ABSTRACT
Leptothrix ochracea creates distinctive iron-mineralized mats that carpet streams and wetlands. Easily recognized by its iron-mineralized sheaths, L. ochracea was one of the first microorganisms described in the 1800s. Yet it has never been isolated and does not have a complete genome sequence available, so key questions about its physiology remain unresolved. It is debated whether iron oxidation can be used for energy or growth and if L. ochracea is an autotroph, heterotroph, or mixotroph. To address these issues, we sampled L. ochracea-rich mats from three of its typical environments (a stream, wetlands, and a drainage channel) and reconstructed nine high-quality genomes of L. ochracea from metagenomes. These genomes contain iron oxidase genes cyc2 and mtoA, showing that L. ochracea has the potential to conserve energy from iron oxidation. Sox genes confer potential to oxidize sulfur for energy. There are genes for both carbon fixation (RuBisCO) and utilization of sugars and organic acids (acetate, lactate, and formate). In silico stoichiometric metabolic models further demonstrated the potential for growth using sugars and organic acids. Metatranscriptomes showed a high expression of genes for iron oxidation; aerobic respiration; and utilization of lactate, acetate, and sugars, as well as RuBisCO, supporting mixotrophic growth in the environment. In summary, our results suggest that L. ochracea has substantial metabolic flexibility. It is adapted to iron-rich, organic carbon-containing wetland niches, where it can thrive as a mixotrophic iron oxidizer by utilizing both iron oxidation and organics for energy generation and both inorganic and organic carbon for cell and sheath production.
IMPORTANCE
Winogradsky's observations of L. ochracea led him to propose autotrophic iron oxidation as a new microbial metabolism, following his work on autotrophic sulfur-oxidizers. While much culture-based research has ensued, isolation proved elusive, so most work on L. ochracea has been based in the environment and in microcosms. Meanwhile, the autotrophic Gallionella became the model for freshwater microbial iron oxidation, while heterotrophic and mixotrophic iron oxidation is not well-studied. Ecological studies have shown that Leptothrix overtakes Gallionella when dissolved organic carbon content increases, demonstrating distinct niches. This study presents the first near-complete genomes of L. ochracea, which share some features with autotrophic iron oxidizers, while also incorporating heterotrophic metabolisms. These genome, metabolic modeling, and transcriptome results give us a detailed metabolic picture of how the organism may combine lithoautotrophy with organoheterotrophy to promote Fe oxidation and C cycling and drive many biogeochemical processes resulting from microbial growth and iron oxyhydroxide formation in wetlands.
KEYWORDS: Leptothrix ochracea, chemolithotrophy, mixotrophy, iron-oxidizing bacteria, iron microbial mats
INTRODUCTION
Leptothrix ochracea was one of the first described microorganisms (1–3), eminently recognizable both microscopically and to the naked eye, yet fundamental questions about its metabolism remain. It is a member of the Leptothrix–Sphaerotilus group within the Gammaproteobacteria, formerly known as the Betaproteobacteria, and its distinguishing characteristic is the prodigious production of iron-mineralized sheaths that are mostly devoid of cells. The cells produce sheaths rapidly (4), which become felted together into fluffy, orange, iron microbial mats in freshwater systems (3, 5–7). These sheaths represent a reservoir of mineral-associated organic carbon (8–11), which adsorb diverse heavy metals (e.g., U and actinides) and nutrients (e.g., P, N, S, and Si) (12–25). L. ochracea is primarily found in oxic freshwater streams, wetlands, and drainage channels with slowly flowing water, a source of ferrous iron, and low quantities of organic carbon. Winogradsky first hypothesized that L. ochracea grows as a chemolithoautotrophic iron oxidizer based on enrichments and environmental observations (3). He posited that its main energy source is ferrous iron based on observations that it requires iron for growth and produces a massive amount of iron oxides relative to a small cell yield. However, Winogradsky’s culture media contained organics from natural water, so neither a strictly autotrophic metabolism nor growth via iron oxidation was demonstrated. To this day, investigations have not confirmed the role of iron in L. ochracea metabolism (26–29).
Because L. ochracea has resisted isolation (30, 31), our understanding of its metabolism is based on enrichment or xenic cultures. Typical culturing methods have used organics, e.g. soil extract, and the repeated difficulty in demonstrating the role of iron oxidation has fueled the idea that L. ochracea is heterotrophic like characterized isolates of Leptothrix and Sphaerotilus (which also oxidize metals, but for unclear purposes). These isolates generally respond strongly to addition of organics, which is not true of L. ochracea (32), suggesting distinct differences in carbon metabolism and growth strategy. Recent laboratory-based studies on enrichment cultures of L. ochracea demonstrated that it can assimilate limited amounts of carbon from bicarbonate (4). Its enrichment from environmental samples required organic-containing environmental water and Fe(II), suggesting that it may use Fe(II) as an energy source (4). Furthermore, in an ecological time-series study, its abundance corresponded to higher quantities of dissolved organic carbon than the autotrophic iron oxidizers in the family Gallionellaceae (4, 32). Its association with higher organic carbon than Gallionellaceae and lower organic carbon than other Leptothrix–Sphaerotilus members gives it a distinct niche from these other metal-oxidizing bacteria.
Genomes can provide much insight into metabolic potential if they contain genes characteristic of specific energy and nutrient pathways. While there are genomes of other Leptothrix and Sphaerotilus isolates (29), those genomes are unlikely to be representative of L. ochracea because of its distinct responses to organic carbon. The first and only genome of L. ochracea was sequenced from a single cell, and as is typical of a single-cell amplified genome (SAG), the L. ochracea L12 SAG is only a partial genome, 0.51 Mb in size (4). Based on its tRNAs, the full genome size is roughly estimated to be 2.2 Mb (4), suggesting the majority of the genome is missing. Thus, the L. ochracea L12 SAG gives limited insights into the metabolism of L. ochracea. It contains genes for a cytochrome bd terminal oxidase, Form II RuBisCO, and electron transport, including alternative complex III, which could enable growth via chemolithoautotrophic iron oxidation. However, these components are not unique to iron oxidation, and the SAG lacks genes for known iron oxidases (e.g., cyc2 and mtoA). The L12 SAG also contains genes for formate dehydrogenase, but lacks organic carbon utilization genes; therefore, whether it can gain energy or biomass from organics is unresolved. L. ochracea’s lifestyle suggests its role as a mixotrophic iron oxidizer, akin to an intermediate between the autotrophic iron-oxidizing Gallionellaceae and the heterotrophic metal-oxidizing Leptothrix–Sphaerotilus group. A complete genome would allow us to more thoroughly discern its metabolisms and evaluate its identity as an autotrophic, heterotrophic, or mixotrophic iron oxidizer.
To this end, we sequenced and analyzed the first near-complete Leptothrix ochracea genomes, reconstructed from metagenomes of freshwater iron mats sampled at the Savannah River Site (SRS) in South Carolina and Spruce Point in Boothbay Harbor, Maine. Nine high-quality, near-complete genomes were reconstructed from stream and wetland mats. These genomes demonstrate the potential for L. ochracea to grow as a mixotrophic iron oxidizer. It has the genomic potential to combine chemolithotrophy and organoheterotrophy to generate iron oxyhydroxides and biomass carbon. Metatranscriptomes and metabolic models of these genomes further support the role of L. ochracea as a mixotrophic iron oxidizer. Our results reveal metabolic connections between iron and carbon cycling within L. ochracea; these relationships help our understanding of the drivers of biogeochemical transformations catalyzed by iron-oxidizing microbes in wetlands.
RESULTS AND DISCUSSION
Iron microbial mat samples
Sampling was performed in the Tims Branch stream and its surrounding wetlands at the Savannah River Site (SRS) in Jackson, SC, and at Spruce Point in Boothbay Harbor, ME. Across these two locations, four sites representing the typical environments for L. ochracea were sampled. These include two freshwater wetlands (SRS MN and DE sites), one freshwater stream (SRS ISCO site), and a drainage channel with slow-moving water (Spruce Point SP site) (Fig. 1). Mats sampled from the SRS wetland sites were dense, dark orange, and flocculent, whereas mats sampled from the SRS stream site and Spruce Point were orange, fluffy, and most prevalent around the stream/channel edges and in areas protected from high flow. In addition, a thin layer of dense, brown mat was observed at the SRS stream site on the surface of the sediment spanning the center of Tims Branch. The sampled mats had morphology matching the typical descriptions of L. ochracea mats (32–34).
Fig 1.
Iron microbial mat sampling locations. Images A–D are from the Savannah River Site; image E is from the Spruce Point site. (A) Extensive iron mat at the DE wetland site; (B) iron mat at the ISCO stream site; (C) fluffy Leptothrix-type iron mat at the ISCO site in the Tims Branch stream; (D) iron mat at the MN wetland site; (E) iron mat in the Spruce Point site drainage channel.
L. ochracea mats are typically found within aerobic, circumneutral to slightly acidic freshwater environments with abundant Fe2+. To evaluate the geochemistry of these sampling sites, pH, oxygen, and Fe2+ were measured (Table 1). The surface water pH near these mats was circumneutral to slightly acidic. Oxygen concentrations ranged widely, with highest values in the ISCO stream site mats and at the surface of DE wetland mats. To evaluate oxygen gradients within these mats, dissolved oxygen measurements were taken near the mat surface and 1.5–2.5 cm below the mat surface. At the ISCO site, oxygen decreased inside the mats compared to the surface, yet mats remained aerobic. In contrast, mats at the MN and DE sites became anoxic at depth. The concentration of dissolved Fe2+ ranged from 1 to 79 µM in mat samples, though lower values may be due to mixing of surface water with mat samples. At the MN3 location, we used dialysis samplers to measure dissolved Fe2+ in sediment pore water directly below mats (Table S1). Here, Fe2+ was highest in sediment pore water near the mat/sediment interface (1250 µM, ~1 cm below mat surface), suggesting that there is a significant flux of ferrous groundwater into the surface water to support growth by iron oxidation.
TABLE 1.
Geochemical parameters from the SRS and Spruce Pointd
| Sampling site | Sample | pH | O2 in surface water (µM) | O2 inside mat (µM) | [Fe2+] (µM) |
|---|---|---|---|---|---|
| SRS | ISCOA1K | 6.1 | 153 | 109a | – |
| ISCOB2 | 6.2 | 249 | – | – | |
| ISCOC1 | 6.2 | 205 | – | 79.4 | |
| MN2 | – | – | – | 33.3 | |
| MN3 | 5.3 | 78.1 | 0.0b | 8.02 | |
| DE1c | 5.5 | 219 | 0.0b | 1.27 | |
| SP | 4SP | 6.1 | 20–80 | – | 9.50 |
Measurement was taken from 2.5 cm below the surface of the mat.
Measurement was taken from 1.5 cm below the surface of the mat.
Sample DE2 was a bulk sample taken over a large area, taken from the same location as site DE1. The geochemistry of DE2 is approximately the same as DE1.
–, not measured.
The SRS mats were characterized via microscopy to demonstrate the presence of sheaths typical of L. ochracea. Sheath-rich mats sampled intact from Spruce Point were previously characterized microscopically (34). This analysis (Fig. S1) and additional phase contrast imaging (Fig. 2D) showed that the Spruce Point mats were largely composed of empty sheaths, with the cells occupying sheath ends at the surface of the mat. The SRS mats also contained abundant sheaths characteristic of Leptothrix ochracea, some with chains of cells and others empty and abandoned by cells (Fig. 2). Such characteristics have previously been described in Leptothrix ochracea mats and are distinct from other Leptothrix and Sphaerotilus (5, 27). Both Spruce Point and SRS samples are consistent with typical morphological descriptions of L. ochracea and suggest that L. ochracea is a prominent and active iron oxidizer in these microbial mats. The massive production of empty mineralized sheath is analogous to the production of iron oxyhydroxide stalks by Gallionella and Mariprofundus. Sheath and stalk structures serve multiple purposes: (1) acting as a holdfast to position cells where they can access Fe(II), nutrients, and O2; (2) a repository for oxidized iron waste products; and (3) sheath production coupled to mineral deposition may assist and direct cell filament motility (34, 35). The extensive formation of Fe-mineralized sheaths suggests that Fe oxidation is an important part of L. ochracea metabolism and growth.
Fig 2.
Microscopy of Leptothrix iron mats. (A) SEM of the L. ochracea sheath structure from the DE site mat; (B) SEM of the iron mat structure showing abundant L. ochracea sheaths in the DE site mat; (C) combined phase contrast and fluorescence microscopy of the mat from the DE site shows a chain of L. ochracea cells (visualized with Syto13 nucleic acid stain) within a sheath and several empty sheaths; (D) phase contrast light micrography of the mat from Spruce Point shows a chain of L. ochracea cells within a sheath and empty sheaths.
Presence of Leptothrix ochracea 16S rRNA gene sequences
The microbial community composition of iron mat samples from the SRS was assessed by 16S rRNA gene analysis. After QC, there were 266,445 reads for eight samples (23,565–46,362 per sample), which clustered into 90,818 operational taxonomic units (OTUs, at 99% similarity) (Table S2). Based on initial SILVA classification, Leptothrix appeared to be either absent or at a very low level in these mats (0.01%–0.47%). However, we checked the SILVA classification of the L. ochracea sequences from the study by Fleming et al. (7) and found that they are incorrectly classified as the genus Paucibacter. Three abundant (>0.5%) OTUs classified as Paucibacter were identified across the eight iron mat samples, and all three OTU sequences are >99% identical to the Fleming sequence, meeting the species delineation threshold for 16S rRNA gene identity (>98.6% (36)) (Table S4). After correcting the classification, we find that L. ochracea is present in all eight samples (0.99% to 6.68%; Table 2; Table S3), consistent with microscopy and mat morphotype observations. Furthermore, these OTUs are by far the most abundant members of the Leptothrix–Sphaerotilus group in all eight samples.
TABLE 2.
Percent abundance of Leptothrix and Sphaerotilus OTUs at the Savannah River Sitea
| OTU | Classification | ISCOA1K | ISCOB2 | ISCOC1 | ISCOC1bead | MN2 | MN3 | DE1 | DE2 |
|---|---|---|---|---|---|---|---|---|---|
| OTU000006 | L. ochracea | 0.016 | 2.22 | 6.17 | 0.012 | 0.004 | 0.009 | 0.012 | 0.015 |
| OTU000001 | L. ochracea | 0.012 | 0.013 | 0.044 | 0.003 | – | 5.48 | 3.60 | 1.43 |
| OTU000011 | L. ochracea | 1.82 | 0.013 | – | 4.74 | 0.940 | 0.015 | 0.005 | 0.004 |
| OTU000215 | L. ochracea | – | – | 0.004 | 0.009 | – | 0.239 | 0.026 | 0.007 |
| OTU000130 | L. ochracea | 0.024 | – | 0.037 | 0.106 | 0.008 | 0.011 | 0.019 | 0.009 |
| OTU000236 | Other Leptothrix | – | – | – | – | – | – | 0.007 | 0.282 |
| OTU000257 | Other Leptothrix | – | – | – | – | – | 0.002 | 0.151 | 0.015 |
| OTU004977 | Sphaerotilus | – | 0.004 | – | – | – | 0.002 | – | – |
| OTU090995 | Sphaerotilus | – | – | – | 0.003 | – | – | – | – |
| OTU040448 | Sphaerotilus | – | – | – | – | – | 0.002 | – | – |
| OTU113053 | Sphaerotilus | – | – | – | – | – | 0.002 | – | – |
| OTU124633 | Sphaerotilus | – | – | – | – | – | 0.002 | – | – |
| OTU132238 | Sphaerotilus | – | – | – | – | – | 0.002 | – | – |
| Total | |||||||||
| L. ochracea | 2.10 | 2.37 | 6.68 | 5.17 | 0.989 | 6.41 | 4.17 | 1.61 | |
| Other Leptothrix | 0.139 | 0.115 | 0.015 | 0.022 | 0.000 | 0.082 | 0.349 | 0.472 | |
| Sphaerotilus | 0.000 | 0.004 | 0.000 | 0.003 | 0.000 | 0.011 | 0.000 | 0.000 | |
Relative abundance of Leptothrix and L. ochracea OTUs above 0.1% and Sphaerotilus OTUs above 0.01%. Total abundance of all L. ochracea, other Leptothrix, and Sphaerotilus OTUs. –, not detected.
Metagenomic sequencing was performed on one iron mat sample from Spruce Point and eight iron mat samples from the SRS. We used BLAST+ to search for matches to the Fleming 16S rRNA sequence for L. ochracea and identified full-length 16S sequences with >99% identity to the Fleming sequence in all nine assemblies. No 16S genes were associated with metagenome-assembled genomes (MAGs) described below. However, the presence of L. ochracea 16S genes in the assemblies confirms that L. ochracea was sequenced in all nine metagenomes.
A phylogenetic tree was constructed using the 16S rRNA sequences of the Leptothrix–Sphaerotilus group from our samples (16S rRNA amplicon sequences and metagenome sequences) and additional publicly available sequences from the Leptothrix–Sphaerotilus group (Fig. 3). Eighteen previously unclassified Leptothrix sequences were identified from SILVA and GenBank by a BLAST search against the L. ochracea L12 sequence. These sequences come from terrestrial freshwater iron-rich environments, including iron microbial mats and iron seeps in mines, stream and river bacterioplankton communities, and a rhizosphere (7, 37–41). Sixteen of the unclassified sequences from SILVA and GenBank were classified as L. ochracea based on >98.6% identity to the L. ochracea L12 sequence. The sequences from this study and the sequences identified by BLAST cluster with the L. ochracea L12 sequence, further demonstrating that they are closely related to L. ochracea (Fig. 3).
Fig 3.
Phylogeny of 16S ribosomal RNA gene sequences in the Leptothrix–Sphaerotilus clade. Maximum likelihood tree of 16S rRNA gene sequences generated using RAxML with 1,000 bootstraps. Metagenome assembly and 16S amplicon sequences from this study are shown in bold. All sequences are full-length, except those denoted by asterisks, representing 465-bp 16S rRNA gene amplicon sequences. Closed circles denote bootstrap support between 75% and 100%. Open circles denote bootstrap support between 50% and 75%. Rhodoferax ferrireducens outgroup not shown. Accession numbers for the collapsed clade of iron microbial mat clones: LN870973.1, LN870963.1, LN870961.1, LN870953.1, LN870947.1, LN870913.1, LN870912.1, LN870911.1, LN870861.1, LN870849.1, LN870848.1, AB722229.1, AB600433.1, HQ290516.1.1404, and HQ290516.1.
Leptothrix–Sphaerotilus genomes
Metagenome-assembled genomes (MAGs) were reconstructed from the metagenome assemblies and manually curated using Anvio (42). One MAG from Spruce Point and twelve MAGs from the SRS were classified by GTDB-tk (43, 44) (Table 3) to the order level as Burkholderiales, which contains the Leptothrix–Sphaerotilus group, but none were specifically classified as Leptothrix. GTDB contains only two Leptothrix genomes (L. cholodnii and L. mobilis), which does not span the full diversity of Leptothrix, so GTDB may have difficulty classifying L. ochracea. Eleven of these Burkholderiales MAGs were deemed to be of high quality using CheckM (>90% completeness, <5% contamination) (43) and were used for further classification.
TABLE 3.
Leptothrix–Sphaerotilus genome attributes
| Classification | Genome name | Completeness | Contamination | Sequence size (Mb) | No. of contigs | GC content (%) | N50 | L50 | No. of CDS | Source |
|---|---|---|---|---|---|---|---|---|---|---|
| Leptothrix ochracea | ISCOC1.001 | 99.03 | 1.03 | 2.90 | 113 | 60.9 | 52206 | 15 | 2720 | Stream |
| ISCOA1K.005 | 97.13 | 2.25 | 2.75 | 291 | 61.1 | 16360 | 49 | 2678 | Stream | |
| ISCOC1bead.006 | 99.03 | 0.47 | 2.91 | 114 | 60.8 | 64115 | 14 | 2718 | Stream | |
| ISCOB2.007 | 98.18 | 1.87 | 2.59 | 248 | 61.3 | 19293 | 39 | 2453 | Stream | |
| DE2.004 | 97.94 | 0.00 | 2.94 | 42 | 60.8 | 92174 | 8 | 2677 | Wetland | |
| MN3.008 | 95.75 | 0.86 | 2.62 | 215 | 61.1 | 15561 | 49 | 2508 | Wetland | |
| MN2.012 | 99.50 | 0.00 | 3.00 | 63 | 60.7 | 138756 | 7 | 2793 | Wetland | |
| DE1.021 | 99.27 | 0.00 | 2.96 | 50 | 60.8 | 114507 | 9 | 2703 | Wetland | |
| 4SP.002 | 99.35 | 0.00 | 3.04 | 47 | 60.6 | 86114 | 11 | 2786 | Drainage channel | |
| Leptothrix ochracea L12 | 23.28 | 0.00 | 0.51 | 36 | 58.0 | 20545 | 8 | 562 | Freshwater | |
| Other Leptothrix |
DE1.014 | 100.00 | 0.59 | 4.69 | 65 | 65.1 | 121859 | 11 | 4441 | Wetland |
| DE1.018_1 | 34.99 | 8.89 | 2.07 | 276 | 65.2 | 9573 | 56 | 2230 | Wetland | |
| DE1.018_2 | 61.21 | 34.48 | 5.07 | 792 | 69.6 | 7506 | 204 | 5173 | Wetland | |
| Leptothrix cholodnii SP-6 | 100.00 | 0.00 | 4.91 | 1 | 68.9 | N/A | 1 | 4446 | Isolate | |
| Leptothrix mobilis | 100.00 | 0.47 | 4.65 | 15 | 69.0 | 719329 | 3 | 4141 | Isolate | |
| Sphaerotilus | MN3.004 | 90.47 | 1.43 | 3.94 | 418 | 68.0 | 12007 | 101 | 3954 | Wetland |
| Sphaerotilus natans | 99.97 | 0.00 | 4.63 | 57 | 69.9 | 161150 | 9 | 4222 | Isolate |
These MAGs are all relatively abundant in their respective assemblies (Table 4), and their relative abundance matches that of the L. ochracea OTUs in the 16S rRNA gene data. Given that L. ochracea represented the most prevalent Leptothrix or Sphaerotilus OTUs, we performed further classification to investigate whether these abundant MAGs represented L. ochracea. None of these MAGs contain a 16S rRNA gene, so we analyzed average amino acid identity (AAI) to determine their relatedness to L. ochracea L12 (Fig. 4). Nine MAGs were identified as L. ochracea based on the AAI values (95.3%–95.7%) above the species delineation cutoff (>95% (41)). Furthermore, the AAI values between all nine MAGs are >99%, demonstrating that these MAGs are all highly related to each other. This is consistent with findings of the study by Fleming et al. (7) who found very limited microdiversity in L. ochracea 16S rRNA gene sequences. The high relatedness of the MAGs from this study is especially striking, given their reconstruction from diverse environments at four different sampling sites from two geographically distinct locales.
TABLE 4.
Percent abundance of Leptothrix and Sphaerotilus MAGs in their respective metagenome assembliesa
| Metagenome assembly | MAG | Percent abundance |
|---|---|---|
| 4SP | 4SP.002 | 65.9 |
| ISCOA1K | ISCOA1K.005 | 4.65 |
| ISCOB2 | ISCOB2.007 | 4.57 |
| ISCOC1 | ISCOC1.001 | 12.9 |
| ISCOC1bead | ISCOC1bead.006 | 12.5 |
| MN2 | MN2.012 | 2.42 |
| MN3 | MN3.008 | 19.2 |
| DE2 | DE2.004 | 7.90 |
| DE1 | DE1.021 | 14.0 |
| DE1.014 | 4.24 | |
| DE1.018_1 | 1.38 | |
| DE1.018_2 | 1.26 | |
| MN3 | MN3.004 | 2.51 |
Percent abundance values represent estimated percent community abundance including unbinned contigs.
Fig 4.
Heatmap of amino acid identity (%) between Leptothrix and Sphaerotilus genomes. Asterisks denote isolate genomes.
We further resolved the phylogeny of these MAGs within the Leptothrix–Sphaerotilus group using a tree of 18 concatenated ribosomal proteins (Fig. 5). The concatenated tree confirmed the AAI results; the same nine MAGs clustered together and formed a monophyletic group with Leptothrix ochracea L12, while the remaining two clustered with Sphaerotilus natans and Leptothrix cholodnii SP-6 (Fig. 4 and 5). There is 100% bootstrap support for the branch separating L. ochracea L12 and the nine L. ochracea MAGs from the other members of the Leptothrix–Sphaerotilus group (Fig. 5). Taken together, these results confirm that L. ochracea is a distinct species within the Leptothrix–Sphaerotilus clade. The metagenome abundance, phylogenetic tree, and amino acid identity results agree with morphological and 16S rRNA gene evidence that L. ochracea is present and abundant in these samples and confirm that these MAGs are in fact L. ochracea. Thus, these nine MAGs represent the first near-complete genomes of L. ochracea.
Fig 5.
Phylogeny of concatenated ribosomal protein sequences from the Leptothrix–Sphaerotilus group. Maximum likelihood tree of genes from metagenome-assembled genomes and reference genomes in the Leptothrix–Sphaerotilus group, using Rhodoferax ferrireducens T118 as an outgroup. Generated using RAxML with 1,000 bootstraps from alignment of ribosomal proteins L2, L3, L4, L6, L13, L17, L19, L20, L27, L28, L35, S2, S3, S8, S9, S11, S13, and S16. Closed circles denote bootstrap support between 75% and 100%. Open circles denote bootstrap support between 50% and 75%.
The genomes of L. ochracea reconstructed here are similar in size (2.6–3.0 Mb) to those of the autotrophic iron oxidizers in the family Gallionellaceae. In contrast, other members of the Leptothrix–Sphaerotilus group have larger genomes of 4.4–5.1 Mb. The smaller genome size of L. ochracea is consistent with it having a distinct, more limited metabolism compared to its closest relatives.
L. ochracea metabolic potential
Genomes of L. ochracea were analyzed to explore their energy metabolisms and metabolic contributions to elemental cycling with a focus on genes for metal oxidation, oxygen tolerance, electron transport, and carbon assimilation. This allowed us to understand the niche of L. ochracea by identifying its major carbon and energy sources and comparing and contrasting these with other Leptothrix–Sphaerotilus. We also investigated genes for nitrogen and sulfur metabolisms to understand the breadth of biogeochemical transformations mediated by L. ochracea. Genes are summarized in Fig. 6 and Table S5.
Fig 6.
Genomic potential of the Leptothrix–Sphaerotilus group. Shaded boxes indicate gene presence. White boxes indicate gene absence. The asterisk indicates that mofA may play a role in Fe oxidation.
Metal oxidation
Iron oxidation
Functionally, L. ochracea has always been categorized as a member of the iron-oxidizing bacteria (3, 45). Although it catalyzes biotic iron oxidation, it has never been shown definitively to conserve energy from iron oxidation. Furthermore, the L. ochracea L12 SAG lacks known iron oxidase genes (4, 46, 47). We analyzed these near-complete genomes for genes that may be responsible for iron oxidation coupled to energy conservation. Seven of the L. ochracea MAGs contain an iron oxidase gene, including a cluster 1 cyc2 and/or mtoA, whereas the other members of the Leptothrix–Sphaerotilus group contain only cluster 2 cyc2 genes (Fig. 6) (clusters defined by McAllister et al., (48)). The cluster 1 Cyc2 is functionally validated as an iron oxidase in neutrophilic iron oxidizers (47), while the cluster 2 Cyc2 function was demonstrated in an acidophile (49). Several of the Leptothrix genomes also have homologs of the gene that encodes the periplasmic electron carrier Cyc1 (Fig. 6), which carries electrons from the outer membrane protein Cyc2 to the inner membrane and electron transport chain. This gene is not as prevalent among iron oxidizers as the iron oxidase genes, likely because iron-oxidizing bacteria use diverse periplasmic electron carriers. The presence of iron oxidases and periplasmic electron carrier genes gives L. ochracea the potential to connect iron oxidation to the electron transport chain with the same mechanism used by Gallionellaceae. This is compelling evidence that L. ochracea has the means to use iron oxidation for energy and growth.
The presence of these validated iron oxidase genes also makes L. ochracea distinct from the isolate L. cholodnii SP-6. Although this isolate demonstrates iron oxidation activity in laboratory cultures (46, 47), its genome lacks known iron oxidase genes, and there is no good evidence thus far for iron oxidation in L. cholodnii being an energy-generating process. It is unclear why L. ochracea and L. cholodnii would have different iron oxidation mechanisms, but it is likely due to the distinct roles of Fe oxidation in their metabolisms.
The presence of multiple iron oxidase genes (cyc2 and mtoA) in L. ochracea genomes may enable the cells to utilize different ferrous iron substrates. Gallionellaceae genomes also commonly contain both cyc2 and mtoA (50), and their expression differs during oxidation of dissolved and solid Fe(II) substrates (51, 52). Cyc2 is a monoheme outer membrane cytochrome–porin that most likely interacts with aqueous Fe2+ (47). MtoA is a decaheme cytochrome, also in the outer membrane, with a structure that could theoretically contact a solid substrate (46). In fact, mtoA has been shown to be expressed specifically when the Gallionellaceae Sideroxydans lithotrophicus ES-1 oxidizes solid Fe(II) in smectite clay (52). In L. ochracea iron mats, the primary iron source is aqueous Fe2+ coming from anoxic groundwaters, but this Fe2+ may become bound to organic matter (humics and extracellular polymers) and minerals (20, 53, 54). Having different iron oxidases would allow L. ochracea to use these varied Fe(II) forms as sources of electrons for growth.
Manganese oxidation
Representatives from Leptothrix and Sphaerotilus have been shown to oxidize Mn (55–58); however, it is unknown whether L. ochracea can also oxidize Mn. Multicopper oxidases (MCOs) can be Mn and Fe oxidases in bacteria (59–61), among other functions. We searched for MCOs in the L. ochracea genomes using a custom MCO detection script to identify MCOs based on a set of copper-binding amino acid motifs (62) and then clustered hits with known MCO sequences within L. cholodnii SP-6 for identification. Among these, mofA was present in all of the L. ochracea MAGs, with one to three copies per genome (Fig. 6; Table S5). L. cholodnii SP-6 possesses several distinct MCOs that have been shown to oxidize Mn, including mnxG and mcoA (59); however, the L. ochracea genomes only encode mofA (Fig. 6; Table S5). The ubiquity of mofA across all L. ochracea genomes, often in multiple copies, suggests that it is essential to growth and survival in iron-oxidizing conditions, but it is unclear whether MofA functions as an Fe or Mn oxidase in L. ochracea. MofA is implicated in Mn oxidation in L. cholodnii SP-6 (63, 64), though MCOs also possess iron oxidation activity (59–61), so its role in iron oxidation cannot be ruled out.
Energy metabolisms
Oxygen
All genomes in this study possess genes for the cbb3-type cytochrome c oxidase (ccoNOPQ), and most of them also encode genes for a cytochrome bd ubiquinol oxidase complex (cydABX) (Fig. 6; Table S5). These terminal oxidases have high affinity for oxygen and therefore are widely understood to be used under microaerobic conditions (65, 66). The L. ochracea MAGs only encode these two terminal oxidases, whereas the other Leptothrix and Sphaerotilus genomes possess genes for the aa3-type cytochrome c oxidase (coxABCD) as well, which has low affinity for oxygen and is highly expressed under oxic conditions (67–69). This suggests that L. ochracea tends to occupy more microaerobic niches, while other members of the Leptothrix–Sphaerotilus group can grow under a wider range of oxygen concentrations.
Electron transport chain
All of the genomes analyzed in this study have genes for complete electron transport chains, including the nuoABCDEFGHIJKLMN gene cluster encoding Complex I, sdhABCD encoding Complex II, a complete cytochrome bc1 complex (Complex III), at least one cytochrome c oxidase (Complex IV), and all five subunits of an F-type ATPase (Complex IV) (Fig. 6; Table S5). We confirmed the presence of genes for ACIII (actABCDEF) in the L. ochracea L12 genome, as reported by Fleming et al. (4) and identified its presence in eight of the nine L. ochracea MAGs. It is absent in all of the other Leptothrix and Sphaerotilus genomes. In all of the L. ochracea genomes with ACIII, actA was truncated. Fleming et al. hypothesized that this was due to the incomplete nature of the L. ochracea L12 assembly, but the actA genes in the MAGs were of the same length. The act gene order (actABCDEF) is conserved among all the L. ochracea genomes possessing ACIII; the only exception is genome MN3.008, where these genes were found split between two separate contigs. There is evidence that ACIII participates in reverse electron transport (RET) during autotrophic iron oxidation by Gallionellaceae (51); however, we hypothesize that L. ochracea does not require RET due to its utilization of organics for energy. Organic carbon oxidation generates NADH, and therefore the energetically costly process of RET would not be required to produce reducing equivalents. Thus, the L. ochracea ACIII, with the truncated actA gene, may have a distinct function from ACIII in the chemolithoautotrophic iron oxidizers.
Carbon fixation and heterotrophy
Here, we examine the genomes of L. ochracea to understand its carbon metabolism, including which substrates it is able to use for biomass or energy generation.
Carbon fixation and storage
All of the L. ochracea MAGs contain genes for Form II RuBisCO (rbcL/cbbM) and genes for a full Calvin–Benson–Bassham (CBB) cycle (Fig. 6; Table S5), which suggests that they are capable of fixing CO2 into organic carbon. This demonstrates that, despite the general understanding of members of the Leptothrix–Sphaerotilus group as heterotrophs, L. ochracea has the potential to use inorganic carbon sources. L. ochracea was shown to assimilate CO2 at a small fraction of its total carbon demand (4). This implies that, while it may obtain some carbon by CO2 fixation, it can also assimilate organic carbon. Combined, this genomic and experimental evidence points to a mixotrophic metabolism.
The genes for RuBisCO and the CBB cycle are not unique to L. ochracea, as the other members of the Leptothrix–Sphaerotilus group also possess the majority of the pathway (Fig. 6; Table S5). This reveals that they also have the potential to use inorganic carbon sources; however, they thrive in environments with higher quantities of organics than L. ochracea (32). Thus, they could alternatively utilize the CBB cycle to recycle CO2 produced during heterotrophic metabolism (70–72).
Organic carbon utilization
Simple sugars and polysaccharides
All complete genomes in this study possess genes for glycolysis and gluconeogenesis and a number of simple sugar transporters (Fig. 6; Table S5). These include genes for a glucose/mannose transport system (gtsABC), a fructose transport system (frcABC), and at least one gene for a putative multiple sugar or simple sugar transport system. Additionally, L. ochracea contains genes for the import and degradation of maltohexose (maltodextrin), including a maltoporin for import, and alpha glucosidase and glycogen phosphorylase for degradation of maltodextrin into D-glucose monomers. The ability to import this polysaccharide would afford L. ochracea the ability to use carbon present from decomposing organic matter in wetlands; therefore, these genes suggest that L. ochracea is able to capitalize on simple sugars and polysaccharides from organic matter degradation and use them for energy and/or biosynthesis pathways.
In addition to genes for sugar import, the L. ochracea genomes encode for numerous genes with CAZy (carbohydrate active enzymes) annotations including polysaccharide lyases (PL9) and glycoside hydrolases (GH13) (Fig. 6; Table S5). Despite these annotations, BLAST searches did not reveal close homologs to these sequences with verified functions. Representatives from category PL9 can degrade major plant cell wall polysaccharides (73) and the sheaths of Sphaerotilus natans (74). Genomes from the other Leptothrix and Sphaerotilus representatives possess higher copy numbers of the PL9 and GH13 CAZy genes, in addition to numerous genes in the category auxiliary activities (AA3), and glycoside hydrolases (GH16 and GH5) (Table S5). Overall, L. ochracea has more limited organic carbon utilization pathways than other Leptothrix–Sphaerotilus, consistent with differences in growth responses to organics. Isolates of the other Leptothrix–Sphaerotilus have strong growth responses to organic carbon and also produce more robust organic sheaths composed of polysaccharides, in contrast to L. ochracea, in which sheaths are primarily iron oxyhydroxide (9, 13, 19, 35, 75–77). L. ochracea sheath synthesis may demand less organic input and therefore fewer organic utilization pathways.
Organic acids and other substrates
All L. ochracea MAGs have genes for L-lactate permease (lctP) and L-lactate dehydrogenase (ykgEFG), which transform L-lactate into pyruvate (Fig. 6; Table S5). This indicates that L. ochracea is able to import lactate and feed it directly into central metabolism. All of these genomes also have a predicted lactate-responsive regulator gene (iclR), which may indicate that they are able to detect the presence of lactate and express these genes when it is present, and use lactate for energy and/or biosynthesis. Genes for acetate permease (actP) and acetate kinase (ackA) are also present in all nine L. ochracea MAGs (Fig. 6; Table S5), suggesting that acetate can be imported and shuttled into pyruvate metabolism. These genes suggest that L. ochracea has the potential to use both lactate and acetate for energy generation and to assimilate organic carbon from them via TCA cycle intermediates.
All Leptothrix and Sphaerotilus genomes contain numerous transporters for peptides and amino acids and peptidases (Table S5), suggesting that they are capable of utilizing peptides and amino acids as sources of carbon. All genomes in the Leptothrix–Sphaerotilus group contain genes for NAD-dependent formate dehydrogenase (Fig. 6; Table S5). Formate is often present in wetlands as a product of fermentation, and formate dehydrogenase can regenerate NADH using formate (78). This suggests that formate can be an energy-generating substrate for L. ochracea and other Leptothrix and Sphaerotilus members.
Other metabolisms
Sulfur oxidation
All of the complete Leptothrix and Sphaerotilus genomes possess genes for thiosulfate oxidation to sulfur (soxABXYZ), except Sphaerotilus natans, and most also have soxCD for oxidation to sulfate (79) (Fig. 6; Table S5). This is consistent with the ability of other iron oxidizers to oxidize thiosulfate (e.g., Sideroxydans lithotrophicus ES-1 and Sideroxyarcus emersonii MIZ01) (51, 80). These genes give L. ochracea the ability to use sulfur species as electron donors for energy conservation, in addition to iron and organics. Furthermore, these genes suggest that L. ochracea contributes to biogeochemical sulfur cycling via thiosulfate oxidation.
Nitrogen metabolism
None of the L. ochracea MAGs contain genes for nitrogen fixation (nifHDK) (Fig. 6; Table S5), indicating that L. ochracea is not capable of nitrogen fixation. Genes for denitrification (napA, narGHI, nirK, nirS, norBC, and nosZ) are largely absent from Leptothrix ochracea (Fig. 6; Table S5); however, all complete genomes in this study contain genes for a respiratory nitrite reductase (nirBD), suggesting that the Leptothrix–Sphaerotilus group may conduct dissimilatory nitrite reduction during aerobic growth (81). All Leptothrix and Sphaerotilus genomes also possess several genes for nitrogen assimilation, including nasA for assimilatory nitrate reduction, genes for ammonia import and assimilation (ammonia transporter, glutamine synthetase, and glutamate synthase), and genes encoding urease (ureABCDEFG) for urea assimilation (Fig. 6; Table S5). In summary, these results demonstrate that the main contribution of L. ochracea to nitrogen cycling is through dissimilatory nitrite reduction and through assimilation of nitrate, ammonia, and urea.
Hydrogen oxidation
Genes for [NiFe]-hydrogenases (hypABCDEF, hupUV, hoxFU, hoxHY) are absent in all Leptothrix ochracea genomes, but are encoded in the genomes of other members of Leptothrix and Sphaerotilus (Table S5).
Vitamin B12
It has been widely reported that vitamin B12 is a growth requirement for the Leptothrix–Sphaerotilus group and must be supplemented in growth media (5, 27, 55, 82). Thus, we looked for vitamin B12 import pathways in these genomes. A vitamin B12 transporter gene (btuB) was identified in all of the Leptothrix and Sphaerotilus genomes, except for L. ochracea L12 and L. cholodnii SP-6 (Fig. 6; Table S5). This transporter performs active transport of vitamin B12 via coupling with the protein TonB (83–85). The tonB gene was present in the majority, though not all, of the Leptothrix and Sphaerotilus genomes that contained btuB. L. cholodnii SP-6 lacks the btuB transporter and is the only Leptothrix or Sphaerotilus genome that possesses genes involved in biosynthesis of vitamin B12 (Fig. 6; Table S5). These results support the suggestion that vitamin B12 is a requirement for most members of this group, but that it cannot be considered a defining trait of the Leptothrix–Sphaerotilus group.
Transcriptome
Metatranscriptome sequencing was performed on SRS mat samples DE1, MN3, and ISCOA1K. We mapped transcript reads to the L. ochracea MAGs from these three samples (DE1.021, MN3.008, and ISCOA1K.005) to gain an insight into the metabolic activity of L. ochracea in its typical environments. The transcriptomes are consistent with our interpretation of L. ochracea as a metal-oxidizing, sheath-forming organism that can tolerate oxygen and oxidative stress (e.g., mofA, epsABP, and glutathione peroxidase gene, Table 5). The expression of genes for iron oxidation, as well as carbon fixation and utilization, supports our characterization of L. ochracea as a mixotrophic iron oxidizer. MAGs DE1.021 and ISCOA1K.005 highly expressed iron oxidation genes cyc2 and cyc1, while all three MAGs show the expression of the multiheme iron oxidase gene mtoA and various other cytochromes (Table 5; Table S6 to S8). The MCO mofA was one of the top expressed genes in all three MAGs (Table 5). Although the role of mofA in L. ochracea is unclear, we can gain insight by examining the environmental chemistry. Iron concentrations (measured by ICP-MS) were much higher than Mn in surface water at sites DE1 and MN3 and in sediment porewater at site MN3. Mn concentrations were 0.7–1.2 µM in surface water and 1.4–2.0 µM in porewater, whereas Fe concentrations were 4–11 µM in surface water and 13–27 mM in sediment (Table S1). The abundance of Fe relative to Mn implies the utility of mofA in iron-rich environments and may suggest its role in Fe oxidation. Furthermore, the cbb3-type cytochrome c oxidase genes ccoNO are expressed highly in all three MAGs (Table 5; Table S6 to S8). A number of organic carbon utilization genes are expressed by L. ochracea, including high expression of genes for lactate permease, lactate dehydrogenase, and a sodium/acetate symporter, and moderate expression of the glucose/mannose transport system gtsABC (Table 5; Table S6 to S8). On the other hand, rbcL (RuBisCO) expression is low to moderate (Table 5; Table S6 to S8). These function-based results provide strong evidence that L. ochracea is growing as an aerobic, mixotrophic iron oxidizer in the environment.
TABLE 5.
In situ expression of key genes in L. ochracea MAGs from the three SRS sampling sites
| Function | Gene | Percentile expressiona | ||
|---|---|---|---|---|
| DE1.021 | ISCOA1K.005 | MN3.008 | ||
| Iron oxidation | cyc2 | 99.6 | 99.3 | N/Ac |
| Iron oxidation | cyc1 | 90.7 | 94.4 | N/Ac |
| Iron oxidation | mtoA | 82.3 | 60.9 | 31.3 |
| cbb3-type cytochrome c oxidase | ccoN | 97.2 | 98.8 | 96.5 |
| cbb3-type cytochrome c oxidase | ccoO | 96.7 | 99.1 | 96.8 |
| Glutathione peroxidase/oxidative stress response | btuE | 91.8 | 92.0 | 84.8 |
| Lactate permease | lctP | 89.9 | 88.6 | 98.0 |
| Lactate dehydrogenase | ykgEFG | 82.0 | 74.2 | 64.5 |
| Na+/acetate symport | actP | 81.3 | 89.1 | 88.8 |
| Glucose/mannose transport | gtsA | 74.0 | 73.8 | 68.4 |
| Glucose/mannose transport | gtsB | 60.1 | 57.5 | 54.4 |
| Glucose/mannose transport | gtsC | 35.4 | 30.9 | 21.4 |
| RuBisCO | rbcL | 28.2 | 50.1 | 56.1 |
| Polysaccharide export protein wzab | epsA | 93.5 | 93.6 | 92.4 |
| Polysaccharide export protein wzbb | epsP | 53.5 | 46.5 | 41.8 |
| Polysaccharide export protein wzcb | epsB | 69.7 | 81.4 | 70.3 |
| Metal oxidation | mofA copy 1 | 97.2 | 99.4 | 97.7 |
| Metal oxidation | mofA copy 2 | 76.4 | 90.7 | 3.56 |
Percentile expression represents the rank of each gene’s mapped read count in comparison to all expressed genes (read counts > 0). Values represent the percentage of genes with read counts lower than that gene.
Putative involvement in sheath formation.
N/A indicates gene absence.
Leptothrix ochracea metabolic model
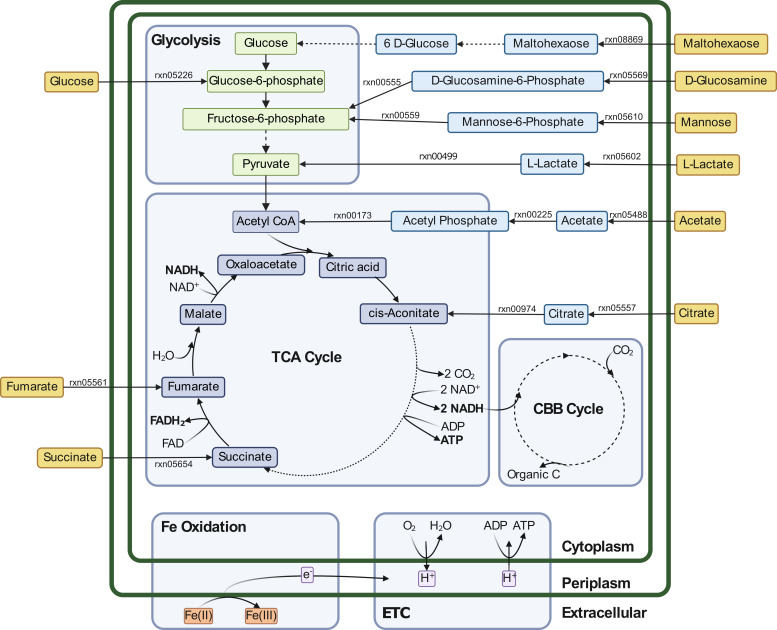
In order to gain an additional insight into the activity and metabolism of L. ochracea, in silico stoichiometric metabolic models of L. ochracea MAG DE1.021 were computed using the ModelSEED tools in KBase. To narrow down which organic carbon compounds L. ochracea is capable of importing and utilizing for growth, we constructed the first model using complete media (i.e., media containing all available compounds in the ModelSEED database) and manually added the energy-generating iron oxidation pathway to the model. When L. ochracea DE1.021 was given complete media, the model displayed flux through the import of numerous organic carbon substrates: lactate, fumarate, glucose, succinate, glucosamine, mannose, maltohexaose, citrate, and all 20 amino acids. These compounds are fed into central metabolism and used for energy and biosynthesis (Fig. 7). Importantly, genes for these import reactions and downstream pathways are indeed present in the L. ochracea genome and not an artifact of gapfilling the model. Furthermore, adding iron oxidation to the complete media model resulted in an increase in theoretical biomass yield (Table 6; objective value).
Fig 7.
In silico metabolic model of Leptothrix ochracea MAG DE1.021 on complete media. Reactions pictured illustrate selected import and central metabolic pathways to represent a subset of reactions captured in the full model. Reaction numbers (rxn#) correspond to unique reaction identifiers in the ModelSEED biochemistry database. Created with BioRender.com.
TABLE 6.
Objective values and exchange flux for L. ochracea metabolic models
| Corg source | Objective value (without Fe oxidation) |
Objective valuea (with Fe oxidation) |
Corg substrate exchange fluxb |
|---|---|---|---|
| Complete media | 72.5 | 82.5 | N/Ac |
| Glycolate | 0.603 | 0.577 | 1 |
| Maltohexaose | 1.47 | 1.41 | 1 |
| Formate | 0.603 | 0.577 | 1 |
| D-Glucose | 0.747 | 0.715 | 1 |
| Succinate | 0.675 | 0.658 | 1 |
| Fumarate | 0.663 | 0.646 | 1 |
| Acetate | 0.603 | 0.577 | 1 |
| D-Mannose | 0.603 | 0.715 | 1 |
| L-Lactate | 0.603 | 0.623 | 1 |
Objective value is a proxy for biomass yield based on the calculated flux through the Gram negative biomass reaction (bio1).
Exchange flux for Corg substrates is set to a maximum value of 1 based on media composition.
N/A indicates that there are variable exchange fluxes for the multiple organic carbon substrates within the model.
To evaluate the growth yield of L. ochracea on diverse organic substrates individually, metabolic models of L. ochracea were constructed using minimal media with Fe2+ and single carbon sources, which were chosen based on its genomic content and the model on complete media. Models on glucose, mannose, formate, succinate, fumarate, maltohexaose, glycolate, acetate, and lactate each demonstrated biomass production, which is consistent with the presence of multiple transporters for organic acids, sugars, and dicarboxylic acids. Furthermore, iron oxidation increased the theoretical biomass yield for the models grown on mannose and lactate, suggesting that iron oxidation supplements energy production in these models (Table 6). All of these compounds were transformed into intermediates of either glycolysis or the tricarboxylic acid (TCA) cycle (Fig. 7); the flux through these central metabolic reactions in these models indicates that these compounds are being used for both energy generation and biosynthesis (Tables S11 to S14; https://figshare.com/articles/dataset/_i_Leptothrix_ochracea_i_metabolic_models_flux_balance_analysis_tables/25977355).
These results demonstrate that it is theoretically possible for L. ochracea to produce biomass while growing mixotrophically using these carbon sources and iron oxidation.
Implications and conclusions
Resolving the identity and niche of L. ochracea requires an understanding of its carbon and energy sources. In the absence of a L. ochracea isolate, we used a metagenomic approach on samples from three of its typical environments at two sites. These yielded the first near-complete genomes of L. ochracea from which we could interpret potential metabolic capabilities and connect these to our understanding of its niche. We also used metatranscriptomics to gain an insight into the activity of L. ochracea in its typical environments.
Historically, L. ochracea has primarily been classified by the presence of sheaths in iron-rich environments (i.e., by morphological observations), but the sheaths are not live material, rather an indication of previous presence. Molecular methods are required for more precise tracking and quantification, though reliable identification requires sufficient reference sequences. Fleming et al. (7) identified a 16S rRNA gene sequence from L. ochracea using FISH and reconstructed a partial single-cell amplified genome of L. ochracea, providing a molecular basis with which to identify L. ochracea. Here, we have recovered 16S sequences from both 16S rRNA amplicon sequencing and metagenome assemblies that are closely related to the Fleming 16S sequence (>99% identity). Furthermore, we recovered nine near-complete genomes that are closely related to the L. ochracea L12 partial single-cell amplified genome, clustering together in concatenated ribosomal protein trees. Overall, our work expands the known 16S diversity of L. ochracea, as well as providing high-quality genomes, resulting in a robust reference molecular data set from multiple sites and environments, with which further L. ochracea can be identified. Altogether, our phylogenetic analyses demonstrate that L. ochracea forms a distinct group within the Leptothrix–Sphaerotilus clade. It is notable how closely related both the L. ochracea MAGs and 16S rRNA gene sequences are, despite their reconstruction from diverse sites. This suggests there may be selective pressures that resist accumulation of genetic variability.
L. ochracea has been shown to oxidize iron biotically, but the role of iron oxidation in its metabolism has not been established. Although Leptothrix and Sphaerotilus isolates are known as metal oxidizers, it has been debated whether Fe or Mn oxidation can be used for energy and growth (26–29). The full genomes of L. ochracea presented here provide the first evidence that any member of the Leptothrix–Sphaerotilus group has at least two characterized iron oxidase genes, cyc2 and mtoA, along with other genes required to form an energy-conserving electron transport chain based on iron oxidation. These same genes are used by known autotrophic iron oxidizers to conserve energy from iron oxidation (47, 49, 51, 52). This strongly suggests that L. ochracea can conserve energy from iron oxidation and use this energy for growth, which could make L. ochracea distinct from other Leptothrix and Sphaerotilus in this ability.
L. ochracea appears to combine the ability to oxidize Fe for energy with the capabilities of carbon fixation and heterotrophy, making it an iron-oxidizing mixotroph. The carbon metabolism of L. ochracea has long been a source of debate and confusion, and thus an important outcome of this work is the multiple lines of evidence for mixotrophy based on its genome content, environmental transcriptomes, and metabolic modeling. The mixotrophic Fe-oxidizing metabolism is consistent with the work of Fleming et al. that showed an Fe(II) requirement for growth and incorporation of both acetate and CO2 into cell biomass (4). While it is unclear if L. ochracea is an obligate or facultative mixotroph, we suggest that its niche is tied to mixotrophy, both for carbon (autotrophy/heterotrophy) and for energy (lithotrophy/organotrophy). This would help explain seemingly disparate observations that it behaves like an autotrophic Fe-oxidizer but has a propensity for organic carbon (27, 32). The ability to use organics may enable its prodigious sheath production and therefore its role as an ecosystem engineer. Further study could elucidate the balance of energy and carbon inputs that allow L. ochracea to thrive in terrestrial wetland environments alongside autotrophs and heterotrophs, taking advantage of organic breakdown products and creating massive iron mats that host communities and sequesters various nutrients, carbon, and metals. An important example is Leptothrix in Arctic tundra wetlands (86), which can play a significant role in controlling nutrient P dynamics in these critical ecosystems (87) via binding P to its sheaths (14). Ultimately, elucidating the metabolic connections between L. ochracea and iron mat communities will improve our understanding and predictions of wetland biogeochemical cycles.
MATERIALS AND METHODS
Sampling sites
Iron microbial mats were sampled from Spruce Point (Boothbay Harbor, ME, USA) in June of 2016 and from wetland and stream sites at the Savannah River Site (SRS) (Jackson, SC, USA) in May of 2021 and January of 2022. Within the Savannah River watershed, Tims Branch is a second-order stream surrounded by forested riparian wetlands. In the stream, prolific iron mats are found in low-flow areas along the edges of the stream where iron-rich groundwater discharges into surface water. Iron mats in Tims Branch grow in patches of between a few cm to ~1 m in size. In the SRS wetlands, mat growth occurs in low-flow areas and standing water. In some sections of these wetlands, mat growth extended continuously across areas of up to 60 square meters. The second site, the Spruce Point site, is a narrow rock-lined 25-meter-long roadside drainage channel adjacent to a wetland. The water is shallow (10–20 cm), and the entire trench is often filled with iron mats dominated by the sheaths of L. ochracea.
Samples were taken from the following three sites at the SRS and from one site at Spruce Point:
MN site (33.3375°N 81.7186°W): The MN site at the SRS is a wetland adjacent to Tims Branch with pockets of iron mat growth, especially in low-flow areas downgradient from trees.
DE site (33.3392°N 81.7181°W): The DE site at the SRS is a wetland adjacent to Tims Branch, upstream from the MN site, which displays extensive iron mat growth 5–8 cm thick.
ISCO site (33.3164°N 81.7138°W): The ISCO site is an SRS stream site within Tims Branch that displays several areas of Leptothrix-type mats.
SP site (43.8294°N 69.6256°W): Spruce Point is a shallow freshwater drainage channel with slow water flow, which displays fluffy mat growth in puffballs and small channels.
Geochemical analyses
Savannah River site
A Hanna HI9829 multiparameter meter was used to record GPS coordinates and measure pH, oxidation reduction potential, percent dissolved oxygen, electrical conductivity, and temperature in the surface water. Oxygen was also measured in the surface water and below the surface of iron mats using a HI9142 dissolved oxygen meter.
Surface water samples were filtered in the field using 0.2-µm nylon filters. Filtered water samples for Fe(II) measurements were acidified in 40 mM sulfamic acid in the field to limit Fe oxidation. The following chemical analyses were conducted the same day at the on-site laboratory: (1) nitrite measurements using a Hach Spectrophotometer with the NitriVer 3 Nitrite Reagent at 507 nm (2); dissolved Fe2+ measurements using a ferrozine assay (88) read at 562 nm on a Hach Spectrophotometer. Additional surface water samples were filtered in the field using 0.45-µm nylon filters. Filtered samples for analysis of anions and metals were preserved at 4°C and later analyzed at the University of Delaware Advanced Materials Characterization Laboratory. Anion concentrations were measured by ion chromatography using the Metrohm IC Pro. Filtered samples were acidified in 2% nitric acid, and metal concentrations were measured via ICP-MS.
A semipermanent passive diffusion sampler was placed in the wetlands surrounding Tims Branch at the MN3 site in April 2021, as described by MacDonald et al. (89) and Kaplan et al. (90). The samplers consisted of 60-mL chambers stacked vertically with a 0.22-µm pore size polyethersulfone membrane separating the chambers from the sediment. Each sampling chamber was connected aboveground by two 0.16-cm Tygon tubes, which were used to inject deoxygenated, deionized water into each chamber. Sediment porewater and chamber water were allowed to equilibrate for 6 months before withdrawing water samples using the Tygon tubes. One advantage of this sampler is that multiple samples can be readily collected at the same location without disturbing the sediment. Diffusion samplers placed at 46, 43, 41, and 35 cm below the sediment surface were sampled in this study. Samples from each chamber were preserved for ion chromatography, ICP-MS, and dissolved Fe2+ measurements as described above.
Spruce Point site
The surface water pH was 6.05, and the water temperature was 15°C. Dissolved oxygen was measured by using a Pyroscience Firesting optical oxygen probe. Dissolved Fe2+ measurements were performed using a ferrozine assay (88). Water was filtered through a 0.22-µm syringe filter and diluted 1:10 directly into ferrozine.
Biological sample collection and preservation
Iron mats were sampled from wetlands and streams using sterile serological pipets and dispensed into sterile 50-mL conical tubes. Mat material was allowed to settle for several minutes. The supernatant was then decanted, and the remaining mat material was homogenized. One-mL aliquots of the homogenized mat were placed into 2-mL tubes and flash-frozen on dry ice in the field for DNA extraction. One 17-L bulk mat sample (DE2) was collected into a carboy using a Global Water SP200 Variable Speed, Peristaltic, Fluid Sampling Pump. The bulk mat was allowed to settle overnight before further sampling. The settled mat material was homogenized, sampled, and flash-frozen on dry ice. At the SP site, approximately 15 mL of the light-colored mat material was carefully collected using a sterile 1-mL pipette tip with the end cut-off to enlarge the opening. This was done to ensure that the most active L. ochracea cells at the leading edge of the mat were captured (34).
Microscopy
Iron mats were visualized using light microscopy at the on-site laboratory to check that mats contained abundant sheath material, indicative of L. ochracea. After confirmation, mat samples were preserved in 4% paraformaldehyde for further microscopic analysis. Upon return to the laboratory, aliquots of these samples were treated with SYTO-13 green fluorescent nucleic acid stain and were visualized under phase contrast and fluorescence microscopy to confirm the presence of sheaths and chains of cells within the sheaths. Additional aliquots of the samples were fixed in 4% glutaraldehyde for scanning electron microscopy. Glutaraldehyde-fixed samples were washed three times and then dehydrated in an ethanol dilution series of increasing concentrations (25%, 50%, 75%, 95%, and 100%). Following dehydration, the samples were placed into a Tousimis Autosamdri-815B critical point dryer. Dried samples were mounted on aluminum stubs and coated with platinum on a Leica EM ACE600. Imaging was performed on a Thermo Scientific Apreo Volumescope SEM at 2kV.
DNA extraction
Savannah River site
DNA was extracted from flash-frozen field samples using the Qiagen DNeasy Powersoil Pro kit according to the manufacturer’s instructions. To ensure that flash freezing did not impact the recovery of L. ochracea, two additional extractions (MN3 and DE1) were performed on fresh (never frozen) iron mat at the field site within hours of sampling. These were compared to the frozen samples based on the 16S rRNA gene sequencing data in order to select samples for metagenome sequencing. For samples DE1 and MN3, both extractions were used for 16S rRNA amplicon sequencing. The communities in frozen and fresh samples were comparable, so the fresh, unfrozen extractions were used for metagenome sequencing.
Spruce Point site
DNA was extracted using the Mo Bio Powersoil DNA extraction kit according to the manufacturer’s instructions, with 200 ul 25:24:1 phenol:chloroform:isoamyl alcohol solution (Sigma Aldrich) added to each bead tube, as described in Scott et al. (91).
16S rRNA amplicon and metagenome sequencing
Savannah River site
All 16S rRNA gene amplicon sequencing and metagenome sequencing were performed at the University of Delaware Sequencing and Genotyping Center. 16S rRNA gene libraries were prepared using the Illumina 16S Sample Preparation Guide. Amplicons were prepared using the lllumina 16S b341F and lllumina 16S 805R primers, with all preparations done at half the volume (92). Libraries for ISCO and MN2 samples were prepared with 2 ng input DNA and 40 amplicon PCR cycles. Libraries for MN3 and DE samples were prepared with 12.5 ng input DNA and 30 PCR cycles. Libraries were sequenced on an Illumina MiSeq using a Nano 500 cycle sequencing kit.
Mothur (v1.48.0) was used to process the raw 16S rRNA data (93, 94). Data were quality-controlled by filtering out low-quality sequences, removing chimeras, and removing any non-bacterial sequences classified as chloroplasts, mitochondria, archaea, or eukaryotes. OTUs were generated using a reference free approach via clustering the sequences at a 99% identity threshold. Sequences were classified taxonomically using the SILVA database (v. 138.1) (95–98). The 16S rRNA data were analyzed for the abundance of known iron-oxidizing bacteria, including Leptothrix OTUs. Results of the 16S rRNA analysis were then used to select samples for metagenomic sequencing.
For the metagenomic sequencing, libraries were prepared using the Illumina DNA prep library preparation kit according to the manufacturer’s instructions. Libraries for ISCO and MN2 samples were prepared with reactions done at one-third the volume (92), 11.5-minute tagmentation reaction, and eight PCR cycles. Sequencing was done via Illumina NextSeq 2000 using a 300 cycle P3 sequencing kit. Libraries for MN3 and DE samples were prepared with reactions done at one-third the volume (92), 11-minute tagmentation reaction, and 10 PCR cycles (99). Sequencing was done via Illumina NextSeq 550 using a 300 cycle high output sequencing kit. Both rounds of metagenome sequencing obtained 151-bp paired end reads.
Spruce Point site
Metagenomic sequencing was done via Illumina NextSeq 550 at the Integrated Microbiome Resource sequencing center. One nanogram of each fluorescently quantified sample was subjected to NexteraXT (Illumina) library preparation, as per the manufacturer’s instructions, except clean-up and normalization were completed using the Just-a-Plate 96 PCR Purification and Normalization Kit (Charm Biotech). Equal amounts of all barcoded samples (Nextera XT CDIs) were then pooled and sequenced in a shared 150 + 150 bp PE NextSeq550 run using a High-Output v2 kit. Sequencing obtained 151-bp paired-end reads.
Metagenome assembly, binning, and annotation
Raw reads were trimmed then filtered to a minimum length of 75 bp and minimum quality of 28 using Cutadapt (100). Quality of the trimmed and filtered reads was checked using FastQC v0.11.9 (101). Paired-end reads were merged using FLASh v1.2.11 (102) with a maximum overlap read length of 151 bp. Metagenome assemblies were generated using metaSPAdes v3.15.3 with kmer lengths of 21, 33, 55, 77, 99, 111, and 127 bases (103). Binning was performed in KBase using MaxBin2.0 v2.2.4 (104), CONCOCT v1.1 (105), and MetaBAT2 v1.7 (106) with minimum contig lengths of 1,000, 2,500, and 2,500 bp, respectively. Resulting bins were optimized, and the best non-redundant bins from each program were selected, using DASTool v1.1.2 (107). CheckM v1.0.18 (108) was used to evaluate bin quality. Bin taxonomy was classified using GTDB-tk v1.7.0 (44, 109, 110).
Unclassified Burkholderiales bins were further placed into a concatenated ribosomal protein tree with known Leptothrix–Sphaerotilus representatives to determine which, if any, belonged to the Leptothrix genus. The concatenated alignments of 18 large and small ribosomal protein genes (L2, L3, L4, L6, L13, L17, L19, L20, L27, L28, L35, S2, S3, S8, S9, S11, S13, and S16) were generated, trimmed, and masked (sites > 70% gaps) in Geneious v10.2.6. These proteins were selected based on the ribosomal proteins present in the L. ochracea L12 genome and those used by Olm et al. (111) for species-level delineation. The final maximum-likelihood tree was generated using RaxML-NG v1.2.0 with the LG + G4 m model and 1,000 bootstraps (112, 113). Visualizations and annotations were created using iTOL v6.7.2 (114).
Bins of interest were curated using Anvi’o v.7.1 by removing contigs with incongruent GC content and/or depth of coverage (42). Post-curation bin quality was assessed with CheckM. In addition, the CoverM v0.6.1 (115) genome command was used to calculate the abundance of each bin in its respective assembly and to estimate its percent abundance in the community, including unbinned contigs.
Genomes were annotated and screened for functional genes of interest using DRAM v0.1.2 (116), FeGenie (117), RAST-tk (118–120), METABOLIC-C (121), and BLAST (122). Putative multicopper oxidase (MCO) sequences were identified and collected using a custom python script that detected conserved MCO motifs as defined by Gräff et al. (62). This generated a list of potential MCOs, and these sequences were clustered at 50% identity using UCLUST (123). Clusters were identified as MCOs using BLAST. Amino acid identity (AAI) was analyzed using EzAAI v1.2.2 (124), and the results were verified against results from the Kostas lab AAI calculator (125).
Metatranscriptomics
RNA was extracted from in situ frozen iron mat samples from sites ISCOA1K, DE1, and MN3 using the Qiagen RNA PowerSoil Total RNA Isolation Kit according to the manufacturer’s instructions. Fragment analysis was performed at the University of Delaware Sequencing and Genotyping Center using an Agilent fragment analyzer. All meta-transcriptome samples were sent to the DOE Joint Genome Institute (JGI) for library preparation and sequencing. An Illumina Low Input (RNA) library was constructed and sequenced 2 × 151 using the Illumina NovaSeq platform. Raw reads were processed with BBDuk (version 39.01) (126) to remove contaminants, trim adapter sequences, remove homopolymer tails with ≥5 Gs, and right quality trim reads where quality drops to 0. BBDuk was also used to remove reads that contained one or more 'N' bases, had an average quality score below 10, or had a minimum length <51 bp. Reads mapped with BBMap to contaminants (human, cat, dog, mouse, common microbial contaminants, ribosomal RNA, or known spike-ins) were also removed. Final, quality-controlled metatranscriptome reads were mapped back to their respective metagenome assemblies using Bowtie2 v.2.1.0 (127). Anvio v.8 was used to combine functional annotations from Anvio hmm sources, COG, pfam, kofam, CAZy, and FeGenie with gene calls for each metagenome assembly. R packages (readr v2.1.4; dplyr v1.1.4) were used to combine Anvio read counts and functional annotations to calculate TPM counts for each gene call and to quantify the percent rank of expressed genes (read counts > 0) (128–136).
Metabolic model generation
Metabolic models for Leptothrix ochracea MAG DE1.021 were generated using ModelSEED and the KBase toolkit (137, 138). Binned genomes were annotated in KBase using RASTtk (118–120), and metabolic models were generated in the Build Metabolic Model app (v2.0.0) using the automatic Gram-negative template for reconstruction and aerobic pyruvate media for ATP gapfilling. Initial models were built using complete media, which can incorporate all compounds in the ModelSEED database. Subsequent models were built using modified glucose minimal media with Fe2+ and a single carbon source with the default constraints on uptake flux (glucose, mannose, formate, succinate, fumarate, maltohexaose, glycolate, acetate, and lactate; Table S10) The ModelSEED biochemistry database (v.2.6.1) was used to generate and gapfill metabolic models (137). Manual curation of these models was performed by manual addition of reactions associated with iron oxidation and electron transport in Sideroxydans lithotrophicus ES-1 and Mariprofundus ferrooxidans (49, 51), and flux through these reactions was set at a minimum of 0.1. Flux through biomass generation and reactions of interest were quantified using Flux balance analysis (v2.0.0).
ACKNOWLEDGMENTS
We thank John Seaman of the Savannah River Ecology laboratory for hosting us in his lab during our SRS field work. We acknowledge Bruce Kingham and Mark Shaw at the University of Delaware DNA Sequencing and Genotyping Center for performing the SRS 16S rRNA amplicon and metagenome sequencing, Andre Comeau at the Integrated Microbial Resource sequencing center for assistance with Spruce Point metagenome sequencing, Tijana Galvina del Rio and Natasha Brown from the Joint Genome Institute for assistance with the metatranscriptome sequencing, Deborah Powell at the University of Delaware Bioimaging Center for performing SEM sample preparation and imaging, and Austin Chambers and Christopher Blanda for writing a custom Python script for detecting MCO motifs. We are grateful to Cara Santelli and Crystal Ng for the use of their diffusion sampler equipment at the Savannah River Site. We thank Chris Henry, Jose Faria, Filipe Liu, and Andrew Freiburger for access to and assistance with KBase metabolic modeling tools. We thank Jarrod Scott for performing DNA isolation and organizing metagenome sequencing for the Spruce Point sample and Emily Fleming for discussions and feedback.
This work was supported by a DOE ESS Grant DE-SC0021010 to C.S.C., NSF EAR- 2243577 and EAR-18833525 to C.S.C., DOE ESS Contract DE-AC02-06CH11357 to P.B.W. and D.I.K., and NSF grant OIA-1826734 to D.E. G.K.T. was also supported by a fellowship from the University of Delaware Microbiology Graduate Program and Unidel Foundation. Support from the University of Delaware Center for Bioinformatics and Computational Biology Core Facility, including the use of the BIOMIX computer cluster, was made possible through funding from Delaware INBRE (NIH P20GM103446), the State of Delaware, and the Delaware Biotechnology Institute. Support was also received from DOE-EM through the Cooperative Agreement DE-EM0005228 to SREL/UGA.
Contributor Information
Clara S. Chan, Email: cschan@udel.edu.
Arpita Bose, Washington University in St. Louis, St. Louis, Missouri, USA.
DATA AVAILABILITY
The data that support the findings of this study are publicly available in the NCBI and IMG. Metagenome reads, metagenome-assembled genomes, 16S rRNA amplicon sequences, and metatranscriptome reads are available under NCBI BioProject PRJNA1117470. Metatranscriptome reads are also available in IMG (IMG Genome IDs: ISCOA1K: 3300063950; MN3: 3300063952; DE1: 3300067170).
SUPPLEMENTAL MATERIAL
The following material is available online at https://doi.org/10.1128/aem.00599-24.
Confocal images from Spruce Point mat showing sheaths and sheath-forming cells.
Tables S1 to S9.
ASM does not own the copyrights to Supplemental Material that may be linked to, or accessed through, an article. The authors have granted ASM a non-exclusive, world-wide license to publish the Supplemental Material files. Please contact the corresponding author directly for reuse.
REFERENCES
- 1. Roth AW. 1797. Catalecta botanica: quibus plantae novae et minus cognitae descriuntur atque illustratur. I.G. Müller, Lipsiae, Lipsiae. [Google Scholar]
- 2. Kutzing FT. 1843. Phycologia generalis oder anatomie, physiologie und systemkunde der tange. Vol. 1. Leipzig, F. A. Brockhaus. [Google Scholar]
- 3. Winogradsky S. 1888. Uber eisenbakterien. Bot Zeitschr 46:261–270. [Google Scholar]
- 4. Fleming EJ, Woyke T, Donatello RA, Kuypers MMM, Sczyrba A, Littmann S, Emerson D. 2018. Insights into the fundamental physiology of the uncultured Fe-oxidizing bacterium Leptothrix ochracea. Appl Environ Microbiol 84:e02239-17. doi: 10.1128/AEM.02239-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mulder EG, Van Veen WL. 1963. Investigations on the Sphaerotilus-Leptothrix group. Antonie Van Leeuwenhoek 29:121–153. doi: 10.1007/BF02046045 [DOI] [PubMed] [Google Scholar]
- 6. Emerson D, Weiss JV. 2004. Bacterial iron oxidation in circumneutral freshwater habitats: findings from the field and the laboratory. Geomicrobiol J. 21:405–414. doi: 10.1080/01490450490485881 [DOI] [Google Scholar]
- 7. Fleming EJ, Langdon AE, Martinez-Garcia M, Stepanauskas R, Poulton NJ, Masland EDP, Emerson D. 2011. What’s new is old: resolving the identity of Leptothrix ochracea using single cell genomics, pyrosequencing and FISH. PLoS One 6:e17769. doi: 10.1371/journal.pone.0017769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Romano AH, Peloquin JP. 1963. Composition of the sheath of Sphaerotilus natans. J Bacteriol 86:252–258. doi: 10.1128/jb.86.2.252-258.1963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Emerson D, Ghiorse WC. 1993. Ultrastructure and chemical composition of the sheath of Leptothrix discophora SP-6. J Bacteriol 175:7808–7818. doi: 10.1128/jb.175.24.7808-7818.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takeda M, Nakano F, Nagase T, Iohara K, Koizumi J. 1998. Isolation and chemical composition of the sheath of Sphaerotilus natans. Biosci Biotechnol Biochem 62:1138–1143. doi: 10.1271/bbb.62.1138 [DOI] [PubMed] [Google Scholar]
- 11. Chan CS, Fakra SC, Edwards DC, Emerson D, Banfield JF. 2009. Iron oxyhydroxide mineralization on microbial extracellular polysaccharides. Geochimica et Cosmochimica Acta 73:3807–3818. doi: 10.1016/j.gca.2009.02.036 [DOI] [Google Scholar]
- 12. Duff MC, Coughlin JU, Hunter DB. 2002. Uranium co-precipitation with iron oxide minerals. Geochimica et Cosmochimica Acta 66:3533–3547. doi: 10.1016/S0016-7037(02)00953-5 [DOI] [Google Scholar]
- 13. Hashimoto H, Yokoyama S, Asaoka H, Kusano Y, Ikeda Y, Seno M, Takada J, Fujii T, Nakanishi M, Murakami R. 2007. Characteristics of hollow microtubes consisting of amorphous iron oxide nanoparticles produced by iron oxidizing bacteria, Leptothrix ochracea. J Magn Magn Mater 310:2405–2407. doi: 10.1016/j.jmmm.2006.10.793 [DOI] [Google Scholar]
- 14. Rentz JA, Turner IP, Ullman JL. 2009. Removal of phosphorus from solution using biogenic iron oxides. Water Research 43:2029–2035. doi: 10.1016/j.watres.2009.02.021 [DOI] [PubMed] [Google Scholar]
- 15. Borch T, Kretzschmar R, Kappler A, Cappellen PV, Ginder-Vogel M, Voegelin A, Campbell K. 2010. Biogeochemical redox processes and their impact on contaminant dynamics. Environ Sci Technol 44:15–23. doi: 10.1021/es9026248 [DOI] [PubMed] [Google Scholar]
- 16. Hohmann C, Winkler E, Morin G, Kappler A. 2010. Anaerobic Fe(II)-oxidizing bacteria show as resistance and immobilize as during Fe(III) mineral precipitation. Environ Sci Technol 44:94–101. doi: 10.1021/es900708s [DOI] [PubMed] [Google Scholar]
- 17. Stewart BD, Amos RT, Nico PS, Fendorf S. 2011. Influence of uranyl speciation and iron oxides on uranium biogeochemical redox reactions. Geomicrobiol J 28:444–456. doi: 10.1080/01490451.2010.507646 [DOI] [Google Scholar]
- 18. Hao L, Guo Y, Byrne JM, Zeitvogel F, Schmid G, Ingino P, Li J, Neu TR, Swanner ED, Kappler A, Obst M. 2016. Binding of heavy metal ions in aggregates of microbial cells, EPS and biogenic iron minerals measured in-situ using metal- and glycoconjugates-specific fluorophores. Geochimica et Cosmochimica Acta 180:66–96. doi: 10.1016/j.gca.2016.02.016 [DOI] [Google Scholar]
- 19. Kunoh Tatsuki, Hashimoto H, McFarlane IR, Hayashi N, Suzuki T, Taketa E, Tamura K, Takano M, El-Naggar MY, Kunoh H, Takada J. 2016. Abiotic deposition of Fe complexes onto Leptothrix sheaths. Biology (Basel) 5:26. doi: 10.3390/biology5020026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kunoh T, Nakanishi M, Kusano Y, Itadani A, Ando K, Matsumoto S, Tamura K, Kunoh H, Takada J. 2017. Biosorption of metal elements by exopolymer nanofibrils excreted from Leptothrix cells. Water Res. 122:139–147. doi: 10.1016/j.watres.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 21. Sowers TD, Harrington JM, Polizzotto ML, Duckworth OW. 2017. Sorption of arsenic to biogenic iron (oxyhydr)oxides produced in circumneutral environments. Geochimica et Cosmochimica Acta 198:194–207. doi: 10.1016/j.gca.2016.10.049 [DOI] [Google Scholar]
- 22. Whitaker A, Duckworth O. 2018. Cu, Pb, and Zn sorption to biogenic iron (oxyhydr)oxides formed in circumneutral environments. Soil Systems 2:18. doi: 10.3390/soilsystems2020018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Whitaker AH, Peña J, Amor M, Duckworth OW.. 2018. Cr(vi) uptake and reduction by biogenic iron (oxyhydr)oxides. Environ Sci Processes Impacts 20:1056–1068. [DOI] [PubMed] [Google Scholar]
- 24. Sowers TD, Holden KL, Coward EK, Sparks DL. 2019. Dissolved organic matter sorption and molecular fractionation by naturally occurring bacteriogenic iron (oxyhydr)oxides. Environ Sci Technol 53:4295–4304. doi: 10.1021/acs.est.9b00540 [DOI] [PubMed] [Google Scholar]
- 25. Sipos P, Németh T, Kis VK, Mohai I. 2008. Sorption of copper, zinc and lead on soil mineral phases. Chemosphere 73:461–469. doi: 10.1016/j.chemosphere.2008.06.046 [DOI] [PubMed] [Google Scholar]
- 26. Winogradsky S. 1922. Eisenbakterien als anorgoxydanten. Zbl Bakt II Abt 57:1–21. [Google Scholar]
- 27. van Veen WL, Mulder EG, Deinema MH. 1978. The Sphaerotilus-Leptothrix group of bacteria. Microbiol Rev 42:329–356. doi: 10.1128/mr.42.2.329-356.1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghiorse WC. 1984. Biology of iron- and manganese-depositing bacteria. Annu Rev Microbiol 38:515–550. doi: 10.1146/annurev.mi.38.100184.002503 [DOI] [PubMed] [Google Scholar]
- 29. Spring S. 2006. The genera Leptothrix and Sphaerotilus, p 758–777. In Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E (ed), The Prokaryotes. Springer, New York, New York, NY. [Google Scholar]
- 30. Cholodny N. 1926. Die eisenbakterien. Beiträge zu einer monographie Pflanzenforsch G fischer jena, Germany, Vol. 4, p 1–162. [Google Scholar]
- 31. Charlet E, Schwartz W. 1954. Beiträge zur biologie der eisenmikroben. I: untersuchungen über die lebensweise von Leptothrix ochracea und einigen begleitenden eisenmikroben schweiz. Z Hydrol 16:318–341. doi: 10.1007/BF02483639 [DOI] [Google Scholar]
- 32. Fleming EJ, Cetinić I, Chan CS, Whitney King D, Emerson D. 2014. Ecological succession among iron-oxidizing bacteria. ISME J 8:804–815. doi: 10.1038/ismej.2013.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Emerson D, Revsbech NP. 1994. Investigation of an iron-oxidizing microbial mat community located near Aarhus, Denmark: laboratory studies. Appl Environ Microbiol 60:4032–4038. doi: 10.1128/aem.60.11.4032-4038.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chan CS, McAllister SM, Leavitt AH, Glazer BT, Krepski ST, Emerson D. 2016. The architecture of iron microbial mats reflects the adaptation of chemolithotrophic iron oxidation in freshwater and marine environments. Front Microbiol 7. doi: 10.3389/fmicb.2016.00796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vesenka J, Havu J, Hruby K, Emerson D. 2018. A model for sheath formation coupled to motility in Leptothrix ochracea. Geomicrobiol J 35:366–374. doi: 10.1080/01490451.2017.1370516 [DOI] [Google Scholar]
- 36. Kim M, Oh H-S, Park S-C, Chun J. 2014. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int J Syst Evol Microbiol 64:346–351. doi: 10.1099/ijs.0.059774-0 [DOI] [PubMed] [Google Scholar]
- 37. Crump BC, Hobbie JE. 2005. Synchrony and seasonality in bacterioplankton communities of two temperate rivers. Limnol Oceanogr 50:1718–1729. doi: 10.4319/lo.2005.50.6.1718 [DOI] [Google Scholar]
- 38. Haaijer SCM, Harhangi HR, Meijerink BB, Strous M, Pol A, Smolders AJP, Verwegen K, Jetten MSM, Op den Camp HJM. 2008. Bacteria associated with iron seeps in a sulfur-rich, neutral pH, freshwater ecosystem. ISME J 2:1231–1242. doi: 10.1038/ismej.2008.75 [DOI] [PubMed] [Google Scholar]
- 39. Kato S, Kikuchi S, Kashiwabara T, Takahashi Y, Suzuki K, Itoh T, Ohkuma M, Yamagishi A. 2012. Prokaryotic abundance and community composition in a freshwater iron-rich microbial mat at circumneutral pH. Geomicrobiol J 29:896–905. doi: 10.1080/01490451.2011.635763 [DOI] [Google Scholar]
- 40. Kato S, Chan C, Itoh T, Ohkuma M. 2013. Functional gene analysis of freshwater iron-rich flocs at circumneutral pH and isolation of a stalk-forming microaerophilic iron-oxidizing bacterium. Appl Environ Microbiol 79:5283–5290. doi: 10.1128/AEM.03840-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zeitvogel F, Adaktylou I, Ingino P, Schmid G, Roehler S, Burkhardt C, Byrne JM, Halama M, Emerson D.. More than rust and mud: internal structure and metal retention of environmental Fe-rich microbial mats. Standard Nucleotide BLAST, NCBI Genbank. [Google Scholar]
- 42. Eren AM, Kiefl E, Shaiber A, Veseli I, Miller SE, Schechter MS, Fink I, Pan JN, Yousef M, Fogarty EC, et al. 2021. Community-led, integrated, reproducible multi-omics with anvi’o. Nat Microbiol 6:3–6. doi: 10.1038/s41564-020-00834-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bowers RM, Kyrpides NC, Stepanauskas R, Harmon-Smith M, Doud D, Reddy TBK, Schulz F, Jarett J, Rivers AR, Eloe-Fadrosh EA, et al. 2017. Minimum information about a single amplified genome (MISAG) and a metagenome-assembled genome (MIMAG) of bacteria and archaea. Nat Biotechnol 35:725–731. doi: 10.1038/nbt.3893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chaumeil P-A, Mussig AJ, Hugenholtz P, Parks DH. 2019. GTDB-Tk: a toolkit to classify genomes with the genome taxonomy database. Bioinformatics 36:1925–1927. doi: 10.1093/bioinformatics/btz848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Harder EC. 1919. Iron-depositing bacteria and their geologic relations. US Government Printing Office. [Google Scholar]
- 46. Liu J, Wang Z, Belchik SM, Edwards MJ, Liu C, Kennedy DW, Merkley ED, Lipton MS, Butt JN, Richardson DJ, Zachara JM, Fredrickson JK, Rosso KM, Shi L. 2012. Identification and characterization of MtoA: a decaheme c-type cytochrome of the neutrophilic Fe(II)-oxidizing bacterium Sideroxydans lithotrophicus ES-1. Front Microbio 3. doi: 10.3389/fmicb.2012.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Keffer JL, McAllister SM, Garber AI, Hallahan BJ, Sutherland MC, Rozovsky S, Chan CS. 2021. Iron oxidation by a fused cytochrome-porin common to diverse iron-oxidizing bacteria. mBio 12:e0107421. doi: 10.1128/mBio.01074-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. McAllister SM, Polson SW, Butterfield DA, Glazer BT, Sylvan JB, Chan CS. 2020. Validating the Cyc2 neutrophilic iron oxidation pathway using meta-omics of Zetaproteobacteria iron mats at marine hydrothermal vents. mSystems 5:e00553–19. doi: 10.1128/mSystems.00553-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Castelle C, Guiral M, Malarte G, Ledgham F, Leroy G, Brugna M, Giudici-Orticoni M-T. 2008. A new iron-oxidizing/O2-reducing supercomplex spanning both inner and outer membranes, isolated from the extreme acidophile Acidithiobacillus ferrooxidans. J Biol Chem 283:25803–25811. doi: 10.1074/jbc.M802496200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hoover RL, Keffer JL, Polson SW, Chan CS. 2023. Gallionellaceae pangenomic analysis reveals insight into phylogeny, metabolic flexibility, and iron oxidation mechanisms. bioRxiv:2023.01.26.525709. doi: 10.1101/2023.01.26.525709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhou N, Keffer JL, Polson SW, Chan CS. 2022. Unraveling Fe(II)-oxidizing mechanisms in a facultative Fe(II) oxidizer, Sideroxydans lithotrophicus strain ES-1, via culturing, transcriptomics, and reverse transcription-quantitative PCR. Appl Environ Microbiol 88:e0159521. doi: 10.1128/AEM.01595-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhou N, Kupper RJ, Catalano JG, Thompson A, Chan CS. 2022. Biological oxidation of Fe(II)-bearing smectite by microaerophilic iron oxidizer Sideroxydans lithotrophicus using dual Mto and Cyc2 iron oxidation pathways. Environ Sci Technol 56:17443–17453. doi: 10.1021/acs.est.2c05142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Weber T, Allard T, Tipping E, Benedetti MF. 2006. Modeling iron binding to organic matter. Environ Sci Technol 40:7488–7493. doi: 10.1021/es0607077 [DOI] [PubMed] [Google Scholar]
- 54. Tapia JM, Muñoz J, González F, Blázquez ML, Ballester A. 2013. Sorption of ferrous and ferric iron by extracellular polymeric substances (EPS) from acidophilic bacteria. Prep Biochem Biotechnol 43:815–827. doi: 10.1080/10826068.2013.805624 [DOI] [PubMed] [Google Scholar]
- 55. Johnson AH, Stokes JL. 1966. Managanese oxidation by Sphaerotilus discophorus. J Bacteriol 91:1543–1547. doi: 10.1128/jb.91.4.1543-1547.1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Balikungeri A, Robin D, Haerdi W. 1985. Manganese in natural waters II. Evidence for microbiological oxidation of Mn (II). Toxicol Environ Chem 9:309–325. doi: 10.1080/02772248509357086 [DOI] [Google Scholar]
- 57. Boogerd FC, de Vrind JP. 1987. Manganese oxidation by Leptothrix discophora. J Bacteriol 169:489–494. doi: 10.1128/jb.169.2.489-494.1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Siering PL, Ghiorse WC. 1996. Phylogeny of the Sphaerotilus-Leptothrix group inferred from morphological comparisons, genomic fingerprinting, and 16S ribosomal DNA sequence analyses. Int J Syst Bacteriol 46:173–182. doi: 10.1099/00207713-46-1-173 [DOI] [PubMed] [Google Scholar]
- 59. Huston WM, Jennings MP, McEwan AG. 2002. The multicopper oxidase of Pseudomonas aeruginosa is a ferroxidase with a central role in iron acquisition. Mol Microbiol 45:1741–1750. doi: 10.1046/j.1365-2958.2002.03132.x [DOI] [PubMed] [Google Scholar]
- 60. Zhang Z, Zhang Z, Chen H, Liu J, Liu C, Ni H, Zhao C, Ali M, Liu F, Li L. 2015. Surface Mn(II) oxidation actuated by a multicopper oxidase in a soil bacterium leads to the formation of manganese oxide minerals. Sci Rep 5:10895. doi: 10.1038/srep10895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kaur K, Sharma A, Capalash N, Sharma P. 2019. Multicopper oxidases: biocatalysts in microbial pathogenesis and stress management. Microbiol Res 222:1–13. doi: 10.1016/j.micres.2019.02.007 [DOI] [PubMed] [Google Scholar]
- 62. Gräff M, Buchholz PCF, Le Roes-Hill M, Pleiss J. 2020. Multicopper oxidases: modular structure, sequence space, and evolutionary relationships. Proteins 88:1329–1339. doi: 10.1002/prot.25952 [DOI] [PubMed] [Google Scholar]
- 63. Brouwers GJ, Vijgenboom E. 2000. Bacterial Mn2+ oxidizing systems and multicopper oxidases: an overview of mechanisms and functions. Geomicrobiol J 17:1–24. doi: 10.1080/014904500270459 [DOI] [Google Scholar]
- 64. Corstjens PL, de Vrind JP, Westbroek P, de Vrind-de Jong EW. 1992. Enzymatic iron oxidation by Leptothrix discophora: identification of an iron-oxidizing protein. Appl Environ Microbiol 58:450–454. doi: 10.1128/aem.58.2.450-454.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ducluzeau A-L, Ouchane S, Nitschke W. 2008. The cbb3 oxidases are an ancient innovation of the domain bacteria. Mol Biol Evol 25:1158–1166. doi: 10.1093/molbev/msn062 [DOI] [PubMed] [Google Scholar]
- 66. Miura H, Mogi T, Ano Y, Migita CT, Matsutani M, Yakushi T, Kita K, Matsushita K. 2013. Cyanide-insensitive quinol oxidase (CIO) from Gluconobacter oxydans is a unique terminal oxidase subfamily of cytochrome bd. J Biochem 153:535–545. doi: 10.1093/jb/mvt019 [DOI] [PubMed] [Google Scholar]
- 67. Bosma G, Braster M, Stouthamer AH, van Verseveld HW. 1987. Isolation and characterization of ubiquinol oxidase complexes from Paracoccus denitrificans cells cultured under various limiting growth conditions in the chemostat. Eur J Biochem 165:657–663. doi: 10.1111/j.1432-1033.1987.tb11491.x [DOI] [PubMed] [Google Scholar]
- 68. Gabel C, Maier RJ. 1993. Oxygen-dependent transcriptional regulation of cytochrome aa3 in Bradyrhizobium japonicum. J Bacteriol 175:128–132. doi: 10.1128/jb.175.1.128-132.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Flory JE, Donohue TJ. 1997. Transcriptional control of several aerobically induced cytochrome structural genes in Rhodobacter sphaeroides. Microbiology (Reading) 143 (Pt 10):3101–3110. doi: 10.1099/00221287-143-10-3101 [DOI] [PubMed] [Google Scholar]
- 70. McKinlay JB, Harwood CS. 2010. Carbon dioxide fixation as a central redox cofactor recycling mechanism in bacteria. Proc Natl Acad Sci U S A 107:11669–11675. doi: 10.1073/pnas.1006175107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yang C-H, Liu E-J, Chen Y-L, Ou-Yang F-Y, Li S-Y. 2016. The comprehensive profile of fermentation products during in situ CO2 recycling by Rubisco-based engineered Escherichia coli. Microb Cell Fact 15:133. doi: 10.1186/s12934-016-0530-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pang J-J, Shin J-S, Li S-Y. 2020. The catalytic role of RuBisCO for in situ CO2 recycling in Escherichia coli. Front Bioeng Biotechnol 8:543807. doi: 10.3389/fbioe.2020.543807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Seyedarabi A, To TT, Ali S, Hussain S, Fries M, Madsen R, Clausen MH, Teixteira S, Brocklehurst K, Pickersgill RW. 2010. Structural insights into substrate specificity and the anti beta-elimination mechanism of pectate lyase. Biochemistry 49:539–546. doi: 10.1021/bi901503g [DOI] [PubMed] [Google Scholar]
- 74. Kondo K, Takeda M, Ejima W, Kawasaki Y, Umezu T, Yamada M, Koizumi J, Mashima T, Katahira M. 2011. Study of a novel glycoconjugate, thiopeptidoglycan, and a novel polysaccharide lyase, thiopeptidoglycan lyase. Int J Biol Macromol 48:256–262. doi: 10.1016/j.ijbiomac.2010.11.009 [DOI] [PubMed] [Google Scholar]
- 75. Takeda M, Makita H, Ohno K, Nakahara Y, Koizumi J. 2005. Structural analysis of the sheath of a sheathed bacterium, Leptothrix cholodnii. Int J Biol Macromol 37:92–98. doi: 10.1016/j.ijbiomac.2005.09.002 [DOI] [PubMed] [Google Scholar]
- 76. Takeda M, Kondo K, Yamada M, Koizumi J-I, Mashima T, Matsugami A, Katahira M. 2010. Solubilization and structural determination of a glycoconjugate which is assembled into the sheath of Leptothrix cholodnii. Int J Biol Macromol 46:206–211. doi: 10.1016/j.ijbiomac.2009.12.006 [DOI] [PubMed] [Google Scholar]
- 77. Suzuki T, Hashimoto H, Ishihara H, Kasai T, Kunoh H, Takada J. 2011. Structural and spatial associations between Fe, O, and C in the network structure of the Leptothrix ochracea sheath surface. Appl Environ Microbiol 77:7873–7875. doi: 10.1128/AEM.06003-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kim BH, Gadd GM, Gadd GM. 2008. Bacterial physiology and metabolism. Cambridge Univ. Press, Cambridge. [Google Scholar]
- 79. Friedrich CG, Bardischewsky F, Rother D, Quentmeier A, Fischer J. 2005. Prokaryotic sulfur oxidation. Curr Opin Microbiol 8:253–259. doi: 10.1016/j.mib.2005.04.005 [DOI] [PubMed] [Google Scholar]
- 80. Kato S, Itoh T, Iino T, Ohkuma M. 2022. Sideroxyarcus emersonii gen. nov. sp. nov., a neutrophilic, microaerobic iron- and thiosulfate-oxidizing bacterium isolated from iron-rich wetland sediment. Int J Syst Evol Microbiol 72. doi: 10.1099/ijsem.0.005347 [DOI] [PubMed] [Google Scholar]
- 81. Akhtar S, Khan A, Sohaskey CD, Jagannath C, Sarkar D. 2013. Nitrite reductase NirBD is induced and plays an important role during in vitro dormancy of Mycobacterium tuberculosis. J Bacteriol 195:4592–4599. doi: 10.1128/JB.00698-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Okrend H, Dondero NC. 1964. Requirement of Sphaerotilus for cyanocobalamin. J Bacteriol 87:286–292. doi: 10.1128/jb.87.2.286-292.1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Heller K, Mann BJ, Kadner RJ. 1985. Cloning and expression of the gene for the vitamin B12 receptor protein in the outer membrane of Escherichia coli. J Bacteriol 161:896–903. doi: 10.1128/jb.161.3.896-903.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Gudmundsdottir A, Bell PE, Lundrigan MD, Bradbeer C, Kadner RJ. 1989. Point mutations in a conserved region (TonB box) of Escherichia coli outer membrane protein BtuB affect vitamin B12 transport. J Bacteriol 171:6526–6533. doi: 10.1128/jb.171.12.6526-6533.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Cadieux N, Kadner RJ. 1999. Site-directed disulfide bonding reveals an interaction site between energy-coupling protein TonB and BtuB, the outer membrane cobalamin transporter. Proc Natl Acad Sci U S A 96:10673–10678. doi: 10.1073/pnas.96.19.10673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Michaud AB, Massé RO, Emerson D. 2023. Microbial iron cycling is prevalent in water-logged Alaskan Arctic tundra habitats, but sensitive to disturbance. FEMS Microbiol Ecol 99:fiad013. doi: 10.1093/femsec/fiad013 [DOI] [PubMed] [Google Scholar]
- 87. Berens MJ, Michaud AB, VanderJeugdt E, Miah I, Sutor FW, Emerson D, Bowden WB, Kinsman-Costello L, Weintraub MN, Herndon EM. 2024. Phosphorus interactions with microbial Fe mats at terrestrial-aquatic interfaces in Arctic tundra ecosystems. Environ Sci Technol 58:11400–11410. doi: 10.1021/acs.est.3c09072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Stookey LL. 1970. Ferrozine---a new spectrophotometric reagent for iron. Anal. Chem 42:779–781. doi: 10.1021/ac60289a016 [DOI] [Google Scholar]
- 89. MacDonald LH, Paull JS, Jaffé PR. 2013. Enhanced semipermanent dialysis samplers for long-term environmental monitoring in saturated sediments. Environ Monit Assess 185:3613–3624. doi: 10.1007/s10661-012-2813-8 [DOI] [PubMed] [Google Scholar]
- 90. Kaplan DI, Buettner SW, Li D, Huang S, Koster van Groos PG, Jaffé PR, Seaman JC. 2017. In situ porewater uranium concentrations in a contaminated wetland: effect of seasons and sediment depth. Appl Geochem 85:128–136. doi: 10.1016/j.apgeochem.2016.11.017 [DOI] [Google Scholar]
- 91. Scott JJ, Breier JA, Luther GW, Emerson D. 2015. Microbial iron mats at the Mid-Atlantic Ridge and evidence that Zetaproteobacteria may be restricted to iron-oxidizing marine systems. PLoS One 10:e0119284. doi: 10.1371/journal.pone.0119284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lai J, Lesnik J, Ruppert N.. 2017. Effective miniaturization of Illumina Nextera XT library prep for multiplexed whole genome sequencing and microbiome applications. Beckman Coulter Life Sciences. [Google Scholar]
- 93. Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB, Parks DH, Robinson CJ, Sahl JW, Stres B, Thallinger GG, Van Horn DJ, Weber CF. 2009. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol 75:7537–7541. doi: 10.1128/AEM.01541-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kozich JJ, Westcott SL, Baxter NT, Highlander SK, Schloss PD. 2013. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl Environ Microbiol 79:5112–5120. doi: 10.1128/AEM.01043-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J, Glöckner FO. 2007. SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 35:7188–7196. doi: 10.1093/nar/gkm864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glöckner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41:D590–6. doi: 10.1093/nar/gks1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Yilmaz P, Parfrey LW, Yarza P, Gerken J, Pruesse E, Quast C, Schweer T, Peplies J, Ludwig W, Glöckner FO. 2014. The SILVA and “all-species living tree project (LTP)” taxonomic frameworks. Nucleic Acids Res 42:D643–D648. doi: 10.1093/nar/gkt1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Glöckner FO, Yilmaz P, Quast C, Gerken J, Beccati A, Ciuprina A, Bruns G, Yarza P, Peplies J, Westram R, Ludwig W. 2017. 25 years of serving the community with ribosomal RNA gene reference databases and tools. J Biotechnol 261:169–176. doi: 10.1016/j.jbiotec.2017.06.1198 [DOI] [PubMed] [Google Scholar]
- 99. Bruinsma S, Burgess J, Schlingman D, Czyz A, Morrell N, Ballenger C, Meinholz H, Brady L, Khanna A, Freeberg L, Jackson RG, Mathonet P, Verity SC, Slatter AF, Golshani R, Grunenwald H, Schroth GP, Gormley NA. 2018. Bead-linked transposomes enable a normalization-free workflow for NGS library preparation. BMC Genomics 19:722. doi: 10.1186/s12864-018-5096-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet j 17:10. doi: 10.14806/ej.17.1.200 [DOI] [Google Scholar]
- 101. Andrews S. 2010. FastQC: a quality control tool for high throughput sequence data.
- 102. Magoč T, Salzberg SL. 2011. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963. doi: 10.1093/bioinformatics/btr507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Prjibelski A, Antipov D, Meleshko D, Lapidus A, Korobeynikov A. 2020. Using SPAdes de novo assembler. CP Bioinf 70. doi: 10.1002/cpbi.102 [DOI] [PubMed] [Google Scholar]
- 104. Wu Y-W, Simmons BA, Singer SW. 2016. MaxBin 2.0: an automated binning algorithm to recover genomes from multiple metagenomic datasets. Bioinformatics 32:605–607. doi: 10.1093/bioinformatics/btv638 [DOI] [PubMed] [Google Scholar]
- 105. Alneberg J, Bjarnason BS, de Bruijn I, Schirmer M, Quick J, Ijaz UZ, Lahti L, Loman NJ, Andersson AF, Quince C. 2014. Binning metagenomic contigs by coverage and composition. Nat Methods 11:1144–1146. doi: 10.1038/nmeth.3103 [DOI] [PubMed] [Google Scholar]
- 106. Kang DD, Froula J, Egan R, Wang Z. 2015. MetaBAT, an efficient tool for accurately reconstructing single genomes from complex microbial communities. PeerJ 3:e1165. doi: 10.7717/peerj.1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Sieber CMK, Probst AJ, Sharrar A, Thomas BC, Hess M, Tringe SG, Banfield JF. 2018. Recovery of genomes from metagenomes via a dereplication, aggregation and scoring strategy. Nat Microbiol 3:836–843. doi: 10.1038/s41564-018-0171-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. 2015. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 25:1043–1055. doi: 10.1101/gr.186072.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Parks DH, Chuvochina M, Waite DW, Rinke C, Skarshewski A, Chaumeil P-A, Hugenholtz P. 2018. A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol 36:996–1004. doi: 10.1038/nbt.4229 [DOI] [PubMed] [Google Scholar]
- 110. Parks DH, Chuvochina M, Chaumeil P-A, Rinke C, Mussig AJ, Hugenholtz P. 2020. A complete domain-to-species taxonomy for Bacteria and Archaea. Nat Biotechnol 38:1079–1086. doi: 10.1038/s41587-020-0501-8 [DOI] [PubMed] [Google Scholar]
- 111. Olm MR, Crits-Christoph A, Diamond S, Lavy A, Matheus Carnevali PB, Banfield JF. 2020. Consistent metagenome-derived metrics verify and delineate bacterial species boundaries. mSystems 5:e00731-19. doi: 10.1128/mSystems.00731-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313. doi: 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Kozlov AM, Darriba D, Flouri T, Morel B, Stamatakis A. 2019. RAxML-NG: a fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics 35:4453–4455. doi: 10.1093/bioinformatics/btz305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Letunic I, Bork P. 2021. Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49:W293–W296. doi: 10.1093/nar/gkab301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Aroney STN, Newell RJP, Nissen J, Camargo AP, Tyson GW, Woodcroft BJ. 2024. CoverM: read coverage calculator for metagenomics (v0.7.0)
- 116. Shaffer M, Borton MA, McGivern BB, Zayed AA, LaRosa SL, Solden LM, Liu P, Narrowe AB, Rodríguez-Ramos J, Bolduc B, Gazitúa MC, Daly RA, Smith GJ, Vik DR, Pope PB, Sullivan MB, Roux S, Wrighton KC. 2020. DRAM for distilling microbial metabolism to automate the curation of microbiome function. Nucleic Acids Res. 48:8883–8900. doi: 10.1093/nar/gkaa621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Garber AI, Nealson KH, Okamoto A, McAllister SM, Chan CS, Barco RA, Merino N. 2020. FeGenie: a comprehensive tool for the identification of iron genes and iron gene neighborhoods in genome and metagenome assemblies. Front Microbiol 11:37. doi: 10.3389/fmicb.2020.00037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, et al. 2008. The RAST server: rapid annotations using subsystems technology. BMC Genomics 9:75. doi: 10.1186/1471-2164-9-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Overbeek R, Olson R, Pusch GD, Olsen GJ, Davis JJ, Disz T, Edwards RA, Gerdes S, Parrello B, Shukla M, Vonstein V, Wattam AR, Xia F, Stevens R. 2014. The SEED and the rapid annotation of microbial genomes using subsystems technology (RAST). Nucleic Acids Res 42:D206–14. doi: 10.1093/nar/gkt1226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Brettin T, Davis JJ, Disz T, Edwards RA, Gerdes S, Olsen GJ, Olson R, Overbeek R, Parrello B, Pusch GD, Shukla M, Thomason JA, Stevens R, Vonstein V, Wattam AR, Xia F. 2015. RASTtk: a modular and extensible implementation of the RAST algorithm for building custom annotation pipelines and annotating batches of genomes. Sci Rep 5:8365. doi: 10.1038/srep08365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Zhou Z, Tran PQ, Breister AM, Liu Y, Kieft K, Cowley ES, Karaoz U, Anantharaman K. 2022. METABOLIC: high-throughput profiling of microbial genomes for functional traits, metabolism, biogeochemistry, and community-scale functional networks. Microbiome 10:33. doi: 10.1186/s40168-021-01213-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. 1990. Basic local alignment search tool. J Mol Biol 215:403–410. doi: 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- 123. Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. doi: 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- 124. Kim D, Park S, Chun J. 2021. Introducing EzAAI: a pipeline for high throughput calculations of prokaryotic average amino acid identity. J Microbiol 59:476–480. doi: 10.1007/s12275-021-1154-0 [DOI] [PubMed] [Google Scholar]
- 125. Rodriguez-R LM, Konstantinidis KT. 2014. Bypassing cultivation to identify bacterial species: culture-independent genomic approaches identify credibly distinct clusters, avoid cultivation bias, and provide true insights into microbial species. Microbe Mag 9:111–118. doi: 10.1128/microbe.9.111.1 [DOI] [Google Scholar]
- 126. Bushnell B, Rood J, Singer E. 2017. BBMerge – accurate paired shotgun read merging via overlap. PLoS One 12:e0185056. doi: 10.1371/journal.pone.0185056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Langmead B, Salzberg SL. 2012. Fast gapped-read alignment with Bowtie 2. Nat Methods 9:357–359. doi: 10.1038/nmeth.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. R Core Team . 2023. R: a language and environment for statistical computing. Vienna, Austria [Google Scholar]
- 129. Wickham H. 2016. ggplot2: elegant graphics for data analysis. Springer-Verlag, Cham. [Google Scholar]
- 130. Sievert C. 2020. Interactive web-based data visualization with R, plotly, and shiny. Chapman and Hall/CRC, Florida. [Google Scholar]
- 131. Love MI, Huber W, Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Wickham H, Hester J, Bryan J. 2023. readr: read rectangular text data (2.1.4).
- 133. Robinson MD, McCarthy DJ, Smyth GK. 2010. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26:139–140. doi: 10.1093/bioinformatics/btp616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Thompson J, Brett C, Neuhaus I, Thompson R. 2022. DGEobj.utils: differential gene expression (DGE) analysis utility toolkit (1.0.6).
- 135. Wickham H, Francois R, Henry L, Muller K, Vaughan D. 2023. Dplyr: a grammar of data manipulation (1.1.4).
- 136. Wickham H. 2007. Reshaping data with the reshape package. J Stat Soft 21. doi: 10.18637/jss.v021.i12 [DOI] [Google Scholar]
- 137. Henry CS, DeJongh M, Best AA, Frybarger PM, Linsay B, Stevens RL. 2010. High-throughput generation, optimization and analysis of genome-scale metabolic models. Nat Biotechnol 28:977–982. doi: 10.1038/nbt.1672 [DOI] [PubMed] [Google Scholar]
- 138. Allen BH, Gupta N, Edirisinghe JN, Faria JP, Henry CS. 2022. Application of the metabolic modeling pipeline in KBase to categorize reactions, predict essential genes, and predict pathways in an isolate genome, p 291–320. In Navid A (ed), Microbial systems biology. Springer US, New York, NY. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Confocal images from Spruce Point mat showing sheaths and sheath-forming cells.
Tables S1 to S9.
Data Availability Statement
The data that support the findings of this study are publicly available in the NCBI and IMG. Metagenome reads, metagenome-assembled genomes, 16S rRNA amplicon sequences, and metatranscriptome reads are available under NCBI BioProject PRJNA1117470. Metatranscriptome reads are also available in IMG (IMG Genome IDs: ISCOA1K: 3300063950; MN3: 3300063952; DE1: 3300067170).