Abstract
The usefulness of restriction endonuclease analysis of chromosomal DNA as a typing method for Clostridium difficile was tested. Over four months all faecal samples were routinely cultured for C difficile. DNA of all isolated strains was isolated and tested with the restriction endonuclease Hind III. The patterns obtained after electrophoresis in agarose gels seemed to be strain specific. Antibiotic susceptibility profiles agreed with the results of the restriction endonuclease analysis, though they were much less discriminating. Analysis of the results indicated that restriction endonuclease analysis is a suitable typing method for C difficile, which may be very valuable in epidemiological studies where a highly discriminating typing method is needed.
Full text
PDF


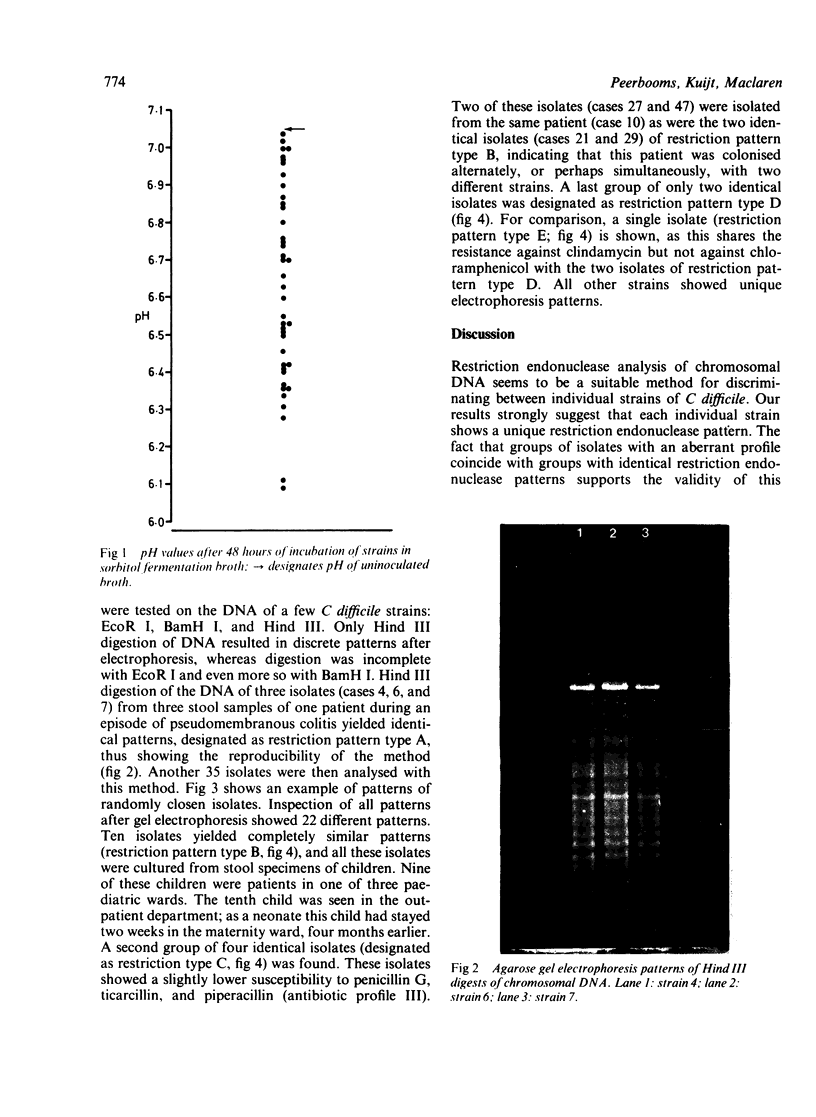
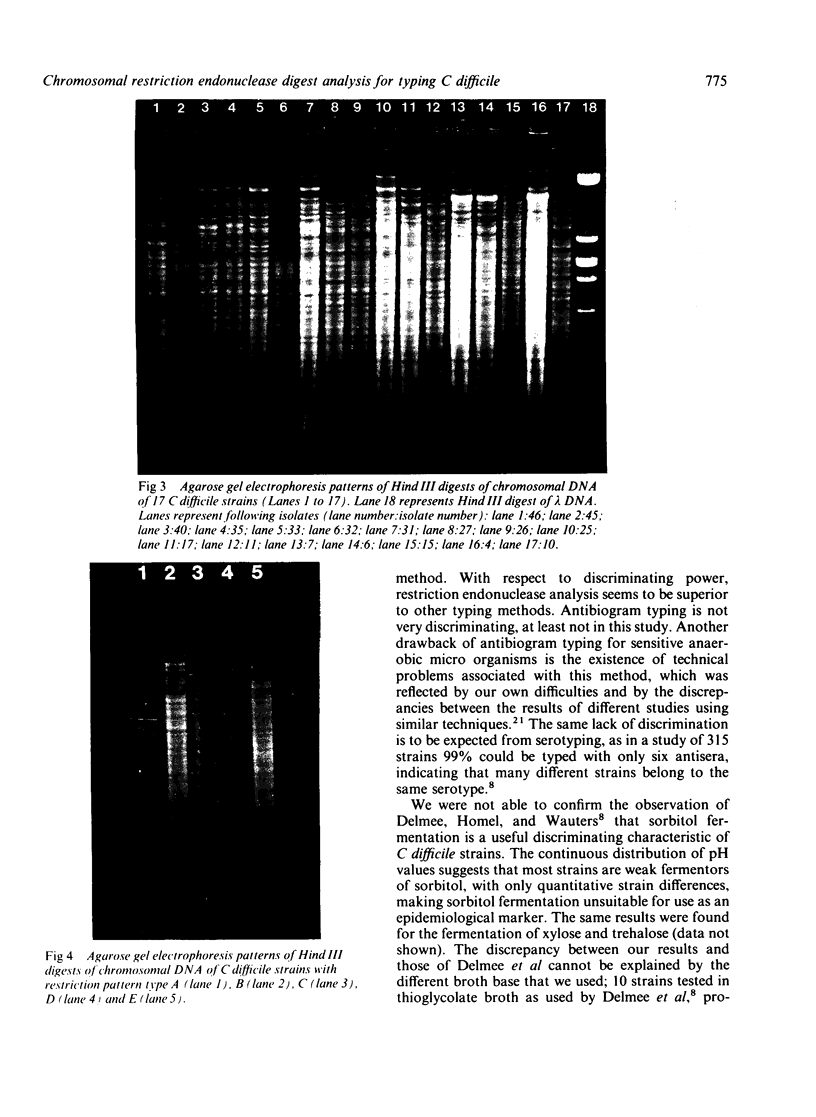

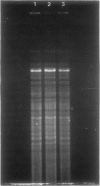
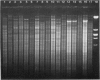
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradbury W. C., Pearson A. D., Marko M. A., Congi R. V., Penner J. L. Investigation of a Campylobacter jejuni outbreak by serotyping and chromosomal restriction endonuclease analysis. J Clin Microbiol. 1984 Mar;19(3):342–346. doi: 10.1128/jcm.19.3.342-346.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. W., Lauermann M., Bartlett J. G. Cytotoxicity assay in antibiotic-associated colitis. J Infect Dis. 1979 Nov;140(5):765–770. doi: 10.1093/infdis/140.5.765. [DOI] [PubMed] [Google Scholar]
- Delmee M., Homel M., Wauters G. Serogrouping of Clostridium difficile strains by slide agglutination. J Clin Microbiol. 1985 Mar;21(3):323–327. doi: 10.1128/jcm.21.3.323-327.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzink J., Bartlett J. G. In vitro susceptibility of Clostridium difficile isolates from patients with antibiotic-associated diarrhea or colitis. Antimicrob Agents Chemother. 1980 Apr;17(4):695–698. doi: 10.1128/aac.17.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K., DuPont H. L., Pickering L. K. Outbreaks of diarrhea associated with Clostridium difficile and its toxin in day-care centers: evidence of person-to-person spread. J Pediatr. 1983 Mar;102(3):376–382. doi: 10.1016/s0022-3476(83)80652-0. [DOI] [PubMed] [Google Scholar]
- Larson H. E., Barclay F. E., Honour P., Hill I. D. Epidemiology of Clostridium difficile in infants. J Infect Dis. 1982 Dec;146(6):727–733. doi: 10.1093/infdis/146.6.727. [DOI] [PubMed] [Google Scholar]
- Marshall R. B., Wilton B. E., Robinson A. J. Identification of Leptospira serovars by restriction-endonuclease analysis. J Med Microbiol. 1981 Feb;14(1):163–166. doi: 10.1099/00222615-14-1-163. [DOI] [PubMed] [Google Scholar]
- Poxton I. R., Aronsson B., Möllby R., Nord C. E., Collee J. G. Immunochemical fingerprinting of Clostridium difficile strains isolated from an outbreak of antibiotic-associated colitis and diarrhoea. J Med Microbiol. 1984 Jun;17(3):317–324. doi: 10.1099/00222615-17-3-317. [DOI] [PubMed] [Google Scholar]
- Richardson S. A., Alcock P. A., Gray J. Clostridium difficile and its toxin in healthy neonates. Br Med J (Clin Res Ed) 1983 Sep 24;287(6396):878–878. doi: 10.1136/bmj.287.6396.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabaqchali S., Holland D., O'Farrell S., Silman R. Typing scheme for Clostridium difficile: its application in clinical and epidemiological studies. Lancet. 1984 Apr 28;1(8383):935–938. doi: 10.1016/s0140-6736(84)92392-4. [DOI] [PubMed] [Google Scholar]
- Viscidi R., Willey S., Bartlett J. G. Isolation rates and toxigenic potential of Clostridium difficile isolates from various patient populations. Gastroenterology. 1981 Jul;81(1):5–9. [PubMed] [Google Scholar]
- Wexler H., Mulligan M. E., Finegold S. M. Polyacrylamide gel electrophoresis patterns produced by Clostridium difficile. Rev Infect Dis. 1984 Mar-Apr;6 (Suppl 1):S229–S234. doi: 10.1093/clinids/6.supplement_1.s229. [DOI] [PubMed] [Google Scholar]
- Wilson K. H., Kennedy M. J., Fekety F. R. Use of sodium taurocholate to enhance spore recovery on a medium selective for Clostridium difficile. J Clin Microbiol. 1982 Mar;15(3):443–446. doi: 10.1128/jcm.15.3.443-446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wüst J., Sullivan N. M., Hardegger U., Wilkins T. D. Investigation of an outbreak of antibiotic-associated colitis by various typing methods. J Clin Microbiol. 1982 Dec;16(6):1096–1101. doi: 10.1128/jcm.16.6.1096-1101.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ketel R. J., ter Schegget J., Zanen H. C. Molecular epidemiology of Legionella pneumophila serogroup 1. J Clin Microbiol. 1984 Sep;20(3):362–364. doi: 10.1128/jcm.20.3.362-364.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]