Abstract
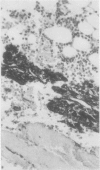
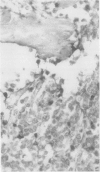
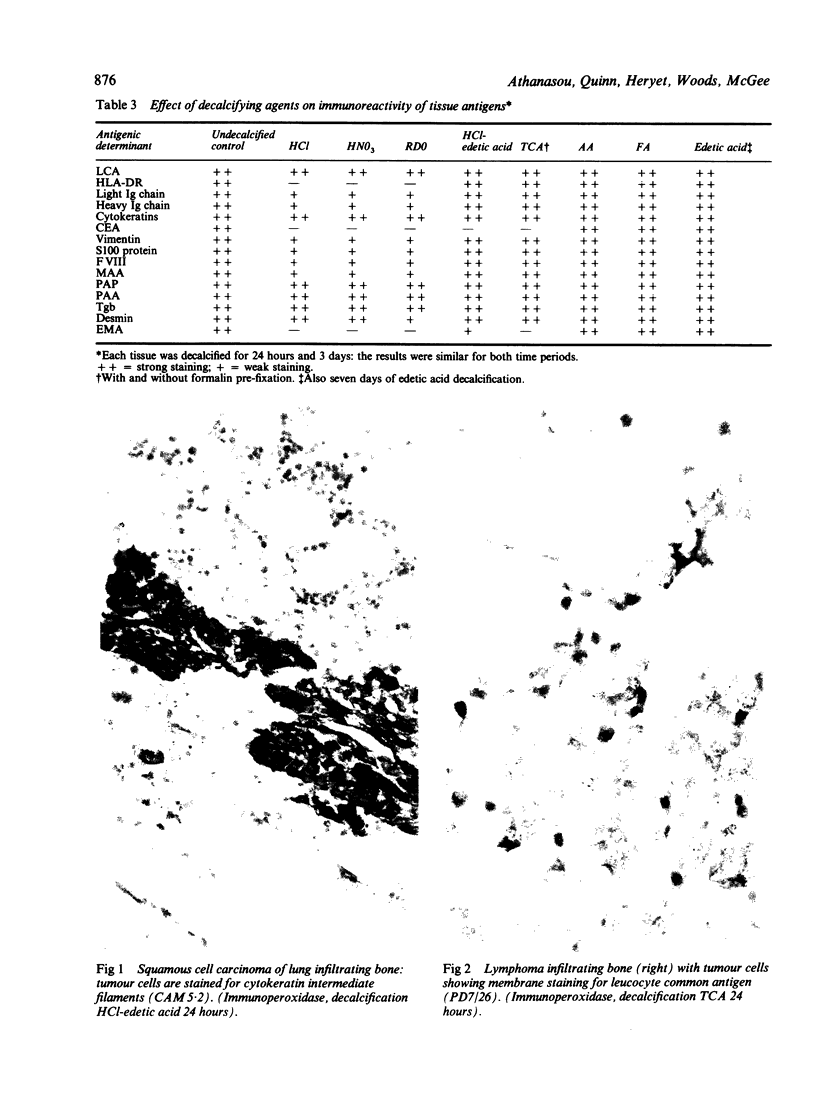
The effects of several strong acids, weak acids, a proprietary decalcifier, and edetic acid on the immunohistochemical staining of cryostat and fixed paraffin embedded sections of tissue from a variety of normal and pathological calcified and uncalcified specimens were studied. Even decalcification in strong acids (HCl, HN03, 5% trichloracetic acid, HCl-edetic acid did not diminish the reactivity of many useful antigens (including leucocyte common antigen, intermediate filaments, S100 protein and epithelial membrane antigen). Weaker acids (formic acid, acetic acid) and edetic acid decalcified more slowly and generally showed greater preservation of antigenic reactivity with better morphology and staining quality. Trichloracetic acid was also useful as a quick one step fixation and decalcifying agent for both cryostat and routinely processed sections. Knowledge of the preservation of antigenic reactivity in decalcified tissue will be useful in the diagnosis of tumours of uncertain histogenesis and origin which affect calcified tissues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burt A., Goudie R. B. Diagnosis of primary thyroid carcinoma by immunohistological demonstration of thyroglobulin. Histopathology. 1979 Jul;3(4):279–286. doi: 10.1111/j.1365-2559.1979.tb03009.x. [DOI] [PubMed] [Google Scholar]
- Cordell J., Richardson T. C., Pulford K. A., Ghosh A. K., Gatter K. C., Heyderman E., Mason D. Y. Production of monoclonal antibodies against human epithelial membrane antigen for use in diagnostic immunocytochemistry. Br J Cancer. 1985 Sep;52(3):347–354. doi: 10.1038/bjc.1985.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran R. C., Gregory J. The unmasking of antigens in paraffin sections of tissue by trypsin. Experientia. 1977 Oct 15;33(10):1400–1401. doi: 10.1007/BF01920206. [DOI] [PubMed] [Google Scholar]
- Debus E., Weber K., Osborn M. Monoclonal antibodies to desmin, the muscle-specific intermediate filament protein. EMBO J. 1983;2(12):2305–2312. doi: 10.1002/j.1460-2075.1983.tb01738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatter K. C., Abdulaziz Z., Beverley P., Corvalan J. R., Ford C., Lane E. B., Mota M., Nash J. R., Pulford K., Stein H. Use of monoclonal antibodies for the histopathological diagnosis of human malignancy. J Clin Pathol. 1982 Nov;35(11):1253–1267. doi: 10.1136/jcp.35.11.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyderman E. Immunoperoxidase technique in histopathology: applications, methods, and controls. J Clin Pathol. 1979 Oct;32(10):971–978. doi: 10.1136/jcp.32.10.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyderman E., Neville A. M. A shorter immunoperoxidase technique for the demonstration of carcinoembryonic antigen and other cell products. J Clin Pathol. 1977 Feb;30(2):138–140. doi: 10.1136/jcp.30.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöbsis A. C., De Vries G. P., Anholt R. R., Sanders G. T. Demonstration of the prostatic origin of metastases: an immunohistochemical method for formalin-fixed embedded tissue. Cancer. 1978 May;41(5):1788–1793. doi: 10.1002/1097-0142(197805)41:5<1788::aid-cncr2820410521>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Mackie R. M., Campbell I., Turbitt M. L. Use of NK1 C3 monoclonal antibody in the assessment of benign and malignant melanocytic lesions. J Clin Pathol. 1984 Apr;37(4):367–372. doi: 10.1136/jcp.37.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin C. A., Bobrow L. G., Bodmer W. F. Monoclonal antibody to cytokeratin for use in routine histopathology. J Clin Pathol. 1984 Sep;37(9):975–983. doi: 10.1136/jcp.37.9.975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews J. B. Influence of decalcification on immunohistochemical staining of formalin-fixed paraffin-embedded tissue. J Clin Pathol. 1982 Dec;35(12):1392–1394. doi: 10.1136/jcp.35.12.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukai K., Yoshimura S., Anzai M. Effects of decalcification on immunoperoxidase staining. Am J Surg Pathol. 1986 Jun;10(6):413–419. doi: 10.1097/00000478-198606000-00006. [DOI] [PubMed] [Google Scholar]
- Taylor C. R. Immunoperoxidase techniques: practical and theoretical aspects. Arch Pathol Lab Med. 1978 Mar;102(3):113–121. [PubMed] [Google Scholar]
- Vanstapel M. J., Peeters B., Cordell J., Heyns W., De Wolf-Peeters C., Desmet V., Mason D. Production of monoclonal antibodies directed against antigenic determinants common to the alpha- and beta-chain of bovine brain S-100 protein. Lab Invest. 1985 Feb;52(2):232–238. [PubMed] [Google Scholar]
- Viac J., Reano A., Brochier J., Staquet M. J., Thivolet J. Reactivity pattern of a monoclonal antikeratin antibody (KL1). J Invest Dermatol. 1983 Oct;81(4):351–354. doi: 10.1111/1523-1747.ep12519941. [DOI] [PubMed] [Google Scholar]
- Wang M. C., Papsidero L. D., Kuriyama M., Valenzuela L. A., Murphy G. P., Chu T. M. Prostate antigen: a new potential marker for prostatic cancer. Prostate. 1981;2(1):89–96. doi: 10.1002/pros.2990020109. [DOI] [PubMed] [Google Scholar]
- Warnke R. A., Gatter K. C., Falini B., Hildreth P., Woolston R. E., Pulford K., Cordell J. L., Cohen B., De Wolf-Peeters C., Mason D. Y. Diagnosis of human lymphoma with monoclonal antileukocyte antibodies. N Engl J Med. 1983 Nov 24;309(21):1275–1281. doi: 10.1056/NEJM198311243092102. [DOI] [PubMed] [Google Scholar]