Abstract
Reovirus virions are internalized into cells by receptor-mediated endocytosis. Within the endocytic compartment, the viral outer capsid undergoes acid-dependent proteolysis leading to degradation of ς3 protein and proteolytic cleavage of μ1/μ1C protein. E64 is a specific inhibitor of cysteine-containing proteases that blocks disassembly of reovirus virions. To identify domains in reovirus proteins that influence susceptibility to E64-mediated inhibition of disassembly, we selected variant viruses by serial passage of strain type 3 Dearing (T3D) in murine L929 cells treated with E64. E64-adapted variant viruses (D-EA viruses) produced 7- to 17-fold-greater yields than T3D did after infection of cells treated with 100 μM E64. Viral genes that segregate with growth of D-EA viruses in the presence of E64 were identified by using reassortant viruses isolated from independent crosses of E64-sensitive strain type 1 Lang and two prototype D-EA viruses. Growth of reassortant viruses in the presence of E64 segregated with the S4 gene, which encodes outer-capsid protein ς3. Sequence analysis of S4 genes of three D-EA viruses isolated from independent passage series revealed a common tyrosine-to-histidine mutation at amino acid 354 in the deduced amino acid sequence of ς3. Proteolysis of D-EA virions by endocytic protease cathepsin L occurred with faster kinetics than proteolysis of wild-type T3D virions. Treatment of D-EA virions, but not T3D virions, with cathepsin D resulted in proteolysis of ς3, a property that also was found to segregate with the D-EA S4 gene. These results indicate that a region in ς3 protein containing amino acid 354 influences susceptibility of ς3 to proteolysis during reovirus disassembly.
Many viruses require endocytic uptake and exposure to acid-dependent proteases or acidic pH to productively infect host cells. Reoviruses are nonenveloped viruses that enter cells by receptor-mediated endocytosis (5, 6, 27, 31). Within late endosomes or lysosomes, viral outer-capsid proteins ς3 and μ1/μ1C are subject to proteolysis by endocytic proteases, resulting in generation of infectious subvirion particles (ISVPs) (2, 6, 10, 30, 31). During this process, ς3 is degraded and lost from virions, viral attachment protein ς1 undergoes a conformational change, and μ1/μ1C is cleaved to form particle-associated fragments μ1δ/δ and φ (reviewed in reference 24). ISVPs are obligate intermediates in reovirus disassembly that mediate penetration of the virus into the cytoplasm (5, 15, 16, 20, 32).
Treatment of cells with E64, a specific inhibitor of proteases containing active-site cysteine residues (3), blocks steps in reovirus disassembly required for generation of ISVPs (1, 9). Likewise, treatment of cells with the weak base ammonium chloride (12, 31) or inhibitors of the vacuolar proton ATPase, such as bafilomycin or concanamycin A (21), also blocks conversion of virions to ISVPs. These observations suggest that the proteolysis of ς3 and μ1/μ1C during virion-to-ISVP conversion is an acid-dependent process mediated by cysteine-containing proteases.
Persistent reovirus infection of murine L929 (L) cells selects mutant cells (LX cells) that do not support viral disassembly within the endocytic pathway. LX cells are permissive for reovirus growth when infection is initiated with ISVPs but not when infection is initiated with virions (12). These findings indicate that LX cells have a defect in virion-to-ISVP processing. Parental L cells and mutant LX cells do not differ in the capacity to internalize reovirus virions, nor do they differ in intravesicular pH. However, in contrast to parental L cells, mutant LX cells do not express the mature, proteolytically active form of cathepsin L, a lysosomal cysteine protease (2). Treatment of reovirus virions with purified cathepsin L leads to formation of particles that have the biochemical and growth properties of ISVPs generated by treatment of virions with intestinal proteases (2). These findings provide strong evidence that cathepsin L is sufficient to mediate reovirus disassembly in murine L cells.
In contrast to wild-type (wt) viruses, viruses isolated from persistently infected L-cell cultures (PI viruses) can grow in cells treated with either ammonium chloride (12, 34) or E64 (1). These findings suggest that PI viruses have altered requirements for acidic pH and proteolysis to complete steps in entry required to generate ISVPs. Mutations in PI viruses that confer growth in ammonium chloride-treated cells segregate with either the S1 or S4 gene, depending on the PI virus studied (34). These results suggest that there are at least two acid-dependent disassembly events during conversion of virions to ISVPs, one involving viral attachment protein ς1, which is encoded by the S1 gene, and another involving outer-capsid protein ς3, which is encoded by the S4 gene. Mutations in PI viruses that confer growth in E64-treated cells segregate exclusively with the S4 gene (1), which suggests that the ς3 protein alone is the primary determinant of susceptibility of the viral outer capsid to proteolytic cleavage during viral entry.
Results of studies using mutant reoviruses selected during persistent infection have identified viral structural proteins that mediate requirements for acidification and proteolysis during viral disassembly. However, the selective pressures acting during a persistent infection are likely to be complex, and it is possible that mutations in PI viruses alter entry steps in addition to those dependent on acidification and proteolysis. Therefore, to select reovirus variants altered specifically in proteolytic events, we isolated variant viruses by serial passage in the presence of protease inhibitor E64. We used reassortant genetics and nucleotide sequence analysis to determine the molecular basis for adaptation of reovirus to growth in the presence of E64. The results indicate that a single mutation in the carboxy terminus of ς3 protein strongly influences the susceptibility of ς3 to proteolysis during the disassembly of reovirus virions.
MATERIALS AND METHODS
Cells and viruses.
Murine L cells were grown in either suspension or monolayer cultures in Joklik's modified Eagle's minimal essential medium (Irvine Scientific, Santa Ana, Calif.) supplemented to contain 5% fetal bovine serum (Intergen, Purchase, N.Y.), 2 mM l-glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 0.25 μg of amphotericin B per ml (Irvine). Reovirus strains type 1 Lang (T1L) and type 3 Dearing (T3D) are laboratory stocks. Purified virion preparations were made using second-passage (P2) L-cell lysate stocks of twice-plaque-purified reovirus as previously described (13). Purified virions containing 35S-labeled proteins were obtained by adding Easy Tag Express-[35S] protein labeling mix (NEN, Boston, Mass.) to cell suspensions (∼12.5 μCi per ml) at the initiation of infection.
Selection of reovirus variants adapted to growth in the presence of E64.
Independent cultures of L cells (6 × 106) in 25-cm2 flasks (Costar, Cambridge, Mass.) were preincubated for 1 h in growth medium containing 100 μM E64 (Sigma Chemical Co., St. Louis, Mo.) prior to viral adsorption. Cultures were inoculated with P2 stocks of reovirus strain T3D generated from independent plaque picks at a multiplicity of infection MOI of 10 PFU per cell. After 1 h of virus adsorption at room temperature, fresh medium containing 100 μM E64 was added, and the cells were incubated at 37°C for either 7 days (passage series 1) or 48 h (passage series 2 and 3). Cultures were frozen and thawed twice, and 0.5 ml of culture lysate was used to infect a fresh culture of E64-treated L cells. This procedure was repeated for either 3 (passage series 1) or 10 (passage series 2 and 3) passages. T3D-derived, E64-adapted (D-EA) viruses were isolated by two rounds of plaque purification on L cells from 3rd-passage lysate stocks of passage series 1 and 10th-passage lysate stocks of passage series 2 and 3. Working stocks of D-EA viruses were prepared using L cells that were not treated with E64.
Growth of reovirus in the presence and absence of E64.
Monolayers of L cells (5 × 105) in 24-well plates (Costar) were preincubated for 4 h in medium supplemented to contain from 0 to 200 μM E64. The medium was removed, and the cells were adsorbed with reovirus strains at an MOI of 2 PFU per cell. After a 1-h incubation at 4°C, the inoculum was removed, cells were washed twice with phosphate-buffered saline, and 1 ml of fresh medium supplemented with 0 to 200 μM E64 was added. After incubation at 37°C for various intervals, cells were frozen and thawed twice, and viral titers in cell lysates were determined by plaque assay (33). Independent experiments were performed using single wells of cells, which were subjected to titer determination in duplicate.
Infection of cells with radiolabeled reovirus virions.
Monolayers of L cells (107) in 75-cm2 flasks (Costar) were preincubated for 4 h in medium supplemented to contain 0 to 200 μM E64. The medium was removed, and cells were adsorbed with purified, 35S-labeled reovirus virions at an MOI of 10,000 particles per cell. After incubation at 4°C for 1 h, the inoculum was removed, cells were washed twice with phosphate-buffered saline, and 3 ml of fresh medium supplemented with 0 to 200 μM E64 was added. After incubation at 37°C for various intervals, cells were scraped and collected by centrifugation at 528 × g for 5 min. Cells were resuspended in 0.5 ml of lysis buffer (150 mM NaCl, 10 mM Tris [pH 7.4], 0.5% Nonidet P-40, 1 mM EDTA, 1 mM benzamidine [Sigma], 100 mM leupeptin [Sigma], 2.5 mM phenylmethylsulfonyl fluoride) and placed on ice for 10 min, and 4.5 ml of homogenization buffer (250 mM NaCl, 10 mM Tris [pH 7.4], 0.067% 2-mercaptoethanol) was added. Samples were sonicated for 1 min, 2.5 ml of Freon (EM Science, Gibbstown, N.J.) was added, and samples were again sonicated for 1 min. Samples were centrifuged at 9,700 × g for 10 min, and the aqueous fraction was placed into 14- by 89-mm centrifuge tubes (Beckman, Palo Alto, Calif.). Virus particles were pelleted by centrifugation in an SW50.1 rotor (Beckman) at 210,000 × g for 1 h.
SDS-PAGE of reovirus structural proteins.
Discontinuous sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed as previously described (19). Viral particles were solubilized by incubation in sample buffer (125 mM Tris, 2% 2-mercaptoethanol, 1% SDS, 0.01% bromophenol blue) at 100°C for 5 min. Samples were loaded into wells of 10% polyacrylamide gels and electrophoresed at 200 V (constant voltage) for 1 h. Following electrophoresis, gels were fixed, dried onto filter paper (Bio-Rad Laboratories, Richmond, Calif.) under vacuum, and exposed to BioMax film (Eastman Kodak Co., Rochester, N.Y.). Alternatively, gels were stained with Coomassie blue R-250 (Sigma) and dried between cellophane sheets.
Isolation and characterization of T1L × D-EA reassortant viruses.
Reassortant viruses were isolated as previously described (35). L-cell monolayers were coinfected with either T1L and D-EA1 or T1L and D-EA3 at various ratios for a total MOI of 10 PFU per cell. After development of significant cytopathic effect (approximately 48 h), putative reassortant viruses were isolated from infected cell lysates by plaque purification twice in L cells. Genotypes of reassortant viruses were determined by SDS-PAGE of viral double-stranded RNA purified from P2 stocks as previously described (35).
Statistical analysis.
The association of reovirus gene segments with growth in L cells treated with E64 was determined by using both the nonparametric Mann-Whitney (MW) test and the parametric two-sample, two-tailed t test assuming unequal variance. MW tests were performed as described previously (26), and the t tests were calculated using Excel 97 (Microsoft, Redmond, Wash.).
Nucleotide sequence analysis of D-EA S4 genes.
The ς3-encoding S4-gene cDNAs of D-EA1, D-EA2, and D-EA3 were generated using reverse transcription (RT) (Boehringer Mannheim Biochemicals, Indianapolis, Ind.) and PCR with primers specific for the noncoding regions of the T3D S4 gene by previously described techniques (11, 34). Resultant S4-gene cDNAs were cloned into the pCR2.1 vector (Invitrogen, San Diego, Calif), and nucleotide sequences of cloned cDNAs from two independent RT-PCR amplifications were determined by dideoxy chain termination reactions as previously described (11).
Treatment of reovirus virions with purified cathepsins.
Purified reovirus virions at a concentration of 2.4 × 1012 particles per ml in reaction buffer L (100 mM NaCl, 15 mM MgCl2, 50 mM sodium acetate [pH 5.0]) were treated with 100 μg of purified, recombinant, human cathepsin L (8) per ml in the presence of 5 mM dithiothreitol at 37°C for various intervals. Virions at a concentration of 2.67 × 1012 particles per ml in reaction buffer D (5 mM MgCl2, 10 mM cysteine, 100 mM potassium acetate [pH 3.8]) were treated with various concentrations of purified bovine cathepsin D (Sigma) per ml at 37°C for 1 h. Protease treatment was terminated by adding either 500 μM E64 to the cathepsin L reactions or 100 μg of pepstatin A (Sigma) per ml to the cathepsin D reaction mixtures and freezing at −20°C. Aliquots of the treatment mixtures were mixed 5:1 with 6× sample buffer (350 mM Tris [pH 6.8], 9.3% DTT, 10% SDS, 0.012% bromophenol blue) and incubated at 100°C for 5 min. Samples were loaded into wells of 10% polyacrylamide gels and electrophoresed at 200 V (constant voltage) for 1 h.
Densitometric analysis of reovirus outer-capsid proteins.
Dried gels containing 35S-labeled reovirus virions were exposed to an imaging plate, and the band intensity was quantitated by determining photostimulus luminescence units using a Fuji2000 phosphorimager (Fuji Medical Systems, Inc., Stamford, Conn.). Alternatively, gels containing Coomassie blue-stained proteins were scanned using Adobe Photoshop 5.0 (Adobe Systems Inc., San Jose, Calif.), and bands were quantitated using the program Scion Image Beta 3b (Scion Corp., Frederick, Md.). For each interval of protease treatment or concentration of protease used, mean densities were determined for bands corresponding to the ς2 protein and the ς3 protein. Densities of bands corresponding to ς3 were divided by densities of bands corresponding to ς2 as a control for loading. Core protein ς2 is not degraded during protease treatment of virions to generate ISVPs (6, 10, 23, 30, 31, 34).
RESULTS
Selection of E64-resistant reovirus variants.
To identify domains in reovirus outer-capsid proteins responsive to proteolysis during viral entry, three independent stocks of reovirus strain T3D were passaged serially in L cells treated with 100 μM E64. This concentration of E64 has been shown to inhibit reovirus growth by blocking the proteolytic disassembly of the viral outer capsid (1). Cells cultivated in passage series 1 were incubated in the presence of E64 for 7 days between passages, with a boost of fresh E64 provided on days 2 and 4; cells cultivated in passage series 2 and 3 were incubated for 48 h between passages. Following the first cycle of viral passage, 0.5 ml of culture lysate was used to infect fresh, E64-treated L-cell cultures. Passage series 1 was continued for 3 passages, and passage series 2 and 3 were continued for 10 passages.
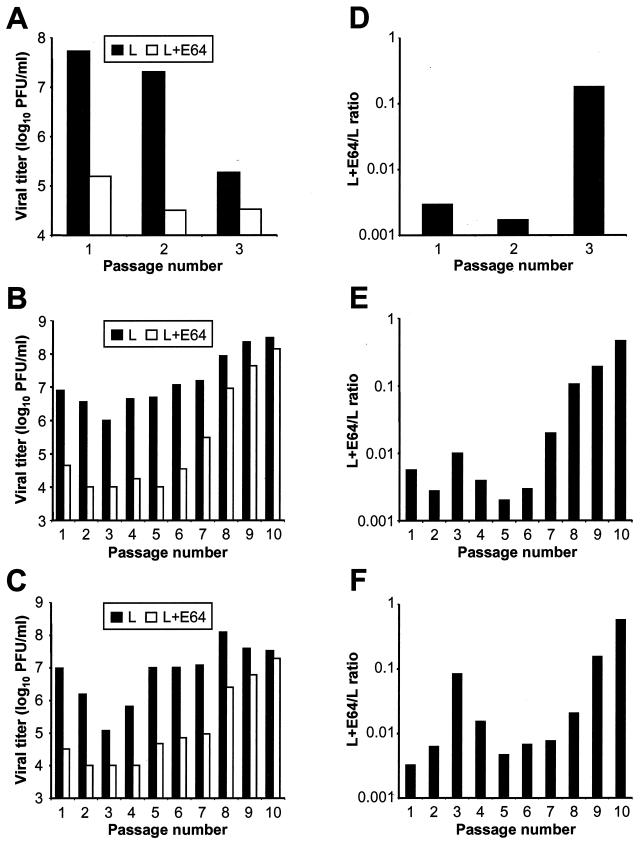
To determine whether E64-resistant viruses were selected during serial passage in cells treated with E64, we tested passage series lysates from all three passage series for growth in the presence and absence of 100 μM E64 (Fig. 1A to C). To standardize for possible differences in viral growth, titers in E64-treated cells were divided by those in untreated cells to calculate L+E64/L ratios for each passage lysate (Fig. 1D to E). We found that lysates from the 3rd passage of passage series 1 and the 10th passage of passage series 2 and 3 displayed a greater than 60-fold increase in resistance to E64 compared to lysates from the first passage. As a control, T3D was passaged 10 times in untreated L cells, with each passage lasting 48 h. Passage series lysates were then tested for growth in the presence and absence of 100 μM E64. L+E64/L ratios for passage lysates obtained from untreated cells did not differ from those for unpassaged T3D (data not shown), suggesting that serial passage of T3D does not select E64-resistant variant viruses. These findings suggest that variant viruses altered in requirements for proteolysis are selected during serial passage in L cells treated with protease inhibitor E64.
FIG. 1.
Selection of reovirus variants capable of growth in L cells treated with E64. Cultures of L cells (5 × 106), preincubated with 100 μM E64, were infected with independent P2 stocks of reovirus strain T3D at an MOI of 10 PFU per cell. Cultures were incubated for either 7 days (passage series 1) or 48 h (passage series 2 and 3) and then lysed by freezing and thawing twice. A 0.5-ml aliquot of culture lysate was used to infect a fresh culture of E64-treated L cells, and the process was repeated for a total of either 3 (passage series 1) or 10 (passage series 2 and 3) passages. Monolayers of L cells (5 × 105), following a 1-h preincubation in medium supplemented with 100 μM E64 or not supplemented, were infected with lysate stocks from each passage series at an MOI of 2 PFU per cell. After a 1-h adsorption period, the inoculum was removed, fresh medium with or without 100 μM E64 was added, and cells were incubated at 37°C for 24 h. Cells were then frozen and thawed twice, and titers of virus in cell lysates were determined by plaque assay. (A to C) Results for passage series 1 (A), passage series 2 (B), and passage series 3 (C) are presented as mean viral titer for two independent experiments. (D to F) L+E64/L ratios for passage series 1 (D), passage series 2 (E), and passage series 3 (F) were calculated by dividing the viral titer in L cells treated with E64 by the viral titer in untreated L cells. D-EA variant viruses were isolated from the passage 3 stock of passage series 1 and the passage 10 stocks of passage series 2 and 3 by plaque purification twice on L-cell monolayers.
Isolation of variant viruses adapted to growth in E64-treated cells.
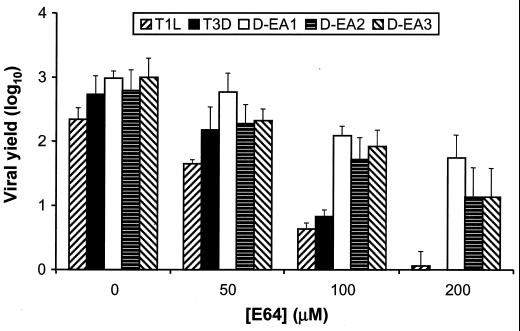
To facilitate studies of the mechanism of viral adaptation to growth in the presence of E64, we isolated independent viral clones from each passage series. D-EA1 was isolated from a 3rd-passage lysate stock of passage series 1, D-EA2 was isolated from a 10th-passage lysate stock of passage series 2, and D-EA3 was isolated from a 10th-passage lysate stock of passage series 3. We determined yields of the cloned D-EA viruses and wt strains T1L and T3D after 24 h of growth in L cells treated with increasing concentrations of E64 from 0 to 200 μM (Fig. 2). Yields of wt T1L and T3D were only four- and sevenfold greater than viral input, respectively, after growth in cells treated with 100 μM E64. In sharp contrast, yields of D-EA1, D-EA2, and D-EA3 were 120-, 50-, and 80-fold greater than viral input, respectively. Growth of D-EA variant viruses was inhibited in cells treated with increasing concentrations of E64, but the magnitude of the inhibition was less than that observed for the wt strains. Other clones isolated from the third passage of passage series 1 were found to have wt sensitivity to E64 (data not shown). However, this was not the case for clones isolated from the 10th passage of passage series 2 and 3. In these passage series, all clones analyzed displayed E64 resistance (data not shown). Therefore, E64-resistant reovirus variants amenable for studies of reovirus disassembly were selected during serial passage of T3D in cells treated with E64.
FIG. 2.
Effect of E64 concentration on growth of wt reovirus strains T1L and T3D and E64-adapted reoviruses D-EA1, D-EA2, and D-EA3. Monolayers of L cells (4 × 105) were preincubated for 1 h in medium supplemented with E64 at the concentrations shown or not supplemented. The medium was removed, and cells were adsorbed with each virus strain at an MOI of 2 PFU per cell. After 1 h, the inoculum was removed, fresh medium with or without E64 was added, and cells were incubated for 24 h. Viral titers in cell lysates were determined by plaque assay. The results are presented as the mean viral yield, calculated by dividing the titer at 24 h by the titer at 0 h for each concentration of E64, for four independent experiments. Error bars indicate standard deviations of the means.
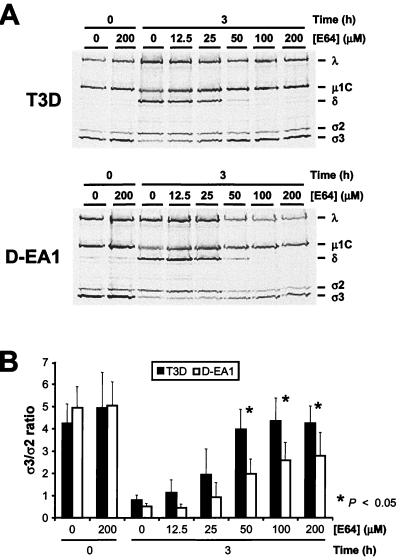
To determine whether differences in growth of wt and D-EA viruses in the presence of E64 are linked to differences in viral disassembly, 35S-labeled virions of wt T3D and D-EA1 were adsorbed to L cells treated with concentrations of E64 from 25 to 200 μM. After 3 h of incubation, viral structural proteins were resolved by SDS-PAGE and visualized by autoradiography (Fig. 3A). Proteolysis of the ς3 and μ1C proteins was apparent after infection of untreated cells with either T3D or D-EA1. After infection of cells treated with E64, we observed a concentration-dependent inhibition of the proteolysis of outer-capsid proteins of both strains; however, proteolysis of outer-capsid proteins of D-EA1 was less sensitive to E64 inhibition. The extent of D-EA1 ς3 cleavage was significantly greater than that of T3D ς3 at 50, 100, and 200 μM E64 (Fig. 3B), which parallels the differences exhibited by these strains in growth in E64-treated cells. These findings show that in the presence of E64, D-EA viruses differ substantially from wt viruses in their capacity to complete disassembly steps leading to generation of ISVPs.
FIG. 3.
(A) Electrophoretic analysis of viral structural proteins of reovirus strains T3D and D-EA1 after infection of L cells in the presence and absence of E64. Monolayers of L cells (107) were preincubated for 4 h in medium supplemented with E64 at the concentrations shown or not supplemented. The medium was removed, and cells were adsorbed with purified 35S-labeled virions of each virus strain at 10,000 particles per cell. After 1 h, the inoculum was removed, fresh medium with or without E64 was added, and cells were incubated at 37°C for either 0 or 3 h. Viral particles in cell lysates were subjected to SDS-PAGE. The E64 concentration and incubation times postadsorption are shown at the top of each autoradiograph. Viral proteins are labeled on the right. (B) Quantitation of ς3 band intensity. The densities of bands corresponding to the ς3 and ς2 proteins were determined, and the results are expressed as the mean ς3/ς2 ratios for three independent experiments. Using a two tailed, two-sample t test, the difference in ς3/ς2 ratio between T3D and D-EA1 was statistically significant (∗, P < 0.05) after infection of cells treated with 50, 100, and 200 μM E64.
Mutant LX cells selected during persistent reovirus infection of L cells do not express the enzymatically active form of the endocytic protease cathepsin L (2). Mutant PI viruses selected during persistent reovirus infection can infect cells treated with E64 (1). Since both PI viruses and D-EA viruses are capable of growth in E64-treated cells, we tested D-EA1 and D-EA3 for the capacity to infect LX cells. In contrast to prototype PI virus PI 3-1, the D-EA viruses were incapable of growth in mutant LX cells (data not shown). These findings indicate that the selection pressures operant during persistent infection differ from those during serial passage in E64-treated cells.
Identification of viral genes that segregate with D-EA virus growth in the presence of E64.
To determine mechanisms by which mutations selected during serial passage of reovirus in E64-treated cells alter proteolytic susceptibility of the viral outer capsid, we used reassortant genetics to identify viral genes associated with growth of DEA viruses in the presence of E64. Reassortant viruses were isolated from independent crosses of wt T1L and D-EA viruses D-EA1 and D-EA3 and tested for growth in the presence and absence of 100 μM E64. This concentration of E64 was chosen to maximize differences in growth between wt and D-EA viruses (Fig. 2). Viral titers in the presence and absence of E64 were determined after 24 h of viral growth, and L+E64/L ratios were calculated for each reassortant virus by dividing the viral titer in E64-treated cells by the viral titer in untreated cells (Tables 1 and 2). Since pretreatment of cells with E64 does not alter viral adsorption (data not shown), titers of reassortant viruses after 24 h of viral growth could be directly compared. Reassortant viruses were ranked by L+E64/L ratio from highest to lowest. While the data form a continuum, in each case, reassortant viruses containing an S4 gene derived from the D-EA virus parent had the highest L+E64/L ratios, ranging from 0.0337 to 0.400 for T1L × D-EA1 reassortants and from 0.0194 to 0.165 for T1L × D-EA3 reassortants, whereas those with an S4 gene from T1L had the lowest ratios, ranging from 0.00230 to 0.0259 for T1L × D-EA1 reassortants and from 0.00500 to 0.0169 for T1L × D-EA3 reassortants. No other reovirus genes were associated with the differences in L+E64/L ratios exhibited by these reassortant viruses.
TABLE 1.
Growth of T1L × D-EA1 reassortant viruses in the presence and absence of E64
| Virus strain | Origin of gene segmenta:
|
Viral titer inb:
|
L+E64/L ratioc | Rankd | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | L2 | L3 | M1 | M2 | M3 | S1 | S2 | S3 | S4 | E64-treated cells | Untreated cells | |||
| Parental | ||||||||||||||
| T1L | L | L | L | L | L | L | L | L | L | L | 4.18 × 105 | 2.20 × 107 | 0.0190 | |
| D-EA1 | E | E | E | E | E | E | E | E | E | E | 2.70 × 107 | 9.91 × 107 | 0.273 | |
| Reassortant | ||||||||||||||
| 1-31 | L | L | L | L | L | L | E | L | E | E | 4.80 × 106 | 1.20 × 107 | 0.400 | 1 |
| 1-6 | E | E | L | L | E | L | L | E | L | E | 1.05 × 106 | 8.28 × 106 | 0.127 | 2 |
| 35 | E | E | E | E | L | E | L | E | E | E | 2.55 × 106 | 2.20 × 107 | 0.116 | 3 |
| 1-57 | L | L | L | L | E | L | L | E | E | E | 1.35 × 106 | 1.21 × 107 | 0.112 | 4 |
| 1-29 | E | E | E | E | E | E | L | E | E | E | 1.17 × 106 | 1.40 × 107 | 0.0836 | 5 |
| 33 | E | E | E | L | E | E | L | E | E | E | 2.40 × 105 | 2.90 × 106 | 0.0828 | 6 |
| 1-25 | E | E | E | L | E | E | L | E | E | E | 1.32 × 106 | 1.90 × 107 | 0.0692 | 7 |
| 1-28 | E | E | L | E | E | E | E | E | L | E | 3.35 × 106 | 5.30 × 107 | 0.0632 | 8 |
| 29 | E | E | E | E | E | E | L | E | E | E | 6.85 × 105 | 1.19 × 107 | 0.0576 | 9 |
| 1-1 | E | E | E | E | E | E | L | E | L | E | 7.75 × 105 | 2.30 × 107 | 0.0337 | 10 |
| 1-11 | E | E | E | L | E | L | L | E | E | L | 4.53 × 105 | 1.75 × 107 | 0.0259 | 11 |
| 1-9 | E | E | E | L | E | L | L | E | E | L | 4.08 × 105 | 1.63 × 107 | 0.0251 | 12 |
| 1-56 | E | E | E | E | L | L | L | E | E | L | 2.25 × 104 | 1.05 × 106 | 0.0214 | 13 |
| 1-10 | L | L | L | L | L | E | L | L | E | L | 2.95 × 105 | 2.20 × 107 | 0.0134 | 14 |
| 47 | E | E | E | L | E | E | L | E | E | L | 3.15 × 104 | 2.85 × 106 | 0.0111 | 15 |
| 1-27 | E | E | E | E | E | E | L | E | E | L | 9.25 × 104 | 1.03 × 107 | 0.00902 | 16 |
| 1-30 | E | E | L | E | E | E | E | L | E | L | 4.00 × 105 | 5.40 × 107 | 0.00741 | 17 |
| 22 | L | E | L | E | E | E | L | E | E | L | 8.05 × 104 | 1.33 × 107 | 0.00608 | 18 |
| 1-44 | E | E | L | E | E | E | L | E | E | L | 3.80 × 104 | 1.23 × 107 | 0.00309 | 19 |
| 37 | E | E | E | L | E | E | E | E | E | L | 4.95 × 104 | 2.15 × 107 | 0.00230 | 20 |
Parental origin of each gene segment: L, gene segment derived from T1L; E, gene segment derived from D-EA1.
L cells (4 × 105) were infected with virus strains at an MOI of 2 PFU per cell. After a 1-h adsorption period, the inoculum was removed, fresh medium with or without 100 μM E64 was added, and cells were incubated at 37°C for 24 h. Cells were frozen and thawed twice, and viral titers in cell lysates were determined by plaque assay. The results are presented as mean viral titer at 24 h for two to four independent experiments for the reassortant viruses and six independent experiments for the parental viruses.
The mean viral titer at 24 h in E64-treated cells was divided by that in untreated cells to calculate an L+E64/L ratio for each virus strain.
Reassortant viruses are ranked from highest to lowest based on their L+E64/L ratios.
TABLE 2.
Growth of T1L × D-EA3 reassortant viruses in the presence and absence of E64
| Virus strain | Origin of gene segmenta:
|
Viral titer inb:
|
L+E64/L ratioc | Rankd | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | L2 | L3 | M1 | M2 | M3 | S1 | S2 | S3 | S4 | E64-treated cells | Untreated cells | |||
| Parental | ||||||||||||||
| T1L | L | L | L | L | L | L | L | L | L | L | 4.18 × 105 | 2.20 × 107 | 0.0190 | |
| D-EA3 | E | E | E | E | E | E | E | E | E | E | 1.25 × 107 | 6.93 × 107 | 0.181 | |
| Reassortant | ||||||||||||||
| 3-45 | E | E | L | E | E | E | L | E | E | E | 1.08 × 105 | 6.50 × 105 | 0.165 | 1 |
| 3-40 | E | E | E | E | E | E | L | E | L | E | 7.80 × 105 | 4.75 × 106 | 0.164 | 2 |
| 3-35 | E | E | L | L | E | L | L | E | E | E | 1.20 × 106 | 7.70 × 106 | 0.156 | 3 |
| 3-28 | E | L | E | E | E | E | E | E | E | E | 1.63 × 106 | 1.73 × 107 | 0.0946 | 4 |
| 3-25 | E | E | E | E | E | E | L | E | E | E | 3.60 × 106 | 4.55 × 107 | 0.0790 | 5 |
| 3-2 | L | E | L | E | E | E | L | E | E | E | 3.20 × 106 | 5.05 × 107 | 0.0634 | 6 |
| 3-32 | E | E | L | E | E | E | L | E | E | E | 1.25 × 106 | 3.30 × 107 | 0.0379 | 7 |
| 3-46 | E | E | L | E | E | L | L | L | L | E | 1.34 × 105 | 6.88 × 106 | 0.0194 | 8 |
| 3-37 | L | E | L | L | E | L | L | E | E | L | 2.22 × 106 | 1.31 × 108 | 0.0169 | 9 |
| 3-9 | L | E | L | E | L | L | L | E | E | L | 2.30 × 105 | 1.95 × 107 | 0.0118 | 10 |
| 3-7 | E | E | E | E | E | E | E | E | E | L | 2.25 × 105 | 4.50 × 107 | 0.00500 | 11 |
Parental origin of each gene segment: L, gene segment derived from T1L; E, gene segment derived from D-EA3.
L cells (4 × 105) were infected with virus strains at an MOI of 2 PFU per cell. After a 1-h adsorption period, the inoculum was removed, fresh medium with or without 100 μM E64 was added, and cells were incubated at 37°C for 24 h. Cells were frozen and thawed twice, and viral titers in cell lysates were determined by plaque assay. The results are presented as mean viral titer at 24 h for two to four independent experiments for the reassortant viruses and six independent experiments for the parental viruses.
The mean viral titer at 24 h in E64-treated cells was divided by that in untreated cells to calculate an L+E64/L ratio for each virus strain.
Reassortant viruses are ranked from highest to lowest based on their L+E64/L ratios.
To confirm the association of the S4 gene with growth of T1L × D-EA reassortants in E64-treated cells, we analyzed the results using nonparametric and parametric statistical techniques. The results of these analyses demonstrated a significant association between growth of reassortant viruses in the presence of E64 and the S4 gene (T1L × D-EA1 reassortants: MW test, P < 0.0001, and t test, P = 0.013; T1L × D-EA3 reassortants: MW test, P = 0.0061, and t test, P = 0.0043). No other viral genes were significantly associated with growth of the T1L × D-EA reassortants in E64-treated cells by these tests (P > 0.05 for both MW and t test except for the S2, M2, and L2 genes of T1L × D-EA3 reassortants, in which a t test assuming unequal variance could not be calculated; a t test assuming equal variance gave P values of >0.05 for these genes). Therefore, these results strongly suggest that mutations in the S4 gene selected during passage of reovirus in E64-treated cells determine the capacity of D-EA viruses to generate higher viral yields than wt viruses after infection of cells treated with E64.
S4 gene nucleotide sequences of D-EA reovirus variants.
To identify mutations associated with the capacity of D-EA viruses to infect cells treated with E64, we determined the S4 gene nucleotide sequences of wt T3D, D-EA1, D-EA2, and D-EA3. The S4 gene is 1,196 nucleotides in length and encodes the 365-amino-acid ς3 protein in a single open reading frame (14). RT-PCR amplifications using oligonucleotide primers complementary to the 5′ and 3′ nontranslated regions of the S4 gene were used to generate cDNA clones corresponding to full-length coding regions of the S4 gene for the four virus strains (Table 3). We also determined the S4 gene nucleotide sequence of an additional virus strain, termed T3D-derived, E64-non-adapted (D-ENA), that exhibited wt sensitivity to E64-mediated growth inhibition. D-ENA was isolated from the same lysate stock of passage series 1 from which D-EA1 was isolated. The S4 gene nucleotide sequences of wt T3D and D-ENA were identical. The S4 sequences of D-EA1 and D-EA2 had a single, identical nucleotide substitution at position 1092, which results in a tyrosine-to-histidine mutation at residue 354 in the deduced amino acid sequence of ς3. The S4 sequence of D-EA3 also contained the nucleotide substitution at position 1092 that results in a tyrosine-to-histidine mutation at amino acid 354 in ς3 and in addition contained a nucleotide substitution at position 624, which results in a glycine-to-arginine mutation at amino acid 198. Thus, serial passage of reovirus strain T3D in the presence of E64 selects few mutations in the S4 gene, and mutations in the deduced amino acid sequence of all viral variants pinpoint a single residue in the carboxy terminus of ς3.
TABLE 3.
Mutations in S4 gene nucleotide sequences of D-EA reovirus variants and corresponding mutations in deduced amino acid sequences of their ς3 proteins
| Virus | Location and nature of mutationsa in:
|
GenBank accession no. | |
|---|---|---|---|
| S4 gene | ς3 protein | ||
| D-EA1 | 1092, U → C | 354, Y → H | AF332135 |
| D-EA2 | 1092, U → C | 354, Y → H | AF332136 |
| D-EA3 | 624, G → A | 198, G → R | AF332137 |
| 1092, U → C | 354, Y → H | ||
Mutations in the S4 gene refer to nucleotide position; mutations in the ς3 protein refer to amino acid position. Mutations were confirmed by sequencing two independent cDNA clones for each virus.
Treatment of wt and D-EA reoviruses with purified endocytic proteases.
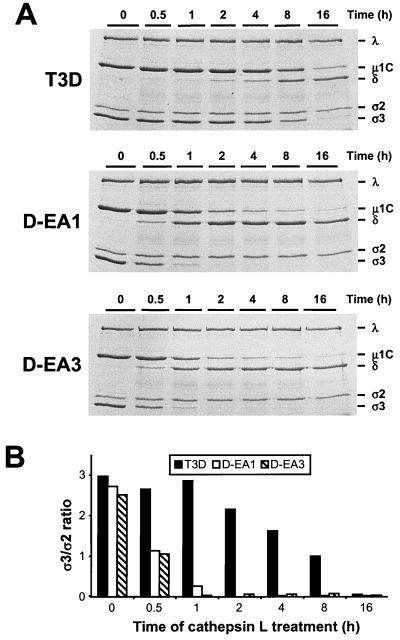
To determine whether altered sensitivity to protease inhibitor E64 during endocytic processing of D-EA viruses correlates with altered susceptibility to protease treatment in vitro, virions of wt and D-EA viruses were treated with either of the endocytic proteases cathepsin L or cathepsin D. Cathepsin L is a cysteine protease that productively cleaves wt virions to functional ISVPs (2), whereas cathepsin D is an aspartic protease that does not digest wt virions (18). Virions were treated with purified recombinant human cathepsin L (8) for various intervals, and digestion of viral outer-capsid proteins was monitored by SDS-PAGE (Fig. 4A). Treatment of D-EA1 and D-EA3 with cathepsin L resulted in proteolytic digestion of outer-capsid proteins indicative of ISVP generation—removal of ς3 and cleavage of μ1/μ1C to μ1δ/δ—with significantly faster kinetics than did treatment of wt T3D. In particular, the viral protein profile (Fig. 4A) and the ς3/ς2 ratio (Fig. 4B) produced by treatment of D-EA1 and D-EA3 with cathepsin L for 1 h resembled those of T3D following cathepsin L treatment for 16 h. Therefore, the D-EA variant viruses are substantially more susceptible to cleavage by an endocytic protease that is capable of mediating reovirus disassembly.
FIG. 4.
Electrophoretic analysis of viral structural proteins of wt and D-EA reoviruses after treatment with cathepsin L. (A) Purified virions of reovirus strains T3D, D-EA1, and D-EA3 were treated with 100 μg of human cathepsin L (pH 5.0) per ml at 37°C for the times shown. Equal numbers of viral particles were loaded into wells of 10% polyacrylamide gels. After electrophoresis, gels were stained with Coomassie blue. Viral proteins are labeled on the right. (B) Quantitation of ς3 band intensity. Densities of bands corresponding to the ς3 and ς2 proteins were determined, and the results are presented as the ς3/ς2 ratios.
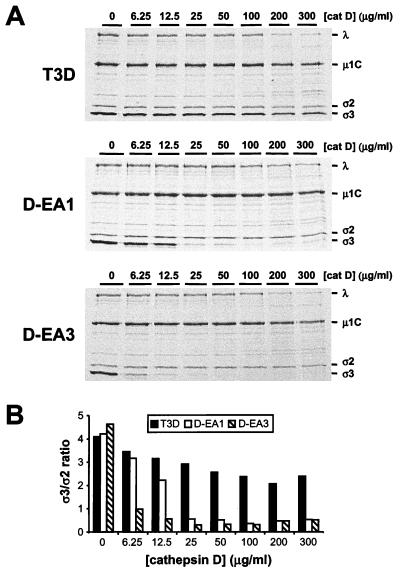
To determine whether the D-EA variant viruses are sensitive to proteolytic degradation by proteases that are incapable of digesting virions of wt reovirus, virions of wt and D-EA viruses were treated with increasing concentrations of purified bovine cathepsin D for 1 h (Fig. 5). Virions of wt reovirus are not susceptible to cathepsin D cleavage in vitro, and inhibition of cathepsin D does not affect reovirus entry in cell culture (18). As previously reported (18), cathepsin D treatment of T3D virions did not result in generation of ISVPs. At the highest concentrations of cathepsin D used, degradation of all T3D proteins was observed; however, there was minimal enhancement of the ς3 and μ1/μ1C cleavage indicative of ISVP formation. In contrast, cathepsin D treatment of D-EA1 and D-EA3 virions resulted in proteolysis of ς3; at concentrations of cathepsin D of 25 μg per ml or higher, the ς3 proteins of D-EA1 and D-EA3 were completely degraded. Treatment of virions with 6.25 μg of cathepsin D per ml resulted in substantial degradation of D-EA3 ς3 but minimal degradation of D-EA1 ς3. Therefore, in comparison to D-EA1 ς3, D-EA3 ς3 appears to be modestly more susceptible to cathepsin D proteolysis. However, cathepsin D treatment of D-EA1 or D-EA3 did not result in cleavage of μ1/μ1C as is observed following treatment with either cathepsin L (Fig. 4) or the intestinal protease chymotrypsin (5, 23, 30, 31). These results indicate that the ς3 proteins of D-EA viruses are susceptible to cleavage by an endocytic protease that does not cleave ς3 of wt reovirus.
FIG. 5.
Electrophoretic analysis of viral structural proteins of wt and D-EA reoviruses after treatment with cathepsin D. (A) Purified 35S-labeled virions of reovirus strains T3D, D-EA1, and D-EA3 were treated with cathepsin D (pH 3.8) at the concentrations shown at 37°C for 1 h. Equal numbers of viral particles were loaded into wells of 10% polyacrylamide gels. Viral proteins are labeled on the right. (B) Quantitation of ς3 band intensity. Densities of bands corresponding to the ς3 and ς2 proteins were determined, and the results are presented as the ς3/ς2 ratios.
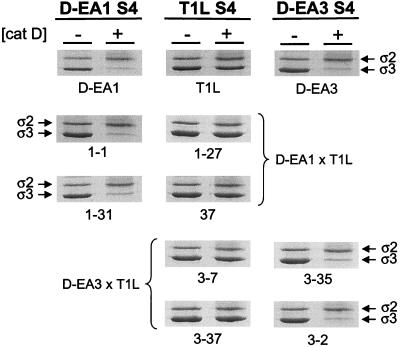
Identification of viral genes that segregate with susceptibility of D-EA virus ς3 proteins to cleavage by cathepsin D.
The observation that the ς3-encoding S4 gene segregates with resistance of T1L × D-EA reassortant viruses to growth inhibition by E64 led us to hypothesize that susceptibility of D-EA viruses to cleavage by cathepsin D is mediated by mutations in the S4 gene. To test this hypothesis, four T1L × D-EA1 reassortant viruses and four T1L × D-EA3 reassortant viruses were treated for 1 h with 100 μg of cathepsin D per ml, and viral proteins were resolved by SDS-PAGE (Fig. 6). Cathepsin D treatment of virions that contained a T1L-derived S4 gene resulted in no degradation of ς3 (Fig. 6, middle column). In contrast, cathepsin D treatment of virions that contained an S4 gene derived from either D-EA1 (left column) or D-EA3 (right column) resulted in proteolysis of ς3. These results indicate that the S4 gene segregates with susceptibility of T1L × D-EA reassortant virus ς3 proteins to cathepsin D cleavage. Moreover, these observations suggest that the tyrosine-to-histidine mutation at amino acid 354 of the ς3 protein is sufficient to confer susceptibility of ς3 to proteolysis by cathepsin D.
FIG. 6.
Identification of viral genes that segregate with susceptibility of D-EA ς3 proteins to proteolysis by cathepsin D. Purified virions of T1L × D-EA1 reassortant viruses and T1L × D-EA3 reassortant viruses were treated with 100 μg of cathepsin D (pH 3.8) at 37°C for 1 h. Equal numbers of viral particles were loaded into wells of 10% polyacrylamide gels. After electrophoresis, gels were stained with Coomassie blue. Viral strains are labeled at the bottom of each gel, and bands corresponding to the ς2 and ς3 proteins are labeled. The parental derivation of the S4 gene for each column is indicated at the top.
DISCUSSION
Following attachment to cell surface receptors, reovirus virions are taken into cells by receptor-mediated endocytosis. Within an endocytic compartment, the viral outer capsid is subject to acid-dependent proteolysis leading to generation of ISVPs. ISVPs are thought to interact directly with vacuolar membranes, resulting in delivery of transcriptionally active cores into the cytoplasm (reviewed in reference 24). Previous studies of PI reoviruses indicate that mutations in the S4 gene selected during persistent infection determine the capacity of PI viruses to grow in cells treated with protease inhibitor E64 (1). In this study, we used a genetic approach, in which reovirus variants were selected by serial passage in L cells treated with E64, to define domains in reovirus outer-capsid proteins that specifically determine viral susceptibility to proteolysis during formation of ISVPs.
Serial passage of reovirus in L cells treated with E64 selected viral variants resistant to E64-mediated growth inhibition. Passage series 2 and 3 had a biphasic gain in E64 resistance, as indicated by the growth of passage-series lysates in the presence and absence of E64. The L+E64/L ratios increased to intermediate levels at passage 3, decreased to a nadir at passage 5, and then became maximal at passage 10. This biphasic gain in E64 resistance suggests that the initial selection of E64-resistant viruses included many defective viral particles that did not survive further propagation. Additional passages likely facilitated expansion and selection of a stable population of E64-resistant viral variants. Of note, generation of defective viruses during serial passage has been reported previously for T3D (7), the parental strain used in these experiments. We did not maintain reovirus in E64-treated cells for more than 10 passages; however, it is possible that additional passages in the presence of E64 would have selected variant viruses with even greater resistance to E64-mediated growth inhibition. It is unlikely that passage in cells treated with higher concentrations of E64 would have yielded selection of variants with enhanced E64 resistance. Attempts to select E64-resistant variants by passage in cells treated with 200 μM E64 resulted in loss of viable virus (data not shown).
The three E64-adapted viruses characterized in this study, which were cloned from independent passage series, produced 7- to 17-fold-greater yields than did wt T3D after growth in the presence of 100 μM E64. The D-EA viruses were not completely resistant to growth inhibition by E64; rather, these viruses were less sensitive to E64-mediated growth inhibition than were wt strains. These findings suggest that D-EA viruses remain dependent on E64-sensitive proteases for maximal viral yield. It is possible that D-EA viruses, like wt viruses, require cysteine-containing proteases for viral disassembly. However, the level of cysteine protease activity required for productive uncoating of D-EA viruses is likely to be substantially lower than that required for wt viruses. Alternatively, the D-EA viruses may not require cysteine protease activity. Within endosomes of cells treated with 200 μM E64, noncysteine proteases may mediate D-EA virus disassembly, though at a lower efficiency than cysteine proteases, resulting in lower viral yields.
Analysis of T1L × D-EA1 and T1L × D-EA3 reassortant viruses indicated that resistance to E64 is determined primarily by mutations in the S4 gene. However, it is possible that other viral genes contributed to the growth differences exhibited by these reassortant viruses in E64-treated cells. Viral yields are influenced by many steps subsequent to viral entry, and viral genes that influence these steps might act to moderate differences in growth of reassortants that have altered entry phenotypes. Therefore, a continuum in the L+E64/L ratios observed for the T1L × D-EA reassortant viruses in this study is not surprising. However, using both parametric and nonparametric statistical techniques, the S4 gene was the only viral gene associated with the differences in L+E64/L ratios exhibited by the T1L × D-EA reassortant viruses. Therefore, the S4 gene likely plays the dominant role in determining the E64-resistance phenotype. The S4 gene encodes outer-capsid protein ς3, a protein which is removed from virions during conversion of virions to ISVPs (6, 10, 30, 31). Viruses adapted to growth in the presence of E64 contain either one (D-EA1 and D-EA2) or two (D-EA3) mutations in the S4 gene. These findings suggest that serial passage in the presence of E64 selects viral variants altered in requirements for proteolytic cleavage of ς3 to complete entry steps.
We directly tested the susceptibility of D-EA viruses to proteolysis by comparing the kinetics of cleavage of outer-capsid proteins ς3 and μ1/μ1C of wt and D-EA viruses after treatment with endocytic proteases in vitro. Treatment of reovirus virions with the cysteine endocytic protease cathepsin L results in generation of ISVPs (2); however, treatment of virions with the aspartic endocytic protease cathepsin D does not (18). Cathepsin L treatment of D-EA viruses resulted in cleavage of ς3 and μl proteins with substantially faster kinetics than did treatment of wt virus. In contrast to wt virus, cathepsin D treatment of D-EA viruses resulted in cleavage of ς3 protein. Consistent with the capacity to infect E64-treated cells, susceptibility of D-EA virus ς3 proteins to cleavage by cathepsin D segregated with the D-EA S4 gene. We think it likely that susceptibility of D-EA virus ς3 proteins to cleavage by cathepsin D represents a general enhancement in the susceptibility of D-EA ς3 proteins to proteolysis rather than a specific adaptation to cleavage by cathepsin D. Similar increases in ς3 cleavage susceptibility also were noted after treatment of D-EA viruses with the intestinal protease chymotrypsin (data not shown). Thus, E64-resistant reovirus variants appear to manifest enhanced susceptibility of ς3 to proteolysis by a variety of proteases.
Strikingly, the deduced amino acid sequences of the ς3 proteins of all three E64-adapted viruses contain a tyrosine-to-histidine mutation at amino acid 354. This was the only mutation observed in D-EA1 and D-EA2; D-EA3 contains an additional mutation, glycine to arginine, at amino acid 198. The tyrosine-to-histidine mutation at amino acid 354 is identical to a mutation found in PI viruses that are resistant to E64 (1). A region of ς3 protein adjacent to amino acid 220 is sensitive to several proteases (22, 28), and this region of the protein is postulated to be cleaved by endocytic proteases during viral entry (29). The mutation at amino acid 354 could enhance susceptibility of ς3 to proteolysis by influencing a cleavage event occurring either locally or at a distance in primary sequence from amino acid 354. The sensitivity of D-EA ς3 to proteolysis by cathepsin D, a protease incapable of cleaving wt ς3 (18), suggests that the mutation at amino acid 354 alters cleavage events occurring at a distance in primary sequence. Cathepsin D cleaves preferentially between amino acids with large hydrophobic side chains (4, 25). Therefore, it is unlikely that the tyrosine-to-histidine mutation at amino acid 354 in ς3, which is flanked by amino acids GDLN(Y354H)PVMI, generates a de novo cathepsin D cleavage site at amino acid 354, especially since the histidine residue likely will be protonated at acidic pH. Instead, we think it probable that the mutation at amino acid 354 unveils an occult cathepsin D cleavage site elsewhere in ς3. The additional glycine-to-arginine mutation at amino acid 198 in D-EA3 ς3 is adjacent in primary sequence to a proteolytically sensitive region surrounding amino acid 220 and may also contribute to the enhanced susceptibility of ς3 to proteolysis. Indeed, in comparison to D-EA1 ς3, D-EA3 ς3 is degraded at lower concentrations of cathepsin D. However, the ς3 proteins of D-EA1 and D-EA3 have similar sensitivities to cathepsin L, which suggests that the additional mutation at amino acid 198 does not significantly enhance susceptibility to all proteases in the context of a mutation at amino acid 354. Further understanding of the mechanism of enhanced proteolysis of D-EA viruses awaits an atomic resolution structure of ς3.
Results presented in this report indicate that reovirus variants can be selected specifically for resistance to inhibitors of outer capsid proteolysis. We show that the E64-adapted viruses contain one or two amino acid substitutions in outer-capsid protein ς3 and that a mutation at amino acid 354 is identical to mutations found in reovirus mutants selected during persistent infection. The fact that such diverse selection pressures—serial passage in E64-treated cells and persistent infection—result in selection of identical mutations at amino acid 354 in ς3 strongly suggests that sequences in the ς3 carboxy terminus influence the susceptibility of reovirus virions to proteolysis by endocytic proteases. However, since D-EA viruses are incapable of infecting mutant LX cells, it is clear that other mutations are required for growth of PI reoviruses in mutant cells selected during persistent infection (35). Nonetheless, the remarkable consistency of the ς3 mutation at amino acid 354 lends supports to a model in which a region of ς3 including amino acid 354 plays a key regulatory role in removal of the reovirus outer capsid during viral entry. This model also is supported by experiments using ISVPs recoated with recombinant ς3 proteins in which a strain-specific polymorphism in ς3 cleavage susceptibility was mapped to amino acids 266 to 365 (17). It is possible that this region of ς3 is altered by changes in pH as the virus traverses the endocytic pathway, influences access to the protease cleavage site, or serves as an initial site of proteolysis. Regardless of the mechanism, results reported here identify amino acid 354 in ς3 protein as a critical determinant of reovirus disassembly.
ACKNOWLEDGMENTS
We express our appreciation to Greg Wilson for careful review of the manuscript.
This work was supported by Public Health Service award T32 GM07347 from the National Institute of General Medical Studies for the Vanderbilt Medical-Scientist Training Program (D.H.E. and G.S.B.), Public Health Service award AI32539 from the National Institute of Allergy and Infectious Diseases, the Amos Christie Society (L.E.S.), and the Elizabeth B. Lamb Center for Pediatric Research.
REFERENCES
- 1.Baer G S, Dermody T S. Mutations in reovirus outer-capsid protein ς3 selected during persistent infections of L cells confer resistance to protease inhibitor E64. J Virol. 1997;71:4921–4928. doi: 10.1128/jvi.71.7.4921-4928.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baer G S, Ebert D H, Chung C J, Erickson A H, Dermody T S. Mutant cells selected during persistent reovirus infection do not express mature cathepsin L and do not support reovirus disassembly. J Virol. 1999;73:9532–9543. doi: 10.1128/jvi.73.11.9532-9543.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett A J, Kembhavi A A, Brown M A, Kirschke H, Knight C G, Tamai M, Hanada K. l-trans-Epoxysuccinyl-leucylamido(4-guanidino) butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982;201:189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barrett A J, Rawlings N D, Woessner J F, editors. Handbook of proteolytic enzymes. New York, N.Y: Academic Press, Inc.; 1998. [Google Scholar]
- 5.Borsa J, Morash B D, Sargent M D, Copps T P, Lievaart P A, Szekely J G. Two modes of entry of reovirus particles into L cells. J Gen Virol. 1979;45:161–170. doi: 10.1099/0022-1317-45-1-161. [DOI] [PubMed] [Google Scholar]
- 6.Borsa J, Sargent M D, Lievaart P A, Copps T P. Reovirus: evidence for a second step in the intracellular uncoating and transcriptase activation process. Virology. 1981;111:191–200. doi: 10.1016/0042-6822(81)90664-4. [DOI] [PubMed] [Google Scholar]
- 7.Brown E G, Nibert M L, Fields B N. The L2 gene of reovirus serotype 3 controls the capacity to interfere, accumulate deletions and establish persistent infection. In: Compans R W, Bishop D H L, editors. Double-stranded RNA viruses. New York, N.Y: Elsevier Biomedical; 1983. pp. 275–287. [Google Scholar]
- 8.Carmona E, Dufour É, Plouffe C, Takebe S, Mason P, Mort J S, Ménard R. Potency and selectivity of the cathepsin L propeptide as an inhibitor of cysteine proteases. Biochemistry. 1996;35:8149–8157. doi: 10.1021/bi952736s. [DOI] [PubMed] [Google Scholar]
- 9.Chandran K, Nibert M L. Protease cleavage of reovirus capsid protein μl/μ1C is blocked by alkyl sulfate detergents, yielding a new type of infectious subvirion particle. J Virol. 1998;762:467–475. doi: 10.1128/jvi.72.1.467-475.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang C T, Zweerink H J. Fate of parental reovirus in infected cell. Virology. 1971;46:544–555. doi: 10.1016/0042-6822(71)90058-4. [DOI] [PubMed] [Google Scholar]
- 11.Dermody T S, Chappell J D, Hofler J G, Kramp W, Tyler K L. Eradication of persistent reovirus infection from a B-cell hybridoma. Virology. 1995;212:272–276. doi: 10.1006/viro.1995.1483. [DOI] [PubMed] [Google Scholar]
- 12.Dermody T S, Nibert M L, Wetzel J D, Tong X, Fields B N. Cells and viruses with mutations affecting viral entry are selected during persistent infections of L cells with mammalian reoviruses. J Virol. 1993;67:2055–2063. doi: 10.1128/jvi.67.4.2055-2063.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furlong D B, Nibert M L, Fields B N. Sigma 1 protein of mammalian reoviruses extends from the surfaces of viral particles. J Virol. 1988;62:246–256. doi: 10.1128/jvi.62.1.246-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giantini M, Seliger L S, Furuichi Y, Shatkin A J. Reovirus type 3 genome segment S4: nucleotide sequence of the gene encoding a major virion surface protein. J Virol. 1984;52:984–987. doi: 10.1128/jvi.52.3.984-987.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hooper J W, Fields B N. Monoclonal antibodies to reovirus ς1 and μ1 proteins inhibit chromium release from mouse L cells. J Virol. 1996;70:672–677. doi: 10.1128/jvi.70.1.672-677.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hooper J W, Fields B N. Role of the μ1 protein in reovirus stability and capacity to cause chromium release from host cells. J Virol. 1996;70:459–467. doi: 10.1128/jvi.70.1.459-467.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jane-Valbuena J, Nibert M L, Spencer S M, Walker S B, Baker T S, Chen Y, Centonze V E, Schiff L A. Reovirus virion-like particles obtained by recoating infectious subvirion particles with baculovirus-expressed ς3 protein: an approach for analyzing ς3 functions during virus entry. J Virol. 1999;73:2963–2973. doi: 10.1128/jvi.73.4.2963-2973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kothandaraman S, Hebert M C, Raines R T, Nibert M L. No role for pepstatin-A-sensitive acidic proteinases in reovirus infections of L or MDCK cells. Virology. 1998;251:264–272. doi: 10.1006/viro.1998.9434. [DOI] [PubMed] [Google Scholar]
- 19.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 20.Lucia-Jandris P, Hooper J W, Fields B N. Reovirus M2 gene is associated with chromium release from mouse L cells. J Virol. 1993;67:5339–5345. doi: 10.1128/jvi.67.9.5339-5345.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinez C G, Guinea R, Benavente J, Carrasco L. The entry of reovirus into L cells is dependent on vacuolar proton-ATPase activity. J Virol. 1996;70:576–579. doi: 10.1128/jvi.70.1.576-579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller J E, Samuel C E. Proteolytic cleavage of the reovirus sigma 3 protein results in enhanced double-stranded RNA-binding activity: identification of a repeated basic amino acid motif within the C-terminal binding region. J Virol. 1992;66:5347–5356. doi: 10.1128/jvi.66.9.5347-5356.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nibert M L, Chappell J D, Dermody T S. Infectious subvirion particles of reovirus type 3 Dearing exhibit a loss in infectivity and contain a cleaved ς1 protein. J Virol. 1995;69:5057–5067. doi: 10.1128/jvi.69.8.5057-5067.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nibert M L, Schiff L A, Fields B N. Reoviruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1557–1596. [Google Scholar]
- 25.Offermann M K, Chlebowski J F, Bond J S. Action of cathepsin D on fructose-1,6-bisphosphate aldolase. Biochem J. 1983;211:529–534. doi: 10.1042/bj2110529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pagano M, Gauvreau K. Principles of biostatistics. Belmont, Calif: Duxbury Press; 1993. [Google Scholar]
- 27.Rubin D H, Weiner D B, Dworkin C, Greene M I, Maul G G, Williams W V. Receptor utilization by reovirus type 3: distinct binding sites on thymoma and fibroblast cell lines result in differential compartmentalization of virions. Microb Pathog. 1992;12:351–365. doi: 10.1016/0882-4010(92)90098-9. [DOI] [PubMed] [Google Scholar]
- 28.Schiff L A, Nibert M L, Fields B N. Characterization of a zinc blotting technique: evidence that a retroviral gag protein binds zinc. Proc Natl Acad Sci USA. 1988;85:4195–4199. doi: 10.1073/pnas.85.12.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shepard D A, Ehnstrom J G, Schiff L A. Association of reovirus outer capsid proteins ς3 and μ1 causes a conformational change that renders ς3 protease sensitive. J Virol. 1995;69:8180–8184. doi: 10.1128/jvi.69.12.8180-8184.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Silverstein S C, Astell C, Levin D H, Schonberg M, Acs G. The mechanism of reovirus uncoating and gene activation in vivo. Virology. 1972;47:797–806. doi: 10.1016/0042-6822(72)90571-5. [DOI] [PubMed] [Google Scholar]
- 31.Sturzenbecker L J, Nibert M, Furlong D, Fields B N. Intracellular digestion of reovirus particles requires a low pH and is an essential step in the viral infectious cycle. J Virol. 1987;61:2351–2361. doi: 10.1128/jvi.61.8.2351-2361.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tosteson M T, Nibert M L, Fields B N. Ion channels induced in lipid bilayers by subvirion particles of the nonenveloped mammalian reoviruses. Proc Natl Acad Sci USA. 1993;90:10549–10552. doi: 10.1073/pnas.90.22.10549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Virgin H W, IV, Bassel-Duby R, Fields B N, Tyler K L. Antibody protects against lethal infection with the neurally spreading reovirus type 3 (Dearing) J Virol. 1988;62:4594–4604. doi: 10.1128/jvi.62.12.4594-4604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wetzel J D, Wilson G J, Baer G S, Dunnigan L R, Wright J P, Tang D S H, Dermody T S. Reovirus variants selected during persistent infections of L cells contain mutations in the viral S1 and S4 genes and are altered in viral disassembly. J Virol. 1997;71:1362–1369. doi: 10.1128/jvi.71.2.1362-1369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson G J, Wetzel J D, Puryear W, Bassel-Duby R, Dermody T S. Persistent reovirus infections of L cells select mutations in viral attachment protein ς1 that alter oligomer stability. J Virol. 1996;70:6598–6606. doi: 10.1128/jvi.70.10.6598-6606.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]