Abstract
Congenital anomalies (CA) encompass all morphological or functional alterations originating prenatally and present at birth. The prenatal diagnosis of these anomalies can significantly impact the overall health of the pregnant individual and may influence her decision regarding the continuation of the pregnancy. In contexts where safe pregnancy termination is not guaranteed by the state, it can lead to unsafe procedures with severe consequences. In our research, we analyzed epidemiological information on CA to develop potential indicators of inequity in access to safe abortion prior to the legalization of legal termination of pregnancy in Argentina. We included cases from 13 public hospitals and 9 non-public subsector hospitals, from the period 2013–2020. Two groups of specific CA were selected: 1) CA capable of being prenatally diagnosed, and 2) CA related to vascular disruptive events. 10/18 of the selected CA capable of being prenatally diagnosed had a significantly higher prevalence in public hospitals (anencephaly, encephalocele, spina bifida, microcephaly, hydrocephalus, holoprosencephaly, hydranencephaly, diaphragmatic hernia, gastroschisis, bilateral renal agenesis). Non public hospitals had higher prenatal detection. Birth prevalence of CA related with vascular disruptive events (limb reduction, Moebius syndrome, amniotic band sequence) were significantly higher in public hospitals. These results suggest disparities in access to prenatal diagnosis and safe abortion based on socioeconomic status. There was a significant gap in access to prenatal diagnosis for CA and possibly to safe elective abortion depending on the type of institution (public vs. non-public).
Keywords: Congenital anomalies, Prenatal diagnosis, Unsafe abortion, Health inequities
Introduction
Congenital anomalies (CA) encompass morphological or functional defects, both sporadic and hereditary, originating prenatally (WHO 1996). CA often result in varying degrees of disability, significantly impacting affected individuals, their families, the healthcare system, and society at large. CA have a diverse etiology, involving environmental and genetic factors, with many cases stemming from multifactorial origins due to complex gene-environment interactions. The prevalence of CA in newborns in Argentina is 1 to 3% (RENAC 2023). Improved control of infectious and nutritional diseases has elevated the relative importance of CA in child mortality rates (Christianson et al. 2006). In Argentina, the infant mortality rate (IMR) stood at 8.0 per 1,000 live births in 2021, with CAs accounting for 1,249 (29%) of the total 4,238 infant deaths (DEIS 2021).
The diagnosis of CA often marks a significant disruption in individuals' life trajectories, particularly when made prenatally. This diagnosis affects various dimensions of the pregnant woman's comprehensive health, including psychological, physical, and social aspects. The primary aim of prenatal CA detection is to furnish early information about embryo-fetal health, enabling the provision of appropriate support and a structured follow-up plan for pregnant women and couples (Dukhovny and Norton 2018; Jelin et al. 2019). This encompasses offering prognostic guidance, exploring potential prenatal interventions, discussing delivery options, and providing the choice of elective termination of pregnancy if desired. Prenatal diagnosis has notably increased CA detection rates during pregnancy in high-income countries since the 1980s, where available resources and equipment facilitate routine prenatal testing for the entire population (Heaney et al. 2022). However, in Argentina, access to prenatal diagnosis remains highly variable across socioeconomic sectors, with access primarily concentrated among women of higher social classes (Bidondo et al. 2020; Bronberg et al. 2020).
Until 2020, legal interruption of pregnancy in Argentina was permitted under two circumstances: if the pregnancy resulted from rape, or if the life or health of the pregnant individual was at risk. In January 2021, National Law No. 27610 on access to Voluntary Interruption of Pregnancy came into effect, expanding the rights associated with this practice. It guarantees voluntary access until the 14th week of gestation and allows for it under the two specific circumstances mentioned above after this period. Following the World Health Organization's definition, health must be seen as "a state of complete physical, mental, and social well-being, and not merely the absence of diseases or infirmities" (WHO 1948). In this context, comprehensive health is considered at risk when any of its dimensions—physical, mental, or social well-being—is affected. Therefore, pregnancy interruption should be legally feasible in all such cases. In other words, there is a call for a comprehensive understanding of health as an irrevocable right, aligning with international declarations of human rights.
Considering that the majority of CA are typically detected during the second trimester of pregnancy, it would be logical to consider pregnancy termination as a viable option when maternal health concerns arise, under a broad interpretation of comprehensive health. However, various obstacles and oppositions have hindered this right, including legal challenges directed at both national and provincial regulations, along with limitations placed on the availability of Legal Interruption of Pregnancy services. These constraints often arise from arbitrary criteria set forth by health authorities or specific healthcare teams, thus undermining the realization of this fundamental reproductive right (Tiseyra et al. 2022).
In countries where pregnancy termination is legally permitted, the decisions made by pregnant individuals facing fetal anomalies vary greatly, influenced by their beliefs and values (Barbero et al. 2018). Nonetheless, a significant proportion of pregnant individuals undergoing prenatal screenings express a willingness to terminate the pregnancy in the event of adverse results (Paolini et al. 2009). Prior to the enactment of Law 27610 in Argentina, research indicated a notable incidence of induced abortions, underscoring that state prohibition of voluntary termination fails to deter pregnant individuals from seeking clandestine procedures (Mario and Pantelides 2009). The criminalization of induced abortion perpetuates discriminatory practices and social injustices, fueling an underground market that violates the human rights of individuals with the capacity to conceive, particularly those from disadvantaged backgrounds (López 2014; Ramos and Fernández Vázquez 2020).
When termination of pregnancy occurs under unsafe conditions, the most serious consequence is the risk of maternal morbidity and mortality (Romero and Moisés 2020). A lesser-known consequence of illegal abortion is the risk of developing specific fetal anomalies when the abortion attempt fails (Pöhls et al. 2000). Unsafe abortion has been particularly associated with the occurrence of disruptive anomalies (Pastuszak et al. 1998; Dal Pizzol et al. 2008; Barbero et al. 2011; Hall 2012; Vauzelle et al. 2013). These types of defects involve the interruption of normal embryo-fetal development processes, where embryonic structures that had formed normally are affected by exposure to external factors, including the inappropriate use of abortifacients (Van Allen 1992; Holmes et al. 2018).
The healthcare system in Argentina is divided into public and non-public subsectors. The non-public subsector includes social security and private insurance. The public sector is funded through taxes and provides services free of charge to the entire population, serving approximately 46% of the inhabitants, primarily those with lower incomes. To investigate the relationship between the prevalence of AC and socioeconomic status, we compared cases born in public hospitals versus non-public hospitals in Buenos Aires City. The hospital´s healthcare sector (public versus non-public) was considered as a proxy variable for socioeconomic status.
Through the analysis of data from the period prior to the legalization of abortion in Argentina, the present study proposed the following objectives a) to detect differences in the prevalence of CA with potential prenatal diagnosis according to birth institution (public vs. non-public), serving as an indicator of inequality in access to pregnancy termination, and b) to identify disparities in the prevalence of CA of disruptive origin according to birth institution (public vs. non-public), serving as an indicator of unsafe abortion.
Material and methods
Design and procedures
The case definition in RENAC includes all live births and stillbirths with major morphological CA, whether externally or internally located. These anomalies are identified from birth until hospital discharge, utilizing methods such as physical examination, complementary tests, surgical interventions, or autopsy. RENAC reports provide a verbatim description of the observed CA in the affected newborn, along with a core set of variables (Groisman et al. 2013). Each anomaly is assigned a code from chapter 17 (codes Q00.0 to Q99.9) of the International Classification of Diseases, 10th Revision (ICD-10), with adaptation by the Royal College of Pediatrics and Child Health.
The present study is a cross-sectional study utilizing RENAC reports from the period 2013–2020. We included cases from a total of 22 maternity hospitals in Buenos Aires City, comprising 13 public hospitals of the public subsector (Álvarez, Argerich, Clínicas, Durand, Fernández, Penna, Piñero, Pirovano, Ramos Mejía, Rivadavia, Santojanni, Sardá, and Vélez Sarsfield) and 9 non-public subsector hospitals (Alemán, Anchorena, Italiano, Mater Dei, Otamendi, Santa Isabel, Suizo Argentina, Trinidad de Palermo, and Churruca).
For the present study, two groups of specific CAs were selected: 1) CAs capable of being prenatally diagnosed, and 2) CAs related to vascular disruptive events, anomalies can occur from use of abortifacients when interruption is unsuccessful (Pöhls et al. 2000). CAs in group 1 included: anencephaly (Q00), encephalocele (Q01), spina bifida (Q05), microcephaly (Q02), hydrocephalus (Q03), holoprosencephaly (Q04.1–04.2), hydranencephaly (Q04.35), critical congenital heart disease (Q20.0, Q20.3, Q20.4, Q21.3, Q21.82, Q22.00, Q22.40, Q22.5, Q23.4, Q25.1-Q25.19, Q25.2, Q26.2, Q26.20), diaphragmatic hernia (Q79.0-Q79.01), gastroschisis (Q79.3), omphalocele (Q79.2), bilateral renal agenesis (Q60.1), renal cysts (Q61.1-Q61.90), conjoined twins (Q89.4), and the following syndromic conditions: Down (Q90.0-Q90.9), Edwards (Q91.0-Q91.2), and Patau (Q91.4-Q91.6). CAs in group 2 were: limb reduction defects (Q71, Q72, Q73), Moebius syndrome (Q87.06), and amniotic band sequence (Q79.80).
Data analysis
The birth prevalence of selected CA was determined as the proportion of cases relative to the total number of births in the participating facilities. A 95% confidence interval (CI) was calculated based on the Poisson distribution. Birth prevalence of selected CAs was calculated considering Poisson’s distribution, with a 95% confidence interval, utilizing STATA 12® software. Prenatal diagnosis was estimated using the prenatal detection rate (PDR), which was calculated as the quotient of prenatally detected cases (numerator) to the total number of cases (denominator), with only isolated cases being included.
To compare the frequency of specific CAs between the two healthcare subsectors, the prevalence ratio was calculated as the quotient of the prevalence in the public sector over the prevalence in the non-public sector. To compare the PDR of both subsectors, the rate ratio was calculated as the quotient of the PDR in the public sector and in the non-public sector. Finally, the temporal trend of the prevalences of the most common CAs and the PDR throughout the period 2013–2020 was evaluated. For this purpose, a non-parametric trend test among ordered groups developed by Cuzick (1985) was used, which is an extension of the Wilcoxon rank-sum test. A value of p < 0.05 was considered significant. Stata statistical software version 13.0 was used for these calculations.
Results
The study evaluated a total of 3,444 cases with CAs, detected among 300,011 births examined in 22 hospitals of the healthcare institutions in the City of Buenos Aires. Of these, 192,354 cases were from the 13 public hospitals, and 107,657 cases were from the 9 non-public maternity hospitals.
We observed that 10/18 of the selected CAs capable of being prenatally diagnosed had a significantly higher prevalence in public hospitals. The greatest differences were observed for hydranencephaly, holoprosencephaly, anencephaly, and gastroschisis. Additionally, a higher prevalence of conjoined twins was observed, although the prevalence ratio was not statistically significant, likely due to the low number of cases. Only two anomalies showed a higher prevalence in non-public institutions: Patau syndrome and severe heart disease, although the differences were not statistically significant (Table 1).
Table 1.
Birth prevalence of selected congenital anomalies in public and Non-public health subsectors hospitals, Buenos Aires City, Argentina, RENAC 2013–2020
| Congenital anomalies | Total | Public Hospitals | Non-public hospitals | Prevalence Ratio | |||
|---|---|---|---|---|---|---|---|
| N | Prevalence (CI 95%) | N | Prevalence (CI 95%) | N | Prevalence (CI 95%) | ||
| Anencephaly | 101 | 3.37 (2.74 – 4.09) | 91 | 4.73 (3.81 – 5.81) | 10 | 0.93 (0.44 – 1.71) | 5.09 * |
| Encephalocele | 40 | 1.33 (0.95—1.82) | 35 | 1.82 (1.27—2.53) | 5 | 0.46 (0.15—1.08) | 3.96* |
| Spina bifida | 227 | 7.57 (6.61 – 8.62) | 184 | 9.57 (8.23 – 11.05) | 43 | 3.99 (2.89 – 5.38) | 2.40* |
| Microcephaly | 48 | 1.60 (1.18 – 2.12) | 40 | 2.08 (1.48 – 2.83) | 8 | 0.74 (0.32 – 1.46) | 2.81* |
| Hydrocephalus | 303 | 10.10 (8.99 -11.30) | 229 | 11.91 (10.41 – 13.55) | 74 | 6.87 (5.40 – 8.63) | 1.73* |
| Holoprosencephaly | 55 | 1.83 (1.38 – 2.39) | 51 | 2.65 (1.97 – 3.49) | 4 | 0.37 (0.10 – 0.95) | 7.16* |
| Hydranencephaly | 22 | 0.70 (0.43 – 1.07) | 21 | 1.09 (0.68 – 1.67) | 1 | 0.09 (0.01 – 0.51) | 12.11* |
| Severe heart disease | 560 | 18.66 (17.15 – 20.28) | 331 | 17.21 (15.40 – 19.16) | 229 | 21.27 (18.60 – 24.21) | 0.81 |
| Diaphragmatic hernia | 207 | 6.90 (5.99 – 7.91) | 171 | 8.89 (7.61 – 10.33) | 36 | 3.34 (2.34 – 4.63) | 2.66* |
| Gastroschisis | 335 | 11.17 (10.00 – 12.43) | 296 | 15.39 (13.68 – 17.24) | 39 | 3.62 (2.58 – 4.95) | 4.25* |
| Omphalocele | 91 | 3.03 (2.44 – 3.72) | 68 | 3.54 (2.74 – 4.48) | 23 | 2.14 (1.35 – 3.21) | 1.65 |
| Bilateral renal agenesis | 36 | 1.20 (0.84 – 1.66) | 31 | 1.61 (1.09 – 2.29) | 5 | 0.46 (0.15 – 1.08) | 3.50* |
| Renal cysts | 194 | 6.46 (5.59 – 7.44) | 137 | 7.12 (5.98 – 8.42) | 57 | 5.3 (4.01 – 6.86) | 1.34 |
| Siameses | 15 | 0.50 (0.28 – 0.82) | 14 | 0.73 (0.40 – 1.22) | 1 | 0.09 (0.01 – 0.51) | 8.11 |
| Down syndrome | 675 | 22.50 (20.83 – 24.26) | 448 | 23.29 (21.18 – 25.55) | 227 | 21.09 (18.43 – 24.01) | 1.10 |
| Patau syndrome | 27 | 0.90 (0.59 – 1.31) | 13 | 0.68 (0.36 – 1.15) | 14 | 1.3 (0.71 – 2.18) | 0.52 |
| Edwards syndrome | 66 | 2.20 (1.70 – 2.80) | 43 | 2.24 (1.62 – 3.01) | 23 | 2.14 (1.35 – 3.21) | 1.05 |
PR prevalence ratio public versus private; *Statistically significant
The PDR varied across different CAs. Non-public hospitals generally exhibited higher PDRs compared to public hospitals. However, the only statistically significant difference was observed in Down syndrome. In non-public hospitals, the PDR for Down syndrome was 47.1% (95% CI: 40—54), whereas in public hospitals, it was 16.9% (95% CI: 13 – 20), resulting in a rate ratio of 2.78 (Table 2).
Table 2.
Prenatal detection rate (PDR) of selected congenital anomalies in public and Non-public health subsector hospitals, Buenos Aires City, Argentina, RENAC 2013–2020
| Congenital anomalies | Public Hospitals | Non-public Hospitals | PDR Ratio | ||||
|---|---|---|---|---|---|---|---|
| N | PDR (CI 95%) | N | PDR (CI 95%) | ||||
| Total | Prenatal detected | Total | Prenatal detected | ||||
| Anencephaly | 91 | 75 | 82.4% (73.0 – 89.6) | 10 | 10 | 100.0% (69.1—100.0) | 1.21 |
| Encephalocele | 35 | 26 | 74.3% (56.7 – 87.5) | 5 | 2 | 40.0% (5.2—85.3) | 0.54 |
| Spina bifida | 184 | 150 | 81.5% (75.1 – 86.8) | 43 | 36 | 83.7% (69.3—93.2) | 1.03 |
| Microcephaly | 40 | 22 | 55.0% (38.5 – 70.7) | 8 | 4 | 50.0% (15.7—84.3) | 0.91 |
| Hydrocephalus | 229 | 187 | 81.7% (76.0 – 86.4) | 74 | 61 | 82.4% (71.8—90.3) | 1.01 |
| Holoprosencephaly | 51 | 41 | 80.4% (66.9 – 90.2) | 4 | 3 | 75.0% (19.4—99.3) | 0.93 |
| Hydranencephaly | 21 | 18 | 85.7% (63.6 – 96.9) | 1 | 1 | 100.0% (25.0—100.0) | 1.17 |
| Severe heart disease | 331 | 214 | 64.7% (59.2 – 69.8) | 229 | 159 | 69.4% (63.0—75.3) | 1.07 |
| Diaphragmatic hernia | 171 | 155 | 90.6% (85.2 – 94.5) | 36 | 27 | 75.0% (57.8—87.9) | 0.83 |
| Gastroschisis | 296 | 261 | 88.2% (83.9 – 91.6) | 39 | 37 | 94.9% (82.6—99.3) | 1.08 |
| Omphalocele | 68 | 55 | 80.9% (69.5 – 89.4) | 23 | 19 | 82.6% (61.2—95.0) | 1.02 |
| Bilateral renal agenesis | 31 | 27 | 87.1% (70.1 – 96.4) | 5 | 4 | 80.0% (28.3—99.5) | 0.92 |
| Renal cysts | 137 | 118 | 86.1% (79.2 – 91.4) | 57 | 50 | 87.7% (76.3—94.9) | 1.02 |
| Siameses | 14 | 13 | 92.9% (66.1 – 99.8) | 1 | 1 | 100.0% (25.0—100.0) | 1.08 |
| Down syndrome | 448 | 76 | 16.9% (13.6 – 20.7) | 227 | 107 | 47.1% (40.5—53.8) | 2.78* |
| Patau syndrome | 13 | 10 | 76.9% (46.2 – 94.9) | 14 | 12 | 85.7% (57.2—98.2) | 1.11 |
| Edwards syndrome | 43 | 36 | 83.7% (69.3 – 93.2) | 23 | 20 | 86.9% (66.4—97.2) | 1.04 |
PDR Ratio private versus public; *Statistically significant
The temporal trend of the combined prevalence of all CAs with potential prenatal diagnosis showed a positive trajectory for both health sectors, although it did not reach statistical significance (Fig. 1). The temporal trend analysis of the prenatal detection rate (PDR) revealed an increase in the percentage of prenatally detected CAs in both sectors (public with z = 2.08 and non-public with z = 1.84). However, this increase was statistically significant only for the public sector (p < 0.038 for public; p > 0.066 for non-public) (Fig. 1).
Fig. 1.
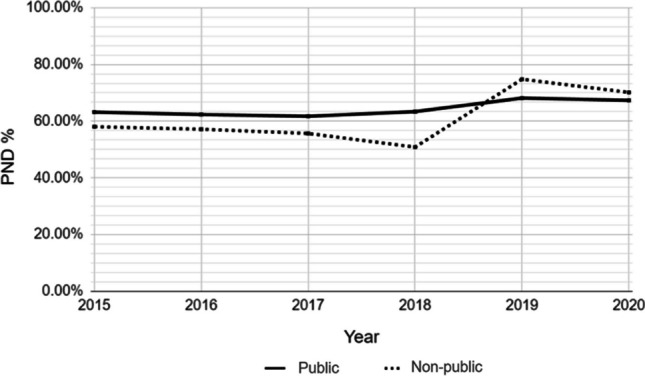
Temporal trend of the prenatal diagnosis rate (PDR) of cases with congenital anomalies with potential prenatal diagnosis for each health subsector, Buenos Aires City, 2013–202
The prevalence of the total selected disruptive anomalies was significantly higher in public institutions. In the cases with limb reduction and amniotic band sequences there were no statistically significant difference. The prevalence ratio showed a high trend in the public sector, however, the confidence intervals were overlapped. All cases of Moebius syndrome were observed in public hospitals (Table 3).
Table 3.
Birth prevalence of congenital anomalies related with vascular disruptive events in the public and non-public health subsectors hospitals, Buenos Aires City, Argentina, RENAC 2013–2020
| Congenital anomalies | Total | Public Hospitals | Non-public hospitals | Prevalence Ratio | |||
|---|---|---|---|---|---|---|---|
| N | Prevalence (CI 95%) | N | Prevalence (CI 95%) | N | Prevalence (CI 95%) | ||
| Limb reduction | 173 | 5.77 (4.94—6.70) | 126 | 6.55 (5.46 – 7.80) | 47 | 4.37 (3.21 – 5.80) | 1.50 |
| Mobius syndrome | 26 | 0.87 (0.56—1.27) | 26 | 1.35 (0.88 – 1.98) | 0 | - | - |
| Amniotic band sequence | 22 | 0.73 (0.46—1.11) | 19 | 0.99 (0.59 – 1.54) | 3 | 0.28 (0.05 – 0.80) | 3.54 |
| Total | 221 | 7.37 (6.42—8.40) | 171 | 8.89 (7.60 – 10.32) | 50 | 4.64 (3.44 – 6.12) | 1.91 |
Discussion
The present study reveals that the prevalence of anencephaly, encephalocele, spina bifida, microcephaly, hydrocephalus, holoprosencephaly, hydranencephaly, diaphragmatic hernia, and bilateral renal agenesis at birth was significantly higher in public institutions in Buenos Aires City. This finding aligns with a prior study conducted on a smaller sample and over a more limited timeframe (Bronberg et al. 2020). Additionally, another study utilizing nationwide data also demonstrated higher prevalence rates in public institutions (Bronberg et al. 2021).
The association between the frequency of specific CAs and low socioeconomic status has been investigated in previous research. In Argentina, Pawluk and colleagues examined the correlation between 25 specific CAs and adverse social determinants. Their case–control study revealed a significant association between cleft lip with or without cleft palate, ventricular septal defect, and poverty indicators (Pawluk et al. 2014). However, they did not find a similar relationship with other CAs, as observed in our research. One potential explanation for this difference could be that Pawluk and colleagues used different socioeconomic status indicators in their analysis.
Studies from other countries have also noted a higher prevalence of CAs in vulnerable populations. For instance, a case–control study conducted in the United States compared the frequency of CAs in pregnant African American women born in the United States versus those born abroad (Hoyt et al. 2020). The study found that those born in the United States, who typically belonged to lower-income groups, exhibited a higher prevalence of CAs. In a meta-analysis conducted by Yu and colleagues, which examined three parameters of maternal socioeconomic status (education, family income, and maternal occupation), a significant association was found between low socioeconomic status and the occurrence of congenital heart disease (Yu et al. 2014). Additionally, Canfield and colleagues studied variations in specific CAs according to ethnicity in the United States and observed a higher occurrence of severe CAs with potential prenatal diagnosis in African American and foreign white pregnant women (Canfield et al. 2006).
In our study, we observed significantly higher prevalences of all CAs affecting the central nervous system (anencephaly, encephalocele, spina bifida, holoprosencephaly, microcephaly, hydrocephalus, and hydranencephaly) in public hospitals. These severe anomalies, which are associated with high morbidity, mortality and disability, have heterogeneous etiology, so their increased prevalence in the public sector cannot be attributed to a single causal factor. Anencephaly, encephalocele, and spina bifida are neural tube defects, and their occurrence has been associated with folic acid deficiency. In Argentina, the prevalence of these defects has decreased following the mandatory fortification of wheat flour with folic acid implemented in 2002 (Lopez-Camelo et al. 2010; Sargiotto et al. 2015; Bidondo et al. 2015). According to data from the National Nutrition Survey (ENNyS 2007), individuals from lower socioeconomic backgrounds tend to have higher folate levels, possibly due to increased consumption of wheat flour-derived foods. Therefore, one would expect the prevalence of neural tube defects to be lower in this population group; however, our results indicate the opposite.
Holoprosencephaly arises from incomplete division of the forebrain, and in most cases, it has a genetic basis. There is no evidence suggesting that environmental factors associated with socioeconomic status could account for differences in prevalence across different healthcare sectors. Microcephaly, hydrocephalus, and hydranencephaly are heterogeneous neurological anomalies influenced by both genetic and environmental factors. We cannot dismiss the possibility that variations in prevalence could be attributed to environmental factors such as congenital infections, which have been associated with lower socioeconomic status in previous studies (Cannon et al. 2010; Torgerson and Mastroiacovo 2013).
Studies conducted in countries where abortion is legal have observed that fetal anomalies affecting the nervous system are among those most associated with elective abortion (Pryde et al. 1992; Schechtman et al. 2002; Johnson et al. 2012). A global consortium of surveillance programs on CA revealed that during the years 2007–2009, no cases of anencephaly were detected in live births in certain regions such as Cuba, Wales, Tuscany, and the northern Netherlands. However, records indicate an increase in the prevalence of this anomaly in products of elective terminations, indicating that such CAs still occur. In many cases, timely prenatal diagnosis and access to safe elective terminations are available (WHO-CDC-ICBDSR 2020).
These background findings suggest that the higher prevalence of CA affecting the central nervous system in public hospitals could be partly explained by a higher proportion of prenatal diagnosis and greater access to termination among the population in the non-public healthcare subsector.
Bilateral renal agenesis and diaphragmatic hernia are both conditions associated with high lethality that also exhibited higher prevalence in public institutions. Regarding diaphragmatic hernia, its etiology is heterogeneous and no environmental risk factors have been identified to date. Although the etiology of agenesis remains unidentified in most cases, pregestational diabetes is known to increase the risk (Davis et al. 2010). The diagnosis of diabetes necessitates actions that require self-care, significant adherence to treatment, and engagement in medical care, which may pose greater challenges for women from socially disadvantaged backgrounds. However, the majority of cases of renal agenesis are not linked to diabetes. Once again, disparities in access to prenatal detection and elective termination of affected pregnancies could partly elucidate the lower prevalence of diaphragmatic hernia and bilateral renal agenesis in non-public institutions.
In the present study, gastroschisis exhibited a prevalence 4.25 times higher in public institutions. The etiology of gastroschisis is still largely unknown, but several studies have identified its strong association with young maternal age (Castilla et al. 2008; Skarsgard et al. 2015; Baldacci et al. 2020). This consistent association suggests that gastroschisis could be caused by the exposure to environmental factors more frequent among adolescent mothers. Our findings likely reflect differences in the distribution of maternal age according to socioeconomic status. Data from Buenos Aires City from the National Health Statistics and Information department (DEIS 2021), revealed that the proportion of pregnant women under 19 years of age was 14.16% in public hospitals, compared to 2.88% in non-public hospitals. Therefore, the observed differences are likely attributable largely to variations in the age distribution of populations rather than to elective terminations.
On the contrary, given that advanced maternal age is the primary risk factor for Down syndrome. It was, one would expect that the prevalence was to be higher in non-public institutions, taking to account that. Official statistics from Buenos Aires City for the period 2011–2021 (DEIS 2021) indicated that the proportion of pregnant women aged 35 and above was 13.27% in public hospitals, compared to 30.77% in non-public hospitals (DEIS 2021). However, the present study revealed a higher prevalence of Down syndrome in public institutions, although the differences were not statistically significant. This result could be attributed to greater access among the population served by non-public hospitals to prenatal diagnosis and elective termination of pregnancy. Previous research has demonstrated that socioeconomic disparities in access to prenatal diagnosis have led to discrepancies in the prevalence of Down syndrome (Khoshnood et al. 2006). In our country, prenatal diagnosis for Down syndrome is routinely available in non-public institutions, whereas it is practically non-existent in the public healthcare subsector (Bidondo et al. 2020). This finding is consistent with other observations from the present study: 47.1% of cases born with Down syndrome in the non-public sector underwent prenatal diagnosis, whereas only 16.9% of those born in public hospitals received prenatal diagnosis.
We observed significant heterogeneity in the PDR of different CA. As anticipated, the PDR was higher for CAs that significantly impact normal fetal morphology and in cases involving multiple anomalies. While we noted a higher PDR for 13 out of 18 CAs in non-public institutions, the only statistically significant difference was observed for Down syndrome. Encephalocele was an exception, exhibiting a higher PDR in public hospitals. However, since fewer than 10 cases were registered in non-public institutions, this difference may be attributable to a bias resulting from the low number of cases rather than a genuine discrepancy.
The variations in PDR across different populations and their potential causes have been the subject of numerous studies investigating various social determinants such as health coverage, socioeconomic status, maternal residence (rural/urban), maternal race, and ethnicity. While some of these determinants may overlap within the same population, socioeconomic status appears to be the variable exerting the greatest impact (Peiris et al. 2009; Hill et al. 2015). For example, a study conducted by Kaur and colleagues explored potential barriers to prenatal diagnosis among pregnant women in the province of Alberta, Canada. Despite the Canadian healthcare system providing universal healthcare coverage, the study found that individuals in the lowest quintile of socioeconomic status had the lowest rates of prenatal diagnosis, and that when prenatal diagnosis was performed, it was often delayed (Kaur et al. 2022).
Pérez et al. investigated the impact of social vulnerability and the timing of prenatal care in a cohort of pregnant women diagnosed with congenital heart disease in fetuses from five hospitals in Boston. They found that pregnant women with higher vulnerability scores tended to undergo their first ultrasound later, and the diagnosis of heart disease was more likely to occur after 24 weeks of gestation. Additionally, lower rates of pregnancy termination were observed in this group. However, the authors noted that when prenatal diagnosis was conducted early in pregnancy, the proportion of elective abortions did not differ based on socioeconomic status (Pérez et al. 2022). This study highlighted that delays in diagnosis significantly limit women's ability to make informed decisions regarding the continuation or termination of their pregnancy.
Regarding trends, our study revealed an increasing prevalence of all CAs with potential prenatal diagnosis in both healthcare subsectors, with statistically significant increases observed in the non-public subsector. This trend could be attributed to greater access to prenatal diagnosis techniques and advancements in this field, resulting in improved detection rates over time.
While the PDR was higher in the non-public sector, the increasing temporal trend observed in the public sector suggests a narrowing of the gap in access to diagnosis between both subsectors. This trend indicates that improvements in access to prenatal diagnosis may be occurring more rapidly in the public sector, potentially reducing disparities in healthcare access over time.
CAs associated with disruptive events, such as limb reduction defects, Moebius syndrome, and amniotic band sequence, exhibited a significantly higher prevalence in public institutions. These anomalies may be linked to attempts to terminate pregnancy in unsafe conditions when the abortion is unsuccessful (Pöhls et al. 2000).
Misoprostol is an analogue of prostaglandin E1 that was initially developed for the prevention and treatment of gastric and duodenal ulcers but later on was repurposed for inducing pregnancy termination (Clark et al. 2007; Shannon and Winikoff 2004). It became widely used as an abortifacient since the late 1980s, even in some Latin American countries where abortion was illegal. The standardization of its use demonstrated safety and led to a reduction in maternal morbidity and mortality by replacing previous, more invasive, unsafe, and ineffective termination methods. This marked progress improved access to termination and minimized health risks. However, its use in the context of illegality had multiple consequences. Firstly, there was a lack of clinical practice guidelines for counseling and monitoring by the health system. Moreover, the criminalization of abortion restricted access to adequate formulations (dosage, combination with other drugs, routes of administration, etc.). Consequently, in conditions where abortion was illegal, the effectiveness of misoprostol as an abortifacient may have been lower than expected, leading to some pregnancies continuing despite its use.
Previous Various studies have demonstrated an association between prenatal exposure to misoprostol and the occurrence of vascular disruptive defects (Castilla and Orioli 1994; Gonzalez et al. 1998; Pastuszak et al. 1998; Vargas et al. 2000; Dal Pizzol et al. 2008; Barbero et al. 2011; Vauzelle et al. 2013). For instance, in a study conducted by Vargas et al. in Brazil, 93 cases with disruptive anomalies and 279 controls were evaluated. The researchers observed a highly significant association between prenatal exposure to misoprostol and total disruptive CA (odds ratio [OR]: 22.0; 95% confidence interval [CI]: 7.3 – 81.3). Notably, all cases with exposure to misoprostol corresponded to failed attempts at termination. Furthermore, the study found highly significant associations for specific disruptive CA, including Moebius syndrome (OR: 49; 95% CI: 7.07 – 1,907) and distal transverse defects of limbs (OR: 24; 95% CI: 3.00 – 99.1) (Vargas et al. 2000).
Moebius syndrome is a disruptive CA characterized by paralysis of the abducens and facial cranial nerves, often accompanied by involvement of other cranial nerves and additional congenital defects. The etiology of Moebius syndrome is heterogeneous and not yet fully understood; it is postulated to result from abnormal brainstem development, which may be due to intrauterine hypoxia, exposure to teratogens (such as misoprostol), or genetically caused rhombencephalic vascular anomalies. However, the majority of cases occur as sporadic events not associated with a defined genetic cause (Bell et al. 2019). In a case series study of Moebius syndrome conducted in a pediatric hospital in Argentina, it was noted that in 7 out of 30 cases, mothers reported using misoprostol as an abortifacient (López et al. 2015).
Comparatively, the prevalence of Moebius syndrome detected in a study conducted in the Netherlands, where abortion has been legal since the 1980s, was 0.21 per 10,000 births, which is approximately seven times lower than the prevalence observed in our research (1.46 per 10,000) (Verzijl et al. 2003). Notably, all Moebius syndrome cases in our study occurred in public institutions, with no cases reported in non-public institutions. This significant disparity could be attributed to differences in access to safe abortion services, influenced by the socioeconomic status of the population.
Limb reduction defects and amniotic bands also exhibited a higher prevalence in public institutions, which could be associated with similar factors as those mentioned for Moebius syndrome.
Limitations
Our study had some limitations. Socioeconomic status was determined based on the hospital subsector of birth, which may not necessarily be associated with the individual socioeconomic status of the cases. However, a recent study conducted in Argentina, involving approximately 5800 households, found that the population living in poverty (measured by income) primarily sought healthcare in public institutions, unlike the higher-income population, which predominantly sought care in non-public institutions (Paternó Manavella et al. 2023). Secondly, differences in the observed prevalences of prenatally detectable CA may be associated, as previously mentioned, with different etiological risk factors between health subsectors and not necessarily with differential access to prenatal diagnosis and termination. Additionally, populations in the two healthcare subsectors may have differing attitudes towards elective abortion decisions.
Finally, the data source was the AC surveillance system, not a study designed to evaluate causality. Consequently, other exposures and possible etiologies would need to evaluate to use disruptive CA as indicator of differential access to prenatal termination in other countries.
Conclusion
Our study revealed a higher prevalence of selected CAs in public institutions compared to non-public institutions in the Autonomous City of Buenos Aires. These differences were mainly observed for CAs affecting the nervous system. The prevalence of Down syndrome in non-public institutions was lower despite the expected higher frequency in those institutions. These observed differences in prevalence between both subsectors could be associated with greater access to prenatal diagnosis and subsequent elective termination of pregnancy in more advantaged populations.
Disruptive anomalies showed a markedly higher prevalence in public institutions, which could be associated with unsafe abortion practices.
Equity constitutes a social value and a guiding principle of health policy action. Building an equitable health system would imply that all prevention opportunities are within reach of all pregnant individuals, including preconception healthcare, pregnancy planning, counseling to avoid exposure to teratogenic factors, timely prenatal diagnosis, and assistance in cases where termination is chosen.
It is estimated that 40% of women of reproductive age worldwide live in countries with restrictive abortion laws (OECD 2023). The inequitable situation observed in our study could possibly be replicated in other countries where abortion is illegal.
The legalization of abortion in December 2020 in Argentina represented a significant advancement in the rights of pregnant individuals. Following the approval of the new regulations, the national and provincial governments have implemented healthcare policies to achieve equity and access to this new right, ensuring its exercise in all healthcare coverages. Standardized protocols have been developed, and authorization has been granted for the commercialization of other effective abortion medications (such as mifepristone). We estimate that this new legal framework will likely allow pregnant individuals greater access to the right to decide the continuation or termination of pregnancy following the prenatal detection of CAs, and it may also impact a reduction in the occurrence of disruptive defects associated with failed abortions.
The results of our study provide new evidence on the need to democratize access to prenatal diagnosis and implement screening studies for CA during pregnancy for all pregnant individuals.
Future epidemiological research in Argentina will allow us to evaluate the impact of the new legislation and changes in health policies.
Author contributions
All authors have contributed equally to the work and have jointly supervised the work.
Data availability
The epidemiological data that support the findings of this study were taken from the databases of the congenital anomalies surveillance system of Argentina (RENAC) and have been published in annual reports that can be found on the website: https://ine.gov.ar/index.php/otrasareas/renac.
Declarations
Ethical approval
The authors have no ethical conflicts to disclose.
Consent to participate
The study was conducted using non-nominalized data, and comparisons were made based on aggregate data. These data fall within the exceptions outlined in Resolution 1480/2011 of the Argentina Ministry of Health, which provides guidelines for research involving human subjects.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Baldacci S, Santoro M, Coi A, Mezzasalma L, Bianchi F, Pierini A (2020) Lifestyle and sociodemographic risk factors for gastroschisis: a systematic review and meta-analysis. Arch Dis Child 105(8):756–764 [DOI] [PubMed] [Google Scholar]
- Barbero P, Liascovich R, Tellechea AL, Piola A, Bidondo MP, Groisman B (2018) Inequidad en el diagnóstico prenatal y la interrupción del embarazo por anomalía fetal en Argentina. Arch Argent Pediatr 116(6):e813–e815 [Google Scholar]
- Barbero P, Liascovich R, Valdez R, Moresco A (2011) Misoprostol teratogenicity: a prospective study in Argentina. Arch Argent Pediatría 109(3):226–231 [DOI] [PubMed] [Google Scholar]
- Bell C, Nevitt S, McKay VH, Fattah AY (2019) Will the real Moebius syndrome please stand up? A systematic review of the literature and statistical cluster analysis of clinical features. Am J Med Genet A 179(2):257–265 [DOI] [PubMed] [Google Scholar]
- Bidondo MP, Groisman B, Gili JA, Liascovich R, Barbero P, Pingray V (2015) Study on the prevalence and neonatal lethality in patients with selected congenital anomalies as per the data of the National Registry of Congenital Anomalies of Argentina. Arch Argent Pediatr 113(4):295–302 [DOI] [PubMed] [Google Scholar]
- Bidondo MP, Groisman B, Duarte S, Tardivo A, Liascovich R, Barbero P (2020) Prenatal detection of congenital anomalies and related factors in Argentina. J Community Genetics 11(3):313–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronberg RA, Groisman B, Bidondo MP, Barbero P, Liascovich R (2020) Birth prevalence of congenital anomalies in the City of Buenos Aires Argentina, according to socioeconomic level. J Community Genet 11(3):303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronberg R, Groisman B, Bidondo MP, Barbero P, Liascovich R (2021) Birth prevalence of congenital anomalies in Argentina, according to socioeconomic level. J Community Genet 12(3):345–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canfield MA, Honein MA, Yuskiv N, Xing J, Mai CT, Collins JS, Devine O, Petrini J, Ramadhani TA, Hobbs CA, Kirby RS (2006) National estimates and race/ethnic-specific variation of selected birth defects in the United States, 1999–2001. Birth Defects Res A 76(11):747–756 [DOI] [PubMed] [Google Scholar]
- Cannon MJ, Schmid DS, Hyde TB (2010) Review of cytomegalovirus seroprevalence and demographic characteristics associated with infection. Rev Med Virol 20(4):202–213 [DOI] [PubMed] [Google Scholar]
- Castilla EE, Orioli IM (1994) Teratogenicity of misoprostol: data from the Latin-American collaborative study of congenital malformations (ECLAMC). Am J Med Genet 51:161–162 [DOI] [PubMed] [Google Scholar]
- Castilla EE, Mastroiacovo P, Orioli M (2008) Gastroschisis: international epidemiology and public health perspectives. Am J Med Genet C Semin Med Genet 148C(3):162–179 [DOI] [PubMed] [Google Scholar]
- Christianson A, Howson CP, Modell B (2006) Global Report on Birth Defects. The Hidden Toll of Dying and Disabled Children. White Plains (NY): March of Dimes Birth Defects Foundation p 1–85
- Clark W, Shannon C, Winikoff B (2007) Misoprostol for uterine evacuation in induced abortion and pregnancy failure. Expert Rev Obstet Gynecol 2(1):67–108 [Google Scholar]
- Cuzick J (1985) A wilcoxon-type test for trend. Statistics in Medicine 4(1):87–90. 10.1002/SIM.4780040112 [DOI] [PubMed]
- Dal Pizzol TS, Sanseverino MTV, Mengue SS (2008) Exposure to misoprostol and hormones during pregnancy and risk of congenital anomalies. Cad Saúde Pública 24(6):1447–53 [DOI] [PubMed] [Google Scholar]
- Davis EM, Peck JD, Thompson D, Wild RA, Langlois P (2010) Maternal diabetes and renal agenesis/dysgenesis. Birth Defects Res A Clin Mol Teratol 88(9):722–727 [DOI] [PubMed] [Google Scholar]
- DEIS Dirección de Estadísticas e Información de Salud. Ministerio de Salud (2021) Estadísticas vitales información Básica Argentina- Año 2021. ISSN: 1668–9054. Serie 5 Número 65. Buenos Aires, marzo de 2023. Available at: https://www.argentina.gob.ar/sites/default/files/serie_5_nro_65_anuario_vitales_2021_-_web.pdf. Accessed 20 May 2024
- Dukhovny S, Norton ME (2018) What are the goals of prenatal genetic testing? Semin Perinatol 42(5):270–274 [DOI] [PubMed] [Google Scholar]
- ENNyS Encuesta nacional de nutrición y salud (2007) Documento de resultados. Available at: https://bancos.salud.gob.ar/recurso/encuesta-nacional-de-nutricion-y-salud-documento-de-resultados-2007. Accessed 20 May 2024
- Gonzalez CH, Marques-Dias MJ, Kim CA, Sugayama SM, Da Paz JA, Huson SM, Holmes LB (1998) Congenital abnormalities in Brazilian children associated with misoprostol misuse in first trimester of pregnancy. Lancet 351(9116):1624–1627 [DOI] [PubMed] [Google Scholar]
- Groisman B, Bidondo MP, Barbero P, Gili JA, Liascovich R (2013) RENAC: Registro Nacional de Anomalías Congénitas de Argentina. Arch Argent Pediatr 111(6):484–49424196761 [Google Scholar]
- Hall JG (2012) Arthrogryposis (multiple congenital contractures) associated with failed termination of pregnancy. Am J Med Genet A 158A(9):2214–2220. 10.1002/ajmg.a.35531 [DOI] [PubMed] [Google Scholar]
- Heaney S, Tomlinson M, Aventin A (2022) Termination of pregnancy for fetal anomaly: a systematic review of the healthcare experiences and needs of parents. BMC Pregnancy Childbirth 22:441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill GD, Block JR, Tanem JB, Frommelt MA (2015) Disparities in the prenatal detection of critical congenital heart disease. Prenat Diagn 35(9):859–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes LB, Westgate MN, Nasri H, Toufaily MH (2018) Malformations attributed to the process of vascular disruption. Birth Defects Res 110:98–107 [DOI] [PubMed] [Google Scholar]
- Hoyt AT, Ramadhani T, Le MT, Shumate CJ, Canfield MA, Scheuerle AE (2020) National birth defects prevention study. Acculturation and selected birth defects among non-Hispanic Blacks in a population-based case-control study. Birth Defects Res 112(7):535–554 [DOI] [PubMed] [Google Scholar]
- Jelin AC, Sagaser KG, Wilkins-Haug L (2019) Prenatal genetic testing options. Pediatr Clin N Am 66(2):281–293 [DOI] [PubMed] [Google Scholar]
- Johnson CY, Honein MA, Dana Flanders W, Howards PP, Oakley GP Jr, Rasmussen SA (2012) Pregnancy termination following prenatal diagnosis of anencephaly or spina bifida: a systematic review of the literature. Birth Defects Res A Clin Mol Teratol 94(11):857–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A, Hornberger LK, Fruitman D, Ngwezi D, Eckersley LG (2022) Impact of location of residence and socioeconomic status on rate and timing of prenatal detection of major congenital heart disease in a jurisdiction of universal health coverage. Ultrasound Obstet Gynecol 60:359–366 [DOI] [PubMed] [Google Scholar]
- Khoshnood B, De Vigan C, Vodovar V, Bréart G, Goffinet F, Blondel B (2006) Advances in medical technology and creation of disparities: the case of Down syndrome. Am J Public Health 96(12):2139–2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López B, Polo C, Martín MC, Mercado G, Groisman B, Bidondo MP, Liascovich R, Barbero P (2015) Secuencia moebius. Análisis retrospectivo de 30 pacientes. Med Infantil XXII N° 2, 76–82
- López E (2014) Aborto inducido: ¿ignorancia o negación de una tragedia? Salud Colectiva 10(2) Lanús ago 2014 [DOI] [PubMed]
- Lopez-Camelo JS, Castilla EE, Orioli IM (2010) Folic acid flourfortifica- tion: impact on the frequencies of 52 congenital anomaly types in three South American countries. Am J Med Genet A 152A:2444–2558 [DOI] [PubMed] [Google Scholar]
- Mario S, Pantelides E (2009) Estimación de la magnitud del aborto inducido en Argentina. Notas de población 35(87):95–120
- OECD (2023) SIGI 2023 global report: gender equality in times of crisis, social institutions and gender index. OECD Publishing, Paris. 10.1787/4607b7c7-en
- Paolini CI, Gadow A, Petracchi F, Igarzabal L, Quadrelli R, Gadow EC (2009) Prenatal screening for chromosome abnormalities in a region with no access to termination of pregnancy. Prenat Diagn 29(7):659–663 [DOI] [PubMed] [Google Scholar]
- Pastuszak AL, Schüler L, Speck-Martins CE, Coelho KE, Cordello SM, Vargas F, Brunoni D, Schwarz IV, Larrandaburu M, Safattle H, Meloni VF, Koren G (1998) Use of misoprostol during pregnancy and Möbius’ syndrome in infants. N Engl J Med 338(26):1881–1885 [DOI] [PubMed] [Google Scholar]
- Paternó Manavella MA, Lafferriere F, Rodríguez Espíndola S (2023) Atención de salud y acceso a la atención médica en la argentina urbana [en línea]. Observatorio de la Deuda Social Argentina. Available at: https://repositorio.uca.edu.ar/handle/123456789/16440. Accessed 20 May 2024
- Pawluk MS, Campaña H, Gili JA, Comas B, Giménez LG, Villalba MI, Scala SC, Poletta FA, López Camelo JS (2014) Adverse social determinants and risk for congenital anomalies. Arch Argent Pediatr 112(3):215–223 [DOI] [PubMed] [Google Scholar]
- Peiris V, Singh TP, Tworetzky W, Chong EC, Gauvreau K, Brown DW (2009) Association of socioeconomic position and medical insurance with fetal diagnosis of critical congenital heart disease. Circ Cardiovasc Qual Outcomes 2(4):354–360 [DOI] [PubMed] [Google Scholar]
- Pérez MT, Bucholz E, Asimacopoulos E, Ferraro AM, Salem SM, Schauer J, Holleman C, Sekhavat S, Tworetzky W, Powell AJ, Sleeper LA, Beroukhim RS (2022) Impact of maternal social vulnerability and timing of prenatal care on outcome of prenatally detected congenital heart disease. Ultrasound Obstet Gynecol 60(3):346–358. 10.1002/uog.24863 [DOI] [PubMed]
- Pöhls UG, Steck T, Dietl J (2000) Fetal complications after failed pregnancy termination in the first trimester. Z Geburtshilfe Neonatol 204(4):153–7 [DOI] [PubMed] [Google Scholar]
- Pryde PG, Isada NB, Hallak M, Johnson MP, Odgers AE, Evans MI (1992) Determinants of parental decision to abort or continue after non-aneuploid ultrasound-detected fetal abnormalities. Obstet Gynecol 80:52–56 [PubMed] [Google Scholar]
- Ramos S, Fernández Vázquez S (2020) ¿Por qué abortan las mujeres? Contexto y biografía en las experiencias de aborto N°12 Mayo 2020 Serie Documentos REDAAS
- Romero M, Moisés S (2020) El aborto en cifras. Serie de documentos REDAAS. REDAAS. Buenos Aires, noviembre 2020
- RENAC (2023) Red Nacional de Anomalías Congénitas (2022) Reporte anual 2022. Available at: https://www.ine.gov.ar/images/docs/RepRENAC2023.pdf. Accessed 20 May 2024
- Sargiotto C, Bidondo MP, Liascovich R, Barbero P, Groisman B (2015) Descriptive study on neural tube defects in Argentina. Birth Defects Res A Clin Mol Teratol 103(6):509–516 [DOI] [PubMed] [Google Scholar]
- Schechtman KB, Gray DL, Baty JD, Rothman SM (2002) Decision-making for termination of pregnancies with fetal anomalies: analysis of 53,000 pregnancies. Obstet Gynecol 99:216–222 [DOI] [PubMed] [Google Scholar]
- Shannon CS, Winikoff B (2004) Misoprostol: an emerging technology for women’s health-report of a seminar. Population Council, New York [Google Scholar]
- Skarsgard ED, Meaney C, Bassil K, Brindle M, Arbour L, Moineddin R (2015) Maternal risk factors for gastroschisis in Canada. Birth Defects Res A Clin Mol Teratol 103:111–118 [DOI] [PubMed] [Google Scholar]
- Tiseyra MV, Vila Ortiz M, Romero M, Abalos E, Ramos S (2022) Barreras de acceso al aborto legal en el sistema público de salud de dos jurisdicciones de Argentina: Rosario y Ciudad Autónoma de Buenos Aires, 2019–2020. Salud Colectiva 18:e4059. 10.18294/sc.2022.4059 [DOI] [PubMed] [Google Scholar]
- Torgerson PR, Mastroiacovo P (2013) The global burden of congenital toxoplasmosis: a systematic review. Bull World Health Organ 91(7):501–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Allen (1992) Structural anomalies resulting from vascular disruption. Pediatr Clin N Am 39:255–277 [DOI] [PubMed] [Google Scholar]
- Vargas FR, Schuler-Faccini L, Brunoni D, Kim C, Meloni VF, Sugayama SM, Albano L, Llerena JC Jr, Almeida JC, Duarte A, Cavalcanti DP, Goloni-Bertollo E, Conte A, Koren G, Addis A (2000) Prenatal exposure to misoprostol and vascular disruption defects: a case-control study. Am J Med Genet 95(4):302–306 [DOI] [PubMed] [Google Scholar]
- Vauzelle C, Beghin D, Cournot M-P, Elefant E (2013) Birth defects after exposure to misoprostol in the first trimester of pregnancy: prospective follow-up study. Reprod Toxicol Elmsford n 36:98–103 [DOI] [PubMed] [Google Scholar]
- Verzijl HTFM, Van der Zwaag B, Cruysberg JRM, Padberg GW (2003) Möbius syndrome redefined : a syndrome of rhombencephalic maldevelopment. Neurology 61(3):327–333 [DOI] [PubMed] [Google Scholar]
- World Health Organization WHO (1948) Preamble to the Constitution of the World as adopted by the International Health Conference. New York, 19-22 June, 1946; signed on 22 July 1946 by the representatives of 61 States (Official Records of the World Health Organization, no. 2, p. 100) and entered into force on 7 April 1948
- WHO World Health Organization (1996) Control of hereditary diseases. Report of a WHO scientific group. World Health Organ Tech Rep Ser 865:1–84 [PubMed] [Google Scholar]
- WHO-CDC-ICBDSR (2020) Birth defects surveillance: a manual for programme managers, 2nd edn. Geneva: World Health Organization
- Yu D, Feng Y, Yang L, Da M, Fan C, Wang S, Mo X (2014) Maternal socioeconomic status and the risk of congenital heart defects in offspring: a meta-analysis of 33 studies. PLoS One 9(10):e111056 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The epidemiological data that support the findings of this study were taken from the databases of the congenital anomalies surveillance system of Argentina (RENAC) and have been published in annual reports that can be found on the website: https://ine.gov.ar/index.php/otrasareas/renac.


