Abstract
Objective
This systematic review summarises the studies that have investigated the relationship between dimensions of social cognition (i.e., Theory of Mind – ToM, emotion recognition, and empathy) and alexithymia in the general adult non-clinical population.
Method
PubMed, PsycINFO, and Scopus databases were screened, using the following strings: (“alexithymi*”) AND (“theory of mind” OR “ToM”); (“alexithymi*”) AND (“empath*”); (“alexithymi*”) AND (“emotion recognition”); (“alexithymi*”) AND (“social cognition”).
Results
A total of 117 studies met the inclusion criteria and were included in this review. The total number of participants included in the reviewed studies was 40,231. Mixed results were found for alexithymia and ToM, while the relationship between emotion recognition or empathy and alexithymia was more homogeneous. Alexithymia was found to be significantly associated with both a reduced ability to recognise emotions and empathy.
Conclusions
These results support the existence of significant relationships between alexithymia and altered social cognitive abilities. Future research is needed to confirm the present findings and further elucidate the complex relationship between these processes. Suggestions are made on how to overcome some of the theoretical and methodological problems in the literature.
Keywords: alexithymia, social cognition, theory of mind, emotion recognition, empathy, systematic review
1. Introduction
Alexithymia is a multidimensional construct characterised by difficulties in identifying and describing feelings, difficulties in distinguishing between feelings and bodily sensations of emotional arousal, restricted imagination processes, and an externally oriented cognitive style (Taylor et al., 1997).
Available data support the presence of alexithymia in both clinical and non-clinical populations. In particular, high levels of alexithymia have been found in various clinical and psychosocial conditions (e.g., Benfante & Romeo, 2023; Di Tella et al., 2018, 2023a; Farina et al., 2021; Isoardo et al., 2024; Miniati et al., 2022, 2023; Veggi et al., 2024). Conversely, alexithymia is thought to exist on a continuum in the general population, with rates ranging from 9% to 17% in men and 5% to 10% in women (Mattila et al., 2007; Salminen et al., 1999).
Difficulties in the ability to identify and describe one’s own feelings have been associated with alterations in the processing of other people’s cognitive and affective mental states (e.g., Grynberg et al., 2012; Pisani et al., 2021). The ability to decode information about the intentions and emotional states of others belongs to the domain of social cognition (e.g., Becchio et al., 2006; Niedenthal & Brauer, 2012). Social cognition enables individuals to construct mental representations of the relationships between oneself and others and to use these representations flexibly to carry out appropriate social interactions (Adolphs, 2001). This complex domain comprises at least three dimensions. The first component is Theory of Mind (ToM), which is the ability to attribute mental states to other people and use this information to explain and predict human behaviour (Enrici et al., 2019; Frith & Frith, 2005; Premack & Woodruff, 1978). A second dimension is the ability to recognise the emotions of others based on facial and bodily expressions; this is an essential skill for adaptive social behaviour as it guides responses and actions towards potentially friendly or threatening individuals (Stolier & Freeman, 2016). Finally, a third crucial social cognitive ability is empathy, which has been defined as “the ability to experience and understand what others feel without confusing oneself with others” (Decety & Lamm, 2006, p. 1146).
The available evidence appears to show significant and positive associations between alexithymia and reduced social cognitive abilities, in particular ToM processes (Brewer et al., 2015; Di Tella et al., 2018), recognition of other people’s emotions (Demers & Koven, 2015; Subic-Wrana et al., 2010), and empathy (Grynberg et al., 2010). However, not all results are consistent (e.g., Kyranides et al., 2022) and the social cognitive profile of alexithymic individuals should be further analysed.
Clarifying the extent of the relationship between alexithymia and social cognition alterations is also crucial given the prominent role that these socio-emotional competencies play in social interactions. For example, emotional expressions can contribute to the development of relationships between individuals within a social group through their signalling function (e.g., Pichon et al., 2009). Similarly, empathy is seen as a dimension of interpersonal functioning that enables individuals to understand, share, and respond to the emotions, gestures, thoughts, and experiences of others (e.g., Baron-Cohen & Wheelwright, 2004). Unsurprisingly, impairment in these abilities can make it difficult for people to interact effectively in interpersonal contexts, negatively impacting social functioning and quality of life (Di Tella et al., 2023b; Krause et al., 2013).
A few systematic reviews were previously conducted to shed light on the relationship between some aspects of social cognition and alexithymia in the general population (Grynberg et al., 2012; Pisani et al., 2021). In particular, the review by Grynberg et al. (2012) sought to examine the relationship between alexithymia and the processing of emotional facial expressions and showed that alexithymia appears to be associated with deficits in this ability. In contrast, the more recent review by Pisani et al. (2021) aimed to summarise the evidence for the relationship between alexithymia and ToM. They emphasised that alexithymia may be associated with reduced ToM, especially when the ability to recognize emotions is required for the inference of mental states.
However, these reviews focused on only one dimension of the social cognition domain (emotion recognition or ToM, respectively) and neglected the others. As different components of social cognition may be associated with alexithymia in different ways, it is essential to examine and summarise the available results for all domains of social cognition. To the best of our knowledge, no previous systematic review has summarised studies from all domains of social cognition. The present review has therefore attempted to fill an important gap in the literature by integrating research findings from the past several decades and overcoming the limited focus of previous reviews.
Therefore, the main aim of this review was to systematically summarise the available studies that have examined the association between all the main dimensions of social cognition (i.e., ToM, emotion recognition, and empathy) and alexithymia in the general adult non-clinical population. The theoretical framework that inspired our work on the possible relationships between alexithymia and facets of social cognition is based on the so-called “shared-network hypothesis”, according to which the same brain areas that are involved in our own experience of emotions are also active when we recognise the same emotions in other people (Singer et al., 2009; Singer & Lamm, 2009). Interestingly, recent neuroscientific findings extend this perspective and show that even the ToM abilities to consider the mental states and characteristics of others and oneself recruit nearly identical cortical areas (Tan et al., 2022). For these reasons, we hypothesize that there is a significant relationship between the difficulties in identifying and describing one's own mental states that characterize individuals with alexithymia and the difficulties in socio-cognitive abilities described in these individuals.
We decided to focus our systematic review only on those studies that examined the relationship between social cognition and alexithymia in non-clinical participants (i.e., individuals not diagnosed with a clinical disorder) to reduce potential confounding factors. Indeed, despite attempts to control for the effect of alexithymia alone, it may be difficult to assess its unique contribution to explaining performance on social cognition tasks in clinical populations, as other neurological and/or psychological symptoms may play a role in social cognition abilities in the context of comorbidity (Grynberg et al., 2012).
2. Methods
A systematic review of the literature was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analysis Protocols (PRISMA) guidelines (Page et al., 2021a, 2021b) to summarise the evidence for the relationship between alexithymia and ToM and/or empathy and/or emotion recognition (social cognitive skills). The protocol was registered with PROSPERO (CRD42023461559).
2.1. Search method
An initial literature search was carried out in December 2022. The following databases were selected based on their characteristics and relevance for the purposes of this study: PubMed, PsycINFO, and Scopus. To cover all aspects of social cognition, four simple strings with Boolean operators were used to query the databases: (“alexithymi*”) AND (“theory of mind” OR “ToM”); (“alexithymi*”) AND (“empath*”); (“alexithymi*”) AND (“emotion recognition”); (“alexithymi*”) AND (“social cognition”).
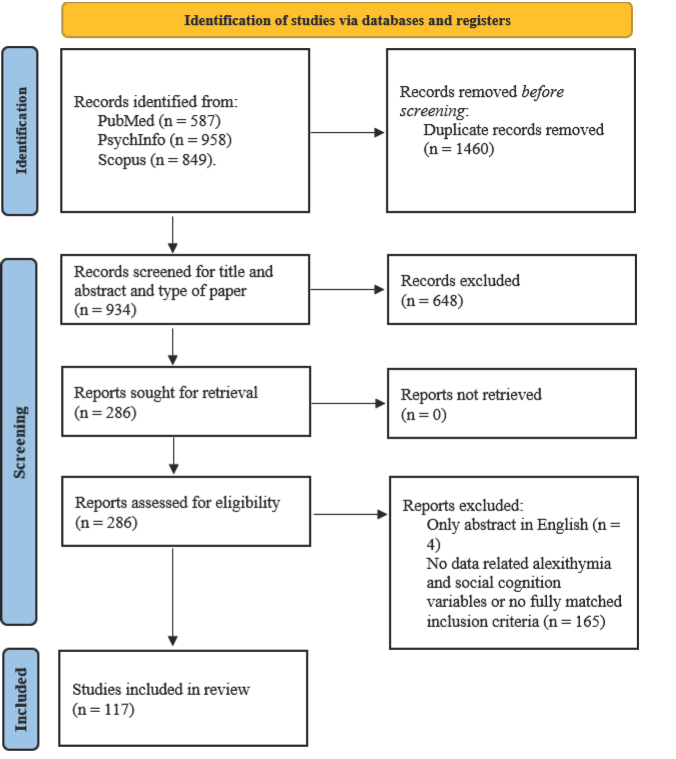
The final search was performed on 20 January 2023. As shown in the flow diagram of article selection (figure 1), a total of 2394 records were identified, ranging from 1979 to 2023. After screening, no further articles were found via cross-references.
Figure 1.
Flow diagram of articles selection
From: Page et al., 2021.
2.2. Eligibility criteria
The inclusion criteria were defined as follows:
Articles published in English and/or Italian (languages known by the authors);
Peer-reviewed papers (such as original articles and brief report) and original studies evaluating alexithymia and social cognition;
Studies that have been published in full-text;
Quantitative studies;
Studies that have used psychometrically validated instruments to assess alexithymia;
Studies that have used psychometrically validated instruments or ad hoc constructed-tasks to assess aspects of social cognition;
Studies that examined the behavioural relationship between alexithymia and dimensions of social cognition;
Studies that included only human participants;
Studies that included only the general population;
Studies that included only adult participants.
Conversely, the following exclusion criteria were established:
Articles in languages not included in the inclusion criteria;
Articles not peer reviewed (i.e., grey literature) or under review at the time the search was conducted;
Articles such as case reports, study protocols, and meeting abstracts that did not contain complete information;
Qualitative studies;
Articles that used qualitative methods or not validated instruments to assess alexithymia;
Studies that reported only neurophysiological data on the relationship between alexithymia and dimensions of social cognition;
Studies that did not include human participants;
Studies that included clinical samples or group patients;
Studies that included children or adolescent participants.
2.3. Studies screening and selection
Two authors (AB and MDT) performed the first step of study selection by screening the articles based on their titles and abstracts. Subsequently, AB, MA, MDT, and RBA read the full texts of the selected articles to identify those that were finally included in the review.
Another author (LC) conducted the literature search again, following the same steps as previously described to ensure that the results of this review were replicable.
All cases where authors disagreed on the inclusion or exclusion of an article were discussed by all authors together until agreement was reached.
2.4. Data extraction
All authors decided what information to extract from the included studies that was relevant to this review. AB, MA, and MDT collected the data independently and then discussed the results interactively. The following information was extracted from the selected studies: authors and year of publication of the article, participants (number and mean age), measures of alexithymia and social cognition, and the main findings related to the specific topic of this review, i.e., the relationship between alexithymia and social cognition.
The studies included in this review were grouped according to the three main components of the social cognition domain (i.e., ToM, emotion recognition, and empathy) associated with alexithymia.
2.5. Quality assessment
The quality assessment of the included studies was performed by two independent reviewers (MDT and AB) using the Joanna Briggs Institute (JBI; Munn et al., 2020) critical appraisal tool for cross-sectional studies (studies with only one target group) and case-control studies (studies with at least one control group – in the present systematic review, those studies that divided the non-clinical sample into two subgroups). These checklists contain 8 and 10 questions, respectively. Possible answers and scores for each question are Yes (1), No (0), Unclear (0), and Not applicable (0). The scores for overall quality range from 0 to 8 for cross-sectional studies and from 0 to 10 for case-control studies. Disagreements about the score to be assigned were discussed by all authors until agreement was reached.
3. Results
3.1. Study selection
Based on our search strategy, 2,394 records were found in the searched databases. After removing duplicates, AB and MDT checked 934 records for title and abstract. Articles were excluded because they did not fulfil the eligibility criteria (n = 648).
AB, MA, MDT, and RBA reviewed 286 full-text documents. Of these, 169 articles were excluded for the following reasons: only the abstract was in English (the text was in a language other than English or Italian) (n = 4), no data on alexithymia or social cognition were available, or the inclusion criteria were not fully met (n = 165). Finally, 117 studies were included in this review.
3.2. Study characteristics
A summary of the main characteristics and results of the 117 studies included in the present systematic review can be found in tables 1, 2, and 3.
Table 1.
Summary of the selected studies concerning the association between alexithymia (Alex) and Theory of Mind (ToM)
| Authors (year) | Participants | Measures | Main results (ToM and Alex) | (QA JBI) | ||
|---|---|---|---|---|---|---|
| N | Age | ToM | Alex | |||
| Al Aïn et al. (2013) | 107 | 23.9 (3.4) | RMET | TAS-20 | Significant correlations were found between the RMET total score and the TAS-20 total (r = -0.26, p < .05), DIF (r = -0.19, p < .05), and DDF (r = -0.26, p < .05) subscale scores. | 50% |
| Alaimo & Schimmenti (2013) | 335 | 22.2 (6.9) | RMET | TAS-20 | The following correlation between the TAS-20 total score and the RMET was found: r = -0.31 (p-value is not available). | 88% |
| Benau et al. (2020) | 279 (102 men; 177 women) | 19.3 (2.2) | RMET | TAS-20 | The TAS-20 DIF subscale was significantly associated with the RMET in men (r = -0.27, p < .01), but not in women (r = -0.07, p = NS). The DDF subscale was not correlated with the RMET either in men (r = -0.11, p = NS) or in women (r = -0.137, p = NS). | 88% |
| Chinello et al. (2020) | 43 parents (20 fathers; 23 mothers) | 52 (5) | RMET | TAS-20 | No significant correlations were found between the RMET and TAS-20 total (p = .053), DIF (p = .290), DDF (p = .136), and EOT (p = .088) subscale scores, even separately for mothers or fathers (all p-values > .182). | 75% |
| Demers & Koven (2015) | 86 | 18.9 (1.1) | RMET | TAS-20 | Significant correlations were detected between the RMET and the TAS-20 total (r = -0.36, p < .005) and EOT subscale scores (r = -0.48, p < .005); no significant associations were found between the RMET and the DIF (r = -0.06; p = NS) and DDF (r = 0.01, p = NS) subscales. | 75% |
| Di Tella et al. (2020) | 206 | 21.2 (2.1) | Strange Stories Test RMET | TAS-20 | No significant association was found between the TAS-20 total score and either the RMET (β = -0.05, p = NS) or the Strange Stories task (β = -0.04, p = NS). | 100% |
| Eddy & Hansen (2020) | 176 | 19.7 (1.3) | RMET CET | TAS-20 | Alex was not found to be a significant predictor of either RMET or CET (p = NS). | 75% |
| Gökçen et al. (2016) | 121 | 18.4 (1.9) | RMET MASC | TAS-20 | Alex was negatively associated with MASC (r = -0.40, p < .001) and RMET (r = -0.30, p = .001) performance. | 88% |
| Herrero-Fernández et al. (2022) | 395 | 36.3 (12.5) | RMET | TAS-20 | The RMET was significantly related to the EOT subscale of the TAS-20 (r = -0.18, p < .001). Conversely, no significant associations were found between the RMET and either the DIF (r = -0.03, p = NS) or DDF (r = -0.07, p = NS) subscales. | 75% |
| Jakobson & Pearson (2021) | 70 (34 males; 36 females) | Males: 20.6 (4.0) Females: 20.5 (5.3) | Videos from RISC database | TAS-20 | The TAS-20 DIF subscale was a significant predictor of unbiased recognition scores (β = 0.32, p = 0.014) and median reaction times (β = 0.39, p = 0.002) in the no context condition. | 50% |
| Lee et al. (2020) | 200 | 23.1 (2.7) | RMET | TAS-20 | The RMET was negatively correlated with the TAS-20 total (r = -.18, p < .05) and DIF (r = -0.18, p = .010) and EOT (r = -0.18, p = .013) subscale scores. | 88% |
| Lockwood et al. (2013) | 110 | 21.9 (3.7) | Animated Triangles Task | TAS-20 | Alex was not significantly associated with the cognitive ToM task (r = -0.120, p = NS). | 75% |
| Lyvers et al. (2017) | 102 | 22.2 | RMET | TAS-20 | Only the TAS-20 EOT subscale was significantly related to the RMET (r = -0.28, p < .01). Conversely, no significant correlations were found between the RMET and the total (r = -0.20, p = NS), DIF (r = -0.14, p = NS) and DDF (r = -0.07, p = NS) subscale scores. | 88% |
| Lyvers et al. (2018) | 161 | 22.6 (7.2 | RMET | TAS-20 | The RMET was significantly associated with the TAS-20 DIF (r = -0.19, p < .05) and EOT (r = -0.31, p < .001) subscales. Conversely, no significant correlation was found between the RMET and the DDF subscale (r = -0.02, p = NS). | 88% |
| Lyvers et al. (2019a) | 291 | 26 (9) | RMET | TAS-20 | A significant association between the RMET and the TAS-20 total score was detected (r = -0.24, p < .001). | 100% |
| Lyvers et al. (2019b) | 242 | 23.2 (4.5) | RMET | TAS-20 | The RMET was found to be significantly associated with the TAS-20 total (r = -0.24, p < .001) and DIF subscale (r = -0.15, p < .05) scores. Conversely, no significant correlations were found between the RMET and the DDF (r = -0.12, p = NS) or EOT (r = -0.03, p = NS) subscales. | 100% |
| Martinez- Sanchez et al. (2017) | 1645 | 31.3 (9.7) | RMET | TAS-20 | Alex was significantly correlated with the RMET performance (r = -0.09, p < .01). Moreover, a significant effect for alexithymia group factor was found [F(2, 1389) = 8.96; p < .001; ηp2 = .011], with the “non-alex” group that obtained higher scores at the RMET compared to the “alex” and “probable” samples (Non-alex: 26.8 ± 3.4; Alex = 25.6 ± 4.1; Prob-alex: 25.9 ± 3.8; p < .01). | 63% |
| Moriguchi et al. (2006) | 30 (14 non- alex;16 alex) | Total: 20.4 (0.9) Non-alex: 20.8 (0.9) Alex: 20.2 (1.0) | Animated Triangles Task | TAS-20 SIBIQ | The alex group scored significantly lower than the non-alex group on ToM intentionality (14.9 ± 3.5 vs. 17.2 ± 1.9, t = 2.31, p = .030) and appropriateness (7.9 ± 1.9 vs. 9.5 ± 1.7, t = 2.34, p = .026). | 80% |
| Nam et al. (2020) | 200 (89 males; 111 females; 129 non-alex;71 alex) | Male: 23.8 (2.5) Female: 22.5 (2.7); Non-alex: 23.3 (2.6) Alex: 22.7 (2.7) | RMET | TAS-20 | In the female group, no significant correlations were found between the RMET and the TAS-20 total score (r = -0.13, p = NS), DIF (r = -0.14, p = NS), DDF (r = -0.03, p = NS), and EOT (r = -0.14, p = NS) subscale scores. Conversely, a significant correlation was found in the male group for the TAS-20 total score (r = -0.25, p < .05), the DIF (r = -0.29, p < .01), and the EOT (r = -0.21, p < .05) subscale scores. Alexithymic male showed poorer performance than non- alexithymic male in the RMET [F(1,43) = 4.55, = .04, p ηp2 = .10). Conversely, alexithymic female did not show poorer performance than non-alexithymic female [F(1,62) = 0.39, p = .54, ηp2 = .01]. The alex group (male + female) did not differ from the non-alex group on RMET performance [26.1 ± 2.8 vs. 26.8 ± 3.1, F(1,198) = 2.26, p = .14, ηp2 = .01]. | 80% |
| Olkoniemi et al. (2019) | 60 | 24.2 (4.2) | Short paragraphs | TAS-20 | High alex levels were associated with longer reading times on sarcastic paragraphs, compared with literal ones (no data are available). | 37.5% |
| Pahnke et al. (2020) | 119 | 21.5 (0.4) | RMET RME-C-T | TAS-20 | A significant correlation between the RME-C-T and the TAS-20 EOT subscale was found (r = -0.22, p = .002). Conversely, no significant associations were detected between the RME-C-T and the DIF (r = -0.10, p = .297) or DDF (r = 0.00, p = .961) subscales. Moreover, non-alex participants were found to perform better on the RME-C-T than alex participants (z = -2.24, p = .009, r = -0.22, 95% CI [-0.38, -0.04]). | 63% |
| Schimmenti (2017) | 792 | 35.8 (11.0) | RMET | TAS-20 | A TAS-significant 20 total association score was found between (r = -the 0.30RMET , p < .and 01). the | 75% |
| Schimmenti et al. (2019) | 799 | 35.8 (11.0) | RMET | TAS-20 | A TAS-significant 20 total association score was found between (r = -the 0.30RMET , p < .and 01). the | 88% |
| Sunahara et al. (2022) | 1473 | 25.8 (11.7) | RMET | TAS-20 | A significant association between the RMET and the TAS-20 total score was revealed (b = -0.19, 95% CI [-0.25, -0.14]). | 88% |
| Swart et al. (2009) | 34 (16 alex; 18 non- alex) | Alex: 20.1 (1.7) Non-alex: 19.3 (1.0) | Conflicting Beliefs and Emotions | BVAQ | A significant difference between alex and non-alex participants was found on the first order emotion question of the ToM task (85.2 ± 9.9 vs. 95.1 ± 8.7, Χ2 = 9.46, p = .002). Conversely, no differences emerged in the other ToM conditions (first order cognition: 93.8 ± 9.1 vs. 98.6 ± 3.4, Χ2 = 2.72, p = .10; second order cognition: 95.3 ± 7.7 vs. 98.6 ± 4.0, Χ2 = 2.17, p = 1.14; second order emotion: 75.4 ± 15.4 vs. 78.5 ± 24.2, Χ2 = 1.12, p = .29). | 70% |
| Vellante et al. (2013) | 200 | 24.1 (2.8) | RMET | TAS-20 | No significant associations between the RMET and the TAS-20 total score were detected in the total (r = -0.12, p = NS) or female (r = 0.02, p = NS) sample. Conversely, a significant correlation was found in the male group (r = -0.22, p < .01). | 63% |
| Wastell & Taylor (2002) | 45 | 22.2 (6.8) | FBPST | TAS-20 | No significant difference between alex and non-alex* participants were revealed on the FBPST scores (Social script: 5.9 ± 0.4 vs. 5.9 ± 0.2, p ≥ .13; Mechanical: 5.8 ± 0.5 vs. 5.8 ± 0.4, p ≥ .13; Capture: 4.6 ± 1.1 vs. 4.5 ± 0.9, p ≥ .13; False belief: 5.6 ± 0.6 vs. 5.4 ± 0.8, p ≥ .13). | 63% |
| Zimmermann et al. (2021) | 32 (10 high alex; 10 | High alex: 27.9 (10.1) | RMET | TAS-20 | No significant correlations were found between alex and the number of errors in the RMET (TAS-20 total score: ρ = -0.01, p = .469; EOT suscale: ρ = -0.19, p = .143). | 70% |
QA (JBI) = Quality Assessment (Joanna Briggs Institute); RMET = Reading the Mind in the Eyes Test; TAS = Toronto Alexithymia Scale; TAS DIF = Difficulty Identifying Feelings; TAS DDF = Difficulty Describing Feelings; TAS EOT = Externally Oriented Thinking; CET = Cat Eyes Test; MASC = Movie for the Assessment of Social Cognition; RME-C-T = Reading the Mind in the Eyes of Children Test; TSIA = Toronto Structured Interview for Alexithymia; BVAQ = Bermond-Vorst Alexithymia Questionnaire; FBPST = False Belief Picture Sequencing Task.
* Non-alexithymic participants were taken from the study by Langdon & Cooltheart, 1999.
Note. Age is expressed in years; NS = not significant.
Table 2.
Summary of the selected studies concerning the association between alexithymia (Alex) and emotion recognition (ER)
| Authors (year) | Participants | Measures | Main results (ER and Alex) | (QA JBI) | ||
|---|---|---|---|---|---|---|
| N | Age | ER | Alex | |||
| Bani et al. (2023) | 342 | 46.4 (12.2) | Modified version of the DANVA2‐ AF | TAS‐20 | A significant association was found between alex and ER accuracy in the unmasked condition (r = 0.15, p < .05); no significant association emerged in the masked condition (r = 0.06; p = NS). | 100% |
| Bègue et al. (2019) | 34 | 23.8 (N/A) | Ad hoc constructed paradigm | TAS-20 | No significant correlation was found between alex and metacognitive ability index (values N/A). | 75% |
| Brewer et al. (2015) | 34 (15 alex; 19 controls) | Alex: 28.7 (14.9) Controls: 22.68 (3.13) | Pictures of Facial Affect Karolinska Directed Emotional Faces Database | TAS‐20 | The alex group exhibited lower sensitivity than the control group to changes in facial emotion [5.6 ± 2.3 vs. 8.8 ± 3.7, t(32) = 2.94, p = .003] (Experiment 1). Alex participants showed reduced inter-rater consistency when judging the character traits, F(3, 861) = 18.49, p < .001, η2 = .037 (Experiment 2), and emotions, F(10, 2790) = 4.83, p < .001, η2 = .017 (Experiment 3), of emotionally neutral models. | 70% |
| Coll et al. (2019) | 42 (20 alex; 22 controls) | Alex: 29.4 (12.0) Controls: 30.1 (10.9) | Radboud Faces Database Implicit facial expression discrimination task | TAS-20 | Alex participants were able to detect physical differences between facial expressions in the explicit emotion discrimination task (p= .66). Conversely, perm alex individuals showed no difference between oddball responses to upright and inverted faces in the mixed-emotions paradigm (p= .21, Cohen’s d = .47; 95% CI: perm -1.12, -0.18). | 70% |
| Connolly et al. (2020a) | 308 | 38.1 (N/A) | FEEST Point-light displays Montreal Affective Voices | TAS‐20 | A negative correlation between supramodal ER ability (measured with faces, bodies, and voices) and alex (r = -0.33; p <.001) was found. | 63% |
| Connolly et al. (2020b) | Study 1a 389 Study 1b 318 | Study 1a 37.0 (11.7) Study 1b 35.9 (N/A) | FEEST Point-light displays Montreal Affective Voices | TAS-20 | Negative correlations between supramodal ER ability and alex (Study 1a: r = -0.18, p = .003; Study 1b: r = -0.36, p <.001) were detected. | 63% |
| Di Tella et al. (2020) | 260 | 21.23 (2.06) | MPAFC | TAS-20 | In the regression model, alex (β = -0.22, p = .005), among all the predictors, was found to be the only significant contributor of ER accuracy. | 100% |
| Hakala et al. (2015) | 40 | 28.1 (9.5). | Stereoscopic photographs | TAS-20 | Facial expression was significantly associated with alex, both for negative, F(2, 3476) = 10.1, p < .001, and positive valences, F(2, 3475) = 32.2, p < .001. | 63% |
| Halberstadt et al. (2021) | Sample 1 183 Sample 2 74 Sample 3 177 Sample 4 43 | Sample 1 19.31 (N/A) Sample 2 19.77 (N/A) Sample 3 22.48 (N/A) Sample 4 37.42 (N/A) Men: 23.7 | PerCEIVED Increasingly Clear Emotions Task DANVA-2-CF | TAS-20 | Performance at the Increasingly Clear Emotions task was found to be associated with the DIF subscale of the TAS-20 [r(183) = 0.16, p = .03]. No other significant correlations were found. | 37.5% |
| Hovey et al. (2018) | 492 (182 men; 310 women) | (3.1) Women: 23.0 (3.2) | ERAM | TAS-20 | A significant association between alex and the audiovisual ER score was found only in female (r = -0.14, p = .01), but not in male (r = -0.11, p = .16) participants. | 88% |
| Hsinga et al. (2013) | 115 | 18.95 (N/A) | Emostroop task | TAS-20 | No significant differences were found between high and low alex groups on either reaction time or accuracy in classifying emotional faces (angry and sad) [F(1, 113) = .10, p = .749; F(1, 113) < .20, p > .60, respectively]. | 50% |
| Jessimer & Markham (1997) | 180 | N/A | Chimeric stimuli (Pictures of Facial Affect) | TAS-20 | A significant difference between high and low alex groups was found on the ER test [F(1, 34) = 15.24, p < .001], with high alex participants showing poorer ER than low alex ones. The high alex group reported a poorer performance on all the six basic emotions [happiness, t(34) = 2.45, p < .01; surprise, t(34) = 2.57, p < .01; sadness, t(34) = 3.5, p < .001; fear, t(34) = 3.5, p < .001; disgust, t(34) = 3.15, p < .01; and anger t(34) = 3.06, p < .01] compared to low alex participants. | 50% |
| Jongen et al. (2014) | 40 (20 alex; 20 non-alex) | Alex: 26.5 (7.7) Non-alex: 25.8 (6.7) | FEEL | TAS-20 | Participants high in alex performed significantly worse than individuals low in alex (t = -2.40; p = .022) in the facial ER task. | 70% |
| Kafetsios & Hess (2019)s | 108 | 25.87 (5.04) | ACE | TAS-20 | Emotion perception bias (perceiving emotions additional to those communicated), but not accuracy (perceiving the emotions communicated), was associated with alex [r(108) = 0.30, p < .01]. Emotion perception bias and accuracy were also associated with DIF [r(108) = 0.34, p < .01; r(108) = −0.49, p < .001] and DDF [r(108) = 0.26, p < .01; r(108) = −0.26, p < .01] scores. | 25% |
| Keightley et al. (2006) | 60 (30 younger adults; 30 older adults) | Younger adults: 25.7 (5.1) Older adults: 72.5 (7.8) | JACNeuF | TAS-20 | In older adults only, a greater performance in the recognition of anger faces was associated with lower alex scores (β = -0.17, p < .01). | 80% |
| Koelkebeck et al. (2015) | 42 | 30.0 (9.5) | Noh mask test | TAS-26 | A significant positive association between alex scores and mean reaction times on the ER test was found (r = 0.32, p = .038). | 90% |
| Kyranides et al. (2022) | 110 | 24.9 (2.8) | MPAFC | TAS-20 | Individuals high in alex did not perform worsley in the facial ER task (either in accuracy or in response times) compared to participants low in alex (p = .58). | 63% |
| Lane et al. (1995) | 318 | N/A | Perception of Affect Task | TAS-20 | A significant association was found between higher alex levels and lower accuracy rates at the ER task (r = -0.32, p < .001). | 63% |
| Lane et al. (2000) | 379 | N/A | Perception of Affect Task | TAS-20 | A significant difference between participants high vs. low in alex in ER accuracy was found (F = 19.8, p < .001). | 75% |
| Larwood al. (2021et ) | 162 | 21.5 (1.9) | Musical stimuli | TAS-20 | Alex was not associated with the number of emotion words generated (r = -0.05, p = NS), but was related to valence-the DDF specific factor (affect r = -0.19judgements , p < .05). Participants of music at least higher for in alex rated sad, angry, and fearful pieces as more neutral in valence and arousal. | 100% |
| Laukka et al. (2021) | 593 (226 men; 367 women) | Men: 23.4 (3.3) Women: 22.9 (3.2) | ERAM | TAS-20 | A significant negative correlation between overall ER accuracy and alex was found (r = -0.19, p < .001). | 50% |
| Lewis et al. (2016) | 389 | 37 (11.7) | FEEST | TAS-20 | Facial ER accuracy was negatively associated with alex total score (r = -0.32, p < .001), DIF (r = 0.21 p = .004), DDF (r = 0.24 p = .01), and EOT (r = 0.36 p < .001) subscale scores. | 63% |
| Maiorana et al. (2022) | 31 | 32 (11) | NimStim Face Stimulus Set | TAS‐20 | Mean reaction times correlated with alex in the mouth-only (r = 0.48, p = .006), unmasked (r = 0.48, p = .007), and eyes-only (r = 0.37, p = .038) conditions. No correlation was found in the masked condition (r = 0.08, p = .656). | 37.5% |
| Malykhin et al. (2023) | 140 | 48.3 (18.4) | Penn Emotion Recognition task | TAS-20 | Alex negatively correlated with the accurate recognition of sad images (r = -0.17, p = .04). This association was driven by an increased number of errors when sad images were assigned to the neutral (r = 0.20, p = .016) and happy (r = 0.16, p = .057) categories. | 75% |
| Mann et al. (1994) | 62 | 31.5 (10.3) | Pictures of Facial Affect | TAS-26 | Participants high in alex performed less accurately overall on the ER test compared to individuals low in alex (top third: 25.9 ± 2.6; second third: 27.2 ± 2.2; lowest third: 27.7 ± 1.3; Χ2(2) = 7.2, p < .05) | 50% |
| Martingano et al. (2022) | 1253 | 27.6 (N/A) | FACS-verified | TAS-20 | No significant associations between the performance on the ER test and alex total, DIF, DDF, and EOT scores were found (all p-values = NS). | 88% |
| Mayer et al. (1990) | 139 | N/A | Pictures of Facial Affect | TAS-26 | Alex was associated with a greater emotional range [r(128) = 0.16, p < .05] and a higher perception of emotion (generally negative) [r(128) = 0.20, p < .01] in response to the emotional stimuli. | 50% |
| McCubbin et al. (2014) | 96 | 22.4 (6.81) | Perception of Affect Task | TAS-20 | A significant association between alex and ER accuracy was found [r(88) = -0.34, p = .001]. ER intensity was not correlated with alex (r = -0.12, p = NS). | 88% |
| Montebarocci et al. (2011) | 91 | 25.3 (4.7) | Pictures of Facial Affect | TAS-20 | High alex group obtained a significantly lower ER accuracy score than the low alex group [F(1.33) = 4.35, p < .05]. | 90% |
| Murphy et al. (2019) | 134 | 55.0 (19.5) | Emotion-identity recognition task | TAS-20 | A negative association between alex and the performance on the ER task was found (r = -0.21, p < .05). | 75% |
| Nook et al. (2015) * | 82 | 22.9 (5.72) | NimStim IASLab | TAS-20 | Higher alex was associated with impaired performance for face-face trials [r(35) = 0.34, p = .04] but not for face-word trials [r(32) = 0.01, p = .96]. | 50% |
| Parker et al. (1993) | 216 (131 women; 85 men) | Women: 20.6 (2.1) Men: 21.1 (1.8) | Photographs from Izard | TAS-20 | A main effect for alex group (low, moderate, high alex) was found [F(2,210) = 4.73, p = .010], as well as a significant interaction between the alex group and the type of emotion [F(8,1680) = 2.16, p = .005]. The low alex group reported significantly higher ER total scores than the high alex sample. | 63% |
| Parsons et al. (2021) | 610 | 32 (4.6) | Infant Facial Emotion Perception Task KDEF-dyn Database | TAS-20 | No significant associations between alex and ratings of arousal or valence across the infant emotion categories were found (all r < .08), with the only exception of a negative correlation between arousal ratings for the muted negative faces and EOT scores (r = 0.11, p = .009). Conversely, the correlations between alex and accuracy for adult faces were significant for the sad (r = 0.10, p = .02) and angry faces (r = 0.14, p < .001), and the overall accuracy scores (r = 0.09, p = .02). Also, EOT scores correlated with accuracy for the sad (r = 0.19, p = .0001) and angry faces (r = 0.16, p = .0001), and overall accuracy (r = 0.15, p = .001). | 63% |
| Radoš et al. (2021) | 426 | 22.5 (4.6) | City Infant Faces Database | TAS-20 | Greater total accuracy on the ER test was related to lower levels of alex total (r = -0.15, p = .009) and EOT (r = -0.19, p = .001) scores. | 63% |
| Ridout et al. (2010) | 45 high (23 EDI; 22 low EDI) | High 19.6 EDI: (1.7) Low EDI: 19.1 (0.9) | TASIT - Emotion Evaluation | TAS-20 | A significant negative correlation between ER accuracy and alex scores was detected [r(45) = -0.54, p < .001]. | 80% |
| Ridout et al. (2021) | Study 1 39 Study 2 38 | Study 1: 19.5 (1.1) Study 2: 19.63 (2.7) | Karolinska and Nimstim face sets TASIT | TAS-20 | Alex did not predict ER accuracy in both tasks (p > .05). | 88% |
| Rosenberg et al. (2020) | 49 | 23.3 (2.8) | Pictures of Facial Affect | TAS-20 BVAQ TSIA | The TAS-20 and BVAQ total scores were significantly correlated with the priming score for angry faces (r = -0.30, p < .05; r = -0.29, p < .05, respectively), whereas no significant association emerged between the TSIA and any of the emotions. Also, the BVAQ Identifying was associated with fearful faces (r = -0.34, p < .05), while the TSIA imaginal processes subscale correlated with happy faces (r = -0.38, p < .01). | 63% |
| Rus-Calafell et al. (2013) | 98 | 32.6 (9.2) | Penn Emotion Recognition Test Virtual Faces | TAS-20 | Positive correlations were found between alex and committed errors in both presentation conditions (static images, r = 0.32, p <.01; virtual reality, r = 0.43, p <.01). | 75% |
| Schlegel et al. (2019) | 70 | 26.0 (4.9) | GERT | TAS-20 | Accuracy in facial emotion recognition was negatively correlated with alex (r = -0.20 , p < .01). | 63% |
| Senior et al. (2020) | 83 | 19.7 (N/A) | Pictures of Facial Affect | TAS-20 | Accuracy in facial ER was negatively correlated with alex [r(75) = -0.4, p < .001]. | 75% |
| Sharpe et al. (2016) | 52 | 22.1 (2.5) | BU-3DFE database | TAS-20 | Alex was not a significant predictor of ER accuracy (p > .05) in the regression model. | 80% |
| Sunahara et al. (2022) | 1756 Biological task; 384 Penn test | Biological task: 24.8 (10.9) Penn test: 19.7 (1.8) | Biological Motion Task Penn Emotion Recognition Test | TAS-20 | Higher alex levels predicted lower ER accuracy on the biological motion test (b = -0.07, 95% CI [-0.12, -0.02]), but not on the Penn Emotion Recognition test (b = -0.04, 95% CI [-0.15, 0.07]). | 88% |
| Swart et al. (2009) | 34 (16 alex; 18 non-alex) | Alex: 20.1 (1.7) Non-alex: 19.3 (1.0) | Micro expression training tool Affective Prosody task | BVAQ | Alex participants scored significantly lower on recognizing brief emotional expressions [F(1,31) = 9.60, p = .004] compared to non-alex individuals. No difference between alex and non-alex participants on accuracy in either the prosody or semantic task was found [F(4,29) = 1.77, p = 0.16; F(4,29) = 0.32, p = 0.86, respectively]. | 70% |
| Taruffi et al. (2017) | 120 | 30.4 (9.5) | Musical stimuli | TAS-20 | Only the EOT subscale of the TAS-20 was a significant predictor of musical emotion recognition total score (β = 0.21, p < .05). | 63% |
QA (JBI) = Quality Assessment (Joanna Briggs Institute); DANVA2‐AF = Diagnostic Analysis of Nonverbal Accuracy FACES 2‐Adult Faces; TAS = Toronto Alexithymia Scale; TAS DIF = Difficulty Identifying Feelings; TAS DDF = Difficulty Describing Feelings; TAS EOT = Externally Oriented Thinking; FEEST = Facial Expressions of Emotion: Stimuli and Tests; MPAFC = Montréal Pain and Affective Face Clips; PerCEIVED = Perceptions of Children’s Emotions in Videos, Evolving and Dynamic task; DANVA2‐CF = Diagnostic Analysis of Nonverbal Accuracy FACES 2‐Children Faces; ERAM = Emotion Recognition Assessment in Multiple modalities; FEEL = Facially Expressed Emotion Labelling; ACE = Assessment of Contextualized Emotions-faces; JACNeuF = Japanese and Caucasian Facial Expressions of Emotions and Neutral Faces; FACS-verified = Facial Action Coding System–verified University of California set of Emotion Expressions; KDEF = Karolinska Directed Emotional Faces; EDI = Eating Disorder Inventory; TASIT = The Awareness of Social Inference Test; BVAQ = Bermond-Vorst Alexithymia Questionnaire; TSIA = Toronto Structured Interview for Alexithymia; GERT = Geneva Emotion Recognition Test.
* Only the data from Study 2 were considered, as in Study 1 the total alexithymia score was calculated by summing up solely the subscales “identifying emotions” and “describing emotions” of the TAS-26.
Note. Age is expressed in years; NS = not significant.
Table 3.
Summary of the selected studies concerning the association between alexithymia (Alex) and empathy
| Authors (year) | Participants | Measures | Main results (Empathy and Alex) | (QA JBI) | ||
|---|---|---|---|---|---|---|
| N | Age | Empathy | Alex | |||
| Al Aïn et al. (2013) | 107 | 23.9 (3.4) | BES | TAS-20 |
Empathy total score was negatively associated with alex total score (r = -0.28, p < .0.5). The following additional results were detected: TAS-20 DIF and BES total (r = 0.02, p = NS), affective (r = -0.17, p < .0.5), and cognitive (r = 0.13, p = NS). TAS-20 DDF and BES total (r = -0.33, p < .0.5), affective (r = -0.39, p < .0.5), and cognitive (r = -0.20, p < .0.5). TAS-20 EOT and BES total (r = -0.33, p < .0.5), affective (r = -0.38, p < .0.5), and cognitive (r = -0.21, p < .0.5). |
50% |
| Alkan Härtwig et al. (2020) | 34 (24 alex; 26 non-alex) |
Alex: 35.0 (10.5) Non-alex: 34.7 (10.1) |
IRI Multifaceted Empathy Task |
TAS-20 BVAQ OAS |
High alex participants reported significantly lower scores on empathy subscales of the IRI [Fantasy t(48) = -2.93, p = .011; Empathy t(48) = -3.24, p < .001; Perspective taking t(48) = -2.69, p = .047; Personal distress t(48) = 2.64 p = .045; Competence t(48) = -0.75, p = .456]. Moreover, high alex participants presented significantly lower emotional empathy than the controls, as shown in the main effect of group in ANOVA [F(1,46) = 8.25, p = .006] and in post hoc t-tests of Empathy-condition [t(48) = −2.59; p = .012]. | 80% |
| Aslan et al. (2021) | 376 | 20.9 (1.9) | Empathy Tendency Scale | TAS-20 | Empathy was negatively associated with alex (r = -0.34, p < .001). | 63% |
| Bogdanov et al. (2013) | 21 | N/A | Multidimensional Emotional Empathy Scale | TAS-20 | No significant relationship between empathy and alex was found (no data available). | 63% |
| Carré et al. (2013) | 370 | 26.1 (12.4) | BES | TAS-20 | No correlations between the BES-Affective and TAS-20 scores were found (all p = NS). Cognitive Empathy subscale correlated with TAS-20 total (r = -0.17, p < .05), DIF (r = -0.18, p < .05), and DDF (r = -0.21, p < .05) scores. | 75% |
| Christensen et al. (2018) | 40 (20 dancers; 20 non-dancers) |
Dancers: 25.4 (4.6) Non- dancer: 24.3 (53.9) |
IRI EES |
TAS-20 BVAQ |
No significant association between the IRI and TAS-20 and the EE and TAS-20 total scores was found (all p = NS). The IRI and EE total scores were negatively associated with the BVAQ total score (IRI, r = -0.35, p < .05; EE, r = -0.51, p < .001). | 70% |
| Colombarolli et al. (2019) | 850 | 31 (10.8) | QCAE | TAS-20 | Significant associations between alex and the following empathy scores were found: QCAE-Perspective taking: r = −0.27, p < .001; QCAE-Online simulation: r = -0.35, p < .001; QCAE-Proximal Responsivity: r = -0.09, p < .01; QCAE-Peripheral Responsivity: r = -0.16, p < .001; QCAE-Cognitive Empathy: r = -0.38, p < .001; QCAE-Total: r = -0.30, p < .001); QCAE-Emotion contagion: r = 0.22, p < .001). Significant associations between the TAS-20 subscale and QCAE subscale scores were found (all correlation results are not shown for space reasons.). | 88% |
| Demers & Koven (2015) | 86 | 18.9 (1.1) | QMEE | TAS-20 | Empathy was associated with two alex subscale scores (TAS-20 DIF: r = 0.27, p < .05; TAS-20 EOT: r = -0.41, p < .005). | 75% |
| Di Girolamo et al. (2019) | 285 | 26.4 (7.0) | QCAE | TAS-20 | Empathy total score was significantly associated with alex total (r = -0.27, p < .01), DDF (r = -0.22, p < .05), and EOT (r = -0.43, p < .01) scores. Cognitive empathy was significantly associated with alex total (r = -0.35 , p < .01), DDF (r = -0.25, p < .01), and EOT (r = -0.43, p < .01) scores. Afective empathy was significantly associated only with EOT scores (r = -0.26, p < .01). | 88% |
| Di Tella et al. (2020) | 260 | 21.2 (2.1) | IRI | TAS-20 | Alex was positively correlated with the Personal Distress subscale (r = 0.39, p < .01) and negatively correlated with the Perspective-Taking subscale (r = -0.35, p < .01) of the IRI. Results of the hierarchical regressions showed a significant predictive role of alex for all the IRI subscales (all p < .05). | 100% |
| Dierckx et al. (2021) | 506 (271 Black sample; 235 Muslim sample) |
Black sample: 27.9 (8.50) Muslim sample: 27.2 (8.50) |
IRI | TAS-20 | Empathy (Empathic Concern and Perspective Taking dimensions) was negatively associated with alex in both Black and Muslim samples (Black sample: Empathic Concern, r = -0.25, p < .001; Perspective Taking, r = -0.30, p < .001. Muslim sample: Empathic concern, r = -0.28, p < .001; Perspective Taking: r = -0.43, p < .001). | 60% |
| Diotaiuti et al. (2021) | 300 | 22 (2.6) | IRI | TAS-20 |
A significant correlation was found between IRI total and TAS-20 subscale scores (DIF, r = -0.19, p < .01; DDF, r = -0.11, p < .05; EOT, r = -0.48, p < .01). The TAS-20 total score was significantly correlated with the IRI Perspective Taking (r = -0.22, p < .01), Personal Distress (r = 0.35, p < .01), and Empathic Concern (r = -0.28, p < .05) subscale scores. Significant correlations also emerged between the DIF subscale and the IRI Personal Distress (r = -0.42, p < .01), Empathic Concern (r = -0.11, p < .05), and Fantasy (r = -0.23, p < .01) subscale scores and between the DDF and Personal Distress (r = -0.28, p < .01). The TAS-20 EOT subscale was significantly associated with the IRI Perspective Taking (r = -0.46, p < .01), Empathic Concern (r = -0.37, p < .05), and Fantasy (r = -0.32, p < .01) subscale scores. |
50% |
| Eddy & Hansen (2021) | 297 | 19.2 (1.2) | IRI | TAS-20 | Alex was associated with Perspective Taking (r = -0.33, p < .0001), Empathic Concern (r = -0.30, p < .0001), and Personal Distress (r = 0.21, p < .0001) subscales of the IRI. | 50% |
| Gleichgerrcht & Decety (2013) | 7584 | 44.6 (12.1) | IRI | TAS-20 | Significant diferences were found between physicians with alex, borderline and without alex on the Empathic Concern (F(2,1878) = 4.62, p < .01, d = .15), Personal Distress (F(2,1878) = 36.2, p < .001, d = .39), and Perspective Taking (F(2,1878) = 48.9, p < .001, d = .46), with alex participants showing less empathic skills than non-alex ones. Moreover, alex was associated with all empathy subscales (Empathic Concern: r = 0.21, p <.001; Perspective Taking: r = 0.23, p < .001; Personal Distress, r = 0.30, p < .001). | 88% |
| Goerlich et al. (2017) | 45 | 24.1 (3.2) | EQ | TAS-20 | Alex total score was negatively correlated with EQ total (r = -0.74, p < 0.001) and subscale scores (Cognitive empathy: r = -0.74; Emotional empathy: r = -0.66; Social empathy: r = -0.72; all p < .001). Significant associations were also found between the EQ total and the three subscales of the TAS-20 (DIF: r = -0.57; DDF: r = -0.69; EOT: r = -0.51; all p < .001). | 88% |
| Goerlich- Dobre et al. (2015) | 125 (70 women; 55 men) |
Women: 25.2 (5.6) Men: 25.6 (5.6) |
IRI | BVAQ | The cognitive dimension of alex was associated with both cognitive (Perspective Taking and Fantasy) (women: r = -0.75, p < .001; men: r = −0.59, p < .001) and affective (Empathic Concern and Personal Distress) empathy (women: r = -0.68, p < .001; men: r = -0.43, p < .001). The affective alex dimension was not significantly related to either empathy dimension, neither in women nor in men (p = NS). | 88% |
| Gökçen et al. (2016) | 121 | 18.4 (1.9) | Self- Assessment Manikin Faces Task | TAS-20 | A not significant association was found between alex and affective empathy performance (r = -0.11, p = 0.249). | 88% |
| Gossen et al. (2014) | 35 (15 high-EQ; 20 low- EQ) |
High-EQ: 23.5 (2.3) Low-EQ: 24.7 (6.0) |
EQ | TAS-20 | Significant differences were found in the TAS-20 total score between the high and low empathy groups, with the former reporting lower levels of alex than the latter (31.5 ± 10.7 vs. 49.2 ± 8.9, p < .0001, η2 = 0.46). | 80% |
| Grynberg et al. (2010) | 645 | 21.2 (3.0) | IRI | TAS-20 |
Alex total was associated with Personal Distress (r = 0.25, p < .001), Empathic Concern (r = -0.18, p < .001), and Perspective Taking (r = -0.28, p < .001) subscales. Significant correlations also emerged between the DIF subscale and the IRI Personal Distress (r = 0.32, p < .001), Perspective Taking (r = -0.14, p < .001), and Fantasy (r = 0.12, p < .01) subscale scores and between the DDF and both Personal Distress (r = 0.22, p < .01) and Perspective Taking (r = -0.18, p < .001). The TAS-20 EOT subscale was significantly associated with the IRI Perspective Taking (r = -0.37, p < .001), Empathic Concern (r = -0.24, p < .001), and Fantasy (r = -0.21, p < .001) subscale scores. |
75% |
| Hao et al. (2020) | 674 | 20 (1.2) | IRI | TAS-20 | A positive association was found between alex and both cognitive (Perspective Taking) (r = 0.09, p < .05) and affective (Empathic Concern) empathy (r = 0.09, p < .05). | 88% |
| Herrero-Fernández et al. (2022) | 395 | 36.3 (12.5) | IRI | TAS-20 | The DDF subscale of TAS-20 was associated with all the IRI subscales (Perspective Taking: r = -0.13, p < .01; Fantasy: r = 0.12, p < .05; Empathic Concern: r = 0.15, p < .01; Personal Distress: r = 0.45, p < .001); the DIF subscale was associated with Perspective Taking (r = -0.20, p < .001) and Personal Distress (r = 0.31, p < .001); the EOT subscale was associated with all the IRI subscales (Perspective Taking: r = -0.51, p < .001; Fantasy: r = -0.30, p < .001; Empathic Concern: r = -0.28, p < .001; Personal Distress: r = 0.18, p < .001). | 75% |
| Himichi et al. (2021) | 516 | 39.5 (11.1) | IRI | TAS-20 | Alex was associated with the Personal Distress (r = 0.44, p < .001), Empathic Concern (r = -0.28, p < .001), Perspective Taking (r = -0.09, p < .05), and Fantasy (r = 0.08, p < .10) subscales of the IRI. | 88% |
| Ignatova et al. (2022) | 210 | 25 (3.0) | TEQ | TAS-20 | A negative association between alex and empathy total scores (r = -0.18, p < .05) was found. | 63% |
| Jonason & Krause (2013) | 320 | 24.2 (7.3) | BES | TAS-20 | The cognitive component of empathy was associated with all facets of alex (DDF: r = -0.32, p < .01; DIF: r = -0.21, p < .01; EOT: r = -0.46, p < .01), while affective empathy correlated only with the EOT subscale of the TAS-20 (r = -0.46, p < .01). | 50% |
| Kamel (2013) | 332 | 34.7 (12.0) | MDEES | TAS-20 | Significant associations were found between empathy total and TAS-20 DDF (r = -0.06, p < .05) and EOT (r = -0.20, p < .05) subscale scores. | 63% |
| Karras et al. (2022) | 550 | 40.3 (15.5) | TEQ IRI | TAS-20 | Alex total score was significantly associated with the TEQ total score (r = -0.13, p < .01) and the Perspective Taking (r = -0.30, p < .001) and Personal Distress (r = 0.32, p < .001) subscale scores of the IRI. | 100% |
| Konrath et al. (2018) | 270 | 33.5 (11.6) | SITES | TAS-20 | Higher empathy scores were associated with lower scores on alex [TAS-20 total: r(270) = -.25, p < .001; DIF: r(270) = -.19, p = .002; DDF: r(270) = -.22, p < .001; EOT: r(270) = -.25, p < .001]. | 75% |
| Law et al. (2004) | 418 | N/A | EES | TAS-20 | The following associations between the TAS-20 DIF, DDF, and EOT subscale scores and the EES total score were detected: r = -0.09, r = -0.04, r = -0.40 (p-values are not available), respectively. | 75% |
| Lee et al. (2020) | 200 | 23.1 (2.7) | IRI | TAS-20 | A significant association between alexithymia total score and the cognitive empathy domain (Perspective Taking and Fantasy subscales) of the IRI was found (r = -0.22, p < .01). Cognitive empathy was also significantly correlated with the DDF (r = -0.19, p < .01) and EOT (r = -0.24, p < .01) subscales of the TAS-20. | 88% |
| Li et al. (2023) | 142 | 21.7 (2.3) | QCAE | TAS-20 | A significant association was found between the alex total score and the QCAE Cognitive empathy subscale score (r = -0.44, p < .001), whereas no significant correlation was detected between the former and the QCAE Affective empathy subscale (r = 0.18, p = NS). | 88% |
| Lockwood et al. (2013) | 110 | 21.9 (3.7) | Self-assessment manikin faces task | TAS-20 | A significant and negative association was found between the alex total score and the performance on the empathy task (r = -0.25, p < .05). | 75% |
| Lyvers et al. (2017) | 102 | 22.2 (N/A) | IRI | TAS-20 |
Significant associations were found between alex total score and the Perspective Taking (r = -0.40, p < .001), Empathic Concern (r = -0.38, p < .001), and Personal Distress (r = 0.31, p < .01) subscale scores of the IRI. Significant correlations also emerged between the TAS-20 DIF and DDF subscales and the IRI Personal Distress (r = 0.27, p < .01; r = 0.25, p < .05), Perspective Taking (r = -0.23, p < .05; r = -0.36, p < .001), and Empathic Concern (r = -0.22, p < .05; r = -0.34, p < .001) subscale scores. The TAS-20 EOT subscale was significantly correlated with all the IRI subscales: Perspective Taking (r = -0.40, p < .001), Fantasy (r = -0.32, p < .01), Empathic Concern (r = -0.37, p < .001), and Personal Distress (r = 0.21, p < .05). |
88% |
| Lyvers et al. (2018) | 161 | 22.6 (7.2) | IRI | TAS-20 | Significant associations were found between the TAS-20 DIF, DDF, and EOT subscales and the Personal Distress subscale of the IRI (r = 0.31, p < .001; r = 0.20, p < .05; r = 0.22, p < .01, respectively). In addition, significant correlations were detected between the EOT subscale score and the Perspective Taking (r = -0.30, p < .001), Fantasy (r = -0.29, p < .001), and Empathic Concern (r = -0.36, p < .001) subscale scores. | 88% |
| Lyvers et al. (2020a) | 205 | N/A | IRI | TAS-20 | No significant association was found between alex and empathy total scores (r = -0.03, p = NS). | 100% |
| Lyvers et al. (2020b) | 253 | 21.6 (3.4) | TEQ | TAS-20 | A significant and negative correlation was detected between alex and empathy total scores (r = -0.40, p < .01). | 88% |
| MacDonald & Price (2017) | 616 | 19.2 (1.4) | QCAE | TAS-20 | A significant and negative association was found between the TAS-20 total score and the QCAE Cognitive empathy subscale score (r = -0.31, p < .001), whereas no significant correlation was detected between the former and the QCAE Affective empathy subscale (r = -0.01, p = NS). | 100% |
| Martínez-Velázquez et al. (2017) | 49 (19 non-alex; 14 affective alex; 19 cognitive alex) |
Non-alex:22.4 (2.7) Affective alex: 22.1 (1.8) Cognitive alex: 21.0 (1.6) |
IRI | TAS-20 BVAQ | Significant differences between the affective, cognitive, and non- alex groups were found on the cognitive sub-score (Perspective Taking) of the IRI [F(2,46) = 8.39, p < .001, η2 = 0.27], particularly between cognitive and affective alex groups (p <.001). The affective sub-score (Fantasy, Empathic Concern, and Personal Distress) of the IRI [F(2,46) = 4.99, p < .011, η2 = 0.18] characterized the affective alex group: non-alex and cognitive alex groups reported greater scores than affective alex sample (p = .036). | 80% |
| Martínez-Velázquez et al. (2020) | 60 (31 women; 29 men) | Women: 20.9 (1.8) Men: 21.1 (2.4) | IRI | TAS-20 | The low-empathy group reported significantly higher alex total scores than the high-empathy group [t(58) = -4.94, p ≤ .001, d = -1.27]. Additionally, a negative correlation was observed between the IRI and TAS-20 total scores [r(58) = -0.58, p ≤ .001]. | 60% |
| Martingano et al. (2022) | 1253 | 27.6 (N/A) | IRI | TAS-20 |
Alex total score was significantly associated with the Perspective Taking (r = -0.25, p < .01), Empathic Concern (r = -0.29, p < .01), and Personal Distress (r = 0.35, p < .01) subscale scores of the IRI. Significant correlations were also found between the DIF subscale and the Perspective Taking (r = -.26, p < .01), Empathic Concern (r = -0.14, p < .01), Fantasy (r = -0.14, p < .01), and Personal Distress (r = 0.37, p < .01) subscales. The DDF facet was significantly related to the Perspective Taking (r = -.14, p < .01) and Personal Distress (r = 0.30, p < .01) subscales. Significant correlations were found between the EOT subscale and the Perspective Taking (r = -.23, p < .01), Empathic Concern (r = -0.39, p < .01), Fantasy (r = -0.24, p < .01), and Personal Distress (r = 0.06, p < .05) subscales. |
88% |
| Mayer et al. (1990) | 139 | N/A | EES | TAS-26 | No significant association between alex and empathy total scores were found (r = 0.01, p = NS). | 50% |
| Mensi et al. (2023) | 58 families | N/A | EQ | TAS-20 | Alex total score was negatively associated with the EQ total score in both fathers (r = -0.41, p = .003) and mothers (r = -0.29, p = .039) of children with autism spectrum disorders. | 63% |
| Morice-Ramat et al. (2018) | 137 | 26.5 (1.3) | JSPE | TAS-20 | A negative correlation between alex and empathy total scores was found [r(135) = -0.38, p < .001]. | 63% |
| Moriguchi et al. (2006) | 30 (14 non-alex;16 alex) |
Total: 20.4 (0.9) Non-alex: 20.8 (0.9) Alex: 20.2 (1.0) |
IRI | TAS-20 SIBIQ | Alex participants reported significantly lower scores on the Perspective Taking (14.6 ± 3.5 vs. 18.5 ± 4.7, t = 2.64, p = .014) and Empathic Concern (16.1 ± 5.0 vs. 20.2 ± 3.7, t = 2.59, p = .015) subscales, while they reported higher scores on the Personal Distress (15.6 ± 4.1 vs. 12.2 ± 3.8, t = -2.37, p = .025) subscale of the IRI compared to non-alex individuals. | 80% |
| Moriguchi et al. (2007) | 30 (14 non- alex;16 alex) |
Total: 20.4 (0.9) Non-alex: 20.8 (0.9) Alex: 20.2 (1.0) |
EES IRI |
TAS-20 SIBIQ | Participants high in alex reported significantly lower scores on the Perspective Taking (14.6 ± 3.4 vs. 18.5 ± 4.9, t = 2.61, p < .05) and Empathic Concern (16.1 ± 4.9 vs. 20.0 ± 3.7, t = 2.48, p < .05) subscales of the IRI and on the Warmth subscale of the EES (49.2 ± 7.9 vs. 58.0 ± 3.2, t = 3.93, p < .05) compared to non-alex individuals. Conversely, alex participants reported higher scores, compared to non-alex ones, on the Personal Distress (15.8 ± 4.1 vs. 12.5 ± 3.7, t = -2.31, p < .05) subscale of the IRI. | 80% |
| Nam et al. (2020) | 200 (129 non- alex;71 alex) |
Non-alex: 23.3 (2.6) Alex: 22.7 (2.7) |
EQ IRI |
TAS-20 |
Participants high in alex reported significantly different scores on the Perspective Taking (17.93 ± 3.75 vs. 19.13 ± 4.11, t = 4.16, p = .043, η2 = .02) and Personal distress (16.21 ± 4.80 vs. 12.56 ± 5.54, t = 21.84, p <.001, η2 = .10) subscales of the IRI. Also, alex participants showed significantly lower scores on the Perception and expression of emotion (34.90 ± 5.06 vs. 38.56 ± 3.99, t = 31.64, p <.001, η2 = .14), Integrate emotion to facilitate thought (35.15 ± 5.22 vs. 36.87 ± 4.71, t = 5.61, p = .019, η2 = .03), Use of emotions (33.86 ± 4.32 vs. 35.26 ± 3.84, t = 5.53, p = .020, η2 = .03), and Regulation of emotions (31.46 ± 5.47 vs. 34.26 ± 5.21, t = 12.74, p <.001, η2 = .06) subscales of the EQ compared to non-alex individuals. Significant associations between the TAS-20 subscale and both the IRI and EQ subscale scores were found (all correlation results are not shown for space reasons). |
80% |
| Patil & Silani (2014a) | 295 | 25.0 (N/A) | IRI | TAS-20 | Alex total score was associated with reduced scores on the Empathic Concern (odds ratio = 0.9704, 95% CI [0.95, 0.99]) and Perspective Taking (odds ratio = 0.9724, 95% CI [0.95, 0.99]), as well as increased scores on the Personal Distress (odds ratio = 1.0434, 95% CI [1.03,1.07]) subscales of the IRI. | 100% |
| Patil & Silani (2014b) | 331 | 24.1 (5.5) | IRI | TAS-20 | Alex total score was associated with higher likelihood of reporting both lower scores on the Perspective Taking (odds ratio = 0.982, 95% CI [0.96,1.00]) and Empathic Concern (odds ratio = 0.979, 95% CI [0.96,1.00]), as well as higher scores on the Personal Distress (odds ratio = 1.051, 95% CI [1.03,1.07]) subscales of the IRI. | 100% |
| Pellicano et al. (2020) | 34 | 23.4 (2.7) | IRI | TAS-20 | Significant associations were found only between the IRI Personal Distress subscale and the TAS-20 total (r = 0.70, p < .001), DIF (r = 0.55, p < .001), and DDF (r = 0.61, p < .001) scores. | 75% |
| Preti et al. (2011) | 256 | 24 (4.5) | EQ | TAS-20 | Alex total score was significantly associated with the EQ Cognitive Empathy (r = -0.20, p < .01) and Emotional Reactivity (r = -0.51, p < .001) subscale scores. | 50% |
| Riccio et al. (2020) | 391 | 20.4 (4.9) | BES | TAS-20 | A significant association between alexithymia total score and the BES Cognitive empathy subscale score (r = -0.39, p < .001) was found. | 63% |
| Saito et al. (2016) | 78 | 20.5 (1.4) | Affective response questionnaire | Galex | High alex individuals were able to provide significantly higher other-oriented affective responses (advanced affective empathy) when they had (vs. had not) been instructed to distinguish others from themselves (β = 0.36, p = .02). | 63% |
| Schimmenti et al. (2019) | 799 | 35.8 (11.0) | EQ | TAS-20 |
Alex total score was significantly associated with the EQ total (r = -0.36, p < .01) and subscale (Cognitive Empathy: r = -0.15, p < .01; Emotional Reactivity: r = -0.37, p < .001; Social Skills: r = -0.39, p < .01) scores. Significant associations were also detected between the TAS-20 DIF, DDF, and EOT subscales and the EQ total (r = -0.29, p < .01; r = -0.24, p < .01; r = -0.24, p < .01) and subscale (Cognitive Empathy: r = -0.12, p < .01; r = -0.08, p < .05; r = -0.11, p < .01. Emotional Reactivity: r = -0.26, p < .001; r = -0.24, p < .001; r = -0.27, p < .001. Social Skills: r = -0.33, p < .01; r = -0.30, p < .01; r = -0.20, p < .01) scores. |
88% |
| Senese et al. (2018) | 633 | 24.3 (5.9) | EQ | TAS-20 | Alex total score was significantly associated with the EQ total (r = -0.31, p < .001) and subscale (Cognitive Empathy: r = -0.13, p < .01; Emotional Reactivity: r = -0.16, p < .001; Social Skills: r = -0.36, p < .001) scores. | 88% |
| Shah (2019) | 306 | 34.0 (11.9) | QCAE | TAS-20 | Significant and negative associations were found between alex total score and the QCAE total (r = -0.39, p < .001) and subscale (Cognitive: r = -0.44, p < .001; Affective: r = -0.19, p < .01) scores. | 50% |
| Shalev & Uzefovsky (2020) | 671 | 24.5 (2.5) |
EQ IRI |
TAS-20 | Significant and negative associations were detected between alex total score and both the EQ (r = -0.49, p < .001) and IRI (r = -0.17, p < .001) total scores. | 100% |
| Sonnby-Borgström (2009) | 102 | 24 (N/A) | IRI | TAS-20 | Negative associations were found between alex total score and the following IRI subscale scores: Perspective Taking (r = -0.25, p < .05), Fantasy (r = -0.20, p < .05), and Empathic Concern (r = -0.20, p < .05). | 63% |
| Stinson et al. (2022) | 824 | N/A | QCAE | TAS-20 | Negative associations were detected between alex total score and the following empathy scores: QCAE-Cognitive Empathy (r = -0.38, p < .001) and QCAE-Responsiveness to Others (r = -0.15, p < .001). | 88% |
| Swart et al. (2009) | 34 (18 non-alex; 16 alex) |
Non-alex: 19.3 (1.0) Alex: 20.1 (1.7) |
EQ | BVAQ | Alex individuals reported lower empathy scores than non-alex ones [34.2 ± 14.5 vs. 45.7 ± 10.1, F(1,32) = 7.31, p < .01]. | 70% |
| Tremblay et al. (2021) |
Study 1: 59 Study 2: 56 |
Study 1: 25.7 (9.1) Study 2: 22.1 (3.5) |
IRI | TAS-20 | A non-significant association was found between alex and empathy scores (r = -0.15, p > .05) in the first sample of participants (aged between 18 and 60), while a significant correlation between these two constructs was detected (r = -0.37, p < .01) in the second sample of young adults only. | 63% |
| Vellante et al. (2013) | 200 | 24.1 (2.8) | EQ | TAS-20 | A negative association was detected between alex and empathy (r = -0.41 , p < .001). | 63% |
| Yang et al. (2020) | 820 | 20.0 (1.3) | IRI | TAS-20 | Positive edges (network analysis approach) were found only between the DIF subscale of the TAS-20 and the Personal Distress subscale of the IRI. | 50% |
| Yang (2022et ) al. | 114 | 20.2 (0.2) | QCAE | TAS-20 | Alex empathy total total score score was significantly (r = 0.30, p < associated .01). with | 75% |
| Zhang W. et al. (2023) | 100 | 20.5 (2.3) | Picture-based and text- based pain empathy task | TAS-20 | Alex was not significantly associated with empathy for others’ pain in either condition (p > .05). | 88% |
| Zhang Y. et al. (2022) | 888 | 21.1 (1.6) | IRI | TAS-20 | A significant association was found between alex total and empathy total scores (r = -0.34, p < .01). | 88% |
QA (JBI) = Quality Assessment (Joanna Briggs Institute); BES = Basic Empathy Scale; TAS‐20 = Toronto Alexithymia Scale; TAS- 20 DIF = Difficulty Identifying Feelings; TAS-20 DDF = Difficulty Describing Feelings; TAS-20 EOT = Externally Oriented Thinking; IRI = Interpersonal Reactivity Scale; OAS = Observer Alexithymia Scale; BVAQ = Bermond Vorst Alexithymia Scale; EES = Emotional Empathy Scale; QCAE = Questionnaire for Cognitive and Affective Empathy; QMEE = Questionnaire Measure of Emotional Empathy; EQ = Empathy Quotient; MDEES = Multidimensional Emotional Empathy Scale; TEQ = Toronto Empathy Questionnaire; SITES = Single Item Trait Empathy Scale; JSPE = Jefferson Scale of Physicians Empathy; Galex = Gotow Alexithymia Questionnaire.
Note. Age is expressed in years; NS = not significant.
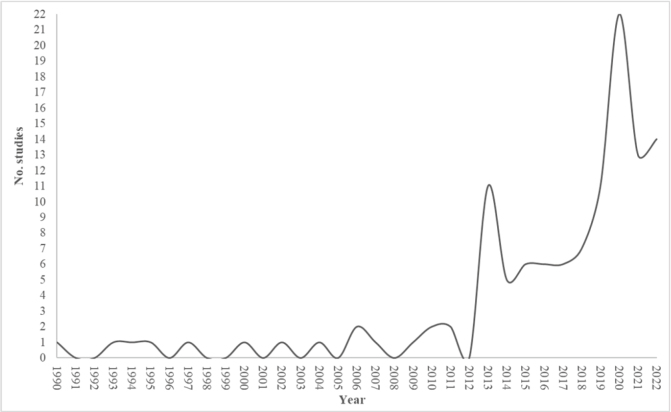
The included studies were cross-sectional studies (96) and case-control studies (21). The year of publication ranges from 1990 to 2022. Figure 2 shows the increase in studies investigating the relationship between alexithymia and social cognition over time.
Figure 2.
Temporal distribution of the studies included in this systematic review
In terms of study area, studies have been conducted across the world, with samples recruited in both Western and Eastern countries.
Taking all the studies together, the total number of participants included in the studies is 40,231, with sample sizes ranging from 21 (Bogdanov et al., 2013) to 7,584 (Gleichgerrcht & Decety, 2013).
The majority of studies assessed the presence of alexithymia using the 20 item Toronto Alexithymia Scale (TAS-20; Bagby et al., 2020; Taylor et al., 2003). The TAS-20 is a self-report instrument that provides a total score and three subscale scores: difficulty identifying feelings (DIF), difficulty describing feelings (DDF), and externally-oriented thinking (EOT). The TAS-20 cut-off scores are as follows: ≤ 51 no alexithymia, 52–60 borderline alexithymia, ≥ 61 alexithymia. Other self-report instruments used to assess alexithymia were the Bermond Vorst Alexithymia Questionnaire (BVAQ; Vorst & Bermond, 2001) and the Gotow Alexithymia Questionnaire (Galex; Gotow et al., 1999). Third-party assessment or structured interviews, in particular the Observer Alexithymia Scale (OAS; Haviland et al., 2001), the Structured Interview by the modified edition of Beth Israel hospital psychosomatic Questionnaire for alexithymia (SIBIQ; Arimura, 2002; Sriram et al., 1988) and the Toronto Structured Interview for Alexithymia (TSIA; Bagby et al., 2006) were also used to assess alexithymia (see supplementary material 1 for a more detailed description of these measurement tools).
Regarding social cognitive skills, heterogeneous measures were used to assess their components, with validated instruments or ad hoc constructed tasks used in the included studies. A brief description of these measures (with the corresponding references) can be found in supplementary material 1.
Based on the different components of social cognition on which the selected articles focused, we distinguished between studies that investigated: the relationship between ToM and alexithymia (n = 28); the association between emotion recognition and alexithymia (n = 44); and the relationship between empathy and alexithymia (n = 64). Some studies (n = 19) examined multiple aspects of social cognition. Therefore, the total sum of studies divided by each domain component is greater than the total number of studies included in this systematic review (n = 117). The article categories are presented separately below.
3.3. Theory of Mind and alexithymia
The results of the studies on the relationship between alexithymia and ToM are shown in table 1.
Most of the included studies, 22 out of 28, used the Reading the Mind in the Eyes Test (RMET) to assess ToM. Although some of these studies have used the RMET to assess the ability to recognize emotion, in the present systematic review the RMET was included in the ToM tasks, as intended by the original authors of the instrument (Baron-Cohen et al., 2001). Of these 22 studies, 13 found a negative correlation between the RMET and the total score of the TAS-20 (Al Aïn et al., 2013; Alaimo & Schimmenti, 2013; Demers & Koven, 2015; Gökçen et al., 2016; Lee et al., 2020; Lyvers et al., 2019a, 2019b; Martinez-Sanchez et al., 2017; Nam et al., 2020; Schimmenti, 2017; Schimmenti et al., 2019; Sunahara et al., 2022; Vellante et al., 2013). Five studies found only a negative correlation between the RMET and one or two of the three subscales of the TAS-20 (but not with the total score) (Benau et al., 2020; Herrero-Fernández et al., 2022; Lyvers et al., 2017, 2018; Pahnke et al., 2020). Finally, 4 of the 22 studies that used the RMET and the TAS-20 simultaneously found no correlation with the total score of the TAS-20 or with at least one of its subscales (Chinello et al., 2020; Di Tella et al., 2020; Eddy & Hansen, 2020; Zimmermann et al., 2021).
Interestingly, two studies have compared alexithymic and non-alexithymic participants on RMET performance and found opposite results (Martinez-Sanchez et al., 2017; Nam et al., 2020). Indeed, Martinez-Sanchez et al. (2017) showed a significant effect for alexithymia (TAS-20) group factor, with the “non-alexithymic” (TAS-20 total score ≤ 51) group scoring higher on the RMET compared to the “alexithymic” (TAS-20 total score ≥ 61) and “probable alexithymic” (TAS-20 total score ≥ 52-60 ≤) samples. In contrast, the study by Nam et al. (2020) found that the alexithymic group (TAS-20 total score ≥ 52) did not differ in RMET performance from the non-alexithymic group (TAS-20 total score ≤ 51). However, when Nam et al. (2020) divided their experimental group into males and females, they found that the RMET performance of the alexithymic males (but not that of the alexithymic females) was worse than that of the non-alexithymics. This finding is consistent with the results of two other studies comparing male and females. Namely, Benau et al. (2020) and Vellante et al. (2013) conducted specific analyses of RMET performance to investigate the differences between the sexes (Benau et al., 2020; Vellante et al., 2013). In particular, the study by Benau et al. (2020) found that the TAS-20 DIF subscale was significantly associated with RMET in males, but not in female (the authors excluded the TAS-EOT from the analyses, as this subscale is not normally associated with eating disorders). Similarly, Vellante et al. (2013) found no significant correlation between RMET and the TAS-20 total score in the female sample. However, a significant association was found in the male group.
Two studies used modified versions of the RMET to assess ToM abilities (Eddy & Hansen, 2020; Pahnke et al., 2020). The study by Eddy and Hansen (2020) found that alexithymia (TAS-20) was not a significant predictor of the Cat Eyes Test. In contrast, Pahnke et al. (2020) found a significant correlation only between the Reading the Mind in the Eyes of Children Test (RME-C-T) and the TAS-20 EOT subscale. They also found that non-alexithymic participants (TAS-20 total score > 51) performed better on the RME-C-T than alexithymic participants (TAS-20 total score ≤ 51).
The Animated Triangles Task was used in two studies to assess ToM (Lockwood et al., 2013; Moriguchi et al., 2006). Lockwood et al. (2013) showed that alexithymia (TAS-20) was not significantly associated with the ToM task. Conversely, the study by Moriguchi et al. (2006) found that the group with alexithymia (TAS-20 and SIBIQ) performed significantly worse on the intentionality and appropriateness dimensions of the Animated Triangles task than the group without alexithymia.
Three studies (Di Tella et al., 2020; Olkoniemi et al., 2019; Wastell & Taylor, 2002) used different instruments (the Strange Stories test, an ad hoc task - short paragraphs, and the False Belief Picture Sequencing Task, respectively) to assess ToM. In particular, Di Tella et al. (2020) found no significant association between the results of the Strange Stories test and the TAS-20 total score. Similarly, the study by Wastell and Taylor (2002) found no significant difference between alexithymic (TAS-20 total score > 68) and non-alexithymic (TAS-20 total score < 68) participants on the False Belief Picture Sequencing Task. In contrast, the study by Olkoniemi et al. (2019) found that high alexithymia levels (TAS-20) were associated with longer reading times for sarcastic paragraphs, compared to literal paragraphs.
Finally, three studies used different instruments to assess ToM (Gökçen et al., 2016; Jakobson & Pearson, 2021; Swart et al., 2009). Specifically, Gökçen et al. (2016) showed that alexithymia (TAS-20) was negatively associated with performance on the Movie for the Assessment of Social Cognition (MASC). Similarly, Jakobson and Pearson’s (2021) study, using videos from the RISC database, found that the TAS-20 DIF subscale was a significant predictor of unbiased recognition performance and median reaction time in the no-context condition only. Swart et al. (2009) also found significant differences between alexithymic (BVAQ total score ≥ 26) and non-alexithymic participants (BVAQ total score ≤ 17) only on the first-order emotion question of the Conflicting Beliefs and Emotions.
3.4. Emotion recognition and alexithymia
The results of the studies on the relationship between alexithymia and emotion recognition are shown in table 2.
Most of the included studies (33 out of 44) used static images of emotional facial expressions to assess participants’ ability to recognise emotions, and most showed a significant relationship between this ability and alexithymia (Bani et al., 2023; Brewer et al., 2015; Coll et al., 2019; Connolly et al., 2020a, 2020b; Hakala et al., 2015; Jessimer & Markham, 1997; Jongen et al., 2014; Kafetsios & Hess, 2019; Keightley et al., 2006; Koelkebeck et al., 2015; Lane et al., 1996, 2000; Lewis et al., 2016; Maiorana et al., 2022; Malykhin et al., 2023; Mann et al., 1994; Mayer et al., 1990; McCubbin et al., 2014; Montebarocci et al., 2011; Murphy et al., 2019; Nook et al., 2015; Parker et al., 1993; Parsons et al., 2021; Radoš et al., 2021; Rosenberg et al., 2020; Rus-Calafell et al., 2013; Senior et al., 2020).
Of these 33 studies, seven (Brewer et al., 2015; Jessimer & Markham, 1997; Mann et al., 1994; Mayer et al., 1990; Montebarocci et al., 2011; Rosenberg et al., 2020; Senior et al., 2020) investigated the recognition of facial emotions using stimuli from the Pictures of Facial Affect and showed consistent results. Specifically, individuals high in alexithymia were found to be less accurate in recognizing facial emotions (highest and lowest 10% of scores on the TAS-20 DIF and DDF; Jessimer & Markham, 1997; TAS-26 total score ≤ 49 for low alexithymia, 50-57 for middle alexithymia, ≥ 58 for high alexithymia, Mann et al., 1994; TAS-20 total score ≤ 51 for low alexithymia, TAS-20 total score ≥ 61 for high alexithymia, Montebarocci et al., 2011) and to have lower sensitivity to subtle changes in facial emotions (anger and disgust; TAS-20 total score > 60 for high alexithymia and TAS-20 < 60 for low alexithymia; Brewer et al., 2015) than individuals with low alexithymia. Similarly, the studies by Senior et al. (2020) and Rosenberg et al. (2020) found that accuracy in facial emotion recognition was negatively correlated with total alexithymia scores (TAS-20 and BVAQ). However, Rosenberg et al. (2020) found no significant association between the TSIA and emotion recognition scores. Finally, in the study by Mayer et al. (1990), alexithymia (TAS-26) was associated with a greater emotional range and a higher perception of emotions (generally negative) in response to emotional stimuli.
In three studies, stimuli from the Perception of Affect Task were used to assess the ability to recognise emotions. In all of these studies (Lane et al., 1996, 2000; McCubbin et al., 2014), a significant association was found between higher alexithymia scores (TAS-20) and lower accuracy rates on the emotion recognition task.
Two studies (Malykhin et al., 2023; Rus-Calafell et al., 2013) used the Penn Emotion Recognition task, which showed that total alexithymia score (TAS-20) correlated negatively with facial emotion recognition accuracy.
Two studies used facial expressions of emotions on images of infants (Parsons et al., 2021; Radoš et al., 2021). Specifically, using the City Infant Faces Database, Radoš et al. (2021) showed that higher total accuracy on the emotion recognition test was related to lower levels of alexithymia (TAS-20). Conversely, Parsons et al. (2021), using a previously developed task with infant photos, found no significant relationships between alexithymia (TAS-20) and ratings of arousal or valence across the infant emotion categories, while a positive correlation was found between alexithymia and overall accuracy on adult faces (KDEF-dyn).
In two studies conducted by the same research group (Connolly et al., 2020a, 2020b), a moderate negative correlation was found between supramodal emotion recognition ability (measured with faces - Facial Expressions of Emotion: Stimuli and Tests set; bodies - Point-light Bodily Emotion Recognition Ability; and voices - Montreal Affective Voices set) and alexithymia (TAS‐20).
Two studies used modified stimuli to assess participants’ ability to recognize facial emotion expressions under masked and unmasked conditions (Bani et al., 2023; Maiorana et al., 2022). Specifically, using facial emotion stimuli from the Diagnostic Analysis of Nonverbal Accuracy Faces 2‐Adult Faces, Bani et al. (2023) found that lower scores on the facial emotion recognition task in the unmasked condition were correlated with higher alexithymia (TAS-20), while no significant relationship was found in the masked condition. Similarly, the study by Maiorana et al. (2022) using modified images from the NimStim Face Stimulus Set showed that mean reaction times correlated positively with alexithymia scores (TAS-20) in the mouth-only condition, the unmasked condition, and the eyes-only condition. No significant correlations were found in the masked condition.
Another study (Nook et al., 2015) used images from the NimStim Face Stimulus Set and Interdisciplinary Affective Science Laboratory and found that alexithymia (TAS-20) correlated negatively with sensitivity in the face-face condition, but not with sensitivity in the face-word condition.
Nine studies used other tasks (one for each of these studies) to assess emotion recognition ability. In particular, the studies by Hakala et al. (2015), Kafetsios and Hess (2019), Keightley et al. (2006), Koelkebeck et al. (2015), Lewis et al. (2016), Murphy et al. (2019) used an ad hoc developed set of images, the Assessment of Contextualized Emotions-faces, Japanese and Caucasian Facial Expressions of Emotions and Neutral Faces, Noh mask test, Facial Expression of Emotion: Stimuli and Tests, an ad hoc emotion-identity recognition task, respectively. In all of these studies, a significant and negative relationship was found between alexithymia (TAS-20 and TAS-26) and performance on the emotion recognition task.
Three other of these nine studies compared alexithymic and non-alexithymic participants (TAS-20) on emotion recognition performance (Coll et al., 2019; Jongen et al., 2014; Parker et al., 1993). In particular, the study by Coll et al. (2019) found that alexithymic individuals (TAS-20 total score ≥ 61) showed no difference between oddball responses to upright and inverted faces (Radboud Faces Database) in the mixed-emotions paradigm, suggesting difficulties in processing the emotional content of faces. Finally, the studies by Jongen et al. (2014) and Parker et al. (1993) found that participants with high alexithymia (TAS-20 total score ≥ 51 and 66th percentile, respectively) performed significantly worse on the facial emotion recognition task (using the Facially Expressed Emotion Labelling test and photographs from Izard, respectively) than those with low alexithymia (TAS-20 total score ≤ 50 and 33rd percentile, respectively).
Of the 33 studies that used static images of emotional facial expressions, only six found no significant relationship between alexithymia scores and emotion recognition performance (Bègue et al., 2019; Hsing et al., 2013; Martingano et al., 2022; Ridout et al., 2021; Sharpe et al., 2016; Sunahara et al., 2022).
Notably, the study by Bègue et al. (2019), which used an ad hoc constructed paradigm in which participants were asked to identify static facial expressions as happy or angry and indicate how confident they were in their responses, found no significant correlation between alexithymia (TAS‐20) and the metacognitive ability index. Similarly, in their study, Hsing et al. (2013) found no significant differences between high and low alexithymia groups (identified as a median split on the total TAS-20 score) in either reaction time or accuracy in classifying emotional faces (angry and sad; Emostroop task). Finally, the studies by Martingano et al. (2022), Ridout et al. (2021), Sharpe et al. (2016) and Sunahara et al. (2022), which used static stimuli from different sets (Facial Action Coding System-verified University of California set of Emotion Expressions; Karolinska and Nimstim face sets; Binghamton University 3D Facial Expression database; Penn Emotion Recognition Test, respectively), all found that the total alexithymia score (TAS-20) was not significantly associated with emotion recognition accuracy.
Other studies (11 out of 44) used dynamic images of emotional facial expressions to assess participants’ ability to recognise emotions. Most of these studies (9) confirmed the above findings and showed that there was a significant relationship between the presence of alexithymia and the ability to correctly recognize the emotions of others.
In particular, two studies (Hovey et al., 2018; Laukka et al., 2021) using a newly developed test (Emotion Recognition Assessment in Multiple modalities) found a negative correlation between the alexithymia total score (TAS-20) and accuracy in emotion recognition, the study by Hovey et al. (2018) only in female participants. Similar results were obtained in the studies by Di Tella et al. (2020), Ridout et al. (2010), Rus-Calafell et al. (2013), Schlegel et al. (2019) and Sunahara et al. (2022), using the Montréal Pain and Affective Face Clips (MPAFC), The Awareness of Social Inference Test (TASIT), the Penn Emotion Recognition Test-96 Faces version, the Geneva Emotion Recognition Test, and a biological motion task, respectively.
In contrast, in the study by Halberstadt et al. (2021), which used both the Increasingly Clear Emotions task and a newly developed instrument (Perceptions of Children’s Emotions in Videos, Evolving and Dynamic task), only a positive association was found between performance on the adult emotion recognition task and the DIF subscale of the TAS-20. Conversely, no significant association was found between emotion recognition accuracy on the infant stimuli and alexithymia.
Finally, the study by Swart et al. (2009), using the micro expression training tool, found that alexithymic participants (BVAQ total score ≥ 26) performed significantly worse than non-alexithymic individuals (BVAQ total score ≤ 17) in recognizing brief emotional expressions.
Only two studies came to the opposite conclusion and showed no significant association between alexithymia and the accuracy of emotion recognition (Kyranides et al., 2022; Ridout et al., 2010). In particular, in contrast to Di Tella et al. (2020), the study by Kyranides et al. (2022), using the MPAFC, showed that individuals with high alexithymia (TAS-20) did not perform worse in facial emotion recognition (neither in accuracy nor in response times) than participants with low alexithymia. In contrast to Ridout et al. (2010), the study by Ridout et al. (2021), which used the TASIT, also found that the total alexithymia score (TAS-20) did not predict emotion recognition accuracy.
Finally, three studies used emotional audio stimuli to assess emotion recognition ability and came to quite diferent conclusions (Larwood et al., 2021; Swart et al., 2009; Tarufi et al., 2017). In particular, Larwood et al.’s (2021) study, which used 10 pieces of music representing five emotions (happiness, tenderness, anger, fear, and sadness), found that alexithymia (TAS-20) was not associated with the number of emotion words generated, but with valence-specific afect judgements of the music (participants high in alexithymia rated sad, angry, and fearful pieces as more neutral in terms of valence and arousal). Similarly, using the same musical emotion stimuli, the study by Tarufi et al. (2017) found that only the EOT subscale of the TAS-20 was a significant predictor of musical emotion recognition total score. Finally, the study by Swart et al. (2009), using the Afective Prosody task, found no difference between alexithymic (BVAQ total score ≥ 26) and non-alexithymic (BVAQ total score ≤ 17) participants (BVAQ) in accuracy in the prosody or semantic task.
3.5. Empathy and alexithymia
The results of the studies on the relationship between alexithymia and empathy are shown in table 3.
About half of the included studies (31 out of 64) used the Interpersonal Reactivity Index (IRI) to assess empathy. Most of these studies that used this self-report instrument showed significant associations between alexithymia and empathy (Di Tella et al., 2020; Dierckx et al., 2021; Diotaiuti et al., 2021; Eddy & Hansen, 2021; Gleichgerrcht & Decety, 2013; Grynberg et al., 2010; Himichi et al., 2021; Karras et al., 2022; Lee et al., 2020; Lyvers et al., 2017, 2020a; Martínez-Velázquez et al., 2020; Martingano et al., 2022; Patil & Silani, 2014a, 2014b; Pellicano et al., 2020; Shalev & Uzefovsky, 2020; Sonnby-Borgström, 2009; Y. Zhang et al., 2022). In particular, a positive association was found between the total score of alexithymia and the IRI dimension of Personal Distress, while a negative association was found between the IRI dimensions of Perspective-Taking and Empathic Concern and the total score of alexithymia. In most studies, no significant relationship was found between the total score of alexithymia and the Fantasy subscale of the IRI (e.g., Di Tella et al., 2020; Eddy & Hansen, 2021; Grynberg et al., 2010).
Other studies attempted to shed light on the relationship between alexithymia and empathy by grouping the subscales of the IRI into two main dimensions: affective empathy, which includes Empathic Concern and/or Personal Distress, and cognitive empathy, which includes Perspective Taking and/or Fantasy (Goerlich-Dobre et al., 2015; Hao et al., 2020; Lee et al., 2020). In particular, the study by Goerlich-Dobre et al. (2015) found that the cognitive dimension of alexithymia (BVAQ) was negatively associated with both cognitive and affective empathy, while the affective dimension of alexithymia was not significantly associated with empathy dimensions. Similarly, Lee et al. (2020) found a significant relationship only between the total score of alexithymia (TAS-20) and the cognitive empathy domain of the IRI. In contrast, the study by Hao et al. (2020) found a positive relationship between the total score of alexithymia (TAS-20) and both cognitive and affective empathy.
Other studies only investigated the relationships between the IRI dimensions and the TAS-20 subscale scores (Herrero-Fernández et al., 2022; Lyvers et al., 2018; Yang et al., 2020). In particular, the study by Herrero-Fernández et al. (2022) found that the DDF and EOT subscales of the TAS-20 were associated with all IRI subscales, while the DIF subscale was associated with the Perspective Taking and Personal Distress dimensions. Similarly, the study by Lyvers et al. (2018) found significant associations between the scores of the DIF and DDF subscales of the TAS-20 and the score of the Personal Distress subscale of the IRI. In addition, similar to the study by Herrero-Fernández et al. (2022), significant correlations were found between the EOT subscale score of the TAS-20 and all IRI dimensions. In contrast, in the study by Yang et al. (2020), only a positive association was found between the DIF factor of the TAS-20 and the Personal Distress subscale of the IRI.
A similar pattern of results was found in those studies that compared alexithymic and non-alexithymic participants and found that the former reported significantly lower empathy scores than the latter (Alkan Härtwig et al., 2020; Gleichgerrcht & Decety, 2013; Martínez-Velázquez et al., 2017, 2020; Moriguchi et al., 2006, 2007; Nam et al., 2020). For example, the study by Alkan Härtwig et al. (2020) found that participants with high alexithymia (TAS-20 total score > 56; BVAQ; OAS) had significantly lower scores on all subscales of the IRI, with the sole exception of the Personal Distress dimension, on which alexithymic individuals scored higher than non-alexithymic individuals (TAS-20 total score < 40). Similarly, the study by Nam et al. (2020) found significant differences between alexithymic (TAS-20 total score ≥ 52) and non-alexithymic (TAS-20 total score ≤ 51) participants on the IRI Perspective Taking and Personal Distress subscales. The study by Gleichgerrcht and Decety (2013) also found significant diferences between physicians with alexithymia (TAS-20 total score ≥ 61), borderline (TAS-20 total score 52-60) and without alexithymia (TAS-20 total score ≤ 51) on the Empathic Concern and Perspective Taking dimensions of the IRI. Yet the study by Martínez-Velázquez et al. (2017) found significant diferences between the affective (Affective-BVAQ score > 44 and Cognitive-BVAQ score ≤ 64), cognitive (Afective-BVAQ score ≤ 44 and Cognitive-BVAQ score > 64), and non-alexithymic (TAS-20 total score ≤ 44, Afective-BVAQ score ≤ 44, and Cognitive-BVAQ score ≤ 64) groups on the Perspective Taking subscale of the IRI, particularly between the cognitive and afective alexithymic groups. On the Empathic Concern, Personal Distress, and Fantasy dimensions of the IRI, the groups without alexithymia and with cognitive alexithymia had higher scores than the group with afective alexithymia. Partially diferent comparisons were made in the study by Martínez-Velázquez et al. (2020), which found that the low empathy group (one standard deviation below the mean) had significantly higher alexithymia scores (TAS-20 total) than the high empathy group (one standard deviation above the mean).
Although most studies found significant relationships between alexithymia and empathy as measured by the IRI, some studies came to opposite conclusions, finding non-significant relationships or mixed results (Christensen et al., 2018; Tremblay et al., 2021). In particular, the study by Christensen et al. (2018) found no significant association between the IRI and the TAS-20, while negative correlations were found between empathy and alexithymia as measured by the BVAQ. Similarly, the study by Tremblay et al. (2021) found a non-significant association between the total scores for alexithymia (TAS-20) and empathy (IRI) in the first sample of participants (aged 18 to 60 years), while a significant correlation between these two constructs was only found in the second sample of young adults.
The second most frequently used self-report questionnaire to assess empathy was the Empathy Quotient (EQ). Of the 10 studies that assessed empathy with the EQ, six found a significant and negative relationship between alexithymia (TAS-20) and empathy (Goerlich et al., 2017; Mensi et al., 2023; Schimmenti et al., 2019; Shalev & Uzefovsky, 2020; Senese et al., 2018; Vellante et al., 2013). One study only investigated the relationship between alexithymia and empathy dimensions (Preti et al., 2011) and found that the total score of alexithymia (TAS-20) was significantly associated with the results of the EQ subscales Cognitive Empathy and Emotional Reactivity. Finally, three studies compared groups of participants based on their alexithymia or empathy levels (Gossen et al., 2014; Nam et al., 2020; Swart et al., 2009). In particular, the study by Gossen et al. (2014) found higher scores for alexithymia (TAS-20) in the high empathy group (EQ total score ≥ 50) compared to the low empathy group (EQ total score < 30). The studies by Swart et al. (2009) and Nam et al. (2020) also found that alexithymic individuals (BVAQ total score ≥ 26 and TAS-20 total score ≥ 52, respectively) had lower empathy scores than non-alexithymic individuals (BVAQ total score ≤ 17 and TAS-20 total score ≤ 51, respectively).
Another frequently used self-report instrument in the studies included in the present systematic review (7 out of 64 studies) was the Questionnaire of Cognitive and Affective Empathy (QCAE). Using this instrument, the majority of included studies showed the presence of significant and negative associations between alexithymia (TAS-20) and empathy total scores (Colombarolli et al., 2019; Di Girolamo et al., 2019; Li et al., 2023; MacDonald & Price, 2017; Shah et al., 2019; Yang et al., 2022). With regard to the QCAE dimensions, alexithymia was found to be negatively associated with cognitive empathy, while no significant associations were found between the affective component of the QCAE and the total score of alexithymia (Di Girolamo et al., 2019; Li et al., 2023; MacDonald & Price, 2017; Stinson et al., 2022). Other results were obtained by Colombarolli et al. (2019) and Shah et al. (2019), who found a significant association between alexithymia (TAS-20) and the two empathy dimensions of the QCAE.
Another self-report instrument used in four studies included in this systematic review was the Basic Empathy Scale (BES). Three studies showed that the alexithymia total score (TAS-20) was negatively related to the Cognitive Empathy subscale of the BES, but not to the affective dimension (Al Aïn et al., 2013; Carré et al., 2013; Riccio et al., 2020). The fourth study found that the cognitive component of empathy was associated with all aspects of alexithymia, while affective empathy correlated only with the EOT subscale of the TAS-20 (Jonason & Krause, 2013).
Similar to the BES, the Emotional Empathy Scale (EES), a self-report instrument, was used in four studies (Christensen et al., 2018; Law et al., 2004; Mayer et al., 1990; Moriguchi et al., 2007). In particular, the study by Christensen et al. (2018) found that the EES was negatively associated with alexithymia as measured by the BVAQ, while no significant relationship was found between the EES and the TAS-20. The same pattern of results was found by Mayer et al. (1990), who found no significant relationship between the TAS-26 and the EES. When examining the different facets of alexithymia, Law et al. (2004) reported the presence of negative correlations (p-values for significance are not available) between the results of the DIF, DDF, and EOT subscales of the TAS-20 and the total score of the EES. Finally, the study by Moriguchi et al. (2007) found that alexithymic participants (SIBIQ total score > 47) and participants without alexithymia (SIBIQ total score < 25) reported lower empathy scores compared to non-alexithymic participants.
Three studies used a self-report instrument, the Toronto Empathy Questionnaire, and showed a significant and negative association between alexithymia (TAS-20) and empathy total scores (Ignatova et al., 2022; Karras et al., 2022; Lyvers et al., 2020b).
Two studies used a self-report instrument, the Multi-Dimensional Emotional Empathy Scale to assess empathy. In one of these studies (Bogdanov et al., 2013), no significant relationship was found between the total scores of empathy and alexithymia (TAS-20), while in the other study (Kamel, 2013) a significant and negative relationship was found between the total score of empathy and the DDF and EOT subscales of the TAS-20.
Five studies used other self-report instruments (one specific to each of these studies) to assess empathy. Three studies found significant and negative correlations between the different empathy scales (Empathy Tendency Scale; Single Item Trait Empathy Scale; Jefferson Scale of Physicians Empathy) and alexithymia (TAS-20) (Aslan et al., 2021; Konrath et al., 2018; Morice-Ramat et al., 2018). In contrast, the study by Demers and Koven (2015), using the Questionnaire Measure of Emotional Empathy, found that empathy was only associated with the TAS-20 DIF and EOT subscales, but not with the total score. A different paradigm was used in the fifth study by Saito et al. (2016), who employed the Affective Response Questionnaire. The authors found that individuals with high levels of alexithymia (Galex) were able to make significantly more other-oriented affective responses (advanced affective empathy) when instructed (as opposed to not instructed) to discriminate others from themselves.
Finally, three studies used performance-based instruments to assess different aspects of empathy. In particular, two studies used the Self-Assessment Manikin Faces Task (SAM) to assess individual affective responses to emotional faces (Gökçen et al., 2016; Lockwood et al., 2013; W. Zhang et al., 2023). Of these two studies, one (Lockwood et al., 2013) found a significant and negative correlation between the alexithymia total score (TAS-20) and performance in the SAM, while the other (Gökçen et al., 2016) found no significant association between the TAS-20 total score and the SAM. The last study by W. Zhang et al. (2023), which used a picture-based and a text-based pain empathy task, found that alexithymic traits (TAS-20) were not significantly associated with empathy for others’ pain in either condition.
3.6. Quality assessment
The results of the quality assessment (JBI tool) for cross-sectional and case-control studies are presented in tables 1, 2 and 3, and in Supplementary material 2. The quality ratings ranged from 2 (25%) to 8 (100%) for cross-sectional studies (n = 96; M = 5.83, SD = 1.39; median = 6) and from 5 (50%) to 9 (90%) for case-control studies (n = 21; M = 7.33, SD = 1.11; median = 8).
4. Discussion
The main aim of this review was to systematically summarise the available studies that have investigated the association between alexithymia and each of the social cognition skills (i.e., ToM, emotion recognition, and empathy) in the general adult non-clinical population.
With regard to alexithymia and ToM, the present systematic review yielded contradictory results, similar to the review by Pisani et al. (2021). In fact, most of the included studies that used the RMET to assess ToM came to opposite conclusions. While about half of the studies showed negative associations between the presence of alexithymia (assessed by the TAS-20 total score) and performance on the RMET (e.g., Al Aïn et al., 2013; Demers & Koven, 2015), the other half found no significant relationship (e.g., Chinello et al., 2020; Di Tella et al., 2020) or only relationships with one or two TAS-20 subscales (e.g., Herrero-Fernández et al., 2022; Lyvers et al., 2017). Similarly contradictory results were found in those studies that used other instruments to assess ToM (e.g., Lockwood et al., 2013; Wastell & Taylor, 2002).
One possible explanation for these discrepancies could lie in methodological and individual differences. For example, the three studies that investigated the relationship between alexithymia and ToM in males and females separately showed that the relationship was only significant in the male participants (Benau et al., 2020; Nam et al., 2020; Vellante et al., 2013). Sex differences in ToM abilities were often not adequately accounted for in the studies reviewed here, but such differences do exist and may explain some of the inconsistencies observed in the literature. For example, in one of the largest studies of RMET to date, Greenberg et al. (2023) found a female advantage in RMET that persisted throughout life, from age 16 to age 70, with a peak in scores at age 20 and an inflection point at age 50. The authors suggest that these findings may have important implications for research addressing the possible role of hormones in the development of ToM in adolescence and the shallow decline in adulthood. As for the present review, these findings suggest that at least part of the conflicting results from the literature examined here may be due to the fact that too few studies have directly examined the role of sex differences in the relationship between ToM and alexithymia, and even fewer studies have considered the possible influence of hormone levels on ToM levels by comparing, for example, women in the follicular and luteal phases of their menstrual cycle.
Another point that may explain the relative heterogeneity of data in the literature on the relationship between ToM and alexithymia is the fact that the overwhelming majority of studies (22 out of 28, i.e. almost 80%) were conducted using the RMET, a task for which psychometric concerns have recently been raised. For example, Higgins et al. (2023) failed to identify an appropriate factor structure for the RMET, and Black (2019) applied item response theory to show that the RMET may be an appropriate instrument to detect ToM deficits in individuals with autism spectrum disorders but is not a good measure to distinguish individuals with normal to high ToM abilities. However, it is worth noting that these psychometric criticisms are in contrast to the fact that the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) lists the RMET as one of the two recommended tests for measuring individual differences in “understanding mental states” (NIMH, 2016).
Given the conflicting claims about the appropriateness or otherwise of using the RMET to measure ToM, we suggest that future work interested in analysing the relationship between alexithymia and ToM should use the RMET at least in conjunction with other ToM tasks, especially tasks that meet stringent criteria, such as those proposed by Quesque and Rossetti (2020), i.e., the success of the task must be attributable with certainty to mental state ascription and to the ability to distinguish between one's own mental states and the mental states of others. Tasks that seem to adequately fulfil these two criteria are the MASC (Dziobek et al., 2006) and the Strange Stories (Happé et al., 1999).
The studies that investigated the relationship between emotion recognition or empathy and alexithymia came to more consistent results.
Specifically, with regard to the relationship between alexithymia and emotion recognition, the majority of included studies that used static or dynamic images of emotional facial expressions showed a negative relationship between the ability to recognise others’ emotions and alexithymia, which is consistent with the findings of Grynberg et al. (2012). Only 8 studies (out of 44) found no significant relationship between alexithymia and performance on facial emotion recognition tasks (e.g., Bègue et al., 2019; Hsing et al., 2013; Kyranides et al., 2022). One possible explanation for these contrasting results could lie in the use of heterogeneous instruments to assess the ability to recognise emotions. Namely, while similar self-report instruments were used to assess alexithymia (e.g., TAS-20 or BVAQ), different instruments were used to assess emotion recognition, which may have led to some inconsistent results. The use of such different tasks (which in many cases were designed ad hoc by the authors) may also make it difficult to compare results between studies. To overcome this problem, future research should make greater use of standardised and ecological tasks (e.g., based on dynamic images of emotional facial expressions) that can reflect the characteristics of social interactions that individuals have in their interpersonal context.
Finally, regarding the relationship between alexithymia and empathy, the results of the selected studies showed that alexithymia appears to be negatively associated with empathy, regardless of which instrument was used to assess these two constructs.
Some differences emerged in the studies that investigated the specific relationship between alexithymia and the affective and cognitive components of empathy (e.g., Di Girolamo et al., 2019; Li et al., 2023). In most cases, significant correlations were only found between alexithymia and the cognitive dimension of empathy.
Although cognitive and affective empathy are part of the same construct, they involve different processes. Namely, affective empathy is about the ability to experience the emotions of another person, whereas cognitive empathy is about understanding the emotional experiences and feelings of others (Jolliffe & Farrington, 2006). It can be assumed that the ability to correctly identify emotions in oneself is closely related to the ability to recognise the emotional states of others (Li et al., 2023). This assumption is supported by the “shared-network hypothesis”: the same areas of the brain that are involved in our own experience of emotions are also active when we process and understand the same emotions in other people (Singer et al., 2009; Singer & Lamm, 2009). This network includes regions such as the anterior insula or the medial and anterior cingulate cortex, which also play an important role in the representation of one’s own emotional states (Singer & Lamm, 2009). Indeed, reduced activation in limbic areas (i.e., anterior insula, anterior cingulate cortex, amygdala) and the prefrontal cortex has been found in individuals with high levels of alexithymia (Kano & Fukudo, 2013). However, further studies are needed to confirm this pattern of results and to better understand the specific relationship between alexithymia and the various components of empathy.
Only six studies (out of 64) came to opposite conclusions by finding non-significant relationships or mixed results (e.g., Christensen et al., 2018; Tremblay et al., 2021). This could be due to some methodological limitations of these studies. Namely, in most cases, the sample sizes were small and less commonly used instruments (e.g., the Self-Assessment Manikin Faces Task or the Picture-based and text-based pain empathy task) were used to assess empathy. For these measurement instruments, there seems to be limited (Self-Assessment Manikin Faces Task; Bradley & Lang, 1994) or no evidence (Picture-based and text-based pain empathy task) for their psychometric properties, making these instruments potentially less valid and reliable. In addition, studies with small sample sizes may not be powerful enough to detect small effects or to compensate for confounding variables (especially with non-normally distributed data). Therefore, future studies should consider these methodological issues when planning research.
As the results of the quality assessment show, the studies included in this review are heterogeneous in terms of the quality of the methodology used and the description of the procedures reported. This is particularly evident in studies that investigated the relationship between alexithymia and both emotion recognition and ToM (with minimum scores of 25% and 37.5%, respectively). Conversely, studies that have investigated the relationship between alexithymia and empathy show a higher methodological quality with a minimum score of 50%. The poor quality of the studies makes their results scarcely robust and generalisable and impairs their replicability.
The present systematic review also has some limitations that need to be considered. First, most of the included studies chose a cross-sectional design; longitudinal studies would allow a better understanding of the interaction between the current target variables. Second, most of the studies analysed only one component of social cognition related to alexithymia. Therefore, the present results cannot be generalised to the domain of social cognition as a whole. Finally, the large heterogeneity of the measures used by the included studies (e.g., for the assessment of emotion recognition) may be the cause of the conflicting results highlighted in this systematic review.
Despite these limitations, to our knowledge, the present systematic review is the first contribution to summarise the available evidence on the relationship between alexithymia and the different components of social cognition, while providing clarity on the constructs included in this domain.
Overall, the current findings appear to support the existence of significant relationships between alexithymia and altered social cognition abilities. The results appear to be more consistent for emotion recognition and empathy, while the evidence for ToM is more contrasting.
Therefore, future research is needed to corroborate the present findings and further elucidate the complex relationship between these processes. In particular, subsequent studies should make greater use of standardised instruments to assess social cognitive abilities, especially emotion recognition, adopt common definitions of these constructs, and apply a rigorous methodology that they report in detail to improve their replicability and quality. Indeed, the complexity of these concepts sometimes leads to a mixed use of instruments. For example, in the studies included in the present systematic review, the RMET was used interchangeably to assess both ToM processes (e.g., Schimmenti, 2017; Sunahara et al., 2022) and emotion recognition (e.g., Benau et al., 2020; Chinello et al., 2020). In addition, some authors have changed the definition of RMET over time, initially characterizing this task as a measure of emotion recognition (e.g., Lyvers et al., 2018: “The RMET is a unidimensional measure that uses 36 black-and white-photographs of male and female eyes to assess facial emotion recognition via eye gaze”) and then as a measure of ToM (e.g., Lyvers et al., 2019b: “The RMET-R is a theory of mind measure that assesses emotion recognition”). Similarly, there appears to be confusion about how the presence or absence of alexithymia should be determined, as many different cut-off scores were used in the studies reviewed here.
The difficulties often associated with the study of complex and interrelated psychological constructs are well known. However, the application of a more rigorous and accurate methodology could help to overcome some of the limitations currently identified in the literature. In addition, further research (including systematic reviews and meta-analyses) should be conducted to further investigate the neural basis of the social cognition processes associated with alexithymia. Indeed, the present evidence seems to show that alexithymic individuals exhibit reduced activation in brain areas involved in social cognitive abilities (Kano & Fukudo, 2013). Similarly, subsequent studies should investigate the role that other psychological variables (e.g., individual differences in attachment style) may play in the relationship between alexithymia and social cognitive abilities.
An in-depth investigation of the relationship between alexithymia and social cognitive abilities could help researchers and clinicians gain a more comprehensive understanding of the mechanisms underlying the ability to understand one’s own emotions as well as the emotions and mental states of others. One dimension that has been little explored in the clinical literature in this context is the relationship between alexithymia, social isolation, and psychological distress. Although high levels of alexithymia have been identified in various clinical and psychosocial disorders, little is known about the impact that the altered social cognition abilities described here have on the ability or, in the specific case of individuals with alexithymia, the inability to establish and maintain qualitatively satisfying social relationships. Given that good quality of social relationships is known to be directly related to good quality of life (e.g., Marazziti et al., 2024; Umberson & Montez, 2010), while social isolation and loneliness are associated with numerous clinical outcomes (e.g., Buecker et al., 2024; Ghiggia et al., 2024), it can be hypothesized that at least part of the clinical difficulties faced by alexithymic individuals are due to the difficulties these individuals have in managing the complex intersubjective dynamics that characterize social life. If this were the case, improving the subjectively perceived quality of the interpersonal dimension would become an important area of clinical treatment and prevention. In this way, it would be possible to plan better psychological interventions that take into account individual differences in social and emotional competences.
References
- Adolphs, R. (2001). The neurobiology of social cognition. Current opinion in neurobiology, 11(2), 231–239. 10.1016/s0959-4388(00)00202-6 [DOI] [PubMed] [Google Scholar]
- Al Aïn, S., Carré, A., Fantini-Hauwel, C., Baudouin, J. Y., & Besche-Richard, C. (2013). What is the emotional core of the multidimensional Machiavellian personality trait?. Frontiers in psychology, 4, 454. 10.3389/fpsyg.2013.00454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaimo, S. M., & Schimmenti, A. (2013). Metacognitive functions screening scale--30 items (mfss-30): un nuovo strumento per lo screening del funzionamento metacognitivo. Psichiatria & Psicoterapia, 32. [Google Scholar]
- Alkan Härtwig, E., Aust, S., Heekeren, H. R., & Heuser, I. (2020). No Words for Feelings? Not Only for My Own: Diminished Emotional Empathic Ability in Alexithymia. Frontiers in behavioral neuroscience, 14, 112. 10.3389/fnbeh.2020.00112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimura, T. (2002). Development of the Structured Interview by the modified edition of Beth Israel Hospital Psychosomatic Questionnaire (SIBIQ) in Japanese edition to evaluate alexithymia. Japanese Journal of Psychosomatic Medicine, 42, 259. [Google Scholar]
- Aslan, G., Bakan, A. B., & Yildiz, M. (2021). An investigation of the relationship between alexithymia and empathy tendency in university students receiving health education. Perspectives in psychiatric care, 57(2), 709–716. 10.1111/ppc.12602 [DOI] [PubMed] [Google Scholar]
- Bagby, R. M., Parker, J. D. A., & Taylor, G. J. (2020). Twenty-five years with the 20-item Toronto Alexithymia Scale. Journal of psychosomatic research, 131, 109940. Advance online publication. 10.1016/j.jpsychores.2020.109940 [DOI] [PubMed] [Google Scholar]
- Bagby, R. M., Taylor, G. J., Parker, J. D. A., & Dickens, S. E. (2006). Toronto Structured Interview for Alexithymia (TSIA) [Database record]. APA PsycTests. 10.1037/t65571-000 [DOI]
- Bani, M., Ardenghi, S., Rampoldi, G., Russo, S., & Strepparava, M. G. (2023). Impact of facemasks on psychotherapy: Clinician's confidence and emotion recognition. Journal of clinical psychology, 79(4), 1178–1191. 10.1002/jclp.23468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen, S., & Wheelwright, S. (2004). The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. Journal of autism and developmental disorders, 34(2), 163–175. 10.1023/b:jadd.0000022607.19833.00 [DOI] [PubMed] [Google Scholar]
- Baron-Cohen, S., Wheelwright, S., Hill, J., Raste, Y., & Plumb, I. (2001). The "Reading the Mind in the Eyes" Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. Journal of child psychology and psychiatry, and allied disciplines, 42(2), 241–251. [PubMed] [Google Scholar]
- Becchio, C., Adenzato, M., & Bara, B. G. (2006). How the brain understands intention: different neural circuits identify the componential features of motor and prior intentions. Consciousness and cognition, 15(1), 64–74. 10.1016/j.concog.2005.03.006 [DOI] [PubMed] [Google Scholar]
- Bègue, I., Vaessen, M., Hofmeister, J., Pereira, M., Schwartz, S., & Vuilleumier, P. (2019). Confidence of emotion expression recognition recruits brain regions outside the face perception network. Social cognitive and affective neuroscience, 14(1), 81–95. 10.1093/scan/nsy102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benau, E. M., Wiatrowski, R., & Timko, C. A. (2020). Difficulties in emotion regulation, alexithymia, and social phobia are associated with disordered eating in male and female undergraduate athletes. Frontiers in Psychology, 11, 1646. 10.3389/fpsyg.2020.01646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benfante, A., & Romeo, A. (2023). Alexithymia Among People Living with HIV: A Scoping Review. AIDS and behavior, 27(6), 1926–1941. 10.1007/s10461-022-03926-9 [DOI] [PubMed] [Google Scholar]
- Black, J. E. (2019). An IRT Analysis of the Reading the Mind in the Eyes Test. Journal of personality assessment, 101(4), 425–433. 10.1080/00223891.2018.1447946 [DOI] [PubMed] [Google Scholar]
- Bogdanov, V. B., Bogdanova, O. V., Gorlov, D. S., Gorgo, Y. P., Dirckx, J. J., Makarchuk, M. Y., Schoenen, J., & Critchley, H. (2013). Alexithymia and empathy predict changes in autonomic arousal during afective stimulation. Cognitive and behavioral neurology : oficial journal of the Society for Behavioral and Cognitive Neurology, 26(3), 121–132. 10.1097/WNN.0000000000000002 [DOI] [PubMed] [Google Scholar]
- Bradley, M. M., & Lang, P. J. (1994). Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. Journal of behavior therapy and experimental psychiatry, 25(1), 49–59. 10.1016/0005-7916(94)90063-9 [DOI] [PubMed] [Google Scholar]
- Brewer, R., Collins, F., Cook, R., & Bird, G. (2015). Atypical trait inferences from facial cues in alexithymia. Emotion, 15(5), 637–643. 10.1037/emo0000066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buecker, S., Horstmann, K. T., & Luhmann, M. (2024). Lonely today, lonely tomorrow: Temporal dynamics of loneliness in everyday life and its associations with psychopathological symptoms. Social Psychological and Personality Science, 15, 170-181. 10.1177/19485506231156061 [DOI] [Google Scholar]
- Carré, A., Stefaniak, N., D'Ambrosio, F., Bensalah, L., & Besche-Richard, C. (2013). The Basic Empathy Scale in adults (BES-A): factor structure of a revised form. Psychological assessment, 25(3), 679–691. 10.1037/a0032297 [DOI] [PubMed] [Google Scholar]
- Chinello, A., Redaelli, M., Parma, F., Faraci, G., Bertelli, S., & Zappa, L. E. (2020). The lack of a «Normative Male Alexithymia» in parents of daughters with anorexia nervosa: A pilot study by using the Reading the Mind in the Eyes test (RME) and the Toronto Alexithymia Scale TAS-20. Psicoterapia Cognitiva e Comportamentale, 26. 10.14605/PCC2612001 [DOI] [Google Scholar]
- Christensen, J. F., Gaigg, S. B., & Calvo-Merino, B. (2018). I can feel my heartbeat: Dancers have increased interoceptive accuracy. Psychophysiology, 55(4), 10.1111/psyp.13008. 10.1111/psyp.13008 [DOI] [PubMed] [Google Scholar]
- Coll, M. P., Murphy, J., Catmur, C., Bird, G., & Brewer, R. (2019). The importance of stimulus variability when studying face processing using fast periodic visual stimulation: A novel 'mixed-emotions' paradigm. Cortex, 117, 182–195. 10.1016/j.cortex.2019.03.006 [DOI] [PubMed] [Google Scholar]
- Colombarolli, M. S., Zuanazzi, A. C., Koich Miguel, F., & Giromini, L. (2019). Psychometric properties of the Toronto Alexithymia Scale (TAS-20) in Brazil. Transcultural psychiatry, 56(5), 992–1010. 10.1177/1363461519847312 [DOI] [PubMed] [Google Scholar]
- Connolly, H. L., Lefevre, C. E., Young, A. W., & Lewis, G. J. (2020a). Emotion recognition ability: Evidence for a supramodal factor and its links to social cognition. Cognition, 197, 104166. 10.1016/j.cognition.2019.104166 [DOI] [PubMed] [Google Scholar]
- Connolly, H. L., Young, A. W., & Lewis, G. J. (2020b). Consistent evidence of a link between Alexithymia and general intelligence. Cognition & emotion, 34(8), 1621–1631. 10.1080/02699931.2020.1789850 [DOI] [PubMed] [Google Scholar]
- Decety, J., & Lamm, C. (2006). Human empathy through the lens of social neuroscience. The Scientific World Journal, 6, 1146–1163. 10.1100/tsw.2006.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demers, L. A., & Koven, N. S. (2015). The Relation of Alexithymic Traits to Afective Theory of Mind. The American journal of psychology, 128(1), 31–42. 10.5406/amerjpsyc.128.1.0031 [DOI] [PubMed] [Google Scholar]
- Di Girolamo, M., Giromini, L., Winters, C. L., Serie, C. M. B., & de Ruiter, C. (2019). The Questionnaire of Cognitive and Affective Empathy: A Comparison Between Paper-and-Pencil Versus Online Formats in Italian Samples. Journal of personality assessment, 101(2), 159–170. 10.1080/00223891.2017.1389745 [DOI] [PubMed] [Google Scholar]
- Di Tella, M., Adenzato, M., Catmur, C., Miti, F., Castelli, L., & Ardito, R. B. (2020). The role of alexithymia in social cognition: Evidence from a non-clinical population. Journal of affective disorders, 273, 482–492. 10.1016/j.jad.2020.05.012 [DOI] [PubMed] [Google Scholar]
- Di Tella, M., Benfante, A., Airale, L., Castelli, L., & Milan, A. (2023a). Alexithymia and Hypertension: Does Personality Matter? A Systematic Review and Meta-analysis. Current cardiology reports, 25(7), 711–724. 10.1007/s11886-023-01894-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Tella, M., Clerico, M., & Castelli, L. (2023b). Associations between socioemotional alterations, quality of life, and social functioning in multiple sclerosis: A scoping review. Current Psychology, 42, 11143–11154. 10.1007/s12144-021-02387-y [DOI] [Google Scholar]
- Di Tella, M., Enrici, I., Castelli, L., Colonna, F., Fusaro, E., Ghiggia, A., Romeo, A., Tesio, V., & Adenzato, M. (2018). Alexithymia, not fibromyalgia, predicts the attribution of pain to anger-related facial expressions. Journal of affective disorders, 227, 272–279. 10.1016/j.jad.2017.10.048 [DOI] [PubMed] [Google Scholar]
- Dierckx, K., Van Hiel, A., Johnson, J. D., & Valcke, B. (2021). The relationship between emotional intelligence and prejudice among two European minorities. Personality and Individual Diferences, 174, 110660. 10.1016/j.paid.2021.110660 [DOI] [Google Scholar]
- Diotaiuti, P., Valente, G., Mancone, S., Grambone, A., & Chirico, A. (2021). Metric Goodness and Measurement Invariance of the Italian Brief Version of Interpersonal Reactivity Index: A Study With Young Adults. Frontiers in psychology, 12, 773363. 10.3389/fpsyg.2021.773363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziobek, I., Fleck, S., Kalbe, E., Rogers, K., Hassenstab, J., Brand, M., Kessler, J., Woike, J. K., Wolf, O. T., & Convit, A. (2006). Introducing MASC: a movie for the assessment of social cognition. Journal of autism and developmental disorders, 36(5), 623–636. 10.1007/s10803-006-0107-0 [DOI] [PubMed] [Google Scholar]
- Eddy, C. M., & Hansen, P. C. (2020). Predictors of performance on the Reading the Mind in the Eyes Test. PloS one, 15, e0235529. 10.1371/journal.pone.0235529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy, C. M., & Hansen, P. C. (2021). Alexithymia Is a Key Mediator of the Relationship Between Magical Thinking and Empathy. Frontiers in psychiatry, 12, 719961. 10.3389/fpsyt.2021.719961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enrici, I., Bara, B. G., & Adenzato, M. (2019). Theory of Mind, pragmatics, and the brain: Converging evidence for the role of intention processing as a core feature of human communication. Pragmatics & Cognition, 26(1), 5-38. 10.1075/pc.19010.enr [DOI] [Google Scholar]
- Farina, B., Imperatori, C., Adenzato, M., & Ardito, R. B. (2021). Perceived parental over-protection in non clinical young adults is associated with affective vulnerability: A cross-sectional study. Journal of affective disorders, 292, 496–499. 10.1016/j.jad.2021.05.071 [DOI] [PubMed] [Google Scholar]
- Frith, C., & Frith U. (2005). Theory of Mind. Current Biology, 15(17), R644-R646. 10.1016/j.cub.2005.08.041 [DOI] [PubMed] [Google Scholar]
- Ghiggia, A., Castelli, L., Adenzato, M., & Di Tella, M. (2024). Emotional competencies and psychological distress: Is loneliness a mediating factor? Scandinavian journal of psychology, 65(2), 359–368. 10.1111/sjop.12987 [DOI] [PubMed] [Google Scholar]
- Gleichgerrcht, E., & Decety, J. (2013). Empathy in clinical practice: how individual dispositions, gender, and experience moderate empathic concern, burnout, and emotional distress in physicians. PloS one, 8(4), e61526. 10.1371/journal.pone.0061526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerlich, K. S., Votinov, M., Lammertz, S. E., Winkler, L., Spreckelmeyer, K. N., Habel, U., Gründer, G., & Gossen, A. (2017). Effects of alexithymia and empathy on the neural processing of social and monetary rewards. Brain structure & function, 222(5), 2235–2250. 10.1007/s00429-016-1339-1 [DOI] [PubMed] [Google Scholar]
- Goerlich-Dobre, K. S., Lamm, C., Pripfl, J., Habel, U., & Votinov, M. (2015). The left amygdala: A shared substrate of alexithymia and empathy. NeuroImage, 122, 20–32. 10.1016/j.neuroimage.2015.08.014 [DOI] [PubMed] [Google Scholar]
- Gökçen, E., Frederickson, N., & Petrides, K. V. (2016). Theory of Mind and Executive Control Deficits in Typically Developing Adults and Adolescents with High Levels of Autism Traits. Journal of autism and developmental disorders, 46(6), 2072–2087. 10.1007/s10803-016-2735-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen, A., Groppe, S. E., Winkler, L., Kohls, G., Herrington, J., Schultz, R. T., Gründer, G., & Spreckelmeyer, K. N. (2014). Neural evidence for an association between social proficiency and sensitivity to social reward. Social cognitive and affective neuroscience, 9(5), 661–670. 10.1093/scan/nst033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotow, K., Kodama, M., & Sasaki, Y. (1999). Is alexithymia unidimensional? - Development of a 2-factor model alexithymia questionnaire. Tsukuba Psychological Research, 21, 163–172. [Google Scholar]
- Greenberg, D. M., Warrier, V., Abu-Akel, A., Allison, C., Gajos, K. Z., Reinecke, K., Rentfrow, P. J., Radecki, M. A., & Baron-Cohen, S. (2023). Sex and age differences in "theory of mind" across 57 countries using the English version of the "Reading the Mind in the Eyes" Test. Proceedings of the National Academy of Sciences of the United States of America, 120(1), e2022385119. 10.1073/pnas.2022385119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynberg, D., Chang, B., Corneille, O., Maurage, P., Vermeulen, N., Berthoz, S., & Luminet, O. (2012). Alexithymia and the processing of emotional facial expressions (EFEs): systematic review, unanswered questions and further perspectives. PloS one, 7(8), e42429. 10.1371/journal.pone.0042429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynberg, D., Luminet, O., Corneille, O., Grèzes, J., & Berthoz, S. (2010). Alexithymia in the interpersonal domain: A general deficit of empathy? Personality and Individual Diferences, 49(8), 845–850. 10.1016/j.paid.2010.07.013 [DOI] [Google Scholar]
- Hakala, J., Kätsyri, J., & Häkkinen, J. (2015). Stereoscopy Amplifies Emotions Elicited by Facial Expressions. i-Perception, 6(6), 2041669515615071. 10.1177/2041669515615071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberstadt, A. G., Cooke, A. N., Hagan, C. A., & Liu, X. (2021). PerCEIVED: Perceptions of children's emotions in videos, evolving and dynamic task. Emotion, 21(8), 1781–1795. 10.1037/emo0001019 [DOI] [PubMed] [Google Scholar]
- Hao, Z., Jin, L., Lyu, R., & Rabia Akram, H. (2020). Problematic mobile phone use and altruism in Chinese undergraduate students: The mediation effects of alexithymia and empathy. Children and Youth Services Review, 118, Article 105402. 10.1016/j.childyouth.2020.105402 [DOI] [Google Scholar]
- Happé, F., Brownell, H., & Winner, E. (1999). Acquired 'theory of mind' impairments following stroke. Cognition, 70(3), 211–240. 10.1016/s0010-0277(99)00005-0 [DOI] [PubMed] [Google Scholar]
- Haviland, M. G., Warren, W. L., Riggs, M. L., & Gallacher, M. (2001). Psychometric properties of the Observer Alexithymia Scale in a clinical sample. Journal of personality assessment, 77(1), 176–186. 10.1207/S15327752JPA7701_12 [DOI] [PubMed] [Google Scholar]
- Herrero-Fernández, D., Parada-Fernández, P., Rodríguez-Arcos, I., Amaya-Carrillo, L., González-Sáez, M. E., & Rubio-González, M. (2022). The mediation effect of mentalization in the relationship between attachment and aggression on the road. Transportation Research Part F: Traffic Psychology and Behaviour, 86, 345–355. 10.1016/j.trf.2022.03.009 [DOI] [Google Scholar]
- Higgins, W. C., Ross, R. M., Langdon, R., & Polito, V. (2023). The "Reading the Mind in the Eyes" Test Shows Poor Psychometric Properties in a Large, Demographically Representative U.S. Sample. Assessment, 30(6), 1777–1789. 10.1177/10731911221124342 [DOI] [PubMed] [Google Scholar]
- Himichi, T., Osanai, H., Goto, T., Fujita, H., Kawamura, Y., Smith, A., & Nomura, M. (2021). Exploring the Multidimensional Links Between Trait Mindfulness and Trait Empathy. Frontiers in psychiatry, 12, 498614. 10.3389/fpsyt.2021.498614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovey, D., Henningsson, S., Cortes, D. S., Bänziger, T., Zettergren, A., Melke, J., Fischer, H., Laukka, P., & Westberg, L. (2018). Emotion recognition associated with polymorphism in oxytocinergic pathway gene ARNT2. Social cognitive and afective neuroscience, 13(2), 173–181. 10.1093/scan/nsx141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsing, C. K., Mohr, A. H., Stansfield, R. B., & Preston, S. D. (2013). Alexithymia slows performance but preserves spontaneous semantic decoding of negative expressions in the Emostroop task. International Journal of Psychological Research, 6, 56–67. 10.21500/20112084.719 [DOI] [Google Scholar]
- Ignatova, D., Joseph, S., Priebe, L., Kamenova, I., & Vladimirova, R. (2022). Prevalence and correlation between childhood trauma and negative mental health outcomes in medical students. Bulgarian Journal of Psychiatry, 7, 21-32. [Google Scholar]
- Isoardo, G., Adenzato, M., Ciullo, S., Fontana, E., Stura, I., Migliaretti, G., Titolo, P., Matteoni, E., Calvo, A., Laino, F., Palumbo, F., & Ardito, R. B. (2024). Emotion Processing in Peripheral Neuropathic Pain: An Observational Study. Medical sciences (Basel, Switzerland), 12(2), 27. 10.3390/medsci12020027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobson, L. S., & Pearson, P. M. (2021). Alexithymic traits predict the speed of classifying non-literal statements using nonverbal cues. Cognition & emotion, 35(3), 569–575. 10.1080/02699931.2020.1715346 [DOI] [PubMed] [Google Scholar]
- Jessimer, M., & Markham, R. (1997). Alexithymia: a right hemisphere dysfunction specific to recognition of certain facial expressions?. Brain and cognition, 34(2), 246–258. 10.1006/brcg.1997.0900 [DOI] [PubMed] [Google Scholar]
- Jollife, D., & Farrington, D. P. (2006). Development and validation of the Basic Empathy Scale. Journal of adolescence, 29(4), 589–611. 10.1016/j.adolescence.2005.08.010 [DOI] [PubMed] [Google Scholar]
- Jonason, P. K., & Krause, L. (2013). The emotional deficits associated with the Dark Triad traits: Cognitive empathy, affective empathy, and alexithymia. Personality and Individual Differences, 55, 532–537. 10.1016/j.paid.2013.04.027 [DOI] [Google Scholar]
- Jongen, S., Axmacher, N., Kremers, N. A., Hoffmann, H., Limbrecht-Ecklundt, K., Traue, H. C., & Kessler, H. (2014). An investigation of facial emotion recognition impairments in alexithymia and its neural correlates. Behavioural brain research, 271, 129–139. 10.1016/j.bbr.2014.05.069 [DOI] [PubMed] [Google Scholar]
- Kafetsios, K., & Hess, U. (2019). Seeing mixed emotions: Alexithymia, emotion perception bias, and quality in dyadic interactions. Personality and Individual Differences, 137, 80–85. 10.1016/j.paid.2018.08.014 [DOI] [Google Scholar]
- Kamel, N. M. F. (2013). The relationship between emotional awareness and empathetic response among psychiatric hospital staff. Life Science Journal, 10, 1272-1284. [Google Scholar]
- Kano, M., & Fukudo, S. (2013). The alexithymic brain: the neural pathways linking alexithymia to physical disorders. BioPsychoSocial medicine, 7(1), 1. 10.1186/1751-0759-7-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karras, M., Delhomme, P., & Csillik, A. (2022). French drivers' behavior: Do psychological resources and vulnerabilities matter?. Journal of safety research, 80, 235–242. 10.1016/j.jsr.2021.12.005 [DOI] [PubMed] [Google Scholar]
- Keightley, M. L., Winocur, G., Burianova, H., Hongwanishkul, D., & Grady, C. L. (2006). Age effects on social cognition: faces tell a different story. Psychology and aging, 21(3), 558–572. 10.1037/0882-7974.21.3.558 [DOI] [PubMed] [Google Scholar]
- Koelkebeck, K., Kohl, W., Luettgenau, J., Triantafillou, S., Ohrmann, P., Satoh, S., & Minoshita, S. (2015). Benefits of using culturally unfamiliar stimuli in ambiguous emotion identification: A cross-cultural study. Psychiatry research, 228(1), 39–45. 10.1016/j.psychres.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Konrath, S., Meier, B. P., & Bushman, B. J. (2018). Development and validation of the Single Item Trait Empathy Scale (SITES). Journal of research in personality, 73, 111–122. 10.1016/j.jrp.2017.11.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause, I., Kern, S., Horntrich, A., & Ziemssen, T. (2013). Employment status in multiple sclerosis: impact of disease-specific and non-disease-specific factors. Multiple sclerosis, 19(13), 1792–1799. 10.1177/1352458513485655 [DOI] [PubMed] [Google Scholar]
- Kyranides, M. N., Christofides, D., & Çetin, M. (2022). Dificulties in facial emotion recognition: taking psychopathic and alexithymic traits into account. BMC psychology, 10(1), 239. 10.1186/s40359-022-00946-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, R. D., Sechrest, L., Riedel, R., Shapiro, D. E., & Kaszniak, A. W. (2000). Pervasive emotion recognition deficit common to alexithymia and the repressive coping style. Psychosomatic medicine, 62(4), 492–501. 10.1097/00006842-200007000-00007 [DOI] [PubMed] [Google Scholar]
- Lane, R. D., Sechrest, L., Reidel, R., Weldon, V., Kaszniak, A., & Schwartz, G. E. (1996). Impaired verbal and nonverbal emotion recognition in alexithymia. Psychosomatic medicine, 58(3), 203–210. 10.1097/00006842-199605000-00002 [DOI] [PubMed] [Google Scholar]
- Larwood, J. L., Vanman, E. J., & Dingle, G. A. (2021). Negative valence specific deficits in judgements of musical afective quality in alexithymia. Cognition & emotion, 35(3), 500–509. 10.1080/02699931.2019.1707514 [DOI] [PubMed] [Google Scholar]
- Laukka, P., Bänziger, T., Israelsson, A., Cortes, D. S., Tornberg, C., Scherer, K. R., & Fischer, H. (2021). Investigating individual differences in emotion recognition ability using the ERAM test. Acta psychologica, 220, 103422. 10.1016/j.actpsy.2021.103422 [DOI] [PubMed] [Google Scholar]
- Law, K. S., Wong, C. S., & Song, L. J. (2004). The Construct and Criterion Validity of Emotional Intelligence and Its Potential Utility for Management Studies. Journal of Applied Psychology, 89(3), 483–496. 10.1037/0021-9010.89.3.483 [DOI] [PubMed] [Google Scholar]
- Lee, H. R., Nam, G., & Hur, J. W. (2020). Development and validation of the Korean version of the Reading the Mind in the Eyes Test. PloS one, 15(8), e0238309. 10.1371/journal.pone.0238309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis, G. J., Lefevre, C. E., & Young, A. W. (2016). Functional architecture of visual emotion recognition ability: A latent variable approach. Journal of experimental psychology. General, 145(5), 589–602. 10.1037/xge0000160 [DOI] [PubMed] [Google Scholar]
- Li, W., Lou, W., Zhang, W., Tong, R. K., Jin, R., & Peng, W. (2023). Gyrus rectus asymmetry predicts trait alexithymia, cognitive empathy, and social function in neurotypical adults. Cerebral cortex, 33(5), 1941–1954. 10.1093/cercor/bhac184 [DOI] [PubMed] [Google Scholar]
- Lockwood, P. L., Bird, G., Bridge, M., & Viding, E. (2013). Dissecting empathy: high levels of psychopathic and autistic traits are characterized by difficulties in different social information processing domains. Frontiers in human neuroscience, 7, 760. 10.3389/fnhum.2013.00760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyvers, M., Cotterell, S., & Thorberg, F. A. (2020a). “Music is my drug”: Alexithymia, empathy, and emotional responding to music. Psychology of Music, 48(5), 626–641. 10.1177/0305735618816166 [DOI] [Google Scholar]
- Lyvers, M., Kohlsdorf, S. M., Edwards, M. S., & Thorberg, F. A. (2017). Alexithymia and Mood: Recognition of Emotion in Self and Others. The American journal of psychology, 130(1), 83–92. 10.5406/amerjpsyc.130.1.0083 [DOI] [PubMed] [Google Scholar]
- Lyvers, M., Mayer, K., Needham, K., & Thorberg, F. A. (2019a). Parental bonding, adult attachment, and theory of mind: A developmental model of alexithymia and alcohol-related risk. Journal of clinical psychology, 75(7), 1288–1304. 10.1002/jclp.22772 [DOI] [PubMed] [Google Scholar]
- Lyvers, M., McCann, K., Coundouris, S., Edwards, M. S., & Thorberg, F. A. (2018). Alexithymia in relation to alcohol use, emotion recognition, and empathy: The role of externally oriented thinking. The American Journal of Psychology, 131(1), 41–51. 10.5406/amerjpsyc.131.1.0041 [DOI] [Google Scholar]
- Lyvers, M., Randhawa, A., & Thorberg, F. A. (2020b). Self-compassion in relation to alexithymia, empathy, and negative mood in young adults. Mindfulness, 11(7), 1655–1665. 10.1007/s12671-020-01379-6 [DOI] [Google Scholar]
- Lyvers, M., Scott, K., & Thorberg, F. A. (2019b). Social anxiety and alexithymia in relation to problematic drinking and theory of mind. The American Journal of Psychology, 132(3), 325–341. 10.5406/amerjpsyc.132.3.0325 [DOI] [Google Scholar]
- MacDonald, H. Z., & Price, J. L. (2017). Emotional understanding: Examining alexithymia as a mediator of the relationship between mindfulness and empathy. Mindfulness, 8(6), 1644–1652. 10.1007/s12671-017-0739-5 [DOI] [Google Scholar]
- Maiorana, N., Dini, M., Poletti, B., Tagini, S., Rita Reitano, M., Pravettoni, G., Priori, A., & Ferrucci, R. (2022). The Effect of Surgical Masks on the Featural and Configural Processing of Emotions. International journal of environmental research and public health, 19(4), 2420. 10.3390/ijerph19042420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malykhin, N., Pietrasik, W., Aghamohammadi-Sereshki, A., Ngan Hoang, K., Fujiwara, E., & Olsen, F. (2023). Emotional recognition across the adult lifespan: Effects of age, sex, cognitive empathy, alexithymia traits, and amygdala subnuclei volumes. Journal of neuroscience research, 101(3), 367–383. 10.1002/jnr.25152 [DOI] [PubMed] [Google Scholar]
- Mann, L. S., Wise, T. N., Trinidad, A., & Kohanski, R. (1994). Alexithymia, affect recognition, and the five-factor model of personality in normal subjects. Psychological reports, 74(2), 563–567. 10.2466/pr0.1994.74.2.563 [DOI] [PubMed] [Google Scholar]
- Marazziti, D., Fantasia, S., Palermo, S., Arone, A., Massa, L., Gambini, M., & Carmassi, C. (2024). Main Biological Models of Resilience. Clinical neuropsychiatry, 21(2), 115–134. 10.36131/cnfioritieditore20240201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Sanchez, F., Fernández-Abascal, E. G., & Sánchez-Pérez, N. (2017). Recognition of emotional facial expressions in alexithymia. Studia Psychologica, 59(3), 206–216. 10.21909/sp.2017.03.741 [DOI] [Google Scholar]
- Martínez-Velázquez, E. S., Ahuatzin González, A. L., Chamorro, Y., & Sequeira, H. (2020). The Influence of Empathy Trait and Gender on Empathic Responses. A Study With Dynamic Emotional Stimulus and Eye Movement Recordings. Frontiers in psychology, 11, 23. 10.3389/fpsyg.2020.00023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Velázquez, E. S., Honoré, J., de Zorzi, L., Ramos-Loyo, J., & Sequeira, H. (2017). Autonomic Reactivity to Arousing Stimuli with Social and Non-social Relevance in Alexithymia. Frontiers in psychology, 8, 361. 10.3389/fpsyg.2017.00361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martingano, A. J., Konrath, S., Zarins, S., & Okaomee, A. A. (2022). Empathy, narcissism, alexithymia, and social media use. Psychology of Popular Media, 11(4), 413–422. 10.1037/ppm0000419 [DOI] [Google Scholar]
- Mattila, A. K., Ahola, K., Honkonen, T., Salminen, J. K., Huhtala, H., & Joukamaa, M. (2007). Alexithymia and occupational burnout are strongly associated in working population. Journal of psychosomatic research, 62(6), 657–665. 10.1016/j.jpsychores.2007.01.002 [DOI] [PubMed] [Google Scholar]
- Mayer, J. D., DiPaolo, M., & Salovey, P. (1990). Perceiving affective content in ambiguous visual stimuli: a component of emotional intelligence. Journal of personality assessment, 54(3-4), 772–781. 10.1080/00223891.1990.9674037 [DOI] [PubMed] [Google Scholar]
- McCubbin, J. A., Loveless, J. P., Graham, J. G., Hall, G. A., Bart, R. M., Moore, D. D., Merritt, M. M., Lane, R. D., & Thayer, J. F. (2014). Emotional dampening in persons with elevated blood pressure: affect dysregulation and risk for hypertension. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine, 47(1), 111–119. 10.1007/s12160-013-9526-2 [DOI] [PubMed] [Google Scholar]
- Mensi, M. M., Gasparini, L., Chiappedi, M., Guerini, F. R., Orlandi, M., Rogantini, C., & Balottin, U. (2023). How parental levels of empathy and alexithymia influence their perception of child's behavior. Minerva pediatrics, 75(5), 719–726. 10.23736/S0026-4946.20.05609-1 [DOI] [PubMed] [Google Scholar]
- Miniati, M., Marazziti, D., & Palagini, L. (2022). Is Alexithymia the Link Between Anorexia and Autism Spectrum Disorders?. Clinical neuropsychiatry, 19(3), 137–149. 10.36131/cnfioritieditore20220302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miniati, M., Poidomani, C., Conversano, C., Orrù, G., Ciacchini, R., Marazziti, D., Perugi, G., Gemignani, A., & Palagini, L. (2023). Alexithymia and Interoceptive Confusion in Covid-19 Pandemic Distress. Clinical neuropsychiatry, 20(4), 264–270. 10.36131/cnfioritieditore20230405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montebarocci, O., Surcinelli, P., Rossi, N., & Baldaro, B. (2011). Alexithymia, verbal ability and emotion recognition. The Psychiatric quarterly, 82(3), 245–252. 10.1007/s11126-010-9166-7 [DOI] [PubMed] [Google Scholar]
- Morice-Ramat, A., Goronflot, L., & Guihard, G. (2018). Are alexithymia and empathy predicting factors of the resilience of medical residents in France?. International journal of medical education, 9, 122–128. 10.5116/ijme.5ac6.44ba [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi, Y., Decety, J., Ohnishi, T., Maeda, M., Mori, T., Nemoto, K., Matsuda, H., & Komaki, G. (2007). Empathy and judging other's pain: an fMRI study of alexithymia. Cerebral cortex, 17(9), 2223–2234. 10.1093/cercor/bhl130 [DOI] [PubMed] [Google Scholar]
- Moriguchi, Y., Ohnishi, T., Lane, R. D., Maeda, M., Mori, T., Nemoto, K., Matsuda, H., & Komaki, G. (2006). Impaired self-awareness and theory of mind: an fMRI study of mentalizing in alexithymia. NeuroImage, 32(3), 1472–1482. 10.1016/j.neuroimage.2006.04.186 [DOI] [PubMed] [Google Scholar]
- Munn, Z., Barker, T. H., Moola, S., Tufanaru, C., Stern, C., McArthur, A., Stephenson, M., & Aromataris, E. (2020). Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evidence Synthesis, 18, 2127–2133. 10.11124/JBISRIR-D-19-00099 [DOI] [PubMed] [Google Scholar]
- Murphy, J., Millgate, E., Geary, H., Catmur, C., & Bird, G. (2019). No effect of age on emotion recognition after accounting for cognitive factors and depression. Quarterly journal of experimental psychology (2006), 72(11), 2690–2704. 10.1177/1747021819859514 [DOI] [PubMed] [Google Scholar]
- Nam, G., Lee, H., Lee, J. H., & Hur, J. W. (2020). Disguised Emotion in Alexithymia: Subjective Difficulties in Emotion Processing and Increased Empathic Distress. Frontiers in Psychiatry, 11, 698. 10.3389/fpsyt.2020.00698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Mental Health. (2016). Research Domain Criteria. https://www.nimh.nih.gov/about/advisory-boards-and-groups/namhc/reports/behavioral-assessment-methods-for-rdoc-constructs (accessed 15 March 2024).
- Niedenthal, P. M., & Brauer, M. (2012). Social functionality of human emotion. Annual review of psychology, 63, 259–285. 10.1146/annurev.psych.121208.131605 [DOI] [PubMed] [Google Scholar]
- Nook, E. C., Lindquist, K. A., & Zaki, J. (2015). A new look at emotion perception: Concepts speed and shape facial emotion recognition. Emotion (Washington, D.C.), 15(5), 569–578. 10.1037/a0039166 [DOI] [PubMed] [Google Scholar]
- Olkoniemi, H., Strömberg, V., & Kaakinen, J. K. (2019). The ability to recognise emotions predicts the time-course of sarcasm processing: Evidence from eye movements. Quarterly journal of experimental psychology (2006), 72(5), 1212–1223. 10.1177/1747021818807864 [DOI] [PubMed] [Google Scholar]
- Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaf, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., Moher . . ., D. (2021a). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ, 372, n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., Shamseer, L., Tetzlaff, J. M., Akl, E. A., Brennan, S. E., Chou, R., Glanville, J., Grimshaw, J. M., Hróbjartsson, A., Lalu, M. M., Li, T., Loder, E. W., Mayo-Wilson, E., McDonald, S., . . . McKenzie, J. E. (2021b). PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ, 372, n160. 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahnke, R., Mau-Moeller, A., Hamm, A. O., & Lischke, A. (2020). Reading the Mind in the Eyes of Children Test (RME-C-T): Development and Validation of a Complex Emotion Recognition Test. Frontiers in psychiatry, 11, 376. 10.3389/fpsyt.2020.00376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker, J. D., Taylor, G. J., & Bagby, R. M. (1993). Alexithymia and the recognition of facial expressions of emotion. Psychotherapy and psychosomatics, 59(3-4), 197–202. 10.1159/000288664 [DOI] [PubMed] [Google Scholar]
- Parsons, C. E., Nummenmaa, L., Sinerva, E., Korja, R., Kajanoja, J., Young, K. S., Karlsson, H., & Karlsson, L. (2021). Investigating the effects of perinatal status and gender on adults' responses to infant and adult facial emotion. Emotion (Washington, D.C.), 21(2), 337–349. 10.1037/emo0000698 [DOI] [PubMed] [Google Scholar]
- Patil, I., & Silani, G. (2014a). Reduced empathic concern leads to utilitarian moral judgments in trait alexithymia. Frontiers in psychology, 5, 501. 10.3389/fpsyg.2014.00501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil, I., & Silani, G. (2014b). Alexithymia increases moral acceptability of accidental harms. Journal of Cognitive Psychology, 26(5), 597–614. 10.1080/20445911.2014.929137 [DOI] [Google Scholar]
- Pellicano, G. R., Pierro, L., Altavilla, D., Massaro, G., Marcellino, E., Sambucini, D., Pescegoffi, A., Capone, M. A., & Lai, C. (2020). Lie for me: how empathy, alexithymia and emotional intelligence influence the ability to conform facial expression to a prosocial untrue verbal message. Rassegna di Psicologia, 37, 47-58. 10.13133/1974-4854/16723 [DOI] [Google Scholar]
- Pichon, S., de Gelder, B., & Grèzes, J. (2009). Two different faces of threat. Comparing the neural systems for recognizing fear and anger in dynamic body expressions. NeuroImage, 47(4), 1873–1883. 10.1016/j.neuroimage.2009.03.084 [DOI] [PubMed] [Google Scholar]
- Pisani, S., Murphy, J., Conway, J., Millgate, E., Catmur, C., & Bird, G. (2021). The relationship between alexithymia and theory of mind: A systematic review. Neuroscience and biobehavioral reviews, 131, 497–524. 10.1016/j.neubiorev.2021.09.036 [DOI] [PubMed] [Google Scholar]
- Premack, D., & Woodruff, G. (1978). Does the chimpanzee have a theory of mind? Behavioral and Brain Sciences, 1(4), 515–526. 10.1017/S0140525X00076512 [DOI] [Google Scholar]
- Preti, A., Vellante, M., Baron-Cohen, S., Zucca, G., Petretto, D. R., & Masala, C. (2011). The Empathy Quotient: a cross-cultural comparison of the Italian version. Cognitive neuropsychiatry, 16(1), 50–70. 10.1080/13546801003790982 [DOI] [PubMed] [Google Scholar]
- Quesque, F., & Rossetti, Y. (2020). What Do Theory-of-Mind Tasks Actually Measure? Theory and Practice. Perspectives on psychological science, 15(2), 384–396. 10.1177/1745691619896607 [DOI] [PubMed] [Google Scholar]
- Radoš, S. N., Matijaš, M., Brekalo, M., Webb, R., & Ayers, S. (2021). Validation of the city infant faces database in student and parent samples. Psihologijske Teme, 30(3), 591-614. 10.31820/pt.30.3.10 [DOI] [Google Scholar]
- Riccio, A., Kapp, S. K., Daou, N., Shane, J., & Gillespie-Lynch, K. (2020). What are replicable aspects of the broader autism phenotype among college students? Collabra: Psychology, 6 (1), 6. 10.1525/collabra.271 [DOI] [Google Scholar]
- Ridout, N., Smith, J., & Hawkins, H. (2021). The influence of alexithymia on memory for emotional faces and realistic social interactions. Cognition & emotion, 35(3), 540–558. 10.1080/02699931.2020.1747991 [DOI] [PubMed] [Google Scholar]
- Ridout, N., Thom, C., & Wallis, D. J. (2010). Emotion recognition and alexithymia in females with non-clinical disordered eating. Eating behaviors, 11(1), 1–5. 10.1016/j.eatbeh.2009.07.008 [DOI] [PubMed] [Google Scholar]
- Rosenberg, N., Ihme, K., Lichev, V., Sacher, J., Rufer, M., Grabe, H. J., Kugel, H., Pampel, A., Lepsien, J., Kersting, A., Villringer, A., & Suslow, T. (2020). Alexithymia and automatic processing of facial emotions: behavioral and neural findings. BMC neuroscience, 21(1), 23. 10.1186/s12868-020-00572-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rus-Calafell, M., Gutiérrez-Maldonado, J., & Frerich, N. (2013). Schizotypy, alexithymia and affect as predictors of facial emotion recognition capability using static and dynamic images. Anuario de Psicología, 43(1), 7–21. [Google Scholar]
- Saito, N., Yokoyama, T., & Ohira, H. (2016). Self-Other Distinction Enhanced Empathic Responses in Individuals with Alexithymia. Scientific reports, 6, 35059. 10.1038/srep35059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen, J. K., Saarijärvi, S., Aärelä, E., Toikka, T., & Kauhanen, J. (1999). Prevalence of alexithymia and its association with sociodemographic variables in the general population of Finland. Journal of psychosomatic research, 46(1), 75–82. 10.1016/s0022-3999(98)00053-1 [DOI] [PubMed] [Google Scholar]
- Schimmenti, A. (2017). The developmental roots of dissociation: A multiple mediation analysis. Psychoanalytic Psychology, 34(1), 96–105. 10.1037/pap0000084 [DOI] [Google Scholar]
- Schimmenti, A., Jonason, P. K., Passanisi, A., La Marca, L., Di Dio, N., & Gervasi, A. M. (2019). Exploring the dark side of personality: Emotional awareness, empathy, and the Dark Triad traits in an Italian sample. Current Psychology, 38, 100–109. 10.1007/s12144-017-9588-6 [DOI] [Google Scholar]
- Schlegel, K., Fontaine, J. R. J., & Scherer, K. R. (2019). The nomological network of emotion recognition ability: Evidence from the Geneva Emotion Recognition Test. European Journal of Psychological Assessment, 35(3), 352–363. 10.1027/1015-5759/a000396 [DOI] [Google Scholar]
- Senese, P. V., De Nicola, A., Passaro, A., & Ruggiero, G. (2018). The factorial structure of a 15-item version of the Italian Empathy Quotient Scale. European Journal of Psychological Assessment, 34(5), 344–351. 10.1027/1015-5759/a000348 [DOI] [Google Scholar]
- Senior, C., Hassel, S., Waheed, A., & Ridout, N. (2020). Naming emotions in motion: Alexithymic traits impact the perception of implied motion in facial displays of affect. Emotion, 20(2), 311–316. 10.1037/emo0000546 [DOI] [PubMed] [Google Scholar]
- Shah, P., Livingston, L. A., Callan, M. J., & Player, L. (2019). Trait Autism is a Better Predictor of Empathy than Alexithymia. Journal of autism and developmental disorders, 49(10), 3956–3964. 10.1007/s10803-019-04080-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev, I., & Uzefovsky, F. (2020). Empathic disequilibrium in two different measures of empathy predicts autism traits in neurotypical population. Molecular autism, 11(1), 59. 10.1186/s13229-020-00362-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe, E., Wallis, D. J., & Ridout, N. (2016). The influence of variations in eating disorder-related symptoms on processing of emotional faces in a non-clinical female sample: An eye-tracking study. Psychiatry research, 240, 321–327. 10.1016/j.psychres.2016.04.065 [DOI] [PubMed] [Google Scholar]
- Singer, T., Critchley, H. D., & Preuschoff, K. (2009). A common role of insula in feelings, empathy and uncertainty. Trends in cognitive sciences, 13(8), 334–340. 10.1016/j.tics.2009.05.001 [DOI] [PubMed] [Google Scholar]
- Singer, T., & Lamm, C. (2009). The social neuroscience of empathy. Annals of the New York Academy of Sciences, 1156, 81–96. 10.1111/j.1749-6632.2009.04418.x [DOI] [PubMed] [Google Scholar]
- Sonnby-Borgström, M. (2009). Alexithymia as related to facial imitation, mentalization, empathy, and internal working models-of-self and -others. Neuropsychoanalysis, 11(1), 111–128. 10.1080/15294145.2009.10773602 [DOI] [Google Scholar]
- Sriram, T. G., Pratap, L., & Shanmugham, V. (1988). Towards enhancing the utility of Beth Israel Hospital Psychosomatic Questionnaire. Psychotherapy and psychosomatics, 49(34), 205–211. 10.1159/000288085 [DOI] [PubMed] [Google Scholar]
- Stinson, J., Wolfe, R., & Spaulding, W. (2022). Social Connectedness in Schizotypy: The Role of Cognitive and Affective Empathy. Behavioral sciences (Basel, Switzerland), 12(8), 253. 10.3390/bs12080253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolier, R. M., & Freeman, J. B. (2016). The neuroscience of social vision. In Absher J.R., & Cloutier, J. (Eds.), Neuroimaging personality, social cognition, and character. Elsevier Academic Press; (pp. 139–157). 10.1016/B978-0-12-800935-2.00007-5 [DOI] [Google Scholar]
- Subic-Wrana, C., Beutel, M. E., Knebel, A., & Lane, R. D. (2010). Theory of mind and emotional awareness deficits in patients with somatoform disorders. Psychosomatic medicine, 72(4), 404–411. 10.1097/PSY.0b013e3181d35e83 [DOI] [PubMed] [Google Scholar]
- Sunahara, C. S., Rosenfield, D., Alvi, T., Wallmark, Z., Lee, J., Fulford, D., & Tabak, B. A. (2022). Revisiting the association between self-reported empathy and behavioral assessments of social cognition. Journal of experimental psychology. General, 151(12), 3304–3322. 10.1037/xge0001226 [DOI] [PubMed] [Google Scholar]
- Swart, M., Kortekaas, R., & Aleman, A. (2009). Dealing with feelings: characterization of trait alexithymia on emotion regulation strategies and cognitive-emotional processing. PloS one, 4(6), e5751. 10.1371/journal.pone.0005751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan, K. M., Daitch, A. L., Pinheiro-Chagas, P., Fox, K. C. R., Parvizi, J., & Lieberman, M. D. (2022). Electrocorticographic evidence of a common neurocognitive sequence for mentalizing about the self and others. Nature communications, 13(1), 1919. 10.1038/s41467-022-29510-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarufi, L., Allen, R., Downing, J., & Heaton, P. (2017). Individual differences in music-perceived emotions: The influence of externally oriented thinking. Music Perception, 34(3), 253-266. 10.1525/mp.2017.34.3.253 [DOI] [Google Scholar]
- Taylor, G. J., Bagby, R. M., & Parker, J. D. A. (1997). Disorders of afect regulation: Alexithymia in medical and psychiatric illness. Cambridge University Press. 10.1017/CBO9780511526831 [DOI] [Google Scholar]
- Taylor, G. J., Bagby, R. M., & Parker, J. D. (2003). The 20-Item Toronto Alexithymia Scale. IV. Reliability and factorial validity in diferent languages and cultures. Journal of psychosomatic research, 55(3), 277–283. 10.1016/s0022-3999(02)00601-3 [DOI] [PubMed] [Google Scholar]
- Tremblay, M. B., Marcoux, A., Turcotte, V., Woods, J., Rouleau, C., Grondin, F., & Jackson, P. L. (2021). I Can But I Shall Not Always Be Empathic. Psychological reports, 124(4), 1634–1672. 10.1177/0033294120945180 [DOI] [PubMed] [Google Scholar]
- Umberson, D., & Montez, J. K. (2010). Social relationships and health: A flashpoint for health policy. Journal of Health and Social Behavior, 51(1, Suppl), S54–S66. 10.1177/0022146510383501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veggi, S., Benfante, A., Di Tella, M., Roveta, F., Castelli, L., & Zara, G. (2024). Intimate Partner Violence and Alexithymia: Do Emotions Matter? A Systematic Review and Meta-Analysis. Trauma, violence & abuse, 25(3), 2521–2534. 10.1177/15248380231217045 [DOI] [PubMed] [Google Scholar]
- Vellante, M., Baron-Cohen, S., Melis, M., Marrone, M., Petretto, D. R., Masala, C., & Preti, A. (2013). The "Reading the Mind in the Eyes" test: systematic review of psychometric properties and a validation study in Italy. Cognitive neuropsychiatry, 18(4), 326–354. 10.1080/13546805.2012.721728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorst, H. C., & Bermond, B. (2001). Validity and reliability of the Bermond–Vorst alexithymia questionnaire. Personality and Individual Differences, 30, 413-434. 10.1016/S0191-8869(00)00033-7 [DOI] [Google Scholar]
- Wastell, C. A., & Taylor, A. J. (2002). Alexithymic mentalising: Theory of mind and social adaptation. Social Behavior and Personality, 30, 141–148. 10.2224/sbp.2002.30.2.141 [DOI] [Google Scholar]
- Yang, H. X., Shi, H. S., Ni, K., Wang, Y., Cheung, E. F. C., & Chan, R. C. K. (2020). Exploring the links between alexithymia, empathy and schizotypy in college students using network analysis. Cognitive neuropsychiatry, 25(4), 245–253. 10.1080/13546805.2020.1749039 [DOI] [PubMed] [Google Scholar]
- Yang, H. X., Zhou, H. Y., Wei, Z., Wan, G. B., Wang, Y., Wang, Y. Y., Yang, T. X., Lui, S. S. Y., & Chan, R. C. K. (2022). Multidimensional Interoception and Autistic Traits Across life Stages: Evidence From a Novel Eye-tracking Task. Journal of autism and developmental disorders, 52(6), 2644–2655. 10.1007/s10803-021-05155-w [DOI] [PubMed] [Google Scholar]
- Zhang, W., Zhuo, S., Li, X., & Peng, W. (2023). Autistic Traits and Empathy for Others' Pain Among the General Population: Test of the Mediating Effects of First-Hand Pain Sensitivity. Journal of autism and developmental disorders, 53(5), 2006–2020. 10.1007/s10803-022-05471-9 [DOI] [PubMed] [Google Scholar]
- Zhang, Y., Liang, T., Gan, X., Zheng, X., Li, H., & Zhang, J. (2022). Social Self-Efficacy and Internet Gaming Disorder Among Chinese Undergraduates: The Mediating Role of Alexithymia and the Moderating Role of Empathy. Frontiers in psychology, 13, 898554. 10.3389/fpsyg.2022.898554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann, K. M., Schmidt, K. D., Gronow, F., Sommer, J., Leweke, F., & Jansen, A. (2021). Seeing things differently: Gaze shapes neural signal during mentalizing according to emotional awareness. NeuroImage, 238, 118223. 10.1016/j.neuroimage.2021.118223 [DOI] [PubMed] [Google Scholar]