbarara 1 and Mbarara 2 are located on the same farm with 36 animals per treatment on both groups), Ugandan government prison (UPS) farms Kiburara and Isimba, NaLIRRI Maruzi and Nabuin ZARDI, number of animals per treatment (Treatment 1, SUB-vaccine, Treatment 2, Control), cattle breed and age. All images are of author´s origin.
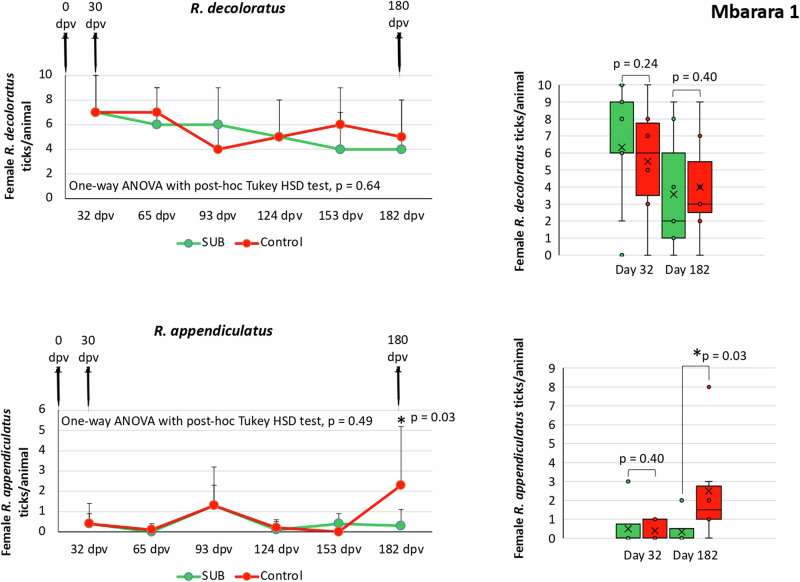
Fig. 2. Female R. decoloratus and R. appendiculatus ticks collected from vaccinated and control cattle in Mbarara 1.
Data collected from 32 dpv to 182 dpv were compared for each tick species between vaccinated and control groups by One-way ANOVA with post-hoc Tukey HSD test (p > 0.05; n = 4–17 animals per group). The number of female ticks per animal at one and six months after vaccination was compared between vaccinated and control groups by Student’s t-test with unequal variance (*p < 0.05; n = 5–8 animals per group). Error bars represent standard deviation. Source data are provided as a Source Data file.
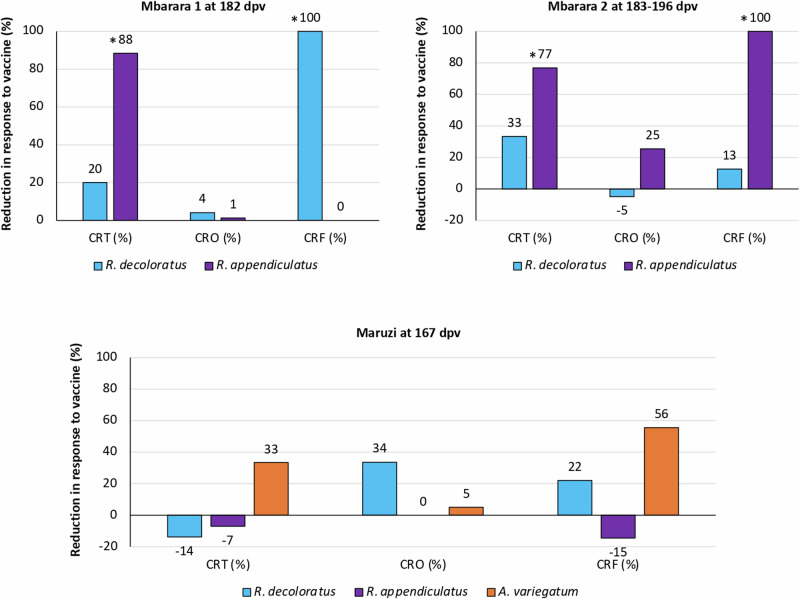
Fig. 4. Effect of SUB vaccine on the reduction in the number of adult female ticks (CRT), oviposition (CRO), and egg fertility (CRF) when compared with the control group.
Data was collected in the different locations at 167–196 dpv. Results were compared between vaccinated and control groups by Student’s t-test with unequal variance (*p < 0.05; n = 3–13 animals per group). Source data are provided as a Source Data file.
Table 1.
Results of SUB vaccine efficacy E on cattle tick infestations and effect on engorged female tick weigh reduction under field conditions in multiple Ugandan locations at around six months after vaccination
| Parameters | Tick species per location | ||
|---|---|---|---|
| R. decoloratus | R. appendiculatus | A. variegatum | |
| Location: Mbarara 1 at 182 dpv | |||
| Mean female ticks | Not present | ||
| TV | 4 | 0.3* | |
| TC | 5 | 2.6 | |
| Mean egg weight | |||
| OV (mg) | 69 | 146 | |
| OC (mg) | 72 | 148 | |
| Mean fertility | |||
| FV (%) | 0* | 0 | |
| FC (%) | 71 | 0 | |
| Efficacy E (%) | 100 | 89 | |
| Engorged female tick weight | |||
| WV (mg) | 131 | 243 | |
| WC (mg) | 130 | 294 | |
| Ew (%) | 0 | 17 | |
| Location: Mbarara 2 at 196 dpv | |||
| Mean female ticks | Not present | ||
| TV | 4 | 0.3* | |
| TC | 6 | 1.3 | |
| Mean egg weight | |||
| OV (mg) | 65 | 120 | |
| OC (mg) | 62 | 161 | |
| Mean fertility | |||
| FV (%) | 69 | 0* | |
| FC (%) | 79 | 59 | |
| Efficacy E (%) | 39 | 100 | |
| Engorged female tick weight | |||
| WV (mg) | 135 | 26* | |
| WC (mg) | 112 | 271 | |
| Ew (%) | 0 | 89 | |
| Location: Maruzi at 167 dpv | |||
| Mean female ticks | |||
| TV | 3.3 | 1.5 | 14 |
| TC | 2.9 | 1.4 | 21 |
| Mean egg weight | |||
| OV (mg) | 458 | 100 | 502 |
| OC (mg) | 690 | 100 | 529 |
| Mean fertility | |||
| FV (%) | 46 | 71 | 20 |
| FC (%) | 59 | 62 | 45 |
| Efficacy E (%) | 41 | 0 | 72 |
| Engorged female tick weight | |||
| WV (mg) | 153 | 265 | 1332 |
| WC (mg) | 112 | 255 | 1711 |
| Ew (%) | 0 | 0 | 22 |
Vaccine efficacy E was calculated based on mean female ticks from the vaccinated (TV) and control (TC) groups, mean egg weight from ticks in the vaccinated (OV) and control (OC) groups, and mean fertility (percent of egg hatching and producing larvae) from ticks in vaccinated (FV) and control (FC) groups. Engorged female tick weigh in vaccinated (WV) and control (WC) were used to calculate the effect on female tick weight (Ew). Results were compared between vaccinated and control groups by Student’s t-test with unequal variance (*p < 0.05; n = 3–13 animals per group).
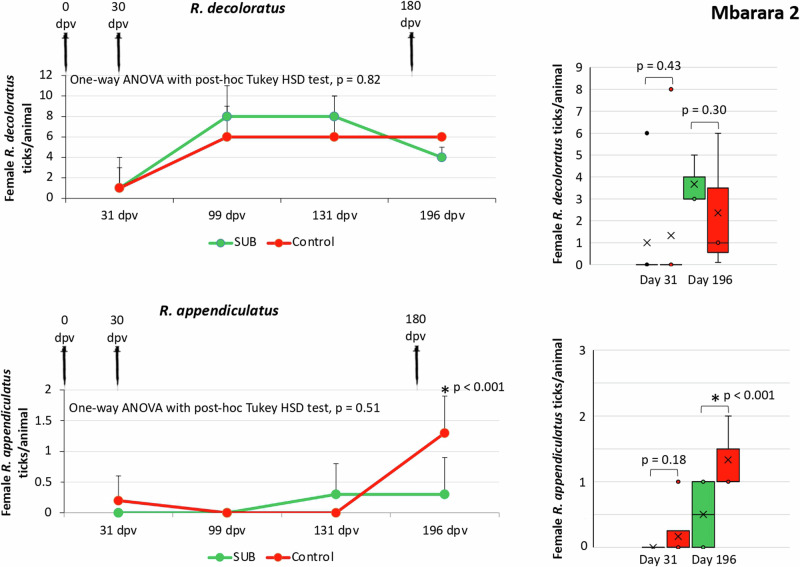
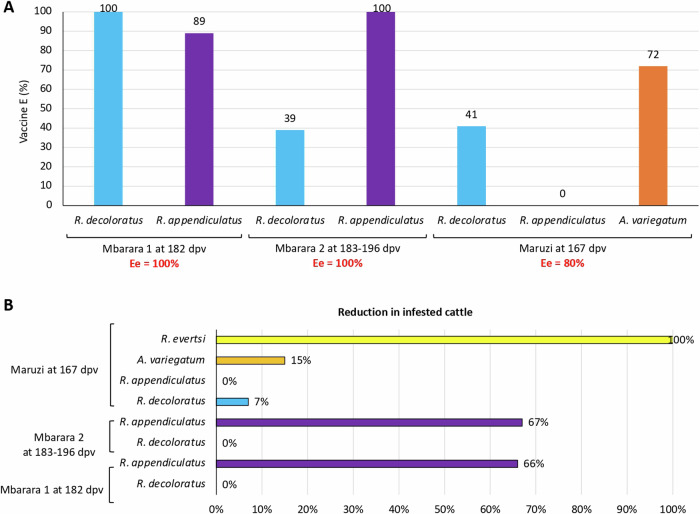
The results showed a significant reduction (p < 0.05) in female tick counts (R. appendiculatus in Mbarara 1 and Mbarara 2; Figs. 2 and 3) and fertility (R. decoloratus in Mbarara 1 and R. appendiculatus in Mbarara 2; Fig. 4 and Table 1). Significant reductions in female tick counts and fertility were not observed for R. appendiculatus, R. decoloratus, and A. variegatum in Maruzi for (Fig. 4 and Table 1). Only 13R. evertsi adult ticks were collected in Maruzi and thus not included in the analysis of vaccine efficacy E. In Kiburara, Isimba and Nabuin, infestations were below 10 ticks per animal throughout the trial. Although not considered in the vaccine efficacy E algorithm, eight dead R. decoloratus were collected at 35 days post-vaccination (dpv) in control but none in SUB-treated group at Maruzi. Despite differences between locations, vaccine efficacy E considering tick infestations, oviposition, and fertility ranged between 39% and 100% with 59.7±44.3% average (59.7±32.8; CI, 26.9–92.5) and without effect only in Maruzi for R. appendiculatus (Table 1).
Fig. 3. Female R. decoloratus and R. appendiculatus ticks collected from vaccinated and control cattle in Mbarara 2.
Data collected from 32 dpv to 182 dpv were compared for each tick species between vaccinated and control groups by One-way ANOVA with post-hoc Tukey HSD test (p > 0.05; n = 3–11 animals per group). The number of female ticks per animal at one and six months after vaccination was compared between vaccinated and control groups by Student’s t-test with unequal variance (*p < 0.05; n = 3–6 animals per group). Error bars represent standard deviation. Source data are provided as a Source Data file.
Vaccine effectiveness Ee
The evaluation of vaccine effectiveness Ee was first focused on the efficacy against multiple tick species feeding on the same host, which reproduces the conditions commonly found in the field. The results showed an effectiveness Ee of 100% for Mbarara 1 and Mbarara 2 and 80% for Maruzi (Fig. 5A) with 93.2±11.7% average (93.2±6.6; CI, 86.6 – 99.8).
Fig. 5. SUB vaccine effectiveness Ee and effect on reduction in infested cattle.
A Vaccine effectiveness Ee against multiple tick species infesting the same host. B Reduction in the number of infested cattle (R). Data was collected in different locations at 167–196 dpv. Source data are provided as a Source Data file.
Additional considerations for the evaluation of vaccine effectiveness Ee included reduction in fed female tick weight as a marker of tick fitness (Ew), total (larvae, nymphs, and adults) tick counts on animals infested with various tick species (Ev), and reduction in the number of infested animals (R) in vaccinated cattle at around 6 months after first vaccine dose administration.
The Ew varied between tick species and locations (Table 1). In Mbarara, the highest Ew was recorded in R. appendiculatus at 182-196 dpv (Mbarara 1, Ew = 17%; Mbarara 2, Ew = 89%). In Maruzi at 167 dpv, as previously shown for E against R. appendiculatus (Table 1), no Ew was shown for R. appendiculatus and R. decoloratus but for A. variegatum (Ew = 22%). For R. evertsi, data was available only for WC = 381 mg in Maruzi.
The Ev was calculated at 156 dpv in Maruzi with cattle infested with A. variegatum, R. appendiculatus and R. decoloratus resulting in 37% efficacy (n = 9-14 animals per group). Regarding R, the results showed reductions higher than 65% in Mbarara 1 and 2 at 182-196 dpv for R. appendiculatus and in Maruzi for R. evertsi (Fig. 5B) with 31.9±40.0% average (31.9±26.1; CI, 5.8 - 58.0). For other tick species, reductions in infested cattle were observed in Maruzi at 167 dpv for A. variegatum (15%) and R. decoloratus (7%) (Fig. 5B).
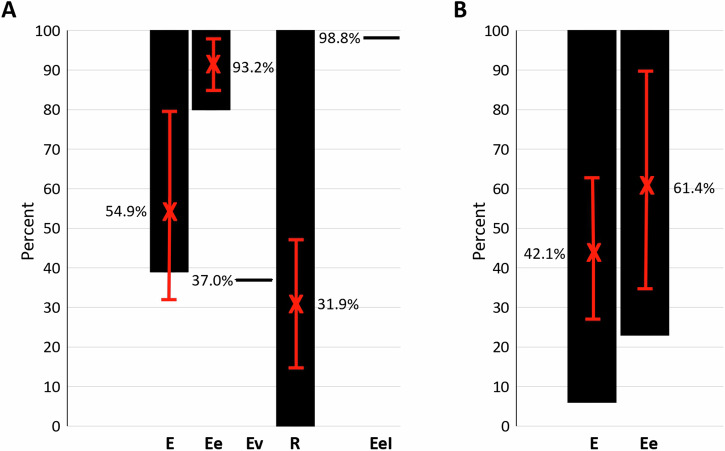
Total integrated vaccine efficacy/effectiveness (E/Ee) was estimated as EeI = 98.8% (55.5±27.2; CI, 28.26–82.74) for all locations with average E = 59.7%, Ee = 93.2%, Ev = 37.0%, and R = 31.9% (Fig. 6A).
Fig. 6. Summary of vaccine efficacy/effectiveness (E/Ee).
A Range, average, and SD are shown for vaccine efficacy E, vaccine effectiveness Ee, total (larvae, nymphs, and adults) tick counts on animals infested with various tick species (Ev), reduction in the number of infested cattle (R), and total integrated vaccine efficacy/effectiveness (EeI) at 167-196 dpv. B Range, average, and SD are shown for vaccine efficacy (E) and vaccine effectiveness (Ee) at 251-327 dpv with data collected in Mbarara 2 (n = 9–11) and Maruzi (n = 12–13). Source data are provided as a Source Data file.
The analysis between vaccine efficacy E, effectiveness Ee, Ev and R and location-related factors was conducted and the only identified correlation was in Maruzi between vaccination schedule and thus dpv at data collection (167 dpv compared to 182-196 in Mbarara) and a tendency towards lower vaccine efficacy E, effectiveness Ee and R. To verify this effect and provide additional support for vaccine efficacy E, effectiveness Ee, tick data was collected at 327 dpv (147 days after the third vaccine dose) and 251 dpv (71 days after the third vaccine dose) in Mbarara 2 and Maruzi, respectively. The results showed efficacy E of 18% and 6% for R. decoloratus and R. appendiculatus, respectively in Mbarara 2 and efficacy E of 38%, 100%, and 48% for R. decoloratus, R. appendiculatus and A. variegatum, respectively in Maruzi. Vaccine effectiveness Ee was 23% in Mbarara 2 and 100% in Maruzi with average for both locations of E = 42.1±36.4% (42.1±15.9; CI, 26.1 – 58.0) and Ee = 61.4±54.7% (61.4±19.6; CI, 41.8–81.0) (Fig. 6B). These results support the absence of location-related factors affecting vaccine efficacy E and effectiveness Ee with data collected after 182 dpv.
To provide additional information, surveillance of tick infestations in Mbarara and Maruzi was continued for 367 and 251 days, respectively without use of acaricides. The results showed a reduction in tick infestations in both SUB-vaccinated and control cattle (Supplementary Fig. 1). These results suggest that vaccination with SUB reduces tick populations in the location and also affecting non-vaccinated coexisting animals. Differences in the dynamics of tick infestations are probably associated with the prevalence of tick species in Mbarara (predominant R. decoloratus) and Maruzi (concomitant R. appendiculatus, R. decoloratus and A. variegatum).
Vaccine safety
During the trial, effects of treatments negatively affecting cattle wellbeing or associated with vaccine safety were not recorded. Cattle did not show local reactions in response to treatments and without records of irregular body temperature and condition, respiration, local reactions-skin coat, feeding, movements, demeanor, or mortality (Supplementary Table 1). Significant alterations were not recorded and without differences between treatments (p > 0.05) in evaluated parameters for nine (Mbarara), eight (Isimba), five (Maruzi and Nabuin), and seven (Kiburara) months after the first treatment (Supplementary Table 1). Cattle body temperature ranged 38.4–38.9 °C and 38.5–38.9 °C (Mbarara) and 38.5–38.8 °C and 38.4-38.9 °C (Nabuin) for SUB vaccinated and control groups, respectively. In Isimba, Maruzi and Kiburara, body temperature ranged 38.7–39.1 °C, 38.5–38.8 °C, and 38.9–39.2 °C, respectively for both SUB vaccinated and control groups. Body condition, feeding, locomotion and respiration were normal for both treatments. Local reactions were not recorded in response to treatments with smooth even/regular hair pattern and kempt. Demeanor was not altered, and all animals were actively involved in all activities such as feeding. Mortality was not recorded. To provide additional information, fecal samples were collected in Nabuin and evaluated with normal consistency in both groups.
Blood samples were collected from SUB-vaccinated and control cattle at Mbarara, Isimba, Maruzi, Kiburara and Nabuin for hematology assessment of different blood and serum parameters associated with animal health (Supplementary Data 3 and Tables 2–5). Differences between animals, locations, number of animals/group, and data availability suggested an analysis that was conducted for all locations throughout the trial.
Hematological analysis was conducted with parameters of blood cells and hemoglobin (Hematology 1; Supplementary Data 3) and blood biomarkers (Hematology 2; Supplementary Data 3). Values for hemoglobin, hematocrits, mean cell volume (MCV), mean cell hemoglobin, and reticulocytes were outside reference values and possibly associated with anemia. Other parameters such as platelets, basophils (basophilia), and eosinophils (eosinopenia) were altered in some locations and related to infection. However, most parameters did not show significant differences between groups and thus were not associated with vaccination. Nevertheless, the vaccine showed a protective capacity against anemia and infection risks. For example, in Nabuin, MCV values were bellow standard (79 fl) but higher in vaccinated (48.8±3.6 fl) than in control animals (45.6±4.0 fl; p = 0.02) while reticulocytes were greater than standard (11-16%) but lower in vaccinated (19.5±3.1%) than in control cattle (21.6±2.3; p = 0.03). Lymphocyte counts were within the normal range (1.6-11 109/l) but lower in Isimba control animals (5.0±1.9 vs. 6.5±2.0; p = 0.03) with a higher risk of infection.
For blood biomarker, the results showed consistent differences at 60 dpv between SUB vaccine and control treatments with higher levels in multiple blood biomarkers in control cattle associated with lipids (cholesterol, high-density lipoprotein cholesterol, triglycerides), liver function (e.g., bilirubin, gamma-glutamyl transferase, alanine aminotransferase, aspartate aminotransferase) and kidney function (urea nitrogen, creatinine) (Supplementary Table 5). Nevertheless, biomarker hematological levels were within normal range, except for total protein level and albumin which were below reference levels in both groups, and creatinine which was above reference in both groups (Supplementary Table 5). These results support that vaccination with the SUB did not affect organ function or cattle health.
Antibody response to vaccination
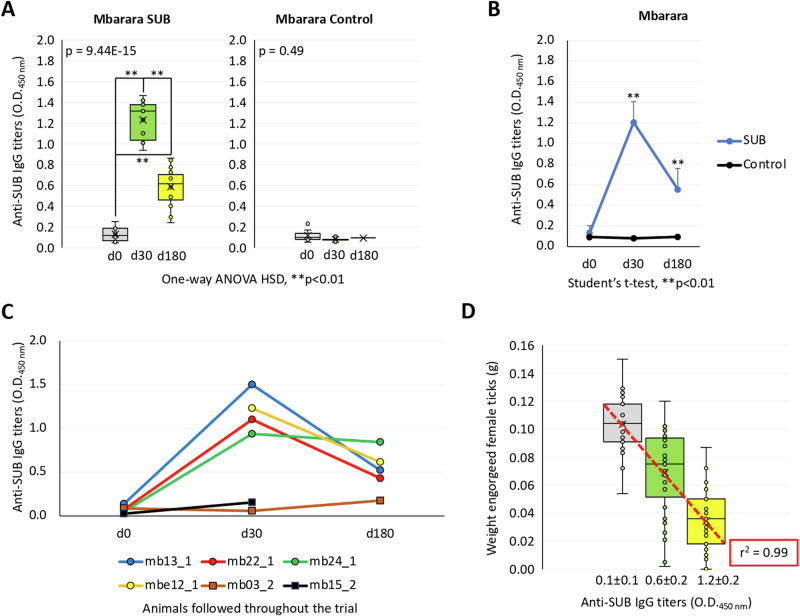
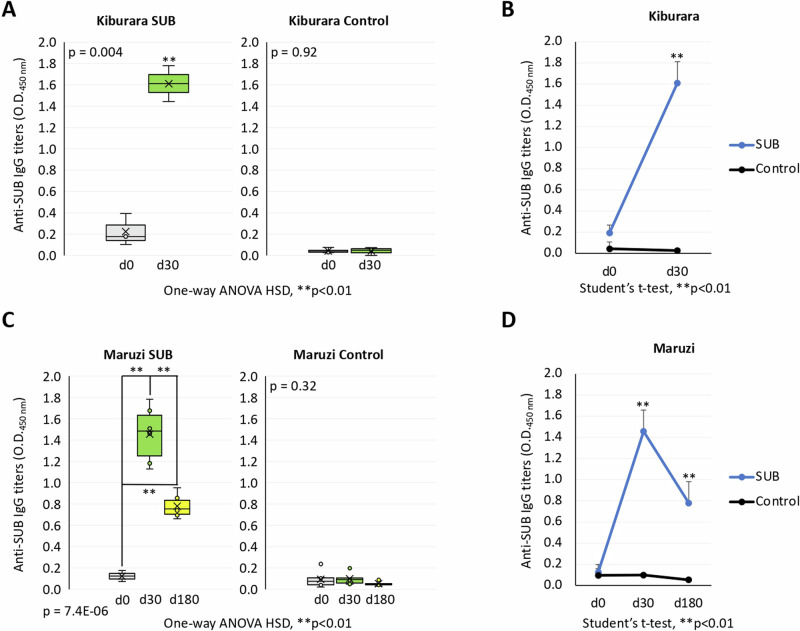
The analysis of anti-SUB IgG antibody titers was conducted at Mbarara, Kiburara, and Maruzi (Figs. 7A–D and 8A–D, Supplementary Data 4). Anti-SUB antibody titers were significantly higher (p < 0.01) in vaccinated animals when compared to controls in Mbarara (Fig. 7A), Kiburara (Fig. 8A) and Maruzi (Fig. 8C). Anti-SUB IgG levels were significantly higher (p < 0.01) in vaccinated cattle at 30 and 180 dpv but not at 0 dpv (Figs. 7B and 8B, D). At the individual level in animals followed throughout the trial, anti-SUB antibody titers increased from zero dpv to 30 dpv and decreased at 180 dpv but were always higher than in PBS-treated control animals (Fig. 7C). These results showed a positive correlation between anti-SUB IgG titers and reduction in Rhipicephalus spp. tick weight (r = 0.99) (Fig. 7D).
Fig. 7. Analysis of anti-SUB antibody titers in cattle in Mbarara.
Results were compared A along the trial for average values for each treatment by One-way ANOVA with post-hoc HSD test (**p < 0.01; n = 8–15 animals per group) and B between treatments at each time point by Student’s t-test with unequal variance (**p < 0.01; n = 8-15 animals per group). C Anti-SUB antibody titers at the individual level in Mbarara animals followed throughout the trial at zero, 30 and 180 dpv in vaccinated (animals mb13_1, mb22_1, mb24_1, and mb12.1) and control (animals mb03_2, and mb15_2) groups. D Correlation between anti-SUB IgG titers and decrease in Rhipicephalus spp. tick weight in Mbarara. Error bars represent standard deviation. Source data are provided as a Source Data file.
Fig. 8. Analysis of anti-SUB antibody titers in cattle in Kiburara and Maruzi.
Results were compared A, C along the trial for average values for each treatment by One-way ANOVA with post-hoc HSD test (**p < 0.01; n = 3 animals per group) and B, D between treatments at each time point by Student’s t-test with unequal variance (**p < 0.01; n = 3-6 animals per group). Error bars represent standard deviation. Source data are provided as a Source Data file.
Tick-borne pathogens in cattle
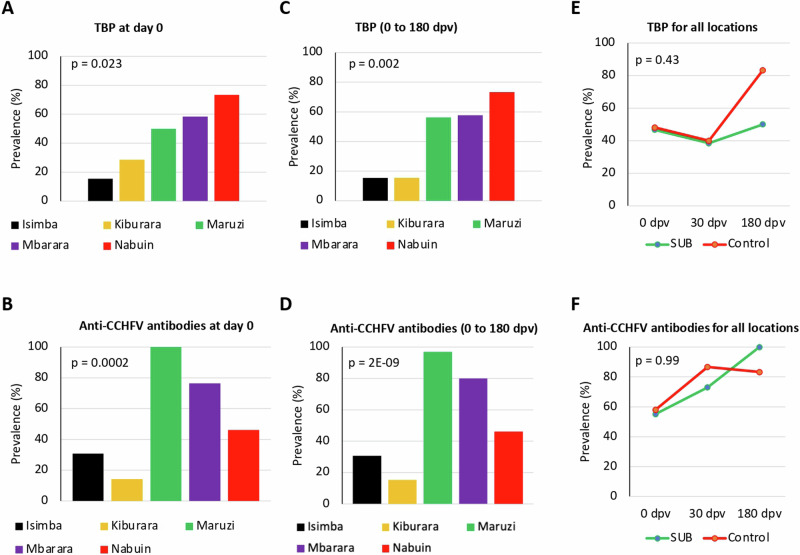
A preliminary analysis of tick-borne pathogens was conducted with a limited number of animals (n = 12-57; 99 total) at different locations and time (zero, 30, 180 dpv) in cattle blood samples using PCR for Anaplasma, Rickettsia, Ehrlichia and piroplasmids and serum antibodies against CCHFV (Supplementary Data 4 and Fig. 9A–F). Of the 99 cattle tested, 48 (48.5%, 95% CI: 38.6 – 58.3) were positive for at least one tick-borne pathogen. The prevalence was higher for piroplasmid infection (39/99, 39.4%; CI, 29.8 – 49.0), followed by Anaplasma spp. (25/99, 25.3%; CI, 16.7–33.8), Rickettsia spp. (0/99, 0.0%) and Ehrlichia spp. (0/99, 0.0%). Sixteen of the 48 positive animals were co-infected with Anaplasma spp. and piroplasmids. Identified pathogens at species level included Anaplasma marginale (PP101631), A. centrale (PP101632), Theileria parva (PP091624, PP091625), and T. mutants (PP091626). The results showed significant differences between different locations in pathogens DNA/seroprevalence before vaccination (day zero; p < 0.05) and throughout the trial (zero to 180 dpv; p < 0.01) (Fig. 9A–D). However, significant differences were not observed between SUB-vaccinated and control groups (p > 0.05). Nevertheless, a non-significant tendency was observed toward a decrease in pathogen DNA prevalence in response to vaccination for all locations at 180 dpv (Fig. 9E). As expected, seroprevalence for CCHF did not vary between groups (Fig. 9F).
Fig. 9. Prevalence of tick-borne pathogens in cattle.
A–D DNA prevalence for Anaplasma, Rickettsia, Ehrlichia, and piroplasmids was evaluated by PCR, and seroprevalence of anti-CCHFV antibodies was evaluated by ELISA at different locations and sample times (zero, 30, 180 dpv). Results were compared between different locations at zero dpv by Fisher’s exact test (p < 0.05; n = 7–17 animals per location) and at zero to 180 dpv by Pearson’s chi-squared test (p < 0.05; n = 13-32 animals per location). E–F Prevalence of TBP and anti-CCHFV antibodies for all locations were compared at different sample times (0–180 dpv) by One-way ANOVA with post-hoc HSD test (p < 0.05; n = 6–32 animals per group). Source data are provided as a Source Data file.
Discussion
Gavac with tick midgut proteins Bm86/Bm95 is the only commercially available vaccine for the control of cattle tick infestations in some Latin-American countries13,21. Results with Gavac have shown vaccine efficacy E between 28% and 100% against R. microplus, R. annulatus, R. decoloratus, Hyalomma dromedarii and H. anatolicum13. However, Gavac vaccine has not been effective (E = 0) against other tick species such as R. appendiculatus and Amblyomma variegatum13. Furthermore, differences in vaccine efficacy E between different locations for the same tick species are probably associated with genetic diversity of tick strains22.
These results highlight the importance of developing vaccines with a personalized vaccine antigen approach focused on regional tick species and genotypes as shown in our study17. Additionally, the experience with Gavac highlighted the importance of government organizations to be involved in vaccine implementation policies and technical support for including vaccines in integrated programs for the control of tick infestations21,23,24.
Subolesin proteins are involved in the regulation of multiple cellular processes15. Subolesin-based vaccine efficacy E against multiple tick species has been reported between 37% and 94%6,17. Under field conditions, a SUB-based vaccine showed an effect on the reduction of tick infestations, number of infested animals, female tick weight and the prevalence of tick-borne pathogens16. Herein, the results of vaccine efficacy E were similar between trials previously conducted under pen-controlled conditions (E = 47–90%)17 and the field trial in our study (E = 39–100%; 59.7±32.8% at 95% CI). The only exception was for R. appendiculatus in Maruzi at 167 dpv. Regarding vaccine effectiveness Ee, as previously reported16, vaccination with SUB reduced for some locations and tick species the female tick weight and the number of infested cattle. Furthermore, vaccine efficacy E on the total (larvae, nymphs and adults) number of ticks (Ev) on animals infested with A. variegatum, R. appendiculatus and R. decoloratus in Maruzi resulted in 37% efficacy, thus supporting a cross-species tick protection with the R. appendiculatus SUB vaccine antigen under natural field conditions.
Differences in vaccine efficacy E and effectiveness Ee were not related to tick species but to other factors associated with locations (higher efficacy E and effectiveness Ee in Mbarara 1 and 2 than in Maruzi) and vaccination schedule and thus dpv at data collection (167 dpv in Maruzi compared to 182-196 in Mbarara). Differences related to location-related factors affecting efficacy E and effectiveness Ee were not present after 182 dpv, thus supporting vaccine efficacy and effectiveness at different locations. Nevertheless, in Mbarara 2 a higher E was obtained for R. appendiculatus than for R. decoloratus, which may correlate with the use of R. appendiculatus cross-protective SUB in the vaccine formulation. Accordingly, the effect of the vaccine on female tick weight associated with tick fitness (17–89% reduction) and a number of infested cattle (66-67%) were also higher for R. appendiculatus.
The most relevant results are associated with vaccine effectiveness Ee evaluated under natural field conditions. The results showed Ee = 93.2±6.6% at 167–196 dpv and Ee = 42.1±15.9% at 251–327 dpv with 95% CI. Additionally, integrated vaccine efficacy/effectiveness E/Ee was estimated for all locations as EeI = 98.8% (55.5±27.2% with 95% CI). Regarding safety analysis, the SUB vaccine was safe and showed a protective capacity against risks of anemia and pathogen infection.
As shown for Gavac25, the correlation between anti-SUB IgG antibody titers and reduction in tick weight provided additional support for the cause-effect relationship between vaccination and the reduction of tick fitness and infestations. Furthermore, using results from this study, anti-SUB IgG antibody titers higher than 0.4 O.D.450 nm reduced tick fitness with vaccine efficacy E higher than 39% and effectiveness Ee between 80%–100% at 167-196 dpv in different locations. As in previous experiments with Gavac10,25, antibody levels should increase after revaccination at day 180 and stay above protective levels for one year, when the booster dose should be administered. Accordingly, it is important to test anti-SUB antibody titers if cattle holders raise concerns about vaccine effectiveness.
Adjuvants play a key role in vaccine formulations, even when using effective antigens. In this study, Montanide ISA 50 V2 (Seppic, France) was used for water in oil (W/O) emulsion vaccine formulation. Montanide ISA 61 VG is the most common adjuvant in vaccine formulations for cattle (e.g.,26). However, Montanide ISA 50 V2 adjuvant has been previously used in commercial Gavac anti-tick vaccine27, and in a SUB-based vaccine formulations with efficacy against tick infestations in cattle and sheep16,17. The W/O emulsion formulations are recommended for cattle vaccines with induction of long-term immunity and higher cost-effectiveness due to reduction of the vaccine dose or antigen concentration28,29. Accordingly, SUB vaccine showed an effect on tick control in cattle up to 367 days after vaccination in Mbarara.
Under One Health perspective, cattle impact on TBD risk to humans and animals30. As previously reported15,16, preliminary results on tick-borne pathogens suggested a trend in reducing infection prevalence in response to SUB vaccine. A follow-up over time will provide conclusive information on the vaccine effect on TBD25. The absence of acaricide applications after the beginning of the trial supports the effect of the vaccine in reducing the use of these compounds and the associated health risks. Nevertheless, a rational combination between acaracides of chemical or natural origin and vaccination may be applied as part of integrated tick management approaches to reduce tick infestations together with acaricide use16,31.
The conclusions of the study support that the SUB-based vaccine designed with genetic information from regional tick strains is protective against cattle tick infestations under field conditions at different Ugandan locations. Vaccination with SUB was safe with a protective capacity against anemia and infection, and reduced the number of infested cattle, tick feeding, and reproduction with vaccine effectiveness against multiple tick species infesting the same host with an average Ee of 93.2% at 167-196 dpv and 61.4% at 251-327 dpv. Total integrated vaccine EeI was estimated as 98.8%. The SUB vaccine was administered with three doses in prime immunization with vaccine effectiveness Ee higher than 60% at 251-327 dpv, thus supporting the application of booster dose yearly at 12 months after first vaccination. Based on data with TickGARD and Gavac23–25,32, the results of the field trial at 251-367 dpv (Ee = 61.4±19.6% at 251-327 dpv) predict a reduction in tick infestations to less than one tick/animal after 4 years of vaccination together with a reduction in acaricide applications. These results support the registration and commercialization of the evaluated SUB vaccine formulation to reduce tick populations and associated risks for humans and animals of TBD and chemical acaracide applications under conditions in Uganda and probably in other countries of Sub-Saharan Africa33.
Methods
Authorization of the tick vaccine field trial
The clinical vaccine field trial was authorized by the National Biosafety Committee (NBC 01/2022) of Uganda National Council for Science and Technology (UNCST-A191ES), and National Drug Authority (NDA - VTC 01/2022).
Selection of trial sites and experimental animals
The selection of trial sites was undertaken in compliance with NDA guidelines on the conduct of ectoparasiticide field trials in 2017 and National Guidelines for Use of Animals in Research and Teaching 2021. The guidelines provide as implemented in this field trial that (a) trial sites may be located in at least two agro-ecological zones to cater for tick diversity and trial product stability under the different climatic conditions and the choice of five sites was done to care of the thermolability of vaccine, (b) inclusion of the different cattle breeds commonly reared in Uganda in their traditional localities, notably Shorthorn Zebu, Boran, Ankole longhorn, and Friesian crosses, (c) use of government-owned cattle farms by National Agricultural Research Organization (NARO) and Minister of Internal Affairs (UPS) to permit the availability of trial cattle up to the end of the study period and qualified veterinary personnel employed by the government. The field trial was designed at different Ugandan locations in collaboration between NaLIRRI/NARO (Uganda) and SaBio/IREC (Spain) (Fig. 1). Locations included Mbarara ZARDI (Mbarara 1 and 2 are located on the same farm Mbarara ZARDI but in Mbarara 1 cattle was confined in grazing paddocks while Mbarara 2 is not fully confined and thus cattle were allowed to access pasture and water resources beyond trial paddocks and those only some analyses were conducted separately for Mbarara 1 and 2), Ugandan government prison (UPS) farms Kiburara and Isimba, NaLIRRI Maruzi and Nabuin ZARDI (Fig. 1). Experimental cattle were selected using the following inclusion (healthy cattle with more than two-years-old and including both sexes) and exclusion (sick/unhealthy cattle or planned for disposal sales before the trial ends) criteria. The trial cattle confinement was undertaken in compliance with the National Guidelines for Confinement for Regulation of Research with Genetically Modified Organisms and Microbes (2007). Cattle management includes confined paddock with supervised relaxation for higher exposure to ticks (Mbarara ZARDI, Maruzi NaLIRRI, Nabuin ZARDI) or strictly in paddock that mimics intensive management (UPS Kiburara, UPS Isimba).
Recombinant SUB production and vaccine treatment formulations
The Rhipicephalus appendiculatus SUB (MT241515; https://www.ncbi.nlm.nih.gov/nuccore/MT241515), selected based on the highest tick cross-species protection in Bos indicus and crossbred cattle in Uganda, was manufactured under Good Laboratory Practice (GLP) conditions at SaBio, IREC, Spain following the previously described protocol17. Vaccine treatments with SUB recombinant protein or PBS control were formulated in Montanide ISA 50 V2 (Seppic, Paris, France) in a stable water in oil (W/O) emulsion at a concentration of 50 μg SUB per ml and stored at 4 °C.
Experimental design
Treatments produced under GLP conditions were coded as Treatment 1 and Treatment 2 at SaBio/IREC. Treatment, sampling, and processing were conducted in a randomized double-blind multi-site field trial by NaLIRRI/NARO in collaboration with personnel at cattle farms. Sample and data analysis were conducted for comparison between treatments by SaBio/IREC and NaLIRRI/NARO. Then, at the completion of the trial, treatments were uncoded by SaBio/IREC to complete comparative analysis between SUB vaccine (Treatment 1) and control (Treatment 2) efficacy, effectiveness, and safety.
For treatment, cattle were injected intramuscularly in the neck muscles with 2 ml vaccine (100 μg SUB per dose) or PBS control with 3 doses on days zero, 30±1 and 181±1 at 27/10/2022, 30/11/2022, 30/04/2023 (Mbarara), 9/11/2022, 10/12/2022, 10/05/2023 (Kiburara), 10/11/2022, 11/12/2022, 12/05/2023 (Isimba), 15/03/2023, 17/04/2023, 18/09/2023 (Maruzi), and 16/03/2023, 18/04/2023, 20/09/2023 (Nabuin).
At different days post-vaccination (dpv; administration of the first dose at zero dpv), tick data (32, 65, 93, 124, 153, 182 dpv at Mbarara 1; 31, 99, 131, 196 dpv at Mbarara 2; 35, 63, 73, 167 dpv at Maruzi), blood samples (zero, 30, 180 dpv at Mbarara, Kiburara, and Maruzi) and vaccine safety associated with cattle wellbeing records (zero, two, four, 13, 28, 30 dpv followed by every two weeks for nine months post-vaccination (mpv) at Mbarara 1 and 2; six days prior treatment, two, four, 18, 33, 35 dpv followed by every two weeks for eight mpv at Isimba; 41, 90, 92 dpv followed by every two weeks for five mpv at Maruzi; six days prior treatment, two, four, 19, 33, 35 dpv followed by every two weeks for seven mpv at Kiburara; 49, 81, 83 dpv followed by every two weeks for five mpv at Nabuin) were collected for analysis. Additionally, tick data was collected at 327 dpv and 251 dpv at Mbarara 2 and Maruzi, respectively. Samples and data were always collected before vaccination when treatments were applied to cattle (Supplementary Data 1-3).
Acaricide use was stopped at 0 dpv and before the beginning of the trial included Duodip (Chlorpyrifos 500 g/l and cypermethrin 50 g/l) twice a week except for Nabuin ZARDI where it was applied only once a week. The incidence of TBD included between 40 (Maruzi and Nabuin) and 50 (Mbarara, Kiburara and Isimba) cases per year of TBD. The reported TBD included East Coast fever (ECF, Theileria parva), babesiosis (Babesia bigemina and Babesia bovis), anaplasmosis (Anaplasma marginale and Anaplasma centrale), heartwater (also known as cowdriosis; Ehrlichia ruminantium, formerly Cowdria ruminantium), and CCHF (CCHFV34). Cattle in contact with wildlife only occurred at Mbarara and Kiburara with buffalo.
Tick infestations
Tick species with highest infestations and prevalence were analyzed in the study according to locations and included one-host tick species, Rhipicephalus decoloratus, two-host tick species, Rhipicephalus evertsi, and three-host tick species, R. appendiculatus and Amblyomma variegatum. As previously described17, adult engorged ticks were collected, counted, weighed individually and incubated for oviposition. The eggs mass per female tick was weighed and incubated for hatching. The recovered larvae per egg batch were weighed.
Collection of cattle blood samples for serum and DNA extraction
Blood samples were collected from individual animals at different locations. From these samples, 400 µl were added with a micropipette to a labeled filter paper (Whatman grade 4 filter paper; Whatman, Maidstone, UK) and let it dry for 10 h. One filter was prepared for each serum and DNA extraction. For serum extraction, filter papers were folded into a 1.5 ml Eppendorf tube (Merck KGaA, Darmstadt, Germany) with 400 µl of sterile 1X phosphate-buffered saline (PBS) and incubated at 4 °C for 12 h. Then, tubes were agitated to cut out the bottom and put it on an empty capless blood collection tube (Merck KGaA) for centrifugation at 1500 × g for 10 min to collect serum at the bottom of the tube. For DNA extraction, phenol-chloroform protocol using Tri Reagent (Sigma-Aldrich, Burlington, MA, USA) was used according to the manufacturer’s instructions. Briefly, a one quarter of each filter paper was cut and transferred into a 1.5 ml tube containing 250 µl Tri Reagent solution. The filter papers were soaked and incubated for 10 min at room temperature (RT). Then, the tip of the tubes was cut-off and placed into 5 ml collection tubes to centrifuge at 750 × g for one min. Filter papers were discarded and the flow-through with blood and Tri Reagent solution was used for DNA extraction. The concentration, quality, and purity of DNA were checked using a spectrophotometer (NanoDrop One, Thermo Scientific, Waltham, MA, USA) and then stored at –80 °C until analysis.
Characterization of tick-borne pathogens in cattle blood DNA and serum samples
Conventional PCR assays with forward (F) and reverse (R) primers (5´- 3´) were used for the detection of tick-borne Anaplasma spp. RpoB 16S rRNA gene (RpoB 16SF (F):
GCTGTTCCTAGGCTYTCTTACGCGA, RpoB 16SR (R):
AATCRAGCCAVGAGCCCCTRTAWGG), Rickettsia spp. 16S rRNA gene (FD1 (F):
AGAGTTTGATCCTGGCTCAG, Rc16 (R):
AACGTCATTATCTTCCTTGC), Ehrlichia spp. 16S rRNA gene (EHR16SF (F):
GGTACCYACAGAAGAAGTCC, EHR16SR (R):
TAGCACTCATCGTTTACAGC), and piroplasmids 18S rRNA gene (PIRO A (F):
AATACCCAATCCTGACACAGGG, PIRO B (R):
TTAAATACGAATGCCCCCAAC)35–38 in cattle blood samples from 99 animals that were collected at 0 dpv (n = 57), 30 dpv (n = 41) and 180 dpv (n = 12) (Supplementary Table 6). Of them, 88 cattle were sampled once and 11 were longitudinally surveyed at d0 and d30. The reaction volume was 25 μl, including 12.5 μl of PCR Master Mix (Promega Corporation, Madison, WI, USA), one μl of each primer at 10 μM, one μl of DNA and 9.5 μl of nuclease-free water. The PCR products were visualized through electrophoresis in 1.5% agarose gels using GelRed® Nucleic Acid Gel Stain (Biotium, Fremont, CA, USA). A selection of the positive samples was sequenced by Sanger sequencing (Secugen S.L., Madrid, Spain). Sequences were compared with those available in GenBank by using a Basic Local Alignment Search Tool (BLAST) search (http://www.ncbi.nlm.nih.gov/blast). Sequences of pathogens identified at the species level were submitted to GenBank (https://www.ncbi.nlm.nih.gov/genbank/; Supplementary Data 4).
The detection of antibodies against Crimean-Congo haemorrhagic fever virus (CCHFV) was performed using the IDScreen CCHF Double Antigen Multispecies commercial ELISA kit (IDVet, Grabels, France) in cattle serum samples from 101 animals. Samples were collected at zero dpv (n = 60), 30 dpv (n = 41) and 180 dpv (n = 12), including 89 cattle sampled once and 12 cattle longitudinally surveyed at d0 and d30. The test was performed according to the manufacturer’s instructions. Briefly, 30 μl of each serum sample, and positive and negative controls were diluted with 50 μl of the kit diluent and incubated for 45 min at 25 °C. After a washing procedure, 50 μl of conjugate was added to each well, followed by an incubation for 30 min at 25 °C. A second washing procedure was performed and 100 μl of substrate solution was added to each well. After incubation for 15 min in the dark at 25 °C, the reaction was stopped with the provided stop solution. Using a SmartSpecTM Plus spectrophotometer (Bio-Rad Laboratories Inc., Hercules, CA, USA), the optical density of each well was measured at 450 nm. Determination of cut-off for sero-positive and sero-negative samples for CCHFV was performed according to the kit criteria.
Infection prevalence was estimated from the proportion of positive samples to the total number of samples tested. Bivariate associations between the presence of tick-borne pathogens and explanatory variables (location and group) were analyzed using the Pearson’s chi-squared test or Fisher’s exact test, as appropriate. Additionally, the McNemar’s test was used to investigate the association between PCR positivity and sampling time for animals that were longitudinally surveyed. Analyses were performed using R software version 4.1.339 and differences were significant with p < 0.05 for a double‐sided test. Confidence interval (CI) at 95% confidence level (Z-value = 1.96) was calculated as CI = Average ± Z x standard deviation (SD)/√n using the Confidence Interval Calculator (Calculator.net, https://www.calculator.net/confidence-interval-calculator.html).
Vaccine efficacy E
Allen and Humphreys8 proposed the analysis of variables associated with tick life cycle using vaccine formulations with midgut protein extracts. This approach was later revised and applied for vaccines with recombinant antigens for the control of tick infestations under experimental and natural conditions9,10.
Currently, vaccine efficacy E for the control of tick species in pen trials under controlled conditions is based on the effect in the reduction of tick infestations, oviposition, and fertility as E (%) = 100 [l - (CRT x CRO x CRF)], where CRT, CRO, and CRF are the reduction in the number of adult female ticks, oviposition and egg fertility compared with the control group40. The formula was then adapted to three-host tick species as E (%) = 100 [1 - (RL x VL x RN x VN x CRT x CRO x CRF), where RL and VL are the reduction in engorged and molting of tick larvae and RN and VN are the reduction in engorged and molting of tick nymphs41,42.
Accordingly, the final calculation of vaccine efficacy E considers available information on tick immature stages as:
E (%) = 100 x [1 – (TV/TC x OV/OC x FV/FC x LV/LC x NV/NC)], where TV and TC are female ticks from vaccinated and control groups, respectively, OV and OC are egg weight from ticks in vaccinated and control groups, respectively, FV and FC are fertility (percent of egg hatching and producing larvae) from ticks in vaccinated and control groups, respectively, LV and LC are engorged larvae from vaccinated and control groups, respectively, and NV and NC are engorged nymphs from vaccinated and control groups, respectively.
Vaccine effectiveness Ee
Vaccine effectiveness Ee is the evaluation of efficacy under field conditions considering locations with different characteristics and infestations by multiple tick species41,42. This information is essential for the approval of vaccine formulations for registration and commercialization to improve livestock health and production.
For the evaluation of vaccine effectiveness Ee against multiple tick species infesting the same host, the combined effect was calculated as:
Ee (%) = 100 x [1 – (TVCsp1 x TVCsp2 x TVCspN)], where (TVC)sp = TV/TC x OV/OC x FV/FC x LV/LC x NV/NC for each tick species sp1, sp2, … spN.
Additional considerations for evaluation of effectiveness Ee include:
Fed female tick weight as a marker of tick fitness, Ew (%) = 100 x (1- WV/WC), where WV and WC are mean female tick weight from the vaccinated and control groups, respectively.
Total (larvae, nymphs and adults) tick counts for all tick species infesting the same host, Ev (%) = 100 x [1 – (TV/TC x LV/LC x NV/NC)].
Reduction in the number of infested cattle (R) after comparison of vaccinated and control animals at around 6 months after first vaccine dose administration.
Total integrated vaccine efficacy/effectiveness E/Ee
The average for all locations of vaccine efficacy E, effectiveness Ee, Ev, and R was used for the estimation of total integrated vaccine efficacy/effectiveness (EeI) as EeI (%) = 100 x [1 - [(1 - (E/100)) x (1 - (Ee/100)) x (1 - (Ev/100)) x (1 - (R/100))]]43,44.
Data analysis for tick infestations and vaccine efficacy and effectiveness
The confidence interval was calculated as described above for tick-borne pathogens. As previously described for SUB vaccine field trial16, tick infestations (female ticks/animal) were compared between vaccinated and control groups throughout the trial using a One-way ANOVA with post-hoc Tukey Honestly Significant Difference HSD test (https://astatsa.com/OneWay_Anova_with_TukeyHSD/) (p = 0.05). The number of female ticks per animal at one and around 6 months after vaccination was compared between vaccinated and control groups by Student’s t-test with unequal variance (p = 0.05).
Vaccine safety
During the trial, cattle were maintained with freedom to roam around and access to water and feed. Vaccine safety and cattle wellbeing were evaluated by recording in response to treatments (n = 36-37 animals/treatment at dates disclosed in the experimental design; Supplementary Data 3) (a) local reactions/skin coat (score 10-smooth even/regular hair pattern and kempt, 20-rough shaggy irregular hair pattern; score 10 is considered normal), (b) body temperature (normal, 38.5–39.0 °C), (c) body condition (score 1-extremely thin, 2-thin, 3-moderate, 4-fat, 5-obese; scores 2-3 are considered normal), (d) respiration/breeding (score 10-normal, 20-not normal/labored), (e) feeding (score 10-normal grazing consistently, 20-limited when animal takes long breaks not feeding while lying down or standing, 30-abnormaly increased intake), (f) locomotion/movement (score 0-normal even walking, 1-slightly lame uneven walking, 2-lame with arched back and head bob when walking, 3-severely lame with great difficulty when walking), (g) demeanor (score 0-dull when animal generally not feeding and lays down with limited response to stimuli, 1-active when animal generally involves actively in all activities such as feeding, 2-retless/hyper when animal makes frequent movements and charging; score 1 is considered normal), and (h) mortality. In Nabuin, fecal samples were collected and evaluated (score 1-normal consistence, 2-diarrhoea, 3-hard). Additionally, blood samples were collected at 0, 30, 60 and 90 dpv from cattle at different locations (Mbarara, Isimba, Kiburara, Nabuin, Maruzi) and submitted to Lancet Laboratories Uganda Limited (Nakasero Hill Lab, Plot 1 Kyadondo Rd., Kampala, Uganda) for hematology analysis in blood using a chemical analyzer (COBAS Integra 400; Roche Holding AG, Basel, Switzerland) and serum (Hitachi 717 Chemistry Analyzer; Roche Holding AG). Blood and serum biomarkers included WBC, white blood cells; lymphocytes; RBC, red blood cells; hemoglobin; hematocrit; MCV, mean cell volume; MCH, mean cell hemoglobin; MCHC, mean cell hemoglobin concentration; RET/RDW, reticulocytes; platelets; neutrophils; basophils; eosinophils; monocytes; TBIL, total bilirubin; CBIL, conjugated bilirubin; ALP, alkaline phosphatase; GGT, gamma-glutamyl transferase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TP, total protein level; ALB, albumin; CHOL, cholesterol; LDLCHOL, low-density lipoprotein (LDL) cholesterol; HDLCHOL, high-density lipoprotein (HDL) cholesterol; NONHDL, non-HDL cholesterol; CHOL/HDL, CHOL to HDL ratio; TRIG, triglycerides; UREA, urea nitrogen; CREA, creatinine. References values were provided by Lancet Laboratories Uganda Limited. Results were compared between treatments at each time point by Student’s t-test with unequal variance (p = 0.05) and throughout the trial for each treatment by One-way ANOVA with post-hoc HSD test (https://astatsa.com/OneWay_Anova_with_TukeyHSD/) (p = 0.05).
Antibody titers and correlation with engorged tick weight
Anti-SUB IgG antibody titers were determined as previously described16 (Supplementary Data 4). The 96-well ELISA microplates (Merck KGaA) were coated with 0.1 μg/well SUB in carbonate/bicarbonate buffer and incubated overnight at 4 °C. Plates were washed with 100 μl/well of washing buffer (PBS, 0.05% Tween 20, pH 7.4), blocked for 1 h at RT with 100 μl/well of blocking buffer (PBS, 2.5% skim milk, pH 7.2), and washed for three times with 100 μl/well washing buffer. Then, 100 µl of bovine serum diluted 1:100 in blocking buffer was added to the wells and the plate was incubated at 37 °C for one h. Plates were washed as before and 100 μl/well of anti-bovine IgG-HRP conjugates (Merck KGaA) diluted 1:10000 in blocking buffer were added to the wells and incubated for 1 h at RT. Plates were washed again as before and 100 µl/well of 3,3′,5,5′-Tetramethylbenzidine (TMB; Abcam, Waltham, Boston, USA) were added and incubated in the dark for 15 min at RT. The reaction was inhibited with the addition of 50 μl H2SO4 3N and the absorbance was measured at 450 nm optical density (O.D.). For data analysis, average of control O.D. values (plate wells without SUB; n = 5) were subtracted from each sample and results compared for each timepoint (0, 30 and 180 pdv) between SUB-vaccinated and PBS-treated groups by Student´s t-test with unequal variance (p = 0.05) and between different timepoints by One-way ANOVA with post-hoc HSD test (https://astatsa.com/OneWay_Anova_with_TukeyHSD/) (p = 0.05). Correlation analysis between anti-SUB IgG antibody titers and engorged Rhipicephalus spp. tick weight was conducted as previously described6,25,31. A Pearson correlation coefficient (r) was calculated in Mbarara animals with data available at different dpv (https://www.socscistatistics.com/tests/pearson/).
Source data
Supplementary Data 1. Evaluation of tick life cycle.
Supplementary Data 2. Total tick counts.
Supplementary Data 3_Evaluation of vaccine safety.xlsx
Supplementary Data 4_Anti-SUB antibodies and prevalence of tick-borne pathogens.xlsx
Acknowledgements
We thank Yona Baguma (Director General NARO), Swidiq Mugerwa (Deputy Director General Research NARO), Halid Kirunda (Director Mbarara ZARDI), and William Nanyeenya (Acting Director of Research at NARO) and Justus Rutaisire coordinator vaccine research for their support to this initiative. We are grateful to Johnson Byabashaija the Commissioner General - Uganda Prisons Service for accepting to host part of the trials. We are grateful to National Drug Authority (NDA) and Uganda National Council for Science and Technology (UNCST) for their regulatory support towards this work. We thank members of our laboratories for their contribution and support to this work. This work was funded by Government of Uganda.
Author contributions
Conception and design: F.K., M.C., J.S., S.M., P.K., H.K., J.R., M.Matovu., M.Moses, M.D., G.N., J.B., C.G. and J.F. Acquisition of data (trial implementation, data collection, provided resources and/or facilities): M.C., R.F.-M., F.K., J.S., S.M., H.K., J.R., M.Matovu., M.Moses, I.K., M.D., G.N., J.B., P.B., A.N., P.O., N.S. and O.E.A. Data analysis: J.F., F.K., J.S., M.Matovu, M.Moses, I.K., M.D., J.B., M.S.-S., C.M.-H., G.F., I.G.F., M.R. and C.G. Manuscript preparation and revisions: J.F., F.K., J.R. and C.G. with inputs from all authors. Study supervision: F.K. and J.R.
Data availability
All data supporting the findings of this study are available within the paper and supplementary information. The source data underlying Figs. 2–9 and Supplementary Figure 1 are provided as a Source Data file.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Fredrick Kabi, Marinela Contreras.
Contributor Information
Fredrick Kabi, Email: freddykabi@gmail.com.
José de la Fuente, Email: jose_delafuente@yahoo.com.
Supplementary information
The online version contains supplementary material available at 10.1038/s41541-024-00966-1.
References
- 1.de la Fuente, J. et al. Perception of ticks and tick-borne diseases worldwide. Pathogens12, 1258 10.3390/pathogens12101258 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adesola, R. O., Warsame, A. A. A. & Idris, I. Current status of Crimean-Congo hemorrhagic fever outbreaks in Uganda and other African countries. Health Sci. Rep.6, e1383 10.1002/hsr2.1383 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Telford, C., Nyakarahuka, L., Waller, L., Kitron, U. & Shoemaker, T. Spatial prediction of Crimean Congo hemorrhagic fever virus seroprevalence among livestock in Uganda. One Health17, 100576 10.1016/j.onehlt.2023.100576 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nyakarahuka, L. et al. Seroepidemiological investigation of Crimean Congo hemorrhagic fever virus in livestock in Uganda, 2017. PLoS One18, e0288587 10.1371/journal.pone.0288587 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kasaija, P. D., Estrada-Peña, A., Contreras, M., Kirunda, H. & de la Fuente, J. Cattle ticks and tick-borne diseases: a review of Uganda’s situation. Ticks Tick. Borne Dis.12, 101756 10.1016/j.ttbdis.2021.101756 (2021). [DOI] [PubMed] [Google Scholar]
- 6.de la Fuente, J. & Contreras, M. Tick vaccines: current status and future directions. Expert Rev. Vaccines14, 1367–1376 10.1586/14760584.2015.1076339 (2015). [DOI] [PubMed] [Google Scholar]
- 7.Kasaija, P. D. et al. Inspiring anti-tick vaccine research, development and deployment in tropical Africa for the control of cattle ticks: review and insights. Vaccines11, 99 10.3390/vaccines11010099 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen, J. R. & Humphreys, S. J. Immunisation of guinea pigs and cattle against ticks. Nature280, 491–493 10.1038/280491a0 (1979). [DOI] [PubMed] [Google Scholar]
- 9.Willadsen, P. et al. Immunologic control of a parasitic arthropod. Identification of a protective antigen from Boophilus microplus. J. Immunol.143, 1346–1351 (1989). [PubMed] [Google Scholar]
- 10.Rodríguez, M. et al. Effect of vaccination with a recombinant Bm86 antigen preparation on natural infestations of Boophilus microplus in grazing dairy and beef pure and cross-bred cattle in Brazil. Vaccine13, 1804–1808 10.1016/0264-410x(95)00119-l (1995). [DOI] [PubMed] [Google Scholar]
- 11.Poland, G. A. Pharmacology, vaccinomics, and the second golden age of vaccinology. Clin. Pharmacol. Ther.82, 623–626 10.1038/sj.clpt.6100379 (2007). [DOI] [PubMed] [Google Scholar]
- 12.de la Fuente, J. & Contreras, M. Vaccinomics: a future avenue for vaccine development against emerging pathogens. Expert Rev. Vaccines20, 1561–1569 10.1080/14760584.2021.1987222 (2021). [DOI] [PubMed] [Google Scholar]
- 13.de la Fuente, J. & Kocan, K. M. Advances in the identification and characterization of protective antigens for recombinant vaccines against tick infestations. Expert Rev. Vaccines2, 583–593 10.1586/14760584.2.4.583 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Abbas, M. N., Jmel, M. A., Mekki, I., Dijkgraaf, I. & Kotsyfakis, M. Recent advances in tick antigen discovery and anti-tick vaccine development. Int. J. Mol. Sci.24, 4969 10.3390/ijms24054969 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Artigas-Jerónimo, S. et al. Functional evolution of Subolesin/Akirin. Front. Physiol.9, 1612 10.3389/fphys.2018.01612 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torina, A. et al. Control of tick infestations and pathogen prevalence in cattle and sheep farms vaccinated with the recombinant Subolesin-Major Surface Protein 1a chimeric antigen. Parasit. Vectors7, 10 10.1186/1756-3305-7-10 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasaija, P. D., Contreras, M., Kabi, F., Mugerwa, S. & de la Fuente, J. Vaccination with recombinant Subolesin antigens provides cross-tick species protection in Bos indicus and crossbred cattle in Uganda. Vaccines8, 319 10.3390/vaccines8020319 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Estrada-Peña, A. & Fuente, dela J. Evolution of tick vaccinology highlights changes in paradigms in this research area. Vaccines11, 253 10.3390/vaccines11020253 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de la Fuente, J. & Estrada-Peña, A. Why new vaccines for the control of ectoparasite vectors have not been registered and commercialized? Vaccines7, 75 10.3390/vaccines7030075 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabi, F. et al. Monitoring the Subolesin vaccine field trial for safer control of cattle ticks amidst increasing acaricide resistance in Uganda. Vaccines10, 1594 10.3390/vaccines10101594 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rodríguez-Mallon, A. The Bm86 discovery: a revolution in the development of anti-tick vaccines. Pathogens12, 231 10.3390/pathogens12020231 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Díaz-Sánchez, S. et al. Low genetic diversity of the only clade of the tick Rhipicephalus microplus in the Neotropics. Pathogens12, 1344 10.3390/pathogens12111344 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valle, M. R. et al. Integrated control of Boophilus microplus ticks in Cuba based on vaccination with the anti-tick vaccine Gavac. Exp. Appl. Acarol.34, 375–382 10.1007/s10493-004-1389-6 (2004). [DOI] [PubMed] [Google Scholar]
- 24.de la Fuente, J. et al. A ten-year review of commercial vaccine performance for control of tick infestations on cattle. Anim. Health Res. Rev.8, 23–28 10.1017/S1466252307001193 (2007). [DOI] [PubMed] [Google Scholar]
- 25.de la Fuente, J. et al. Field studies and cost-effectiveness analysis of vaccination with Gavac against the cattle tick Boophilus microplus. Vaccine16, 366–373 10.1016/s0264-410x(97)00208-9 (1998). [DOI] [PubMed] [Google Scholar]
- 26.Khorasani, A., Madadgar, O., Soleimanjahi, H., Keyvanfar, H. & Mahravani, H. Evaluation of the efficacy of a new oil-based adjuvant ISA 61 VG FMD vaccine as a potential vaccine for cattle. Iran. J. Vet. Res17, 8–12 (2016). [PMC free article] [PubMed] [Google Scholar]
- 27.Perez Heredia, C. et al. Using Montanide™ ISA 50 V2 as adjuvant for the formulation of the anti-tick Gavac® vaccine. Biotecnol. Apl.34, 4201–4205. http://scielo.sld.cu/pdf/bta/v34n4/bta01417.pdf (2017).
- 28.Aucouturier, J., Dupuis, L. & Ganne, V. Adjuvants designed for veterinary and human vaccines. Vaccine19, 2666–2672 10.1016/s0264-410x(00)00498-9 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Moreira, C. Jr. et al. Evaluation of long-term immune response in cattle to botulism using a recombinant E. coli bacterin formulated with Montanide™ ISA 50 and aluminum hydroxide adjuvants. Micro. Pathog.189, 106596 10.1016/j.micpath.2024.106596 (2024). [DOI] [PubMed] [Google Scholar]
- 30.Chakraborty, S., Gao, S., Allan, B. F. & Smith, R. L. Effects of cattle on vector-borne disease risk to humans: A systematic review. PLoS Negl. Trop. Dis.17, e0011152 10.1371/journal.pntd.0011152 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.García-García, J. C. et al. Control of ticks resistant to immunization with Bm86 in cattle vaccinated with the recombinant antigen Bm95 isolated from the cattle tick, Boophilus microplus. Vaccine18, 2275–2287 10.1016/s0264-410x(99)00548-4 (2000). [DOI] [PubMed] [Google Scholar]
- 32.Lodos, J., Ochagavia, M. E., Rodríguez, M. & de la Fuente, J. A simulation study of the effects of acaricides and vaccination on Boophilus cattle-tick populations. Prev. Vet. Med38, 47–63 10.1016/s0167-5877(98)00113-5 (1999). [DOI] [PubMed] [Google Scholar]
- 33.de la Fuente, J. et al. Increasing access to biotech products for animal agriculture in Sub-Saharan Africa through partnerships. Nat. Biotechnol.42, 1013–1014 10.1038/s41587-024-02300-5 (2024). [DOI] [PubMed] [Google Scholar]
- 34.Mirembe, B. B. et al. Sporadic outbreaks of Crimean-Congo haemorrhagic fever in Uganda, July 2018-January 2019. PLoS Negl. Trop. Dis.15, e0009213 10.1371/journal.pntd.0009213 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dahmani, M., Davoust, B., Rousseau, F., Raoult, D., Fenollar, F. & Mediannikov, O. Natural Anaplasmataceae infection in Rhipicephalus bursa ticks collected from sheep in the French Basque Country. Ticks Tick. Borne Dis.8, 18–24 10.1016/j.ttbdis.2016.09.009 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Parola, P., Roux, V., Camicas, J. L., Baradji, I., Brouqui, P. & Raoult, D. Detection of ehrlichiae in African ticks by polymerase chain reaction. Trans. R. Soc. Trop. Med. Hyg.94, 707–708 10.1016/s0035-9203(00)90243-8 (2000). [DOI] [PubMed] [Google Scholar]
- 37.Weisburg, W. G. et al. Phylogenetic diversity of the Rickettsiae. J. Bacteriol.171, 4202–4206 10.1128/jb.171.8.4202-4206.1989 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olmeda, A. S. et al. A subtropical case of human babesiosis. Acta Trop.67, 229–234 10.1016/s0001-706x(97)00045-4 (1997). [DOI] [PubMed] [Google Scholar]
- 39.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2018). Available online at https://www.R-project.org/.
- 40.Aguirre Ade, A., Garcia, M. V., Szabó, M. P., Barros, J. C. & Andreotti, R. Formula to evaluate efficacy of vaccines and systemic substances against three-host ticks. Int. J. Parasitol.45, 357–359 10.1016/j.ijpara.2015.02.003 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Ndawula, C. Jr. From bench to field: a guide to formulating and evaluating anti-tick vaccines delving beyond efficacy to effectiveness. Vaccines9, 1185 10.3390/vaccines9101185 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shim, E. & Galvani, A. P. Distinguishing vaccine efficacy and effectiveness. Vaccine30, 6700–6705 10.1016/j.vaccine.2012.08.045 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Toledo-Romaní, M. E. et al. Safety and efficacy of the two doses conjugated protein-based SOBERANA-02 COVID-19 vaccine and of a heterologous three-dose combination with SOBERANA-Plus: a double-blind, randomised, placebo-controlled phase 3 clinical trial. Lancet Reg. Health Am.18, 100423 10.1016/j.lana.2022.100423 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.WHO (World Health Organization). Design of vaccine efficacy trials to be used during public health emergencies – Points of considerations and key principles. Accessed November 2023. https://www.who.int/docs/default-source/blue-print/working-group-for-vaccine-evaluation-(4th-consultation)/ap1-guidelines-online-consultation.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Data 1. Evaluation of tick life cycle.
Supplementary Data 2. Total tick counts.
Supplementary Data 3_Evaluation of vaccine safety.xlsx
Supplementary Data 4_Anti-SUB antibodies and prevalence of tick-borne pathogens.xlsx
Data Availability Statement
All data supporting the findings of this study are available within the paper and supplementary information. The source data underlying Figs. 2–9 and Supplementary Figure 1 are provided as a Source Data file.