Abstract
One of the primary concerns in the field of green synthesis of nanoparticles (NPs) utilizing plant materials is the scarcity of high purity, challenges in achieving large-scale production, and limited global accessibility. Hygienic preparation and safe storage of plant extracts are also considerable challenges in this field. So, an investigation was started to overcome these limitations. Virgin coconut oil (VCO) in its purest form is available commercially all over the world. Also, it has high medicinal value with excellent biomedical applications. Very limited work has been reported on oils as bio reducers and stabilizers. In those reports, they used a few chemicals as mediators in the processes of synthesis and cleaning. So, to the best of our knowledge, for the first time, zinc oxide (ZnO) NPs were synthesized using VCO as a reducing and capping agent with zero chemical mediators. A comprehensive investigation of the structural, microstructural, and optical properties was reported. X-ray diffraction confirms the formation of VCO-ZnO NPs with an average crystallite size of 32.81 nm in a hexagonal structure. UV characteristics confirm quantum confinement through a well-defined SPR near 223 nm with fwhm of 67 nm and a direct band gap at 3.96 eV. FTIR reveals the capping of VCO through carboxylic functional groups, particularly the −COO– group of coconut oil at 1770 cm–1 with a shift of about 30 cm–1 compared to plain VCO. TEM confirms the polycrystalline nature with nearly spherical and 10–22 nm particle size. The zeta potential of −15.4 ± 5.0 mV signifies the stability and antiagglomeration properties. FESEM with EDS results confirms morphological excellence, the purity level of synthesized NPs (99.5%), and the prominent scalability of NPs (84.38% yield). Finally, as-synthesized VCO-ZnO NPs showed very good antioxidant (IC50 78.991, 51.464, and 4.677 μg/mL in DPPH, ABTS, and FRAP assays, respectively), anti-inflammatory (IC50 22.42 μg/mL in protein denaturation), antimicrobial (MIC 0.156 mg/mL for Pseudomonas and 0.316 mg/mL for S. aureus), and antidiabetic properties (IC50 88.45 and 147.67 μg/mL for α-amylase and α-glucosidase assays, respectively).
1. Introduction
The contribution of nanomaterials to society has been increasing day by day in many ways. Particularly, metal nanoparticles have enormous importance in various branches of industries and fields due to their size-dependent physical and chemical properties. The synthesis of these nanoparticles (NPs) in a physical method involves the use of costly equipment and high temperatures and pressures even though it gives stable and well-defined nanostructures. The fastest chemical approach may be suitable for nonmedical fields but not preferable for medical fields due to the chemical hazards. Also, at the end of the product’s lifetime, it may lead to environmental harmfulness.
To limit the use of expensive and toxic chemicals and to reduce undesirable side effects, the best method is a green method. It has its own significance in saving energy and nature from hazardous chemicals when compared to physical and chemical methods. In this green method, which is carried out in a bottom-up approach, first, it is necessary to have an organic/inorganic precursor source. Next green reducing agents (biomolecules) are needed for the conversion of ionic precursors to atoms. This conversion is called bioreduction. Lastly, nucleation is where the atoms are joined together, forming several stable NPs with a shield of bioactivity called capping.
Over the past decade, major papers have reported plant materials as biological extracts. Most of these involve the extraction of various parts of plants. However, these plants and their extracts are globally and commercially unavailable and require energy and time in their preparation. Also, it is difficult to get organic plant materials on a large scale.1,2 Plant products such as vegetable oils might overcome these types of limitations. Very few papers have reported the involvement of these oils in bioactive reduction. Bioactive components present in the plant extracts are mostly polyphenol compounds and flavonoids, whereas in vegetable oils bioactive components are fatty acids. Pure and unrefined virgin coconut oil (VCO) is commercially available in many countries around the world. Its fatty acid profile contains saturated (90%) and medium chain fatty acids (60%) (mainly lauric acid).3 This abundant lauric acid next to Mother’s Milk present in VCO has a few health benefits. The immediate burning capacity of triglycerides in VCO makes it not convert into cholesterol or fat. Hence, they protect against heart disease and stroke. It is a good antioxidant and is good as an anti-inflammatory, antidiabetic, etc. as per previous reports.4,5 It is the healthiest edible oil.6
Among metal oxides, zinc oxide (ZnO) is quite interesting due to its high medicinal value, high thermal stability, and low cost. ZnO has been used as a mineral supplement for zinc, an essential nutrient for the human body. It is effective against diabetes mellitus as reported.7 The acid buffering capacity of ZnO enables its use in water treatment.8 ZnO as a direct band gap material with high exciton binding energy has excellent optical properties and applications in the semiconductor industry.9 The high ionization energy for oxygen makes the Zn–O bond stronger. It has excellent electrical and catalytic activity, chemical stability, and environmental friendliness.10,11 With high biocompatibility and low toxicity, ZnO has many biological applications and can be used in antimicrobial treatment, sunscreen lotions, cosmetics, etc.12 Being a good catalytic material, ZnO has been used in many industrial products such as paints, rubber, coatings, and textiles.
Very limited research has been conducted on synthesizing NPs using oils as bioreducers and stabilizers. The reporters used acetone as a mediator in the treated oils (essential and edible oils) with precursor solutions and a few chemicals in the reduction of NPs and the cleaning process.13−17 Zamiri reported size-controlling and antiagglomeration capacity among the ablated silver NPs in the laser ablation method.6 However, this top-down green method has a limitation of high preparation cost in practical applications.18 So, to the best of our knowledge in a literature review, no work has been done on the synthesis of cost-effective metal oxides using a green method without using any chemical mediators.
So this novel fabrication of VCO-capped ZnO.(VCO-ZnO) NPs with its simple method, without any chemical mediators and with high purity, would make the nanoworld healthier in the medical field. According to WHO Global Health Estimates, ischemic heart disease is terrifying the world by occupying the top spot of global causes of death. Cancer occupies the second position as the leading disease. Antioxidants play an important role in preventing these degenerative diseases.19,20 Diabetes mellitus is also in the top 10 lists. Hence, the current study aimed to investigate the antioxidant, antidiabetic, and anti-inflammatory effects of VCO-ZnO NPs and compare them with standard medications such as ascorbic acid, metformin, and diclofenac sodium, respectively.21 Additionally, a comparative analysis was conducted with pure ZnO to assess these biomedical activities. The pure ZnO NPs were synthesized using a conventional chemical precipitation method. Furthermore, the study explored the antimicrobial properties of these NPs, which could be beneficial in various applications, including the textile industry, water treatment, and the treatment of bacterial and fungal infection.
2. Materials and Methods
2.1. Materials
99.99% pure zinc sulfate heptahydrate (ZnSO4·7H2O) was obtained from Sigma-Aldrich and virgin coconut oil from the nearby manufacturing company Konaseema Agro Products, A.P., India. Bovine serum albumin (BSA), trypsin, blood suspension, phosphate buffer, sodium acetate, tris HCl buffer, dimethyl sulfoxide (DMSO), 2,2-diphenyl-1-picrylhydraxyl (DPPH), methanol, 2,4,6-tripyridyl-S-triazine (TPTZ), ferric chloride (FeCl3), azino-bis(3-ethyl benzothiazoline-6-sulfonic acid) (ABTS), potassium persulfate, ascorbic acid, diclofenac sodium, Luria–Bertani broth (LB Broth), triphenyl tetrazolium chloride (TTC), dinitrosalicylic acid (DNSA), and para-nitrophenyl-α-d-glucopyranoside (PNDGP) were obtained from Himedia Laboratories. Staphylococcus aureus (S. aureus) and Pseudomonas bacterial and Candida fungal cultures were obtained from MTTC, Chandigarh, India.
2.2. Characterization
For confirming quantum confinement of VCO-ZnO NPs, UV–vis optical absorption spectra in the wavelength range of 200–800 nm were obtained using a Shimadzu UV-2600 instrument. For functional group analysis and hence the confirmation of VCO functional groups present in VCO-ZnO NPs, Fourier transform infrared spectra were obtained at room temperature using the FTIR instrument Bruker Alpha-II with a liquid-nitrogen-cooled HgCdTe detector at 4 cm–1, and the spectra were averaged over 500 scans in the mid-IR range (400–4000 cm–1). Both instruments are available at the Central Instrumentation Lab, GC(A), Rajahmundry, AP, India. To determine the phase purity and morphology of the VCO-ZnO NPs, XRD and TEM characterizations were performed at Sprint Testing Solutions, Mumbai, India. To study the size distribution and zeta potential, a DLS study report of the synthesized NPs was obtained from MNIT, Jaipur, India. To get the surface morphology, purity, and percent yield, FESEM with EDS characterizations were done at GITAM, AP, India.
2.3. Methods
2.3.1. Synthesis of VCO-ZnO NPs
10 g of ZnSO4·7H2O precursor was added to 100 mL of VCO directly and stirred thoroughly for 1 h at room temperature. With its rich amounts of saturated fats, unrefined VCO has a high boiling point of ∼220 °C and has a smoking point of 1700 °C. So the solution mixture was heated below the smoking point at ∼140 °C for 4 h in a hot air oven and allowed to cool to room temperature. A gel-like formation was obtained at the bottom of the solution. The gel was collected using filter paper and dried at 220 °C for 15 min or until the oil completely evaporated. This approach of synthesis is called the gel combustion route method.22 Finally, the sample was calcinated to 300 °C for 3 h, ground into a fine powder, and stored safely for further use.
2.3.2. In Vitro Antioxidant Activity
The synthesized pure ZnO and VCO-ZnO NPs (samples) were adequately assessed for in vitro antioxidant activity by three different methods, namely a free radical scavenging DPPH assay, a ferric reducing antioxidant power (FRAP) assay, and a cationic radical scavenging ABTS assay.23,24 All these activities were carried out in gradient concentrations of samples from 10 to 40 μg/mL.
In the DPPH method, methanol solutions of samples at selected concentrations were added to a 3 × 10–2 mM methanol solution of DPPH. DPPH with methanol was used as a control. In the FRAP method, the FRAP reagent was prepared using 0.01 M TPTZ in 0.04 M HCl, 0.02 M FeCl3 and 0.3 M acetate buffer (pH 3.5) in 1:1:10 (v/v/v) proportion. Then, selected concentrations of acetate solutions of samples in acetate buffer were added to the prepared FRAP reagent. In this assay the control was FRAP with acetate buffer. In the ABTS method, the first ABTS reagent was prepared using 7 mM ABTS and 2.45 mM potassium persulfate in a 1:1 ratio. The reagent was preincubated overnight in the dark and diluted in methanol. Then the selected concentrations of aqueous solutions of samples after 15 min of sonication were added to that reagent. Distilled water with ABTS reagent was used as a control.
All three method reaction mixtures including ascorbic acid as a standard were subjected to 30 min of incubation in the dark at ambient temperature and centrifuged at 5000 rpm for 5 min. UV absorbances of the mixture as well as of the control at 517, 593, and 734 nm for DPPH, FRAP, and ABTS assays, respectively, were noted. The inhibition percentage was calculated using a standard formula.25 Further interpretation of results was done by determining an important biological parameter known as efficient concentration 50 (EC50) or inhibitory concentration 50 (IC50) using a standard function four-parameter (A, B, C, D) logistic function (sigmoidal) in an online IC50 calculator.26 The higher the antioxidant activity, the lower the value of IC50.25
2.3.3. In Vitro Anti-inflammatory Activity
The anti-inflammatory activity of as-synthesized samples was also tested by three different in vitro methods, namely protein denaturation, proteinase denaturation, and heat-induced RBC hemolysis assays,.27 All these activities were carried out in gradient concentrations of samples from 10 to 40 μg/mL. Diclofenac sodium was used as a standard, and phosphate buffer solution was used as the control for all three experiments.
In the proteinase denaturation inhibition assay, the reagents 0.05 mg of trypsin and Tris HCl (pH 7.4) were added with selected concentrations of samples and standard along with control and incubated at 37 °C for 5 min. Then, 48 μg of casein hydrolysate was added to all the mixtures and again kept at 20 min incubation at the same temperature. Finally, after incubation, the mixtures were added to 70% perchloric acid and allowed to cool at room temperature. Later, they were centrifuged at 3000 rpm for 5 min.
In the protein denaturation inhibition assay, the reaction mixtures (phosphate buffer saline (pH 6.4), 1% BSA, and various concentrations of test samples) were subjected to 15 min of incubation at 37 °C and then 5 min of heat (70 °C) was further supplied. The solutions were cooled to room temperature. Then, they were centrifuged at 3000 rpm for 5 min.
In heat-induced hemolysis, heparinized chicken blood was centrifuged at 5000 rpm for 5 min; the supernatant was removed and washed thrice with an equal volume of physiological saline (0.9% NaCl). The obtained blood volume was resuspended in 10% PBS (7.4) (v/v). Then, the reaction mixtures (blood suspension, PBS 7.4, and various concentrations of sample NPs) were incubated at 54 °C for 20 min in a shaking water bath and centrifuged at 2500 rpm for 5 min. The supernatant mixtures’ UV absorbances at 210, 660, and 540 nm for protein denaturation, proteinase denaturation, and heat-induced RBC hemolysis assays, respectively, were noted. The percent inhibition in three assays was calculated by the standard formula. The Inhibitory concentration 50 (IC50) values were also determined as per the reference given in the previous section.
2.3.4. Antimicrobial Activity
The evaluation of antimicrobial activity was performed by an agar-based Kirby–Bauer disc diffusion method.28 The method is simpler and faster than the broth-based method.29 The prepared potato dextrose agar (PDA) medium an nutrient agar medium underwent sterilization at 121 °C and 15 lb pressure for 20 min by using an autoclave. The molten medium was placed in sterile Petri dishes and allowed to cool. The selected bacterial and fungal cultures were spread on the prepared solid medium. To test the significant antimicrobial activity of synthesized NPs against the selected Gram-positive (S. aureus) and Gram-negative (Pseudomonas) pathogenic bacteria and fungal culture (Candida), various concentrations were chosen based on minimum inhibitory concentration values. Sterilized Whatman filter paper discs of around 6 to 8 mm size were coated with sample solutions (sonicated for better mixing of sample in water) and placed on the culture. Bacterial culture plates were incubated at 37 °C for 24 h, and the fungal culture was incubated at 24 °C for 48 h.
2.3.5. In Vitro Antidiabetic Activity
To determine the activity against one of the growing challenging diseases, diabetes, the synthesized VCO-ZnO NP samples were tested at selected concentrations (20–100 μg/mL) in both α-amylase and α -glucosidase inhibition assays as per previous works.30,31 First, 0.02 and 0.1 M sodium phosphate buffers (SPBs) were prepared and their pH was adjusted to 6.9 by adding 6 mM NaCl. For the α-amylase (α-glucosidase) inhibition assay, 1 mL of sample was mixed in respective mounts of 0.02 M (0.1 M for α-glucosidase) SPB and 250 μL (150 μL for α-glucosidase) of 1U alpha-amylase (0.1 U α-glucosidase). The mixture was incubated for 20 min at 37 °C. After that 250 μL (50 μL for α-glucosidase) of 1% starch solution (2 mM para-nitrophenyl for α-glucosidase) in 0.02 M (0.1 M for α-glucosidase) SPB was added to the above mixture and again incubated for 15 min at 37 °C. The reaction was terminated by adding 1 mL of DNSA (50 μL of 0.1 M sodium carbonate for α-glucosidase). The α-amylase mixture was incubated for 10 min in a water bath at 100 °C. The same was done for selected concentrations, and the absorbance at 540 nm (405 nm) was noted. Metformin was used as a standard for a comparative study. The inhibition percentage of both assays was calculated using the standard formula.
3. Results and Discussion
3.1. UV–Vis Data Interpretation
Novel optical characteristics emerge when the size of particles becomes smaller than 100 nm. UV–visible absorption spectroscopy is a well-known, fast technique to study the optical properties of newly synthesized nanomaterials. In the present paper, quantum confinement, type, and value of band gap of as prepared VCO-ZnO NPs were reported as the UV–vis absorption data.
As the minimum UV region of transparency for water is 190 nm, a sonicated aqueous solution of VCO-ZnO NPs with a concentration of 0.1 mM was taken for the UV–vis absorption spectrum and was obtained as shown in (Figure 1). It shows a surface plasmon resonance (SPR) band at 220–260 nm with a peak position at about 223 nm. This intense well-defined SPR near 223 nm with a full width at half-maximum (fwhm) of about 80 nm shows the use of VCO in reducing the particle size distribution. An fwhm of less than 100 nm is normally considered as the signature of narrow particle distribution.13
Figure 1.
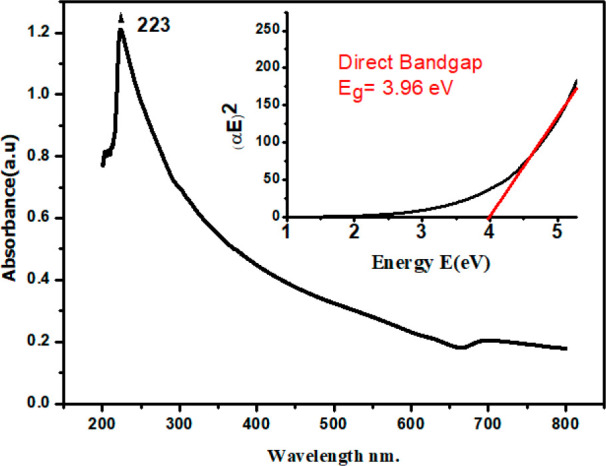
UV–vis spectrum of biosynthesized ZnO NPs using virgin coconut oil.
This excitonic absorption peak at about 223 nm for VCO-ZnO NPs lies much below the excitonic absorption peak at 373 nm for bulk ZnO.32 This blue shift in the absorption clearly indicates the quantum confinement of the VCO-ZnO NPs. In the quantum confinement range, as the particle size decreases, discrete energy levels play a role rather than continuous bands. Hence then the band gap of the particles increases. The value of this band gap energy can be accurately determined to explain the photophysical and chemical properties of semiconducting materials. As the prepared semiconducting material shows a negligible absorbance at lower energies, the Tauc plot method may be used to determine the band gap using the formula33
where α is the absorption coefficient, E is the photon energy, A is a constant relative to the material, and γ is either 2 for a direct transition or 1/2 for an indirect transition. Hence, the optical band gap for the absorption peak can be obtained by linear region extrapolation as shown in the inset of Figure 1. The band gap of VCO-ZnO is obtained at ∼3.96 eV. The shift is about 0.59 eV compared with bulk ZnO (Eg = 3.37 eV).34 No linear relation was found for n = 1/2, suggesting that as-synthesized VCO-ZnO nanostructures have a direct transition. The absence of the characteristic absorption of lauric acid (n→π* ≈ 280–290 nm) might also indicate the coordination complex formation of ligands.
3.2. XRD Analysis
The XRD pattern of VCO-ZnO NPs is shown in Figure 2. The well-defined, narrow, high-intensity peaks reveal the nanocrystalline phase formation. The reflected intensities against detector angle 2θ are observed at 31.9, 34.58, 36.38, 47.70, 56.75, and 63.01°, which are indexed as (100), (002), (101), (102), (110), (103), (200), (112), (201), and (202), respectively, in agreement with the Joint Committee on Powder Diffraction Standards (JCPDS) card number 00-036-1451. The calculated cell parameters (using the method of TJB Holland & SAT Redfern 1995 “Unit Cell” software) are 3.2496 and 5.2065 Å, which also coincide well with JCPDS data (Table 1). The percentage of contraction of calculated d-spacing using Bragg’s law from JCPDS d-spacing data was less than 0.5% (Table 2). This qualitative comparison with the standard crystallographic database confirms the formation of ZnO in a hexagonal crystal system with a wurtzite structure and P63mc space group.35 Phase purity was confirmed through the absence of additional peaks within the resolution limit of the diffractometer. Broadening of the XRD peak can be calculated by its full width at half-maximum (fwhm), a suitable XRD parameter for the determination of the average crystalline size. Using a nonlinear Gaussian curve fit, the average fwhm was obtained at 0.4°. Using the Debye–Scherrer formula (D = 0.9 λ/β cos θ, where θ is the diffraction angle, λ = 1.5406 Å, and β is fwhm), the VCO-ZnO crystallite size was determined to be around 32.8 nm.35
Figure 2.
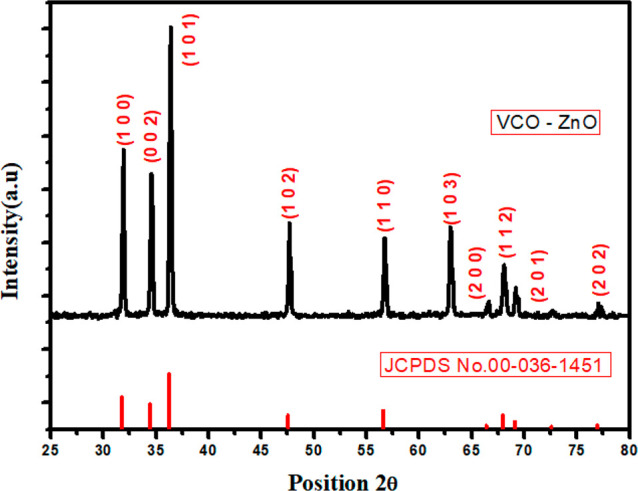
X-ray diffraction pattern of VCO-ZnO NPs and JCPDS reference pattern.
Table 1. Unit Cell Parameters of VCO-ZnO NPs.
| parameter | VCO-ZnO | JCPDS data |
|---|---|---|
| a = b (Å) | 3.2496 | 3.2498 |
| c (Å) | 5.2065 | 5.2066 |
| volume (106 pm3) | 47.61 | 47.62 |
Table 2. Percent Contraction of d-Spacing Values from Calculated and JCPDS Data.
| 2θ (deg) | d-spacing (Å) | JCPDS data (Å) | contraction (%) |
|---|---|---|---|
| 31.90 | 2.8034 | 2.8143 | 0.38 |
| 34.58 | 2.5914 | 2.6033 | 0.45 |
| 36.38 | 2.4674 | 2.4759 | 0.34 |
| 47.70 | 1.9048 | 1.9111 | 0.32 |
| 56.75 | 1.6209 | 1.6247 | 0.23 |
| 63.01 | 1.4739 | 1.4771 | 0.21 |
3.3. FTIR Data Interpretation
The mechanism of bio reduction of NPs in green synthesis is still incompletely determined. So, the present FTIR study tries to identify the contribution of functional groups present in VCO in the formation of ZnO NPs as well as the prevention of NP agglomeration. This qualitative chemical analysis reveals the presence of stretching and bending vibrations of Zn–O, C–O, −CH3, −CH2, C=O, and O–H(C–H) functional groups with absorption peaks at 520, 1130, 1480, 1640, 1770, and broad 2828–2980 cm–1, respectively. When compared with the FTIR spectrum of plain VCO, the sample spectrum almost matches it (Figure 3). A small shift in vibrational frequency and a change in intensity (Table 3) can be interpreted by the interrelation of ZnO NPs with the functional groups in VCO.
Figure 3.
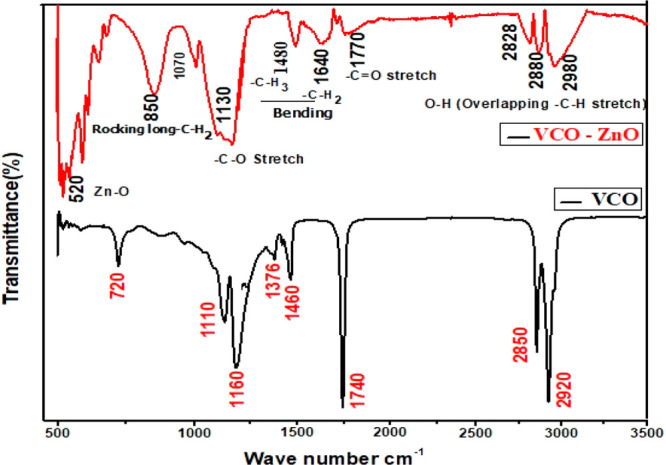
Fourier transform infrared spectra of VCO-ZnO and VCO.
Table 3. Functional Groups Corresponding to FTIR Peak Positions of VCO-ZnO and VCO.
| VCO (cm–1) | VCO-ZNO (cm–1) | shift | functional group/bond |
|---|---|---|---|
| 520, very intense | stretching vibrations of Zn–O bonds | ||
| 720 | 850 | 130 | rocking motion of long chain −CH2 group |
| 1160 | 1130, high intensity | 30 | stretching vibrations of –C–O |
| 1376, 1460 | 1480, 1640, low intensity | 104 | bending vibrations of –C–H3 and –C–H2 |
| 1740 | 1770, low intensity | 30 | stretching vibrations of –C=O |
| 2850, 2920 | 2828, 2880, 2980, medium intensity and broad | 20–60 | stretching vibration of –O–H in acids (usually overlaps –C–H stretching of –C–H3 and –C–H2) |
A very broad O–H stretch band is seen along with the C=O peak, almost certainly indicating carboxylic acid. As reported earlier, the medium-chain triacyl glycerides (TAGs) account for approximately half of all types of TAGs in coconut oil.36 There is a possibility of capping of TAG. However, TAG might decompose into medium chain fatty acids (MCFAs) and glycerol groups during the process.
The rich contributor to MCFAs is lauric acid (CH3–(CH2)10–C–OH), which makes up half of the fatty acids in coconut oil. So, it signifies the possibility of bonding of polar carboxylic groups (mostly lauric acid) with ZnO NPs and hence might be involved in the bioreduction of NPs. These metal carboxylates Zn(COO−) were confirmed through broad, intense absorption peaks at a vibrational frequency of 1130 cm–1.13 Lighter H– ions from −COO–H groups areexpected to share partially with heavy ZnO and hence form capped ZnO NPs (Figure 4).13 The broad absorption band at 2828–2980 cm–1 signifies the symmetric and asymmetric stretching of methyl and methylene groups. The rocking motion of four or more −CH2 groups is associated with the band at 865 cm–1 and is called a long chain band. This long nonpolar carbon chain could prevent the agglomeration of NPs through steric repulsion.
Figure 4.
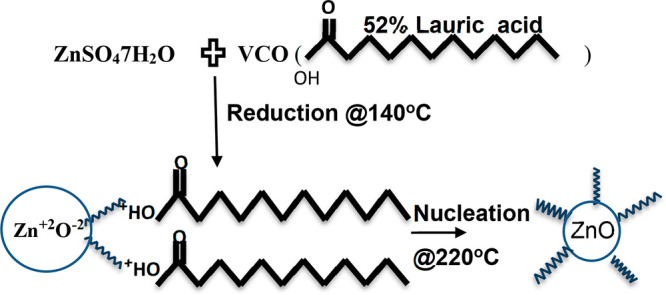
Possible reaction mechanism of the formation of VCO-ZnO NPs.
3.4. TEM and Selected Area Diffraction Analysis
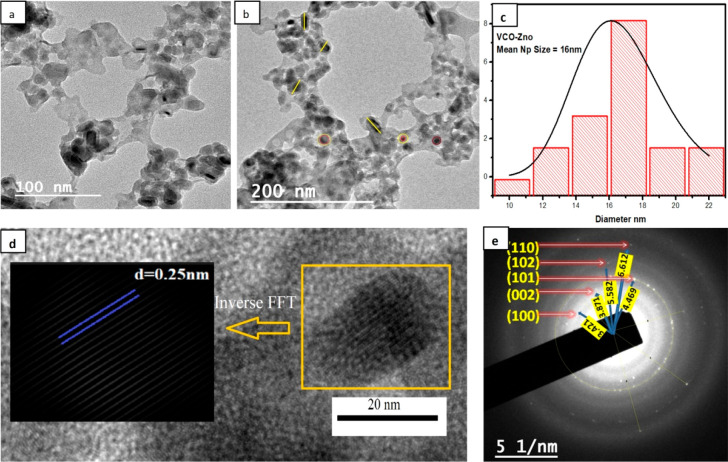
To understand the morphology of VCO-ZnO NPs, a nanosize characterization tool, a transmission electron microscope (TEM), was used to get the lattice fringe width and particle size. Figure 5a,b clearly shows the formation of NPs with sizes ranging from 10 to 22 nm and with spherical and rod-like structures. ImageJ software was used to find the mean particle size and was obtained as 16 nm. These TEM images also depict the capping of VCO on ZnO, which prevents agglomeration of NPs. The particle size distribution is shown in Figure 5c. Using inverse FFT analysis as shown in Figure 5d, the calculated lattice fringe spacing is ∼0.25 nm, which coincides well with the d-spacing value from XRD. The crystallinity of synthesized VCO-ZnO NPs is also confirmed by the SAED pattern as given in Figure 5e. The ring pattern shows the polycrystallinity of the sample material and was indexed by calculating d-spacing values using ImageJ software and by matching with JCPDS card no. 361451 as shown in Table 4
Figure 5.
Transmission electron micrographs of VCO-ZnO NPs: (a) image at 100 nm scale; (b) image at 200 nm scale. (c) Size distribution histogram. (d) Inverse FFT. (e) SAED pattern.
Table 4. Calculated d-Spacing Values and hkl Indexing of SAED Ring Pattern.
| d-spacing (Å) | JCPDS data (Å) | hkl | |
|---|---|---|---|
| R1 | 2.9231 | 2.8143 | 100 |
| R2 | 2.5833 | 2.6033 | 002 |
| R3 | 2.2376 | 2.4759 | 101 |
| R4 | 1.7915 | 1.9111 | 102 |
| R5 | 1.5124 | 1.6247 | 110 |
3.5. In Vitro Antioxidant Activity
To measure the presence and activity of antioxidants in synthesized VCO-ZnO NPs, an in vitro study was done with three types of electron-transfer-based assays, namely DPPH, FRAP, and ABTS.37 Each method has pros and cons.38 To avoid the problem of underestimation and overestimation of total antioxidant capacity, these three standard methods were chosen.39,40 The quantitative details of percent inhibition of DPPH by VCO-ZnO NPs are given in Table 5. Here, we calculated IC50 values directly by taking the mean of percent activity at gradient concentrations of samples using the online calculator mentioned in section 2.3.2. The visual representations of percent of inhibitory activity and IC50 values of the samples along with ascorbic acid as standard for each assay are depicted in Figure 6 and Table 6, respectively. The correlation of IC50 values of VCO among the three assays is matched with that of VCO-ZnO.24 All assays were done in triplicate, and the results were obtained with very minimal standard errors (∼0.001 to 0.5).
Table 5. Triplicate Results of Antioxidant Activity for VCO-ZnO in DPPH Method and IC50.
| inhibition
(%) |
|||||
|---|---|---|---|---|---|
| concentration (μg/mL) | trial 1 | trial 2 | trial 3 | mean ± SD | IC50 (μg/mL) |
| 10 | 06.372 | 5.882 | 6.209 | 6.154 ± 0.144 | 78.991 |
| 20 | 15.359 | 16.503 | 15.523 | 15.795 ± 0.357 | |
| 30 | 23.039 | 22.059 | 22.059 | 22.386 ± 0.327 | |
| 40 | 31.007 | 30.372 | 29.471 | 30.283 ± 0.446 | |
Figure 6.
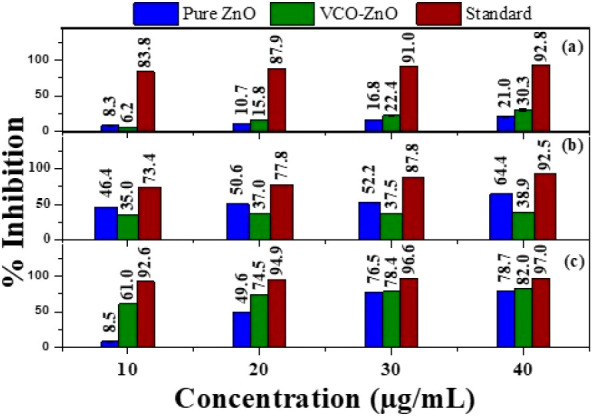
Percentage inhibition of (a) DPPH, (b) ABTS, and (c) FRAP at different concentrations of pure ZnO, VCO-ZnO, and ascorbic acid.
Table 6. IC50 Measures against Various Biological Components of In Vitro Antioxidant Activity.
| IC50 (μg/mL) |
|||
|---|---|---|---|
| sample | DPPH | ABTS | FRAP |
| pure ZnO | 28.632 | 79.440 | 19.054 |
| VCO-ZnO | 78.991 | 51.464 | 04.677 |
| ascorbic acid | 22.945 | 25.835 | 19.492 |
| VCO24 | 93.918 | 43.599 | 10.780 |
In the DPPH assay, the absorption of DPPH at 517 nm decreased slowly with the increase of concentration of synthesized samples in a solution containing DPPH. It is shown in Figure 6 in terms of visual representation of the mean and standard deviations of percent of inhibition. Nearly comparable percents of free radical scavenging activity were observed for pure ZnO (21.0%) and VCO-ZnO (30.3%) at 40 μg/mL but were not comparable with that of the standard (92.8%). In the ABTS assay, a higher percentage of inhibitory activity was shown by pure ZnO (64.4%) than by VCO-ZnO (38.9%) at 40 μg/mL. Whereas in the FRAP method, VCO-ZnO showed a higher radical inhibition capacity of 82% at 40 μg/mL which is nearly comparable with the percemt inhibition of ascorbic acid. The calculated IC50 values of VCO-ZnO were obtained at 78.991 and 51.464 μg/mL in both DPPH and ABTS assays, respectively, whereas in the FRAP assay, the IC50 value of VCO-ZnO was obtained at a much lower value, 4.6 μg/mL, which proved that VCO-ZnO is a good antioxidant.
3.6. In Vitro Anti-inflammatory Activity
The in vitro inhibition activity in protein/albumin denaturation, proteinase denaturation, and heat-reduced RBC hemolysis assays were used to measure the anti-inflammatory properties of pure ZnO and VCO-ZnO which are compared to the standard diclofenac sodium. The quantitative details of percent inhibition activity of VCO-ZnO for Protein denaturation are given in Table 7. Here also, we calculated IC50 values directly by taking the mean of percent activity at gradient concentrations of samples using an online calculator.26 The visual representation of the mean and standard deviations of percent of inhibition and IC50 values are represented in Figure 7 and Table 8, respectively. All assays were done in triplicate and the results obtained with very minimal standard errors (∼0.000 to 0.300).
Table 7. Triplicate Results of Anti-inflammatory Activity in Protein Denaturation Method for VCO-ZnO NPs and and IC50.
| inhibition
(%) |
|||||
|---|---|---|---|---|---|
| concentration (μg/mL) | trial 1 | trial 2 | trial 3 | mean ± SD | IC50 (μg/mL) |
| 10 | 41.935 | 41.935 | 41.935 | 41.935 ± 0.000 | 22.423 |
| 20 | 50.000 | 50.000 | 50.000 | 50.000 ± 0.000 | |
| 30 | 63.014 | 63.014 | 63.758 | 63.262 ± 0.248 | |
| 40 | 66.038 | 66.460 | 65.823 | 66.107 ± 0.187 | |
Figure 7.
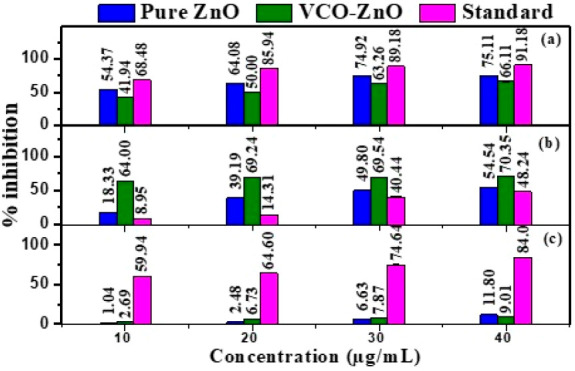
Percentage inhibition of (a) protein denaturation, (b) proteinase denaturation, and (c) heat-induced hemolysis at different concentrations of pure ZnO, VCO-ZnO, and diclofenac sodium.
Table 8. IC50 Measures against Various Biological Components of In Vitro Anti-inflammatory Activity.
| IC50 (μg/mL) |
|||
|---|---|---|---|
| sample | protein denaturation | proteinase denaturation | heat-induced RBC hemolysis |
| pure ZnO | 20.218 | 16.477 | 38.109 |
| VCO-ZnO | 22.423 | 04.467 | 5.099 |
| diclofenac sodium | 04.696 | 25.508 | 33.393 |
In a comparison of anti-inflammatory activity of VCO-ZnO with that of pure ZnO, the highest percent of inhibitory activity was shown by pure ZnO for protein denaturation (75.11%) and heat-induced RBC hemolysis assays (11.80%), whereas for the proteinase denaturation assay the highest percent of inhibitory activity (70.35%) was shown by VCO-ZnO at 40 μg/mL. In the protein denaturation assay the IC50 values for pure ZnO and VCO-ZnO are found to be 20.21 and 22.42 μg/mL, respectively. VCO-ZnO showed lower IC50 values for both proteinase denaturation and heat-induced RBC hemolysis, which are 4.46 and 5.099 μg/mL, respectively, than for pure ZnO. Contradictory results for percent of inhibition and IC50 values were found in heat-induced RBC hemolysis. With these lower IC50 values in all three assays, it is observed that synthesized VCO-ZnO NPs show strong anti-inflammatory properties.
3.7. Antimicrobial Activity
The antimicrobial activity of pure ZnO and VCO-ZnO NPs with different concentrations was applied against clinically significant pathogenic microorganisms, and their sensitivity in terms of zone of inhibition (ZOI) was tested in triplicate. From Figure 8, the ZOI that appeared around the disc coated with VCO-ZnO NPs clearly showed a significantly higher growth of inhibition against pure ZnO for Pseudomonas and Candida. However, a growth comparable with that if pure ZnO was found for S. aureus bacteria at 0.5 mg/mL. Increased antimicrobial activity was observed proportionally with the higher concentration. The bar graph representation of the mean and standard deviation of ZOI is shown in Figure 9. The synthesized VCO-ZnO NPs were equally active in the inhibition of the selected pathogens Pseudomonas (21.6 mm), S. aureus (25.00 mm), and Candida (17.9 mm) at 1 mg/mL concentration. These outcomes concurred with those of ZnO NPs from previous reports.41,42 The observed white annulus might indicate the spread of fatty acid functional groups present in synthesized VCO-ZnO NPs.
Figure 8.
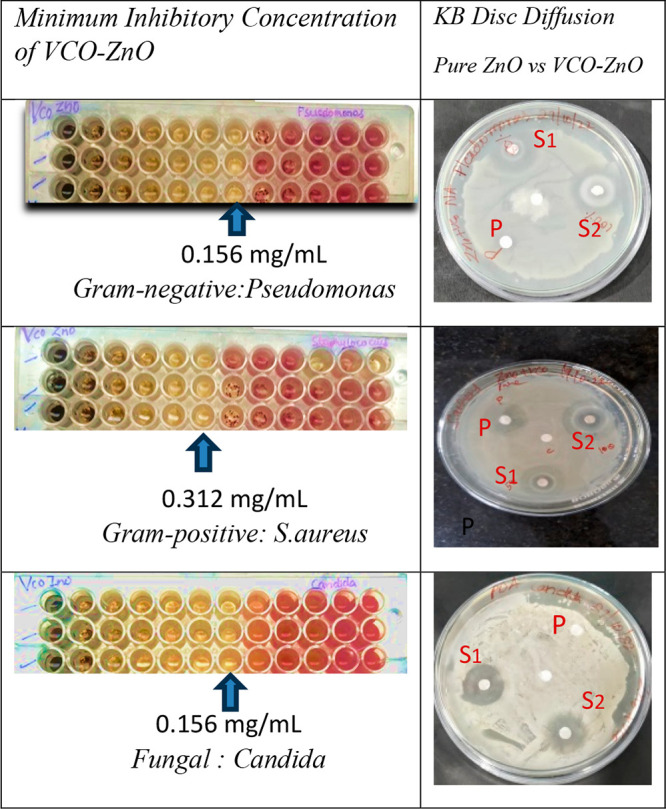
MIC results and visual representation of zone of inhibition of pure ZnO at 0.5 mg/mL (P) and VCO-ZnO at 0.5 mg/mL (S1) and 1 mg/mL (S2) concentrations against selected pathogens.
Figure 9.
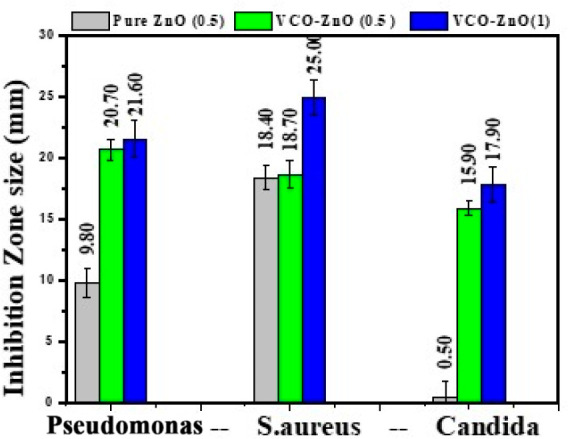
Zone of inhibition of pure ZnO and VCO-ZnO NP activity at different concentrations against the bacteria Pseudomonas and S. aureus and fungus Candida.
3.8. In Vitro Antidiabetic Activity
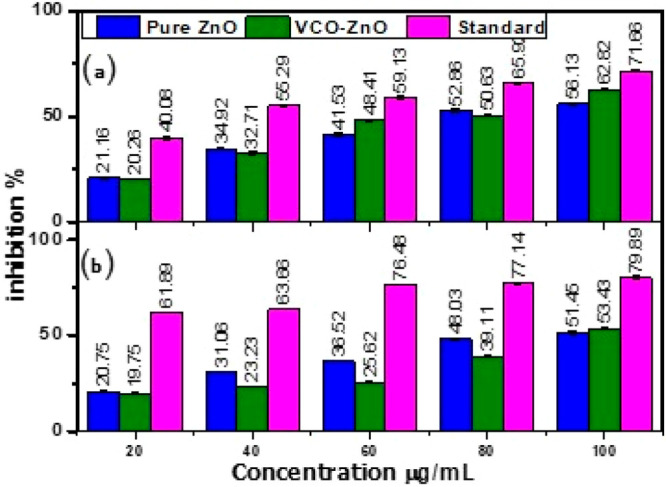
α-Amylase and α-glucosidase enzyme inhibition is necessary to control blood glucose levels in diabetic patients. The inhibitory activity of VCO-ZnO NPs was assessed by calculating the percentage of inhibition in both assays conducted in triplicate. The quantitative information detailing the percentage of inhibition activity of VCO-ZnO for α-amylase assays can be found in Table 9. The visual representation of the mean and standard deviations of percent of inhibition is shown in Figure 10. The percentage inhibition increased with increased concentration of VCO-ZnO NPs, ranging from 20.26% (20 μg/mL) to 62.82% (100 μg/mL) for α-amylase and from 19.75% (20 μg/mL) to 53.43% (100 μg/mL) for α-glucosidase. The inhibitory potential of VCO-ZnO NPs on α-amylase is nearly equivalent to that of metformin at a concentration of 100 μg/mL. However, for α-glucosidase, a concentration of more than 100 μg/mL was needed. At the highest selected concentration, VCO-ZnO showed more antidiabetic activity than pure ZnO. The calculated IC50 values by taking the mean of percent activity at gradient concentrations of samples using an online calculator26 are given in Table 10, indicating that VCO-ZnO showed better α-amylase enzyme inhibition than α-glucosidase enzyme inhibition.
Table 9. Triplicate Results of Antidiabetic Activity in α-Amylase Assay for VCO-ZnO and IC50.
| inhibition
(%) |
|||||
|---|---|---|---|---|---|
| concentration (μg/mL) | trial 1 | trial 2 | trial 3 | mean ± SD | IC50 (μg/mL) |
| 20 | 20.196 | 19.816 | 20.756 | 20.256 ± 0.273 | 88.452 |
| 40 | 33.273 | 31.813 | 33.053 | 32.713 ± 0.454 | |
| 60 | 47.706 | 48.706 | 48.806 | 48.406 ± 0.351 | |
| 80 | 50.726 | 51.226 | 49.926 | 50.626 ± 0.378 | |
| 100 | 63.522 | 62.322 | 62.622 | 62.822 ± 0.360 | |
Figure 10.
Percentage inhibition in (a) α-amylase and (b) α-glucosidase assays at different concentrations of pure ZnO, VCO-ZnO, and metformin.
Table 10. IC50 Measures against α-Amylase and α-Glucosidase of In Vitro Antidiabetic Activity.
| IC50 (μg/mL) |
||
|---|---|---|
| sample | α-amylase | α-glucosidase |
| pure ZnO | 46.35 | 99.56 |
| VCO-ZnO | 88.45 | 147.67 |
| metformin | 49.56 | 114.19 |
3.9. Stability, Purity, and Scalability of synthesized VCO-ZnO NPs
3.9.1. Stability of VCO-ZnO NPs: DLS Analysis
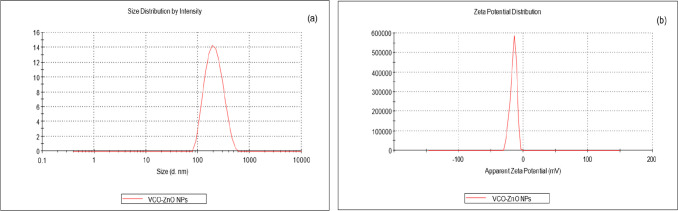
The DLS study (Malvern Instruments Ltd.) is utilized to investigate the size distribution, polydispersity index (PDI), and zeta potential of VCO-ZnO NPs in the colloidal solution quantitatively. The average size distribution is obtained as ∼200 ± 60 nm (Figure 11a). This larger size distribution is attributed to the DLS, providing a hydrodynamic diameter of NPs in the hydrated state or capping of VCO over the surface of ZnO NPs. The lower PDI (0.141) indicates the monodisperse nature of VCO-ZnO NPs and also implies control over the synthesis process, resulting in VCO-ZnO NPs with uniform sizes.43 The zeta potential is a measurement of the electric charge that is on the surface of particles and is used to determine the colloidal stability. The zeta potential of VCO-ZnO NPs in distilled water is −15.4 ± 5 mV as shown in Figure 11b, indicating its high stability and prevention of agglomeration due to the strong electrostatic repulsive forces between NPs.
Figure 11.
Dynamic light scattering (DLS) analysis of VCO-ZnO NPs: (a) size distribution by intensity and (b) zeta potential distribution.
3.9.2. Purity and Scalability
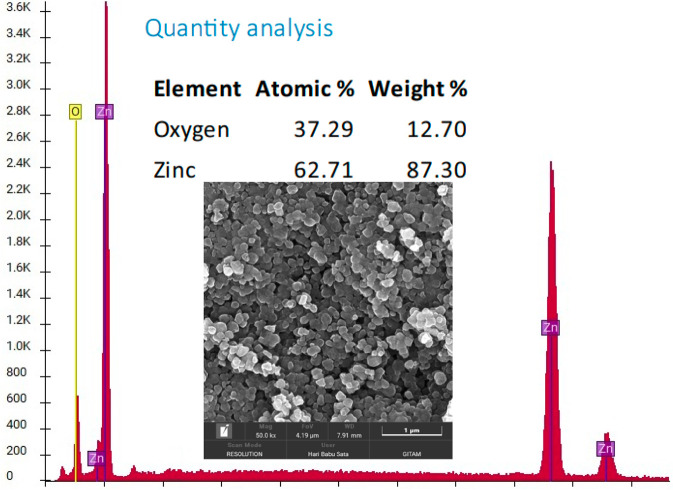
One of the best techniques to determine the purity of a sample is energy dispersive spectroscopy (EDS). The quantity analysis and EDS labeled peaks show an almost 99.5% purity of the sample. Using this percentage of purity, the precursor (ZnSO4·7H2O) weight (10 g) and its molecular weight (287.56 U), VCO-ZnO NP weight after drying (2.4 g) and molar mass of ZnO (81.38 U), we calculated the percent yield of NPs in the online Nanoparticle Yield Calculator InstaNANO (https://instanano.com/all/characterization/other/yield/ (accessed April 7, 2024)). The results are presented in Table 11. The quantification study shows a yield of 84.38% of ZnO NPs, indicating the potential for commercial production of ZnO NPs on a larger scale without causing harm to the environment through the disposal of unreacted metals, making it a promising prospect.44 The FESEM image in Figure 12 depicts the clear and distinct images of the synthesized VCO-ZnONPs. They are almost spherical with few rodlike morphologies. The size of NPs was calculated using ImageJ software and showed them to be in the range 60–90 nm.
Table 11.
| precursor weight (mg) | 10000 |
| precursor molecular weight | 287.56 |
| nanoparticle molar mass | 81.38 |
| nanoparticles weight after drying (mg) | 2400 |
| weight (%) of NPs from EDS | 99.5 |
| yield (%) | 84.38 |
Figure 12.
SEM and EDS analysis of VCO-ZnO NPs.
3.10. Comparative Study with Results from Previous Literature
In the Introduction, the benefits of utilizing VCO as a reducing agent for the synthesis of ZnO NPs are highlighted. Unlike many plant extracts, the commercial availability of VCO in its purest form enhances efficiency and purity and saves time and energy. Conducting a comparative analysis with various synthesis methods for ZnO NPs as in Table 12 can offer valuable insights into the advantages of employing VCO over other biological or green reducing agents. This analysis may encompass factors such as method of extract preparation and NP synthesis, morphological comparisons, and effectiveness in biomedical applications.
Table 12. Comparative Study of Benefits/Features of VCO over Other Reducing Agents in NP Synthesis with the Aid of Previous Literaturea.
| reducing agent | extract method of reducing agent | synthesis method of NPs | particle size (nm) | application study | ref |
|---|---|---|---|---|---|
| VCO | cold pressed-commercially available | green gel combustion route | 16 | *IC50, 78.9 μg/mL (DPPH); *POI, 38.9% at 40 μg/mL(DPPH); #IC50, 22.4229 μg/mL (PDI); @IC50, 88.45 μg/mL (α-amylase); ϕMIC, 0.156 mg/mL (PA), 0.316 mg/mL (SA); ϕZOI, 21.60 mm at 1 mg/mL (PA), 25.00 mm at 1 mg/mL (SA) | present work |
| almond oil | cold pressed-commercially available | chemical precipitation | 35 | *IC50, 328.5 μg/mL (DPPH); ϕMIC, 0.3 mg/mL (PA) | (45) |
| Eucalyptus globulus Essential oil | hydrodistilled in a Clevenger apparatus | green precipitation using acetone dilution | 24 | ϕMIC, 2 mg/mL (PA) | (46) |
| clove buds | rotary evaporation method | green hydrothermal | 50 | *POI, 85% (DPPH); ϕZOI, 12 mm (SA) | (47) |
| Mentha pulegium essential oil | hydrodistillation using a Clevenger apparatus | chemical sol–gel method | nanofluid | ϕMIC, 3.3 ppm (SA) | (48) |
| M. fragrans fruit (nutmeg) | 63 | *POI, 66% at 0.4 mg/mL (DPPH); ϕZOI, 17 ± 1.66 mm (PA), 21 ± 1.73 mm (SA) at 1 mg/mL; @POI,73.23 ± 0.42 (α-amylase), 65.21 ± 0.49 (α-glucosidase) at 400 μg/mL | (49) | ||
| Medicago sativa L. (alfalfa) | boiling method | green combustion route method | 10 | *POI, 42.3% (DPPH); ϕMIC, 0.0093 mg/mL (Candida) | (50) |
| Aerva persica roots | rotary evaporation method | hydrothermal route | 45 | *POI, 91% at 0.25 mg/mL (DPPH); #POI, 79.76% at 36.5 mg/mL (carrageenan induced paw edema model) | (51) |
| aloe vera leaf | boiling and crude method | green precipitation | 25–35 | (52) | |
| leaves of H. fomes (HF) and S. apetala (SA) | boiling method | green precipitation method | 40–50 | #IC50, 63.29 μg/mL for SA-ZnO and 72.35 μg/mL for HF-ZnO (PDI); @IC50, 334.4 μg/mL for SA-ZnO and 394.38 μg/mL for HF-ZnO (α-amylase) | (53) |
Abbreviations: POI, percentage of inhibition; PDI, protein denaturation inhibition; PA, Pseudomonas aeruginosa SA, S. aureus; ZOI, zone of inhibition. Symbols: *, characteristic values of antioxidant study; #, characteristic values of antiinflammatory study; @, characteristic values of antidiabetic study; ϕ, characteristic values of antimicrobial study.
In order to represent all of the studies in one table, various symbols and abbreviations have been adopted as shown in the footnote to Table 12.
3.11. Conclusion
In conclusion, metal nanoparticles have gained prominence across industries for medical applications due to their size-dependent properties. Conventional methods often entail high costs and environmental risks; therefore, a promising alternative is a green synthesis method. Choosing vegetable oils in the purest form like virgin coconut oil (VCO) may help overcome the limitations of green synthesis such as difficulties in plant collection and extract preparation. By utilizing bioreduction by biomolecules from VCO, renowned for its health benefits, it serves as an effective reducer and stabilizer for nanoparticles. The functional groups within VCO contribute to the reduction process and hinder nanoparticle agglomeration. Overall, the highlights of the work are the successful synthesis of ZnO NPs using VCO as a reducing as well as capping agent with zero chemical mediators and their biological properties which are comparable with those of standard medicines. These may make the synthesized ZnO NPs more suitable for use in the medical field. Further, the substantial nanoparticle yield indicates that the method has significant potential for large-scale production and commercialization. These key points open up possibilities for future research into producing other metal nanoparticles using VCO.
Acknowledgments
The authors are thankful to the Central Instrumentation Laboratory (CIL), Government College (A) Rajamahendravaram for providing the necessary facilities during the research work.
Author Contributions
B.D.L.: Iinvestigation, resources, writing the manuscript. P.T.R.: visualization, writing the manuscript. B.V.V.K.: visualization, investigation. A.M.: investigation, data curation, writing the manuscript. E.B.S. and V.K.: visualization, investigation. K.R.R.: supervision, conceptualization, methodology, reviewing the manuscript.
The authors declare no competing financial interest.
References
- Ying S.; Guan Z.; Ofoegbu P. C.; Clubb P.; Rico C.; He F.; Hong J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environmental Technology & Innovation 2022, 26, 102336. 10.1016/j.eti.2022.102336. [DOI] [Google Scholar]
- Nasrullah M.; Gul F. Z.; Hanif S.; Mannan A.; Naz S.; Ali J. S.; Zia M. Green and Chemical Syntheses of CdO NPs: A Comparative Study for Yield Attributes, Biological Characteristics, and Toxicity Concerns. ACS Omega 2020, 5 (11), 5739–5747. 10.1021/acsomega.9b03769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngampeerapong C.; Chavasit V.; Durst R. W.. Bioactive and Nutritional Compounds in Virgin Coconut Oils.Malaysian J. Nutrition 2018, 24, 257–267. [Google Scholar]
- Iranloye B.; Oludare G.; Olubiyi M. Anti-Diabetic and Antioxidant Effects of Virgin Coconut Oil in Alloxan Induced Diabetic Male Sprague Dawley Rats. JDM 2013, 03 (04), 221–226. 10.4236/jdm.2013.34034. [DOI] [Google Scholar]
- Nitbani F. O.; Tjitda P. J. P.; Nitti F.; Jumina J.; Detha A. I. R. Antimicrobial Properties of Lauric Acid and Monolaurin in Virgin Coconut Oil: A Review. ChemBioEng. Reviews 2022, 9 (5), 442–461. 10.1002/cben.202100050. [DOI] [Google Scholar]
- Zamiri R.; Zamiri R.; Sadrolhosseini A. R. Preparation of Silver Nanoparticles in Virgin Coconut Oil Using Laser Ablation. IJN 2011, 71. 10.2147/IJN.S14005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang K. S. The Current and Future Perspectives of Zinc Oxide Nanoparticles in the Treatment of Diabetes Mellitus. Life Sciences 2019, 239, 117011. 10.1016/j.lfs.2019.117011. [DOI] [PubMed] [Google Scholar]
- Spoialǎ A.; Ilie C.-I.; Truşcǎ R.-D.; Oprea O.-C.; Surdu V.-A.; Vasile B. Ş; Ficai A.; Ficai D.; Andronescu E.; Diţu L.-M. Zinc Oxide Nanoparticles for Water Purification. Materials 2021, 14 (16), 4747. 10.3390/ma14164747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D. K.; Shukla S.; Sharma K. K.; Kumar V. A Review on ZnO: Fundamental Properties and Applications. Materials Today: Proceedings 2022, 49, 3028–3035. 10.1016/j.matpr.2020.10.238. [DOI] [Google Scholar]
- Walton I. M.; Cox J. M.; Benson C. A.; Patel D. D. G.; Chen Y.-S.; Benedict J. B. The Role of Atropisomers on the Photo-Reactivity and Fatigue of Diarylethene-Based Metal-Organic Frameworks. New J. Chem. 2016, 40 (1), 101–106. 10.1039/C5NJ01718A. [DOI] [Google Scholar]
- Ansari S. A.; Khan M. M.; Ansari M. O.; Lee J.; Cho M. H. Biogenic Synthesis, Photocatalytic, and Photoelectrochemical Performance of Ag-ZnO Nanocomposite. J. Phys. Chem. C 2013, 117 (51), 27023–27030. 10.1021/jp410063p. [DOI] [Google Scholar]
- Xie J.; Li H.; Zhang T.; Song B.; Wang X.; Gu Z. Recent Advances in ZnO Nanomaterial-Mediated Biological Applications and Action Mechanisms. Nanomaterials 2023, 13 (9), 1500. 10.3390/nano13091500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kshirsagar A.; Khanna T.; Dhanwe V.; Kate K. H.; Khanna P. K. Green Synthesis of Silver Nano-Particles by Use of Edible Oils. j nanosci nanotechnol 2018, 18 (1), 386–393. 10.1166/jnn.2018.14592. [DOI] [PubMed] [Google Scholar]
- Meena Kumari M.; Philip D. Facile One-Pot Synthesis of Gold and Silver Nanocatalysts Using Edible Coconut Oil. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2013, 111, 154–160. 10.1016/j.saa.2013.03.076. [DOI] [PubMed] [Google Scholar]
- Vinicius De Oliveira Brisola Maciel M.; Da Rosa Almeida A.; Machado M. H.; Elias W. C.; Gonçalves Da Rosa C.; Teixeira G. L.; Noronha C. M.; Bertoldi F. C.; Nunes M. R.; Dutra De Armas R.; Manique Barreto P. L. Green Synthesis, Characteristics and Antimicrobial Activity of Silver Nanoparticles Mediated by Essential Oils as Reducing Agents. Biocatalysis and Agricultural Biotechnology 2020, 28, 101746. 10.1016/j.bcab.2020.101746. [DOI] [Google Scholar]
- Khanna P. K.; Nair C. K. K. Synthesis of Silver Nanoparticles Using Cod Liver Oil (Fish Oil): Green Approach to Nanotechnology. International Journal of Green Nanotechnology: Physics and Chemistry 2009, 1 (1), P3–P9. 10.1080/19430870902909759. [DOI] [Google Scholar]
- Kumar B.; Smita K.; Cumbal L.; Debut A. Sacha Inchi (Plukenetia Volubilis L.) Oil for One Pot Synthesis of Silver Nanocatalyst: An Ecofriendly Approach. Industrial Crops and Products 2014, 58, 238–243. 10.1016/j.indcrop.2014.04.021. [DOI] [Google Scholar]
- Jiang Z.; Li L.; Huang H.; He W.; Ming W. Progress in Laser Ablation and Biological Synthesis Processes: “Top-Down” and “Bottom-Up” Approaches for the Green Synthesis of Au/Ag Nanoparticles. IJMS 2022, 23 (23), 14658. 10.3390/ijms232314658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ar G.Antioxidant Activity of Some Medicinal Species Using FRAP Assay. J. Medicinal Plants 2011, 10, 54–60. [Google Scholar]
- Goodarzi S.; Rafiei S.; Javadi M.; Haghighian H. K.; Noroozi S.. A Review on Antioxidants and Their Health Effects. J. Nutrition Food Security 2018, 3, 106–112 [Google Scholar]
- Vamsi Krishna B. V.; Tirupathi Rao P.; Durga Lakshmi B.; Vasudha K.; Esub Basha Sk.; Putra Kumar B.; Kiran P. S. S.; Shreyas Chandra K.; R K R. Green Fabrication of Tinospora Cordifolia-Derived MgO Nanoparticles: Potential for Diabatic Control and Oxidant Protection. Next Materials 2024, 3, 100171. 10.1016/j.nxmate.2024.100171. [DOI] [Google Scholar]
- Ansari S. A.; Khan M. M.; Lee J.; Cho M. H. Highly Visible Light Active Ag@ZnO Nanocomposites Synthesized by Gel-Combustion Route. Journal of Industrial and Engineering Chemistry 2014, 20 (4), 1602–1607. 10.1016/j.jiec.2013.08.006. [DOI] [Google Scholar]
- Khuda F.; Jamil M.; Ali Khan Khalil A.; Ullah R.; Ullah N.; Naureen F.; Abbas M.; Shafiq Khan M.; Ali S.; Muhammad Umer Farooqi H.; Ahn M.-J. Assessment of Antioxidant and Cytotoxic Potential of Silver Nanoparticles Synthesized from Root Extract of Reynoutria Japonica Houtt. Arabian Journal of Chemistry 2022, 15 (12), 104327. 10.1016/j.arabjc.2022.104327. [DOI] [Google Scholar]
- Syafitri E.; Dalimunthe A.; Silalahi J. The Antioxidant Activity of Virgin Coconut Oil Using the FRAP, ABTS, CUPRAC, and DPPH Methods. Pharma Innovation 2023, 12 (11), 21–25. 10.22271/tpi.2023.v12.i11a.23972. [DOI] [Google Scholar]
- Molyneux P.The Use of the Stable Free Radical Diphenylpicryl- Hydrazyl (DPPH) for Estimating Antioxidant Activity. Songklanakarin Journal of Science and Technology 2004, 26 ( (2), ) .1 [Google Scholar]
- AAT Bioquest, Inc., IC50 Calculator, 2024. https://www.aatbio.com/tools/ic50-calculator.
- Gunathilake K.; Ranaweera K.; Rupasinghe H. Influence of Boiling, Steaming and Frying of Selected Leafy Vegetables on the In Vitro Anti-Inflammation Associated Biological Activities. Plants 2018, 7 (1), 22. 10.3390/plants7010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- K V.; D A.; B M.; B K. PHYTOCHEMICAL SCREENING, ANTIMICROBIAL, AND ANTIOXIDANT ACTIVITIES OF ROOT AND LEAF EXTRACTS OF LEUCAS ASPERA. Asian J. Pharm. Clin Res. 2019, 141–147. 10.22159/ajpcr.2019.v12i3.29085. [DOI] [Google Scholar]
- Shalaby E. A.; Shanab S. M. M.; El-Raheem W. M. A.; Hanafy E. A. Biological Activities and Antioxidant Potential of Different Biosynthesized Nanoparticles of Moringa Oleifera. Sci. Rep 2022, 12 (1), 18400. 10.1038/s41598-022-23164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinotha V.; Iswarya A.; Thaya R.; Govindarajan M.; Alharbi N. S.; Kadaikunnan S.; Khaled J. M.; Al-Anbr M. N.; Vaseeharan B. Synthesis of ZnO Nanoparticles Using Insulin-Rich Leaf Extract: Anti-Diabetic, Antibiofilm and Anti-Oxidant Properties. Journal of Photochemistry and Photobiology B: Biology 2019, 197, 111541. 10.1016/j.jphotobiol.2019.111541. [DOI] [PubMed] [Google Scholar]
- Balan K.; Qing W.; Wang Y.; Liu X.; Palvannan T.; Wang Y.; Ma F.; Zhang Y. Antidiabetic Activity of Green Synthesis Silver Nanoparticles Using Lonicera Japonica Leaves Extract. RSC Adv. 2016, 6, 40162. 10.1039/C5RA24391B. [DOI] [Google Scholar]
- Singla M. L.; Shafeeq M M.; Kumar M. Optical Characterization of ZnO Nanoparticles Capped with Various Surfactants. J. Lumin. 2009, 129 (5), 434–438. 10.1016/j.jlumin.2008.11.021. [DOI] [Google Scholar]
- Makuła P.; Pacia M.; Macyk W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9 (23), 6814–6817. 10.1021/acs.jpclett.8b02892. [DOI] [PubMed] [Google Scholar]
- Guo L.; Yang S.; Yang C.; Yu P.; Wang J.; Ge W.; Wong G. K. L. Highly Monodisperse Polymer-Capped ZnO Nanoparticles: Preparation and Optical Properties. Appl. Phys. Lett. 2000, 76 (20), 2901–2903. 10.1063/1.126511. [DOI] [Google Scholar]
- Alamdari S.; Jafar Tafreshi M.; Sasani Ghamsari M.; Majles Ara M. H. Preparation and Characterization of ZnO and CWO Nanopowders for Radiation Sensing. Progress in Physics of Applied Materials 2021, 1, 14–18. 10.22075/ppam.2021.23502.1007. [DOI] [Google Scholar]
- Dayrit F. M.Lauric Acid Is a Medium-Chain Fatty Acid, Coconut Oil Is a Medium-Chain Triglyceride. Phillippine J. Sci. 2014, 143 ( (2), ), 157–166 [Google Scholar]
- Apak R.; Güçlü K.; Demirata B.; Özyürek M.; Çelik S.; Bektaşoǧlu B.; Berker K.; Özyurt D. Comparative Evaluation of Various Total Antioxidant Capacity Assays Applied to Phenolic Compounds with the CUPRAC Assay. Molecules 2007, 12 (7), 1496–1547. 10.3390/12071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P.; Modi H. A.. Comparative Study of DPPH, ABTS and FRAP Assays for Determination of Antioxidant Activity. Chemistry, Environmental Science, Medicine 2015, 3, 1 [Google Scholar]
- Dorsey B. M.Healthy Components of Coffee Processing by-Products In Handbook of Coffee Processing By-Products Elsevier: 2017; Chapter 2, p 27. [Google Scholar]
- Rumpf J.; Burger R.; Schulze M. Statistical Evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu Assays to Assess the Antioxidant Capacity of Lignins. Int. J. Biol. Macromol. 2023, 233, 123470. 10.1016/j.ijbiomac.2023.123470. [DOI] [PubMed] [Google Scholar]
- Naseer M.; Aslam U.; Khalid B.; Chen B. Green Route to Synthesize Zinc Oxide Nanoparticles Using Leaf Extracts of Cassia Fistula and Melia Azadarach and Their Antibacterial Potential. Sci. Rep 2020, 10 (1), 9055. 10.1038/s41598-020-65949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R.; Umar A.; Kumar G.; Nalwa H. S. Antimicrobial Properties of ZnO Nanomaterials: A Review. Ceram. Int. 2017, 43 (5), 3940–3961. 10.1016/j.ceramint.2016.12.062. [DOI] [Google Scholar]
- Meißner T.; Oelschlägel K.; Potthoff A. Implications of the Stability Behavior of Zinc Oxide Nanoparticles for Toxicological Studies. Int. Nano Lett. 2014, 4 (3), 116. 10.1007/s40089-014-0116-5. [DOI] [Google Scholar]
- Nasrullah M.; Gul F. Z.; Hanif S.; Mannan A.; Naz S.; Ali J. S.; Zia M. Green and Chemical Syntheses of CdO NPs: A Comparative Study for Yield Attributes, Biological Characteristics, and Toxicity Concerns. ACS Omega 2020, 5 (11), 5739–5747. 10.1021/acsomega.9b03769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramzan I.; Bashir M.; Saeed A.; Khan B. S.; Shaik M. R.; Khan M.; Shaik B.; Khan M. Evaluation of Photocatalytic, Antioxidant, and Antibacterial Efficacy of Almond Oil Capped Zinc Oxide Nanoparticles. Materials 2023, 16 (14), 5011. 10.3390/ma16145011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obeizi Z.; Benbouzid H.; Ouchenane S.; Yılmaz D.; Culha M.; Bououdina M. Biosynthesis of Zinc Oxide Nanoparticles from Essential Oil of Eucalyptus Globulus with Antimicrobial and Anti-Biofilm Activities. Materials Today Communications 2020, 25, 101553. 10.1016/j.mtcomm.2020.101553. [DOI] [Google Scholar]
- Anvarinezhad M.; Javadi A.; Jafarizadeh-Malmiri H. Green Approach in Fabrication of Photocatalytic, Antimicrobial, and Antioxidant Zinc Oxide Nanoparticles - Hydrothermal Synthesis Using Clove Hydroalcoholic Extract and Optimization of the Process. Green Processing and Synthesis 2020, 9 (1), 375–385. 10.1515/gps-2020-0040. [DOI] [Google Scholar]
- Jahanpanahi M.; Sani A.M.; Sani A. M.; Jahanpanahi M. Antimicrobial Effect of Nanofluid Including Zinc Oxide (ZnO) Nanoparticles and Mentha Pulegium Essential Oil. J. App Biol. Biotech 2016, 1. 10.7324/JABB.2016.40410. [DOI] [Google Scholar]
- Faisal S.; Jan H.; Shah S. A.; Shah S.; Khan A.; Akbar M. T.; Rizwan M.; Jan F.; Wajidullah; Akhtar N.; Khattak A.; Syed S. Green Synthesis of Zinc Oxide (ZnO) Nanoparticles Using Aqueous Fruit Extracts of Myristica Fragrans: Their Characterizations and Biological and Environmental Applications. ACS Omega 2021, 6 (14), 9709–9722. 10.1021/acsomega.1c00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Król A.; Railean-Plugaru V.; Pomastowski P.; Buszewski B. Phytochemical Investigation of Medicago Sativa L. Extract and Its Potential as a Safe Source for the Synthesis of ZnO Nanoparticles: The Proposed Mechanism of Formation and Antimicrobial Activity. Phytochemistry Letters 2019, 31, 170–180. 10.1016/j.phytol.2019.04.009. [DOI] [Google Scholar]
- Fatima K.; Asif M.; Farooq U.; Gilani S. J.; Bin Jumah M. N.; Ahmed M. M. Antioxidant and Anti-Inflammatory Applications of Aerva Persica Aqueous-Root Extract-Mediated Synthesis of ZnO Nanoparticles. ACS Omega 2024, 9 (14), 15882–15892. 10.1021/acsomega.3c08143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangeetha G.; Rajeshwari S.; Venckatesh R. Green Synthesis of Zinc Oxide Nanoparticles by Aloe Barbadensis Miller Leaf Extract: Structure and Optical Properties. Mater. Res. Bull. 2011, 46 (12), 2560–2566. 10.1016/j.materresbull.2011.07.046. [DOI] [Google Scholar]
- Thatoi P.; Kerry R. G.; Gouda S.; Das G.; Pramanik K.; Thatoi H.; Patra J. K. Photo-Mediated Green Synthesis of Silver and Zinc Oxide Nanoparticles Using Aqueous Extracts of Two Mangrove Plant Species, Heritiera Fomes and Sonneratia Apetala and Investigation of Their Biomedical Applications. Journal of Photochemistry and Photobiology B: Biology 2016, 163, 311–318. 10.1016/j.jphotobiol.2016.07.029. [DOI] [PubMed] [Google Scholar]