Abstract
Background
Most older adults use medications that may increase falls, often defined as fall risk increasing drugs or “FRIDs”. Two definitions for FRIDs, the Centers for Disease Control and Prevention’s (CDC) Stopping Elderly Accidents, Deaths & Injuries (STEADI-Rx) and Swedish National Board of Health and Welfare (SNBHW) definitions, are widely accepted, though include different FRIDs in their definitions. Whether factors associated with FRID use in older adults differ by definition is unknown.
Methods
We hypothesized that factors for FRID use will vary by FRID definition in 1,352 community-dwelling older Black and White adults with medication information in the Health, Aging and Body Composition Study (Health ABC; 2007–08 clinic visit; 83.4 ± 2.8 years; 54.1% women; 65.1% White). Multivariable logistic regression and multivariable negative binomial regression, progressively entering groups of covariates (demographics, lifestyle/behavior factors, and multimorbidity), modeled FRID use (yes/no) and count.
Results
Of 87.0% participants using SNBHW FRIDs, 82.9% used cardiac medications, with lower use of all other FRIDs (range:1.1-12.4%). Of 86.6% participants using STEADI-Rx FRIDs, 80.5% used cardiac medications, with lower use of all other FRIDs (range:1.1-16.1%). Participants with FRID use by either definition were more likely to have chronic health conditions, a hospitalization in the prior year, higher non-FRIDs medication counts, higher Center for Epidemiologic Studies Depression Scale (CES-D) scores, and less physical activity (all p < 0.05). Participants with STEADI-Rx FRID use had poorer vision and higher Modified Mini-Mental State (3MS) scores. In multivariable logistic regression for SNBHW use, hypertension, body mass index (BMI), 3MS scores, and non-FRID count were positively associated with FRID use and poorer vision and Digit Symbol Substitution Test (DSST) scores were negatively associated. In addition to SNBHW factors, higher CES-D scores were associated with STEADI-Rx FRID use. In multivariable negative binomial regression, hypertension, higher BMI, CES-D scores, and non-FRID count were associated with higher FRID count and sleep problems with lower FRID count for both definitions. Higher 3MS and lower DSST scores were associated with higher STEADI-Rx FRID count. Women had lower SNBHW FRID count after adjustments.
Conclusions
Risk factors for FRID use in older adults differ slightly by STEADI-Rx and SNBHW FRIDs definition, but are largely similar.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12877-024-05301-w.
Keywords: Geriatrics, FRID, Falls, Medication, STEADI, Older adults, Risk factors
Introduction
Medications with potential to increase a person’s risk of falling are referred to as “fall-risk increasing drugs” (FRIDs). Only two definitions for FRIDs have been used widely. In 2010, the Swedish National Board of Health and Welfare (SNBHW) created a list of 13 clusters of drugs classified as FRIDs [1]. In 2020, the Centers for Disease Control and Prevention (CDC) in partnership with the University of North Carolina Eshelman School of Pharmacy and School of Medicine created a list of 9 categories of drugs that are FRIDs under the program STEADI-Rx (Stopping Elderly Accidents, Deaths & Injuries) [2].
Regardless of definition, the use of FRIDs in older adults (≥65 years) have become increasingly more common. For example, according to data from the Medical Expenditure Panel Survey (MEPS), FRID use showed an increase from 57% in 1999 to 94% in 2017 under the STEADI-Rx definition, possibly driven by the rise in antidepressant, anticonvulsant, and antihypertensive medication use [3]. The increase in FRIDs has also coincided with an approximate linear trend in fall-related mortality from 29.4 per 100,000 in 1999 to 63.3 per 100,000 in 2017, a 115% increase [3, 4]. Current research has not described the reason for these concurrent increases, though FRID use may be a contributing factor.
Additionally, the demographics, lifestyle factors [5], and multimorbidity [5] of older adults who use FRIDs remain largely unexamined, with limited information under the STEADI-Rx definition and no known study using the SNBHW. Based on population data from 1999 to 2017, FRID use was high in those ≥75 years old (79.2%) and those 65–74 years old (72.9%) [3]. Women (77.0%) and men (74.0%) also had a high percentage of receiving ≥1 FRID [3]. Moreover, under-represented ethnic/race groups of older adults that were Native American (80.0%) and Black (78.4%) reported higher FRID use vs. older White (75.8%) and Asian/Pacific Islander (66.1%) adults [3]. However, major gaps remain in understanding sociodemographic risk factors, including education and income, of older adults who use FRIDs, and many demographics associated with FRID use are only available in a non-generalizable subset of the population or in non-U.S. populations, including demographics of individual FRID use [5–13]. These factors are important to describe since the population of older adults who use FRIDs could be the target population for deprescribing efforts to reduce falls and fall injury risk in older adults. Thereby, our results will support future recruitment efforts in deprescribing research.
Therefore, the objective of the current study is to, for the first time, assess the association of a wide range of risk factors, including demographics of age, race, sex, and education, lifestyle characteristics, and disease-related factors, with the use of FRIDs, including both FRID classes and counts of FRID classes, in older Black and White men and women from the Health, Aging and Body Composition Study (Health ABC). We hypothesized that demographics, lifestyle/behavior factors, and types of multimorbidity will be associated with FRID use according to both the CDC’s STEADI-Rx and the SBNHW definitions, and that these factors will vary by FRID definition.
Methods
Participants
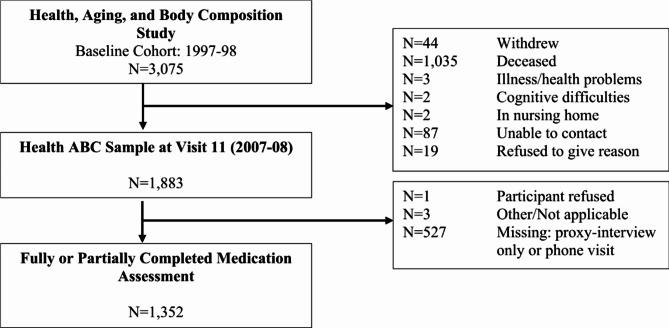
The Health, Aging and Body Composition Study (Health ABC) was a longitudinal cohort study of Black and White, initially well-functioning, community-dwelling older men and women from Pittsburgh, PA and Memphis, TN to investigate body composition and factors related to functional limitations and disability (n = 3,075; aged 70–79 years; 52% women; 42% Black at baseline in 1997-98). Participants were recruited from a random sample of White Medicare Fee-For-Service beneficiaries and all age-eligible Black Medicare beneficiaries and community residents. To be eligible, participants had to report no difficulty walking ¼ mile, climbing 10 steps, or performing activities of daily living; had no cancer or active treatment in <3 years; and planned to remain in the study area for at least 3 years. This study was approved by institutional review boards at the University of Pittsburgh and University of Tennessee Health Science Center, and written informed consent was obtained from each participant prior to participating [14, 15]. Participants were followed for 17 years (until 2012-13) and contacted yearly to obtain information of interest. By the 2007-08 visit, 1,035 participants were deceased, 44 withdrew, and 113 were missing for other reasons. Of the 1,883 participants remaining in the Health ABC Study in 2007-08 (year 11), 527 had either phone or proxy visits with no medication data. Of 1,356 with clinic visits, home visits, or mixed visits, 1,352 participants were included in the analysis with no missing medication data (Fig. 1).
Fig. 1.
Participant flow chart for medication data at the 2007-08 Health ABC visit
Covariates - factors of interest
Several key factors, including lifestyle and behavioral factors, as well as multimorbidity, were adjusted for in our analyses due to past literature associations with the factors or outcomes of interest. All covariates used in our analysis were measured at the 2007-08 clinic visit unless noted otherwise.
Age, race (White or Black), sex, education (less than high school, high school graduate, and postsecondary education), and site (Pittsburgh/Memphis) were self-reported at baseline [16]. Having non-Medicare health insurance (at the 2006-07 clinic visit), family income (<$10k, $10k-$25k, $25k-$50k, >$50k; at the 2001-02 clinic visit), and marital status (married, widowed, divorced, separated, never married; at the 2000-01 clinic visit) were self-reported [16]. Lifestyle and behavioral factors included smoking status (current, past, never), alcohol consumption (drinks/week), physical activity (kilocalories/week walking and stair climbing), and body mass index (BMI; kg/m2) [16]. Weight (kg) was measured on a standard balance beam scale, and height was measured by a stadiometer to the nearest 0.1 cm [17].
Multimorbidity covariates for this analysis included self-reported fall history reported from the question “In the past 12 months, have you fallen and landed on the floor or ground?”, knee or leg pain over the past year (y/n), any difficulty with activities of daily living (y/n), vision (excellent/good, fair, poor/blind), and sleeping problems (y/n) defined by restless sleep at least some of the time (1–2 days) [18–20]. Hospitalization in the previous year was self-reported through a questionnaire [16]. Depressive symptoms were assessed using the Center for Epidemiologic Studies Depression Scale (CES-D) score [21]. Cognitive function was measured using the Digit Symbol Substitution Test (DSST) and Modified Mini-Mental State (3MS) Examination [22]. Diabetes was defined using fasting glucose ≥126 mg/dL and participants taking hypoglycemic medications [19]. Physiological hypertension was defined as measured systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg [15, 20]. Insufficient renal function was defined by cystatin C ≥1 mg/dL [23]. Ankle brachial index <0.9 indicated peripheral arterial disease (PAD) and ≥1.3 indicated arterial stiffening [24].
Medication counts of the number of non-FRIDs were defined by subtracting the count of FRIDs used from the total medications a participant used. Medication inventory occurred during the clinic visits using “brown bag” review method. Participants were asked to bring to clinic all prescription medications that had been taken during the month before the clinic visit. Non-prescription medications were not collected at the 2007-08 clinic visit.
Outcomes: FRID class use & count of FRIDs
Outcomes included either FRID class use (yes/no) or a continuous count of FRIDs under both (1) the SNBHW definition and (2) the STEADI-Rx definition. FRID classes include 13 classes under the SNBHW definition and 9 classes under the STEADI-Rx definition [2, 5]. These medications were coded using the Iowa Drug Information System (IDIS) Drug Vocabulary and Thesaurus codes [25] (Table 1).
Table 1.
FRIDs lists and their medication classes for the SNBHW and the CDC STEADI-Rx definitions
| Swedish National Board for Health and Welfare | CDC STEADI-Rx |
|---|---|
| Antidepressants | Antidepressants |
| Antipsychotics | Antipsychotics |
| Opioids | Opioids |
| Hypnotics and Sedatives | Hypnotics and Sedatives |
| Anxiolytics* | Benzodiazepines |
| Antihypertensives: centrally acting, ganglion-blocker, and peripherally acting antiadrenergic agents, agents acting on arteriolar smooth muscles, and antihypertensives for pulmonary arterial hypertension | Antihypertensives: hypotensive agents, alpha-blockers, vasodilating agents, calcium channel blockers, diuretics, beta-blockers, ACE-inhibitors, and ARBs |
| Vasodilators used in cardiac diseases | Muscle Relaxants |
| Diuretics | Antihistamines |
| Beta-blockers | Anticonvulsants |
| Calcium Channel Blockers | |
| Dopaminergic Agents | |
| Agents acting on the renin-angiotensin system | |
| Alpha adrenoreceptor antagonists |
Differences noted italics
*Though anxiolytics are directly cited as a FRID, only benzodiazepines will be discussed due to the lack of information on anxiolytics in the literature
Abbreviations: Alpha-blockers, Alpha Adrenoreceptor Antagonists; ACE, Angiotensin-Converting-Enzyme; ARBs, Angiotensin II Receptor Blockers
The medication classes in the STEADI-Rx definition included anticonvulsants, antidepressants, antihistamines, antihypertensives (hypotensive agents, alpha adrenoreceptor antagonists (alpha-blockers), vasodilating agents, calcium channel blockers, diuretics, beta-blockers, angiotensin-converting-enzyme (ACE) inhibitors, and angiotensin II receptor blockers (ARBs), antipsychotics, benzodiazepines, muscle relaxants, opioids, and hypnotics and sedatives. Though the STEADI-Rx definition does not list specific drug names that fall under each medication class, the SNBHW definition uses the Anatomical Therapeutic Chemical (ATC) Classification System, which defines drug names under each code. Specifically, the following ATC drug codes are assessed for the SNBHW definition: antidepressants (N06A), antipsychotics (N05A; N05AN excluded), opioids (N02A), anxiolytics (N05B), hypnotics and sedatives (N05C), vasodilators used in cardiac diseases (C01D), antihypertensives (C02), diuretics (C03), beta-blockers (C07), calcium channel blockers (C08), dopaminergic agents (anti-Parkinson drugs; N04B), agents acting on the renin-angiotensin system (RAS acting agents; C09), and alpha-blockers (G04CA) [5]. Antihypertensives classified by code C02 included in our analysis were centrally acting, ganglion-blocker, and peripherally acting antiadrenergic agents, agents acting on arteriolar smooth muscles, and antihypertensives for pulmonary arterial hypertension (Additional file 2 – Supplementary Table 3). The classifications of STEADI-Rx drugs were determined using pre-set drug classes developed in the Health ABC Study along with consultation of the ATC codes and a pharmacist investigator for the study (Additional file 2 – Supplementary Table 2) [15]. Each FRID class was encoded for our study using ingredient codes. All participants with diabetes taking hypoglycemic medications were also using another FRID and therefore were classified as FRID users though hypoglycemic medications were not included in the count of FRIDs.
Statistical methods
Descriptive statistics were calculated and stratified by FRID use (yes/no) for both definitions to determine statistically significant differences. T-tests or Wilcoxon rank-sum tests were used for continuous variables, and Chi-Square tests or Fisher’s Exact tests for categorical variables. The prevalence of each FRID class was calculated for the sample and included overlap in participants taking ≥1 medication. We also calculated the prevalence of each FRID class for participants taking only 1 FRID class. Risk factors associated with FRID class use and count of FRID classes were modeled using multivariable logistic regression and negative binomial regression, respectively. For the primary analysis using multivariable logistic regression modeling FRID class use (yes/no), covariate factors of interest were entered in groups, where covariates were removed at p≥0.1 except for age, race, and sex which were included regardless of significance since these demographic characteristics are associated with other exposures. The secondary analysis using negative binomial regression to model the count of FRIDs entered covariates in groups, with covariates removed at p≥0.1. Age, race, and sex were also included in the count of FRIDs models regardless of significance. For both the primary and secondary analyses, models were built progressively starting with unadjusted models and progressed by adding age, race, and sex for minimally adjusted models. The models were further adjusted by groups of covariates (1) Model 1: other demographics, (2) Model 2: Model 1 + lifestyle and behavioral factors, and finally (3) Model 3: Model 2 + multimorbidity and count of non-FRIDs. Final models included the same covariates for both definitions for comparability of results, including non-significant variables if significant for the final model of either definition. Collinearity of all covariates was assessed prior to the modeling. Two separate sensitivity analyses were done with variables excluded from initial model building due to high missingness: (1) forcing marital status (26% missing) and income (8% missing) into the final models, and (2) forcing cystatin C (10% missing) and peripheral arterial disease (19% missing) into the final models. All analyses were conducted using SAS software (version 9.4; SAS Institute, Cary, NC) with LOGISTIC and GENMOD procedures to obtain the main results.
Results
Of 1,352 participants, 1,176 (87.0%) were using FRIDs under the SNBHW definition, and 1,171 (86.6%) were using FRIDs under the STEADI-Rx definition in Chi-Square analysis. Of users, 23 were classified as ‘yes’ under the SNBHW definition to ‘no’ under the STEADI-Rx definition, and 18 were classified as from ‘yes’ under STEADI-Rx definition to ‘no’ under the SNBHW definition. Univariate descriptive statistics showed participants with FRID use vs. no use under the SNBHW definition were more likely to be married, have a hospitalization in the prior year, lower physical activity from walking and stair climbing, a higher BMI, a higher prevalence of hypertension, poor renal function, diabetes, higher CES-D scores, and take more non-FRIDs. In addition to these factors, except for income, participants with FRID use under the STEADI-Rx definition were less likely to report vison impairment (poor/very poor/blind vs. excellent vision) and have a higher 3MS score (Additional file 1 – Table 1).
Medication prevalence
Of FRID users as defined by the SNBHW, overall cardiac medications were used by 82.9% of participants (Additional file 2 – Supplementary Table 1). More specifically, RAS acting agents were used by 43.5% of participants, with similar prevalence of some other antihypertensive medications: diuretics, beta-blockers, and calcium channel blockers (Table 2). Prevalence of alpha-blocker, antihypertensive, and nitrate use was much lower. Of the other FRID classes defined by the SNBHW, antidepressants were used by 12.4% of participants, followed by opioids, benzodiazepines, sedative hypnotics, anti-Parkinson drugs, and antipsychotics. Moreover, 334 participants (24.7%) were using just one of the SNBHW FRID classes, primarily consisting of antihypertensive medications. A higher number of women than men used SNBHW antihypertensives. Of FRID users as defined by the STEADI-Rx definition, overall cardiac medications were used by 80.5% of participants, which could similarly be described for comparability with the SNBHW definition as antihypertensives (6.5%), RAS acting agents (43.4%), diuretics (40.8%), beta-blockers (38.6%), calcium channel blockers (33.1%), alpha-blockers (9.5%), and nitrates (4.5%) (Additional file 2 – Supplementary Table 1, Table 2). The prevalence for all other FRIDs use like opioids, antidepressants, muscle relaxants, benzodiazepines, anticonvulsants, antihistamines, sedative hypnotics, and antipsychotics was much lower than overall cardiac medication use (Table 2). Lastly, 707 participants (52.3%) were using just one of the STEADI-Rx FRID classes, which also primarily consisted of antihypertensives. A higher number of women than men also used STEADI-Rx antihypertensives.
Table 2.
SNBHW and STEADI-Rx FRID class prevalence (%): Overall and for 1 FRID class only
| Medication Classes, % | SNBHW | STEADI-Rx | ||
|---|---|---|---|---|
| Overall (N = 1,352) | Medication-Specific Use only* (N = 331) | Overall (N = 1,352) | Medication-Specific Use only* (N = 707) | |
| Antidepressants | 12.4 | 1.3 | 11.1 | 1.2 |
| Antipsychotics | 1.1 | 0.1 | 1.1 | 0.1 |
| Opioids | 6.2 | 0.5 | 16.1 | 1.2 |
| Benzodiazepines | 5.9 | 0.8 | 8.1 | 0.7 |
| Sedative hypnotics | 3.6 | 0.1 | 2.1 | 0.1 |
| Anticonvulsants | - | - | 7.0 | 0.3 |
| Muscle Relaxants | - | - | 9.3 | 0.5 |
| Antihistamines | - | - | 3.5 | 0 |
| **Antihypertensives | 6.5 | 0.3 | 6.5 | 0.2 |
| **Diuretics | 40.8 | 3.3 | 40.8 | 3.0 |
| **Beta-Blocker | 38.9 | 5.5 | 38.6 | 4.1 |
| **Calcium Channel Blocker | 33.7 | 4.9 | 33.1 | 3.9 |
| **RAS acting agents | 43.5 | 5.8 | 43.4 | 5.3 |
| **Alpha-blocker | 11.0 | 1.5 | 9.5 | 0.2 |
| **Nitrate | 7.5 | 0.1 | 4.5 | 0 |
| **Anti-Parkinson Drugs | 2.1 | 0.3 | - | - |
*Percentages shown as a proportion of overall use
**The STEADI-Rx cardiac medication list has been modified for comparability with the SNBHW definition, which uses ATC codes to define these categories (see Outcomes: FRID Class Use & Count of FRIDs)
-: Not a FRID for respective definition
Multivariable logistic regression
In the final multivariable analysis using the SNBHW definition (Table 3), higher BMI, physiological hypertension, higher 3MS score, and higher number of non-FRIDs were associated with higher odds for FRID use. Poor vs. excellent vision and higher DSST score were associated with lower odds of FRID use. Except for CES-D score, findings were consistent for the fully adjusted multivariable analysis using the STEADI-Rx definition (Table 3). CES-D score was associated with higher odds of FRID use using the STEADI-Rx definition.
Table 3.
Risk factors for FRID use (yes/no) from the final multivariable logistic regression models
| Risk Factors | SNBHW Definition | STEADI-Rx Definition |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Age (years), per SD | 0.93 (0.76, 1.13) | 0.96 (0.78, 1.17) |
| Black race, y/n | 0.81 (0.52, 1.27) | 0.96 (0.61, 1.51) |
| Women, y/n | 0.96 (0.66, 1.40) | 1.32 (0.91, 1.93) |
| BMI (kg/m2), per SD | 1.53 (1.24, 1.89)** | 1.54 (1.25, 1.90)** |
| Hypertension, y/n | 3.07 (1.98, 4.76)** | 3.05 (1.98, 4.69)** |
| Vision (Poor/Very Poor/Completely Blind vs. Excellent), y/n | 0.32 (0.14, 0.78)** | 0.26 (0.11, 0.59)** |
| CES-D Score, per SD | 1.24 (0.98, 1.57) | 1.36 (1.07, 1.74)** |
| 3MS Score, per SD | 1.60 (1.23, 2.08)** | 1.69 (1.30, 2.19)* |
| DSST Score, per SD | 0.75 (0.57, 0.97)* | 0.76 (0.59, 0.99)* |
| # Non-FRIDs, per SD | 3.67 (2.67, 5.06)** | 3.03 (2.24, 4.08)** |
*p < 0.05
**p < 0.01
Abbreviations: ADL, Activities of Daily Living; CES-D, Centers for Epidemiologic Studies Depression Scale; 3MS, Modified Mini-Mental State Examination; DSST, Digit Symbol Substitution Test
Sensitivity analyses for multivariable logistic regression
Being never married vs. married (OR = 0.34, 95% CI: 0.14, 0.83) was associated with a lower odds of FRID use for the STEADI-Rx definition and for the SNBHW definition (OR = 0.34, 95% CI: 0.14, 0.81) in the final multivariable logistic regression models. Income, insufficient renal function, and PAD were not related to either the SNBHW definition or the STEADI-Rx definition.
Multivariable negative binomial regression
BMI, physiological hypertension, CES-D score, and number of non-FRIDs were associated with higher incidence rates of FRIDs count using both the SNBHW and STEADI-Rx definition (Table 4). Sleep problems were associated with lower incidence rates for both definitions, though women had a lower incidence rate of FRIDs count for only the SNBHW definition. Additionally, under the STEADI-Rx definition, 3MS score was associated with a higher incidence rate of FRIDs count and DSST was associated with a lower incidence rate (Table 4).
Table 4.
Risk factors for Count of FRIDs (#) from the final multivariable negative binomial regression models
| Risk Factors | SNBHW Definition | STEADI-Rx Definition |
|---|---|---|
| IRR (95% CI) | IRR (95% CI) | |
| Age (years), per SD | 1.00 (0.95, 1.04) | 0.99 (0.95, 1.04) |
| Black race, y/n | 0.91 (0.74, 1.11) | 0.86 (0.71, 1.05) |
| Women, y/n | 0.76 (0.64, 0.90)** | 1.04 (0.88, 1.24) |
| BMI (kg/m2), per SD | 1.10 (1.05, 1.15)** | 1.13 (1.09, 1.18)** |
| Hypertension, y/n | 1.36 (1.20, 1.53)** | 1.40 (1.24, 1.58)** |
| Sleep Problems, y/n | 0.91 (0.83, 1.00)* | 0.88 (0.81, 0.96)* |
| CES-D Score, per SD | 1.10 (1.06, 1.15)** | 1.10 (1.06, 1.15)** |
| 3MS Score, per SD | 1.05 (0.99, 1.13) | 1.09 (1.02, 1.16)* |
| DSST Score, per SD | 0.94 (0.89, 1.00) | 0.93 (0.88, 0.99)* |
| # Not FRIDs, per SD | 1.27 (1.22, 1.31)** | 1.27 (1.22, 1.31)** |
*p < 0.05
**p < 0.01
Abbreviations: ADL, Activities of Daily Living; CES-D, Centers for Epidemiologic Studies Depression Scale; 3MS, Modified Mini-Mental State Examination; DSST, Digit Symbol Substitution Test
Sensitivity analyses for multivariable negative binomial regression
No association was found in the final multivariable negative binomial models between marriage or income and the count of FRIDs. However, insufficient renal function was associated with a higher rate of the count of FRIDs using both the SNBHW definition (IRR = 1.21, 95% CI: 1.10, 1.34) and the STEADI-Rx definition (IRR = 1.18, 95% CI: 1.07, 1.30), and PAD was associated with FRIDs count for the SNBHW definition (IRR = 1.15, 95% CI: 1.03, 1.28) but not the STEADI-Rx definition.
Discussion
To our knowledge, our study was the first to describe the association of a wide range of risk factors for FRID use in older Black and White men and women and to compare the risk factor associations across two common FRID definitions, SNBHW and STEADI-Rx. In community-dwelling older Black and White adults, having hypertension and higher BMI, higher 3MS score, and number of non-FRIDs were associated with a higher odds of FRID use and higher rate of FRID counts, suggesting that multimorbidity in older adults is associated with FRID use. Additionally, the number of medications may be higher in older adults with higher BMI [26] due to the comorbidity associated with obesity [27]. Older adults with higher BMI also may be more likely to have hypertension [28]. One previous Health ABC Study showed that BMI, 3MS score, hypertension, and number of non-FRIDs, may be higher with antihypertensive users than non-antihypertensive users, though these comparisons were not statistically tested [15]. The SNBHW and STEADI-Rx definitions of FRIDs classified antihypertensives similarly overall as described in the methods.
We found some differences in statistical significance between factors using the two definitions, although ORs and IRRs were generally similar. Only one factor (women) had different associations for FRID use (y/n) and the count of FRIDs when using the SNBHW and the STEADI-Rx definition, which may be due to differences in the medication classes included within each definition. For example, the SNBHW definition included anti-Parkinson drugs, which were not included in the STEADI-Rx definition, though the prevalence of those using those drugs were low. Medication classes found in the STEADI-Rx but not the SNBHW definition included muscle relaxants, antihistamines, and anticonvulsants, which all had fairly low prevalence also. Higher CES-D scores were associated with STEADI-Rx FRID use, and lower DSST scores were associated with SNBHW FRID use. Women vs. men had a lower SNBHW FRIDs count, which may reflect more antihypertensive-only users for women than men in this definition for our participants (see Table 4). Higher depressive symptoms may be associated with higher FRID counts since depression in older adults is also associated with chronic diseases such as cardiovascular disease, chronic pain and fatigue [29] which have higher FRID use of antihypertensives, opioids and anticonvulsants [30–32]. Participants with sleep problems had a lower rate of FRIDs count, which seems unusual given that antihypertensives were the most common FRID and cardiovascular disease is often associated with sleep issues [33].
Lastly, we found some additional unexpected risk factors for FRID use. Participants with higher 3MS scores had a higher rate of STEADI-Rx FRIDs count, and higher DSST scores associated with a lower rate of FRIDs count. DSST scores were in the expected direction showing participants with higher DSST scores were associated with lower FRIDs count. 3MS scores were in the opposite direction and showed participants with lower cognition were associated with lower FRIDs count. The DSST has often been associated with psychomotor speed, while the 3MS included components of global cognition like orientation, concentration, language, praxis, and immediate and delayed memory [34]. It may have been that the medications for those with lower cognition were more closely scrutinized, resulting in lower FRID use. These results also suggest other FRIDs besides antihypertensives may be responsible for associations as prior Health ABC Study showed no difference in 3MS scores among antihypertensive users vs. non-antihypertensive users [15]. In other studies, opioid and sedative hypnotic use were associated with a higher risk of cognitive impairment [35, 36]. Poor vision was associated with a lower odds of FRID counts for both definitions. One study has suggested that both poor visual input and correcting poor visual input should be monitored in older adults, as both were associated with an increased risk of falls [37]. Therefore, older adults with poor vision are possibly more closely monitored for multiple FRID use to not exacerbate fall risk, resulting in lower use. These unexpected risk factors for FRIDs should be investigated further to confirm our findings.
Only one known study has been published assessing risk factors related to FRID use under the SNBHW definition, and no known studies have assessed risk factors related to use by the STEADI-Rx definition [5]. In the previous study using the SNBHW definition, higher total number of medications, severe falls in the previous year (defined as falls leading to emergency visits at hospitals or hospital admission as a consequence of syncope, contusion, or clinical bone fracture), and women had higher count of FRIDs used, though no other risk factors were assessed [5]. Apart from women having a higher count of FRIDs, these factors from the previous study were similar to ours, but our study additionally reported the total number of non-FRIDs and fall history over the past year instead of severe falls. Importantly, self-reported falls over the past year and severe falls in the past year likely have varying validity. These findings likely show that both less serious and more serious falls lead to higher count of FRIDs. Additionally, the most prevalent FRIDs in our study and the previous study were classes of antihypertensives and antidepressants, thereby providing some comparability between the studies [5]. One major difference between our study and this previous study is our study included only community-dwelling older adults, which may explain why some results did not align. The previous study was comprised primarily of participants living in nursing homes (76%), with community-dwelling older adults (24%) making up a smaller percentage of the participants [5]. Their findings suggest that women in nursing homes may have had higher FRID use than community-dwelling women in our study. Those in nursing homes may have unique risk factors leading to FRID use such as higher multimorbidity vs. community-dwelling older adults. Moreover, higher multimorbidity in the nursing home participants could indicate a higher rate of major depression, which is an indication for antidepressant use [38, 39], or differences in their level of pain severity, which would require different dosages and frequencies of opioid use which may also impact fall risk, with higher dosages and frequencies being associated with higher fall risk [40]. Residency in nursing homes is also associated with severe falls, which may indicate higher multimorbidity or more frailty among residents vs. community-dwelling older adults. Future studies can further explore additional measures of interest related to fall or fall injury risk to assess whether these factors can help further define the populations of older adults who use FRIDs [41]. High-risk populations, like older adults with hypertension, diabetes, chronic kidney disease, and insomnia, also need investigation for FRID use, as these are common indications of FRIDs use by prevalence of disease in older adults.
Neither age, site, nor education were significant risk factors for either definition for either FRID use (yes/no) or the FRIDs count. All of the participants in our sample were aged 79–89 years, therefore a fairly narrowly aged cohort. Future studies using a wider age range of participants are needed to further explore this finding. Additionally, our finding for education was similar to an existing study assessing anticholinergic users vs. non-users [18] but dissimilar to a study assessing antihypertensives users vs. non-users [15]. Therefore, at some of the oldest ages, education may only predict use of certain FRIDs [42].
Though studies have demonstrated that women and White older adults were more likely to fall than men and Black older adults, our study did not find that these demographics were associated with FRID use (yes/no) [43, 44]. The absence of a finding may be due to homogeneity in our populations since all participants in the Health ABC Study were Medicare eligible and able to attend a clinic visit. However, even after the implementation of Medicare Part D, which was introduced to reduce out-of-pocket medication costs and lower the cost of prescription drugs, differences still existed in cost-related nonadherence and benefits from Medicare Part D between non-Hispanic Black and White older adults [45, 46]. This lack of a differential effect for FRID use by race could be explained by differences in Medicare Part D enrollment among the Health ABC Study participants in the 2007-08 visit, leading to a more balanced sample in terms of medication coverage, and consequently medication use, than the general public. It may also be that older White and Black participants in the Health ABC Study were more balanced in indications vs. the general population. Interestingly, women had a lower rate of FRIDs count according to the SNBHW definition, though men (85.1%) and women (85.0%) ≥60 years in the US in 2015–2016 had similar use of all prescription medications (one or more) according to the National Health and Nutrition Examination Survey using the “brown bag” review method [47]. It may be that FRIDs in the SNBHW definitions or the indications of the FRIDs included are more associated with men than women. Since prevalence data for individual FRID use is not published by sex for many of the older adult populations, comparing our results to past studies is not currently possible. Further studies are needed to understand this lower use of FRIDs among women and to evaluate other racial group associations with FRID use, such as Hispanic, Native American, Asian, and Pacific Islander older adults.
Current recommendations on fall prevention provided by the United States Preventive Services Task Force (USPSTF) do not include FRID definitions. USPSTF guidelines focus on exercise interventions while regulating medication management within a level of a multifactorial intervention – multistage interventions in which modifiable risk factors are assessed first and the then subsequent interventions are tailored to each patient or participant based on the first assessment [48]. However, the guidelines do not focus specifically on FRID medication management. Our results inform future interventions on medication management of factors related to FRID use, thus providing foundational knowledge for research on FRIDs and fall/fall injury risk and later allowing for a more targeted approach in recruiting participants.
Our study had some limitations that should be noted. First, participants brought only prescription medications to the clinic visit that were used in the past 30 days. Thus, older adults who use medications as needed, like for opioids, or use over-the-counter seasonal medications, like for antihistamines, may be misclassified as FRID non-users if they had not used the medication in the previous 30 days. The brown bag review itself also may have inadvertently had the effect to reduce inappropriate drug use in participants. Second, cardiac medications constituted the majority of FRID users by either definition, thus our analyses for the counts of FRIDs used is more heavily influenced by the cardiac medications than the other FRID classes of medications. Next, our population is community-dwelling older adults who met the inclusion criteria of the Health ABC Study and were able to attend an in-person visit more than a decade after baseline, which likely resulted in a healthier cohort compared to the general population. Our results may not be generalizable to non-community-based settings, like hospitals or long-term care settings. Finally, STOPPFall (Screening Tool of Older Persons Prescriptions in older adults with high fall risk), a list of 14 medication classes defined as FRIDs that was published in December 2020, fell outside the scope of this analysis [49]. Future analyses could investigate whether the same risk factors from the SNBHW and STEADI-Rx definitions are associated with the STOPPFall definition of FRIDs.
Our study has several strengths. We evaluated many risk factors with two common FRIDs definition at the same time, which has not previously been published. Our medication inventory from the “brown bag” review method was based on participants’ use rather than prescription fill or pharmacy databases, which may be less accurate [50]. Additionally, we assessed numerous potential risk factors, including some with indications for individual FRID use (e.g., physiologic hypertension, depressive symptoms, etc.) due to the wealth of cohort data. Furthermore, the mean age in our study population was ≥80 years old, the population with the highest prevalence of FRID use [3]. Our study population also included a large percentage of Black older adults, a group typically understudied for FRIDs and fall risk factors.
Conclusion
Our study showed that of the risk factors for FRID use in the population, many were same for each definition of FRIDs. Future studies targeting FRID use and fall prevention efforts may benefit from an understanding the population of older adults that uses FRIDs.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Abbreviations
- FRIDs:
Fall-risk increasing drugs
- STEADI-Rx
Stopping Elderly Accidents, Deaths & Injuries
- SNBHW:
Swedish National Board of Health and Welfare
- RAS:
Renin-angiotensin-system
- BMI:
Body mass index
- ADL:
Activities of Daily Living
- CES-D:
Center for Epidemiologic Studies Depression Scale
- 3MS:
Modified Mini-Mental State Examination
- DSST:
Digit Symbol Substitution Test
- PAD:
Peripheral arterial disease
- USPSTF:
United States Preventive Services Task Force
Author contributions
JER: Participated in study concept and design, data analysis and interpretation, and preparation of the manuscript. RMB: Participated in study concept and design, data analysis and interpretation, and preparation of the manuscript. KSF: Participated in data analysis and interpretation and preparation of the manuscript. LX: Participated in study concept and design, data analysis and interpretation, and preparation of the manuscript. KMR: Participated in data interpretation and preparation of the manuscript. JMB: Participated in data interpretation and preparation of the manuscript. JAP: Participated in interpretation of data and preparation of the manuscript. JAC: Participated in study concept and design, data interpretation, and preparation of the manuscript. ESS: Participated in study concept and design, data analysis and interpretation, and preparation of the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by the National Institutes of Health/National Institute of Aging T32AG000181, Training in Epidemiology of Aging and National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and National Institute of Nursing Research grant R01-NR012459; This research was funded in part by the Intramural Research Program of the NIH, National Institute on Aging. This work was also supported by NIH/NIA grant R01-AG061136.
Data availability
This study was based on the dataset from the Health ABC Study online through the National Institute on Aging’s website at https://healthabc.nia.nih.gov. The data is available to approved researchers as noted in the Health ABC Study Publication Guidelines.
Declarations
Ethics approval and consent to participate
This study used de-identified data from the Health, Aging, and Body Composition (Health ABC) Study. The NIA institutional review board approved the study protocol. All participants signed an informed consent form that was approved by the institutional review boards of the University of Pittsburgh and the University of Tennessee.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Sponsor’s role
The funding institutes had no role in the design, methods, subject recruitment, data collection, analysis, and preparation of manuscript or in the decision to submit the manuscript for publication.
Dual publication
The manuscript has not been published elsewhere and reviewed by any other journal. This abstract of this paper was presented in part at the Gerontological Society of America Annual Scientific Meeting in November 2022.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Correa-Pérez A, Delgado-Silveira E, Martín-Aragón S, Cruz-Jentoft AJ. Fall-risk increasing drugs and recurrent injurious falls association in older patients after hip fracture: a cohort study protocol. Ther Adv Drug Saf. 2019;10:2042098619868640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreri SP, Blalock SJ, Robinson JM, Renfro CP, Busby-Whitehead J, Burns ER, et al. STEADI-Rx: guide for community pharmacists. National Center for Injury Prevention and Control of the Centers for Disease Control and Prevention; 2020.
- 3.Shaver AL, Clark CM, Hejna M, Feuerstein S, Wahler RG, Jacobs DM. Trends in fall-related mortality and fall risk increasing drugs among older individuals in the United States,1999–2017. Pharmacoepidemiol Drug Saf. 2021;30(8):1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drew JAR, Xu D. Trends in Fatal and Nonfatal injuries among older americans, 2004–2017. Am J Prev Med. 2020;59(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milos V, Bondesson Å, Magnusson M, Jakobsson U, Westerlund T, Midlöv P. Fall risk-increasing drugs and falls: a cross-sectional study among elderly patients in primary care. BMC Geriatr. 2014;14(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cox N, Ilyas I, Roberts HC, Ibrahim K. Exploring the prevalence and types of fall-risk-increasing drugs among older people with upper limb fractures. Int J Pharm Pract. 2023;31(1):106–12. [DOI] [PubMed] [Google Scholar]
- 7.Doshi A, Miller GE. Trends in the Use of Diuretics to Treat Hypertension, 1997 and 2003. Agency for Healthcare Research and Quality; 2006 Mar [cited 2022 Sep 19]. Report No.: 120. https://meps.ahrq.gov/data_files/publications/st120/stat120.shtml
- 8.Anderson TS, Ayanian JZ, Zaslavsky AM, Souza J, Landon BE. National trends in antihypertensive treatment among older adults by race and presence of comorbidity, 2008 to 2017. J Gen Intern Med. 2022. [DOI] [PMC free article] [PubMed]
- 9.Lin Y-H, Huang W-Y, Chang C-C, Chen Y-F, Wu L-Y, Chang H-C, et al. Trends in the use of antimuscarinics and alpha-adrenergic blockers in women with lower urinary tract symptoms in Taiwan: a nationwide, population-based study, 2007–2012. PLoS ONE. 2019;14(10):e0220615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ham AC, Swart KMA, Enneman AW, van Dijk SC, Oliai Araghi S, van Wijngaarden JP, et al. Medication-related fall incidents in an older, ambulant population: the B-PROOF study. Drugs Aging. 2014;31(12):917–27. [DOI] [PubMed] [Google Scholar]
- 11.Haasum Y, Fastbom J, Johnell K. Use of fall-risk inducing drugs in patients using Anti-parkinson drugs (APD): a Swedish Register-based study. PLoS ONE. 2016;11(8):e0161246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hálfdánarson Ó, Zoëga H, Aagaard L, Bernardo M, Brandt L, Fusté AC, et al. International trends in antipsychotic use: a study in 16 countries, 2005–2014. Eur Neuropsychopharmacol. 2017;27(10):1064–76. [DOI] [PubMed] [Google Scholar]
- 13.Montastruc F, Bénard-Laribière A, Noize P, Pambrun E, Diaz-Bazin F, Tournier M, et al. Antipsychotics use: 2006–2013 trends in prevalence and incidence and characterization of users. Eur J Clin Pharmacol. 2018;74(5):619–26. [DOI] [PubMed] [Google Scholar]
- 14.Newman AB, Haggerty CL, Kritchevsky SB, Nevitt MC, Simonsick EM, Health ABC Collaborative Research Group. Walking performance and cardiovascular response: associations with age and morbidity–the Health, Aging and Body Composition Study. J Gerontol Biol Sci Med Sci. 2003;58(8):715–20. [DOI] [PubMed] [Google Scholar]
- 15.Marcum ZA, Perera S, Newman AB, Thorpe JM, Switzer GE, Gray SL, et al. Antihypertensive Use and Recurrent Falls in Community-Dwelling older adults: findings from the Health ABC Study. J Gerontol Biol Sci Med Sci. 2015;70(12):1562–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sagawa N, Marcum ZA, Boudreau RM, Hanlon JT, Albert SM, O’Hare C, et al. Low blood pressure levels for fall injuries in older adults: the Health, Aging and Body Composition Study. Eur J Ageing. 2018;15(3):321–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbour KE, Sagawa N, Boudreau RM, Winger ME, Cauley JA, Nevitt MC, et al. Knee osteoarthritis and the risk of medically treated Injurious Falls among older adults: A Community-based US Cohort Study. Arthritis Care Res (Hoboken). 2019;71(7):865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marcum ZA, Perera S, Thorpe JM, Switzer GE, Gray SL, Castle NG, et al. Anticholinergic Use and Recurrent Falls in Community-Dwelling older adults: findings from the Health ABC Study. Ann Pharmacother. 2015;49(11):1214–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Introducing the Health ABC Study. The Dynamics of Health, Aging, and Body Composition. [cited 2022 Apr 19]. https://healthabc.nia.nih.gov/
- 20.Xue L, Boudreau RM, Donohue JM, Zgibor JC, Marcum ZA, Costacou T, et al. Persistent polypharmacy and fall injury risk: the Health, Aging and Body Composition Study. BMC Geriatr. 2021;21(1):710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roberts RE, Vernon SW. The Center for epidemiologic studies Depression Scale: its use in a community sample. Am J Psychiatry. 1983;140(1):41–6. [DOI] [PubMed] [Google Scholar]
- 22.Mehta KM, Simonsick EM, Rooks R, Newman AB, Pope SK, Rubin SM, et al. Black and white differences in cognitive function test scores: what explains the difference? J Am Geriatr Soc. 2004;52(12):2120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shlipak MG, Katz R, Sarnak MJ, Fried LF, Newman AB, Stehman-Breen C, et al. Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med. 2006;145(4):237–46. [DOI] [PubMed] [Google Scholar]
- 24.Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation. 2012;126(24):2890–909. [DOI] [PubMed] [Google Scholar]
- 25.Marcum ZA, Perera S, Donohue JM, Boudreau RM, Newman AB, Ruby CM, et al. Analgesic use for knee and hip osteoarthritis in community-dwelling elders. Pain Med. 2011;12(11):1628–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Randhawa AK, Parikh JS, Kuk JL. Trends in medication use by body mass index and age between 1988 and 2012 in the United States. PLoS ONE. 2017;12(9):e0184089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim Y, Boster J. Obesity and comorbid conditions. StatPearls. Treasure Island (FL): StatPearls Publishing; 2024. [PubMed] [Google Scholar]
- 28.Landi F, Calvani R, Picca A, Tosato M, Martone AM, Ortolani E et al. Body Mass Index is strongly Associated with Hypertension: results from the longevity check-up 7 + study. Nutrients. 2018;10(12). [DOI] [PMC free article] [PubMed]
- 29.Goodwin GM. Depression and associated physical diseases and symptoms. Dialogues Clin Neurosci. 2006;8(2):259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosenblum A, Marsch LA, Joseph H, Portenoy RK. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp Clin Psychopharmacol. 2008;16(5):405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castro-Marrero J, Sáez-Francàs N, Santillo D, Alegre J. Treatment and management of chronic fatigue syndrome/myalgic encephalomyelitis: all roads lead to Rome. Br J Pharmacol. 2017;174(5):345–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart J, Manmathan G, Wilkinson P. Primary prevention of cardiovascular disease: a review of contemporary guidance and literature. JRSM Cardiovasc Dis. 2017;6:2048004016687211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagai M, Hoshide S, Kario K. Sleep duration as a risk factor for cardiovascular disease- a review of the recent literature. Curr Cardiol Rev. 2010;6(1):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rosano C, Perera S, Inzitari M, Newman AB, Longstreth WT, Studenski S. Digit symbol substitution test and future clinical and subclinical disorders of cognition, mobility and mood in older adults. Age Ageing. 2016;45(5):688–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neelamegam M, Zgibor J, Chen H, O’rourke K, Bakour C, Rajaram L, et al. The effect of opioids on the cognitive function of older adults: results from the personality and total health through life study. Age Ageing. 2021;50(5):1699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martin P, Tannenbaum C. Use of the EMPOWER brochure to deprescribe sedative-hypnotic drugs in older adults with mild cognitive impairment. BMC Geriatr. 2017;17(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambrose AF, Paul G, Hausdorff JM. Risk factors for falls among older adults: a review of the literature. Maturitas. 2013;75(1):51–61. [DOI] [PubMed] [Google Scholar]
- 38.Dow B, Lin X, Tinney J, Haralambous B, Ames D. Depression in older people living in residential homes. Int Psychogeriatr. 2011;23(5):681–99. [DOI] [PubMed] [Google Scholar]
- 39.Sheffler ZM, Patel P, Abdijadid S, Antidepressants. StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. [PubMed]
- 40.Virnes R-E, Tiihonen M, Karttunen N, van Poelgeest EP, van der Velde N, Hartikainen S. Opioids and falls risk in older adults: a narrative review. Drugs Aging. 2022;39(3):199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu Q, Ou X, Li J. The risk of falls among the aging population: a systematic review and meta-analysis. Front Public Health. 2022;10:902599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naji A, Gatling JW. Muscarinic antagonists. StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 43.Geng Y, Lo JC, Brickner L, Gordon NP. Racial-ethnic differences in fall prevalence among older women: a cross-sectional survey study. BMC Geriatr. 2017;17(1):65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang VC, Do MT. Risk factors for falls among seniors: implications of gender. Am J Epidemiol. 2015;181(7):521–31. [DOI] [PubMed] [Google Scholar]
- 45.Bakk L. Racial/Ethnic differences in cost-related nonadherence and Medicare Part D: a longitudinal comparison. J Health Care Poor Underserved. 2015;26(4):1132–48. [DOI] [PubMed] [Google Scholar]
- 46.Jamison JJ, Wang J, Surbhi S, Adams S, Solomon D, Hohmeier KC, et al. Impact of medicare part D on racial and ethnic minorities. Divers Equal Health Care. 2016;13(5):326–33. [PMC free article] [PubMed] [Google Scholar]
- 47.Martin CB, Hales CM, Gu Q, Cynthia OL. Products - Data Briefs - Number 332 - February 2019. 2019 [cited 2023 Mar 6]. https://www.cdc.gov/nchs/products/databriefs/db334.htm
- 48.Recommendation. Falls Prevention in Community-Dwelling Older Adults: Interventions | United States Preventive Services Taskforce. [cited 2021 Nov 2]. https://uspreventiveservicestaskforce.org/uspstf/recommendation/falls-prevention-in-older-adults-interventions
- 49.Seppala LJ, Petrovic M, Ryg J, Bahat G, Topinkova E, Szczerbińska K, et al. STOPPFall (Screening Tool of older persons prescriptions in older adults with high fall risk): a Delphi study by the EuGMS Task and Finish Group on fall-risk-increasing drugs. Age Ageing. 2021;50(4):1189–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caskie GIL, Willis SL, Warner Schaie K, Zanjani FAK. Congruence of medication information from a brown bag data collection and pharmacy records: findings from the Seattle longitudinal study. Exp Aging Res. 2006;32(1):79–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study was based on the dataset from the Health ABC Study online through the National Institute on Aging’s website at https://healthabc.nia.nih.gov. The data is available to approved researchers as noted in the Health ABC Study Publication Guidelines.