Abstract
Extracellular vesicles (EVs) have emerged as a fascinating area of research in molecular biology, with diverse therapeutic applications. These small membrane-bound structures, released by cells into the extracellular space, play a crucial role in intercellular communication and hold great potential for advancing medical treatments. The aim of this study is to have a narrative review on the use and therapeutic applications of EVs. Their unique characteristics, including stability, biocompatibility, and the ability to traverse biological barriers, make them promising tools for targeted drug delivery. By engineering EVs to encapsulate specific cargo molecules, such as therapeutic proteins, small interfering RNA (siRNA), or anti-cancer drugs, researchers can enhance drug stability and improve targeted delivery to desired cells or tissues. This approach can minimize off-target effects and improve therapeutic efficacy. Based on our literature search, we found that EVs can be used as biomarkers to predict diseases. Although much progress has been made in understanding the biology and function of exosomes, there are still unanswered questions that require further research. This includes identifying appropriate and safe techniques for producing exosomes in large quantities, determining which types of cells are suitable for exosome donor cells for therapeutic purposes, and investigating the safety of exosomes in human studies. Overall, the use of exosomes in clinical therapeutic applications requires a strong understanding of molecular signaling cascades and exosome profiles, as well as the specificity and sensitivity of biomarker and drug delivery methods.
Keywords: Extracellular vesicles (EVs), intercellular communication, targeted drug delivery, regenerative medicine, exosomes, therapeutic applications
Introduction
Extracellular vesicles (EVs) have emerged as a compelling and promising field of study within molecular biology and therapeutics. These minute membrane-bound structures, released by cells into the extracellular space, play a pivotal role in intercellular communication. Containing a diverse range of bioactive molecules, such as proteins, lipids, and nucleic acids, EVs have garnered considerable attention for their therapeutic applications [1].
EVs can be classified into different types based on size and biogenesis pathways, including exosomes, microvesicles, and apoptotic bodies. Exosomes, the smallest EVs with diameters ranging from 30 to 150 nanometers, are of particular interest due to their capacity to transport a wide array of cargo and engage in communication with recipient cells. They are formed through the endosomal pathway, where inward budding of the plasma membrane leads to the creation of multivesicular bodies that subsequently release exosomes upon fusion with the cell membrane [2].
A notable aspect of EVs is their crucial role in intercellular communication. By disseminating their cargo molecules, EVs can transmit vital information to neighboring or distant cells. Acting as carriers of biological messages, EVs transport molecules that regulate fundamental cellular functions, including proliferation, differentiation, and immune responses. Additionally, the ability of EVs to traverse physiological barriers, such as the blood-brain barrier, facilitates communication between cells in different tissues and organs [3].
EVs facilitate intercellular communication by transferring a diverse cargo, including proteins, nucleic acids, and lipids, to recipient cells. This transfer can occur through endocytosis or direct fusion with the plasma membrane of target cells. EVs also carry surface ligands that interact with receptors on recipient cells, triggering downstream signaling pathways [2]. Through these mechanisms, EVs play pivotal roles in immune regulation, tissue homeostasis, and disease progression. Their ability to transmit molecular information over short and long distances underscores their significance in orchestrating cellular responses and maintaining tissue integrity in physiological and pathological contexts [3].
The therapeutic potential of EVs has captivated researchers in recent years. Their unique characteristics, including stability, biocompatibility, and the ability to overcome biological barriers, make them highly promising candidates for targeted drug delivery systems. EVs can be engineered to transport specific cargo molecules, such as therapeutic proteins, small interfering RNAs (siRNAs), or anti-cancer drugs, to targeted cells or tissues. Encapsulation within EVs enhances the stability of these payloads and minimizes undesirable side effects [4].
Furthermore, EVs offer immense potential in the field of regenerative medicine. They have demonstrated the ability to promote tissue repair and regeneration by delivering bioactive molecules to damaged cells or tissues. Stem cell-derived EVs, for instance, exhibit regenerative properties, stimulating tissue healing and modulating immune responses. These regenerative capabilities position EVs as promising tools for developing novel therapies for various diseases, including neurodegenerative disorders, cardiovascular diseases, and inflammatory conditions [5].
In conclusion, extracellular vesicles represent a captivating and promising field of study with significant therapeutic potential. These nanosized structures serve as crucial mediators of intercellular communication, transmitting bioactive molecules and modulating cellular functions. The capacity to engineer EVs for targeted drug delivery and exploit their regenerative properties underscores their appeal for therapeutic applications, as shown in Figure 1 [6]. As our understanding of EV biology and their therapeutic capabilities continues to expand, we anticipate that these minute vesicles will revolutionize the field of medicine, contributing to the development of innovative and highly effective treatments [7].
Figure 1.
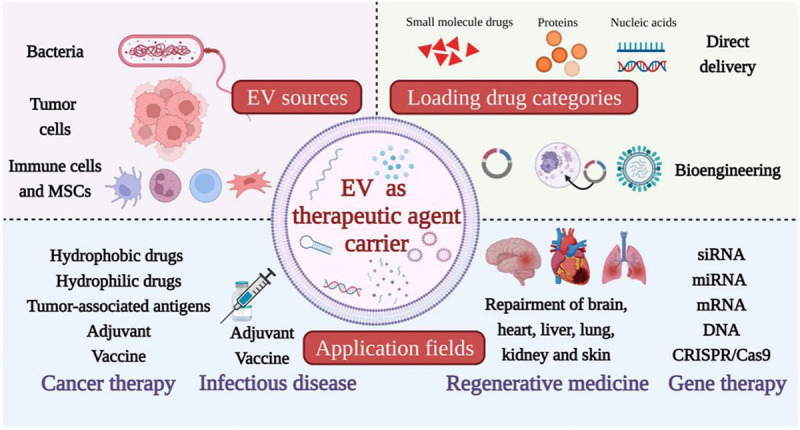
Extracellular vesicles as therapeutic agent carriers.
Materials and methods of work
Study design
The current study employed a simple review methodology to examine relevant literature in the field. The review period spanned three months, from November to February 2018.
Data sources
To gather relevant articles, a comprehensive search was conducted in prominent scientific databases including Scopus, ISI, and PubMed. These databases were chosen due to their extensive coverage of descriptive and analytical studies.
Search strategy
The search strategy involved the use of specific keywords to identify articles related to the study’s focus. In the Scientific Information Database, the following keywords were utilized: extracellular vesicles, exosome, Endosomal Sorting Complex Required for Transport (ESCRT), Multivesicular Body (MVB). These keywords were selected based on their relevance to the research topic and the potential to yield articles of interest.
Article selection criteria
The primary criterion for article selection was their direct connection to exosomes, as well as the presence of at least one of the designated keywords. This criterion ensured that the chosen articles were pertinent to the study’s objectives.
Article collection
A total of 145 articles were initially identified through the database searches. After a thorough screening process, 118 articles were deemed suitable for inclusion in the review. The screening process involved carefully assessing each article’s title, abstract, and full text to ensure alignment with the research goals.
In summary, this review employed a simple methodology over a three-month period, targeting articles indexed in Scopus, ISI, and PubMed. The search strategy involved using specific keywords to identify relevant literature, with a focus on articles related to exosomes. Ultimately, 118 articles were considered suitable for inclusion in the study.
Exosomes and therapeutic goals
Past studies have shown that cell therapy or stem cell transplantation leads to beneficial effects at the transplant site. These discoveries have opened the way for the use of stem cells in medicine and have made significant progress in the last few decades [8].
One of the most familiar and common uses of stem cells in the field of cell therapy is the transplantation of hematopoietic stem cells into the bone marrow. Hematopoietic stem cells can be extracted from the iliac crest of the pelvis or from the blood circulation. These cells possess the ability to self-renew, divide, and differentiate into various types of blood cells. Approximately 2,500 hematopoietic stem cell transplants are performed worldwide each year, in both autologous and allogeneic forms, to treat diseases such as lymphoma, leukemia, immune system disorders, congenital metabolic disorders, hemoglobinopathy, and bone marrow hyperproliferative syndrome.
Furthermore, the utilization of various types of stem cells, such as mesenchymal precursors, bone marrow mononuclear cells, and cardiac stem cells, for transplantation in different organs, including the cardiovascular system, has been explored [9].
For instance, Orlic and her research team demonstrated that the injection of allogeneic c-kit+ bone marrow stem cells into the heart tissue of mice with infarction resulted in the regeneration of the heart muscle and improvement in heart function.
Subsequently, researchers demonstrated the beneficial effects of cell therapy in clinical studies involving patients with cardiovascular diseases. In the field of neurological disease treatment using stem cells, numerous laboratory and clinical studies have been conducted. These studies have explored the use of embryonic stem cells, neural stem cells, mesenchymal stem cells, and induced pluripotent stem cells for treating conditions such as Parkinson’s, Huntington’s, and Alzheimer’s [10].
Nevertheless, there are certain challenges in this field, including ethical considerations, immunogenicity, the risk of tumorigenesis, and the potential formation of unwanted cells.
Mesenchymal stem cells (MSCs) represent another type of stem cell that has been extensively studied for their therapeutic effects in the field of cell therapy. For instance, the transplantation of MSCs has been shown to promote healing and bone regeneration in animal models [11].
Furthermore, beyond animal models, the therapeutic benefits of these cells have been demonstrated in the treatment of osteogenesis imperfecta in children.
Hence, MSCs play a crucial role in the repair and reconstruction of diseases and bone lesions. Moreover, research conducted on animal models of heart infarction and brain damage has revealed that these cells migrate to the site of injury and contribute to tissue reconstruction.
Nevertheless, the study indicates that upon injection, only a small portion of these cells reach the intended target site, with the majority becoming trapped in the pulmonary capillaries. Furthermore, the therapeutic effects of these cells are short-term in nature [12].
Observations suggest that, in addition to the direct differentiation and replacement of injected stem cells in damaged tissues, another mechanism plays a vital role in organ recovery. These alternative mechanisms, often referred to as paracrine effects, are considered responsible for the positive impacts of cell therapy. This perspective aligns perfectly with the fact that living cells in organs communicate with each other and with the extracellular chemical molecules secreted by cells, utilizing cell-cell and cell-matrix mechanisms [13].
These released agents may affect the following: a - on the same cell that produced the molecule (autocrine signaling), b - on nearby target cells (paracrine signaling) or c - on distant target cells (signaling endocrine).
Increasing evidence shows that paracrine effects actually play a major role in cell therapy in the field of regenerative medicine, where stem cells or more differentiated progenitor cells are used to repair damaged organs such as the heart, kidney, or nervous tissue.
Stem cells secrete cytokines, chemokines, many growth factors and other small bioactive molecules that regulate the activity and function of cells in an autocrine and paracrine manner [14].
In this regard, in addition to producing different cytokines, cells secrete various biomolecules in the form of exosomal vesicles to communicate with other cells and also affect biological processes to the extracellular space. As mentioned, exosomes contain different types of proteins, nucleic acids and fats and can be distributed throughout the body. Furthermore, previous studies have demonstrated that stem cell exosomes, including those derived from mesenchymal stem cells, exhibit beneficial effects in the treatment of diverse diseases. As a result, exosome therapy can be considered as an alternative to cell therapy.
These characteristics of exosomes have captured the interest of researchers in the medical nanotechnology field, prompting them to explore exosome therapy as a viable substitute for cell therapy [15].
In general, studies investigating the utilization of exosomes primarily focus on their role as biomarkers and carriers of therapeutic agents for predicting and treating a wide range of diseases. These diseases include cancer, cardiovascular diseases, kidney diseases, liver and metabolic diseases, lung diseases, immune system abnormalities, bone diseases, and neurological diseases [16].
It is important to note that the majority of studies exploring the therapeutic applications of exosomes are currently in the preliminary and preclinical stages. These studies involve investigations conducted on animal models and in vitro models.
Our understanding of the therapeutic and clinical applications of exosomes is expanding, encompassing their use as biomarkers for various diseases. Additionally, exosomes can serve as carriers for therapeutic agents in clinical trials for disease treatment. In this context, a search using the keyword “exosome” in the clinical trials database conducted until April 2019 yielded 99 registered studies related to exosomes. Out of the registered studies, the highest number (52 studies) is related to basic research in the field of exosome biology, while the lowest number (3 studies) is registered for the utilization of exosomes as drug carriers.
In the remaining part of this section, we will discuss the practical roles of exosomes, specifically their significance as 1) biomarkers and 2) carriers of therapeutic agents.
Exosomes as biomarkers
All the molecules contained within exosomes have the potential to be utilized for disease diagnosis. Exosomes serve as carriers of a diverse array of potential biomarkers. Their release into the extracellular space presents an excellent opportunity for investigation in bodily fluids such as blood, urine, and malignant ascites.
Exosomes are commonly found in patients with lung cancer and melanoma. The dual role of exosomes as biomarkers and messengers has created opportunities for researchers to assess the temporal-spatial state of cells and gain a deeper understanding of the role of exosomes in medicine. The isolation and identification of exosomes have facilitated their utilization as biomarkers for pathological conditions, disease severity, or disease stage. Exosomes are extensively employed as biomarkers in cancer-related diseases [17].
These studies are not exclusive to cancer, as similar investigations have been conducted concerning the proteome of exosomes from various cell types and biological fluids. Exploring and comprehending the role of exosomes in cardiovascular physiology has unveiled exosomal biomarkers associated with cardiovascular diseases. Asthma serves as another example of a non-cancerous condition. Analysis of exosomes isolated from patients with asthma revealed distinct expression patterns in miRNA content [18].
The expression profile of the entire miRNA24 was altered, resulting in a substantial decrease in the expression of let-7 and miRNA-200 family members compared to a healthy sample. The researchers indicated that this pattern could serve as a biological marker.
Therefore, the decrease or increase in exosomal content can be regarded as a biomarker for pathological conditions. In the field of cancer, exosomes transport miRNA-18a, an oncogene member of the miR-17-92 branch. It has been observed that the level of this molecule is higher in cancer patients compared to healthy individuals.
The expression level of exosomal miRNA-145, a tumor suppressor, is found to be low in malignant thyroid cancer. Furthermore, the expression pattern of miRNAs within exosomes can serve as a biomarker for early cancer diagnosis. For instance, in breast cancer, one of the most common and aggressive types, researchers have proposed that the expression of exosomal miRNAs such as miR_373, miR_182, miR_21, miR_10, and miR_1246 can be utilized as biomarkers in the early stages of cancer progression.
Exosomes contain various proteins in addition to miRNAs, which provide insights into the cellular conditions. Transcription factors found in urine EVs of patients with acute kidney injury (AKI) are recognized as biomarkers for this condition.
Various proteins found in exosomes can serve as initial diagnostic factors for tumors. In a particular study, proteomic analysis of urinary exosomes successfully identified eight protein biomarkers for the screening and monitoring of bladder-gallbladder cancers.
Jakabsen and colleagues proposed, through protein analysis of exosomes derived from breast cancer cells, that exosome surface proteins like CD317 and EGFR could be regarded as biomarkers for this type of cancer. Furthermore, proteins including CEA, Survivin-2B, Survivin, and Tumorantigen-15 have been identified as biomarkers in breast cancer.
Hence, the representation of miRNA and exosomal protein profiles resembling their tissue of origin can be employed as diagnostic biomarkers for both cancerous and non-cancerous diseases, eliminating the need for cancer tissue samples.
Exosomes as drug-carrying vesicles
Exosomes can serve as carriers for drugs or biological agents to treat diverse diseases. Shetam and colleagues proposed that the utilization of exosomes in combination with siRNA is a valuable approach for manipulating target genes in heterogeneous cell populations. Hence, the application of exosomes as oligonucleotide shuttles in various environments should be given careful consideration [16].
Exosomes rapidly disperse in body fluids like blood, urine, bronchiole fluid, etc. They can enter target cells by interacting with receptors and plasma membranes, delivering their cargo into the cytoplasm and inducing cellular responses.
For instance, exosomes derived from antigen-presenting cells (dendritic cells) can modulate the cellular immune response by transferring major histocompatibility complex (MHC) class I and II molecules to T cells and immune cells. In recent years, numerous studies have demonstrated the usefulness of exosomes for drug delivery in the treatment of various diseases [19].
The growth of colon and breast cancer cells was successfully inhibited by utilizing exosomes containing doxorubicin or exosome-related nanovesicles.
In recent years, several studies have demonstrated the effectiveness of drug delivery via exosomes for treating various diseases. The use of exosomes loaded with doxorubicin or exosome-like nanovesicles has proven successful in inhibiting the growth of colon and breast cancer cells.
Exosomes possess unique features and capabilities that vary depending on the cell type. For instance, exosomes derived from cancer cells can directly deliver paclitaxel (PTX) to primary prostate cancer cells and sensitize tumor stem cells.
The advantages of exosomes derived from stem cells are being increasingly recognized. Mesenchymal stem cells engineered with PTX can effectively deliver exosomes abundant in PTX, resulting in the inhibition of tumor cell growth in laboratory settings [20].
Loading exosomes with anti-cancer compounds has yielded promising results. In an experiment, doxorubicin and PTX were concurrently loaded into exosomes and injected into xenograft cancer cells in the brain of Zebra fish. The findings demonstrated a decrease in tumor growth.
In addition to unique pharmacological and nucleotide sequences, some researchers have explored the use of plant essential oils for loading exosomes. Exosomes containing curcumin effectively attenuated brain inflammation induced by lipopolysaccharide in mice.
Exosomes derived from mouse embryonic stem cells and pre-loaded with curcumin, when cultured with nerve vessels, induce angiogenesis and regeneration responses following ischemia-reperfusion injury in a mouse model. Another advantage of exosomes is their ability to regulate transcription and control gene expression [21].
Lunavat and his colleagues demonstrated that exosomes loaded with siRNAs effectively control gene expression in a laboratory environment.
Exosomes possess unique features and capabilities depending on the cell type. For instance, exosomes derived from cancer cells can directly deliver the anticancer drug PTX to primary central cells of prostate cancer, sensitizing tumor stem cells to its toxicity.
Kim and her colleagues showed that PTX-rich exosomes increase tumor suppression and decrease cancer cell resistance in a mouse model of lung cancer. These results show that exosomes can be used as an important vehicle for carrying medicinal agents. The advantages of exosomes in comparison to other vesicles in transferring therapeutic agents are: exosomes are very similar to transport vesicles and have a lipid-biological membrane that covers the blue core. Additionally, they contain both hydrophilic and lipophilic substances and can deliver their cargo to the intended targets.
These features enable exosomes to overcome some of the limitations of liposomes. Exosomes can easily spread in body fluids, remain in blood circulation for a long time, pass through physiological barriers, and enter cells. Exosomes, which contain a large volume of biomolecules, do not stimulate immunogenic responses and do not accumulate in the liver or lungs instead of the target tissues.
Exosome administration models
Based on the type of disease, exosomes are administered through different methods. Easy access to large amounts of exosomes should be considered in the design of clinical trials, and administration routes should be carefully chosen to achieve effective therapeutic benefits, especially for hidden tumors.
Different methods have been used in experiments to increase the efficiency of drug delivery for specific diseases. In one experiment, both intravenous and intranasal injection methods were used in a mouse model of Parkinson’s disease.
While this review aimed to provide a comprehensive analysis of the existing literature on therapeutic applications of extracellular vesicles, it is important to acknowledge several limitations. Firstly, due to the narrative nature of our review, statistical analysis methods were not employed to compare our findings with those of other reports. Despite these limitations, we believe that our review offers valuable insights into therapeutic applications of extracellular vesicles and provides a foundation for further research in this area.
Conclusion
Exosomes have emerged as a promising tool for the treatment of various diseases. With their ability to transport biomolecules, exosomes can be used as therapeutic agents for the recovery of different diseases by manipulating biological cargoes. They can also serve as biomarkers for predicting diseases. Although much progress has been made in understanding the biology and function of exosomes, there are still unanswered questions that require further research. This includes identifying appropriate and safe techniques for producing exosomes in large quantities, determining which types of cells are suitable as exosome donor cells for therapeutic purposes, and investigating the safety of exosomes in human studies. Overall, the use of exosomes in clinical therapeutic applications requires a strong understanding of molecular signaling cascades and exosome profiles, as well as the specificity and sensitivity of biomarker and drug delivery methods. Further research in this field will be crucial for unlocking the full potential of exosomes in the treatment of various diseases.
Disclosure of conflict of interest
None.
References
- 1.Qiu X, Liu J, Zheng C, Su Y, Bao L, Zhu B, Liu S, Wang L, Wang X, Wang Y, Zhao W, Zhou J, Deng Z, Liu S, Jin Y. Exosomes released from educated mesenchymal stem cells accelerate cutaneous wound healing via promoting angiogenesis. Cell Prolif. 2020;53:e12830. doi: 10.1111/cpr.12830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.An Y, Lin S, Tan X, Zhu S, Nie F, Zhen Y, Gu L, Zhang C, Wang B, Wei W, Li D, Wu J. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021;54:e12993. doi: 10.1111/cpr.12993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krämer-Albers EM. Extracellular vesicles at CNS barriers: mode of action. Curr Opin Neurobiol. 2022;75:102569. doi: 10.1016/j.conb.2022.102569. [DOI] [PubMed] [Google Scholar]
- 4.Dang XTT, Kavishka JM, Zhang DX, Pirisinu M, Le MTN. Extracellular vesicles as an efficient and versatile system for drug delivery. Cells. 2020;9:2191. doi: 10.3390/cells9102191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xing Y, Sun X, Dou Y, Wang M, Zhao Y, Yang Q, Zhao Y. The immuno-modulation effect of macrophage-derived extracellular vesicles in chronic inflammatory diseases. Front Immunol. 2021;12:785728. doi: 10.3389/fimmu.2021.785728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu S, Wu X, Chandra S, Lyon C, Ning B, Jiang L, Fan J, Hu TY. Extracellular vesicles: emerging tools as therapeutic agent carriers. Acta Pharm Sin B. 2022;12:3822–3842. doi: 10.1016/j.apsb.2022.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu H, Liang J, Ye X, Huang M, Ma L, Xie X, Liu D, Cao H, Simal-Gandara J, Rengasamy KRR, Wang Q, Xiao G, Xiao J. The potential role of extracellular vesicles in bioactive compound-based therapy: a review of recent developments. Crit Rev Food Sci Nutr. 2023;63:10959–10973. doi: 10.1080/10408398.2022.2081667. [DOI] [PubMed] [Google Scholar]
- 8.Kharazi U, Badalzadeh R. A review on the stem cell therapy and an introduction to exosomes as a new tool in reproductive medicine. Reprod Biol. 2020;20:447–459. doi: 10.1016/j.repbio.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Nagler A, Labopin M, Dholaria B, Angelucci E, Afanasyev B, Cornelissen JJ, Sica S, Meijer E, Ciceri F, Van Gorkom G, Kröger N, Martin H, Pioltelli P, Risitano A, Canaani J, Savani BN, Sanz J, Mohty M. Comparison of haploidentical bone marrow versus matched unrelated donor peripheral blood stem cell transplantation with posttransplant cyclophosphamide in patients with acute leukemia. Clin Cancer Res. 2021;27:843–851. doi: 10.1158/1078-0432.CCR-20-2809. [DOI] [PubMed] [Google Scholar]
- 10.Alessandrini M, Preynat-Seauve O, De Bruin K, Pepper MS. Stem cell therapy for neurological disorders. S Afr Med J. 2019;109:70–77. doi: 10.7196/SAMJ.2019.v109i8b.14009. [DOI] [PubMed] [Google Scholar]
- 11.Benavides-Castellanos MP, Garzón-Orjuela N, Linero I. Effectiveness of mesenchymal stem cell-conditioned medium in bone regeneration in animal and human models: a systematic review and meta-analysis. Cell Regen. 2020;9:5. doi: 10.1186/s13619-020-00047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guillamat-Prats R. The role of MSC in wound healing, scarring and regeneration. Cells. 2021;10:1729. doi: 10.3390/cells10071729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia H, Li X, Gao W, Fu X, Fang RH, Zhang L, Zhang K. Tissue repair and regeneration with endogenous stem cells. Nat Rev Mater. 2018;3:174–193. [Google Scholar]
- 14.Alvites R, Branquinho M, Sousa AC, Lopes B, Sousa P, Maurício AC. Mesenchymal stem/stromal cells and their paracrine activity-immunomodulation mechanisms and how to influence the therapeutic potential. Pharmaceutics. 2022;14:381. doi: 10.3390/pharmaceutics14020381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Araldi RP, D’Amelio F, Vigerelli H, de Melo TC, Kerkis I. Stem cell-derived exosomes as therapeutic approach for neurodegenerative disorders: from biology to biotechnology. Cells. 2020;9:2663. doi: 10.3390/cells9122663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barile L, Vassalli G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63–78. doi: 10.1016/j.pharmthera.2017.02.020. [DOI] [PubMed] [Google Scholar]
- 17.Deb A, Gupta S, Mazumder PB. Exosomes: a new horizon in modern medicine. Life Sci. 2021;264:118623. doi: 10.1016/j.lfs.2020.118623. [DOI] [PubMed] [Google Scholar]
- 18.Cañas JA, Sastre B, Rodrigo-Muñoz JM, Del Pozo V. Exosomes: a new approach to asthma pathology. Clin Chim Acta. 2019;495:139–147. doi: 10.1016/j.cca.2019.04.055. [DOI] [PubMed] [Google Scholar]
- 19.Lindenbergh MFS, Stoorvogel W. Antigen presentation by extracellular vesicles from professional antigen-presenting cells. Annu Rev Immunol. 2018;36:435–459. doi: 10.1146/annurev-immunol-041015-055700. [DOI] [PubMed] [Google Scholar]
- 20.Kalimuthu S, Gangadaran P, Rajendran RL, Zhu L, Oh JM, Lee HW, Gopal A, Baek SH, Jeong SY, Lee SW, Lee J, Ahn BC. A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front Pharmacol. 2018;9:1116. doi: 10.3389/fphar.2018.01116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoang T, Wang J, Boyd P, Wang F, Santiago C, Jiang L, Yoo S, Lahne M, Todd LJ, Jia M, Saez C, Keuthan C, Palazzo I, Squires N, Campbell WA, Rajaii F, Parayil T, Trinh V, Kim DW, Wang G, Campbell LJ, Ash J, Fischer AJ, Hyde DR, Qian J, Blackshaw S. Gene regulatory networks controlling vertebrate retinal regeneration. Science. 2020;370:eabb8598. doi: 10.1126/science.abb8598. [DOI] [PMC free article] [PubMed] [Google Scholar]


