Abstract
Pain accompanied by depressive symptoms is a common reason for seeking medical assistance, and many chronic pain patients experience comorbid depression. The brain-derived neurotrophic factor (BDNF) is a well-known neurotrophin expressed throughout the nervous system, playing a crucial role in neuronal growth and neuroplasticity. This study aimed to examine the effects of exercise on BDNF expression in the nervous system and reserpine (RSP)-induced pain-depression dyad. RSP (1 mg/kg) was subcutaneously administered once daily for three days in mice. The exercise was performed using a rota-rod tester for seven consecutive days following RSP administration. Pain responses were evaluated using von Frey filaments, and depression-like behaviors were assessed through forced swimming and open field tests. Immunofluorescence staining was performed to examine the changes in BDNF expression in the dorsal root ganglion (DRG), spinal cord, and hippocampus. Administration of RSP reduced mechanical paw withdrawal threshold, increased immobility time in the forced swimming test, and decreased movement in the open field test. The immunoreactivity of BDNF was increased in the DRG and spinal dorsal regions, and decreased in the hippocampus after RSP administration. Physical exercise significantly reduced the RSP-induced mechanical hypersensitivity and depression-like behaviors. In addition, exercise suppressed not only the increased expression of BDNF in the DRG and spinal dorsal regions but also the decreased expression of BDNF in the hippocampus induced by RSP administration. These findings suggest that repetitive exercise could serve as an effective and non-invasive treatment option for individuals experiencing both pain and depression by modulating BDNF expression.
Keywords: Exercise, Brain-derived neurotrophic factor, Reserpine, Pain, Depression
INTRODUCTION
Pain is an unbearable suffering physical condition that affects more than 1.5 billion people globally, and depression is a widespread mood disorder among more than 280 million people worldwide [1, 2]. Severe pain or depression can be the cause of suicide, often accompanied by both symptoms. Additionally, pain and depression lead to a vicious cycle that exacerbates each other, intensifying patients' suffering and drastically reducing their quality of life [3-5]. As such, the complex symptoms of the two diseases are increasingly problematic in individual patients and society, and it is urgent to establish fundamental treatment data for them. Although individual studies on pain and depression have been actively conducted [6-8], there is still a lack of understanding of the complex relationship between the two diseases.
Reserpine (RSP), a potent indole alkaloid extracted from the roots of Rauwolfia serpentina, is traditionally well known for its antihypertensive properties [9, 10]. However, its side effects have been identified to deplete monoamine neurotransmitters such as serotonin, norepinephrine, and dopamine [11]. While the mechanism of action of RSP in this regard is multifaceted and not fully understood, previous studies have shown that it induces pain and depression-like behaviors in animal models [12-17]. Pain and depression models induced by RSP can be used as animal models suitable for understanding the neurobiological mechanisms of these complex disorders.
Exercise, which covers a range of activities from systematic exercise to routine physical activity, is widely known for its numerous health benefits, even though it is non-invasive and non-pharmaceutical. According to various previous studies, exercise has been shown to regulate a variety of physiological and psychological mechanisms, leading to drug-like effects [18]. Recently, there has been an increasing interest in the role of physical exercise as part of non-pharmacological treatment methods for managing pain [19, 20] and depression [21-23]. The results of these previous studies suggest that exercise can be a promising complementary or independent treatment for patients with pain and depression.
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family, is well known to play a crucial role in neuronal survival, differentiation, and synaptic plasticity by acting as a major ligand for the tropomyosin receptor kinase B (TrkB) [24-27]. In addition, it has been shown that dysregulation of BDNF signaling is associated with the pathophysiology of various mental and neurological diseases, including Parkinson's disease, Alzheimer's disease, depression, and chronic pain [25, 28-30]. Several studies have reported that increased levels of BDNF in the DRG and spinal cord affect pain [31, 32], while decreased levels of BDNF in the prefrontal cortex and hippocampus are involved in depression [33, 34]. Based on these backgrounds, the aim of the present study was to investigate the effects of exercise on BDNF expression in the peripheral and central nervous systems in RSP-induced pain-depression dyad animal models. To do this, we investigated whether: (1) subcutaneous administration of RSP induces not only mechanical hypersensitivity and depression-like behaviors but also the changes in BDNF expression of the nervous system; (2) repetitive physical exercise alleviates the RSP-induced pain-depression dyad and the changes in BDNF expression; and finally (3) these actions of physical exercise are more effective than gabapentin, a calcium channel blocker, or fluoxetine, a selective serotonin reuptake inhibitor.
MATERIALS AND METHODS
Animals
Male ICR mice (Samtako, Osan, South Korea) weighing 20 to 25 g at 5 weeks of age were used in this experiment. All animal experimentation adheres to the policy of the Chungnam National University regarding the use and care of animals and this study was conducted with the approval of the Animal Experiment Ethics Committee of Chungnam National University (approval number: 202309A-CNU-140). Animals were housed in a standard environment consisting of a 12-hour light/dark cycle, constant room temperature (maintained between 20°C and 25°C), and 40%~60% humidity. Food and water were supplied ad libitum.
Drug administration
To induce pain and depression, reserpine (RSP, Cat. No. R0875, Sigma, St. Louis, MO, USA) was dissolved in a 1 mg/kg dose of physiological saline solution and injected subcutaneously into the dorsal region once daily for three consecutive days. As a positive control group for pain, gabapentin (GBP, Cat. No. PHR1049, Sigma), known as a calcium channel blocker, was administered intraperitoneally at a dose of 50 mg/kg. Fluoxetine (FLU, Cat. No. PHR1394, Sigma), known as a selective serotonin reuptake inhibitor, was used as a positive control for depression and administered intraperitoneally at a dose of 10 mg/kg. GBP and FLU were dissolved in physiological saline and administered once daily after the last administration of RSP. The doses of drugs used in the present study were selected based on doses used in the previous literatures [16, 35, 36].
Physical exercise
The exercise was performed using the rota-rod Tester (SciTech Korea Inc., Seoul, Korea). Mice were placed on a suspended cylindrical platform (12 cm wide, 6 cm in diameter) positioned 33 cm above the apparatus floor. To prevent mice from escaping, each cylinder was divided by a plastic wall on both sides. The rota-rod exercise was conducted for 30 minutes every day after the last administration of RSP. Rotation was set at two speeds of 15 and 30 revolutions per minute (RPM) to examine the appropriate exercise intensity.
Mechanical allodynia assessments
To assess mechanical sensitivity, the withdrawal threshold of both hind paws was measured using a series of von Frey filaments (0.07, 0.16, 0.4, 0.6, 1, 1.4, 2 g, North Coast Medical, Morgan Hill, CA, USA) with the 'ascending stimuli' method, as previously described [37]. Briefly, mice were placed on a metal mesh grid under a plastic chamber and habituated for at least 30 minutes before the test. The filaments were applied from underneath the metal mesh flooring to each hind paw. Starting with the lowest filament force (0.07 g) as the first stimulus, higher forces were applied if there was no response. The force that produced a 60% withdrawal response was recorded as the withdrawal threshold value. Normal baseline values of paw withdrawal responses to mechanical stimulation were measured before the injection of RSP. In the exercise group and all drug administration groups, measurements were taken 30 minutes after treatment. The resulting analysis values were expressed as the means of both hindpaws.
Open field test
The square black acrylic open-field box (40 cm×40 cm×40 cm), chosen for optimal contrast with the white mouse, was placed in a soundproof testing room equipped with a video camera. Before the test, all mice were allowed to adapt to the testing room for 30 minutes. At the start of the test trial, each mouse was placed in the center of the open-field box for 30 seconds, and the subsequent 5 minutes of behavior were recorded using the EthoVision-XT video tracking system (Noldus Information Technology, Netherlands). In this study, we examined moved distance and crossing to assess the effects of exercise on RSP-induced depression in mice, focusing on anti-anxiety-based depressive behaviors.
Forced swimming test
The forced swimming test was conducted following the method described by Porsolt et al. [38] with minor modifications. One day before the test, mice were placed in a clear plexiglass cylinder (10 cm×25 cm) filled with 15 cm of water (24°C±0.5°C) for 15 minutes. For the main test, conducted 24 hours after the pre-test, mice were again placed in the same system for 6 minutes. After an initial 1-minute period of vigorous activity, the mice showed a period of immobility by stopping climbing or swimming and making only small forelimb movements to keep their head above water. The duration of immobility during the last 5 minutes of the test was recorded as immobility time.
Immunofluorescence and image analysis
Immunofluorescence was performed 10 days after RSP injection. Mice were deeply anesthetized with avertin and perfused transcardially with heparinized phosphate-buffered saline (PBS, pH 7.4) followed by 4% paraformaldehyde (PFA) for 15 minutes. Bilateral dorsal root ganglions (DRGs) and spinal cord from the lumbar extension (L4-L6) region and brain were immediately extracted. Then, collected tissues were incubated in 4% PFA overnight for post-fixation. Following fixation, the tissues were immersed in a 30% sucrose solution with PBS until they sank to the bottom. Subsequently, tissues were frozen after embedding in Surgipath® FSC 22® frozen section compound (Leica Biosystems, Wetzlar, Germany). For immunohistochemical analysis, all procedures were followed as described in the previous report [39]. The compound-embedded tissue arrays were sliced into 10-μm sections, which were then affixed to silane-coated glass slides. To remove the embedding compound, sections were washed three times with a PBS solution, each time for 10 minutes. Nonspecific bindings were blocked with 3% bovine serum albumin in PBS for 1 hour at room temperature. After blocking, the sections were incubated with a 1:1,000 dilution of anti-BDNF antibody (Cat. No. ab108319, Abcam, Cambridge, UK) in the blocking solution overnight at 4°C and then washed with PBST. After the wash, the sections were incubated with a 1:1,000 dilution of Cy3-conjugated secondary antibody (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA) for 1 hour at room temperature. Stained sections were mounted with VECTASHIELD® (Vector Laboratories Inc., Newark, CA, USA) and visualized with an Axiophot microscope (Carl Zeiss, Oberkochen, Germany). The threshold for fluorescence intensity was set based on the values of the control group, and the positive area of immunoreactive cells was measured and analyzed using Image J software.
Statistical analysis
Data values are expressed as the mean±standard error of the mean (SEM). Repeated measures of the two-way analysis of variance (ANOVA) were performed to determine overall effects in the time course of nociceptive behavioral tests. Post hoc analysis used Dunnett’s test to determine the p-value among the experimental groups. Additionally, column analysis employed a Student’s t-test to compare the two means. GraphPad Prism 8.0 (GraphPad Software Inc., San Diego, CA, USA) was used to perform the statistical analysis, and a p-value <0.05 was considered statistically significant.
RESULTS
Moderate-intensity physical exercise reduces the RSP-induced pain and depression-like behaviors in mice
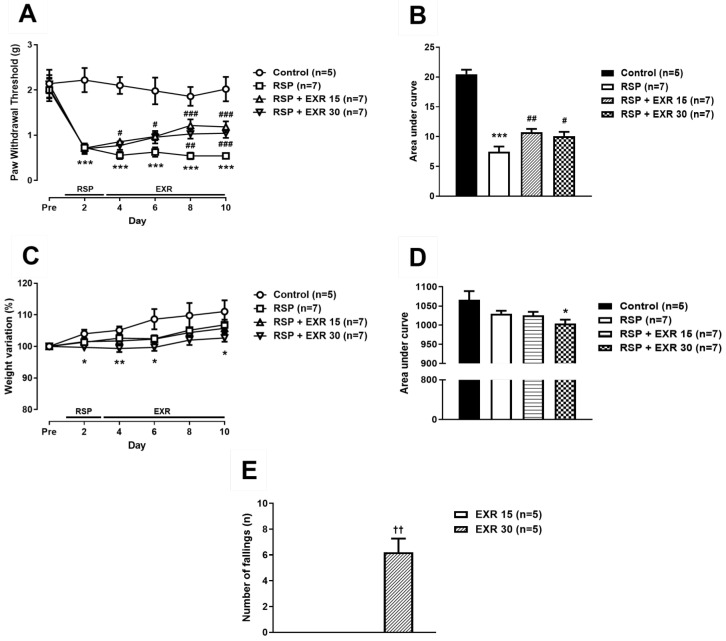
To determine the effect of physical exercise on the RSP-induced pain and depressive symptoms and to set the appropriate exercise intensity, exercise at an intensity of 15 or 30 RPM was performed after subcutaneous administration of RSP in mice. Repeated administration of RSP (1 mg/kg) significantly reduced mechanical paw withdrawal threshold (PWT, g) from the second day after initiation of drug administration as compared to that of the control group (Fig. 1A, B; ***p<0.001 vs Control). This reduction was suppressed by exercise at an intensity of 15 RPM from the second day after initiation of exercise, while exercise at an intensity of 30 RPM inhibited the RSP-induced reduction of PWT from the 6th day after initiation of exercise (#p<0.05, ##p<0.01, ###p<0.001 vs RSP-treated group). As a result of measuring the weight change of mice during the experiment, the control group showed a tendency to increase weight variation (%), while this increase was not detected in the RSP-treated exercise group at an intensity of 30 RPM (Fig. 1C, D; *p<0.05, **p<0.01 vs Control). In addition, the exercise at an intensity of 30 RPM increased the number of falls in rota-rod tester during the 30-minute exercise time as compared to that of the exercise group at an intensity of 15 RPM (Fig. 1E; ††p<0.01 vs EXR15 group). Moreover, the external appearance of the exercised group at an intensity of 30 RPM was not better than that of the exercised group at an intensity of 15 RPM (data not shown).
Fig. 1.
Effects of different intensity (15 or 30 RPM) of exercise on the reserpine (RSP, 1 mg/kg)-induced pain and body weight variation in mice. (A, B) Physical exercise performed using a rota-rod tester at an intensity of 15 or 30 RPM inhibited the RSP-induced reduction of the paw withdrawal threshold (A). Area under curve was analyzed in control, RSP-treated, and RSP-treated exercised (15 or 30 RPM) groups (B). (C, D) The RSP-treated exercised (30 RPM) group of mice display lower body weight variation (%) relative to control mice (C). Area under the curve was analyzed and shown in D. (E) The number of falls in rota-rod tester during exercise was increased in the exercised group at an intensity of 30 RPM. n=5~7 mice/group. *p<0.05, **p<0.01, ***p<0.001 vs Control; #p<0.05, ##p<0.01, ###p<0.001 vs RSP; ††p<0.01 vs RSP+EXR15.
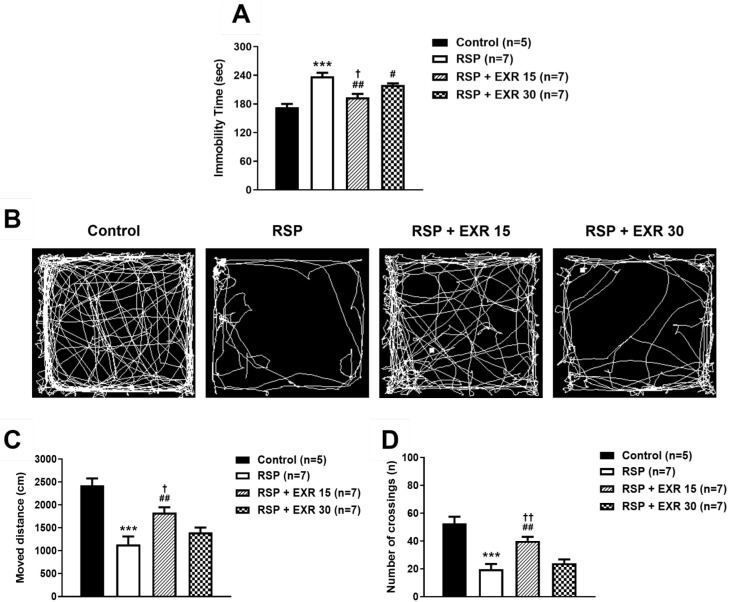
In the forced swimming test, a despair-based behavioral experiment, administration of RSP (1 mg/kg) led to a significant increase in immobility time (sec) as compared to that of the control group (Fig. 2A; *p<0.05 vs Control). This increase was inhibited by exercise at an intensity of 15 or 30 RPM (#p<0.05, ##p<0.01 vs RSP-treated group), and more significant inhibition appeared in the exercise group at an intensity of 15 RPM (††p<0.01 vs RSP+EXR30 group). In the Open field test, an anxiety-based behavioral experiment, administration of RSP decreased the moved distance (cm) and the number of crossings as compared to those of the control group (Fig. 2B~D; *p<0.05 vs Control). This decrease was suppressed by the exercise at an intensity of 15 RPM, while the exercise at an intensity of 30 RPM did not change the effect of RSP in the open field test (#p<0.05 vs RSP-treated group; (†p<0.05 vs RSP+EXR30 group). These results suggest that the exercise at an intensity of 15 RPM is more effective than 30 RPM to reduce the RSP-induced pain and depression-like behaviors. Thus, the exercise at an intensity of 15 RPM was selected and conducted in the next experiments based on the above results.
Fig. 2.
Effects of different intensity (15 or 30 RPM) of exercise on the reserpine (RSP, 1 mg/kg)-induced depression-like behaviors in mice. (A) Physical exercise performed using a rota-rod tester at an intensity of 15 or 30 RPM inhibited the RSP-induced increased immobility time (sec) during the forced swimming test. (B~D) Representative images for visualization of the path of movement during the open field test (B). The RSP-treated exercised (15 RPM) group of mice showed a significant increase in the total moved distance (C) and the number of crossings in the box during the open field test (D). n=5~7 mice/group. *p<0.05, **p<0.01, ***p<0.001 vs Control; #p<0.05, ##p<0.01 vs RSP; †p<0.05, ††p<0.01 vs RSP+EXR30.
Physical exercise inhibits the RSP-induced changes in BDNF immunoreactivity in the DRG, spinal cord, and hippocampus
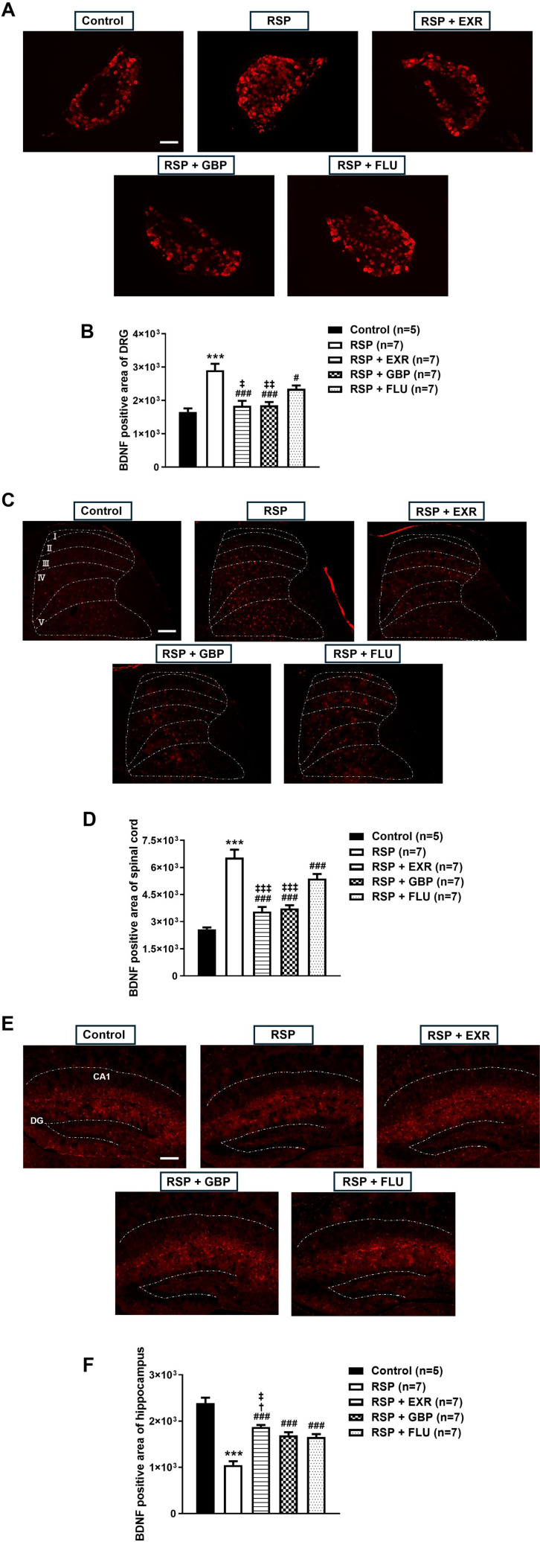
Next, we examined whether RSP administration would change the expression of BDNF in the peripheral and central nervous systems, and whether this change is modulated by physical exercise. For comparison with the exercised group, the effect of gabapentin, an α2δ1 calcium channel blocker, or fluoxetine, a selective serotonin reuptake inhibitor, on BDNF expression was also evaluated as a positive control. Administration of RSP (1 mg/kg) increased BDNF immunoreactivity in the lumbar DRG and spinal cord dorsal horn of mice as compared to that of the control group (Fig. 3A, B; ***p<0.001 vs Control). This increase was significantly inhibited not only by physical exercise at an intensity of 15 RPM but also by GBP (50 mg/kg) or FLU (10 mg/kg) administration (#p<0.05, ###p<0.001 vs RSP-treated group). However, FLU administration was less effective than physical exercise or GBP administration (‡p<0.05, ‡‡p<0.01, ‡‡‡p<0.001 vs RSP+FLU-treated group).
Fig. 3.
Effects of physical exercise (15 RPM), gabapentin (GBP, 50 mg/kg), and fluoxetine (FLU, 10 mg/kg) on BDNF immunoreactivity in the DRG, spinal cord, and hippocampus of reserpine (RSP, 1 mg/kg)-treated mice. (A~F) Physical exercise at an intensity of 15 RPM inhibited the RSP-induced changes in BDNF-immunoreactive area in the DRG (A, B), spinal cord dorsal horn (C, D), and hippocampus (E, F). Administration of GBP or FLU suppressed the RSP-induced modulation of BDNF expression, but less effectively than physical exercise. White dotted lines in C indicate the laminae I-V in the spinal cord dorsal horn, and those in E indicate the hippocampal subfields CA1 and the dentate gyrus (DG). Scale bar=20 µm. n=5~7 mice/group. ***p<0.001 vs Control; #p<0.05, ###p<0.001 vs RSP; †p<0.05 vs RSP+GBP; ‡p<0.05, ‡‡p<0.01, ‡‡‡p<0.001 vs RSP+FLU.
By contrast, administration of RSP decreased BDNF immunoreactivity in the hippocampus of mice as compared to that of the control group (Fig. 3C; ***p<0.001 vs Control). This decrease was significantly restored by physical exercise and GBP or FLU administration (###p<0.001 vs RSP-treated group), but physical exercise was more effective than GBP and FLU administration (†p<0.05 vs RSP+GBP, ‡p<0.05 vs RSP+FLU-treated group). The representative images of BDNF immunostaining in the DRG, spinal cord, and hippocampus of mice shown in Fig. 3D.
Antinociceptive and antidepressive effects of physical exercise are more effective than gabapentin or fluoxetine administration
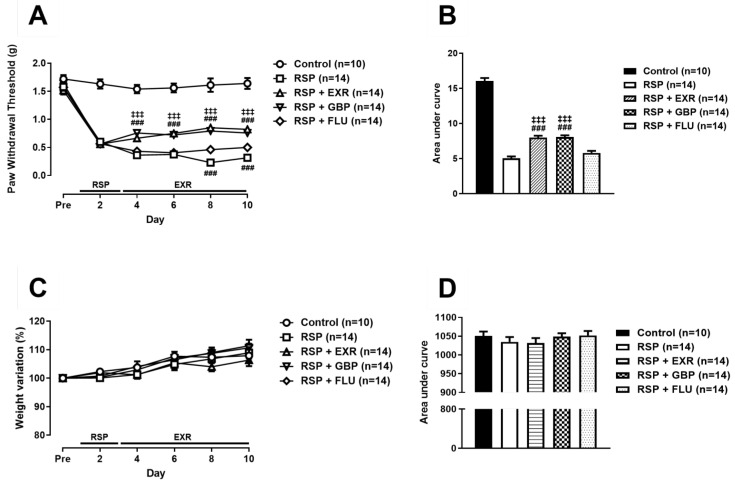
To investigate whether the antinociceptive and antidepressive effects of physical exercise are more effective than those of gabapentin and fluoxetine, these drugs were administrated once daily consistent with the schedule of physical exercise. Administration of GBP (50 mg/kg) or FLU (10 mg/kg) significantly inhibited the RSP-induced reduction of PWT (Fig. 4A, B; ###p<0.001 vs RSP-treated group). Statistical significance of antinociceptive effect of GBP was detected from the second day after initiation of drug administration similarly with that of physical exercise, while FLU administration had an effect on PWT from the 6th day after initiation of drug administration. The result of weight variation analysis during the experiments showed that there was no significant difference in the body weight variation between control and experimental groups (Fig. 4C, D).
Fig. 4.
Effects of physical exercise (15 RPM), gabapentin (GBP, 50 mg/kg), and fluoxetine (FLU, 10 mg/kg) on the reserpine (RSP, 1 mg/kg)-induced pain and body weight variation in mice. (A, B) Not only physical exercise at an intensity of 15 RPM but also GBP or FLU administration inhibited the RSP-induced reduction of the paw withdrawal threshold, but FLU was less effective than physical exercise and GBP administration (A). Area under curve was analyzed in control, RSP-treated, RSP-treated exercised (15 RPM), RSP and GBP-treated, RSP and FLU-treated groups (B). (C, D) There was no difference in body weight variation (%) between control and experimental groups (C). Area under the curve was analyzed and shown in D. n=10~14 mice/group. ###p<0.001 vs RSP; ‡‡‡p<0.001 vs RSP+FLU.
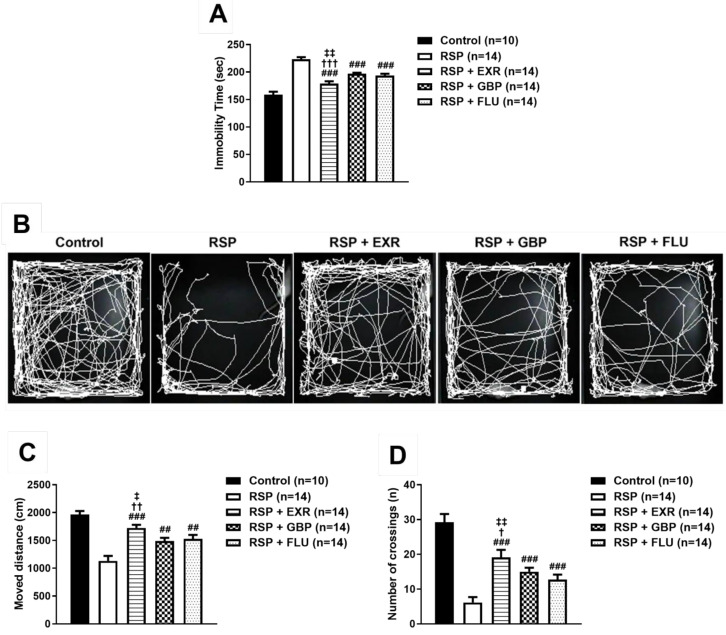
In the forced swimming test, administration of GBP or FLU significantly reduced the immobility time that was increased by RSP administration (Fig. 5A; ###p<0.001 vs RSP-treated group), but physical exercise was more effective than GBP and FLU administration (†††p<0.001 vs RSP+GBP-treated group; ‡‡p<0.01 vs RSP+FLU-treated group). Similar to the results obtained from the forced swimming test, administration of GBP or FLU significantly restored the RSP-induced reduction of the moved distance and the number of crossings (Fig. 5B~D; ##p<0.01, ###p<0.001 vs RSP-treated group). However, the exercised group showed a more significant increase in the moved distance and the number of crossings than the GBP- or FLU-treated group did (†p<0.05, ††p<0.01 vs RSP+GBP-treated group; ‡p<0.05, ‡‡p<0.01 vs RSP+FLU-treated group).
Fig. 5.
Effects of physical exercise (15 RPM), gabapentin (GBP, 50 mg/kg), and fluoxetine (FLU, 10 mg/kg) on the reserpine (RSP, 1 mg/kg)-induced depression-like behaviors in mice. (A) Administration of GBP or FLU inhibited the RSP-induced increased immobility time (sec) during the forced swimming test, but less effectively than physical exercise. (B~D) Representative images for visualization of the path of movement during the open field test (B). Administration of GBP or FLU restored the RSP-induced decrease in the total moved distance (C) and the number of crossings in the box during the open field test, but less effectively than physical exercise (D). n=10~14 mice/group. ##p<0.01, ###p<0.001 vs RSP; †p<0.05, ††p<0.01, †††p<0.001 vs RSP+GBP; ‡p<0.05, ‡‡p<0.01 vs RSP+FLU.
DISCUSSION
Various health benefits of physical exercise have already been validated through numerous studies [18]. Recent studies have shown that exercise can regulate different neurobiological pathways in the nervous system. In animal models of pain, physical exercise has been shown to regulate not only pro-inflammatory cytokines in the central and peripheral nervous systems but also neurotrophins such as nerve growth factor and BDNF [40-43]. Moreover, exercise can enhance the production of mood-related neurotransmitters, promote neurogenesis through increased secretion of various neurotrophic factors, and decrease the release of cortisol, the stress hormone, ultimately leading to antidepressant effects [44-47]. On the other hand, studies have shown that excessive intensity of physical exercise causes mitochondrial dysfunction, oxidative stress, and mood disturbance [48-50]. This suggests that moderate intensity exercise is more effective in ameliorating the pain-depression dyad. As a result, it was confirmed that 15 rpm was the moderate intensity among the exercise intensities used in this study. In the present study, moderate intensity of physical exercise modulated the expression of BDNF in the DRG, spinal cord, and hippocampus as well as the RSP-induced mechanical hypersensitivity and depressive-like behaviors in mice. These results suggest that physical exercise could reduce both pain and depression dyad and play a critical role in the modulation of BDNF-mediated signaling in the nervous system.
There have been various arguments regarding the role of BDNF in the peripheral and central nervous systems in animal models of pain and depression. Previous studies using acute and chronic pain animal models have shown that the increase of BDNF in DRG and spinal cord is directly and/or indirectly involved in pro-nociception [32, 51-53]. In addition, previous studies using various cause-induced depressive animal models have confirmed that BDNF in multiple regions of the brain is reduced, and in particular, BDNF in the hippocampal region has been found to play a key role in depression [54-57]. This is consistent with the present study showing that BDNF immunoreactivity was increased in both the DRG and spinal cord, and decreased in the hippocampus in RSP-administrated mice. Regarding that these changes were inhibited by physical exercise with a similar pattern of suppressive effect on pain and depression-like behaviors, the mechanisms underlying exercise-induced antinociceptive and antidepressive effects may be at least partially involved in the changed BDNF expression.
Previous studies have shown that peripheral inflammation or nerve damage increases BDNF synthesis in DRG neurons and is widely released into the spinal cord via anterograde transport [31, 58, 59]. Relatedly, increased BDNF levels in the DRG and spinal cord may lead to the increase in the phosphorylation of TrkB, N-methyl-D-aspartate (NMDA) receptor, and α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor, which can contribute to central sensitization [51, 60-64]. Thus, it is possible that the RSP-induced increased BDNF expression in the DRG and spinal cord plays an important role in mechanical hypersensitivity. In addition, various biomarkers of the brain region for depression have been identified. Among them, the major neurophysiological changes in depression is the inhibition of neurogenesis, such as decreased hippocampal size, synaptogenesis, and neuronal communications, due to the depletion of BDNF and monoamines [65]. Although the relationship between monoamines and BDNF is not fully understood, it has been suggested that the key factors are monoamine reduction and BDNF alterations across the peripheral and central nervous systems in the pain-depression complex symptoms caused by RSP [66-68]. The detailed mechanisms by which BDNF and monoamines regulate pain and depression need to be further investigated in the future studies.
Studies have been conducted to use various antidepressants as treatments for pain. Among them, selective serotonin reuptake inhibitors (SSRI) showed less pronounced analgesic effects, while tricyclic antidepressants and serotonin noradrenaline reuptake inhibitors (SNRI) showed significant effects [69-71]. In this regard, we also determined that administration of fluoxetine, a type of SSRI, exhibited less analgesic effect on the RSP-induced pain compared to those of exercise or GBP administration. These results suggest a possible role of noradrenaline in the analgesic effects of antidepressants. In the central nervous system, as known to all noradrenaline nuclei are located in the brainstem, and the largest noradrenaline nuclei called locus coeruleus (LC) contain more than 50% of all noradrenaline neurons [72, 73]. The descending noradrenaline pathway, from the ventral LC to α2-adrenergic receptors (α2Rs) in the spinal dorsal horn, plays an important role in endogenous analgesia [74]. In normal physiological conditions, noradrenaline released from the descending axon produces antinociceptive effects by reducing neuronal excitability in the spinal dorsal horn through stimulation of α2R coupled with inhibitory G proteins [75]. Additionally, in the cholinergic interneurons of the spinal cord, the α2R converts inhibitory G proteins to excitatory G proteins (G-protein switch; from Gi to Gs) through the BDNF-induced activation of TrkB receptor. Consequently, stimulation of the α2R with BDNF signaling leads to the release of acetylcholine, resulting in a stronger analgesic effect [76, 77]. Thus, it could be possible that this descending pain suppression pathway plays a critical role in a vicious cycle of pain and depression, which is related to the interaction between BDNF and monoamine, especially noradrenaline.
Reserpine binds to vesicular monoamine transporters, blocking neurotransmitter uptake into the vesicles, and causing irreversible monoamine depletion in the central nervous system, resulting in a variety of side effects including depression [11, 78-80]. In addition, it has been reported that RSP increases the mechanical sensitivity of cutaneous C-fibers, causing alterations in the function of the peripheral nervous system [81]. As such, the animal model administered RSP causes neurological changes in both the peripheral and central nervous systems, making it a suitable model for studying the complex relationship between pain and depression. The administration of RSP causes changes in the peripheral nervous system, increasing BDNF expression in the DRG and spinal cord, which leads to pain. Simultaneously, it irreversibly depletes monoamines in the central nervous system, affecting all related mechanisms of the brain and causing depression-like behaviors. Accordingly, it can be inferred that the abnormal BDNF-monoamine interaction increases due to the impaired noradrenaline-related mechanism, suppressing the endogenous analgesic pathway and activating the signals related to pro-nociception. These results strongly suggest that BDNF may be a key therapeutic target to break the vicious cycle between pain and depression. Although the present study has limitations in that it has only been investigated in animal models administered with RSP, the results obtained from the present study and the previous studies support that selective targeting of BDNF signaling pathway could be an important approach to treat both pain and depression.
In conclusion, moderate-intensity physical exercise regulates BDNF expression in the nervous system as well as has strong analgesic and anti-depressive effects in a mouse model of RSP-induced pain-depression dyad. The results of this study suggest that the mechanisms underlying the regulation of BDNF expression may be a key therapeutic target against pain-depression complex symptoms and show that exercise can be one of the non-pharmacological and non-cost alternative treatments for patients suffering from pain and depression.
ACKNOWLEDGEMENTS
This study was supported by research fund of Chungnam National University and Basic Science Research Program through the National Research Foundation of Korea (NRF, grant number: 2021R1A6A3A01086598). Graphical abstracts were created using the illustration tool at BioRENDER.com.
References
- 1.National Institute of Mental Health (NIH), author Statistics-major depression [Internet] NIH; Bethesda: 2018. Available from: https://www.nimh.nih.gov/health/statistics/major-depression . [Google Scholar]
- 2.World Health Organization (WHO), author Mental disorders [Internet]. WHO; Geneva: 2019. Available from: https://www.who.int/en/news-room/fact-sheets/detail/mental-disorders . [Google Scholar]
- 3.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Arch Intern Med. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 4.Campbell LC, Clauw DJ, Keefe FJ. Persistent pain and depression: a biopsychosocial perspective. Biol Psychiatry. 2003;54:399–409. doi: 10.1016/S0006-3223(03)00545-6. [DOI] [PubMed] [Google Scholar]
- 5.Papadopoulos V, Aghazadeh Y, Fan J, Campioli E, Zirkin B, Midzak A. Translocator protein-mediated pharmacology of cholesterol transport and steroidogenesis. Mol Cell Endocrinol. 2015;408:90–98. doi: 10.1016/j.mce.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gameroff MJ, Olfson M. Major depressive disorder, somatic pain, and health care costs in an urban primary care practice. J Clin Psychiatry. 2006;67:1232–1239. doi: 10.4088/JCP.v67n0809. [DOI] [PubMed] [Google Scholar]
- 7.IsHak WW, Wen RY, Naghdechi L, Vanle B, Dang J, Knosp M, Dascal J, Marcia L, Gohar Y, Eskander L, Yadegar J, Hanna S, Sadek A, Aguilar-Hernandez L, Danovitch I, Louy C. Pain and depression: a systematic review. Harv Rev Psychiatry. 2018;26:352–363. doi: 10.1097/HRP.0000000000000198. [DOI] [PubMed] [Google Scholar]
- 8.Olfson M, Gameroff MJ. Generalized anxiety disorder, somatic pain and health care costs. Gen Hosp Psychiatry. 2007;29:310–316. doi: 10.1016/j.genhosppsych.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Brest AN, Onesti G, Sekine G, Kodama R, Moyer JH. Acetazolamide alone and in combination with reserpine in the treatment of hypertension. Angiology. 1961;12:589–592. doi: 10.1177/000331976101201107. [DOI] [PubMed] [Google Scholar]
- 10.Luxenberg J, Feigenbaum LZ. The use of reserpine for elderly hypertensive patients. J Am Geriatr Soc. 1983;31:556–559. doi: 10.1111/j.1532-5415.1983.tb02201.x. [DOI] [PubMed] [Google Scholar]
- 11.Schuldiner S, Shirvan A, Linial M. Vesicular neurotransmitter transporters: from bacteria to humans. Physiol Rev. 1995;75:369–392. doi: 10.1152/physrev.1995.75.2.369. [DOI] [PubMed] [Google Scholar]
- 12.Arora V, Kuhad A, Tiwari V, Chopra K. Curcumin ameliorates reserpine-induced pain-depression dyad: behavioural, biochemical, neurochemical and molecular evidences. Psychoneuroendocrinology. 2011;36:1570–1581. doi: 10.1016/j.psyneuen.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Leith NJ, Barrett RJ. Effects of chronic amphetamine or reserpine on self-stimulation responding: animal model of depression? Psychopharmacology (Berl) 1980;72:9–15. doi: 10.1007/BF00433801. [DOI] [PubMed] [Google Scholar]
- 14.Liu SB, Zhao R, Li XS, Guo HJ, Tian Z, Zhang N, Gao GD, Zhao MG. Attenuation of reserpine-induced pain/depression dyad by gentiopicroside through downregulation of GluN2B receptors in the amygdala of mice. Neuromolecular Med. 2014;16:350–359. doi: 10.1007/s12017-013-8280-8. [DOI] [PubMed] [Google Scholar]
- 15.Minor TR, Hanff TC. Adenosine signaling in reserpine-induced depression in rats. Behav Brain Res. 2015;286:184–191. doi: 10.1016/j.bbr.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 16.Nagakura Y, Oe T, Aoki T, Matsuoka N. Biogenic amine depletion causes chronic muscular pain and tactile allodynia accompanied by depression: a putative animal model of fibromyalgia. Pain. 2009;146:26–33. doi: 10.1016/j.pain.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 17.Sousa FSS, Birmann PT, Baldinotti R, Fronza MG, Balaguez R, Alves D, Brüning CA, Savegnago L. α- (phenylselanyl) acetophenone mitigates reserpine-induced pain-depression dyad: behavioral, biochemical and molecular docking evidences. Brain Res Bull. 2018;142:129–137. doi: 10.1016/j.brainresbull.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Vina J, Sanchis-Gomar F, Martinez-Bello V, Gomez-Cabrera MC. Exercise acts as a drug; the pharmacological benefits of exercise. Br J Pharmacol. 2012;167:1–12. doi: 10.1111/j.1476-5381.2012.01970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kami K, Tajima F, Senba E. Exercise-induced hypoalgesia: potential mechanisms in animal models of neuropathic pain. Anat Sci Int. 2017;92:79–90. doi: 10.1007/s12565-016-0360-z. [DOI] [PubMed] [Google Scholar]
- 20.Wakaizumi K, Kondo T, Hamada Y, Narita M, Kawabe R, Narita H, Watanabe M, Kato S, Senba E, Kobayashi K, Kuzumaki N, Yamanaka A, Morisaki H, Narita M. Involvement of mesolimbic dopaminergic network in neuropathic pain relief by treadmill exercise: a study for specific neural control with Gi-DREADD in mice. Mol Pain. 2016;12:1744806916681567. doi: 10.1177/1744806916681567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren J, Xiao H. Exercise for mental well-being: exploring neurobiological advances and intervention effects in depression. Life (Basel) 2023;13:1505. doi: 10.3390/life13071505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuch FB, Vancampfort D, Richards J, Rosenbaum S, Ward PB, Stubbs B. Exercise as a treatment for depression: a meta-analysis adjusting for publication bias. J Psychiatr Res. 2016;77:42–51. doi: 10.1016/j.jpsychires.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 23.Szuhany KL, Otto MW. Assessing BDNF as a mediator of the effects of exercise on depression. J Psychiatr Res. 2020;123:114–118. doi: 10.1016/j.jpsychires.2020.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu B, Nagappan G, Guan X, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- 25.Numakawa T, Odaka H, Adachi N. Actions of brain-derived neurotrophin factor in the neurogenesis and neuronal function, and its involvement in the pathophysiology of brain diseases. Int J Mol Sci. 2018;19:3650. doi: 10.3390/ijms19113650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohira K, Hayashi M. A new aspect of the TrkB signaling pathway in neural plasticity. Curr Neuropharmacol. 2009;7:276–285. doi: 10.2174/157015909790031210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. doi: 10.1038/nrn3379. [DOI] [PubMed] [Google Scholar]
- 28.Björkholm C, Monteggia LM. BDNF - a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–79. doi: 10.1016/j.neuropharm.2015.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Merighi A, Salio C, Ghirri A, Lossi L, Ferrini F, Betelli C, Bardoni R. BDNF as a pain modulator. Prog Neurobiol. 2008;85:297–317. doi: 10.1016/j.pneurobio.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Palasz E, Wysocka A, Gasiorowska A, Chalimoniuk M, Niewiadomski W, Niewiadomska G. BDNF as a promising therapeutic agent in Parkinson's disease. Int J Mol Sci. 2020;21:1170. doi: 10.3390/ijms21031170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang DW, Choi JG, Ju Song H, Kim J, Lee M, Kim T, Kang SY, Ryu Y, Seung Yoo H, Sun Lee J, Bong Park J, Do Lee S, Kim HW. Analgesic efficacy of median nerve stimulation in mice with chemotherapy-induced peripheral neuropathymodulation of brain-derived neurotrophic factor expression. J Tradit Chin Med. 2023;43:686–694. doi: 10.19852/j.cnki.jtcm.2023.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yajima Y, Narita M, Usui A, Kaneko C, Miyatake M, Narita M, Yamaguchi T, Tamaki H, Wachi H, Seyama Y, Suzuki T. Direct evidence for the involvement of brain-derived neurotrophic factor in the development of a neuropathic pain-like state in mice. J Neurochem. 2005;93:584–594. doi: 10.1111/j.1471-4159.2005.03045.x. [DOI] [PubMed] [Google Scholar]
- 33.Li P, Huang W, Chen Y, Aslam MS, Cheng W, Huang Y, Chen W, Huang Y, Wu X, Yan Y, Shen J, Tong T, Huang S, Meng X. Acupuncture alleviates CUMS-induced depression-like behaviors by restoring prefrontal cortex neuroplasticity. Neural Plast. 2023;2023:1474841. doi: 10.1155/2023/1474841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng P, Zhang X, Liu TT, Liu J, Luo Y, Xie MX, Yang H, Fang R, Guo DW, Zhong ZY, Wang YH, Ge JW. A whole transcriptome profiling analysis for antidepressant mechanism of Xiaoyaosan mediated synapse loss via BDNF/trkB/PI3K signal axis in CUMS rats. BMC Complement Med Ther. 2023;23:198. doi: 10.1186/s12906-023-04000-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gómez ML, Martínez-Mota L, Estrada-Camarena E, Fernández-Guasti A. Influence of the brain sexual differentiation process on despair and antidepressant-like effect of fluoxetine in the rat forced swim test. Neuroscience. 2014;261:11–22. doi: 10.1016/j.neuroscience.2013.12.035. [DOI] [PubMed] [Google Scholar]
- 36.Kang DW, Moon JY, Choi JG, Kang SY, Ryu Y, Park JB, Lee JH, Kim HW. Antinociceptive profile of levo-tetrahydropalmatine in acute and chronic pain mice models: role of spinal sigma-1 receptor. Sci Rep. 2016;6:37850. doi: 10.1038/srep37850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deuis JR, Dvorakova LS, Vetter I. Methods used to evaluate pain behaviors in rodents. Front Mol Neurosci. 2017;10:284. doi: 10.3389/fnmol.2017.00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 39.Kang DW, Choi JG, Kim J, Park JB, Lee JH, Kim HW. Bee venom reduces burn-induced pain via the suppression of peripheral and central substance P expression in mice. J Vet Sci. 2021;22:e9. doi: 10.4142/jvs.2021.22.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almeida C, DeMaman A, Kusuda R, Cadetti F, Ravanelli MI, Queiroz AL, Sousa TA, Zanon S, Silveira LR, Lucas G. Exercise therapy normalizes BDNF upregulation and glial hyperactivity in a mouse model of neuropathic pain. Pain. 2015;156:504–513. doi: 10.1097/01.j.pain.0000460339.23976.12. [DOI] [PubMed] [Google Scholar]
- 41.Bobinski F, Martins DF, Bratti T, Mazzardo-Martins L, Winkelmann-Duarte EC, Guglielmo LG, Santos AR. Neuroprotective and neuroregenerative effects of low-intensity aerobic exercise on sciatic nerve crush injury in mice. Neuroscience. 2011;194:337–348. doi: 10.1016/j.neuroscience.2011.07.075. [DOI] [PubMed] [Google Scholar]
- 42.Chen YW, Li YT, Chen YC, Li ZY, Hung CH. Exercise training attenuates neuropathic pain and cytokine expression after chronic constriction injury of rat sciatic nerve. Anesth Analg. 2012;114:1330–1337. doi: 10.1213/ANE.0b013e31824c4ed4. [DOI] [PubMed] [Google Scholar]
- 43.López-Álvarez VM, Modol L, Navarro X, Cobianchi S. Early increasing-intensity treadmill exercise reduces neuropathic pain by preventing nociceptor collateral sprouting and disruption of chloride cotransporters homeostasis after peripheral nerve injury. Pain. 2015;156:1812–1825. doi: 10.1097/j.pain.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 44.Michalsen A, Grossman P, Acil A, Langhorst J, Lüdtke R, Esch T, Stefano GB, Dobos GJ. Rapid stress reduction and anxiolysis among distressed women as a consequence of a three-month intensive yoga program. Med Sci Monit. 2005;11:CR555–561. [PubMed] [Google Scholar]
- 45.Ross RE, VanDerwerker CJ, Saladin ME, Gregory CM. The role of exercise in the treatment of depression: biological underpinnings and clinical outcomes. Mol Psychiatry. 2023;28:298–328. doi: 10.1038/s41380-022-01819-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wegner M, Helmich I, Machado S, Nardi AE, Arias-Carrion O, Budde H. Effects of exercise on anxiety and depression disorders: review of meta- analyses and neurobiological mechanisms. CNS Neurol Disord Drug Targets. 2014;13:1002–1014. doi: 10.2174/1871527313666140612102841. [DOI] [PubMed] [Google Scholar]
- 47.Xie Y, Wu Z, Sun L, Zhou L, Wang G, Xiao L, Wang H. The effects and mechanisms of exercise on the treatment of depression. Front Psychiatry. 2021;12:705559. doi: 10.3389/fpsyt.2021.705559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Flockhart M, Nilsson LC, Tais S, Ekblom B, Apró W, Larsen FJ. Excessive exercise training causes mitochondrial functional impairment and decreases glucose tolerance in healthy volunteers. Cell Metab. 2021;33:957–970.e6. doi: 10.1016/j.cmet.2021.02.017. [DOI] [PubMed] [Google Scholar]
- 49.Sánchez Macarro M, Ávila-Gandía V, Pérez-Piñero S, Cánovas F, García-Muñoz AM, Abellán-Ruiz MS, Victoria-Montesinos D, Luque-Rubia AJ, Climent E, Genovés S, Ramon D, Chenoll E, López-Román FJ. Antioxidant effect of a probiotic product on a model of oxidative stress induced by high-intensity and duration physical exercise. Antioxidants (Basel) 2021;10:323. doi: 10.3390/antiox10020323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Takemura A, Eda N, Saito T, Shimizu K. Mild hyperbaric oxygen for the early improvement of mood disturbance induced by high-intensity exercise. J Sports Med Phys Fitness. 2022;62:250–257. doi: 10.23736/S0022-4707.21.11971-1. [DOI] [PubMed] [Google Scholar]
- 51.Groth R, Aanonsen L. Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or trkB, prevent inflammation-induced hyperalgesia. Pain. 2002;100:171–181. doi: 10.1016/S0304-3959(02)00264-6. [DOI] [PubMed] [Google Scholar]
- 52.Zhao J, Seereeram A, Nassar MA, Levato A, Pezet S, Hathaway G, Morenilla-Palao C, Stirling C, Fitzgerald M, McMahon SB, Rios M, Wood JN London Pain Consortium, author. Nociceptor-derived brain-derived neurotrophic factor regulates acute and inflammatory but not neuropathic pain. Mol Cell Neurosci. 2006;31:539–548. doi: 10.1016/j.mcn.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 53.Zhou XF, Deng YS, Xian CJ, Zhong JH. Neurotrophins from dorsal root ganglia trigger allodynia after spinal nerve injury in rats. Eur J Neurosci. 2000;12:100–105. doi: 10.1046/j.1460-9568.2000.00884.x. [DOI] [PubMed] [Google Scholar]
- 54.Deyama S, Kaneda K. The duration of the antidepressant-like effects of a single infusion of brain-derived neurotrophic factor into the medial prefrontal cortex in mice. Behav Brain Res. 2020;394:112844. doi: 10.1016/j.bbr.2020.112844. [DOI] [PubMed] [Google Scholar]
- 55.Monteggia LM, Barrot M, Powell CM, Berton O, Galanis V, Gemelli T, Meuth S, Nagy A, Greene RW, Nestler EJ. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc Natl Acad Sci USA. 2004;101:10827–10832. doi: 10.1073/pnas.0402141101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shirayama Y, Chen AC, Nakagawa S, Russell DS, Duman RS. Brain-derived neurotrophic factor produces antidepressant effects in behavioral models of depression. J Neurosci. 2002;22:3251–3261. doi: 10.1523/JNEUROSCI.22-08-03251.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Siuciak JA, Lewis DR, Wiegand SJ, Lindsay RM. Antidepressant-like effect of brain-derived neurotrophic factor (BDNF) Pharmacol Biochem Behav. 1997;56:131–137. doi: 10.1016/S0091-3057(96)00169-4. [DOI] [PubMed] [Google Scholar]
- 58.Lever IJ, Bradbury EJ, Cunningham JR, Adelson DW, Jones MG, McMahon SB, Marvizón JC, Malcangio M. Brain-derived neurotrophic factor is released in the dorsal horn by distinctive patterns of afferent fiber stimulation. J Neurosci. 2001;21:4469–4477. doi: 10.1523/JNEUROSCI.21-12-04469.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moy JK, Khoutorsky A, Asiedu MN, Dussor G, Price TJ. eIF4E phosphorylation influences Bdnf mRNA translation in mouse dorsal root ganglion neurons. Front Cell Neurosci. 2018;12:29. doi: 10.3389/fncel.2018.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- 61.Duan B, Liu DS, Huang Y, Zeng WZ, Wang X, Yu H, Zhu MX, Chen ZY, Xu TL. PI3-kinase/Akt pathway-regulated membrane insertion of acid-sensing ion channel 1a underlies BDNF-induced pain hypersensitivity. J Neurosci. 2012;32:6351–6363. doi: 10.1523/JNEUROSCI.4479-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garraway SM, Petruska JC, Mendell LM. BDNF sensitizes the response of lamina II neurons to high threshold primary afferent inputs. Eur J Neurosci. 2003;18:2467–2476. doi: 10.1046/j.1460-9568.2003.02982.x. [DOI] [PubMed] [Google Scholar]
- 63.Pezet S, Malcangio M, Lever IJ, Perkinton MS, Thompson SW, Williams RJ, McMahon SB. Noxious stimulation induces Trk receptor and downstream ERK phosphorylation in spinal dorsal horn. Mol Cell Neurosci. 2002;21:684–695. doi: 10.1006/mcne.2002.1205. [DOI] [PubMed] [Google Scholar]
- 64.Slack SE, Pezet S, McMahon SB, Thompson SW, Malcangio M. Brain-derived neurotrophic factor induces NMDA receptor subunit one phosphorylation via ERK and PKC in the rat spinal cord. Eur J Neurosci. 2004;20:1769–1778. doi: 10.1111/j.1460-9568.2004.03656.x. [DOI] [PubMed] [Google Scholar]
- 65.Murawska-Ciałowicz E, Wiatr M, Ciałowicz M, Gomes de Assis G, Borowicz W, Rocha-Rodrigues S, Paprocka-Borowicz M, Marques A. BDNF impact on biological markers of depression-role of physical exercise and training. Int J Environ Res Public Health. 2021;18:7553. doi: 10.3390/ijerph18147553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang DG, Jin SL, Li GY, Li QQ, Li ZR, Ma HX, Zhuo CJ, Jiang RH, Ye MJ. Serotonin regulates brain-derived neurotrophic factor expression in select brain regions during acute psychological stress. Neural Regen Res. 2016;11:1471–1479. doi: 10.4103/1673-5374.191222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martinowich K, Lu B. Interaction between BDNF and serotonin: role in mood disorders. Neuropsychopharmacology. 2008;33:73–83. doi: 10.1038/sj.npp.1301571. [DOI] [PubMed] [Google Scholar]
- 68.Narita M, Aoki K, Takagi M, Yajima Y, Suzuki T. Implication of brain-derived neurotrophic factor in the release of dopamine and dopamine-related behaviors induced by methamphetamine. Neuroscience. 2003;119:767–775. doi: 10.1016/S0306-4522(03)00099-X. [DOI] [PubMed] [Google Scholar]
- 69.Attal N, Cruccu G, Baron R, Haanpää M, Hansson P, Jensen TS, Nurmikko T. EFNS guidelines on the pharmacological treatment of neuropathic pain: 2010 revision. Eur J Neurol. 2010;17:1113–e88. doi: 10.1111/j.1468-1331.2010.02999.x. [DOI] [PubMed] [Google Scholar]
- 70.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, Gilron I, Haanpää M, Hansson P, Jensen TS, Kamerman PR, Lund K, Moore A, Raja SN, Rice AS, Rowbotham M, Sena E, Siddall P, Smith BH, Wallace M. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14:162–173. doi: 10.1016/S1474-4422(14)70251-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150:573–581. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 72.Proudfit HK, Clark FM. The projections of locus coeruleus neurons to the spinal cord. Prog Brain Res. 1991;88:123–141. doi: 10.1016/S0079-6123(08)63803-0. [DOI] [PubMed] [Google Scholar]
- 73.Singewald N, Philippu A. Release of neurotransmitters in the locus coeruleus. Prog Neurobiol. 1998;56:237–267. doi: 10.1016/S0301-0082(98)00039-2. [DOI] [PubMed] [Google Scholar]
- 74.Llorca-Torralba M, Borges G, Neto F, Mico JA, Berrocoso E. Noradrenergic locus coeruleus pathways in pain modulation. Neuroscience. 2016;338:93–113. doi: 10.1016/j.neuroscience.2016.05.057. [DOI] [PubMed] [Google Scholar]
- 75.Pan HL, Wu ZZ, Zhou HY, Chen SR, Zhang HM, Li DP. Modulation of pain transmission by G-protein-coupled receptors. Pharmacol Ther. 2008;117:141–161. doi: 10.1016/j.pharmthera.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hayashida K, Eisenach JC. Spinal α2 -adrenoceptor-mediated analgesia in neuropathic pain reflects brain-derived nerve growth factor and changes in spinal cholinergic neuronal function. Anesthesiology. 2010;113:406–412. doi: 10.1097/ALN.0b013e3181de6d2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hayashida KI, Clayton BA, Johnson JE, Eisenach JC. Brain derived nerve growth factor induces spinal noradrenergic fiber sprouting and enhances clonidine analgesia following nerve injury in rats. Pain. 2008;136:348–355. doi: 10.1016/j.pain.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Delgado PL. Depression: the case for a monoamine deficiency. J Clin Psychiatry. 2000;61(Suppl 6):7–11. [PubMed] [Google Scholar]
- 79.Henry JP, Gasnier B, Roisin MP, Isambert MF, Scherman D. Molecular pharmacology of the monoamine transporter of the chromaffin granule membrane. Ann N Y Acad Sci. 1987;493:194–206. doi: 10.1111/j.1749-6632.1987.tb27201.x. [DOI] [PubMed] [Google Scholar]
- 80.Henry JP, Sagné C, Botton D, Isambert MF, Gasnier B. Molecular pharmacology of the vesicular monoamine transporter. Adv Pharmacol. 1998;42:236–239. doi: 10.1016/S1054-3589(08)60736-X. [DOI] [PubMed] [Google Scholar]
- 81.Taguchi T, Katanosaka K, Yasui M, Hayashi K, Yamashita M, Wakatsuki K, Kiyama H, Yamanaka A, Mizumura K. Peripheral and spinal mechanisms of nociception in a rat reserpine-induced pain model. Pain. 2015;156:415–427. doi: 10.1097/01.j.pain.0000460334.49525.5e. [DOI] [PubMed] [Google Scholar]