Abstract
Biological studies of the determinants of Cryptosporidium infectivity are lacking despite the fact that cryptosporidiosis is a major public health problem. Recently, the 60-kDa glycoprotein (GP60) has received attention because of its high sequence polymorphism and association with host infectivity of isolates and protection against reinfection. However, studies of GP60 function have been hampered by its heavy O-linked glycosylation. Here, we used advanced genetic tools to investigate the processing, fate, and function of GP60. Endogenous gene tagging showed that the GP60 cleavage products, GP40 and GP15, are both highly expressed on the surface of sporozoites, merozoites and male gametes. During invasion, GP40 translocates to the apical end of the zoites and remains detectable at the parasite-host interface. Deletion of the signal peptide, GPI anchor, and GP15 sequences affects the membrane localization of GP40. Deletion of the GP60 gene significantly reduces parasite growth and severity of infection, and replacement of the GP60 gene with sequence from an avirulent isolate reduces the pathogenicity of a highly infective isolate. These results have revealed dynamic changes in GP60 expression during parasite development. They further suggest that GP60 is a key protein mediating host infectivity and pathogenicity.
Subject terms: Parasite development, Parasite biology
The immunodominant surface antigen GP60 undergoes dynamic changes in expression profile and localization during parasite invasion and development and is a key molecule mediating host infectivity and pathogenicity.
Introduction
Cryptosporidiosis is a major public health problem in both developed and developing countries1. It causes moderate to severe diarrhea and associated stunting and mortality in children in low- and middle-income countries and large outbreaks of enteric disease in the general population in high-income countries2,3. Treatment for the disease is limited, with only one partially effective drug, nitazoxanide, approved by the US Food and Drug Administration, and no vaccines against the causative Cryptosporidium pathogen4. This is largely due to a lack of in-depth understanding of the biological factors that control infectivity and virulence5.
The 60-kDa glycoprotein (GP60, also known as GP40/GP15) is one of the earliest molecules identified to be involved in Cryptosporidium-host interactions and possibly virulence. The gene encoding the protein was identified independently by four research groups over 20 years ago by screening expression libraries and N-terminal sequencing of monoclonal antibody identified proteins6–9. Bioinformatic analysis suggests that the protein is a secretory protein with an N-terminal signal peptide and a C-terminal glycophosphatidylinositol (GPI) anchor. The results of these studies suggest that the protein is likely a precursor with a cleavage site that proteolytically processes the protein into two smaller mature fragments, GP40 and GP15 (also known as CP17). A putative furin cleavage site was soon identified in the GP60 sequences, and is used by a subtilisin-like serine protease, CpSUB1, to cleave GP60 into GP40 and GP1510,11. The GP15 fragment of the protein has been identified as an immunodominant antigen and one of the seven Cryptosporidium proteins associated with protection against reinfection in children12. As an immunodominant antigen under selection pressure, GP60 is a variant protein with extensive sequence polymorphism6. As a result, the GP60 gene is the most widely used marker for subtyping human pathogenic C. parvum and C. hominis13. Among the major GP60 subtype families of C. parvum, the IIa, IIc, and IId subtypes differ significantly in host preference14. Similarly, some GP60 subtypes of C. hominis are more virulent than others15.
Despite its potential importance in invasion and virulence, research into the function and action of GP60 in Cryptosporidium-host interactions is hampered by the mucin-like nature of both GP40 and GP15 with heavy O-linked glycosylation and the presence of a GPI anchor in GP15. As a result, recombinant GP60 produced by the prokaryotic expression system lacks proper glycosylation, making it difficult to study its biological processing and functions16. Therefore, although the GP60 gene is the most widely studied Cryptosporidium gene, only a handful of studies have been conducted on the biological properties of the GP60 protein. Even the subcellular location of GP60 is controversial. Some believe that GP60 is a surface protein attached to sporozoites through the GPI anchor6,17, another group suggests that GP60 is associated with the parasitophorous vacuole membrane (PVM)18, while others have used GP60 as a marker for micronemal proteins19.
In the present study, we have performed the first biological characterization of the GP60 protein using genetic manipulation tools. Endogenous gene tagging, deletion, and replacement were performed using CRISPR/Cas9 technology. The results of the study indicate that the multifunctional GP60 is translocated through its unique structural domains to different locations during C. parvum invasion and development, GP60 plays a key role in host infectivity and pathogenicity, and that both GP40 and GP15 are required for GP60-mediated host infectivity.
Results
GP60 is highly expressed in vivo
The results of the whole transcriptome analysis indicated that the GP60 gene was the most highly expressed gene in both the ileum and colon of IFN-γ knockout (GKO) mice infected with C. parvum (Fig. 1A). The high expression of GP60 protein was confirmed by immunofluorescence analysis (IFA) of tissue sections from infected mice using a mAb (IId-67) against the GP40 fragment of the protein, which showed the presence of many green fluorescence-stained parasites at the brush border of the ileum and colon. Some developmental stages were also seen deep in the intestinal tissue on the surface of the crypt of Lieberkuhn (Fig. 1B). The presence of high levels of GP60 expression at both the brush border and crypt of Lieberkuhn was further confirmed by immunohistochemical staining of tissue sections of the ileum and colon from infected animals using the same mAb (Fig. 1C). These results showed that GP60 was expressed throughout most of the developmental stages of Cryptosporidium.
Fig. 1. GP60 is highly expressed in vitro and in vivo.
A Manhattan plots showing the relative expression of all Cryptosporidium genes (by chromosome) in the ileum and colon of IFN-γ knockout (GKO) C57BL/6 mice infected with IIdA20G1-HLJ isolate of Cryptosporidium parvum, as indicated by the FPKM values in RNA-seq analysis of the transcriptome. Each dot represents the expression level of one gene, with the expression of the GP60 gene and two genes (CP2 and LDH) known to have high expression being indicated (n = 4). B Expression of GP60 in ileum and colon sections as detected by immunofluorescence analysis using a mAb against GP40 (mAb IId-67). GP60 expression in parasites within the crypt of Lieberkuhn is indicated by an asterisk symbol. Scale bar = 10 μm. C Expression of GP60 in ileum and colon sections as detected by immunohistochemical analysis using the IId-67 mAb. GP60 expression in parasites within the crypt of Lieberkuhn is indicated by an asterisk symbol. D Violin plots showing the relative GP60 gene expression in the transcriptomes of sporozoites and developmental stages in HCT-8 cells after infection with IIdA20G1-HLJ for different times. Each dot represents one gene, and the expression of the GP60 gene is shown relative to the well-known CP2 and LDH genes (n = 4).
GP60 is a surface protein highly expressed in invasion stages as well as in male gametes
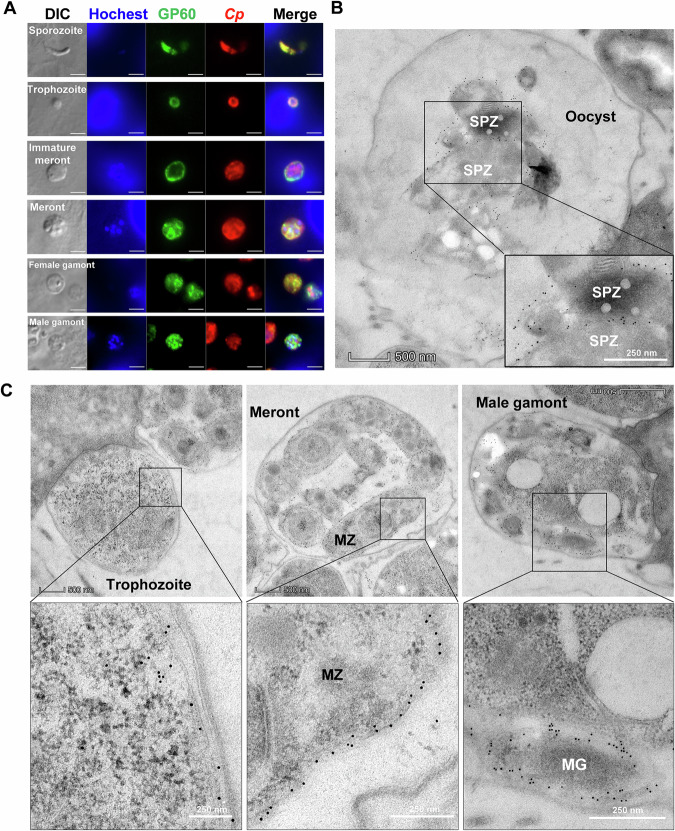
Immunofluorescence analysis of developing stages in HCT-8 cell cultures showed high reactivity of the mAb against GP40 with free sporozoites, developing trophozoites, meronts and male gamonts (Fig. 2A). Immunoelectron microscopy (IEM) of parasites in intestinal tissues of infected mice revealed the presence of gold particles on the surface of late trophozoites, merozoites within meronts, and gametes within male gamonts (Fig. 2B). In contrast, other developmental stages such as early trophozoites and female gametes (Fig. S1) showed lower reactivity to the mAb. In mature oocysts in vivo, GP40 expression was mainly on the surface of sporozoites (Fig. 2C). We did not detect any gold particles in micronemes in IEM analysis.
Fig. 2. GP60 is a surface protein highly expressed in invasion stages as well male gametes.
A Expression of GP60 in developing stages of Cryptosporidium parvum cultured in HCT-8 cells, as indicated by immunofluorescence analysis using an anti-GP40 mAb (IId-67). A polyclonal antibody against sporozoites (Sporo-Glo) is used as the control (Anti-CP). Scale bars = 2 μm. B Expression of GP60 in developing stages of C. parvum in the ileum of IFN-γ knockout (GKO) C57BL/6 mice, as indicated by immunoelectron microscopy using the IId-67 mAb. Gold particles are mainly detected on the surface of late trophozoites, merozoites (MZ) within meronts, and gametes (MG) within the male gamonts. C Expression of GP60 in intravacuolar stage of Cryptosporidium in the ileum of mice as indicated by immunoelectron microscopy using the IId-67 mAb. Gold particles are restricted to the surface of sporozoites (SPZ).
Endogenous gene tagging supports the membrane localization of GP60 protein expression
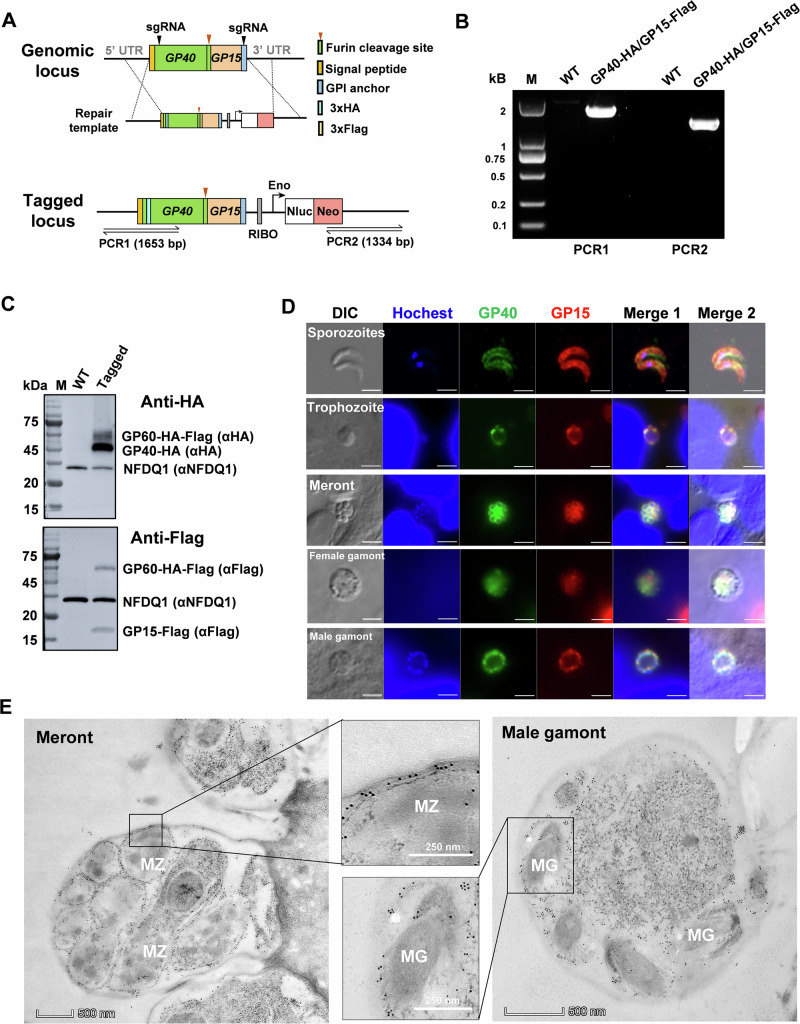
To study the dynamics of the GP60 protein and its cleavage products in the developmental stages of C. parvum, we performed endogenous tagging of the GP60 gene using CRISPR/Cas9 to add a 3× HA tag and a FLAG tag in the gene after the sequence encoding the signal peptide (between amino acids 33 and 34) and the furin cleavage site (between amino acids 212 and 215), respectively, producing the expected GP40 product with an N-terminal HA tag and GP15 product with an N-terminal FLAG tag. Sequences of the neomycin resistance gene (Neo) and nanoluciferase (Nluc) were also included for selection of the tagged line and monitoring of parasite load in mice infected with the transfected C. parvum (Fig. 3A).
Fig. 3. Endogenous gene tagging supports the membrane location GP60 protein expression in Cryptosporidium parvum.
A Strategy used in tagging the N-terminal end of the GP40 fragment with a 3× hemagglutinin (HA) epitope and the N-terminal end of the GP15 fragment with a 3× FLAG epitope using CRISPR/Cas9. B Confirmation of the correct integration of the tagging cassette through PCR analysis of the GP40 fragment tagged with the HA sequence and the GP15 fragment tagged with the FLAG sequence. No product is generated in PCR analysis of the wildtype (WT). C Confirmation of the correct tagging of the GP40 and GP15 products of GP60 by Western blot (WB) analysis of crude protein extracted from C. parvum oocysts using mAbs against the HA and FLAG tags using the expression of NFDQ1 (detected by a polyclonal antibody against recombinant NFDQ1) as control. As expected, both the GP60 precursor and the GP40 product are seen in WB analysis with the HA mAb, and both GP60 and the GP15 product are seen in WB analysis with the FALG mAb. No HA or FLAG-tagged products are seen in WB analysis of the WT. D Expression of the GP40 (tagged with HA) and GP15 (tagged with FLAG) products of GP60 in free sporozoites and developing stages of C. parvum cultured in HCT-8 cells, as indicated by immunofluorescence analysis. GP40 and GP15 colocalize on the surface of sporozoites, later trophozoites, merozoites within mature meront, and gametes within male gamonts, but not on the surface of female gametes. Scale bars = 2 μm. E Confirmation of the surface location of GP40 by immunoelectron microscope analysis of merozoites (MZ) within a meront and gametes (MG) within a male gamont using the HA mAb.
PCR analysis of the transfectant confirmed the correct integration of the tagging cassette into the genome (Fig. 3B). Results of Western blot analysis of oocyst lysate were consistent with this finding; the mAb against HA reacted with two bands of ~65 and 45 kDa, likely representing the HA-tagged GP60 and its furin cleavage product GP40, while the mAb against the FLAG tag detected the full GP60 protein and its furin cleavage product GP15 (Fig. 3C). IFA analysis of life cycle stages of the endogenously tagged mutant using mAbs against HA and FLAG showed abundant expression of GP40 and GP15 on surface sporozoites, trophozoites, merozoites and male gametes. In contrast, the female gametes showed low reactivity to both mAbs. In the IFA analysis, GP40 and GP15 colocalized with each other, allowing comparative studies of their fate during invasion and development of C. parvum (Fig. 3D). This result was confirmed by IEM analysis of the tagged line, which showed a GP40 expression on the surface of these developmental stages (Figs. 3E and S2). Since the native protein of GP60 was not completely cleaved into GP40 and GP15, some of the reactivity of the HA and Flag antibodies in IFA and IEM might have been directed to the intact GP60.
GP60 is translocated to the feeder organelle during invasion and re-synthesized in early intracellular development
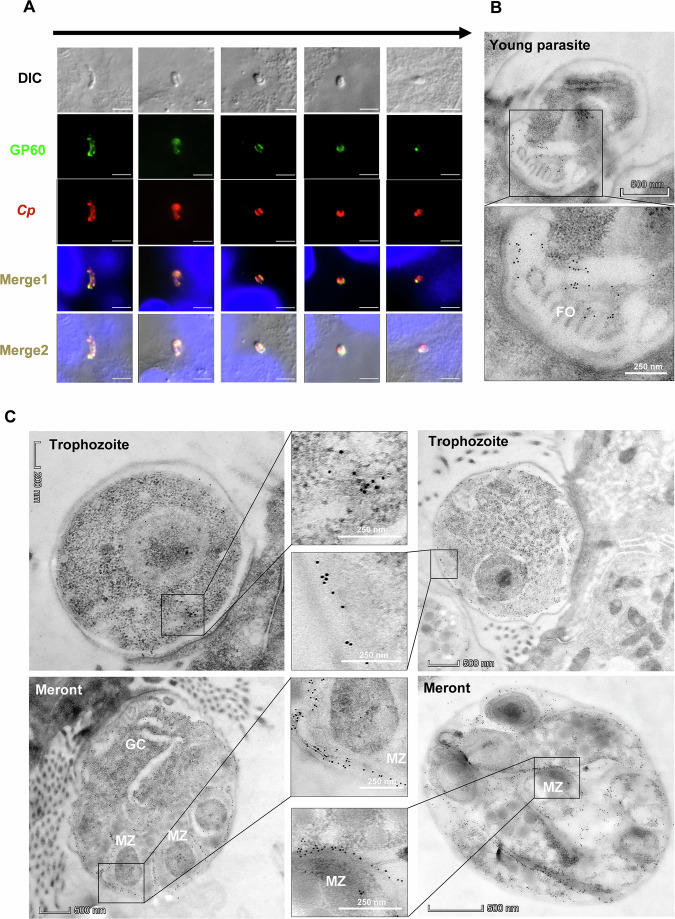
We examined the dynamics of GP60 expression using the endogenously tagged line and the mAb against the HA tag, which was incorporated into the N-terminal end of the GP40. During epithelial cell invasion, GP60 (or its GP40 product) was translocated from the sporozoite surface to the parasite-host interface (Fig. 4A). In enveloped sporozoites and newly formed trophozoites, GP60 expression was largely restricted to the feeder organelle, with no visible detection of the protein in the remaining parts of the developing parasites by IEM analysis (Fig. 4B).
Fig. 4. GP60 moves to the parasite-host interface during invasion and is resynthesized during development.
A Movement of GP60 during invasion of host cells by Cryptosporidium parvum sporozoites as indicated by the HA-tagging of the GP40 product of GP60, using a mAb against the HA tag to track GP40 and a polyclonal antibody against sporozoites (Sporo-Glo) as a control (Anti-CP). GP40 moves to the parasite-host interface as the sporozoite enters the host cell and rounds up. Scale bars = 2 μm. B Confirmation of the localization of GP40 at the C. parvum-host interface (the feeder organelle, FO) by immunoelectron microscopy using the HA mAb. C GP60 is resynthesized in trophozoites and moves to the surface of later trophozoites and merozoites (MZ) within mature meronts. During the early C. parvum development in host cells, only a few gold particles are present in the cytosol of newly formed trophozoites (red arrow). Gold particles increase in number and are mostly seen on the surface of late trophozoites. In 4N meronts, gold particles are mostly seen on the surface of newly formed merozoites (MZ) and within the germinal center (GC). Once the 4N meronts mature to 8N, gold particles are seen only on the surface of the merozoites.
RNA-seq analysis indicated that the GP60 gene was not expressed in oocysts and newly excysted sporozoites. Soon after invasion, GP60 gene expression was first detected, gradually peaking at 12 h and remaining at this level at 24, 36, 48 and 72 h (Fig. 1D). Consistent with this timing, IEM examinations of HA-tagged mutant indicated that GP60 was initially expressed in the cytosol of young trophozoites and subsequently translocated to the surface of mature trophozoites. In 4N meronts, GP60 was mainly found on the surface of developing merozoites and in the germinal center (GC) where new merozoites budded out. As meronts mature to 8N, GP60 was present only on the surface of merozoites (Fig. 4C).
GP60 functions as both a secretory and a membrane protein
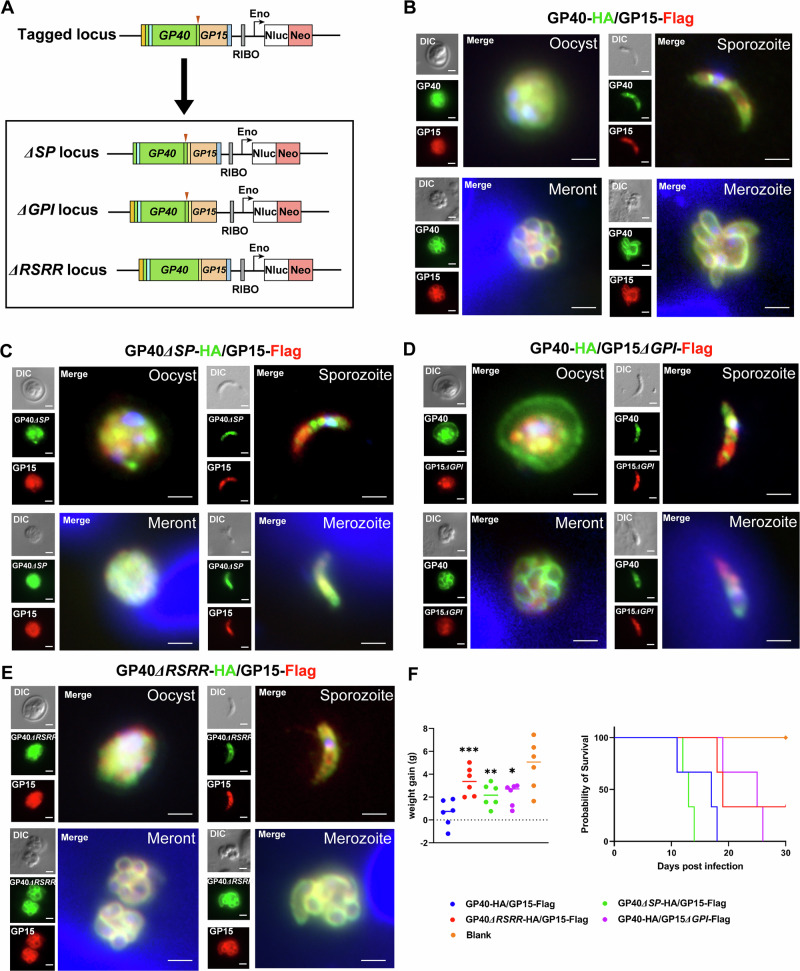
Since GP60 has both a signal peptide and a GPI anchor, we examined the effect of each domain on the translocation of the protein (Fig. 5A). The successful deletion of these sequences in mutant lines was confirmed by PCR analysis using primers franking the insert and 5′and 3′sequences of the native locus, producing the expected PCR products in mutant lines only (Fig. S3A, B). Deletion of the signal peptide prevented the protein from being secreted to the parasite surface. As a result, the synthesized GP60 accumulated in the cytosol of sporozoites, trophozoites and merozoites, as shown by IFA analysis using mAbs against the incorporated HA and FLAG tags (Fig. 5B, C). In contrast, deletion of the GPI anchor sequence resulted in the segregation of newly synthesized GP60 into the PV space between parasite and host membranes (for trophozoites, meronts and male gamonts) and into the space between merozoites (for mature meronts) (Fig. 5B, D). This result was confirmed by comparative IEM analysis of the tagged wildtype (WT) and mutant lines (Fig. S2).
Fig. 5. GP60 functions as both a secretory and a membrane protein.
A Strategy used in deletion of the signal peptide (SP), glycophosphatidylinositol (GPI) anchor, and furin cleavage (RSRR) sequences in Cryptosporidium parvum using CRISPR/Cas9. The GP40-HA/GP15-FLAG construct in Fig. 2A is used as the template in further modifications of the GP60 gene. B Immunofluorescence analysis (IFA) of the expression of the GP40 and GP15 products of GP60 in transgenic C. parvum that has been endogenously tagged at the GP60 locus as shown in Fig. 2A, using mAbs against HA and FLAG. As expected, GP40 (tagged with HA) and GP15 (tagged with FLAG) are expressed on the surface of sporozoites and merozoite and colocalize with each other. Scale bars = 2 μm. C IFA of the expression of GP40 and GP15 products of GP60 in transgenic C. parvum with the signal peptide deleted from GP60. GP40 and GP15 are now expressed in the cytosol and do not colocalize with each other. Scale bars = 2 μm. D IFA analysis of the expression of GP40 and GP15 products of GP60 in transgenic C. parvum with the GPI anchor deleted from GP60. GP40 and GP15 loss the membrane expression and do not colocalize with each other. In particular, GP40 is also detected on the oocysts wall and accumulates in the space between merozoites within meronts. Scale bars = 2 μm. E IFA analysis of the expression of GP40 and GP15 products of GP60 in transgenic C. parvum with the furin cleavage site deleted from GP60. GP40 and GP15 expression is mostly similar to that in (B). Scale bars = 2 μm. F Body weight (DPI 2–DPI 12) and survival (DPI 30) of IFN-γ knockout mice infected with mutant C. parvum lines described above as determined at the end of the infection study (n = 3). ***p = 0.0005, GP40-HA∆RSRR/GP15-FLAG; **p = 0.0030, GP40-HA∆SP/GP15-FLAG; *p = 0.0103, GP40-HA/GP15∆GPI-Flag.
After deletion of the signal peptide or GPI anchor, GP40 (tagged by HA) and GP15 (tagged by FLAG) were no longer co-localized, especially during the oocyst stage (Fig. 5C, D). Furthermore, neither of them co-localized with the membrane protein CP23 at the sporozoite stage (Fig. S4). In oocysts, GP60 was normally expressed only on sporozoites, with colocalization of the GP40 and GP15 products of the processed protein. However, in oocysts of mutants with a deleted GPI anchor in the GP60 gene, GP40 was expressed not only inside sporozoites but also within the oocyst, while GP15 was restricted to inside sporozoites (Fig. 5D). This was particularly evident in the IEM analysis of the mutants with the HA mAb, which showed the presence of gold particles in oocysts space and the space between oocyst wall and PVM (Fig. S2). These data suggested that GP40 was secreted outside the mature stages and anchored to the parasite surface by GP15.
GP60 is proteolytically processed
We replaced the native GP60 gene of C. parvum with a GP60 sequence that had no furin cleavage site (with the RSRR sequence). Targeted sequencing confirmed successful removal of the 12 nucleotides encoding the cleavage sequence (Fig. S5A). Western blot analysis of crude sporozoite proteins using mAbs against the HA and FLAG tags indicated that deletion of the RSRR sequences prevented cleavage of GP60 into GP40 and GP15 (Fig. S5B). However, this lack of processing did not affect the translocation of the GP60 protein, since both GP40 (tagged with HA) and GP15 (tagged with FLAG) co-localized on the surface of sporozoites and merozoites in IFA analysis (Fig. 5E). This result was also confirmed by IEM analysis with the HA mAb (Fig. S5C).
Impaired translocation and processing of GP60 affects the growth and host infectivity of C. parvum
Deletion of the signal peptide, GPI anchor, or furin cleavage sequences had some effect on the growth and pathogenicity of C. parvum. During in vitro infection of HCT-8 cells, deletion of the signal peptide reduced the growth of C. parvum at 24, 36 and 48 h, although no obvious effect was observed when the GPI anchor and furin cleavage sequences were deleted (Fig. S6A). However, deletion of the GPI anchor and furin cleavage sequences moderately reduced the parasite load in infected GKO mice, as indicated by fecal luciferase measurements and oocysts per gram of feces (OPG, Fig. S6B). In addition, mice infected with these mutants had a higher body weight than those infected with the HA-tagged WT line (Fig. 5F), and those infected with mutants lacking the GPI anchor or furin cleavage sequences survived longer (Fig. S6B). The slightly shorter survival time of mice infected with the mutant lacking the signal peptide than those infected with the HA-tagged WT could be due to the inherent variability of GKO mice to C. parvum infection. Histopathologic examination of the ileum was consistent with this observation; mice infected with these mutants had lower parasite loads, fewer changes in intestinal architecture, and a higher villus height to crypt depth ratio than those infected with the HA-tagged WT line (Fig. S6C, D).
GP60 is dispensable
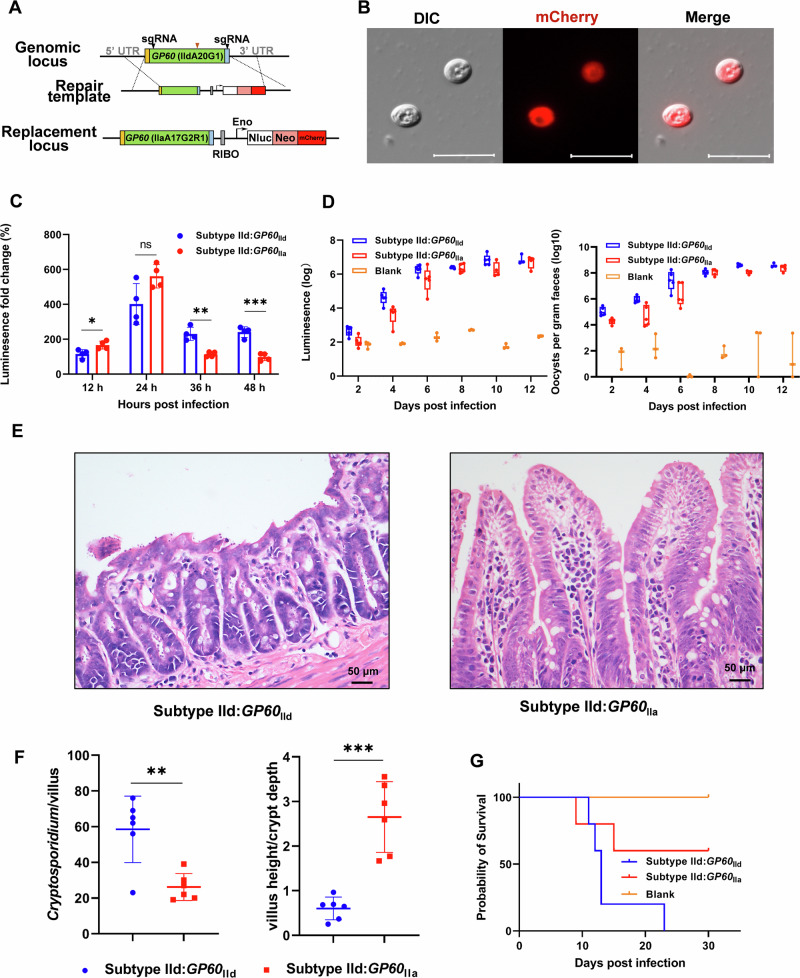
To assess the biological significance of GP60, we replaced the GP60 gene sequence in the C. parvum genome with the mCherry sequence (Fig. 6A). Surprisingly, GP60 was dispensable as we were able to generate a mutant expressing mCherry but lacking the GP60 sequence (Fig. 6B). The complete deletion of the gene was confirmed by PCR analysis of the mutant (Fig. 6C). The oocyst shedding of the mutant was initially low, but increased significantly after a secondary passage in mice (Fig. 6D). IFA analysis of intracellular stages at different cultivation time with the GP40 mAb IId-67 confirmed complete GP60 deletion (Fig. 6E).
Fig. 6. GP60 in Cryptosporidium parvum is dispensable.
A Strategy used in the replacement of the GP60 sequence with sequences encoding mCheery, NanoLuciferase (Nluc), and neomycin resistance gene (Neo). B Microscopy of oocysts from mice infected with the transgenic C. parvum, with almost all oocysts being red. Scale bars = 10 μm. C Confirmation of the correct integration of the knockout cassette through PCR analysis. No product is generated in PCR analysis of the wildtype (WT), except for PCR 3 conducted using GP60-specific primers. D Serial passages of the transgenic line in mice to increase the infection intensity for the characterization of the transgenic line. E Confirmation of GP60 deletion through immunofluorescence analysis using mAb IId-67. No GP60 is expressed on mCherry-positive parasites in HCT-8 cultures. Scale bars = 2 μm. F Effect of GP60 deletion on the growth of C. parvum in HCT-8 cells in comparison with the HA and FLAG-tagged WT (control) line. **p = 0.0094 at 36 h, ***p = 0.0006 at 48 h. G Differences in the infection intensity of C. parvum as measured by fecal luciferase activity between IFN-γ knockout (GKO) mice infected with the GP60-deleted line and those infected with the HA and FLAG-tagged WT line. H Comparison of physical activity between GKO mice infected with GP60-deleted and control lines. ***p = 0.0007. I Differences in the survival of GKO mice infected with GP60-deleted and control lines.
GP60 plays important role in host infectivity of C. parvum
The GP60 deletion had a profound effect on the growth of C. parvum. The GP60-KO mutant grew slower than the HA-tagged WT line, especially during late development in vitro (Fig. 6F). In GKO mice, the mutant line produced a lower infection intensity than the HA-tagged line, as indicated by the measurement of fecal luciferase activity and OPG (Figs. 6G and S7A). As a result, mice infected with the GP60-KO mutant gained weight faster than those infected with the WT line. Moreover, they showed no overt clinical signs of infection, and exhibited normal movement and fecal output. In contrast, mice infected with the HA-tagged line showed lethargy, significant weight loss, and reduced fecal output due to inappetence (Figs. 6H and S7B, C). Consistent with this, mice sacrificed at the peak of C. parvum infection showed differences in parasite load between the two groups. Notably, mice infected with the GP60-KO mutant showed lower numbers of parasites on the surface of the ileum than those infected with the HA-tagged line (Fig. S7D, E). They maintained a largely normal villus structure, with a significantly higher villus height/crypt depth ratio than those infected with the HA-tagged line (Fig. S7F). All mice infected with the GP60-KO mutant survived the infection, while those infected with the HA-tagged lined succumbed by DPI 18 (Fig. 6I).
GP40 and GP15 rely on each other for proper localization
Mutants in which the GP40 or GP15 fragment of the GP60 gene were removed were also successfully generated in vivo (Fig. S8A, B). The correct integration of the gene deletion cassettes was confirmed by allele-specific PCR using primers franking the native and insert sequences (Fig. S3C, D). This was further confirmed by microscopy, as oocysts with GP40 or GP15 deleted were red due to the replacement of gene fragments encoding them with the mCherry sequence. IFA analysis of developmental stages using the mAb against GP40 and a polyclonal antibody against GP15 further confirmed the loss of GP40 or GP15 expression in these mutants (Fig. S8C).
The gene deletion changed the location of the two GP60 fragments. Although oocysts generated by the GP40-KO and GP15-KO mutants were red due to the replacement of the fragments with mCherry, sporozoites excysted from them were colorless, indicating that the association between GP40 and GP15 on the sporozoite membrane was prevented by the replacement of the GP40 or GP15 with mCherry (Fig. S8B). This was expected, as the GP40-KO and GP15-KO mutants both had kept the furin cleavage site, which would hydrolyze the protein expressed by the GP40-KO and GP15-KO mutants to mCherry and GP40 or GP15. Similarly, the GP40 deletion changed the location of GP15 expression in mature meronts from the membrane to the cytosol, probably due to the lack of signal peptide after the furin cleavage. In addition, the GP15 deletion changed the location of GP40 in mature meronts from membrane of merozoites to space between them due to the loss of the GPI anchor after the furin cleavage (Figs. S8C and S9A, B). As a result, GP40 and the mCherry did not co-localize with each other, with mCherry remaining inside merozoites (Fig. S9A, C).
We were unable to accurately assess the effect of GP40 and GP15 deletion on C. parvum growth in vitro and pathogenicity in vivo because of the presence of some WT oocysts in secondary passages of the GP40- and GP15-deleted lines. Approximately 2% (2/121 for the GP40-deleted line and 3/160 for the GP15-deleted line) of the oocysts in these mutant lines were colorless.
GP60 contributes to host infectivity
Previously, we showed that the IIdA20G1-HLJ isolate induced significantly higher oocyst shedding intensity and duration than the IIaA17G2R1-IOWA isolate, leading to lower body weight gain and survival of infected GKO mice20. The GP60 sequences of the two isolates differ mainly in the GP40 region of the protein, with nearly identical sequences of the signal peptide, furin cleavage site, and GPI anchor (Fig. S10). To further evaluate the role of GP60 in host infectivity of C. parvum, we replaced the GP60 gene in the genome of the highly infective IIdA20G1-HLJ isolate with the GP60 sequence of the avirulent IIaA17G2R1-IOWA isolate (Fig. 7A). The gene replacement was confirmed by the ectopic expression of mCherry in transgenic oocysts as revealed by fluorescence microscopy (Fig. 7B), and by DNA sequence analysis (Fig. S3E). It resulted in slower in vitro growth of C. parvum during the sexual phase of development (Fig. 7C). It also reduced the intensity of oocyst shedding in GKO mice compared to those infected with the tagged WT line (Fig. 7D). In addition, mice infected with the replaced GP60 gene survived longer (Fig. 7G). Consistent with this, mice infected with the IIa GP60 mutant had fewer parasites on the ileal surface and less damage to the villus architecture than those infected with the tagged line carrying the WT IId GP60 gene (Fig. 7E, F).
Fig. 7. GP60 contributes to host infectivity as indicated by gene replacement results.
A Strategy used in the replacement of the GP60 gene in the virulent IIdA20G1-HLJ isolate with the GP60 sequence from the a virulent IIaA17G2R1-IOWA isolate, with the incorporation of sequences encoding mCheery, NanoLuc luciferase (Nluc), and neomycin resistance gene (Neo) for the identification and selection of transgenic parasites. B Microscopy of oocysts from mice infected with the transgenic line, with the transgenic oocysts being red. Scale bars = 10 μm. C Effect of the GP60 replacement on the growth of C. parvum in HCT-8 cells in comparison with those infected with the HA and FLAG- tagged WT line. The gene replacement has reduced the growth of C. parvum at 36 h and 48 h. *p = 0.0236 at 12 h, **p = 0.0011 at 36 h, ***p = 0.0002 at 48 h. D Effect of the GP60 replacement on the infection intensity of C. parvum in IFN-γ knockout (GKO) mice as measured by fecal luciferase activity and oocysts per gram of feces, in comparison with the uninfected control. Differences in the ileum architecture (E) and parasite load and villus height to crypt depth ratio (F) in the ileum between mice infected with the virulent WT line (Subtype IId) and those infected with the GP60 replacement line (Subtype IId:GP60IIa). Mice infected with the latter have fewer parasites and less intensive damage in the ileum. **p = 0.0028, ***p = 0.0001. G Differences in the survival of GKO mice after infection with the GP60 replacement line (Subtype IId:GP60IIa) and similarly tagged WT line (Subtype IId).
Discussion
One of the long-standing issues in cryptosporidiosis research is the parasite-host interaction during early infection21. More than a handful of parasite molecules located on the apical end and surface of sporozoites have been implicated in the initial attachment and invasion process of C. parvum22. They include several members of the mucin glycoprotein family such as Muc4, Muc5, GP900, and GP60. Of these, GP60 and its cleavage products GP40 and GP15 have received the most attention. Studies using neutralizing mAbs have implicated GP60 and its GP40 and GP15 products in sporozoite and merozoite attachment to and invasion of enterocytes8,23. Differences in host preference and virulence have been reported between GP60 subtype families of C. parvum and C. hominis, respectively14,15. Mice experimentally infected with different GP60 subtypes of C. parvum have shown significant differences in infection intensity, duration, and pathogenicity20. However, few studies have been undertaken to examine the biological processes of GP60-mediated host cell infection. Underlying the poor understanding of Cryptosporidium infection is the lack of in-depth studies of the parasite-host interactions using advanced genetic tools22. CRISPR/Cas9-based genetic manipulation tools have only recently been used by one research group to systematically characterize the role and process of ROP and MEDLE proteins in early C. parvum-host interactions24,25. These proteins have been shown to be exported into host cells and to modify host cytoskeletal and other responses during C. parvum invasion and infection process.
Data from this study suggest that the GP60 protein likely plays multiple roles in C. parvum-host interactions and contributes to host infectivity. Consistent with its immunodominant nature, GP60 is expressed on the surface of mobile stages (sporozoites, merozoites, and male gametes), suggesting that GP60 may be involved in sporozoite and merozoite invasion and fertilization of C. parvum. Once synthesized in the parasite cytosol, GP60 is secreted outside the developing stages through its signal peptide, cleaved at its furin cleavage site into GP40 and GP15, anchored to the surface of C. parvum developing stages through the GPI anchor at the C-terminal end of GP15, translocated to the apical end of the sporozoites during invasion, and remains detectable only at the pathogen-host interface. Therefore, the GP40 and GP15 products of GP60 are dependent on each other for translocation to the parasite surface. Since the deletion of the GP60 gene and replacement of the gene sequence with that of an avirulent strain both reduced the growth and pathogenicity of a virulent strain of C. parvum, GP60 appears to be a key factor in host infectivity.
We have confirmed the surface location of the GP60 protein. Analyses using mAb staining and endogenous gene tagging have both shown high levels of GP60 on the surface of sporozoites, merozoites, and male gametes. Previously, several studies using mAbs have shown the expression of GP60 on the surface of both sporozoites and merozoites6,8,17. We believe that the previous suggestion of GP60 as a PVM protein18 was the result of a misinterpretation of the surface location of GP60 in late trophozoites and merozoites under immunofluorescence microscopy. We have furthered our understanding of GP60 expression by demonstrating that the GP60 gene is the most highly expressed gene in the C. parvum genome by transcriptome analysis of both in vitro and in vivo infections. Further studies are needed to determine whether GPI-anchored GP60 is transported from the Golgi to the plasma membrane via vesicles following the conventional sorting system for GPI-anchored proteins26, or is first transported into micronemes where GP60 has been identified19.
More importantly, we have shown for the first time that GP60 is highly expressed on the surface of male gametes. To date, only a few proteins, such as α-tubulin and HAP2, are known to be expressed at high levels in male gametes of Cryptosporidium spp.27. Like α-tubulin, GP60 is also expressed in sporozoites and merozoites, but rarely in female gametes. The function of GP60 in male gametes is not yet known, but the fact that GP60 is involved in the initial parasite-host interaction and the surface location of the protein suggest that the protein may be involved in the fertilization of female gametes by the male gametes. This is further supported by the observation that the suppression of parasite growth in vitro by GP60 gene deletion and replacement mainly occurred during the sexual development of C. parvum. Currently, little is known about the sexual development in Cryptosporidium spp. As blockage of male gamete entry into female gametes has been suggested as a cause of incomplete development of C. parvum in vitro, sexual reproduction likely plays an important role in pathogen transmission27. The identification of a surface protein in male gametes opens a new avenue for studies of sexual reproduction in Cryptosporidium spp. To date, studies on gene expression in sexual stages have been limited to female gametes due to lack of surface markers for flow cytometric sorting of male gametes19,27.
In agreement with the multifunctional nature of GP60 protein, the expression of the GP60 gene undergoes dynamic changes during the life cycle of C. parvum. While the GP60 protein is highly expressed in sporozoites, the gene encoding it is not transcribed during this stage. During invasion, GP60 moves to the apical end and is shed by sporozoites and merozoites gliding on host cells6. Shortly after the entry into the host membrane, GP60 is detectable only at the parasite-host cell interface, reinforcing its role during the invasion. Once the newly invaded parasite is rounded off, GP60 is no longer detected, and new GP60 is synthesized in the cytosol of developing trophozoites and translocated to the surface of late trophozoites in preparation for merozoite biogenesis. Both the signal peptide and GPI anchor sequences are required for GP60 translocation, as deletion of the signal peptide resulted in GP60 accumulation in the cytosol, and deletion of the GPI anchor sequence changed the membrane location of the GP40 product to free in the parasitophorous vacuole and oocyst. This finding is expected because in eukaryotic cells the signal peptide is required for proper sorting of secretory proteins in the endoplasmic reticulum28. Similarly, the GPI anchor is required for the sorting of GPI-anchored proteins from other secretory proteins in the trans-Golgi network for transport to the plasma membrane26.
The surface location of GP60 is consistent with its highly variable and immunodominant nature. Since its initial discovery, the GP60 gene has been known as the most polymorphic gene in the Cryptosporidium genome, expressing different families of GP60 proteins with highly divergent sequences6,29. C. parvum isolates from different subtype families differ from each other in their host preference, with IIa being mainly found in calves and humans, IId mainly in lambs, goat kids, and humans, and IIc almost exclusively in humans14. In contrast, C. hominis families appear to differ from each other in virulence, with the Ib subtype family, particularly the IbA10G2 subtype, causing more severe clinical manifestations than other subtype families in some epidemiological studies15. Therefore, GP60 characteristics have been linked to the phenotypic characteristics of Cryptosporidium isolates. This correlation is also supported by the dominance of the C. parvum IIaA15G2R1 subtype worldwide and the C. hominis IbA10G2 subtype in Europe and other high-income countries30,31. As one of the two most dominant antigens in C. parvum and C. hominis32,33 and one of the few protective Cryptosporidium antigens against reinfection12, immune and other adaptive selection has likely occurred in this gene34,35.
In this study, we provide experimental evidence for GP60 as a key factor in C. parvum infectivity and pathogenicity. Despite the biological importance of GP60, the gene encoding it is dispensable, suggesting the existence of alternative mechanisms for zoite invasion. However, deletion of the GP60 gene significantly reduced the growth of C. parvum, and GKO mice infected with the mutant line showed significantly lower parasite loads and no clinical signs of infection, while mice infected with the tagged WT line experienced severe infection and exhibited lethargy, arched backs, wet feces, and weight loss. All mice infected with the WT line died, while those infected with the mutant line survived the infection. More importantly, replacing the IId GP60 sequence with the IIa sequence from an avirulent isolate significantly attenuated the WT line, supporting the involvement of GP60 in host infectivity. The presence of WT oocysts in secondary passages of GP40- and GP15-deleted lines suggests that these deletions may also have a deleterious effect on C. parvum infection. Similarly, disruption of the translocation and processing of the GP60 proteins by deleting the signal peptide, GPI anchor and furin cleavage sequences only moderately reduces the infectivity and pathogenicity of the pathogen. This, together with the disposable nature of the complete GP60 gene, suggests that there are compensatory mechanisms for GP60-mediated infection of host cells. Although yet observed in Cryptosporidium spp., plasticity and functional redundancy are common features of gene families involved in the invasion process of Plasmodium spp. and T. gondii36,37.
In conclusion, we have performed the first biological study of a major immunodominant Cryptosporidium antigen using reverse genetic tools. The data obtained suggest that GP60 likely plays multiple functions during C. parvum invasion and reproduction and is an important factor in host infectivity. Further studies are needed to identify the mechanisms underlying GP60-mediated functions. Such studies should also evaluate the potential effect of the GP60-deletion on immune responses induced by C. parvum infection.
Methods
Ethics statement
The laboratory animals were housed and treated in accordance with the Regulations for the Administration of Affairs Concerning Experimental Animals of the People’s Republic of China. We have complied with all relevant ethical regulations for animal use. All experiments were conducted under the approval of the Laboratory Animal Research Center of Guangdong Province and South China Agricultural University (approval number: 2021C005).
Mice
IFN-γ knockout (GKO) C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, Maine, USA) and bred in the Laboratory Animal Center of the South China Agricultural University. Mice were housed in individually ventilated cages and provided with sterilized food, water, and bedding. C57BL/6J mice (3–5 weeks old) were purchased from the Laboratory Animals Research Center of Guangzhou Province. Most of the infection studies were performed on mice aged 3–5 weeks. Female Kunming mice (6–8 weeks old) used in antibody production were purchased from the Laboratory Animals Research Center of Guangzhou Province.
HCT-8 cell culture and infection
The human ileocecal adenocarcinoma cell line HCT-8 (ATCC CCL-244) was obtained from the Chinese Academy of Sciences for the in vitro cultivation of C. parvum. HCT-8 cells were seeded in 24-well plates and cultured until they reached approximately 80% confluence. The cells were then inoculated with C. parvum oocysts in RPMI 1640 culture medium (Gibco, Grand Island, USA) supplemented with 2% FBS (Gibco, Grand Island, USA) as described38. Invasion stages of parasites were obtained by infecting HCT-8 cells for 5–30 min, while intracellular stages of parasites were obtained by infecting HCT-8 cells for 3–48 h. Monolayers were harvested at different times to observe trophozoites (3 h), meronts (12, 24, and 36 h) and male and female gametes (48 h) using the IFA assay.
Oocyst preparation and excystation
The C. parvum IIdA20G1-HLJ isolate was obtained from dairy calves in Heilongjiang (HLJ) Province, China20, while the C. parvum IIaA17G2R1-IOWA isolate was purchased from Waterborne, Inc. (New Orleans, USA). They were passaged in calves or GKO mice, with oocysts purified as described20 and stored at 4 °C in PBS for up to 6 months before use. Purified oocysts of the similar age were used in comparative studies of different mutant lines. Prior to infection, oocysts were treated with 10% Clorox Household Bleach (Clorox, Oakland, USA) on ice for 10 min, followed by centrifugation at 16,000 × g and 4 °C for 3 min. After discarding the supernatant, the oocysts were washed three times with PBS. The oocysts were then resuspended in 400 μL of 1% BSA-PBS and mixed with 400 μL of 0.2 mM sodium taurodeoxycholate solution (Sigma-Aldrich, Saint Louis, USA) to a final concentration of 0.75%. The mixture was incubated for 1 h at 37 °C in a water bath and free sporozoites obtained were washed three times with PBS by centrifugation at 16,000 × g and room temperature for 3 min.
Transcriptome analysis
For in vivo experiments, GKO mice were infected with 10,000 C. parvum oocysts and their intestines were harvested at DPI 12 for RNA extraction. For in vitro experiments, HCT-8 cells were seeded in 48-well plates and cultured as described above, followed by infection with 4 × 105 bleached C. parvum oocysts per well. RNA extraction from free sporozoites was performed immediately after oocyst shedding, while RNA extraction from parasites-infected cells was performed at 3, 6, 12, 24, 36, 48 and 72 h post infection. Four replicates were performed for each time period and negative controls consisted of uninfected cells. The extracted RNA was sent to Genedenovo Biotechnology Co., Ltd (Guangzhou, China) for transcriptome sequencing using Illumina technology. FastQC (v0.11.9, https://github.com/s-andrews/FastQC) was used for quality control of the sequence reads, while STAR (2.7.9a) was used to map the raw reads to the reference C. parvum IIdA20G1-HLJ genome (SRA, SRR15694560). FPKM values for the GP60 and reference genes were calculated using RSEM (v1.3.1, https://github.com/deweylab/RSEM.git), and gene expression levels were visualized as violin plots using the R package ggplot2 and Manhattan plots using the R package qqman. The RNA-seq data generated in this study have been submitted to NCBI under BioProject No. PRJNA1011005.
Preparation of recombinant proteins and antibodies
Recombinant GP60, GP15, NFDQ1 (encoded by cgd8_10) and CP23 (encoded by cgd4_3620) proteins with a polyhistidine tag but without the signal peptide or glycosylphosphatidylinositol (GPI) were expressed in a prokaryotic expression system, and purified. The GP60, NFDQ, and CP23 genes were amplified and cloned into the pET-28a expression vector. Similarly, the GP15 gene was amplified and cloned into the pE-SUMO expression vector. The recombinant plasmids (pET-28a-GP60, pET-28a-NFDQ, pET-28a-CP23 and pE-SUMO-GP15) were transformed into E. coli BL21 (DE3) strain. The recombinant proteins produced were purified using Ni-Sepharose (General Electric Company, USA) according to the manufacturer-recommended procedures. Recombinant GP40 with HA tag was expressed in a eukaryotic expression system, and purified as described previousely39.
To generate a mAb against GP60, recombinant GP60 was used to immunize three BALB/c mice. Splenocytes from these mice were fused with mouse myeloma cells, and positive hybridoma cells reactive with the recombinant protein were screened by ELISA. A stable hybridoma cell line secreting antibodies reactive with GP60 was obtained by subcloning. The GP60 mAb was produced and purified from ascites of mice intraperitoneally injected with the positive hybridoma using routine procedures. A mAb named IId-67 was found to recognize GP40. In Western blot analysis, IId-67 reacted with recombinant GP40 but not with recombinant GP15 (Fig. S11). Thus, it is a mAb to GP40.
To generate pAbs against GP15, NFDQ and CP23, three Kunming mice were each immunized subcutaneously with 100 μg of purified recombinant proteins in an equal volume of Freund’s complete adjuvant (Sigma-Aldrich, Saint Louis, USA) for the primary immunization and the same antigens in Freund’s incomplete adjuvant (Sigma) 7, 14, 28 and 44 days later. Sera were collected 14 days after the last immunization and the preimmune sera were used as control sera.
Histological examination
Intestines were harvested from parasite-infected GKO mice. Intestinal fragments were fixed in 4% paraformaldehyde for 12 h, embedded in paraffin, and sectioned. The samples were processed for staining with hematoxylin and eosin, immunofluorescence staining with anti-GP40 primary antibody and Alexa Fluor-conjugated secondary antibodies, and immunohistochemistry with anti-GP40. The stained slides were examined under an Olympus BX53 microscope (Olympus, Tokyo, Japan).
Luciferase assay
Luciferase assays were performed using the Nano-Glo Luciferase Assay Kit (Promega, Madison, USA) as previously described40. For mouse fecal samples, one fecal pellet was collected in a 1.5 mL microcentrifuge tube to which eight 3 mm glass beads (Fisher Scientific, Hanover, USA) and 0.8 mL fecal lysis buffer (50 mM Tris pH 7.6, 2 mM DTT, 2 mM EDTA pH 8.0, 10% glycerol, 1% Triton X-100 prepared in water) were added. The tube was vortexed for 1 min and centrifuged at 19,000 × g for 1 min. Then 50 μL of the supernatant was added to a 96-well plate, followed by the addition of 50 μL of a 25:1 Nano-Glo Luciferase Buffer to the Nano-Glo Luciferase Substrate Mix. The plate was then incubated for 3 min at room temperature in the dark.
For luciferase analysis of HCT-8 cell cultures, the medium was replaced with 100 μL Nano-Glo Luciferase Buffer and the mixture was incubated at 37 °C for 10 min. The cells were scraped, the resulting lysate was transferred to 96-well plates and 1 μL Nano-Glo Luciferase Substrate was added. The plate was incubated in the dark at room temperature for 3 min, and luminescence was measured using a Synergy HTX imaging multimode reader (BioTek, Winooski, USA).
Transfection of C. parvum sporozoites
Oocysts (2.5 × 107 per transfection) were excysted as described above, and the released sporozoites were resuspended in 80 μL complete SF buffer (consisting of 65.6 μL Cell Line Solution and 14.4 μL Supplementary 1). Afterwards, 20 μL of plasmid suspension (50 μg Cas9/gRNA expression plasmid and 50 μg homologous repair plasmid) was then added to the mixture, which was transferred to a cuvette (Lonza Cologne, Cologne, Germany) and electroporated using an AMAXA 4D nucleofector system (Lonza Cologne) with program EH100. The transfected sporozoites were diluted in PBS and inoculated into three GKO mice by oral gavage. Prior to infection, the mice were given 100 μL of 8% NaHCO3 solution to neutralize gastric acid. The next day, the drinking water in the animal cages was replaced with a solution containing 16 g/L paromomycin (Yuaye, Shanghai, China) for in vivo selection. Parasite shedding was monitored by measuring nanoluciferase activity in the feces of infected mice.
Immunofluorescence microscopy assay
For IFA analysis of extracellular sporozoites and oocysts, oocysts were excysted and placed on a coverslip, air dried, fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.5% Triton X-100 (Sigma-Aldrich, St. Louis, USA) at 37 °C for 30 min and blocked with 1% BSA at 37 °C for 20 min. For IFA analysis of intracellular parasites in vitro, HCT-8 cells were infected with oocysts and incubated for different periods. The cells were fixed with 4% paraformaldehyde for 15 min, permeabilized with 0.1% Triton X-100 at 37 °C for 15 min and blocked with 1% BSA at 37 °C for 20 min. The following primary antibodies were diluted in 0.1% BSA for staining: rabbit anti-HA (CST, Danvers, USA) at 1:800, mouse anti-FLAG (Beyotime, Shanghai, China) at 1:500, anti-GP40 (purified mouse mAb IId-67) at 1:1000, anti-GP15 (purified mouse pAb) at 1:500, and anti-CP23 (rabbit pAb) at 1:1000. Cells were incubated with the antibodies overnight at 4 °C, followed by 6 washes with PBS. Alexa Fluor-conjugated secondary antibodies (Thermo, Massachusetts, USA) diluted 1:1000 and Hoechst diluted 1:2000 in 0.1% BSA were added to the coverslips and incubated at 37 °C for 30 min. The cells were then washed 6 times in PBS, mounted on slides using Vectashield and sealed with nail varnish. The stained slides were examined under a Zeiss Axioskop Mot 2 fluorescence microscope (Carl Zeiss, Oberkochen, Germany).
Immunoelectron microscopy (IEM)
GKO mice were infected with 1000 transgenic oocysts and the ileum was harvested at DPI 12, which was the time of peak parasite load. The samples were sectioned lengthwise, washed three times with cold PBS and fixed in freshly prepared 0.1% glutaraldehyde and 4% paraformaldehyde mixture for 4 h at 4 °C. After gradient dehydration, infiltration in LR-White resin overnight and a further 24 h in fresh LR-White resin at −20 °C, the samples were transferred to fresh LR-White resin and polymerized under UV light (365 nm wavelength) at −25 °C for 3 to 4 days. The samples were trimmed and cut into 70 nm ultrathin sections using a Leica EM UC7 ultramicrotome (Leica Microsystems Inc.). The sections were blocked with 1% BSA for 20 min and then incubated with rabbit anti-HA antibody (CST) overnight at 4 °C. After washing in 0.1% BSA, the sections were further incubated with 10 nm colloidal gold-conjugated goat anti-rabbit IgG (H + L) for 60 min. Finally, the sections were stained with 2% uranyl acetate (w/v) and examined using a Talos L120C transmission electron microscope (Thermo, Massachusetts, USA). No IEM was performed with the anti-FLAG mAb due to its low affinity, which resulted in the presence of few gold particles after sample processing. IEM was the primary method we used to discern the localization of the GP60 expression.
Scanning electron microscopy (SEM)
The ileum was collected as described above, prefixed in 2.5% glutaraldehyde, postfixed in 0.2 M osmium tetroxide (OsO4) and examined under an EVO MA 15/LS 15 scanning electron microscope (Carl Zeiss).
Western blot analysis
After excystation of 5 × 106 oocysts, sporozoites were lysed with 40 μL RIPA lysis buffer (Thermo) and incubated at 4 °C overnight. The supernatant was collected after centrifugation and mixed with 10 μL 5×SDS loading buffer (TRANS, Beijing, China) before boiling for 10 min. The samples were stored at −20 °C to prevent protein renaturation. The prepared samples were resolved by SDS-PAGE and transferred to a PVDF membrane. The membrane was then blocked in TBST (0.05% Tween 20 in TBS) with 5% skim milk for 2 h at room temperature, followed by three 5 min washes with TBST. The following primary antibodies were diluted in TBST containing 0.5% skim milk: rabbit anti-HA (CST) at 1:800, mouse anti-FLAG (Beyotime) at 1:400, and anti-NFDQ1 (purified rabbit pAb) at 1:1000. The membrane was then incubated with the antibodies overnight at 4 °C, followed by five 5 min washes each with TBST. After overnight incubation at 4 °C, the membrane was washed with TBST and then incubated with HRP-conjugated antibody (Beyotime) diluted 1:2000 in TBST containing 0.5% skim milk for 45 min at room temperature. After washing with PBST, the membrane was incubated with Immobilon Western chemiluminescent HRP substrate (1:1 solution A-solution B; Millipore), viewed on the Chemstudio imaging system (Analytik Jena, Germany) and imaged using VisionWorksLS (Analytik Jena, Germany). Uncropped blots are shown in Fig. S12.
Primers
PCR primers used in the study are listed in Supplementary Data 1 in the Supplementary Material. They were synthesized by Sangon Biotech (Shanghai, China).
Construction of repair templates and CRISPR/Cas9 plasmids for genetic modification of C. parvum
Homology repair templates and CRISPR/Cas9 plasmids were generated as previously described41. Briefly, CRISPR/Cas9 plasmids were generated by adding a single guide RNA (sgRNA) targeting the gene of interest to the linear Cas9 plasmid amplified from pACT:Cas9-GFP, U6:sgINS1 via Gibson assembly cloning. To target the GP60 gene for deletion, a dual sgRNA strategy was used to target regions at the 5’ and 3’ ends of the gene. Primers for CRISPR/Cas9 are listed in Supplementary Data 1.
Endogenous epitope tagging
A dual sgRNA-directed CRISPR/Cas strategy was used for endogenous tagging of the GP60 gene. The plasmid pACT1:Cas9-GFP, U6:sgGP60 was generated by replacing the INS1 sgRNA in the plasmid pACT1:Cas9-GFP, U6:sgINS141 with an sgRNA corresponding to a region 43 bp downstream of the initiation codon and 45 bp upstream of the stop codon in the GP60 gene (CPCDC_6g1080) by Gibson assembly cloning using the ClonExpress II One Step Cloning Kit (Vazyme, Nanjing, China). The GP60 sgRNA was designed using the eukaryotic pathogen CRISPR guide RNA/DNA design tool (http://grna.ctegd.uga.edu). To generate a plasmid capable of tagging both the GP40 fragment with the HA tag and the GP15 fragment with the FLAG tag, a portion of the N-terminal UTR (412 bp), CDS (969 bp) and C-terminal UTR (888 bp) of the GP60 gene was first amplified from C. parvum (IIdA20G1) genomic DNA. The Nluc-P2A-neo reporter and the pUC19 backbone were then amplified from the pINS1-3HA-Nluc-P2A-neo plasmid41. After completion of the plasmid pGP40/GP15-Nluc-P2A-neoby Gibson assembly cloning, 3× HA and 3× FLAG were inserted into the region 99 bp downstream of the initiation codon and the other region 291 bp upstream of the stop codon in the GP60 gene using the same approach. The tagging plasmid pGP40-HA-GP15-FLAG-Nluc-P2A-neo was completed after introduction of the mutant protospacer adjacent motif (PAM) using Gibson assembly cloning.
Gene deletion
To generate a plasmid for GP60 gene deletion, the targeting plasmid pGP60-mCh-Nluc-P2A-neo-GP60 was made by replacing the UPRT homologous flanks in plasmid UPRT-mCh-Nluc-P2A-neo-UPRT with GP60 homologous flanks (1142 bp upstream and 1075 bp downstream of the CPCDC_6g1080 gene) using Gibson assembly of PCR-amplified fragments. To generate plasmids for GP40 and GP15 gene deletion, the targeting plasmids pmCh/GP15-Nluc-P2A-neo and pGP40/mCh-Nluc-P2A-neo were generated by modification of the plasmid pGP40/GP15-Nluc-P2A-neo described above. The mCherry sequence was amplified from UPRT-mCh-Nluc-P2A-neo-UPRT and used to replace the GP40 (GP60 CDS 100–618 bp) or GP15 (GP60 CDS 676–870 bp) gene by Gibson assembly cloning. To knock out the furin-like protease cleavage sites (RSRR), signal peptide (SP) and glycosylphosphatidylinositol (GPI) anchor in the GP60 gene, the targeting plasmid pGP40∆RSRR-HA/GP15-FLAG-Nluc-P2A-neo, pGP40∆SP-HA/GP15-FLAG-Nluc-P2A-neo, and pGP40-HA/GP15∆GPI-FLAG-Nluc-P2A-neo were generated by modification of the plasmid pGP40-HA/GP15-FLAG-Nluc-P2A-neo.
Gene replacement
To replace the GP60 gene in the virulent IIdA20G1-HLJ isolate with the GP60 sequence of the avirulent IIaA17G2R1-IOWA isolate, the targeting plasmid pGP40/GP15 (IIaA17G2R1)-Nluc-P2A-neo-mCh was produced by modification of the plasmid pGP40/GP15 (IIdA20G1)-Nluc-P2A-neo. The GP60-IIdA20G1 sequence in the plasmid pGP40/GP15 (IIdA20G1)-Nluc-P2A-neo was replaced by the GP60-IIaA17G2R1 sequence amplified from DNA extracted from IIaA17G2R1-IOWA. The mCherry sequence and PAM were introduced into the plasmid as described above.
In vitro growth assay of C. parvum
HCT-8 cells were seeded in 48-well plates and cultured as described above, followed by infection with 10,000 oocysts per well. Luminescence was measured at 3, 12, 24, 36 and 48 h after infection. The relative luminescence value was obtained by dividing the luminescence value of the corresponding day by the luminescence value at 3 h. The growth rate was calculated by dividing the following day’s data by the previous day’s data.
In vivo infected assay of C. parvum
IFN-γ KO mice and wild type mice (C57BL/6J mice) aged 3 to 5 weeks were used in the experiment. They were weighed on the day of infection and assigned to groups according to the experimental conditions, with the mean body weight being similar between groups. Mice were housed in individual cages throughout the infection studies. Each mouse was infected with 1000 fresh oocysts by gavage and received paromomycin (16 g/L) in the drinking water during the experiment. Feces were collected every 2 days, starting at DPI 2, and the intensity of oocyst shedding in feces was determined by OPG and luciferase assays. Mice were weighed every 2 days and the time of death was recorded to construct the survival curve.
Quantification of oocyst shedding
DNA was extracted from fecal pellets using a Magbeads FastDNA Kit for Soil (MP Biomedicals, Santa Ana, USA). Oocyst numbers were quantified by SSU rRNA-based qPCR as previously described42. A standard curve for C. parvum genomic DNA was generated by extracting DNA from negative fecal samples spiked with 10, 102, 103, 104, 105, and 106 oocysts of the C. parvum IOWA isolate (Waterborne, New Orleans, USA). The qPCR reactions were performed on a LightCycler 480 II (Roche, Indianapolis, USA) as previously described43.
PCR verification of gene integrations
PCR analysis of the targeted genomic regions was used to confirm the correct integration of the insertion or replacement sequences into the transgenic lines. The PCR reaction contained DNA purified from oocysts, 2× Fine Taq PCR SuperMix (TRANS, Beijing, China) and the primers listed in Supplementary Data 1. PCR products were analyzed by electrophoresis and imaged on the GelDoc XR+ system (BioRad, Hercules, USA). Uncropped gels are shown in Supplementary Data 2.
Statistics
Statistical analyses were performed using GraphPad Prism 9.0 (GraphPad software). An unpaired t-test was used to compare the means of two groups at the same time point. Differences with p values < 0.05 were considered significant, with standard deviations shown as error bars in the figures.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This work was supported by the National Natural Science Foundation of China (32030109, 31972697, 31820103014 and 32150710530), Guangdong Major Project of Basic and Applied Basic Research (2020B0301030007), 111 Project (D20008) and Double First-class Discipline Promotion Project (2023B10564003). We thank Jilei Huang, Chuanhe Liu and Xiaoxian Wu from the Instrumental Analysis & Research Center, South China Agricultural University for technical assistance.
Author contributions
Y.G., L.X. and Y.F. conceived and designed the study. M.L., F.Y., T.H. and X.G. performed the experiments. M.L., N.L. and L.S. participated in data analysis. M.L., F.Y., L.X. and Y.G. prepared the manuscript. All authors read and approved the final version of the manuscript.
Peer review
Peer review information
Communications Biology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editors: Nishith Gupta and Tobias Goris. A peer review file is available.
Data availability
All data supporting the results of this study are available in the paper and as Supplementary Information. Original blot images are shown in Fig. S12, PCR primers used in the study were listed in Supplementary Data 1 and the source data were listed in Supplementary Data 2. The RNA-seq data generated in this study could been obtained on NCBI (BioProject No. PRJNA1011005). Other data are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Muxiao Li, Fuxian Yang.
Contributor Information
Yaoyu Feng, Email: yyfeng@scau.edu.cn.
Lihua Xiao, Email: lxiao1961@gmail.com.
Yaqiong Guo, Email: guoyq@scau.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-024-06885-0.
References
- 1.Checkley, W. et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for Cryptosporidium. Lancet Infect. Dis.15, 85–94 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD Diarrhoeal Diseases Collaborators. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis.17, 909–948 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gharpure, R. et al. Cryptosporidiosis outbreaks—United States, 2009-2017. Morb. Mortal. Wkly. Rep.68, 568–572 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schneider, A., Wendt, S., Lubbert, C. & Trawinski, H. Current pharmacotherapy of cryptosporidiosis: an update of the state-of-the-art. Expert Opin. Pharmacother.22, 2337–2342 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Guerin, A. & Striepen, B. The Biology of the intestinal intracellular parasite Cryptosporidium. Cell Host Microbe28, 509–515 (2020). [DOI] [PubMed] [Google Scholar]
- 6.Strong, W. B., Gut, J. & Nelson, R. G. Cloning and sequence analysis of a highly polymorphic Cryptosporidium parvum gene encoding a 60-kilodalton glycoprotein and characterization of its 15- and 45-kilodalton zoite surface antigen products. Infect. Immun.68, 4117–4134 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winter, G., Gooley, A. A., Williams, K. L. & Slade, M. B. Characterization of a major sporozoite surface glycoprotein of Cryptosporidum parvum. Funct. Integr. Genomics1, 207–217 (2000). [DOI] [PubMed] [Google Scholar]
- 8.Cevallos, A. M. et al. Molecular cloning and expression of a gene encoding Cryptosporidium parvum glycoproteins gp40 and gp15. Infect. Immun.68, 4108–4116 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Priest, J. W., Kwon, J. P., Arrowood, M. J. & Lammie, P. J. Cloning of the immunodominant 17-kDa antigen from Cryptosporidium parvum. Mol. Biochem. Parasitol.106, 261–271 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Wanyiri, J. W. et al. Role of CpSUB1, a subtilisin-like protease, in Cryptosporidium parvum infection in vitro. Eukaryot. Cell8, 470–477 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wanyiri, J. W. et al. Proteolytic processing of the Cryptosporidium glycoprotein gp40/15 by human furin and by a parasite-derived furin-like protease activity. Infect. Immun.75, 184–192 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilchrist, C. A. et al. Specific Cryptosporidium antigens associate with reinfection immunity and protection from cryptosporidiosis. J. Clin. Invest.133, 10.1172/JCI166814 (2023). [DOI] [PMC free article] [PubMed]
- 13.Xiao, L. & Feng, Y. Molecular epidemiologic tools for waterborne pathogens Cryptosporidium spp. and Giardia duodenalis. Food Waterborne Parasitol.8-9, 14–32 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng, Y., Ryan, U. M. & Xiao, L. Genetic diversity and population structure of Cryptosporidium. Trends Parasitol.34, 997–1011 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Cama, V. A. et al. Cryptosporidium species and subtypes and clinical manifestations in children, Peru. Emerg. Infect. Dis.14, 1567–1574 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Connor, R. M., Wanyiri, J. W., Wojczyk, B. S., Kim, K. & Ward, H. Stable expression of Cryptosporidium parvum glycoprotein gp40/15 in Toxoplasma gondii. Mol. Biochem. Parasitol.152, 149–158 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Connor, R. M., Wanyiri, J. W., Cevallos, A. M., Priest, J. W. & Ward, H. D. Cryptosporidium parvum glycoprotein gp40 localizes to the sporozoite surface by association with gp15. Mol. Biochem. Parasitol.156, 80–83 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cui, Z. et al. Cryptosporidium parvum gp40/15 is associated with the parasitophorous vacuole membrane and is a potential vaccine target. Microorganisms8, 10.3390/microorganisms8030363 (2020). [DOI] [PMC free article] [PubMed]
- 19.Guerin, A. et al. Cryptosporidium uses multiple distinct secretory organelles to interact with and modify its host cell. Cell Host Microbe31, 650–664.e656 (2023). [DOI] [PubMed] [Google Scholar]
- 20.Jia, R. et al. High infectivity and unique genomic sequence characteristics of Cryptosporidium parvum in China. PLoS Negl. Trop. Dis.16, e0010714 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward, H. & Cevallos, A. M. Cryptosporidium: molecular basis of host-parasite interaction. Adv. Parasitol.40, 151–185 (1998). [DOI] [PubMed] [Google Scholar]
- 22.Pinto, D. J. & Vinayak, S. Cryptosporidium: host-parasite interactions and pathogenesis. Curr. Clin. Microbiol. Rep. 1–6, 10.1007/s40588-021-00159-7 (2021). [DOI] [PMC free article] [PubMed]
- 23.Cevallos, A. M. et al. Mediation of Cryptosporidium parvum infection in vitro by mucin-like glycoproteins defined by a neutralizing monoclonal antibody. Infect. Immun.68, 5167–5175 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guerin, A. et al. Cryptosporidium rhoptry effector protein ROP1 injected during invasion targets the host cytoskeletal modulator LMO7. Cell Host Microbe29, 1407–1420 e1405 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dumaine, J. E. et al. The enteric pathogen Cryptosporidium parvum exports proteins into the cytosol of the infected host cell. Elife10, 10.7554/eLife.70451 (2021). [DOI] [PMC free article] [PubMed]
- 26.Muniz, M. & Zurzolo, C. Sorting of GPI-anchored proteins from yeast to mammals–common pathways at different sites? J. Cell Sci.127, 2793–2801 (2014). [DOI] [PubMed] [Google Scholar]
- 27.Tandel, J. et al. Life cycle progression and sexual development of the apicomplexan parasite Cryptosporidium parvum. Nat. Microbiol.4, 2226–2236 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiser, M. F. Unique endomembrane systems and virulence in pathogenic protozoa. Life11, 10.3390/life11080822 (2021). [DOI] [PMC free article] [PubMed]
- 29.Gilchrist, C. A. et al. Genetic diversity of Cryptosporidium hominis in a Bangladeshi community as revealed by whole-genome sequencing. J. Infect. Dis.218, 259–264 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caccio, S. M. & Chalmers, R. M. Human cryptosporidiosis in Europe. Clin. Microbiol. Infect.22, 471–480 (2016). [DOI] [PubMed] [Google Scholar]
- 31.Xiao, L. Molecular epidemiology of cryptosporidiosis: an update. Exp. Parasitol.124, 80–89 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Priest, J. W. et al. Detection by enzyme immunoassay of serum immunoglobulin G antibodies that recognize specific Cryptosporidium parvum antigens. J. Clin. Microbiol.37, 1385–1392 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ortega-Mora, L. M., Troncoso, J. M., Rojo-Vazquez, F. A. & Gomez-Bautista, M. Identification of Cryptosporidium parvum oocyst/sporozoite antigens recognized by infected and hyperimmune lambs. Vet. Parasitol.53, 159–166 (1994). [DOI] [PubMed] [Google Scholar]
- 34.Garcia, R. J. & Hayman, D. T. S. Evolutionary processes in populations of Cryptosporidium inferred from gp60 sequence data. Parasitol. Res.116, 1855–1861 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Li, N. et al. Genetic recombination and Cryptosporidium hominis virulent subtype IbA10G2. Emerg. Infect. Dis.19, 1573–1582 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamarque, M. H. et al. Plasticity and redundancy among AMA-RON pairs ensure host cell entry of Toxoplasma parasites. Nat. Commun.5, 4098 (2014). [DOI] [PubMed] [Google Scholar]
- 37.Bushell, E. et al. Functional profiling of a plasmodium genome reveals an abundance of essential genes. Cell170, 260–272.e268 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu, R. et al. Characterization of INS-15, a metalloprotease potentially involved in the invasion of Cryptosporidium parvum. Microorganisms7, 452 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li, M. et al. Stable expression of mucin glycoproteins GP40 and GP15 of Cryptosporidium parvum in Toxoplasma gondii. Parasites Vectors17, 65 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vinayak, S. et al. Genetic modification of the diarrhoeal pathogen Cryptosporidium parvum. Nature523, 477–480 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu, R., Feng, Y., Xiao, L. & Sibley, L. D. Insulinase-like protease 1 contributes to macrogamont formation in Cryptosporidium parvum. mBio12, 10.1128/mBio.03405-20 (2021). [DOI] [PMC free article] [PubMed]
- 42.Li, N. et al. Diarrhoea outbreak caused by coinfections of Cryptosporidium parvum subtype IIdA20G1 and rotavirus in pre-weaned dairy calves. Transbound. Emerg. Dis.69, e1606–e1617 (2022). [DOI] [PubMed] [Google Scholar]
- 43.Hu, S. et al. Age and episode-associated occurrence of Cryptosporidium species and subtypes in a birth-cohort of dairy calves. Transbound. Emerg. Dis.69, e1710–e1720 (2022). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data supporting the results of this study are available in the paper and as Supplementary Information. Original blot images are shown in Fig. S12, PCR primers used in the study were listed in Supplementary Data 1 and the source data were listed in Supplementary Data 2. The RNA-seq data generated in this study could been obtained on NCBI (BioProject No. PRJNA1011005). Other data are available from the corresponding author upon request.