Cardiac involvement in eosinophilic granulomatosis with polyangiitis (EPGA) is increasingly recognized, but it is unusual to be the initial presentation. We report a case of vasculitic myocardial infarction (MI) and cardiogenic shock in an elderly man caused by EPGA.
A 73-year-old man presented with acute chest pain radiating to back for two days. He has a history of asthma and allergic rhinitis for more than ten years well controlled by inhaled corticosteroid. The electrocardiogram showed sinus rhythm, right bundle-branch block and anterolateral ST-segment depression. He had elevated troponin T of 548 ng/L (reference: < 14 ng/L), creatine kinase of 454 IU/L (reference: 39–308 IU/L), white blood cell count of 13.8 × 109/L (reference: 3.7–9.3 × 109/L) and eosinophil count of 12.4 × 109/L (reference: 0.0–0.6 × 109/L). Antineutrophil cytoplasmic, anti-extractable nuclear antigen, and anti-nuclear antibodies were negative. The evolution of laboratory parameters is shown (Figure 1). Transthoracic echocardiogram showed reduced left ventricular ejection fraction of 40%, anterolateral hypokinesia and 1 cm pericardial effusion near left ventricular apex. Aspirin, clopidogrel and low molecular weight heparin were started. Coronary angiogram found normal coronary arteries. The patient developed cardiogenic shock necessitating inotropic support. Computed tomography did not show pulmonary infiltrates. Cardiac magnetic resonance (CMR) showed anterolateral hypokinesia (supplemental material, video), diffuse subendocardial delayed enhancement (Figure 2A), perfusion defect at rest (Figure 2B) and edema (Figure 2C). A small circumferential pericardial effusion was also present (supplemental material, video). Subsequently, a purpuric rash developed on the patient’s shin (Figure 3). Punch skin biopsy confirmed leukocytoclastic vasculitis with eosinophils. Despite the negative serology, the diagnostic criteria for EPGA was fulfilled.[1] Following the institution of high dose steroid and rituximab, there was precipitous fall in troponin, eosinophil count and inflammatory markers (Figure 1). The patient’s hemodynamics improved significantly; he was able to wean off inotropes and started on guideline-directed medical therapy for heart failure. At three months, echocardiogram showed improved left ventricular ejection fraction of 50%, anterolateral hypokinesia, and a small residual pericardial effusion.
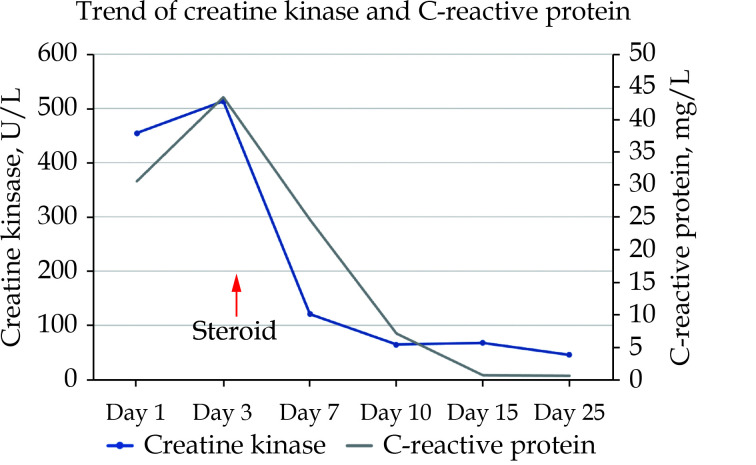
Figure 1.
The levels of creatine kinase and C-reactive protein were plotted against time after presentation.
Both parameters dropped precipitously following institution of immunosuppression.
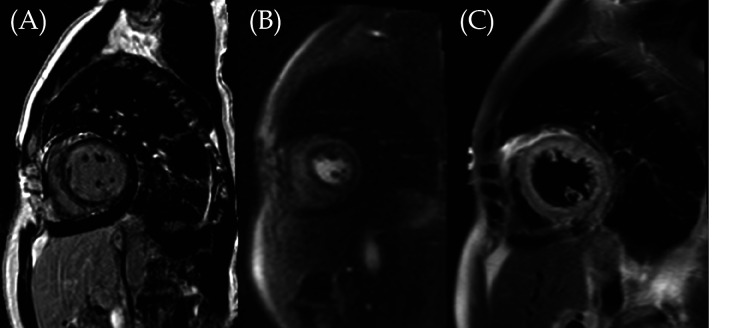
Figure 2.
Diffuse near circumferential subendocardial late gadolinium enhancement was seen at the mid-ventricular short axis (A) associated with perfusion defect at rest (B) and edema (C), signifying acute myocardial infarction in a non-coronary distribution.
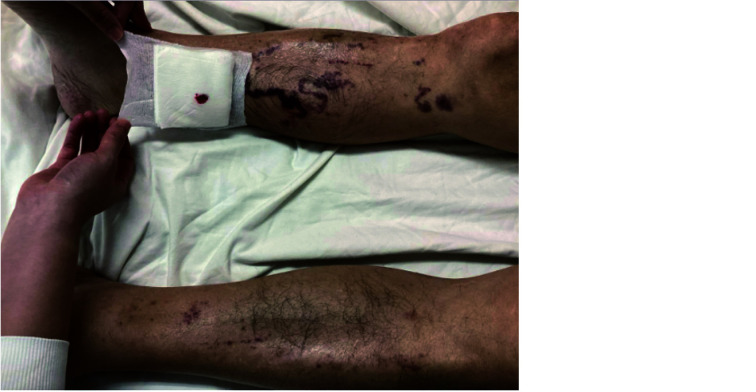
Figure 3.
Palpable purpuric rash characteristic of vasculitis on both legs, more severe on the right.
This case demonstrates the strength of CMR in patients with MI with non-obstructive coronary arteries, of which CMR is the modality of choice.[2] The prevalence of cardiac involvement in EPGA is variable ranging from 16% to 92% in the literature (Table 1). All patterns of late gadolinium enhancement on CMR have been reported, including mid-wall, epicardial, and subendocardial. Apical thrombus is found in the minority of patients. Cardiac involvement is associated with worse disease severity, increased cardiac and total mortality, and it is argued that CMR should be performed in all patients with EPGA.[3] Although corticosteroid is an established treatment of EPGA, use of noncorticosteroid immunosuppression including rituximab and cyclophosphamide limits myocardial damage and contractile dysfunction.[4]
Table 1. Summary of the prevalence and pattern of cardiac involvement by CMR in EPGA in the literature.
| Author | Sample size |
Mean left ventricular ejection fraction |
Presence of scar |
Infarction scar |
Left ventricular thrombus |
| CMR: cardiac magnetic resonance; EPGA: eosinophilic granulomatosis with polyangiitis; NA: not available. | |||||
| Zampieri, et al [5] | 52 | 58% | 13% | 6% | 4% |
| Yune, et al [6] | 16 | 50% | 50% | 44% | 0 |
| Dunogué, et al [7] | 42 | 58% | 60% | 31% | 2% |
| Lagan, et al [8] | 13 | 60% | 92% | 15% | 0 |
| Miszalski-Jamka, et al [4] | 51 | 56% | 16% | NA | NA |
| Hazebroek, et al [3] | 50 | 53% | 61% | 2% | 0 |
In conclusion, we report a case of acute MI caused by EPGA diagnosed with CMR that responded promptly to immunosuppression.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
ACKNOWLEDGMENTS
This study was not supported by any funding. All authors had no conflicts of interest to disclose.
References
- 1.Grayson PC, Ponte C, Suppiah R, et al 2022 American College of Rheumatology/European Alliance of Associations for rheumatology classification criteria for eosinophilic granulomatosis with polyangiitis. Arthritis Rheumatol. 2022;74:386–392. doi: 10.1002/art.41982. [DOI] [PubMed] [Google Scholar]
- 2.Liang K, Bisaccia G, Leo I, et al CMR reclassifies the majority of patients with suspected MINOCA and non MINOCA. Eur Heart J Cardiovasc Imaging. 2023;25:8–15. doi: 10.1093/ehjci/jead182. [DOI] [PubMed] [Google Scholar]
- 3.Hazebroek MR, Kemna MJ, Schalla S, et al Prevalence and prognostic relevance of cardiac involvement in ANCA-associated vasculitis: eosinophilic granulomatosis with polyangiitis and granulomatosis with polyangiitis. Int J Cardiol. 2015;199:170–179. doi: 10.1016/j.ijcard.2015.06.087. [DOI] [PubMed] [Google Scholar]
- 4.Miszalski-Jamka T, Szczeklik W, Sokołowska B, et al Noncorticosteroid immunosuppression limits myocardial damage and contractile dysfunction in eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome) J Am Coll Cardiol. 2015;65:103–105. doi: 10.1016/j.jacc.2014.08.055. [DOI] [PubMed] [Google Scholar]
- 5.Zampieri M, Emmi G, Beltrami M, et al Cardiac involvement in eosinophilic granulomatosis with polyangiitis (formerly Churg-Strauss syndrome): prospective evaluation at a tertiary referral centre. Eur J Intern Med. 2021;85:68–79. doi: 10.1016/j.ejim.2020.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Yune S, Choi DC, Lee BJ, et al Detecting cardiac involvement with magnetic resonance in patients with active eosinophilic granulomatosis with polyangiitis. Int J Cardiovasc Imaging. 2016;32:155–162. doi: 10.1007/s10554-016-0843-y. [DOI] [PubMed] [Google Scholar]
- 7.Dunogué B, Terrier B, Cohen P, et al Impact of cardiac magnetic resonance imaging on eosinophilic granulomatosis with polyangiitis outcomes: a long-term retrospective study on 42 patients. Autoimmun Rev. 2015;14:774–780. doi: 10.1016/j.autrev.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Lagan J, Naish JH, Fortune C, et al Myocardial involvement in eosinophilic granulomatosis with polyangiitis evaluated with cardiopulmonary magnetic resonance. Int J Cardiovasc Imaging. 2021;37:1371–1381. doi: 10.1007/s10554-020-02091-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.