Abstract
Carpal tunnel syndrome (CTS) is a common peripheral canalicular nerve entrapment syndrome in the upper extremities. The compression of or injury to the median nerve at the wrist as it passes through a space-limited osteofibrous carpal canal can cause CTS, resulting in hand pain and impaired function. The present paper reviews the literature on the prevalence, pathology, diagnosis, treatment, and risk factors of CTS in conjunction with the role of genetic factors in CTS etiology. CTS diagnosis is primarily linked with clinical symptoms; still, it is simplified by sophisticated approaches such as magnetic resonance imaging and ultrasonography. CTS symptoms can be ameliorated through conservative and surgical strategies. The exact CTS pathophysiology needs clarification. Genetic predispositions to CTS are augmented by various variants within genes; however, CTS etiology could include risk factors such as wrist movements, injury, and specific conditions (e.g., age, body mass index, sex, and cardiovascular conditions). The high prevalence of CTS diminishes the quality of life of its sufferers and imposes costs on health systems, hence the significance of research and clinical trials to elucidate CTS pathogenesis and develop novel therapeutic targets.
Background
Carpal tunnel syndrome (CTS), the most common peripheral nerve entrapment syndrome, was first studied in 1854 by Paget (Paget 1860). In 1913, Pierre Marie and Charles Foix, two French neurologists, were the first to suggest surgery in a patient with bilateral muscle atrophy (Amadio 1995; Cobb et al. 1992). The term “carpal tunnel syndrome” (CTS) was coined by Moersch in 1938 (Dellon and Kallman 1983). CTS does not lead to mortality; nevertheless, in untreated cases, it can cause irreversible median nerve damage with a severe loss of hand function (Hegmann et al. 2018).
CTS can be caused by the confinement of or damage to the median nerve at the wrist within the constraint of the carpal tunnel. Patients with CTS suffer from pain in the upper extremities and paresthesia, especially in the thumb, middle finger, forefinger, and some parts of the ring finger. The severity rate in patients can be mild to severe, although no correlation has been found between the symptoms and the severity of CTS (Scalise et al. 2021).
Increased thickness of soft connective tissues and inflammation may result in the compression of the median nerve within the carpal tunnel and cause CTS (Ashworth 2014; Wipperman and Goerl 2016). The syndrome comprises approximately 90% of all nerve compression syndromes and is associated with a high economic burden. The prevalence rate of CTS in the general population ranges from 7 to 16% among adults, with women aged between 40 and 60 years bearing the brunt (Padua et al. 2016; Chammas et al. 2014). Furthermore, the age at CTS onset is lower in females than in males (45–54 years vs. 75–84 years), and white people are more susceptible to CTS than black people (Hegmann et al. 2018). The incidence of CTS in the United States and most developed countries is about one in three persons per 1000 per year, with a prevalence of 50 per 1000 (Sevy and Varacallo 2022). According to the United Kingdom General Practice Research Database, 88 males and 193 females per 100,000 individuals in the United Kingdom experience CTS (Burton et al. 2014). The overall prevalence of the syndrome based on physical examinations in Iran was estimated to be 17.53% (Moosazadeh et al. 2018). Additionally, the highest CTS prevalence of 61% was found among industrial workers (Hagberg et al. 1992), and office work-related CTS prevalence has been determined to range from about 5000 to 7500 per 100000 individuals (Lee et al. 2019).
While studies broad in scope have been conducted on CTS pathophysiology, the exact pathogenesis of most CTS cases remains unknown. Several risk factors, such as age, sex (Atroshi et al. 1999), pregnancy (Oliveira et al. 2019), morbid obesity, steady wrist activity, diabetes, rheumatoid inflammation, and genetic heredity (Geoghegan et al. 2004), can be associated with CTS development. In this regard, high pressure due to mechanical impact (fractures or trauma to the wrist), fibrosis, and tendon inflammation tend to squeeze the median nerve as it travels through the carpal tunnel.
We herein present a review of the literature on the various aspects of CTS, including its pathophysiology, diagnosis, treatment, and genetic factors.
Pathophysiology
CTS is the most frequent entrapment neuropathy in the upper extremities. Although the exact pathophysiologic mechanisms underlying CTS have yet to be determined, the interstitial fluid pressure within the carpal canal and the continuous contact pressure on the median nerve from the neighboring tissues have been implicated (Werner and Armstrong 1997). It is speculated that hyper-interstitial pressure within the carpal tunnel promotes the compression and traction of the peripheral median nerve along its course in the carpal tunnel at the wrist and mechanical damage to the myelin sheath. Local ischemia begotten by the mechanical compression of the nerve provokes the demyelination of its small and large fibers, causing paresthesia, pain, and motor and sensory disorders (Millesi et al. 1990; Werner and Andary 2002b).
Considering the normal pressure in the carpal tunnel (2–10 mm Hg), frequent wrist movements can dramatically increase the interstitial fluid pressure in the tunnel to more than tenfold. Pressure is estimated to rise tenfold and eightfold during extension and flexion at the wrist, respectively (Werner and Andary 2002b, 2002a). The tension is spread through the nerves from the start point of compression to intact axons in the internodal segment.
The carpal tunnel contains tendons and other structures made of connective tissue. There are nine flexor tendons and a thick flexor retinaculum in its confined space. Therefore, the pathology of the retinaculum and the tendons may also be involved in the pathogenesis of CTS (Burger et al. 2014, 2015a; Dada et al. 2016).
Symptoms and Diagnosis
Patients are diagnosed by clinicians primarily after symptom appearance. Signs such as numbness in the fingers or arms in various positions or during consecutive activities constitute the first manifestation of CTS (Ghasemi-Rad et al. 2014).
Patients with CTS always experience nocturnal paresthesia, pain, and a sensation of swelling over the median nerve area of the hand. The symptoms may appear with more intensity at night than in the daytime (Papež et al. 2008).
CTS diagnosis is chiefly related to the patient’s clinical history, including the strength and characteristics of symptoms and their dispersion throughout the hand, whether or not accompanied by arm manifestations (Osiak et al. 2021). Electrodiagnostic approaches, including electromyography and nerve conduction studies, can confirm the diagnosis (Jablecki et al. 2002). Diagnosis confirmation is also possible via magnetic resonance imaging, ultrasonography, and provocative tests such as the Tinel sign with 48%–73% sensitivity and 30%–94% specificity and the Phalen maneuver with 67%–83% sensitivity and 40%–98% specificity (Bruske et al. 2002). In these tests, positive results are considered when the repetitive tapping (the Tinel sign) and flexing of the wrist to 90° (the Phalen maneuver) produce the typical symptoms of carpal tunnel.
Ultrasound is also capable of identifying demyelinating CTS with secondary axonal degeneration (Deng et al. 2018; Wipperman and Goerl 2016).
The scratch collapse test is a novel test for CTS diagnosis. In this test, a stimulus is given over a space of nerve compression. The patient is asked to perform bilateral external shoulder rotation. In the case of transient muscle loss, the patient experiences arm collapsing, and the test is considered positive (Kahn et al. 2018; Huynh et al. 2018; Cheng et al. 2008).
Treatment
Two types of treatment strategies, conservative and surgical, are employed to ameliorate CTS symptoms (Wipperman and Goerl 2016).
Nonsurgical treatments or conservative therapies, consisting of hand splinting, laser therapy, pharmacotherapy (e.g., local corticosteroid injections and oral drugs), physical therapy, therapeutic ultrasound, and musculoskeletal manipulations, are utilized mainly in CTS patients with mild or moderate symptoms (Burke et al. 2003; Padua et al. 2016). In addition, patients who undergo conservative therapy benefit in the short term, while the long-term benefits of conservative therapy are still the subject of debate.
Studies have demonstrated that wearing a wrist splint can improve CTS symptoms during rest, whereas it worsens them with activity (Walker et al. 2000; Kruger et al. 1991). Wearing a rigid neutral splint, usually at nighttime for six weeks, can confer clinical improvements in untreated CTS patients with mild or moderate symptoms; nonetheless, no further benefits have been observed in extending splinting for another six weeks (Atroshi et al. 2019). Splinting is a suitable treatment option when employed within three months of disease onset. Improvements in sensory and motor nerve conduction velocities at long-term follow-ups have been observed when splints are used every night (Sevim et al. 2004; Kruger et al. 1991).
CTS patients with mild-to-moderate symptoms benefit from local corticosteroid injections in the carpal tunnel at the wrist, alleviating the pressure within the tunnel by decreasing inflammation and the edema of the tenosynovium passing through it (Ostergaard et al. 2020). Improvements in clinical symptoms have been observed with local corticosteroid injections for patients with severe CTS at one month, while such improvements in patients with mild-to-moderate CTS remain undetermined. In a previous investigation, no additional significant symptom relief was observed after two or more local injections of corticosteroids compared with a single injection (Stark and Amirfeyz 2013). Corticosteroid injection as a conservative management strategy for CTS is controversial, however. Whereas a study reported significant clinical relief in symptoms within one month after corticosteroid injection, another investigation demonstrated no symptom relief following the same treatment modality (Marshall et al. 2007). The application of oral steroids like prednisolone only causes some clinical improvements in symptoms in the short term (Osiak et al. 2021). Nonsteroidal anti-inflammatory drugs, pyridoxine, and diuretics are no longer considered efficacious in CTS treatment (Gerritsen et al. 2002; Ostergaard et al. 2020). Carpal tunnel release is performed as a surgical decompression treatment in patients with severe CTS, and there is no evidence of improvements after conservative treatments with minimal complications. Although 80% of symptoms are decreased by conservative therapy, surgery is required in some cases with a negative response to traditional therapies (Ono et al. 2010). In 70%–90% of the CTS patients assessed in a prior study, carpal tunnel release was associated with acceptable long-term clinical outcomes (Zamborsky et al. 2017).
Carpal tunnel release can be performed via the open approach, defined as a conventional open or mini-open release, or the endoscopic approach, conducted either as a single-portal surgery (Zuo et al. 2015) or a dual-portal technique (Chow and Hantes 2002). Apropos of the long-term functional outcome, no significant differences have been reported between open and endoscopic carpal tunnel release approaches (Chen et al. 2014; Larsen et al. 2013; Michelotti et al. 2014; Sayegh and Strauch 2015; Atroshi et al. 2015). Open surgery is correlated with minimal complications and high treatment success rates for any type of CTS pathology (e.g., any space-occupying lesion and deformity) or even in revision surgery. The endoscopic approach is a costly technique associated with higher rates of transient nerve damage, albeit smaller incisions in this technique contribute to enhanced cosmetic results (Shin 2019; Zamborsky et al. 2017).
Predisposing (Risk) Factors and CTS
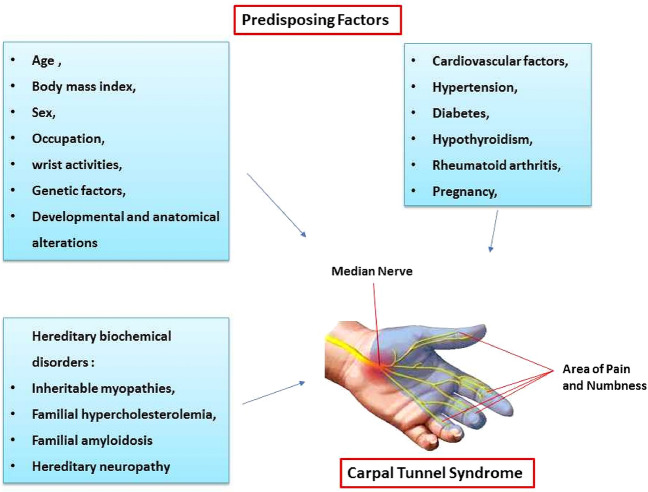
Several investigations have demonstrated a strong association between age and CTS development. Individuals over 55 years of age are more prone to CTS. Several studies have reported diminishing outcomes in post-carpal tunnel release surgery patients as they grow older (Żyluk and Puchalski 2013; Porter et al. 2002; Moschovos et al. 2019; Haghighat et al. 2012; Wilgis et al. 2006) (Fig. 1).
Fig. 1.
Predisposing factors that might be implicated in carpal tunnel syndrome
Various investigations have proven a significant correlation between body mass index and anthropometric hand measurements (as independent risk factors) and the development of CTS and the severity of its symptoms (Sharifi-Mollayousefi et al. 2008; Ünaldı et al. 2015; Kurt et al. 2008; Hassan and Al-Hawary 2013).
Mounting evidence suggests an association between sex and CTS. Some studies have demonstrated a similar rate of CTS between men and women doing the same job. Other studies on CTS as a work-related disorder in both sexes in different populations have reported dissimilarities (Fig. 1). For instance, a study on the Bremen population reported an estimated rate of 33% in men and 15% in women under 65 years old (Giersiepen et al. 2000). Overall, CTS is 10 times more prevalent in females than in males (Hegmann et al. 2018).
Some cardiovascular factors may be associated with CTS. A prior study investigated the association between CTS and atherosclerotic risk factors, carotid artery intima-media thickness, and vascular diseases and demonstrated that obesity, high cholesterol, hypertension, cardiac arrhythmias, and high triglycerides were associated with CTS in individuals between 30 and 44 years of age. Additionally, the findings revealed an association between CTS and coronary artery disease, valvular heart disorders, and carotid artery intima-media thickness in subjects 60 years of age or older (Shiri et al. 2011; Cazares-Manríquez et al. 2020).
Hypertension can be a safekeeping factor for CTS in the early phase, but CTS risk will increase in long-term hypertension (Guan et al. 2018).
Inherent traits like short height, thickness, and obesity; diseases such as diabetes, hypothyroidism, and rheumatoid arthritis; pregnancy; monotonous wrist activity; and developmental and anatomical alterations in the carpal tunnel could be involved in CTS (Atroshi et al. 1999; Gossett and Chance 1998; Padua et al. 2016; Żyluk 2020; Alford et al. 2004). CTS is frequent in patients with rheumatoid arthritis due to its inflammatory process (Fig. 1) (Smerilli et al. 2021). Wrist activity could be a CTS risk factor (Ozcakir et al. 2018). The tissue components of an anatomically convoluted peripheral nerve trunk may react distinctively to various levels of compression. Repetitive movements and continuous compression at the wrist can impair the blood flow in the endoneurial capillary system. Primarily, endoneurial microcirculation is generated by low pressure on a nerve trunk. An escalation in venous pressure may prompt intraneural edema and augmented intrafascicular tissue pressure. Subsequently, endoneurial edema can cause blood–nerve barrier alterations and oxygen deficiency in the nerve, contributing to CTS development (Lundborg et al. 1982) (Fig. 1).
Edema in the carpal tunnel elevates the tunnel volume contents, increases nerve vulnerability, and impairs the nerve blood supply, leading to the manifestation of CTS symptoms (Shiri et al. 2011). On the other hand, damage to the endothelial layer may give rise to vascular permeability and culminate in edema in the carpal tunnel. Nerve degeneration and intraneural fibrosis can follow vascular supply impairment, intensifying the vulnerability of the nerve to mechanical loads and continued tissue ischemia (Shiri et al. 2011).
Genetics and CTS
Numerous studies have provided evidence regarding the role of genetic predispositions in CTS development, but the causative genes have yet to be determined (Burger et al. 2014, 2015a, b; Puchalski et al. 2019; Senel et al. 2010). Although CTS is mostly sporadic and idiopathic, the familial incidence of CTS in 17%–39% of cases suggests the role of genetic susceptibility (Radecki 1994; Puchalski et al. 2019) (Table 1).
Table 1.
Susceptible nucleotide changes previously examined as predisposing genetic factors in the development of carpal tunnel syndrome regarding genome analysis
| Genes | Alterations | Sex | Ethnicity | Clinical significance | Symptoms | References |
|---|---|---|---|---|---|---|
| Genome analysis | ||||||
| TTR | p.Lys90Glu | F | Finland | Pathogenic | Vitreous amyloidosis and CTS | (Raivio et al. 2016) |
| p.Phe33Val | M | British Caucasian | Pathogenic | Bilateral CTS | (Gregory et al. 2008) | |
| p.Val30Met | M | Japan | Pathogenic | Familial amyloid polyneuropathy and CTS | (Kato-Motozaki et al. 2008) | |
| p.Leu58Arg | F | Japan | Pathogenic | Familial amyloid polyneuropathy and CTS | (Kato-Motozaki et al. 2008) | |
| p.Ile84Ser | F | Japan | Pathogenic | Early-onset FAP with features including carpal tunnel syndrome | (Booth et al. 2000; Wallace et al. 1988) | |
| p.Ile84Asn | NA | American | Pathogenic | CTS and cardiomyopathy | (Booth et al. 2000; Skinner et al. 1992) | |
| p.Tyr114His | M | Japan | Pathogenic | Nodular cutaneous amyloidosis and bilateral CTS | (Mochizuki et al. 2001) | |
| p.Tyr69Ile | F | Japan | Pathogenic | Developed CTS and congestive heart failure | (Takei et al. 2003) | |
| p.Tyr78Phe | M | French | Pathogenic | Peripheral neuropathy and CTS | (Magy et al. 2003) | |
| p.Val122 deletion | F&M | Spanish | Pathogenic | CTS | (Munar-Qués et al. 2000) | |
| FBN2 | p.Phe1670Cys | F&M | NA | Pathogenic | Early-onset CTS | (Peeters et al. 2021) |
| COMP | p.Val66Glu | F&M | China | Pathogenic | Familial bilateral CTS | (Li et al. 2020) |
| p.Arg718Trp | F&M | China | Pathogenic | Familial bilateral CTS | (Li et al. 2020) | |
CTS Carpal tunnel syndrome, FAP Familial amyloidotic polyneuropathy, F Female, M Male
The results of some population and family-based investigations have revealed a higher incidence rate of CTS among the relatives of patients. In 1959, inherited CTS among the members of the same family was reported (Tanzer 1959). Thereafter, Phalen (Phalen 1970), Radecki (Radecki 1994), and Puchalski and Szlosser (Senel et al. 2010) confirmed familial predispositions to CTS (Phalen 1970; Radecki 1994). Be that as it may, other investigations have suggested that true inheritable CTS is scarce (Gossett and Chance 1998).
Several studies have reported a high incidence rate of bilateral CTS in patients suffering from familial CTS by comparison with the general population (Alford et al. 2004; Puchalski et al. 2019), related to variations in the size of the carpal tunnel or its contents due to genetic variations (Alford et al. 2004).
Investigations of mutations in the cartilage oligomeric matrix protein (COMP) have revealed the role of extracellular matrix proteins in CTS (Żyluk 2020). A prior study (Li et al. 2020) identified the specific role of 2 missense mutations, p.R718W and p.V66E, in the COMP gene by sequencing the targeted locus in 2 large families with autosomal-dominant inheritance bilateral CTS (Table 1).
Molecular Pathways and Susceptible Polymorphisms Involved in CTS
The genetic factors and molecular pathways of CTS need elucidation. However, several studies have shown the role of polymorphisms as suspected factors in the syndrome.
Although the direct cause of the increased pressure in idiopathic CTS remains unknown, three distinct mechanisms, namely collagen synthesis, collagen degradation, and protection against oxidative stress effects in connective tissues, are considered to be engaged in genetic predispositions to CTS (Dada et al. 2016; Burger et al. 2014, 2015b, 2016).
Many genes involved in the regulation and modulation of the aforementioned mechanisms have exhibited their potential effects on CTS development (Tables 1, 2, 3). Likewise, possible genetic variations that may impact the characteristics, expression, and regulation of collagen fibrils can also be associated with CTS (Wenstrup et al. 2011; Żyluk 2020). Collagen fibrils are the basic elements of tendons, ligaments, and bones, all of which are specialized connective tissues (Burger et al. 2014). Collagen, found in the structure of connective tissues, consists of several subtypes. The principal type of collagen in fibrils is type I, followed by types V, XI, and XII (Dada et al. 2016). These latter types are involved in the regulation of fibrillogenesis, as well as the size and maintenance of fibrils, in the course of tendon development (Wenstrup et al. 2011).
Table 2.
Susceptible alterations formerly investigated as predisposing genetic factors in the development of carpal tunnel syndrome through association analysis
| Genes | Alterations | Sex | Ethnicity | Clinical significance | Symptoms | References |
|---|---|---|---|---|---|---|
| Association analysis | ||||||
| MCP-1 (CCL2) | c.-2518A > G | 231 M/135 F | Japan | Significant |
GG genotype as a risk factor for the development of CTS |
(Omori et al. 2002) |
| TTR | p.Val50Met | F57&7 M | Iraq | Not significant | Associated with familial carpal tunnel syndrome | (Al-Mudhafar et al. 2021) |
| COMT | p.Val158Met | 95 F | Turkey | Not significant |
Development, functional, and clinical status of CTS |
(İnal et al. 2015) |
| p.Val158Met | 120 F | Madrid, Spain | Not significant | CTS | (Fernández-de-Las-Peñas et al. 2018) | |
| FBN2 | p.Phe1670Cys | M/F | NA | Significant | Early-onset CTS | (Peeters et al. 2021) |
| ACAN | c.6833-4299 T > G | 90F/9 M | Western Cape region of South Africa | Not significant | CTS | (Peeters et al. 2021) |
| BGN | p.Ser180Ser | 90F/9 M | Western Cape region of South Africa | Significant | CTS | (Burger et al. 2014) |
| IL-6 | c.-274C > G | 94 F/9 M | South Africa | Not significant | CTS | (Burger et al. 2015b) |
| IL-1β | c.-598 T > C | 94 F/9 M | South Africa | Not significant | CTS | (Burger et al. 2015b) |
| IL-6R | p.Asp358Ala | 94 F/9 M | South Africa | Significant | CTS | (Burger et al. 2015b) |
| VEGFA | c.-2055A > C | 94 F/9 M | South Africa | Not significant | CTS | (Burger et al. 2015b) |
| COL1A1 | c.104-441G > T | 94 F/9 M | South Africa | Significant | CTS | (Dada et al. 2016) |
| COL11A1 | p.Pro1284Leu | 94 F/9 M | South Africa | Significant | CTS | (Dada et al. 2016) |
| p.Ser1496Pro | 94 F/9 M | South Africa | Not significant | CTS | (Dada et al. 2016) | |
| COL11A2 | c.798 + 1569 T > A | 94 F/9 M | South Africa | Not significant | CTS | (Dada et al. 2016) |
| COL12A1 | p.Gly3058Ser | 94 F/9 M | South Africa | Not significant | CTS | (Dada et al. 2016) |
| MMP10 | p.Arg53Lys | 92F/9 M | South Africa | Not significant | CTS | (Burger et al. 2016) |
| MMP1 | c.-1673delG | 92F/9 M | South Africa | Not significant | CTS | (Burger et al. 2016) |
| MMP3 | p.Lys45Glu | 92F/9 M | South Africa | Not significant | CTS | (Burger et al. 2016) |
| MMP12 | c.-124A > G | 92F/9 M | South Africa | Not significant | CTS | (Burger et al. 2016) |
| COL5A1 | c.*83C > T | 92F/9 M | South Africa | Significant | Bilateral or unilateral CTS | (Burger et al. 2015a) |
| c.*267C > T | 92F/9 M | South Africa | Significant | Bilateral or unilateral CTS | (Burger et al. 2015a) | |
| c.*873_*874insGGGA | 92F/9 M | South Africa | Significant | Bilateral or unilateral CTS | (Burger et al. 2015a) | |
| GSTM1 | GSTM1-null | 140F | Turkey | Significant | CTS | (Eroğlu et al. 2016) |
| GSTT1 | GSTM1-null and GSTT1-null combined genotypes | 140F | Turkey | Significant | CTS | (Eroğlu et al. 2016) |
| GSTP1 | p.Ile105Val | 140F | Turkey | Significant | CTS | (Eroğlu et al. 2016) |
| SERPINA1 | p.Glu366Lys | M&F | Iceland, the UK, Denmark, and Finland | Significant | CTS | (Skuladottir et al. 2022) |
CTS Carpal tunnel syndrome, F Female, M Male
Table 3.
All reported gene expression alterations which might be associated with the development of carpal tunnel syndrome
| Genes | Alterations | Sex | Ethnicity | Clinical Significance | Symptoms | References |
|---|---|---|---|---|---|---|
| Expression analysis | ||||||
| ADAMTS17, ADAMTS10, andEFEMP1, | – | M&F | UK | Significant | CTS | (Wiberg et al. 2019) |
| DIRC3 (lncRNA) and IGFBP5 | – | M&F | UK | Significant | Trigger finger and CTS | (Patel et al. 2021) |
| ITGAL, ITGAM, PECAM1, VIL2, TGFBR2, RAB7, RNF5, and NFKB1 | – | 120F | South Korea | Upregulated | CTS | (Kim et al. 2006) |
| PRG5, CASP8, CDH1, IGFBP5, CBX3, HREV107, PIN, and WINT2 | – | 120F | South Korea | Downregulated | CTS | (Kim et al. 2006) |
CTS Carpal tunnel syndrome, F Female, M Male
Alterations in some properties of collagen, such as elasticity and endurance, may translate into changes in carpal tunnel pressure, leading to median nerve compression (Wenstrup et al. 2011; Dada et al. 2016). The nucleotide variations of genes encoding the different collagen types might increase the risk of damage to connective tissues and result in CTS development (Burger et al. 2015a, 2014; Dada et al. 2016; Ficek et al. 2013; Hay et al. 2013).
Previous studies have demonstrated that COL1A1 (encodes the α1 chain of type I collagen), COL5A1 (encodes the α1 chain of type V collagen), and COL11A1 variants might have a significant role in CTS predispositions (Żyluk 2020). The nucleotide variations of COL5A1 in the 3ʹ-untranslated region (3’-UTR) consist of rs13946 (C/T), rs14774622 (C/T)/rs55748801 (G/A) (W/M where W = CG), rs12722 (C/T), and rs71746744 (− /AGGG) and are associated with CTS (Dada et al. 2016). The genotyping of COL1A1 rs1800012 (G/T), COL11A1 rs3753841 (T/C), COL11A1 rs1676486 (C/T), COL11A2 rs1799907 (T/A), and COL12A1 rs970547 (A/G) in CTS patients undergoing surgery showed considerable over-representation in the TT genotype of COL11A1 rs3753841, especially among female patients (Dada et al. 2016) (Table 1).
The T-C haplotype, formed by the rs3753841 and rs1676486 of COL5A1 and COL11A1, significantly modify CTS risk (Dada et al. 2016). These variants change messenger RNA stability and can, thus, alter the synthesis of types V and XI collagen, involved in the regulation of collagen fibril assembly and diameter (Dada et al. 2016; Hay et al. 2013).
The nucleotide alteration G/T (rs1800012) of COL1A1 may be associated with an increased binding affinity of COL1A1 for the Sp1 transcription factor. Accordingly, it may impact gene expression and the overproduction of type I collagen, leading to the alteration of the mechanical properties of connective tissues in the carpal tunnel and the development of CTS. This variant also significantly raises the risk of CTS development in women (Ficek et al. 2013; Dada et al. 2016).
The nucleotide variants of rs1676486 (T allele) and rs3753841 (T allele) in COL11A1 are associated with CTS development. Both variants may affect conformational changes in type XI collagen, impacting the structural and functional characterization of new collagen fibrils. These alterations are involved in CTS development by exerting effects on the connective tissue structures in the carpal tunnel (Hay et al. 2013; Mio et al. 2007).
Mutations in COL1A1 might play a significant role in CTS pathophysiology; nonetheless, nucleotide alterations in COL1A1 are involved in the pathogenesis of osteogenesis imperfecta (Gajko-Galicka 2002), Ehlers–Danlos syndrome (Almatrafi et al. 2020), glaucoma (Mauri et al. 2016), osteoarthritis, and intervertebral disk generation (Zhong et al. 2017).
Tendons consist of aggrecan and biglycan, which are proteoglycans encoded by ACAN and BGN, respectively. The rs1126499(C/T) variant of BGN and COL5A1 and BGN gene–gene interactions are associated with CTS (Burger et al. 2014) (Table 2).
Matrix metalloproteinase (MMP) genes are implicated in collagen fibril degradation and, consequently, the remodeling of connective tissues (Żyluk 2020; Burger et al. 2016; Raleigh et al. 2009). Significant correlations between connective tissue injuries and nucleotide variants within MMP10, MMP1, MMP3, and MMP12 gene clusters have been reported. Still, the results regarding the association between these genes and CTS are controversial. Several studies have demonstrated significant associations between the variants of MMP3 and chronic Achilles tendinopathy and suggested links between haplotypes, including the MMP variants of MMP10 (rs4860655), MMP1 (rs1799750), MMP3 (rs679620), and MMP12 (rs2276109), and anterior cruciate ligament ruptures in the knee joint (Raleigh et al. 2009; Posthumus et al. 2012; Malila et al. 2011). These variants of MMPs might be associated with the risk of CTS development given their associations with other musculoskeletal diseases.
The results of an association study suggested no relationships either between CTS and MMP10 rs486055 (C/T), MMP1 rs1799750 (G/GG), MMP3 rs679620 (A/G), and MMP12 rs2276109 (A/G) or between CTS and any suspected haplotypes (Burger et al. 2016) (Table 2).
Another mechanism that might be related to CTS etiology is oxidative stress, also enacted in the development of systemic inflammatory diseases and other pathologies. The overproduction of hydroxyl free radicals and reactive oxygen as a result of oxidative stress in the synovial connective tissue around the carpal tunnel may lead to edema, tissue damage, and median nerve compression (Kim et al. 2010). Since glutathione S-transferases (GSTs) are implicated in the defense mechanisms against reactive oxidative stress, some nucleotide variants in genes encoding GSTs have been identified to play a role in CTS development (Kim et al. 2010). Polymorphisms and specific nucleotide variations in GST1M and GSTT1 are common and lead to a lack of enzyme function or reduced enzyme activity, respectively.
The results of an association study concerning nucleotide variants in GSTM1, GSTT1, and GSTP1 in Turkish patients with CTS demonstrated a significantly higher incidence rate of the GSTM1-null variant in the patients and a twofold increase in the risk of CTS development (Eroğlu et al. 2016) (Fig. 2). Moreover, functional and clinical status is aggravated in CTS patients carrying the GSTP1-Ile105Val and Val/Val variants compared with CTS patients carrying the GSTP1 Ile/Ile variant (Eroğlu et al. 2016) (Table 2).
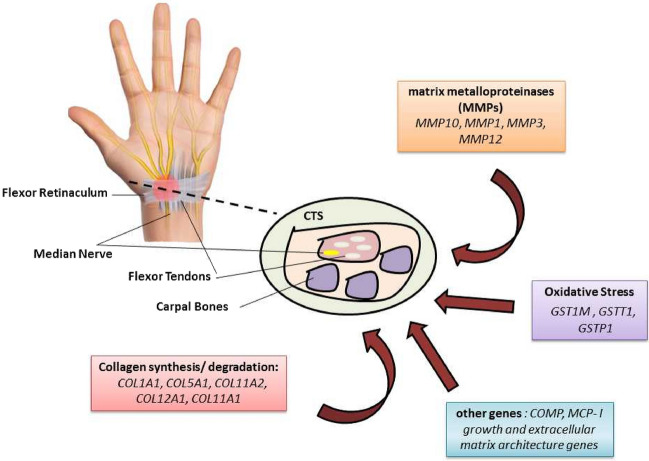
Fig. 2.
Schematic view of the genes implicated in the regulation and modulation of the potential mechanisms involved in carpal tunnel syndrome (CTS)
Research has been conducted on associations between some interleukins, the growth factor, and genes including SERPINA1 and COMT. The results of a study investigating the association between CTS and the gene variants of IL-1β rs16944 (-511C/T), IL-6 rs1800795 (− 174G/C), IL-6R rs2228145 (C/A), and VEGFA rs699947 (− 2578C/A) indicated that the AA genotype of the IL-6R rs2228145 variant significantly reduced the risk of CTS development (Burger et al. 2015b). Another investigation reported no statistically significant association between the interleukin-1 receptor antagonist, angiotensin-converting enzyme I/D polymorphisms, and the risk of CTS development (Cevik et al. 2018) (Fig. 2). A study showed that a missense variant (p.Glu366Lys) in SERPINA1 could confer protection against CTS (Skuladottir et al. 2022). The results of a study on the association between rs4680 genotypes (Val58Met) in the catechol-O-methyltransferase (COMT) gene of female patients with CTS and treatment outcomes demonstrated no association between the Val58Met polymorphism and any outcomes, including pain, symptoms, and the severity of function (Fernández-de-Las-Peñas et al. 2018). An association case–control study found no significant relationship between the Val158Met (rs4680) polymorphism in the COMT gene and CTS severity (İnal et al. 2015). Japanese patients on hemodialysis were analyzed for the genetic polymorphisms of monocyte chemoattractant protein-I (MCP-I) and macrophage inflammatory protein-Iα (MIP-Iα), considered to be the development factors of CTS. Although there was no significant distinction between surgical and nonsurgical CTS patients and the genotype distributions of MCP-I or MIP-Iα, the GG genotype at − 2518 in MCP-I was associated with CTS development (Omori et al. 2002) (Fig. 2 & Table 2).
A genome-wide association study of entrapment neuropathy in the United Kingdom Biobank introduced 16 susceptibility single-nucleotide polymorphism loci significantly associated with CTS. Further, the upshots suggested that nucleotide variations in the genes involved in growth and extracellular matrix architecture led to genetic predispositions to CTS by changing the environment of median nerve transits (Wiberg et al. 2019) (Table 2).
A study on the genetic basis of concomitant CTS and trigger finger, two common nontraumatic hand diseases, in the United Kingdom Biobank determined an association between five independent loci. Further analyses in that study showed that the rs62175241 allele, mapped to the Disrupted in Renal Carcinoma 3 (DIRC3) (a long noncoding RNA) locus, was a disease-protective allele and was associated with the overexpression of both DIRC3 and Insulin-Like Growth Factor Binding Protein 5 (IGFBP5) (Patel et al. 2021).
Hereditary Biochemical Disorders and CTS
Previous investigations have demonstrated associations between liability to pressure palsies and some genetic biochemical disorders, including inheritable myopathy, familial hypercholesterolemia, familial amyloidosis, and hereditary neuropathy, in CTS predispositions (Leifer et al. 1992; Ihara et al. 1991; Murakami et al. 1994). Compared with the general population, in the relatives of individuals with these diseases, the incidence of CTS is high, and the onset of symptoms is early.
CTS was a clinical feature of familial amyloidotic polyneuropathy II in an Indiana family of Swiss descent (Wallace et al. 1988). Various studies have been published concerning transthyretin (TTR) mutations and CTS. In this regard, since one of the first manifestations of amyloidosis is CTS, screening the TTR gene could help diagnose the syndrome early (Karam et al. 2019).
A missense mutation, c.268A > C (p.Lys90Glu), was reported in a Finnish family with vitreous amyloidosis for the first time (Raivio et al. 2016). Heterozygous transversion from A to T in codon 78 (TAC-TTC) in exon 3 of TTR was reported in a patient from a French family of Italian origin with neuropathy and bilateral CTS surgery (Magy et al. 2003). A TTR Phe33Val alteration was detected in a British Caucasian male with familial amyloidotic polyneuropathy and bilateral CTS (Gregory et al. 2008), a TTR Val122 deletion was found in a Spanish family (Munar-Qués et al. 2000), and a point mutation (p.Tyr114His) was detected in a Japanese family with familial CTS (Murakami et al. 1994; Mochizuki et al. 2001). In addition, the significant role of the FBN2 gene was identified by exome sequencing in a family with early-onset CTS (a heterozygous variant, c.5009 T > G; p.Phe1670Cys) (Peeters et al. 2021) (Table 1).
Gene Expression, Noncoding RNAs, and CTS
There is a paucity of information regarding alterations in gene expression in CTS. However, an expression analysis on 120 CTS patients and 30 controls based on electrodiagnosis categorized CTS patients into three groups: mild, moderate, and severe. The results demonstrated upregulation in 20 genes and downregulation in 28 genes. Moreover, the ITGAL, ITGAM, PECAM1, VIL2, TGFBR2, RAB7, RNF5, and NFKB1 genes were upregulated, whereas the PRG5, CASP8, CDH1, IGFBP5, CBX3, HREV107, PIN, and WINT2 genes were downregulated (Table 3). The aforementioned genes were involved predominantly in TGF-β signaling, NF-Kb signaling, antiapoptotic functions, and T-cell receptor signaling. Although the findings indicated no relationship between gene expression alterations and the severity of CTS symptoms, the authors suggested that proinflammatory and antiapoptotic mechanisms might have a role in CTS development (Kim et al. 2006).
An investigation reported the overexpression of TGF-β in the subsynovial connective tissues of patients with idiopathic CTS and concluded that it might be involved in CTS pathogenesis by regulating COX-2 and NGF expression (Nakawaki et al. 2018).
An RNA sequencing analysis of surgically resected tenosynovium from CTS patients demonstrated the expression of ADAMTS17, ADAMTS10, and EFEMP1, related to CTS pathogenesis (Wiberg et al. 2019) (Table 3).
An association study via an expression analysis of the disease-protective rs62175241 allele in fibroblasts from healthy donors (n = 79) and tenosynovium samples from CTS patients (n = 77) reported that the rs62175241 allele was associated with increased expression levels of DIRC3 (a long noncoding RNA) and IGFBP5. Moreover, the effect of the rs62175241 protective allele on DIRC3 expression was tissue-specific, and a positive regulation existed in the stomach and spleen, while there was a negative regulation in the testis and amygdala. Additionally, IGFBP5 was a secreted antagonist of IGF-1 signaling, and the upregulation of IGF-1 was associated with CTS and trigger finger. The authors, therefore, concluded that IGF-1 was a driver of both diseases (Patel et al. 2021) (Table 3).
Conclusions
Although CTS is a well-known entrapment neuropathy, none of the studies conducted to date has demonstrated its exact mechanisms. Indeed, recent years have witnessed considerable improvements in the diagnosis, treatment, and associated risk factors of CTS; still, further research is needed to clarify genetic predispositions to CTS in different populations. Most studies on genetic predispositions to this syndrome have been conducted in South African, Turkish, and Brazilian populations, with little information available regarding other races, including Caucasians. Further research is warranted to probe into the gene expression alterations of all coding and noncoding genes in CTS with a view to exploring its exact molecular signaling pathways and main regulators. We believe that future experimental and bioinformatics analyses should seek to clarify CTS pathogenesis so as to develop appropriate and effective diagnostic and therapeutic strategies in the treatment of this syndrome.
Search Strategy
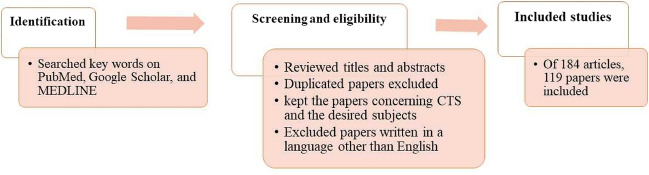
This paper reviews studies published in PubMed, Google Scholar, and MEDLINE on the pathophysiology, diagnosis, treatment, genetics, and underlying molecular mechanisms of carpal tunnel syndrome (CTS). The keywords employed for searching the articles and reviews were as follows: carpal tunnel syndrome, CTS, diagnosis, epidemiology, prevalence, CTS pathomechanisms, pathophysiology, molecular mechanisms, genetics, signaling pathways, polymorphisms, gene expression, treatment, risk factors, symptoms, predisposing factors, hereditary biochemical disorders, and noncoding RNAs. Our selection criteria cover all research and review articles with possible search terms, keywords, and phrases.
First, we reviewed the titles and abstracts of the papers depicted in each database. Next, we identified and excluded duplicated papers and those written in languages other than English. Subsequently, we kept papers on CTS and the desired subjects by reviewing titles and abstracts. Finally, we sought to review all aspects of CTS (Fig. 3).
Fig. 3.
Schematic flow chart of the search strategy
Abbreviations
- CTS
Carpal tunnel syndrome
- DNA
Deoxyribonucleic acid
- RNA
Ribonucleic acid
- MRI
Magnetic resonance imaging
- US
Ultrasonography
- UK
United Kingdom
- NSAIDs
Non-steroidal anti-inflammatory drugs
- OCTR
Open carpal tunnel release
- ECTR
Endoscopic carpal tunnel release
- COMP
Cartilage oligomeric matrix protein
- ECM
Extracellular matrix
- COL1A1
Collagen type I alpha 1 Chain
- COL5A1
Collagen Type V Alpha 1 Chain
- COL11A1
Collagen Type XI Alpha 1 Chain
- 3’-UTR
3ʹ-Untranslated region
- COL12A1
Collagen type XII alpha 1 chain
- mRNA
Messenger ribonucleic acid
- Sp1
Specificity protein 1*
- ACAN
Aggrecan
- BGN
Biglycan
- MMPs
Matrix metalloproteinases
- MMP10
Matrix Metallopeptidase 10
- MMP1
Matrix Metallopeptidase 1
- MMP3
Matrix Metallopeptidase 3
- MMP12
Matrix Metallopeptidase 12
- GST
Glutathione S-transferases
- GSTM1
Glutathione S-Transferase Mu 1
- GSTT1
Glutathione S-Transferase Theta 1
- GSTP1
Glutathione S-Transferase Pi 1
- SERPINA1
Serpin Family A Member 1
- COMT
Catechol-O-Methyltransferase
- IL-1β
Interleukin 1 Beta)
- IL-6
Interleukin 6
- IL-6R
Interleukin 6 Receptor
- VEGFA
Vascular endothelial growth factor A
- (ACE) I/D
Angiotensin-converting enzyme
- MCP-I
Monocyte chemoattractant protein-I
- MIP-Iα
Macrophage inflammatory protein-Iα
- SNPs
Single-nucleotide polymorphisms
- GWAS
Genome-wide association study
- UKB
UK Biobank
- DIRC3
Disrupted In Renal Carcinoma 3
- lncRNA
Long non-coding RNAs
- FAP II
Familial amyloidotic polyneuropathy II
- IGFBP5
Insulin-Like Growth Factor Binding Protein 5
- TTR
Transthyretin
- FCTS
Familial carpal tunnel syndrome
- FBN2
Fibrillin 2
- ITGAL
Integrin Subunit Alpha L
- ITGAM
Integrin Subunit Alpha M
- RAB7
Ras-related protein Rab-7a
- RNF5
Ring Finger Protein 5
- PECAM1
Platelet And Endothelial Cell Adhesion Molecule 1
- VIL2
Villin-2
- TGFBR2
Transforming Growth Factor Beta Receptor 2
- NFKB1
Nuclear Factor Kappa B Subunit 1
- PRG5
Plasticity-related Gene 5
- CASP8
Caspase 8
- PIN
PIN-FORMED
- HREV107
Phospholipase A2 group XVI
- CDH1
Cadherin 1
- CBX3
Chromobox 3
- WNT2
Wingless-Type MMTV Integration Site Family Member 2
- NGF
Nerve Growth Factor
- COX-2
Cyclooxygenase-2
- ADAMTS17
ADAM metallopeptidase with thrombospondin type 1 motif, 17
- ADAMTS10
ADAM metallopeptidase with thrombospondin type 1 motif, 10
- EFEMP1
EGF Containing Fibulin Extracellular Matrix Protein 1
- PLA2G2F
Phospholipase A2 group IIF
- PLA2G4F
Phospholipase A2 group IVF
- PLA2G4D
Phospholipase A2, group IVD
- PLA2G3
Phospholipase A2 group III
- IGF-1
Insulin-Like Growth Factor 1
- PLA2G4E
Phospholipase A2, group IVE
- NF-Kb
Nuclear factor kappa B
- SSCTs
Subsynovial connective tissues
- TGF
Transforming growth factor
Author Contributions
MM contributed to concept, database search, and revision of the first draft, figures, and tables. MS contributed to database search and the writing of the first draft. AGH contributed to database search and the writing of the first draft. MO contributed to concept, the writing of the review, and supervising and revising the whole draft, figures, and tables. All the authors have reviewed the final manuscript.
Funding
This work was funded by a research grant from Bam University of Medical Sciences (Grant No. 4010000028) and the Research Deputyship of Rajaie Cardiovascular Medical and Research Center.
Data Availability
All data reviewed during the present study are included in this published article.
Declarations
Conflict of interest
All the authors have read and approved the data presented in the manuscript and declared that there is no conflict of interest.
Ethical Approval
The study protocol was approved by Bam University of Medical Sciences (IR.MUBAM.REC.1401.029).
Consent for Publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alford JW, Weiss A, Akelman E (2004) The familial incidence of carpal tunnel syndrome in patients with unilateral and bilateral disease. Am J Orthop 33(8):397–400 [PubMed] [Google Scholar]
- Almatrafi A, Hashmi JA, Fadhli F, Alharbi A, Afzal S, Ramzan K, Basit S (2020) Further evidence of a recessive variant in COL1A1 as an underlying cause of ehlers-danlos syndrome: a report of a saudi founder mutation. Glob Med Genet 7(4):109–112. 10.1055/s-0041-1722873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mudhafar RH, Ajeena IM, Al-Awadi IJ, Al-Mudhafar DH, Hadi NR (2021) Transthyretin gene mutation associated with familial carpal tunnel syndrome in sample of Iraqi patients. Acta Informatica Med 29(2):99. 10.5455/aim.2021.29.99-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amadio P (1995) The first carpal tunnel release? J Hand Surg 20(1):40–41. 10.1016/S0266-7681(05)80013-0 [DOI] [PubMed] [Google Scholar]
- Ashworth N (2014) Carpal tunnel. BMJ 349:g6437 [DOI] [PubMed] [Google Scholar]
- Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosén I (1999) Prevalence of carpal tunnel syndrome in a general population. JAMA 282(2):153–158. 10.1001/jama.282.2.153 [DOI] [PubMed] [Google Scholar]
- Atroshi I, Hofer M, Larsson G-U, Ranstam J (2015) Extended follow-up of a randomized clinical trial of open vs endoscopic release surgery for carpal tunnel syndrome. JAMA 314(13):1399–1401. 10.1001/jama.2015.12208 [DOI] [PubMed] [Google Scholar]
- Atroshi I, Tadjerbashi K, McCabe SJ, Ranstam J (2019) Treatment of carpal tunnel syndrome with wrist splinting: study protocol for a randomized placebo-controlled trial. Trials 20(1):1–11. 10.1186/s13063-019-3635-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth D, Stangou A, Williams R, Gillmore J, Tennent G, Hawkins P (2000) Transthyretin Ile84Thr is associated with familial amyloid polyneuropathy. Hum Mutat 16(5):447–447. 10.1002/1098-1004(200011) [DOI] [PubMed] [Google Scholar]
- Bruske J, Bednarski M, Grzelec H, Zyluk A (2002) The usefulness of the Phalen test and the Hoffmann-Tinel sign in the diagnosis of carpal tunnel syndrome. Acta Orthop Belg 68(2):141–145 [PubMed] [Google Scholar]
- Burger MC, De Wet H, Collins M (2014) The BGN and ACAN genes and carpal tunnel syndrome. Gene 551(2):160–166. 10.1016/j.gene.2014.08.051 [DOI] [PubMed] [Google Scholar]
- Burger M, de Wet H, Collins M (2015a) The COL5A1 gene is associated with increased risk of carpal tunnel syndrome. Clin Rheumatol 34(4):767–774. 10.1007/s10067-014-2727-7 [DOI] [PubMed] [Google Scholar]
- Burger MC, de Wet H, Collins M (2015b) Interleukin and growth factor gene variants and risk of carpal tunnel syndrome. Gene 564(1):67–72. 10.1016/j.gene.2015.03.047 [DOI] [PubMed] [Google Scholar]
- Burger MC, De Wet H, Collins M (2016) Matrix metalloproteinase genes on chromosome 11q22 and risk of carpal tunnel syndrome. Rheumatol Int 36(3):413–419. 10.1007/s00296-015-3385-z [DOI] [PubMed] [Google Scholar]
- Burke F, Ellis J, McKenna H, Bradley M (2003) Primary care management of carpal tunnel syndrome. Postgrad Med J 79(934):433–437. 10.1136/pmj.79.934.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton C, Chesterton LS, Davenport G (2014) Diagnosing and managing carpal tunnel syndrome in primary care. Br J Gen Pract 64(622):262–263. 10.3399/bjgp14X679903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazares-Manríquez MA, Wilson CC, Vardasca R, García-Alcaraz JL, Olguín-Tiznado JE, López-Barreras JA, García-Rivera BR (2020) A review of carpal tunnel syndrome and its association with age, body mass index, cardiovascular risk factors, hand dominance, and sex. Appl Sci 10(10):3488. 10.3390/app10103488 [Google Scholar]
- Cevik B, Tekcan A, Inanir A, Kurt SG, Yigit S (2018) The investigation of association between IL-1Ra and ACE I/D polymorphisms in carpal tunnel syndrome. J Clin Lab Anal 32(1):e22204. 10.1002/jcla.22204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chammas M, Boretto J, Burmann LM, Ramos RM, Santos Neto FCd, Silva JB (2014) Carpal tunnel syndrome-Part I (anatomy, physiology, etiology and diagnosis). Revista Brasileira De Ortopedia 49:429–436. 10.1016/j.rboe.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Duan X, Huang X, Lv J, Peng K, Xiang Z (2014) Effectiveness and safety of endoscopic versus open carpal tunnel decompression. Arch Orthop Trauma Surg 134(4):585–593. 10.1007/s00402-013-1898-z [DOI] [PubMed] [Google Scholar]
- Cheng CJ, Mackinnon-Patterson B, Beck JL, Mackinnon SE (2008) Scratch collapse test for evaluation of carpal and cubital tunnel syndrome. J Hand Surg 33(9):1518–1524. 10.1016/j.jhsa.2008.05.022 [DOI] [PubMed] [Google Scholar]
- Chow JC, Hantes ME (2002) Endoscopic carpal tunnel release: thirteen years’ experience with the Chow technique. J Hand Surg 27(6):1011–1018. 10.1053/jhsu.2002.35884 [DOI] [PubMed] [Google Scholar]
- Cobb T, Dalley B, Posteraro R, Lewis R (1992) The carpal tunnel as a compartment. Anat Perspect Orthop Rev 21(4):451–453 [PubMed] [Google Scholar]
- Dada S, Burger MC, Massij F, de Wet H, Collins M (2016) Carpal tunnel syndrome: the role of collagen gene variants. Gene 587(1):53–58. 10.1016/j.gene.2016.04.030 [DOI] [PubMed] [Google Scholar]
- Dellon AL, Kallman CH (1983) Evaluation of functional sensation in the hand. J Hand Surg 8(6):865–870. 10.1016/s0363-5023(83)80083-5 [DOI] [PubMed] [Google Scholar]
- Deng X, Chau LP, Chiu SY, Leung KP, Li SW, Ip WY (2018) Exploratory use of ultrasound to determine whether demyelination following carpal tunnel syndrome co-exists with axonal degeneration. Neural Regen Res 13(2):317–323. 10.4103/1673-5374.226402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eroğlu P, Erkol İnal E, Sağ ŞÖ, Görükmez Ö, Topak A, Yakut T (2016) Associations analysis of GSTM1, T1 and P1 Ile105Val polymorphisms with carpal tunnel syndrome. Clin Rheumatol 35(5):1245–1251. 10.1007/s10067-014-2855-0 [DOI] [PubMed] [Google Scholar]
- Fernández-de-Las-Peñas C, Ambite-Quesada S, Fahandezh-Saddi Díaz H, Paras-Bravo P, Palacios-Cena D, Cuadrado ML (2018) The Val158Met polymorphism of the catechol-O-methyltransference gene is not associated with long-term treatment outcomes in carpal tunnel syndrome: A randomized clinical trial. PLoS ONE 13(10):e0205516. 10.1371/journal.pone.0205516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficek K, Cieszczyk P, Kaczmarczyk M, Maciejewska-Karłowska A, Sawczuk M, Cholewinski J, Leonska-Duniec A, Stepien-Slodkowska M, Zarebska A, Stepto NK (2013) Gene variants within the COL1A1 gene are associated with reduced anterior cruciate ligament injury in professional soccer players. J Sci Med Sport 16(5):396–400. 10.1016/j.jsams.2012.10.004 [DOI] [PubMed] [Google Scholar]
- Gajko-Galicka A (2002) Mutations in type I collagen genes resulting in osteogenesis imperfecta in humans. Acta Biochim Pol 49(2):433–441 [PubMed] [Google Scholar]
- Geoghegan J, Clark D, Bainbridge L, Smith C, Hubbard R (2004) Risk factors in carpal tunnel syndrome. J Hand Surg 29(4):315–320. 10.1016/j.jhsb.2004.02.009 [DOI] [PubMed] [Google Scholar]
- Gerritsen AA, De Krom MC, Struijs MA, Scholten RJ, De Vet HC, Bouter LM (2002) Conservative treatment options for carpal tunnel syndrome: a systematic review of randomised controlled trials. J Neurol 249(3):272–280. 10.1007/s004150200004 [DOI] [PubMed] [Google Scholar]
- Ghasemi-Rad M, Nosair E, Vegh A, Mohammadi A, Akkad A, Lesha E, Mohammadi MH, Sayed D, Davarian A, Maleki-Miyandoab T (2014) A handy review of carpal tunnel syndrome: From anatomy to diagnosis and treatment. World J Radiol 6(6):284. 10.4329/wjr.v6.i6.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giersiepen K, Eberle A, Pohlabeln H (2000) Gender differences in carpal tunnel syndrome? occupational and non-occupational risk factors in a population-based case-control study. Ann Epidemiol 10(7):481. 10.1016/s1047-2797(00)00133-2 [DOI] [PubMed] [Google Scholar]
- Gossett JG, Chance PF (1998) Is there a familial carpal tunnel syndrome? an evaluation and literature review. Muscle Nerve 21(11):1533–1536. 10.1002/(sici)1097-4598(199811) [DOI] [PubMed] [Google Scholar]
- Gregory ME, Carey M, Hawkins PN, Banerjee S, Gillmore JD (2008) Characterisation and management of vitreous and nerve amyloid in familial amyloid polyneuropathy due to variant transthyretin, Phe33Val. Br J Ophthalmol 92(1):34–35. 10.1136/bjo.2007.124123 [DOI] [PubMed] [Google Scholar]
- Guan W, Lao J, Gu Y, Zhao X, Rui J, Gao K (2018) Case-control study on individual risk factors of carpal tunnel syndrome. Exp Ther Med 15(3):2761–2766. 10.3892/etm.2018.5817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg M, Morgenstern H, Kelsh M (1992) Impact of occupations and job tasks on the prevalence of carpal tunnel syndrome. Scandinavian J Work, Environ & Health,. 10.5271/sjweh.1564 [DOI] [PubMed] [Google Scholar]
- Haghighat A, Khosrawi S, Kelishadi A, Sajadieh S, Badrian H (2012) Prevalence of clinical findings of carpal tunnel syndrome in Isfahanian dentists. Adv Biomed Res. 10.4103/2277-9175.96069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MM, Al-Hawary MA (2013) Body mass index and motor distal latency as independent risk factors for recurrent carpal tunnel syndrome following an open release surgery. Egyptian J Neurol, Psychiatry Neurosurg 50(1):13–17 [Google Scholar]
- Hay M, Patricios J, Collins R, Branfield A, Cook J, Handley CJ, September AV, Posthumus M, Collins M (2013) Association of type XI collagen genes with chronic Achilles tendinopathy in independent populations from South Africa and Australia. Br J Sports Med 47(9):569–574. 10.1136/bjsports-2013-092379 [DOI] [PubMed] [Google Scholar]
- Hegmann KT, Merryweather A, Thiese MS, Kendall R, Garg A, Kapellusch J, Foster J, Drury D, Wood EM, Melhorn JM (2018) Median nerve symptoms, signs, and electrodiagnostic abnormalities among working adults. JAAOS 26(16):576–584. 10.5435/JAAOS-D-17-00034 [DOI] [PubMed] [Google Scholar]
- Huynh MN, Karir A, Bennett A (2018) Scratch collapse test for carpal tunnel syndrome: A systematic review and meta-analysis. Plast Reconstr Surg Global Open. 10.1097/GOX.0000000000001933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihara Y, Nobukuni K, Namba R, Kamisaka K, Kibata M, Kajinami K, Fujita H, Mabuchi H, Shirabe T, Ohshima K-I (1991) A family of familial hypercholesterolemia with cerebral infarction and without coronary heart disease: an unusual case with corneal opacity, polyneuropathy and carpal tunnel syndrome in the family: therapy with probucol and tocopherol nicotinate. J Neurol Sci 106(1):10–18. 10.1016/0022-510x(91)90187-c [DOI] [PubMed] [Google Scholar]
- İnal EE, Eroğlu P, Görükmez O, Sağ ŞÖ, Yakut T (2015) Association between the val158met polymorphism with susceptibility and severity of carpal tunnel syndrome. Balkan J Med Genet 18(2):43–48. 10.1515/bjmg-2015-0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablecki C, Andary M, Floeter M, Miller R, Quartly C, Vennix M, Wilson J (2002) Practice parameter: electrodiagnostic studies in carpal tunnel syndrome: report of the American Association of Electrodiagnostic Medicine, American Academy of Neurology, and the American Academy of Physical Medicine and Rehabilitation. Neurology 58(11):1589–1592. 10.1212/wnl.58.11.1589 [DOI] [PubMed] [Google Scholar]
- Kahn LC, Yee A, Mackinnon SE (2018) Important details in performing and interpreting the scratch collapse test. Plast Reconstr Surg 141(2):399–407. 10.1097/PRS.0000000000004082 [DOI] [PubMed] [Google Scholar]
- Karam C, Dimitrova D, Christ M, Heitner SB (2019) Carpal tunnel syndrome and associated symptoms as first manifestation of hATTR amyloidosis. Neurology 9(4):309–313. 10.1212/CPJ.0000000000000640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato-Motozaki Y, Ono K, Shima K, Morinaga A, Machiya T, Nozaki I, Shibata-Hamaguchi A, Furukawa Y, Yanase D, Ishida C (2008) Epidemiology of familial amyloid polyneuropathy in Japan: Identification of a novel endemic focus. J Neurol Sci 270(1–2):133–140 [DOI] [PubMed] [Google Scholar]
- Kim H-W, Kim K-N, Seo S-H, Lee S-H, Sohn S-H, Kim Y-R, HaLee Y-M, Shim J-S, Ahn D-S, Kim M-K (2006) Gene expression profile in carpal tunnel syndrome patients. Mol Cell Toxicol 2(4):266–272 [Google Scholar]
- Kim JK, Koh YD, Kim JS, Hann HJ, Kim MJ (2010) Oxidative stress in subsynovial connective tissue of idiopathic carpal tunnel syndrome. J Orthop Res 28(11):1463–1468. 10.1002/jor.21163 [DOI] [PubMed] [Google Scholar]
- Kruger VL, Kraft GH, Deitz JC, Ameis A, Polissar L (1991) Carpal tunnel syndrome: objective measures and splint use. Arch Phys Med Rehabil 72(7):517–520 [PubMed] [Google Scholar]
- Kurt S, Kisacik B, Kaplan Y, Yildirim B, Etikan I, Karaer H (2008) Obesity and carpal tunnel syndrome: is there a causal relationship? Eur Neurol 59(5):253–257. 10.1159/000115639 [DOI] [PubMed] [Google Scholar]
- Larsen M, Sørensen A, Crone K, Weis T, Boeckstyns M (2013) Carpal tunnel release: a randomized comparison of three surgical methods. J Hand Surg (european Volume) 38(6):646–650. 10.1177/1753193412475247 [DOI] [PubMed] [Google Scholar]
- Lee I-H, Kim Y-K, Kang D-M, Kim S-Y, Kim I-A, Kim E-M (2019) Distribution of age, gender, and occupation among individuals with carpal tunnel syndrome based on the National Health Insurance data and National Employment Insurance data. Annals Occup Environ Med. 10.35371/aoem.2019.31.e31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifer D, Cros D, Halperin JJ, Gallico GG III, Pierce DS, Shahani BT (1992) Familial bilateral carpal tunnel syndrome: report of two families. Arch Phys Med Rehabil 73(4):393–397. 10.1016/0003-9993(92)90017-q [DOI] [PubMed] [Google Scholar]
- Li C, Wang N, Schäffer AA, Liu X, Zhao Z, Elliott G, Garrett L, Choi NT, Wang Y, Wang Y (2020) Mutations in COMP cause familial carpal tunnel syndrome. Nat Commun 11(1):1–16. 10.1038/s41467-020-17378-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundborg G, Gelberman RH, Minteer-Convery M, Lee YF, Hargens AR (1982) Median nerve compression in the carpal tunnel—functional response to experimentally induced controlled pressure. J Hand Surg 7(3):252–259. 10.1016/s0363-5023(82)80175-5 [DOI] [PubMed] [Google Scholar]
- Magy N, Liepnieks JJ, Gil H, Kantelip B, Dupond J-L, Kluve-beckerrnan B, Benson MD (2003) A transthyretin mutation (Tyr78Phe) associated with peripheral neuropathy, carpal tunnel syndrome and skin amyloidosis. Amyloid 10(1):29–33. 10.3109/13506120308995254 [DOI] [PubMed] [Google Scholar]
- Malila S, Yuktanandana P, Saowaprut S, Jiamjarasrangsi W, Honsawek S (2011) Association between matrix metalloproteinase-3 polymorphism and anterior cruciate ligament ruptures. Genet Mol Res 10(4):4158–4165. 10.4238/2011.October.31.1 [DOI] [PubMed] [Google Scholar]
- Marshall SC, Tardif G, Ashworth NL (2007) Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 10.1002/14651858.CD001554.pub2 [DOI] [PubMed] [Google Scholar]
- Mauri L, Uebe S, Sticht H, Vossmerbaeumer U, Weisschuh N, Manfredini E, Maselli E, Patrosso M, Weinreb RN, Penco S, Reis A, Pasutto F (2016) Expanding the clinical spectrum of COL1A1 mutations in different forms of glaucoma. Orphanet J Rare Dis 11(1):108. 10.1186/s13023-016-0495-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelotti B, Romanowsky D, Hauck RM (2014) Prospective, randomized evaluation of endoscopic versus open carpal tunnel release in bilateral carpal tunnel syndrome: an interim analysis. Ann Plast Surg 73:S157–S160. 10.1097/SAP.0000000000000203 [DOI] [PubMed] [Google Scholar]
- Millesi H, Zöch G, Rath T (1990) The gliding apparatus of peripheral nerve and its clinical significance. Ann Chir Main Memb Super 9(2):87–97. 10.1016/s0753-9053(05)80485-5 [DOI] [PubMed]
- Mio F, Chiba K, Hirose Y, Kawaguchi Y, Mikami Y, Oya T, Mori M, Kamata M, Matsumoto M, Ozaki K (2007) A functional polymorphism in COL11A1, which encodes the α1 chain of type XI collagen, is associated with susceptibility to lumbar disc herniation. Am J Hum Genet 81(6):1271–1277. 10.1086/522377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki H, Kamakura K, Masaki T, Hirata A, Tokuda T, Yazaki M, Motoyoshi K, Ikeda S-I (2001) Nodular cutaneous amyloidosis and carpal tDnnel syndrome due to the amyloidogenic transthyretin His 114 variant. Amyloid 8(2):105–110. 10.3109/13506120109007352 [DOI] [PubMed] [Google Scholar]
- Moosazadeh M, Asadi-Aliabadi M, Rostami F, Farshidi F, Karimi N (2018) Prevalence of carpal tunnel syndrome in Iran: A systematic review and meta-analysis. J Mazandaran Univ Med Sci 28(161):144–153 [Google Scholar]
- Moschovos C, Tsivgoulis G, Kyrozis A, Ghika A, Karachalia P, Voumvourakis K, Chroni E (2019) The diagnostic accuracy of high-resolution ultrasound in screening for carpal tunnel syndrome and grading its severity is moderated by age. Clin Neurophysiol 130(3):321–330. 10.1016/j.clinph.2018.12.005 [DOI] [PubMed] [Google Scholar]
- Munar-Qués M, Saraiva M, Ordeig-Calonge J, Moreira P, Pérez-Vidal R, Puig-Pujol X, Monells-Abel J, Badal-Alter J (2000) Familial amyloid polyneuropathy in a Spanish family with a transthyretin deletion (deltaVal 122) presenting with carpal tunnel syndrome. Clin Genet 58(5):411–412. 10.1034/j.1399-0004.2000.580515.x [DOI] [PubMed] [Google Scholar]
- Murakami T, Tachibana S, Endo Y, Kawai R, Hara M, Tanase S, Ando M (1994) Familial carpal tunnel syndrome due to amyloidogenic transthyretin His 114 variant. Neurology 44(2):315–315. 10.1212/wnl.44.2.315 [DOI] [PubMed] [Google Scholar]
- Nakawaki M, Uchida K, Onuma K, Sukegawa K, Matsumoto T (2018) Transforming Growth Factor-Beta (TGF-Β) regulates nerve growth factor and cyclooxygenase-2 expression in sub-synovial connective tissue in patients with carpal tunnel syndrome. Int Arch Orthop Surg. 10.23937/iaos-2017/1710002 [Google Scholar]
- Oliveira GADd, Bernardes JM, Santos EdS, Dias A (2019) Carpal tunnel syndrome during the third trimester of pregnancy: prevalence and risk factors. Arch Gynecol Obstet 300(3):623–631. 10.1007/s00404-019-05233-6 [DOI] [PubMed] [Google Scholar]
- Omori K, Kazama JJ, Song J, Goto S, Takada T, Saito N, Sakatsume M, Narita I, Gejyo F (2002) Association of the MCP-1 gene polymorphism A-2518G with carpal-tunnel syndrome in hemodialysis patients. Amyloid 9(3):175–182. 10.3109/13506120209114819 [DOI] [PubMed] [Google Scholar]
- Ono S, Clapham PJ, Chung KC (2010) Optimal management of carpal tunnel syndrome. Int J Gen Med 3:255. 10.2147/ijgm.s7682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osiak K, Elnazir P, Walocha J, Pasternak A (2021) Carpal tunnel syndrome: State-of-the-art review. Folia Morphol. 10.5603/FM.a2021.0121 [DOI] [PubMed] [Google Scholar]
- Ostergaard PJ, Meyer MA, Earp BE (2020) Non-operative treatment of carpal tunnel syndrome. Curr Rev Musculoskelet Med 13(2):141–147. 10.1007/s12178-020-09616-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcakir S, Sigirli D, Avsaroglu H (2018) High wrist ratio is a risk factor for carpal tunnel syndrome. Clin Anat 31(5):698–701. 10.1002/ca.23198 [DOI] [PubMed] [Google Scholar]
- Padua L, Coraci D, Erra C, Pazzaglia C, Paolasso I, Loreti C, Caliandro P, Hobson-Webb LD (2016) Carpal tunnel syndrome: clinical features, diagnosis, and management. Lancet Neurol 15(12):1273–1284. 10.1016/S1474-4422(16)30231-9 [DOI] [PubMed] [Google Scholar]
- Paget J (1860) Lectures on surgical pathology. Lindsay & Blakiston, Philadelphia [Google Scholar]
- Papež BJ, Palfy M, Turk Z (2008) Infrared thermography based on artificial intelligence for carpal tunnel syndrome diagnosis. J Int Med Res 36(6):1363–1370. 10.1177/147323000903700321 [DOI] [PubMed] [Google Scholar]
- Patel B, Kleeman S, Neavin D, Powell J, Baskozos G, Ng M, Benett D, Schmid A, Furniss D, Wiberg A (2021) DIRC3-IGFBP5 is a shared genetic risk locus and therapeutic target for carpal tunnel syndrome and trigger finger. medRxiv. 10.1016/S2665-9913(22)00180-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters S, Decramer A, Cain SA, Houpt P, Verstreken F, Noyez J, Hermans C, Jacobs W, Lammens M, Fransen E (2021) Delineation of a new fibrillin-2-opathy with evidence for a role of FBN2 in the pathogenesis of carpal tunnel syndrome. J Med Genet 58(11):778–782. 10.1136/jmedgenet-2020-107085 [DOI] [PubMed] [Google Scholar]
- Phalen GS (1970) Reflections on 21 years’ experience with the carpal-tunnel syndrome. JAMA 212(8):1365–1367 [PubMed] [Google Scholar]
- Porter P, Venkateswaran B, Stephenson H, Wray C (2002) The influence of age on outcome after operation for the carpal tunnel syndrome: a prospective study. J Bone Joint Surg Br 84(5):688–691. 10.1302/0301-620x.84b5.12266 [DOI] [PubMed] [Google Scholar]
- Posthumus M, Collins M, Van Der Merwe L, O’cuinneagain D, Van Der Merwe W, Ribbans WJ, Schwellnus M, Raleigh SM (2012) Matrix metalloproteinase genes on chromosome 11q22 and the risk of anterior cruciate ligament (ACL) rupture. Scand J Med Sci Sports 22(4):523–533. 10.1111/j.1600-0838.2010.01270.x [DOI] [PubMed] [Google Scholar]
- Puchalski P, Szlosser Z, Żyluk A (2019) Familial occurrence of carpal tunnel syndrome. Neurol Neurochir Pol 53(1):43–46. 10.5603/PJNNS.a2019.0004 [DOI] [PubMed] [Google Scholar]
- Radecki P (1994) The familial occurrence of carpal tunnel syndrome. Muscle Nerve 17(3):325–330. 10.1002/mus.880170311 [DOI] [PubMed] [Google Scholar]
- Raivio VE, Jonasson J, Myllykangas L, Ala-Mello S, Kankuri-Tammilehto M, Kiuru-Enari S, Westermark P, Tanskanen M, Kivelä T (2016) A novel transthyretin Lys70Glu (p. Lys90Glu) mutation presenting with vitreous amyloidosis and carpal tunnel syndrome. Amyloid 23(1):46–50. 10.3109/13506129.2015.1126574 [DOI] [PubMed] [Google Scholar]
- Raleigh SM, Van der Merwe L, Ribbans WJ, Smith RK, Schwellnus MP, Collins M (2009) Variants within the MMP3 gene are associated with Achilles tendinopathy: possible interaction with the COL5A1 gene. Br J Sports Med 43(7):514–520. 10.1136/bjsm.2008.053892 [DOI] [PubMed] [Google Scholar]
- Sayegh ET, Strauch RJ (2015) Open versus endoscopic carpal tunnel release: a meta-analysis of randomized controlled trials®. Clinic Orthop Relat Res 473(3):1120–1132. 10.1007/s11999-014-3835-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalise V, Brindisino F, Pellicciari L, Minnucci S, Bonetti F (2021) Carpal Tunnel Syndrome: A National Survey to Monitor Knowledge and Operating Methods. Int J Environ Res Public Health 18(4):1995. 10.3390/ijerph18041995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senel S, Ceylaner G, Yuksel D, Erkek N, Karacan C (2010) Familial primary carpal tunnel syndrome with possible skipped generation. Eur J Pediatr 169(4):453–455. 10.1007/s00431-009-1055-4 [DOI] [PubMed] [Google Scholar]
- Sevim S, Dogu O, Çamdeviren H, Kaleagasi H, Aral M, Arslan E, Milcan A (2004) Long-term effectiveness of steroid injections and splinting in mild and moderate carpal tunnel syndrome. Neurol Sci 25(2):48–52. 10.1007/s10072-004-0229-0 [DOI] [PubMed] [Google Scholar]
- Sevy JO, Varacallo M (2022) Carpal Tunnel Syndrome. In: StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC., Treasure Island, FL. PMID: 28846321 [PubMed]
- Sharifi-Mollayousefi A, Yazdchi-Marandi M, Ayramlou H, Heidari P, Salavati A, Zarrintan S (2008) Assessment of body mass index and hand anthropometric measurements as independent risk factors for carpal tunnel syndrome. Folia Morphol 67(1):36–42 [PubMed] [Google Scholar]
- Shin EK (2019) Endoscopic versus open carpal tunnel release. Curr Rev Musculoskelet Med 12(4):509–514. 10.1007/s12178-019-09584-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiri R, Heliövaara M, Moilanen L, Viikari J, Liira H, Viikari-Juntura E (2011) Associations of cardiovascular risk factors, carotid intima-media thickness and manifest atherosclerotic vascular disease with carpal tunnel syndrome. BMC Musculoskelet Disord 12(1):1–12. 10.1186/1471-2474-12-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner M, Harding J, Skare I, Jones LA, Cohen AS, Milunsky A, Skare J (1992) A new transthyretin mutation associated with amyloidotic vitreous opacities: asparagine for isoleucine at position 84. Ophthalmology 99(4):503–508. 10.1016/s0161-6420(92)31949-9 [DOI] [PubMed] [Google Scholar]
- Skuladottir AT, Bjornsdottir G, Ferkingstad E, Einarsson G, Stefansdottir L, Nawaz MS, Oddsson A, Olafsdottir TA, Saevarsdottir S, Walters GB (2022) A genome-wide meta-analysis identifies 50 genetic loci associated with carpal tunnel syndrome. Nat Commun 13(1):1–9. 10.1038/s41467-022-29133-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smerilli G, Di Matteo A, Cipolletta E, Carloni S, Incorvaia A, Di Carlo M, Grassi W, Filippucci E (2021) Ultrasound assessment of carpal tunnel in rheumatoid arthritis and idiopathic carpal tunnel syndrome. Clin Rheumatol 40(3):1085–1092. 10.1007/s10067-020-05293-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark H, Amirfeyz R (2013) Cochrane corner: local corticosteroid injection for carpal tunnel syndrome. J Hand Surg (European Volume) 38(8):911–914. 10.1177/1753193413490848 [DOI] [PubMed] [Google Scholar]
- Takei Y-I, Hattori T, Yazaki M, Tokuda T, Urasawa N, Kanai S, Ikeda S-I (2003) Transthyretin Tyr69-to-Ile mutation (doublenucleotide substitution in codon 69) in a Japanese familial amyloidosis patient with cardiomyopathy and carpal tunnel syndrome. Amyloid 10(1):25–28. 10.3109/13506120308995253 [DOI] [PubMed] [Google Scholar]
- Tanzer RC (1959) The carpal-tunnel syndrome: a clinical and anatomical study. JBJS 41(4):626–634 [PubMed] [Google Scholar]
- Ünaldı HK, Kurt S, Çevik B, Mumcuoğlu İ, Sümbül O (2015) The relationship between waist circumference, wrist circumference, and body mass index in carpal tunnel syndrome. J Turgut Ozal Med Center 22(3):152–157 [Google Scholar]
- Walker WC, Metzler M, Cifu DX, Swartz Z (2000) Neutral wrist splinting in carpal tunnel syndrome: a comparison of night-only versus full-time wear instructions. Arch Phys Med Rehabil 81(4):424–429. 10.1053/mr.2000.3856 [DOI] [PubMed] [Google Scholar]
- Wallace M, Conneally P, Benson M (1988) A DNA test for Indiana/Swiss hereditary amyloidosis (FAP II). Am J Hum Genet 43(2):182 [PMC free article] [PubMed] [Google Scholar]
- Wenstrup RJ, Smith SM, Florer JB, Zhang G, Beason DP, Seegmiller RE, Soslowsky LJ, Birk DE (2011) Regulation of collagen fibril nucleation and initial fibril assembly involves coordinate interactions with collagens V and XI in developing tendon. J Biol Chem 286(23):20455–20465. 10.1074/jbc.M111.223693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner R, Andary M (2002a) Carpal tunnel syndrome incidence in general population. Clin Neurophysiol 113:1373–1381 [DOI] [PubMed] [Google Scholar]
- Werner RA, Andary M (2002b) Carpal tunnel syndrome: pathophysiology and clinical neurophysiology. Clin Neurophysiol 113(9):1373–1381 [DOI] [PubMed] [Google Scholar]
- Werner RA, Armstrong TJ (1997) Carpal tunnel syndrome: ergonomic risk factors and intracarpal. Phys Med Rehabil Clin North Am 8(3):555–569. 10.1016/s1388-2457(02)00169-4 [Google Scholar]
- Wiberg A, Ng M, Schmid AB, Smillie RW, Baskozos G, Holmes MV, Künnapuu K, Mägi R, Bennett DL, Furniss D (2019) A genome-wide association analysis identifies 16 novel susceptibility loci for carpal tunnel syndrome. Nat Commun 10(1):1–12. 10.1038/s41467-019-08993-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilgis E, Burke F, Dubin N, Sinha S, Bradley M (2006) A prospective assessment of carpal tunnel surgery with respect to age. J Hand Surg 31(4):401–406. 10.1016/j.jhsb.2006.03.165 [DOI] [PubMed] [Google Scholar]
- Wipperman J, Goerl K (2016) Carpal tunnel syndrome: diagnosis and management. Am Fam Physician 94(12):993–999 [PubMed] [Google Scholar]
- Zamborsky R, Kokavec M, Simko L, Bohac M (2017) Carpal tunnel syndrome: symptoms, causes and treatment options. Lit Rev Ortop Traumatol Rehabil 19(1):1–8. 10.5604/15093492.1232629 [DOI] [PubMed] [Google Scholar]
- Zhong B, Huang D, Ma K, Deng X, Shi D, Wu F, Shao Z (2017) Association of COL1A1 rs1800012 polymorphism with musculoskeletal degenerative diseases: a meta-analysis. Oncotarget 8(43):75488–75499. 10.18632/oncotarget.20797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo D, Zhou Z, Wang H, Liao Y, Zheng L, Hua Y, Cai Z (2015) Endoscopic versus open carpal tunnel release for idiopathic carpal tunnel syndrome: a meta-analysis of randomized controlled trials. J Orthop Surg Res 10(1):1–13. 10.1186/s13018-014-0148-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Żyluk A (2020) The role of genetic factors in carpal tunnel syndrome etiology: A review. Adv Clin Exp Med 29(5):623–628. 10.17219/acem/118846 [DOI] [PubMed] [Google Scholar]
- Żyluk A, Puchalski P (2013) A comparison of the results of carpal tunnel release in patients in different age groups. Neurol Neurochir Pol 47(3):241–246. 10.5114/ninp.2013.35486 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data reviewed during the present study are included in this published article.