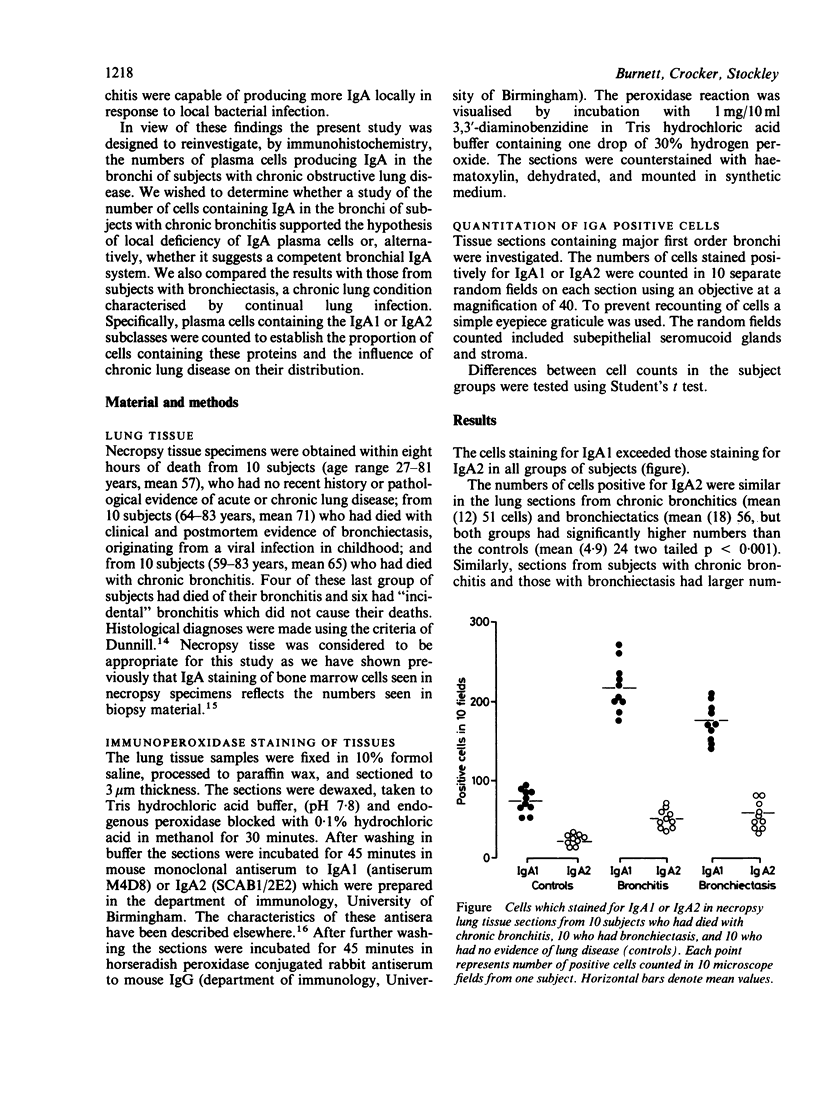
Abstract
Necropsy specimens were obtained from the lungs of 10 subjects who had no history of lung disease, 10 who had died with chronic bronchitis, and 10 with bronchiectasis. Tissue sections were stained for IgA1 or IgA2 using the immunoperoxidase technique, and the number of cells in the bronchi stained for these proteins was counted. The total number of IgA positive cells was increased in bronchitic and bronchiectatic lungs compared with those from control subjects. The number of IgA2 positive cells was similar in those with bronchitis and bronchiectasis and significantly higher than in controls. Similarly, cells containing IgA1 were increased in the lungs of subjects with chest disease but were higher in those with bronchitis than in those with bronchiectasis. The proportion of IgA2:total IgA containing cells was similar in sections from controls (mean (SD) 25 (5.0)%) and those with bronchiectasis (mean (SD) 24 (4)%), but lower in those with bronchitis (mean (SD) 19 (5.0)%). The results show that cells containing IgA1 predominate in the major bronchi but that the proportion of cells containing IgA2 is higher than in non-mucosal lymphoid tissues. Bronchitis and bronchiectasis are associated with greater numbers of cells producing IgA in the bronchi, and this is consistent with increased local production of IgA in the lung secretions of bronchitic subjects.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André C., André F., Fargier C. Distribution of IgA 1 and IgA 2 plasma cells in various normal human tissues and in the jejunum of plasma IgA-deficient patients. Clin Exp Immunol. 1978 Aug;33(2):327–331. [PMC free article] [PubMed] [Google Scholar]
- Burnett D. Immunoglobulins in the lung. Thorax. 1986 May;41(5):337–344. doi: 10.1136/thx.41.5.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacroix D. L., Marchandise F. X., Francis C., Sibille Y. Alpha-2-macroglobulin, monomeric and polymeric immunoglobulin A, and immunoglobulin M in bronchoalveolar lavage. Am Rev Respir Dis. 1985 Oct;132(4):829–835. doi: 10.1164/arrd.1985.132.4.829. [DOI] [PubMed] [Google Scholar]
- Farris M. A., Hardie D., de Lange G., Jefferis R. Immunogenic and antigenic epitopes of immunoglobulins. X: Monoclonal antibodies specific for human IgA, the IgA1 and IgA2 subclasses and an nA2m(2) iso-allotypic epitope. Vox Sang. 1985;48(2):116–121. doi: 10.1111/j.1423-0410.1985.tb00155.x. [DOI] [PubMed] [Google Scholar]
- Haimoto H., Nagura H., Imaizumi M., Watanabe K., Iijima S. Immunoelectronmicroscopic study on the transport of secretory IgA in the lower respiratory tract and alveoli. Virchows Arch A Pathol Anat Histopathol. 1984;404(4):369–380. doi: 10.1007/BF00695221. [DOI] [PubMed] [Google Scholar]
- Kilian M., Mestecky J., Kulhavy R., Tomana M., Butler W. T. IgA1 proteases from Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis, and Streptococcus sanguis: comparative immunochemical studies. J Immunol. 1980 Jun;124(6):2596–2600. [PubMed] [Google Scholar]
- Mostov K. E., Blobel G. A transmembrane precursor of secretory component. The receptor for transcellular transport of polymeric immunoglobulins. J Biol Chem. 1982 Oct 10;257(19):11816–11821. [PubMed] [Google Scholar]
- Soutar C. A. Distribution of plasma cells and other cells containing immunoglobulin in the respiratory tract in chronic bronchitis. Thorax. 1977 Aug;32(4):387–396. doi: 10.1136/thx.32.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley R. A., Burnett D., Afford S. C. The immunological measurements of 'free' secretory piece and its relationship to local IgA production. Clin Exp Immunol. 1981 Jul;45(1):124–130. [PMC free article] [PubMed] [Google Scholar]
- Stockley R. A., Burnett D. Local IgA production in patients with chronic bronchitis: effect of acute respiratory infection. Thorax. 1980 Mar;35(3):202–206. doi: 10.1136/thx.35.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley R. A., Mistry M., Bradwell A. R., Burnett D. A study of plasma proteins in the sol phase of sputum from patients with chronic bronchitis. Thorax. 1979 Dec;34(6):777–782. doi: 10.1136/thx.34.6.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umetsu D. T., Ambrosino D. M., Quinti I., Siber G. R., Geha R. S. Recurrent sinopulmonary infection and impaired antibody response to bacterial capsular polysaccharide antigen in children with selective IgG-subclass deficiency. N Engl J Med. 1985 Nov 14;313(20):1247–1251. doi: 10.1056/NEJM198511143132002. [DOI] [PubMed] [Google Scholar]


