Abstract
Historically, the treatment of anterior cruciate ligament (ACL) injuries shifted from primary repair to reconstruction because the native, intrasynovial location of the ACL precluded the formation of a fibrin-rich clot needed for ligament healing. However, increasing attention has been paid to augmenting the biological environment surrounding the ACL to facilitate its healing after arthroscopic repair. The bridge-enhanced ACL restoration implant uses resorbable collagen mixed with autologous blood to provide a biological scaffold for tissue healing. The short-term results of this procedure are promising, showing noninferiority to traditional ACL reconstruction at 2 years postoperatively and a higher rate of return to sport at 6 months. Our technique for performing the bridge-enhanced ACL repair is efficient, is easy to learn, and achieves excellent fixation of the ACL stump augmented with an internal brace.
Technique Video
Anterior cruciate ligament (ACL) tears are among the most common knee injuries in the United States, with an incidence of about 120,000 cases per year.1,2 The standard of treatment for ACL ruptures has changed over the past 40 to 50 years. Primary ACL repair was once the treatment of choice but was found to have a high failure rate and unacceptable clinical outcomes, particularly in the young athlete.3, 4, 5 Since then, a paradigm shift occurred in which repair was abandoned, regardless of the location of the tear.6 The gold standard of care for ACL ruptures became ACL reconstruction (ACLR) with autograft or allograft, with reliable patient-reported outcomes and return-to-sport data.7,8
The recent literature has shown renewed interest in ACL repair for more proximal tears. However, most studies have reported short-term, nonrandomized data and failure rates not superior to reconstruction.9, 10, 11 This has spawned research efforts around augmenting the ACL repair with biological adjuncts. Approved by the Food and Drug Administration in 2020, the bridge-enhanced ACL restoration (BEAR) implant (Miach Orthopaedics, Westborough, MA) uses a resorbable collagen-based medium that combines with autologous patient blood to create a scaffold that can bridge the gap between the torn ligament and bone. Without the fibrin clot that typically forms at the torn ends of injured ligaments, the intrasynovial ACL is at a biological disadvantage to heal.12 By providing this scaffold with the BEAR implant, the intra-articular environment can be made more favorable for ligament healing.13 This article presents our technique for performing an ACL repair augmented with the BEAR implant (Video 1).
Surgical Technique
Positioning and Equipment
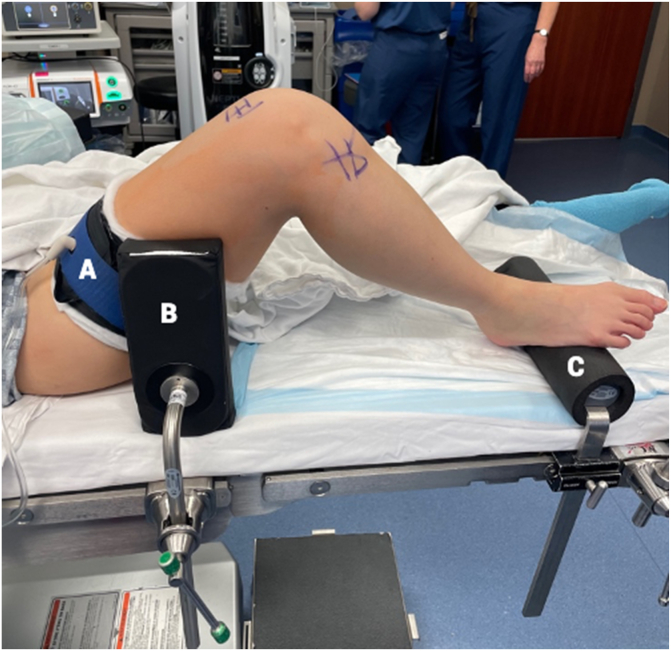
The procedure can be executed using the same positioning that the performing surgeon uses for standard ACLR. Unless contraindicated, an adductor canal regional block is typically performed by the anesthesiologist. The knee is examined with the Lachman and pivot-shift maneuvers. The patient is positioned supine with a tourniquet placed on the upper thigh, a foot bump, and a lateral thigh post (Fig 1). All equipment required is listed in Table 1.
Fig 1.
Preoperative setup. The patient is positioned supine on the operating table with a tourniquet on the right upper thigh (A), a foot bump to maintain the knee at 90° of flexion (C), and a lateral post to balance the knee when flexed (B).
Table 1.
All Specialty Equipment and Instruments Used in Procedure
| Arthroscopic tissue elevator |
| 4.5-mm round burr |
| 2.7-mm cannulated drill with drill sheath |
| Looped nitinol wire |
| 8-mm × 30-mm Passport cannula |
| First Pass Mini suture passer |
| No. 2 Ultrabraid suture and Minitape suture |
| No. 2 looped suture |
| Two titanium cortical buttons (4 mm ×12 mm; Smith & Nephew) |
| BEAR implant |
BEAR, bridge-enhanced anterior cruciate ligament restoration.
Portals and Diagnostic Arthroscopy
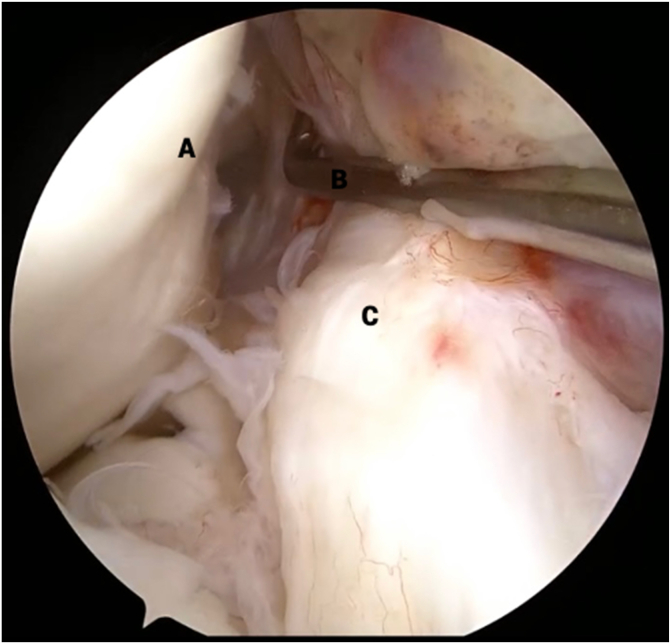
A standard anterolateral portal is made vertically “high and tight” off the lateral edge of the infrapatellar tendon, centered at a line tangential to the inferior pole of the patella. The arthroscope is then introduced atraumatically into the knee. An anteromedial portal is made using spinal needle localization under direct visualization. A diagnostic arthroscopy is performed, and a careful examination of the ACL is conducted, assessing for tear type, tissue height, vascularity, and tibial footprint integrity. If amenable to repair, a decision is made to carry out the following procedure. The ACL stump prior to repair is shown in Figure 2.
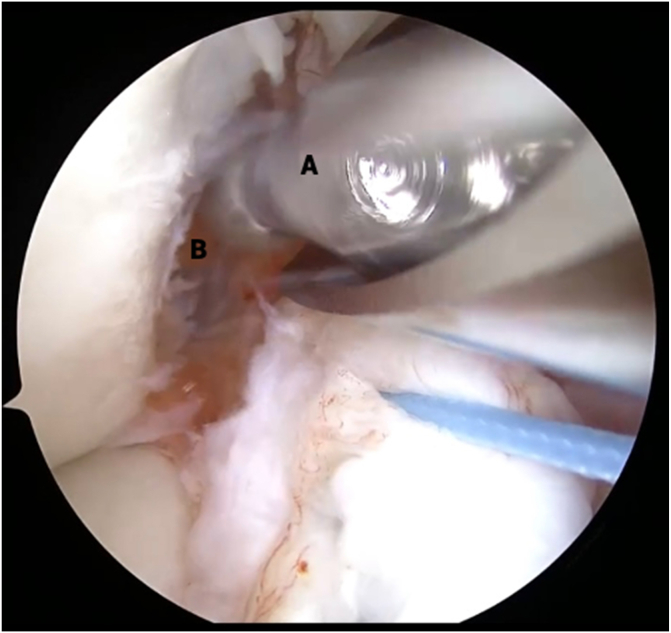
Fig 2.
View from anterolateral portal in right knee with supine positioning. An arthroscopic probe (B) is used to pull the torn anterior cruciate ligament (ACL) stump (C) away from the lateral intercondylar wall (A) to reveal the site of proximal avulsion. The torn ACL remnant tissue (C) is inspected for tear type, tissue height, vascularity, and tibial footprint integrity.
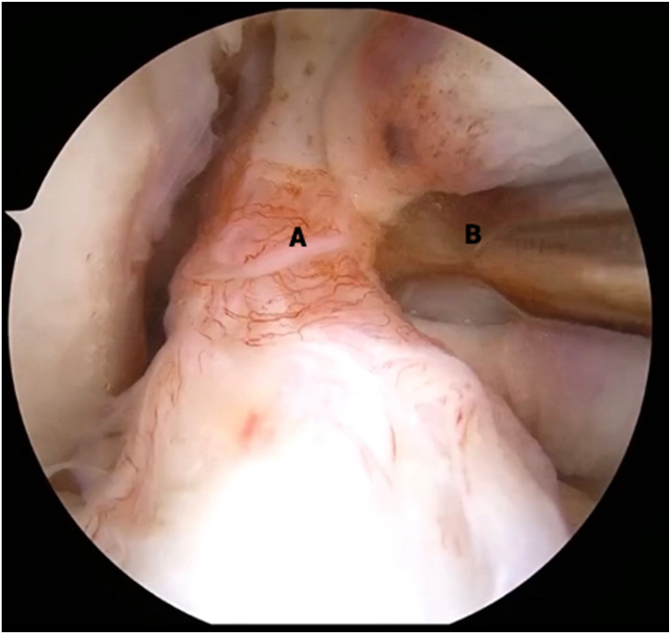
ACL Stump Preparation
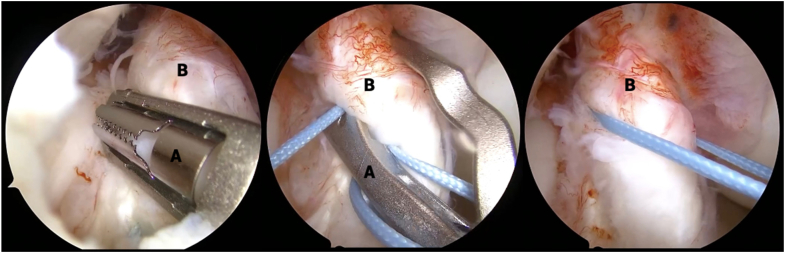
An arthroscopic tissue elevator is used to carefully separate the ACL stump from any scar tissue attachments (Fig 3). Once the ACL stump is mobilized, a Passport cannula (Arthrex, Naples, FL) is inserted into the medial portal; a No. 2 Ultrabraid suture (Smith & Nephew, Andover, MA) is loaded into the First Pass Mini suture passer (Smith & Nephew); and the ACL stump is sutured in an alternating, modified Bunnell configuration from distal to proximal. Once both limbs of suture are exiting from the proximal end of the ACL stump and the tissue is able to be reined with manipulation of the sutures, this step is complete (Fig 4).
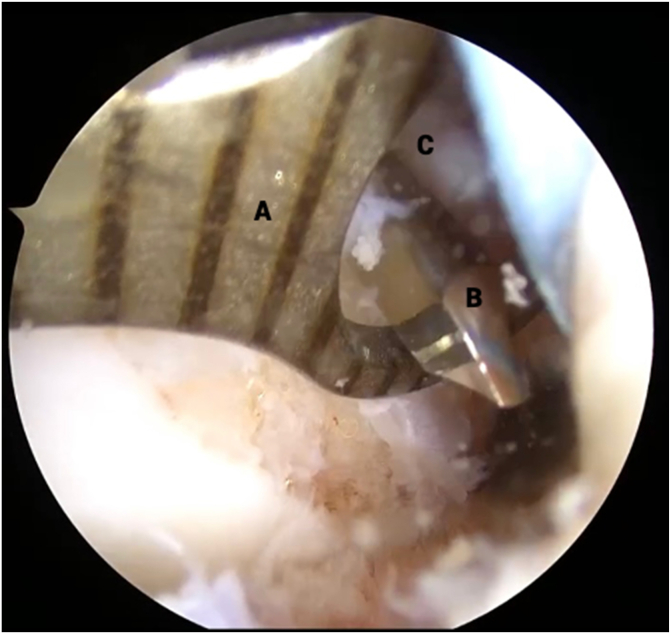
Fig 3.
View from anterolateral portal in right knee with supine positioning. An arthroscopic tissue elevator (B) is used to carefully separate the anterior cruciate ligament (ACL) stump (A) from any scar tissue attachments. This step is critical to allow redirection of the proximal end of the ACL stump back to the native ACL footprint because scar attachments may prohibit superolateral translation of the stump tissue.
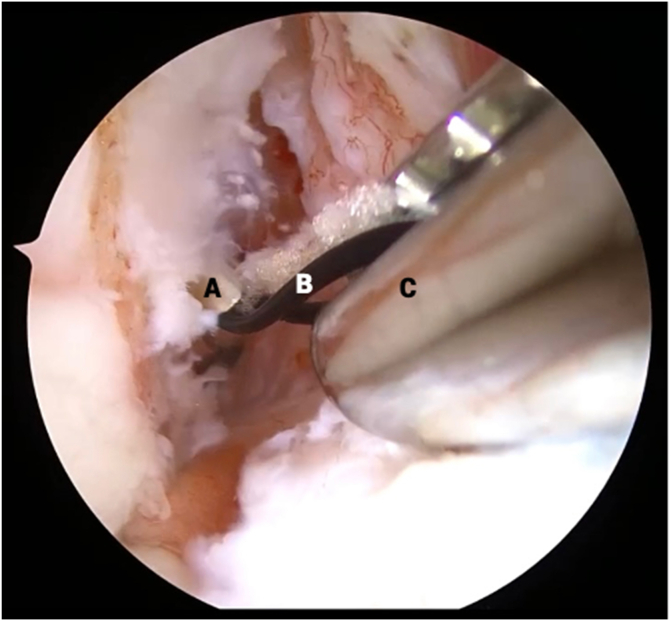
Fig 4.
View from anterolateral portal in right knee with supine positioning. An arthroscopic suture passer (A) is used to suture the stump tissue (B) in an alternating, modified Bunnell configuration, beginning distally (shown on left) and working proximally (shown in middle), until both limbs of suture are exiting from the proximal end of the anterior cruciate ligament stump and the tissue is able to be reined with manipulation of the sutures (shown on right).
Femoral Preparation
A 4.5-mm round burr is used to roughen the femoral footprint to prepare for ligament implantation (Fig 5). An outside-in femoral drill guide is introduced via the lateral portal, and the target is placed on the ACL footprint. The guide is maneuvered through a small slit in the iliotibial band and onto the femur, where the 2.7-mm drill is used to penetrate the bone (Fig 6). The drill is then removed, leaving the sleeve in place, through which a nitinol wire is passed. The looped end of the nitinol wire is retrieved out of the medial portal (Fig 7). The looped end of 1 looped suture and the 2 ACL repair sutures are then passed through the looped end of the nitinol wire and shuttled out of the superolateral incision.
Fig 5.
View from anterolateral portal in right knee with supine positioning. A 4.5-mm round burr (A) is used to roughen the lateral intercondylar ridge (B) to prepare for ligament implantation.
Fig 6.
View from anteromedial portal in right knee with supine positioning. An outside-in drill guide (A) is brought in through the lateral portal and positioned over the femoral anterior cruciate ligament footprint. A small incision is made on the lateral thigh and deep through the iliotibial band so that a 2.7-mm drill (B) can be used to penetrate the lateral intercondylar notch (C) from outside in.
Fig 7.
View from anterolateral portal in right knee with supine positioning. A looped nitinol wire (B) is passed through the drill sleeve (A), and an arthroscopic grasper (C) is used to retrieve the looped end of the wire out of the medial portal. The 2 anterior cruciate ligament repair sutures and 1 additional looped suture are then passed through the nitinol wire and shuttled out of the superolateral incision through which the femoral tunnel was made.
Tibial Preparation
A standard ACL drill guide is inserted through the medial portal, and the target is placed on the tibial footprint. A 2.7-mm cannulated drill is used to penetrate the proximal tibia. The drill is then removed, and the looped nitinol wire is shuttled through the sleeve (Fig 8) and retrieved out of the medial portal.
Fig 8.
View from anterolateral portal in right knee with supine positioning. An outside-in drill guide (A) is positioned over the tibial footprint, and the tibia is penetrated with a 2.7-mm cannulated drill, through which a looped nitinol wire is passed (B) and retrieved with an arthroscopic grasper (C) out of the medial portal.
Internal Brace
The ACL repair sutures are loaded onto a titanium button that is preloaded with a Minitape suture (Smith & Nephew) functioning as an internal brace. The free limbs of the internal brace are passed through the looped passing suture and are shuttled out of the medial portal (Fig 9). This action brings the femoral button through the iliotibial band and onto the femur and aligns the internal brace along the trajectory of the ACL (Fig 10).
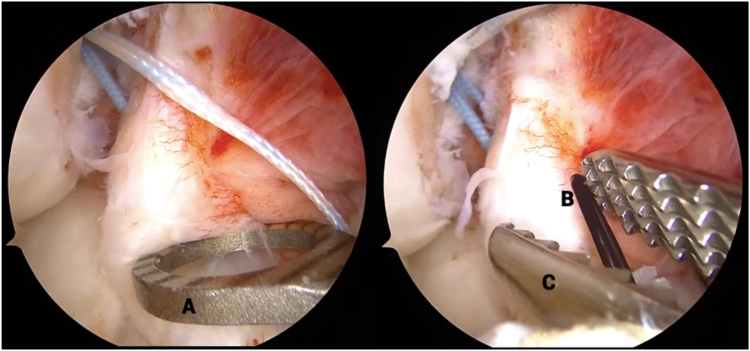
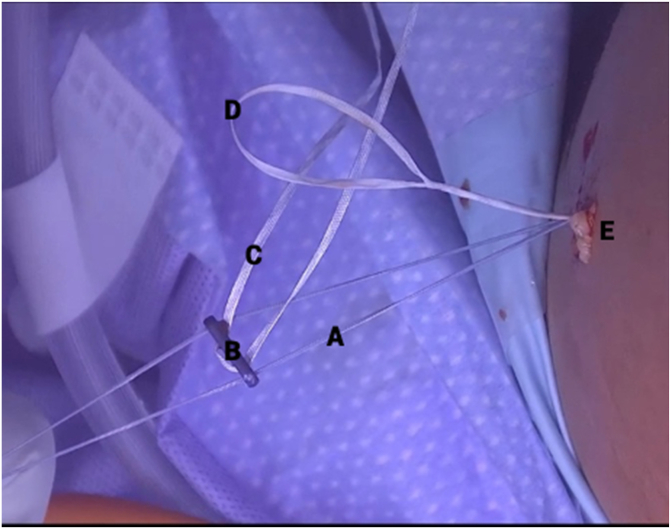
Fig 9.
Clinical view of right knee, positioned supine, during internal brace application. The anterior cruciate ligament repair sutures (A) are loaded onto a titanium button (B) that is preloaded with a Minitape suture (C) functioning as an internal brace. The free limbs of the internal brace are passed through the looped passing suture (D), pulled into the superolateral incision (E), and shuttled out of the medial portal.
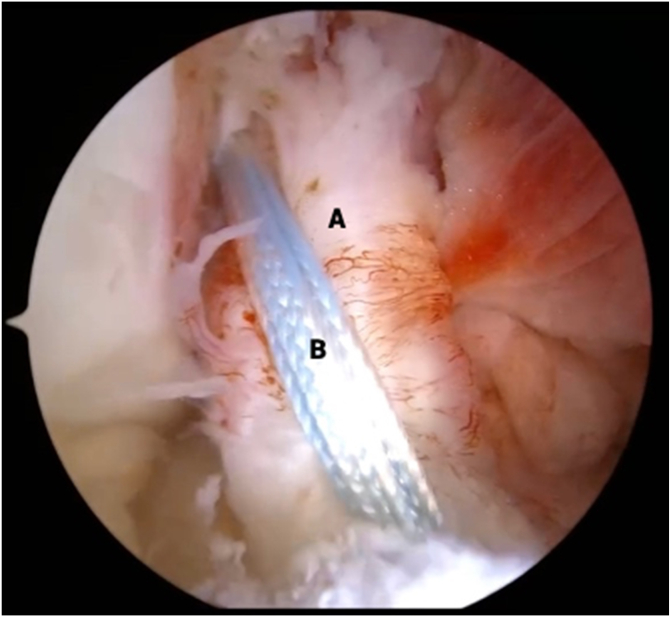
Fig 10.
View from anterolateral portal in right knee with supine positioning. The anterior cruciate ligament (A) and internal brace (B) are seen in tensioned femorotibial orientation prior to bridge-enhanced anterior cruciate ligament restoration (BEAR) implantation.
Arthrotomy
The limb is exsanguinated with an Esmarch bandage, and the tourniquet is inflated to 250 mm Hg. The anteromedial portal is extended proximally roughly 2 to 3 cm to accommodate passage of the BEAR implant (Fig 11). Extra care is taken not to injure the meniscus, medial femoral condyle, or internal brace sutures. The joint is irrigated with an antibiotic rinse prior to BEAR insertion.
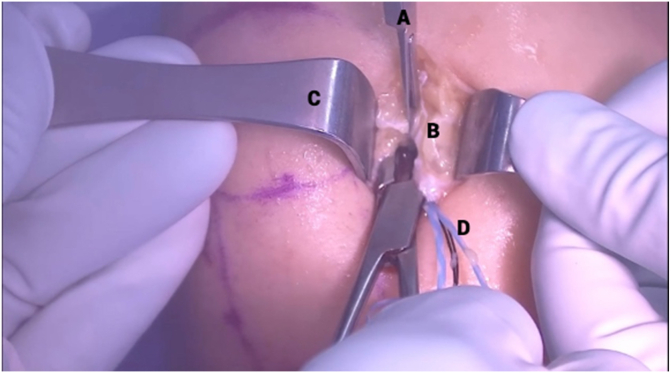
Fig 11.
Clinical view of right knee, positioned supine, during capsulotomy step. A No. 11 blade scalpel (A) is used to extend the medial portal (B) proximally until the notch can be clearly visualized to prepare for bridge-enhanced anterior cruciate ligament restoration (BEAR) insertion. Army-navy retractors (C) are used for careful dissection to avoid injury to the meniscus, medial femoral condyle, or internal brace sutures (D).
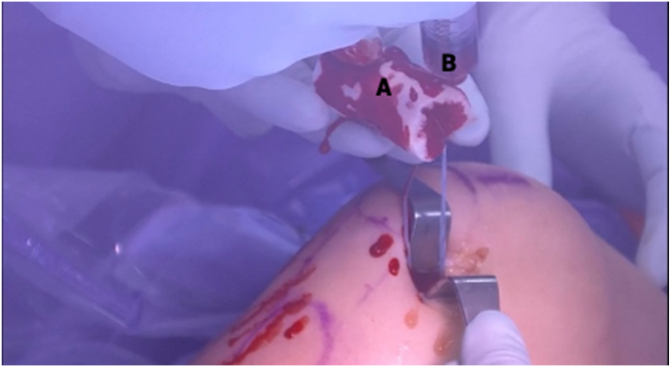
BEAR Preparation and Implantation
Each limb of the internal brace is loaded onto a straight free needle and passed through the center of the BEAR implant (Fig 12). The free ends of the internal brace are then passed through the looped end of the nitinol wire and shuttled out of the tibial incision to prepare for BEAR insertion. The BEAR implant is hydrated with 10 mL of the patient’s blood (Fig 13), beginning with 2 mL centrally, followed by the remaining 8 mL peripherally. The distal end is left dehydrated and is used to push the implant down the internal brace sutures and into the notch while the free ends of suture are pulled distally and the knee is brought into extension. This action positions the BEAR implant along the repaired ACL stump and orients the internal brace across the tibiofemoral ACL footprints.
Fig 12.
Clinical view of right knee, positioned supine, during bridge-enhanced anterior cruciate ligament restoration (BEAR) suture passage. Each limb of the internal brace is loaded onto a straight free needle (A) and individually passed through the center of the BEAR implant (B).
Fig 13.
Clinical view of right knee, positioned supine, during hydration of bridge-enhanced anterior cruciate ligament restoration (BEAR) implant. Ten milliliters of the patient’s blood is collected by the anesthesiologist and handed off in a sterile syringe (B). The BEAR implant (A) is hydrated with the patient’s blood, beginning with 2 mL centrally, followed by the remaining 8 mL peripherally. The distal end of the implant is left dehydrated to allow for a stiffer surface against which to push the implant into the notch.
Closure
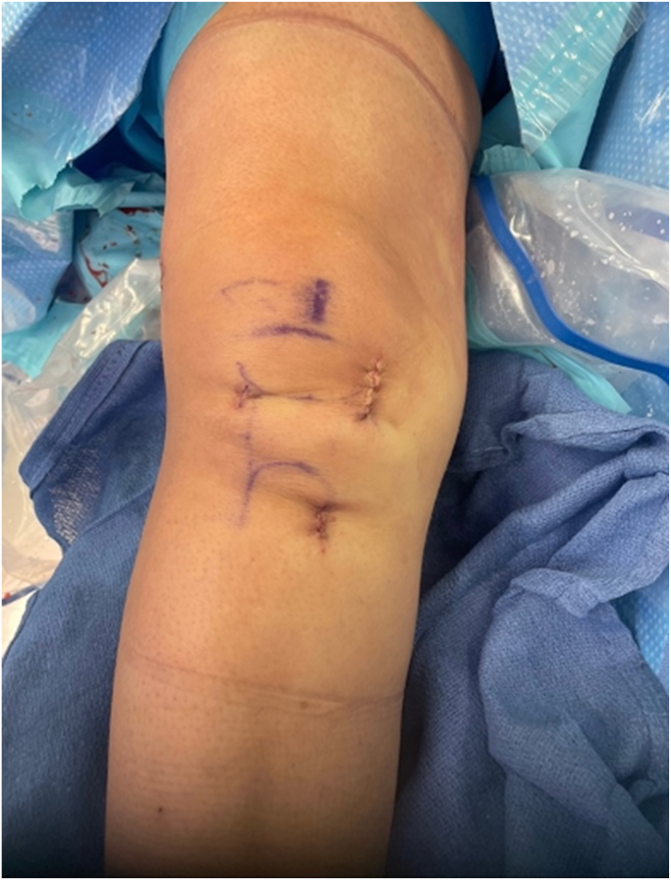
The medial arthrotomy is closed expeditiously with a braided No. 0 Stratafix suture (Ethicon, Somerville, NJ) to prevent extrusion of the liquefied BEAR implant. The internal brace is then tied over a titanium button on the proximal tibia with the help of a knot pusher. The ACL repair sutures are tied over the femoral button using the same technique. The tibial ends of the internal brace can alternatively be secured into a suture anchor. The tibial incision and medial arthrotomy incision are closed with deep dermal interrupted No. 2-0 Vicryl sutures (Ethicon), followed by a running subcuticular No. 3-0 Monocryl suture (Ethicon). The smaller anterolateral and superolateral portals are closed with a buried No. 3-0 Monocryl suture (Fig 14).
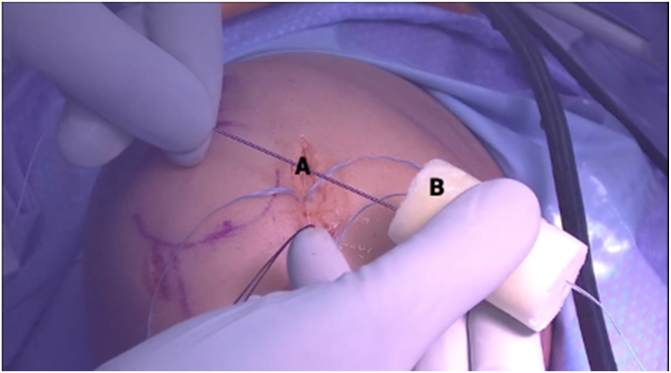
Fig 14.
Clinical view of right knee, positioned supine, postoperatively after closure of incisions.
Postoperative Protocol
Postoperatively in weeks 0 to 2, the patient is allowed range of motion (ROM) of 0° to 45° with partial weight bearing at 50%. In weeks 2 to 4, the patient is permitted ROM of 0° to 90° with partial weight bearing at 75%. After week 4, the patient is allowed weight bearing as tolerated with full ROM.
Discussion
This article provides an efficient and easy-to-learn technique for a bridge-enhanced ACL repair. This procedure is similar to a suture-augmented ACL repair, which has shown durability for proximal avulsions,14 but it adds a biological scaffold15 that expands its indications and may provide superior outcomes compared with traditional ACLR.16
A 2020 randomized controlled trial showed that International Knee Documentation Committee (IKDC) subjective scores and anteroposterior knee laxity values in the BEAR group were noninferior to those in the ACLR group at 2 years after surgery.17 The recent literature has also shown earlier resolution of disability with repair compared with reconstruction,18 as well as higher levels of readiness to return to sport at 6 months.19 Additionally, repairing the native ACL preserves knee anatomy, retains proprioceptive fibers, and may decrease the risk of post-traumatic osteoarthritis.6,20,21
In contrast, a recent 2023 follow-up study of the original BEAR trial cohort reported an overall 15% failure rate of the procedure in the first 2 years postoperatively.22 Multivariate logistic regression analysis identified younger age and increased medial tibial slope as independent predictors of ACL repair failure. There were no failures or revisions in any patients older than 22 years.22 The advantages and disadvantages of the BEAR procedure compared with ACLR are summarized in Table 2. Pearls and pitfalls to consider during the BEAR-augmented ACL repair procedure are shown in Table 3.
Table 2.
Advantages and Disadvantages of BEAR-Augmented ACL Repair Compared With Autograft ACLR
| BEAR-Augmented ACL Repair | Autograft ACLR | |
|---|---|---|
| ACL size and orientation | Restores native ACL cross-sectional area and orientation23 | Graft remains nearly 50% larger than contralateral ACL at 2 yr postoperatively23 |
| Donor-site morbidity | No graft donor-site morbidity | Potential for anterior knee pain, reduced quadriceps or hamstring strength, loss of range of motion, donor-site skin sensitivity, and inability to kneel depending on autograft and harvesting technique used24 |
| Hamstring strength | Increased hamstring strength at 2 yr compared with hamstring autograft ACLR18 | Weaker hamstring strength at 2 yr18 |
| Anterior knee pain | Less anterior knee pain compared with BTB autograft ACLR | Anterior knee pain experienced by 22%-23% of patients undergoing ACLR with BTB autograft25,26 |
| Postoperative recovery | Earlier postoperative resolution of symptoms and return to function18 | Lower patient-reported outcomes at early postoperative time points18 |
| Return to sport | Greater return-to-sport readiness at 6 mo19 | Lower return-to-sport readiness at 6 mo19 |
| Knee proprioception | Preserves native knee kinematics and proprioception6,20,21 | Removes proprioceptive fibers of native ACL and alters knee kinematics and kinetics27,28 |
| Tibial insertion | Preserves native ACL tibial insertion | Alters native ACL tibial insertion |
| Anterior-posterior knee laxity | No statistically significant difference29 | No statistically significant difference29 |
| Revision rate | No statistically significant difference17,22,30, 31, 32 | No statistically significant difference17,22,30, 31, 32 |
ACL, anterior cruciate ligament; ACLR, anterior cruciate ligament reconstruction; BEAR, bridge-enhanced anterior cruciate ligament restoration; BTB, bone-patellar tendon-bone.
Table 3.
Pearls and Pitfalls to Consider During BEAR-Augmented ACL Repair Procedure
| Pearls |
| Spending a deliberate amount of time freeing the ACL stump with the arthroscopic elevator because this will facilitate improved suturing of the stump tissue |
| Suturing the ACL stump as best allowed by the tissue’s anatomy, noting that no strict configuration must be followed |
| Having both limbs of suture exiting from the proximal, medial aspect of the stump tissue, such that pulling the sutures in the femoral tunnel docks the tissue in the osseous cavity |
| Making a large enough medial arthrotomy to allow for easy BEAR implantation |
| Leaving the distal end of the BEAR implant dehydrated to allow for a firm surface against which to push the implant into the notch |
| Expeditiously closing the medial arthrotomy to prevent extrusion of the liquefied BEAR implant |
| Pitfalls |
| Leaving scarred connections to the medial intercondylar notch or the PCL intact |
| Making too small of an opening in the ITB, which may prevent the cortical button from sitting on bone |
| Making too small of a medial arthrotomy and struggling to insert the BEAR implant once it has already been hydrated |
| Hydrating the BEAR implant before the arthrotomy is adequate, the knee is exsanguinated, and all members of the surgical team are ready for implantation because the BEAR implant liquefies rapidly once hydrated |
| Hydrating the entire BEAR implant because this will make insertion difficult |
ACL, anterior cruciate ligament; BEAR, bridge-enhanced anterior cruciate ligament restoration; ITB, iliotibial band; PCL, posterior cruciate ligament.
Additional studies are needed to assess the long-term viability of BEAR in human subjects. However, in the senior author’s (SA) practice, BEAR-augmented ACL repair has become an effective and reproducible technique to provide anterior and rotatory stability while preserving native knee proprioception and eliminating donor-site morbidity, when properly indicated and performed.
Disclosures
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: J.N.G. reports a consulting or advisory relationship with DePuy Synthes Mitek Sports Medicine. S.G.A. reports a consulting or advisory relationship with Miach Orthopaedics. All other authors (D.E.K., C.N.D., E.D.H.) declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Data
Bridge-enhanced arthroscopic anterior cruciate ligament (ACL) repair in right knee. The patient is positioned supine with a tourniquet on the upper thigh, a lateral thigh post, and a foot bump to maintain knee flexion during the procedure. The arthroscope is introduced into the knee via a standard anterolateral portal. The ACL stump is inspected, mobilized, and sutured. The femoral footprint is roughened; then, a tunnel is drilled from outside in, and the repair sutures are retrieved. A tibial tunnel is drilled from outside in as well. The ACL sutures are passed through a cortical button that is preloaded with an internal brace, which is then shuttled out of the medial portal. The internal brace sutures are passed through the bridge-enhanced anterior cruciate ligament restoration (BEAR) implant and then shuttled out of the tibial tunnel. The implant is hydrated with 10 mL of the patient’s blood and then slid down the internal brace sutures and into the notch while the knee is brought into extension. The tibial ends of the internal brace and the ACL repair sutures are tied over the tibial and femoral cortical buttons, respectively.
References
- 1.Sanders T.L., Maradit Kremers H., Bryan A.J., et al. Incidence of anterior cruciate ligament tears and reconstruction: A 21-year population-based study. Am J Sports Med. 2016;44:1502–1507. doi: 10.1177/0363546516629944. [DOI] [PubMed] [Google Scholar]
- 2.Herzog M.M., Marshall S.W., Lund J.L., Pate V., Mack C.D., Spang J.T. Trends in incidence of ACL reconstruction and concomitant procedures among commercially insured individuals in the United States, 2002-2014. Sports Health. 2018;10:523–531. doi: 10.1177/1941738118803616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feagin J.A., Curl W.W. Isolated tear of the anterior cruciate ligament: 5-Year follow-up study. Am J Sports Med. 1976;4:95–100. doi: 10.1177/036354657600400301. [DOI] [PubMed] [Google Scholar]
- 4.Odensten M., Hamberg P., Nordin M., Lysholm J., Gillquist J. Surgical or conservative treatment of the acutely torn anterior cruciate ligament: A randomized study with short-term follow-up observations. Clin Orthop Relat Res. 1985;198:87–93. [PubMed] [Google Scholar]
- 5.Sandberg R., Balkfors B., Nilsson B., Westlin N. Operative versus non-operative treatment of recent injuries to the ligaments of the knee. A prospective randomized study. J Bone Joint Surg Am. 1987;69:1120–1126. [PubMed] [Google Scholar]
- 6.van der List J.P., DiFelice G.S. Primary repair of the anterior cruciate ligament: A paradigm shift. Surgeon. 2017;15:161–168. doi: 10.1016/j.surge.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Lai C.C.H., Ardern C.L., Feller J.A., Webster K.E. Eighty-three per cent of elite athletes return to preinjury sport after anterior cruciate ligament reconstruction: A systematic review with meta-analysis of return to sport rates, graft rupture rates and performance outcomes. Br J Sports Med. 2018;52:128–138. doi: 10.1136/bjsports-2016-096836. [DOI] [PubMed] [Google Scholar]
- 8.Mihelic R., Jurdana H., Jotanovic Z., Madjarevic T., Tudor A. Long-term results of anterior cruciate ligament reconstruction: A comparison with non-operative treatment with a follow-up of 17-20 years. Int Orthop. 2011;35:1093–1097. doi: 10.1007/s00264-011-1206-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonkergouw A., van der List J.P., DiFelice G.S. Arthroscopic primary repair of proximal anterior cruciate ligament tears: Outcomes of the first 56 consecutive patients and the role of additional internal bracing. Knee Surg Sports Traumatol Arthrosc. 2019;27:21–28. doi: 10.1007/s00167-018-5338-z. [DOI] [PubMed] [Google Scholar]
- 10.Van Der List J.P., Vermeijden H.D., Sierevelt I.N., DiFelice G.S., Van Noort A., Kerkhoffs G.M.M.J. Arthroscopic primary repair of proximal anterior cruciate ligament tears seems safe but higher level of evidence is needed: A systematic review and meta-analysis of recent literature. Knee Surg Sports Traumatol Arthrosc. 2020;28:1946–1957. doi: 10.1007/s00167-019-05697-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vermeijden H.D., van der List J.P., Benner J.L., Rademakers M.V., Kerkhoffs G.M.M.J., DiFelice G.S. Primary repair with suture augmentation for proximal anterior cruciate ligament tears: A systematic review with meta-analysis. Knee. 2022;38:19–29. doi: 10.1016/j.knee.2022.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Perrone G.S., Proffen B.L., Kiapour A.M., Sieker J.T., Fleming B.C., Murray M.M. Bench-to-bedside: Bridge-enhanced anterior cruciate ligament repair. J Orthop Res. 2017;35:2606–2612. doi: 10.1002/jor.23632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murray M.M., Flutie B.M., Kalish L.A., et al. The bridge-enhanced anterior cruciate ligament repair (BEAR) procedure: An early feasibility cohort study. Orthop J Sports Med. 2016;4 doi: 10.1177/2325967116672176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douoguih W.A., Apseloff N.A., Murray J.C., Kelly R.L., Svoboda S.J. Suture-augmented ACL repair for proximal avulsion or high-grade partial tears shows similar side-to-side difference and no clinical differences at 2 years versus conventional ACL reconstruction for near-complete and mid-substance tears or poor ACL tissue quality. Arthroscopy. 2024;40:857–867. doi: 10.1016/j.arthro.2023.07.011. [DOI] [PubMed] [Google Scholar]
- 15.Murray M.M., Kalish L.A., Fleming B.C., et al. Bridge-enhanced anterior cruciate ligament repair: Two-year results of a first-in-human study. Orthop J Sports Med. 2019;7 doi: 10.1177/2325967118824356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinkler M.A., Furdock R.J., McMellen C.J., Calcei J.G., Voos J.E. Biologics, stem cells, growth factors, platelet-rich plasma, hemarthrosis, and scaffolds may enhance anterior cruciate ligament surgical treatment. Arthroscopy. 2023;39:166–175. doi: 10.1016/j.arthro.2022.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Murray M.M., Fleming B.C., Badger G.J., et al. Bridge-enhanced anterior cruciate ligament repair is not inferior to autograft anterior cruciate ligament reconstruction at 2 years: Results of a prospective randomized clinical trial. Am J Sports Med. 2020;48:1305–1315. doi: 10.1177/0363546520913532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barnett S.C., Murray M.M., Badger G.J., et al. Earlier resolution of symptoms and return of function after bridge-enhanced anterior cruciate ligament repair as compared with anterior cruciate ligament reconstruction. Orthop J Sports Med. 2021;9 doi: 10.1177/23259671211052530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanborn R.M., Badger G.J., Proffen B., et al. Psychological readiness to return to sport at 6 months is higher after bridge-enhanced ACL restoration than autograft ACL reconstruction: Results of a prospective randomized clinical trial. Orthop J Sports Med. 2022;10 doi: 10.1177/23259671211070542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denti M., Monteleone M., Berardi A., Panni A.S. Anterior cruciate ligament mechanoreceptors: Histologic studies on lesions and reconstruction. Clin Orthop Relat Res. 1994;308:29–32. [PubMed] [Google Scholar]
- 21.Kiapour A.M., Murray M.M. Basic science of anterior cruciate ligament injury and repair. Bone Joint Res. 2014;3:20–31. doi: 10.1302/2046-3758.32.2000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanborn R.M., Badger G.J., Fleming B.C., et al. Preoperative risk factors for subsequent ipsilateral ACL revision surgery after an ACL restoration procedure. Am J Sports Med. 2023;51:49–57. doi: 10.1177/03635465221137873. [DOI] [PubMed] [Google Scholar]
- 23.Kiapour A.M., Ecklund K., Murray M.M., et al. Changes in cross-sectional area and signal intensity of healing anterior cruciate ligaments and grafts in the first 2 years after surgery. Am J Sports Med. 2019;47:1831–1843. doi: 10.1177/0363546519850572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kartus J., Movin T., Karlsson J. Donor-site morbidity and anterior knee problems after anterior cruciate ligament reconstruction using autografts. Arthroscopy. 2001;17:971–980. doi: 10.1053/jars.2001.28979. [DOI] [PubMed] [Google Scholar]
- 25.Rousseau R., Labruyere C., Kajetanek C., Deschamps O., Makridis K.G., Djian P. Complications after anterior cruciate ligament reconstruction and their relation to the type of graft: A prospective study of 958 cases. Am J Sports Med. 2019;47:2543–2549. doi: 10.1177/0363546519867913. [DOI] [PubMed] [Google Scholar]
- 26.Biau D.J., Tournoux C., Katsahian S., Schranz P.J., Nizard R.S. Bone-patellar tendon-bone autografts versus hamstring autografts for reconstruction of anterior cruciate ligament: Meta-analysis. BMJ. 2006;332:995–1001. doi: 10.1136/bmj.38784.384109.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoshino Y., Fu F.H., Irrgang J.J., Tashman S. Can joint contact dynamics be restored by anterior cruciate ligament reconstruction? Clin Orthop Relat Res. 2013;471:2924–2931. doi: 10.1007/s11999-012-2761-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hall M., Stevermer C.A., Gillette J.C. Gait analysis post anterior cruciate ligament reconstruction: Knee osteoarthritis perspective. Gait Posture. 2012;36:56–60. doi: 10.1016/j.gaitpost.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 29.Mansour J., Ghanimeh J., Ghoul A., Estephan M., Khoury A., Daher M. Bridge enhanced ACL repair vs. ACL reconstruction for ACL tears: A systematic review and meta-analysis of comparative studies. SICOT J. 2023;9:8. doi: 10.1051/sicotj/2023007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webster K.E., Feller J.A. Exploring the high reinjury rate in younger patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2016;44:2827–2832. doi: 10.1177/0363546516651845. [DOI] [PubMed] [Google Scholar]
- 31.Cruz AI Jr, Beck J.J., Ellington M.D., et al. Failure rates of autograft and allograft ACL reconstruction in patients 19 years of age and younger. JB JS Open Access. 2020;5 doi: 10.2106/JBJS.OA.20.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cordasco F.A., Black S.R., Price M., et al. Return to sport and reoperation rates in patients under the age of 20 after primary anterior cruciate ligament reconstruction: Risk profile comparing 3 patient groups predicated upon skeletal age. Am J Sports Med. 2019;47:628–639. doi: 10.1177/0363546518819217. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Bridge-enhanced arthroscopic anterior cruciate ligament (ACL) repair in right knee. The patient is positioned supine with a tourniquet on the upper thigh, a lateral thigh post, and a foot bump to maintain knee flexion during the procedure. The arthroscope is introduced into the knee via a standard anterolateral portal. The ACL stump is inspected, mobilized, and sutured. The femoral footprint is roughened; then, a tunnel is drilled from outside in, and the repair sutures are retrieved. A tibial tunnel is drilled from outside in as well. The ACL sutures are passed through a cortical button that is preloaded with an internal brace, which is then shuttled out of the medial portal. The internal brace sutures are passed through the bridge-enhanced anterior cruciate ligament restoration (BEAR) implant and then shuttled out of the tibial tunnel. The implant is hydrated with 10 mL of the patient’s blood and then slid down the internal brace sutures and into the notch while the knee is brought into extension. The tibial ends of the internal brace and the ACL repair sutures are tied over the tibial and femoral cortical buttons, respectively.