Summary
Warm-blooded animals such as birds and mammals are able to protect stable body temperature due to various thermogenic mechanisms. These processes can be facultative (occurring only under specific conditions, such as acute cold) and adaptive (adjusting their capacity according to long-term needs). They can represent a substantial part of overall energy expenditure and, therefore, affect energy balance. Classical mechanisms of facultative thermogenesis include shivering of skeletal muscles and (in mammals) non-shivering thermogenesis (NST) in brown adipose tissue (BAT), which depends on uncoupling protein 1 (UCP1). Existence of several alternative thermogenic mechanisms has been suggested. However, their relative contribution to overall heat production and the extent to which they are adaptive and facultative still needs to be better defined. Here we focus on comparison of NST in BAT with thermogenesis in skeletal muscles, including shivering and NST. We present indications that muscle NST may be adaptive but not facultative, unlike UCP1-dependent NST. Due to its slow regulation and low energy efficiency, reflecting in part the anatomical location, induction of muscle NST may counteract development of obesity more effectively than UCP1-dependent thermogenesis in BAT.
Keywords: Regulation of thermogenesis, Warm-blooded animals, Body temperature, UCP1
Introduction
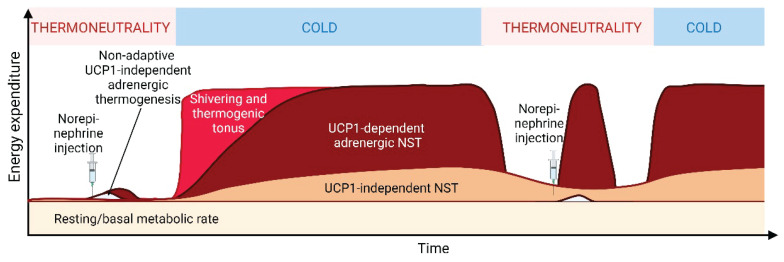
Life is sustained by a constant supply of energy that propels cellular processes. However, no biochemical reaction is 100 % efficient, and portion of energy is inevitably “lost” as a heat [1]. Birds and mammals evolved an ability to utilize this metabolic inefficiency to keep stable body temperature. They are, therefore, endothermic, i.e. able to warm themselves using internal heat sources, and homeothermic, i.e. with stable body temperature. These enabled them to colonize environments with cold climates. Their basal metabolic rate is 5–10 times higher in comparison to other vertebrates with similar body weight but variable body temperature (poikilotherms), which depend mainly on ambient temperature (ectotherms) [2]. In the homeotherms, the heat dissipated by basal metabolism fully covers the cost of maintaining a stable body temperature under conditions of so called thermoneutral zone (i.e. ambient temperature typically in a range 20–25 °C for lightly dressed human, and 29–33 °C for standard laboratory mouse during day and night [3]). At temperatures below the thermoneutral zone, homeotherms turn on several heat-saving and heat-generating processes [4–7]. As ambient temperature can change rapidly, it is essential to control these thermoregulatory mechanisms tightly, switching them on and off as needed to prevent hypothermia or overheating. Thus, the thermogenesis is acutely adjustable, i.e. facultative. It includes shivering and non-shivering thermogenesis (NST; Fig. 1). Shivering is defined as the involuntary contractile activity of skeletal muscles triggered by motor neurons, producing heat but no external work. It is activated especially in response to acute cold stimuli. NST includes all potential facultative heat-producing mechanisms independent on muscle contractile activity. NST is also adaptive: If a cold stimulus persists, the capacity for NST gradually increases, which allows cessation of shivering [7].
Fig. 1.
Two-level regulation of thermogenesis in brown adipose tissue (BAT) and skeletal muscle. UCP1-dependent thermogenesis in BAT is activated by fatty acids released by lipolysis of intracellular triacylglycerols (TAG) in response to norepinephrine from sympathetic nerve endings. The same mechanism causes adaptive rise in Ucp1 expression and UCP1 content. Shivering is acutely stimulated by acetylcholine from motor nerve endings, activation of acetylcholine receptors and consecutive calcium release from sarcoplasmic reticulum through ryanodine receptors (RyR). Efficiency of calcium recycling is affected by interaction between sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) and sarcolipin (SLN). The activity of SLN is probably regulated mostly at the level of gene expression. SLN may represent a mechanism of adaptive, long-term tuning of regulatory pathway of shivering and/or shivering-independent mechanism of NST. Created with Biorender.com.
Thermoregulatory rather than diet-induced thermogenesis in brown fat
Placental mammals developed a unique and particularly efficient mechanism of NST, allowing them to use the maximum amount of dissipated energy for thermoregulation. This NST is mediated by mitochondrial uncoupling protein 1 (UCP1) in brown adipose tissue (BAT; Fig. 1) [7]. UCP1 is activated in the cold, by the sympathetic nervous system and adrenergic receptor signaling via cAMP-dependent lipolysis (Fig. 1). Liberated free fatty acids directly activate the protonophoric activity of UCP1 while overriding the opposite effect of intracellular purine nucleotides (reviewed e.g. in [7]). This results in the uncoupling of respiratory chain activity from ATP synthesis, maximizing respiratory rate and dissipating the energy of the transmembrane electrochemical gradient of protons as heat. The increase in energy expenditure follows the stimulus within dozens of minutes at the latest, depending also on the increased blood flow through BAT, which supplies oxygen and nutrients [7]. Long-term cold exposure also regulates UCP1 at the level of mRNA and protein content, resulting in increased thermogenic capacity of BAT, which also reflects BAT growth and oxidative capacity. Thus, UCP1-mediated NST is both facultative and adaptive (Fig. 1). Adaptation to cold is accompanied by increased lipogenesis [8], possibly in order to replenish lipid stores in BAT.
UCP1 is also present in inducible adipocytes interspersed in white adipose tissue (WAT) depots. These cells, later called brite or beige adipocytes [9,10], were described first by Leslie Paul Kozak, one of the most original and renowned scientists in the field of obesity and energy metabolism (see Table 1) [11,12]. L. P. Kozak has also characterized the mechanism of brown (brite/beige)-adipocyte-specific expression of Ucp1 by discovering the unique enhancer in the 5′-flanking region of Ucp1 [13,14], see also [15]. However, the capacity of these brite/beige cells for UCP1-dependent thermogenesis is somewhat limited compared to classical BAT [16,17].
Table 1.
Studies of Leslie Paul Kozak, PhD (1940 – 2023) focused on mechanism and role of NST.
| 1991 – 1995, early studies focused on UCP1-mediated NST: |
| 1997 – 2006, development of mice with germline inactivation of Ucp1 in adipocytes; results suggested that UCP1 was required for thermoregulatory thermogenesis but not for adaptive induction of NST by cold, resulting in obesity-resistance. UCP1-independent NST involved metabolism in white fat and possibly also other tissues: |
| 1998 – 2011, brown fat cells could be induced in specific depots of white fat by cold: |
2010, physiological role of brown fat is protection against cold but not against obesity:
|
| 2015 – 2016, UCP1 and sarcolipin have complementary but distinct roles in NST: |
It has been repeatedly and wishfully suggested that UCP1 plays a physiological role not only in protection against cold, but also against obesity [18–20]. This hypothesis is based on a concept of “diet-induced thermogenesis”, which supposes that BAT burns excess calories in states of positive energy balance to maintain stable body weight. The existence of such a physiological mechanism was challenged by L. P. Kozak in 2010, in his important but long overlooked article entitled “Brown Fat and the Myth of Diet-Induced Thermogenesis” [21]. There, he brought several arguments to support this skepticism, based mainly on experiments in mice with germline inactivation of Ucp1 in adipocytes (UCP1-KO mice) [22] or ectopic expression of Ucp1 in WAT, which models were developed in his laboratory [23]. Notably, mice with partially ablated BAT develop obesity [24], but this may only be a consequence of hyperphagia. Both hyperphagia and the development of obesity are absent when these mice are reared at thermoneutrality [25]. Similarly, the propensity to obesity of UCP1-KO mice depends on ambient temperature. At laboratory temperature, UCP1-KO mice do not develop obesity [22] or they even show paradoxical obesity resistance [26], while some authors report that these mice develop obesity at thermoneutral temperature [27,28]. An increased level of UCP1, which indicates a high capacity for NST, is not directly associated with obesity resistance unless the biochemical (i.e. protonophoric) activity of the existing UCP1 is stimulated at the same time [29–31]. Thus, while diet-induced thermogenesis may be involved in acute control of food intake [32], UCP1 does not seem to play a physiological role in the prevention of body gain. Generally, it is not even clear to what extent body weight is under homeostatic control [33].
However, as already recognized by L. P. Kozak [21], artificial stimulation of both UCP1-mediated and UCP1-independent NST could be used clinically in body weight management, irrespective of the validity of the traditional concept of diet-induced thermogenesis. Induction of UCP1-mediated thermogenesis using β-adrenergic agonist can reduce obesity in mice [34] and even in humans [35], but cardiovascular side-effects prevented its clinical use [35]. Similarly, obesity development was counteracted by ectopic expression of Ucp1 gene in WAT of mice and pigs [23,36].
In addition to the UCP1-dependent thermogenic mechanism bypassing ATP synthase, also other biochemical pathways could mediate NST in BAT by dissipating the metabolic energy of ATP [37]. Mechanisms such as creatine cycling controlled by creatine kinase b (CKB) [38,39], esterification/lipolysis of triacylglycerols [40] and some others [41,42] may be involved and need to be better characterized. Namely the CKB-dependent pathway was recently suggested to represent a complementary mechanism to that mediated by UCP1 in adipocytes, with functional redundancy providing a robust mechanism for heat production (reviewed in [39]). Similarly to UCP1, these mechanisms are usually believed to be under adrenergic control (e.g. [43]), which challenges the view that only UCP1 could mediate adrenergically regulated facultative and adaptive NST [44].
Mechanisms of thermoregulatory NST in other organs and tissues besides BAT and their control are relatively poorly characterized. Increased energy expenditure in response to adrenergic stimulation [45] or cold [46] has been reported in perigonadal WAT. Moreover, NST could also take place in subcutaneous WAT [10,42,47], or liver [48,49]. It could also result from inter-organ futile metabolic cycling [50]. Although all of these mechanisms need to be further characterized, this short review will focus specifically on the thermogenic mechanisms in skeletal muscle, their contribution to the maintenance of a stable body temperature, and their impact on the propensity to obesity.
Shivering and cold-induced changes in muscle
Skeletal muscles evolved primarily to enable locomotion. However, with the advent of endothermy in birds and mammals, skeletal muscle also became engaged in thermogenesis comprising both shivering and putative NST. Muscle function is based on sliding actin and myosin filaments organized in sarcomeres, which consume ATP and generate force and potentially subsequent changes in muscle length. However, typical muscle is able to convert only 20–30 % of ATP to work, while the most of the remaining part is dissipated as heat (reviewed in [51]), which may contribute to thermos-regulation. A major contribution of muscles to overall thermogenesis could be inferred from their high oxidative capacity. Thus, in adult humans, the capacity of skeletal muscles to burn fat energy stores is several-fold greater than that of BAT [52]. Blood flow through skeletal muscles can substantially increase during the transition from basal state to state of maximal physical activity. Even in the resting state, muscles can account for 20–30 % of the total oxygen consumption [5,7]. Muscle metabolism is one of the major contributors to resting energy expenditure with a clear potential to affect the pathogenesis of obesity [53]. Shivering can increase the whole-body energy expenditure up to five times its basal values, corresponding to approximately 40 % of maximal oxygen consumption (VO2max) during exercise [54]. The putative contribution of muscle NST has not been reliably quantified. However, it is to be inferred that even a slight relative increase in muscle energy expenditure, independent of physical activity or shivering, would have a profound effect on whole body energy balance, potentially affecting fat deposition.
Skeletal muscles are composed of long multinucleated cells (muscle fibers), which differ in their biochemical and functional properties: i) slow-twitch type I fibers have high content of mitochondria (causing dark red color), high oxidative capacity, and high fatigue resistance; ii) fast-twitch type IIa fibers have high content of mitochondria and rely both on glycolysis and oxidative metabolism; iii) fast-twitch type IIb fibers with low mitochondrial content (causing pale color) and high glycolytic activity, which are prone to rapid fatigue; and additionally iv) type IIx muscle fibers [55]. Fiber type composition of individual muscles is optimized for the typical work pattern of the respective muscle groups. It can be partially affected by muscle endurance training, which leads to increased mitochondrial biogenesis, as well as adaptation of mitochondrial network, contractile apparatus, and vasculature [56]. Some of these changes towards increased oxidative capacity have also been seen in animal models during prolonged cold exposure, suggesting a similar effect on muscle metabolism as endurance training and demonstrating a partially adaptive nature of shivering [57].
Skeletal muscles are relatively flexible in the utilization of various fuel mixtures under acute cold exposure [58], while chronic cold exposure leads to a proportional increase in the oxidation of lipids by the muscle [59]. The fiber composition is also a strong predictor of the shivering pattern evoked by acute cold [60]. While short-term activity can rely on glycolytic fast-twitch fibers, prolonged endurance activity requires a higher proportion of oxidative slow-twitch fibers. The changes in muscle metabolism and fiber composition in response to cold exposure may reflect the activation of both shivering and NST. Rapid changes in muscle metabolism are likely associated with shivering, which is activated at the beginning of cold exposure [61]. These changes may vary between individuals and depend on severity of cold exposure (see below). Sustained low-intensity shivering in humans largely depends on lipid oxidation (50 %), while muscle glycogen and plasma glucose are used to a lesser extent (30 % and 10 %, respectively) [59]. Increased energy requirements during the transition from low to moderate shivering intensity are primarily met by increased glycogen consumption in humans [62]. Besides these changes in the biochemistry of muscle shivering, long-term exposure (i.e. acclimation) to cold environment results in an adaptive increase in the capacity for NST in BAT and possibly elsewhere, leading to the cessation of shivering [5–7,16,60,63].
Distinguishing between shivering and putative muscle NST is not straightforward, as the definition of shivering itself is ambiguous. There are at least two different types of muscle contractile thermogenic activities. Burst shivering (“classical shivering”) is associated with fatigued type II muscle fibers and, therefore, cannot be sustained for a prolonged time [63]. On the other hand, muscle tone, also reported as resting muscle mechanical activity [64], shivering microvibrations, minor tremor, or thermoregulatory tonus [65], can be continuous and therefore associated with fatigue resistant, oxidative type I muscle fibers [63]. When assessed by electromyography, burst and continuous shivering in humans typically show frequencies of 0.1–0.2 Hz and 4–8 Hz, respectively ([63]; in general, the smaller the animal, the higher the frequency [64]). Burst shivering can be perceived visually, while continuous shivering may not be visually manifested.
The relative contribution of each of these types of contractile activity to overall shivering thermogenesis is debated. It also exhibits high inter-individual variability, perhaps corresponding to variability in muscle fiber composition [58]. The mutation causing a shift towards higher proportion of slow-twitch muscles results in increased muscle tone and higher resistance to cold [66]. It has been observed that the intensity of continuous shivering increases linearly with decreasing ambient temperature below thermoneutral zone [57,64,65], whereas burst shivering is only triggered at very low temperatures (below 7 °C in rats [57]), when thermogenic capacity of continuous shivering is presumably insufficient. On the other hand, some authors do not report any continuous shivering preceding shivering bursts [63]. Interestingly, contractile activity similar to continuous shivering is also detected after vigorous exercise [65], suggesting a common mechanism linking it to skeletal muscle training.
Assessment of shivering activity is further complicated by a broad range of muscle groups that can be involved in thermogenesis. Extensive continuous shivering was recorded e.g. in m. extensor digitorum longus, deep parts of m. tibialis, deep neck muscles and m. soleus (but not in the superficial part of m. tibialis and m. trapezius) [57]. The involvement of particular muscle groups can also considerably vary among individuals: While some humans rely on shivering in upper body muscles, others use upper leg muscles [54]. Hence, evaluating shivering activity in a specific muscle does not necessarily indicate the overall shivering activity. This complicates the quantification of the contribution of this mechanism to the total body heat production.
As reviewed by Blondin and Haman [63], muscle metabolic activity and the relative importance of individual muscles can be indirectly visualized using positron emission tomography (PET) and the glucose analogue [18F]FDG. Shivering pattern of individual muscles can be characterized using electromyography (assessing regulatory muscle electric activity). While surface electromyography is widely used in humans, it is rarely applied to rodents (e.g. in [67]) because of technical constraints. Placing an electrode directly onto the muscle is more common in rodents [68–70]. However, it can produce misleading results due to the open surgery and prevailing effects of anaesthesia. Another way to collect data on whole-body shivering patterns is through mechanical methods, most commonly mechanomyography [16,71]. This technique is getting increasingly sensitive and provides output corresponding to electromyography [16]. However, a comprehensive comparison of the two techniques is missing. The least reliable and most subjective approach to assessing shivering is visual observation, which may fail to detect less striking forms of contractile activity.
Shivering is thus a phenomenon of under-estimated complexity. Proper quantification of its contribution to thermogenesis and overall energy expenditure is a technically challenging task, which is rarely sufficiently addressed in studies attempting to uncover potential alternative mechanisms of NST. However, without evaluation of at least presence of shivering, results of the whole-body studies focused on NST, conducted at ambient temperatures below thermoneutrality, are difficult to interpret (see below).
Muscle NST: Major role of slippage of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase?
Prior to the identification of BAT as a major site of adrenergic thermogenesis, several investigators, including L. Jansky and his colleagues at the Charles University in Prague, suggested that adaptive NST occurs primarily in skeletal muscle [72–75]. Although the research focus later shifted predominantly towards BAT, several alternative mechanisms were proposed to play a role in NST in skeletal muscle, including i) UCP1-independent mitochondrial proton leak [60,63]; ii) impaired thermodynamic efficiency of Na+/K+-ATPase in the plasma membrane [76,77]; and iii) futile substrate cycling between de novo lipogenesis (DNL) and fatty acid (FA) oxidation (DNA/FAox cycle) controlled by leptin–AMP-activated protein kinase (AMPK) axis [78–81]. Also thermoregulatory tonus (continuous “shivering”) should be considered here (see above). However, major attention has been paid recently to NST resulting from slippage of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) [42,63,82–84].
As stated above, only a relatively small proportion of ATP consumed in muscle is used directly to generate contractile force. Various muscles and organisms differ in contractile efficiency, which is the ratio of ATP consumed for contraction and for regulatory processes, particularly calcium cycling which requires SERCA. The regulatory processes can consume up to 45 % of ATP [51]. Muscle contraction is initiated by a well-established signaling cascade that involves acetylcholine release from motor nerve endings at the neuromuscular junction and the stimulation of nicotinic acetylcholine receptors on the muscle membrane (Fig. 1). Local depolarization triggers action potential, which is further propagated across the plasma membrane and its invaginations (T-tubules) to the vicinity of sarcoplasmic reticulum (SER). Activated voltage-gated dihydropyridine receptors in T-tubules open adjacent ryanodine receptors (RyR) in the membrane of SER, enabling the massive release of calcium from this reservoir to the cytoplasm. Elevated cytoplasmic calcium concentration is the critical signal to initiate the muscle contraction. In particular, calcium binding to regulatory protein troponin causes exposition of binding sites for myosin heads on actin filament and allows cross-bridge cycling, which hydrolyses ATP and generates the contraction force (see e.g. [85] for more detail). The single neural stimulus causes only short twitch contraction because calcium is rapidly removed from the cytoplasm by the activity of SERCA. If the second stimulus follows the first one before SERCA manages to remove calcium from the cytoplasm and reach resting calcium level, the twitches are partially summated, i.e. the cytoplasmic calcium concentration after the second twitch is higher and the generated tension is greater than those after a single twitch. A sequence of stimuli of sufficient frequency leads to complete summation of individual twitches into stronger and longer lasting tetanic contraction. Activity of SERCA is thus critical for regulation of strength of muscle contraction.
Two SERCA genes (Atp2a1 for SERCA1 and Atp2a2 for SERCA2) are expressed in mammalian skeletal muscle, producing several splice variants. Fast kinetic SERCA1a and 1b are found in adult fast-twitch muscles, and SERCA1b also in neonates [86,87]. SERCA2a with slow kinetics is found in slow-twitch fibres [88] and its expression is induced by cold [16,89]. During neonatal development, gradual changes from SERCA2a and SERCA1b to SERCA1a were reported [88,89].
The activity of SERCA is affected by interaction with micropeptides phospholamban (particularly in heart ventricles) and sarcolipin (SLN; both in heart and skeletal muscle). The normal working cycle of SERCA comprises the binding of 2 calcium ions and a molecule of ATP to the cytosolic side of the pump, hydrolysis of ATP, and subsequent conformational change causing the release of both calcium ions to the sarcoplasmic reticulum [90,91]. Phospholamban interaction with SERCA (preferentially SERCA2a [92]) depends on phospholamban phosphorylation [93] and only happens if the cytoplasmic calcium level is low [91,94]. In heart ventricles, adrenergic stimulation leads to phosphor-lamban phosphorylation and subsequent disinhibition of SERCA resulting in prolonged periods of high calcium concentration and therefore in increased cardiac contractility [95]. A similar function was suggested for SLN in heart atria [96] but attention was later shifted to activity of SLN in skeletal muscle. The kinetics of SLN is different from that of phospholamban: SLN binds to SERCA (preferentially SERCA1a [92]) regardless of calcium concentration [94]. The binding of SLN reduces maximal rate of SERCA calcium pumping without affecting SERCA affinity for calcium, resulting in calcium ions slipping back to the cytoplasm and in uncoupling of ATP hydrolysis from calcium pumping; i.e. heat production [91,97]. As an alternative to the effect of SLN on SERCA calcium pumping, calcium leak through RyR has also been suggested to regulate thermogenesis in murine resting skeletal muscle [98].
Mice lacking either UCP1 or SLN can survive in the cold probably due to compensatory upregulation of the remaining thermogenic mechanisms, while UCP1/SLN double knockout mice cannot sustain long-term cold exposure [99]. Thus, both SLN and UCP1 may be involved in thermogenesis in cold [99]. Muscle SLN level is increased in mice with ablation of either interscapular BAT or UCP1 [99–101]. Thus, BAT and muscle may represent synergistic and potentially even partially redundant components of thermoregulatory NST [60,82]. Importantly in this respect, our own data suggest that the relative contribution of the discussed thermogenic mechanisms varies among different murine strains. C57BL/6 mice, which are typically used in the majority of metabolic studies, rely mainly on BAT, while A/J mice, known for their resistance to obesity, use muscle thermogenesis to a greater extent [16]. While the burst shivering rate does not differ between C57BL/6 and A/J mice at cold, A/J mice recruit larger amount of SLN in their muscles [16].
Calcium cycling (involving RyR and SERCA) was shown to be the primary thermogenic mechanism in specialized heater organs of some species of marine fish (e.g. [102]). Although these organs were derived from extraocular muscles, they lost their contractile apparatus. In contrast, in mammals, SLN- and RyR-dependent mechanisms operate in fully functional muscles where the primary role of calcium is to regulate sarcomere contraction. In such a system, proof of functional independence of shivering and calcium cycling is quite methodologically challenging. Similarly to continuous shivering, also SLN-dependent thermogenesis is thought to be localized in slow-twitch oxidative fibers relying primarily on lipid oxidation [89].
Although attempts were made to demonstrate that stimulation of SLN can occur in the absence of corresponding changes in shivering (e.g. [94]), these reports need to be taken with caution considering the above mentioned complexity of shivering (not only burst shivering but also the thermoregulatory tonus) and pitfalls of its assessment. Without any doubt, SLN-induced calcium slippage in SERCA may generate heat, but it is unclear whether this effect is potent enough and can be sufficiently quickly adjusted to play a role in facultative thermogenesis [83]. As regulation of SERCA activity is a common mechanism how to adjust heart contractility (see above), it is possible that the primary function of SERCA regulation in skeletal muscle is also to adjust the calcium level in the long term, which determines the thresholds for muscle contraction. Therefore, SLN may play a role in both the adaptive tuning of shivering activity and muscle NST.
Regulation of the activity of SLN (and other putative muscle NST mechanisms) needs to be better understood (Fig. 2). Adrenergic signaling is for decades accepted as the mean of regulation of facultative NST [5].
Fig. 2.
Metabolic responses to acute and chronic cold exposure in mice (adapted from [7]). General increase of energy expenditure upon cold exposure reflects several thermogenic mechanisms. Muscle shivering and thermoregulatory tonus cover majority of heat production in response to acute cold stimulus. Continuing shivering leads to adaptation resembling exercise training, but shivering is gradually replaced by NST. NST mediated by UCP1 has a low capacity at thermoneutrality. However, if cold persist, capacity of UCP1-dependent NST adaptively rises and it gradually replaces shivering. It is facultative, i.e. it can be rapidly switched on and off due to its control by adrenergic nervous system (adrenergic thermogenesis). NST alternative to UCP1-mediated heat production, reflects several mechanisms, including NST in skeletal muscle. Preferential activation and adaptive increase of NST capacity in BAT or skeletal muscle depends on the genetic background of the mice. Control of muscle NST is probably relatively slow – it may not be facultative. Thus, when the contribution of muscle NST to total energy expenditure is relatively high compared to BAT (such as in A/J mice, see the main text), a substantial amount of energy is lost during the stay of cold-adapted mice in thermoneutral conditions. This phenomenon may contribute to resistance to obesity. Prolonged maintenance of cold-adapted animals at thermoneutral conditions results in reduction of the capacity of NST (not shown). Created with Biorender.com.
It was assumed that the adrenergic system also regulates SLN activity in skeletal muscles [103,104], similar to how phospholamban activity is regulated in the heart (see above). However, to our knowledge, this assumption has never been unambiguously proven. While adrenergic stimulation results also in some UCP1-independent rise in energy expenditure, this induction is not potentiated by cold adaptation in UCP1-KO animals [68]. Interestingly, while cold adaptation recruits adrenergic thermogenesis in C57BL/6 mice, A/J mice adapt to cold without recruiting capacity for adrenergic thermogenesis, presumably relying on muscle mechanisms that are regulated by different means [16]. Cold tolerance requires intact signaling including several hormones, namely leptin and thyroid hormones [78,105]. Similarly, corticotropin was suggested to mediate the thermogenic effect on muscle [106]. Thus, putative muscle NST may be under endocrine rather than sympathetic control. However, whether humoral regulation can be fast enough to govern facultative thermogenesis remains to be elucidated.
Additional research is required to verify the involvement of various potential mechanisms of NST in skeletal muscles, as well as to understand their interdependence, regulation, and functional importance. The extent to which these mechanisms are facultative and adaptive is still a matter of debate. When assessing their contribution to energy balance, it is challenging to separate NST activation from both classic shivering and thermogenic tonus. This is because they occur simultaneously with muscle contractile activity in the same organ.
States of myosin: The grey zone between shivering and NST
During the last decade, one more mechanism for increased muscle energy dissipation has been described in the grey zone between shivering and NST. During cross-bridge cycling, active myosin heads bind to the exposed myosin binding sites of the actin filament (see above) and, by conformational changes (driven by ATP hydrolysis), cause sliding of both filaments on each other, which results in muscle contraction. However, besides this active state, myosin heads can also exist in two different inactive states distinguished experimentally by following the kinetics of release of fluorescent ATP hydrolysis products from myosin [107]. Classical relaxed state (or disordered relaxed state; DRX) is characterized by low basal ATPase activity independent of binding to actin, while the newly identified super relaxed state (SRX) hydrolyzes nearly no ATP [107]. The ratios of these three states vary depending on parameters such as sarcomere length, phosphorylation of regulatory proteins, or calcium level [108]. The transition of part of myosin from SRX to DRX would significantly increase energy expenditure, and it was therefore suggested to play a role in cold adaptation [109]. Considerable effort is currently invested in identifying compounds that would affect the SRX/DRX ratio in order to treat pathologies including obesity by increasing muscle energy expenditure.
Cold-induced adaptive induction of the capacity of NST in skeletal muscles rather than in BAT is associated with obesity resistance
Experiments in mice suggest that putative SLN-dependent NST could affect the propensity to obesity, as SLN-deficient mice gain more weight than the control mice if exposed to high-fat diet [28,94] and muscle-specific Sln overexpression prevents diet-induced obesity [110]. Surprisingly, UCP1 and SLN double KO mice do not develop dietary obesity, possibly because of relying on less efficient mechanisms of NST [28]. Obesity-resistant inbred murine strains (such as A/J mice) exhibit higher muscle Sln expression than obesity-prone C57BL6 mice [16]. Besides SLN, another more generally expressed protein, neuronatin, was also recently claimed to cause SERCA uncoupling [111]. Neuronatin expression is affected by high-fat feeding [111], which is interpreted as an indication that SERCA-mediated NST is involved in a physiological negative feedback loop preventing the development of obesity.
Muscle NST and UCP1-mediated NST in BAT differ in important features that could affect their impact on both thermal and energy homeostasis. To achieve the same level of protection against cold, more energy is required by thermogenesis in skeletal muscle compared to UCP1-mediated thermogenesis in BAT [21,82]. This is consistent with the more recent evolution of BAT as a specialized and precisely controlled thermogenic organ, as compared with muscle. The different energy efficiency of thermoregulatory thermogenesis exerted by BAT and muscle could result from differences in i) location and/or ii) regulation.
Location of BAT depots in the center of the body, close to major arteries, allows relatively efficient transport of heat formed in BAT to the vital organs with minimal losses, as compared with thermogenesis in the muscles, which are mostly somewhat peripherally located [6,21]. However, it was also noted that continuous shivering is often happening in highly vascularized deep muscles where heat losses are more minor, and muscle activity may efficiently heat venous blood returning from peripheral tissues [57].
Regulation of NST in BAT and muscle is very different. Thermogenesis in BAT is strictly activated during i) periods of cold exposure or ii) when animals are eating, i.e. during the dark phase of the day in mice [20]. On the other hand, muscle NST seems to be under a looser and less flexible control. When adaptively increased in response to long-lasting cold exposure, it could dissipate energy independent of actual energy intake (see below).
Collectively, the combined effect of the relative thermogenic inefficiency, and absence of rapid flexible regulation of muscle NST in comparison to BAT, results in relatively high energy cost of NST mediated by skeletal muscles, which could lead to reduced propensity to obesity. Indeed, we have observed that both obesity-prone C57BL/6 and obesity-resistant A/J mice were able to survive in the cold with only a slight decrease in body temperature, using muscle shivering for thermogenesis at the beginning of cold exposure (6 °C). Prolonged maintenance of mice at low temperature led to increased capacity of NST in BAT in obesity-prone mice. Surprisingly, however, obesity-resistant A/J mice failed to activate BAT, but instead increased oxidative capacity in skeletal muscle. This putative muscle NST may be mediated by the SLN-SERCA mechanism, consuming additional ATP and allowing for the acceleration of mitochondrial oxidation [16]. Thus, resistance to obesity was associated with cold-induced rise of the capacity of NST in skeletal muscles rather than in BAT (Fig. 2), which is in line with earlier opinions about the adaptivity of muscle NST [5,6]. That capacity for NST in skeletal muscles could be adaptively induced was also documented in mice with genetic ablation of SLN, in accordance with the notion that UCP1- and SLN-SERCA-mediated NST complement each other [99].
The importance of genetic background (particularly the potential role of heterosis) in cold resistance was earlier reported by Kozak's lab [12,112]. Our results suggest that A/J mice represent a model for characterizing UCP1-independent mechanisms of NST [21,45,113,114] and their physiological role. Reflecting the relatively low thermogenic activity of their BAT, these mice may provide a better model of the situation in humans compared with C57BL/6 mice and mixed-background mice, used in most of the previous studies in this field with the focus on the role of UCP1 [20,68], SERCA-SLN [82,99,101] or other mechanisms [26,40,42,45,78,115,116] of NST. To get further insight into the mechanisms engaged in NST, more inbred strains of mice differing in susceptibility to dietary obesity [117] should be characterized in future studies.
Two common approaches used in the prevention and treatment of obesity are reducing calorie intake and increasing physical activity. However, in both rodents and humans, weight loss imposed by fasting leads to i) decreased leptinaemia and sympathetic nervous system activity and ii) increased muscle energy efficiency, i.e. decreased energy expenditure [118]. This can be reversed by normalizing the leptin levels in the context of reversing the decline in sympathetic activity [118,119]. Besides showing the importance of the genetic background of experimental animals for the study of muscle NST, our results support the notion that NST in skeletal muscle may partially counteract weight gain, and its induction could be used to treat obesity. More studies are required in order to elucidate the (i) mechanisms behind adaptivity of NST in skeletal muscle, and (ii) its quantitative importance with respect to overall energy balance.
Conclusions
Although NST includes several alternative mechanisms, UCP1-dependent NST is widely considered the only means of facultative and adaptive NST involved in the homeostasis of both body temperature and possibly body weight. This traditional view has recently been challenged. The involvement of BAT in the maintenance of a constant body weight and prevention of obesity has become increasingly controversial. Compared to small rodents, humans are rarely exposed to chronic cold and can rely more on muscle shivering. Our understanding of both shivering and putative muscle NST is not sufficient. In addition to classic burst shivering, the muscle can produce significant amounts of heat by increasing tonus, a type of muscle contractile activity that may not be visually manifested. Muscle contractions are rapidly regulated by the release and removal of calcium. The efficiency of calcium removal by SERCA is affected by the peptide SLN, which is up-regulated by cold. We propose here that SLN plays a role in the long-term tuning of shivering activity. SLN decreases the efficiency of calcium pumping by SERCA, and promotes thermogenesis. Even though the mechanisms behind NST in skeletal muscles are not fully characterized, it is to be inferred that the slow regulation of muscle NST and its adaptive induction in response to cold may result in increased muscle thermogenesis even when it is not acutely required for thermal homeostasis. The ability to activate NST in the muscle or in BAT depends on the genetic background, which could contribute to strain-specific differences in propensity to obesity in mice. Lower energy efficiency of muscle thermogenesis relative to that in BAT, reflecting the anatomic location, as well as the large oxidative capacity of the muscles, further augments the potential of muscle NST to reduce body fat. In adult humans, the capacity of skeletal muscle to burn fat energy stores is several-fold greater than in BAT. Thus, only a relatively small increase in thermogenesis in muscle could significantly reduce adipose tissue deposition. We propose that muscle NST represents a more promising target for weight-reducing therapies than the traditionally studied BAT.
Acknowledgements
Supported by project no. 22-07004S of Czech Science Foundation, and by the project National Institute for Research of Metabolic and Cardiovascular Diseases (Programme EXCELES, ID Project No. LX22NPO5104) – Funded by the European Union-Next Generation EU.
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Himms-Hagen J. Role of thermogenesis in the regulation of energy balance in relation to obesity. Can J Physiol Pharmacol. 1989;67:394–401. doi: 10.1139/y89-063. [DOI] [PubMed] [Google Scholar]
- 2.Grigg G, Nowack J, Bicudo J, Bal NC, Woodward HN, Seymour RS. Whole-body endothermy: ancient, homologous and widespread among the ancestors of mammals, birds and crocodylians. Biol Rev Camb Philos Soc. 2022;97:766–801. doi: 10.1111/brv.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Skop V, Guo J, Liu N, Xiao C, Hall KD, Gavrilova O, Reitman ML. Mouse Thermoregulation: Introducing the Concept of the Thermoneutral Point. Cell Rep. 2020;31:107501. doi: 10.1016/j.celrep.2020.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girardier L. The regulation of the biological furnace of warm blooded animals. Experientia. 1977;33:1121–1122. doi: 10.1007/BF01922276. [DOI] [PubMed] [Google Scholar]
- 5.Jansky I. Humoral thermogenesis and its role in maintaining energy balance. Physiol Rev. 1995;75:237–259. doi: 10.1152/physrev.1995.75.2.237. [DOI] [PubMed] [Google Scholar]
- 6.Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol Rev. 2006;86:435–464. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- 7.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 8.Masoro EJ. Role of lipogenesis in nonshivering thermogenesis. Fed Proc. 1963;22:868–873. [PubMed] [Google Scholar]
- 9.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. doi: 10.1016/j.cell.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guerra C, Koza RA, Yamashita H, King KW, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kozak LP. The genetics of brown adipocyte induction in white fat depots. Front Endocrinol (Lausanne) 2011;2:64. doi: 10.3389/fendo.2011.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyer BB, Kozak LP. The mitochondrial uncoupling protein gene in brown fat: correlation between DNase I hypersensitivity and expression in transgenic mice. Mol Cell Biol. 1991;11:4147–4156. doi: 10.1128/mcb.11.8.4147-4156.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozak UC, Kopecky J, Teisinger J, Enerback S, Boyer B, Kozak LP. An upstream enhancer regulating brown-fat-specific expression of the mitochondrial uncoupling protein gene. Mol Cell Biol. 1994;14:59–67. doi: 10.1128/mcb.14.1.59-67.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cassard-Doulcier AM, Gelly C, Fox N, Schrementi J, Raimbault S, Klaus S, Forest C, Bouillaud F, Ricquier D. Tissue-specific and beta-adrenergic regulation of the mitochondrial uncoupling protein gene: control by cis-acting elements in the 5′-flanking region. Mol Endocrinol. 1993;7:497–506. doi: 10.1210/mend.7.4.8388995. [DOI] [PubMed] [Google Scholar]
- 16.Janovska P, Zouhar P, Bardova K, Otahal J, Vrbacky M, Mracek T, Adamcova K, et al. Impairment of adrenergically-regulated thermogenesis in brown fat of obesity-resistant mice is compensated by non-shivering thermogenesis in skeletal muscle. Mol Metab. 2023;69:101683. doi: 10.1016/j.molmet.2023.101683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shabalina IG, Petrovic N, de Jong JM, Kalinovich AV, Cannon B, Nedergaard J. UCP1 in Brite/Beige Adipose Tissue Mitochondria Is Functionally Thermogenic. Cell Rep. 2013;5:1196–1203. doi: 10.1016/j.celrep.2013.10.044. [DOI] [PubMed] [Google Scholar]
- 18.Rothwell NJ, Stock MJ. Similarities between Cold-Induced and Diet-Induced Thermogenesis in the Rat. Can J Physiol Pharmacol. 1980;58:842–848. doi: 10.1139/y80-130. [DOI] [Google Scholar]
- 19.Wijers SL, Saris WH, Marken Lichtenbelt WD. Individual thermogenic responses to mild cold and overfeeding are closely related. J Clin Endocrinol Metab. 2007;92:4299–4305. doi: 10.1210/jc.2007-1065. [DOI] [PubMed] [Google Scholar]
- 20.von Essen G, Lindsund E, Cannon B, Nedergaard J. Adaptive facultative diet-induced thermogenesis in wild-type but not in UCP1-ablated mice. Am J Physiol Endocrinol Metab. 2017;313:E515–E527. doi: 10.1152/ajpendo.00097.2017. [DOI] [PubMed] [Google Scholar]
- 21.Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 2010;11:263–267. doi: 10.1016/j.cmet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- 23.Kopecky J, Clarke G, Enerback S, Spiegelman B, Kozak LP. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J Clin Invest. 1995;96:2914–2923. doi: 10.1172/JCI118363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowell BB, Susulic V, Hamann A, Lawitts JA, Himms-Hagen J, Boyer BB, Kozak LP, Flier JS. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature. 1993;366:740–742. doi: 10.1038/366740a0. [DOI] [PubMed] [Google Scholar]
- 25.Melnyk A, Harper ME, Himms-Hagen J. Raising at thermoneutrality prevents obesity and hyperphagia in BAT-ablated transgenic mice. Am J Physiol. 1997;272:R1088–R1093. doi: 10.1152/ajpregu.1997.272.4.R1088. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Rossmeisl M, McClaine J, Kozak LP. Paradoxical resistance to diet-induced obesity in UCP1-deficient mice1. J Clin Invest. 2003;111:399–407. doi: 10.1172/JCI15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 Ablation Induces Obesity and Abolishes Diet-induced Thermogenesis in Mice Exempt from Thermal Stress by Living at Thermoneutrality. Cell Metab. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Rowland LA, Maurya SK, Bal NC, Kozak L, Periasamy M. Sarcolipin and uncoupling protein 1 play distinct roles in diet-induced thermogenesis and do not compensate for one another. Obesity. 2016;24:1430–1433. doi: 10.1002/oby.21542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Willershauser M, Li Y, Fromme T, Schnabl K, Bast-Habersbrunner A, Ramisch S, Mocek S, Klingenspor M. Uncoupling protein 1 expression does not protect mice from diet-induced obesity. Am J Physiol Endocrinol Metab. 2021;320:E333–E345. doi: 10.1152/ajpendo.00285.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.von Essen G, Lindsund E, Maldonado EM, Zouhar P, Cannon B, Nedergaard J. Highly recruited brown adipose tissue does not in itself protect against obesity. Mol Metab. 2023;76:101782. doi: 10.1016/j.molmet.2023.101782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nedergaard J, von Essen G, Cannon B. Brown adipose tissue: can it keep us slim? A discussion of the evidence for and against the existence of diet-induced thermogenesis in mice and men. Philos Trans R Soc Lond B Biol Sci. 2023;378:20220220. doi: 10.1098/rstb.2022.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Schnabl K, Gabler SM, Willershauser M, Reber J, Karlas A, Laurila S, et al. Secretin-Activated Brown Fat Mediates Prandial Thermogenesis to Induce Satiation. Cell. 2018;175:1561–1574.e12. doi: 10.1016/j.cell.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 33.Speakman JR, Hall KD. Models of body weight and fatness regulation. Philos Trans R Soc Lond B Biol Sci. 2023;378:20220231. doi: 10.1098/rstb.2022.0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Collins S, Daniel KW, Petro AE, Surwit RS. Strain-specific response to beta3-adrenergic receptor agonist treatment of diet-induced obesity in mice. Endocrinology. 1997;138:405–413. doi: 10.1210/endo.138.1.4829. [DOI] [PubMed] [Google Scholar]
- 35.Finlin BS, Memetimin H, Confides AL, Kasza I, Zhu B, Vekaria HJ, Harfmann B, et al. Human adipose beiging in response to cold and mirabegron. JCI Insight. 2018;3:e121510. doi: 10.1172/jci.insight.121510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng Q, Lin J, Huang J, Zhang H, Zhang R, Zhang X, Cao C, et al. Reconstitution of UCP1 using CRISPR/Cas9 in the white adipose tissue of pigs decreases fat deposition and improves thermogenic capacity. Proc Natl Acad Sci U S A. 2017;114:E9474–E9482. doi: 10.1073/pnas.1707853114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindberg O, Prusiner SB, Cannon B, Ching TM, Eisenhardt RH. Metabolic control in isolated brown fat cells. Lipids. 1970;5:204–209. doi: 10.1007/BF02532470. [DOI] [PubMed] [Google Scholar]
- 38.Kazak L. Promoting metabolic inefficiency for metabolic disease. iScience. 2023;26:107843. doi: 10.1016/j.isci.2023.107843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahbani JF, Bunk J, Lagarde D, Samborska B, Roesler A, Xiao H, Shaw A, et al. Parallel control of cold-triggered adipocyte thermogenesis by UCP1 and CKB. Cell Metab. 2024;36:526–540.e7. doi: 10.1016/j.cmet.2024.01.001. [DOI] [PubMed] [Google Scholar]
- 40.Oeckl J, Janovska P, Adamcova K, Bardova K, Brunner S, Dieckmann S, Ecker J, et al. Loss of UCP1 function augments recruitment of futile lipid cycling for thermogenesis in murine brown fat. Mol Metab. 2022;61:101499. doi: 10.1016/j.molmet.2022.101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ikeda K, Kang Q, Yoneshiro T, Camporez JP, Maki H, Homma M, Shinoda K, et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med. 2017;23:1454–1465. doi: 10.1038/nm.4429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ukropec J, Anunciado RP, Ravussin Y, Hulver MW, Kozak LP. UCP1-independent thermogenesis in white adipose tissue of cold-acclimated Ucp1−/− mice. J Biol Chem. 2006;281:31894–31908. doi: 10.1016/S0021-9258(19)84104-2. [DOI] [PubMed] [Google Scholar]
- 43.Rahbani JF, Scholtes C, Lagarde DM, Hussain MF, Roesler A, Dykstra CB, Bunk J, et al. ADRA1A-Galpha(q) signalling potentiates adipocyte thermogenesis through CKB and TNAP. Nat Metab. 2022;4:1459–1473. doi: 10.1038/s42255-022-00667-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Golozoubova V, Cannon B, Nedergaard J. UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. Am J Physiol Endocrinol Metab. 2006;291:E350–E357. doi: 10.1152/ajpendo.00387.2005. [DOI] [PubMed] [Google Scholar]
- 45.Granneman JG, Burnazi M, Zhu Z, Schwamb LA. White adipose tissue contributes to UCP1-independent thermogenesis. Am J Physiol Endocrinol Metab. 2003;285:E1230–E1236. doi: 10.1152/ajpendo.00197.2003. [DOI] [PubMed] [Google Scholar]
- 46.Flachs P, Adamcova K, Zouhar P, Marques C, Janovska P, Viegas I, Jones JG, et al. Induction of lipogenesis in white fat during cold exposure in mice: link to lean phenotype. Int J Obes (Lond) 2017;41:372–380. doi: 10.1038/ijo.2016.228. [DOI] [PubMed] [Google Scholar]
- 47.Meyer CW, Willershauser M, Jastroch M, Rourke BC, Fromme T, Oelkrug R, Heldmaier G, Klingenspor M. Adaptive thermogenesis and thermal conductance in wild-type and UCP1-KO mice. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1396–R1406. doi: 10.1152/ajpregu.00021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Poussin C, Ibberson M, Hall D, Ding J, Soto J, Abel ED, Thorens B. Oxidative phosphorylation flexibility in the liver of mice resistant to high-fat diet-induced hepatic steatosis. Diabetes. 2011;60:2216–2224. doi: 10.2337/db11-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burcelin R, Crivelli V, Dacosta A, Roy-Tirelli A, Thorens B. Heterogeneous metabolic adaptation of C57BL/6J mice to high-fat diet. Am J Physiol Endocrinol Metab. 2002;282:E834–E842. doi: 10.1152/ajpendo.00332.2001. [DOI] [PubMed] [Google Scholar]
- 50.Hui S, Cowan AJ, Zeng X, Yang L, TeSlaa T, Li X, Bartman C, et al. Quantitative Fluxomics of Circulating Metabolites. Cell Metab. 2020;32:676–688.e4. doi: 10.1101/2020.03.02.973669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Barclay CJ, Curtin NA. Advances in understanding the energetics of muscle contraction. J Biomech. 2023;156:111669. doi: 10.1016/j.jbiomech.2023.111669. [DOI] [PubMed] [Google Scholar]
- 52.Blondin DP, Labbe SM, Phoenix S, Guerin B, Turcotte EE, Richard D, Carpentier AC, Haman F. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J Physiol. 2015;593:701–714. doi: 10.1113/jphysiol.2014.283598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zurlo F, Larson K, Bogardus C, Ravussin E. Skeletal muscle metabolism is a major determinant of resting energy expenditure. J Clin Invest. 1990;86:1423–1427. doi: 10.1172/JCI114857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haman F, Blondin DP. Shivering thermogenesis in humans: Origin, contribution and metabolic requirement. Temperature (Austin) 2017;4:217–226. doi: 10.1080/23328940.2017.1328999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schiaffino S. Fibre types in skeletal muscle: a personal account. Acta Physiol (Oxford) 2010;199:451–463. doi: 10.1111/j.1748-1716.2010.02130.x. [DOI] [PubMed] [Google Scholar]
- 56.Booth FW, Ruegsegger GN, Toedebusch RG, Yan Z. Endurance Exercise and the Regulation of Skeletal Muscle Metabolism. Prog Mol Biol Transl Sci. 2015;135:129–151. doi: 10.1016/bs.pmbts.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 57.Lomo T, Eken T, Bekkestad Rein E, Nja A. Body temperature control in rats by muscle tone during rest or sleep. Acta Physiol (Oxford) 2020;228:e13348. doi: 10.1111/apha.13348. [DOI] [PubMed] [Google Scholar]
- 58.Haman F, Legault SR, Rakobowchuk M, Ducharme MB, Weber JM. Effects of carbohydrate availability on sustained shivering II. Relating muscle recruitment to fuel selection. J Appl Physiol. 2004;96:41–49. doi: 10.1152/japplphysiol.00428.2003. [DOI] [PubMed] [Google Scholar]
- 59.Haman F, Peronnet F, Kenny GP, Massicotte D, Lavoie C, Scott C, Weber JM. Effect of cold exposure on fuel utilization in humans: plasma glucose, muscle glycogen, and lipids. J Appl Physiol. 2002;93:77–84. doi: 10.1152/japplphysiol.00773.2001. [DOI] [PubMed] [Google Scholar]
- 60.Blondin DP, Daoud A, Taylor T, Tingelstad HC, Bezaire V, Richard D, Carpentier AC, et al. Four-week cold acclimation in adult humans shifts uncoupling thermogenesis from skeletal muscles to brown adipose tissue. J Physiol. 2017;595:2099–2113. doi: 10.1113/JP273395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castellani JW, Young AJ. Human physiological responses to cold exposure: Acute responses and acclimatization to prolonged exposure. Auton Neurosci. 2016;196:63–74. doi: 10.1016/j.autneu.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 62.Haman F, Peronnet F, Kenny GP, Massicotte D, Lavoie C, Weber JM. Partitioning oxidative fuels during cold exposure in humans: muscle glycogen becomes dominant as shivering intensifies. J Physiol. 2005;566:247–256. doi: 10.1113/jphysiol.2005.086272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blondin DP, Haman F. Shivering and nonshivering thermogenesis in skeletal muscles. Handb Clin Neurol. 2018;156:153–173. doi: 10.1016/B978-0-444-63912-7.00010-2. [DOI] [PubMed] [Google Scholar]
- 64.Dulloo AG, Miles-Chan JL, Montani JP, Schutz Y. Isometric thermogenesis at rest and during movement: a neglected variable in energy expenditure and obesity predisposition. Obes Rev. 2017;18(Suppl 1):56–64. doi: 10.1111/obr.12505. [DOI] [PubMed] [Google Scholar]
- 65.McKay WP, Vargo M, Chilibeck PD, Daku BL. Effects of ambient temperature on mechanomyography of resting quadriceps muscle. Appl Physiol Nutr Metab. 2013;38:227–233. doi: 10.1139/apnm-2011-0358. [DOI] [PubMed] [Google Scholar]
- 66.Wyckelsma VL, Venckunas T, Houweling PJ, Schlittler M, Lauschke VM, Tiong CF, Wood HD, et al. Loss of alpha-actinin-3 during human evolution provides superior cold resilience and muscle heat generation. Am J Hum Genet. 2021;108:446–457. doi: 10.1016/j.ajhg.2021.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scholle HC, Biedermann F, Arnold D, Jinnah HA, Grassme R, Schumann NP. A surface EMG multi-electrode technique for characterizing muscle activation patterns in mice during treadmill locomotion. J Neurosci Methods. 2005;146:174–182. doi: 10.1016/j.jneumeth.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 68.Golozoubova V, Hohtola E, Matthias A, Jacobsson A, Cannon B, Nedergaard J. Only UCP1 can mediate adaptive nonshivering thermogenesis in the cold. FASEB J. 2001;15:2048–2050. doi: 10.1096/fj.00-0536fje. [DOI] [PubMed] [Google Scholar]
- 69.Westerberg R, Mansson JE, Golozoubova V, Shabalina IG, Backlund EC, Tvrdik P, Retterstol K, Capecchi MR, Jacobsson A. ELOVL3 is an important component for early onset of lipid recruitment in brown adipose tissue. J Biol Chem. 2006;281:4958–4968. doi: 10.1074/jbc.M511588200. [DOI] [PubMed] [Google Scholar]
- 70.Chen Y, Ikeda K, Yoneshiro T, Scaramozza A, Tajima K, Wang Q, Kim K, et al. Thermal stress induces glycolytic beige fat formation via a myogenic state. Nature. 2019;565:180–185. doi: 10.1038/s41586-018-0801-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Islam MA, Sundaraj K, Ahmad RB, Ahamed NU. Mechanomyogram for muscle function assessment: a review. PLoS One. 2013;8:e58902. doi: 10.1371/journal.pone.0058902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jansky L, Hart JS. Participation of skeletal muscle and kidney during nonshivering thermogenesisin cold-acclimated rats. Can J Biochem Physiol. 1963;41:953–964. doi: 10.1139/y63-108. [DOI] [PubMed] [Google Scholar]
- 73.Jansky L, Hart JS. Cardiac output and organ blood flow in warm- and cold-acclimated rats exposed to cold. Can J Physiol Pharmacol. 1968;46:653–659. doi: 10.1139/y68-096. [DOI] [PubMed] [Google Scholar]
- 74.Mejsnar J, Jansky L. Means of noradrenalin action during non-shivering thermogenesis in a single muscle. Int J Biometeorol. 1971;15:321–324. doi: 10.1007/BF01803920. [DOI] [PubMed] [Google Scholar]
- 75.Jansky L. Contribution of striated muscles to regulatory heat production. Experientia. 1977;33:1123–1124. doi: 10.1007/BF01922277. [DOI] [PubMed] [Google Scholar]
- 76.Smith TJ, Edelman IS. The role of sodium transport in thyroid thermogenesis. Fed Proc. 1979;38:2150–2153. [PubMed] [Google Scholar]
- 77.Clarke RJ, Catauro M, Rasmussen HH, Apell HJ. Quantitative calculation of the role of the Na(+),K(+)-ATPase in thermogenesis. Biochim Biophys Acta. 2013;1827:1205–1212. doi: 10.1016/j.bbabio.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 78.Ukropec J, Anunciado RV, Ravussin Y, Kozak LP. Leptin is required for uncoupling protein-1-independent thermogenesis during cold stress. Endocrinology. 2006;147:2468–2480. doi: 10.1210/en.2005-1216. [DOI] [PubMed] [Google Scholar]
- 79.Solinas G, Summermatter S, Mainieri D, Gubler M, Pirola L, Wymann MP, Rusconi S, et al. The direct effect of leptin on skeletal muscle thermogenesis is mediated by substrate cycling between de novo lipogenesis and lipid oxidation. FEBS Lett. 2004;577:539–544. doi: 10.1016/j.febslet.2004.10.066. [DOI] [PubMed] [Google Scholar]
- 80.Kus V, Prazak T, Brauner P, Hensler M, Kuda O, Flachs P, Janovska P, et al. Induction of muscle thermogenesis by high-fat diet in mice: association with obesity-resistance. Am J Physiol Endocrinol Metab. 2008;295:E356–E367. doi: 10.1152/ajpendo.90256.2008. [DOI] [PubMed] [Google Scholar]
- 81.Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- 82.Bal NC, Periasamy M. Uncoupling of sarcoendoplasmic reticulum calcium ATPase pump activity by sarcolipin as the basis for muscle non-shivering thermogenesis. Philos Trans R Soc Lond B Biol Sci. 2020;375:20190135. doi: 10.1098/rstb.2019.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Campbell KL, Dicke AA. Sarcolipin Makes Heat, but Is It Adaptive Thermogenesis? Front Physiol. 2018;9:714. doi: 10.3389/fphys.2018.00714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nowack J, Vetter SG, Stalder G, Painer J, Kral M, Smith S, Le MH, et al. Muscle nonshivering thermogenesis in a feral mammal. Sci Rep. 2019;9:6378. doi: 10.1038/s41598-019-42756-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 86.Nowack J, Giroud S, Arnold W, Ruf T. Muscle Non-shivering Thermogenesis and Its Role in the Evolution of Endothermy. Front Physiol. 2017;8:889. doi: 10.3389/fphys.2017.00889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Toth A, Fodor J, Vincze J, Olah T, Juhasz T, Zakany R, Csernoch L, Zador E. The Effect of SERCA1b Silencing on the Differentiation and Calcium Homeostasis of C2C12 Skeletal Muscle Cells. PLoS One. 2015;10:e0123583. doi: 10.1371/journal.pone.0123583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- 89.Pant M, Bal NC, Periasamy M. Cold adaptation overrides developmental regulation of sarcolipin expression in mice skeletal muscle: SOS for muscle-based thermogenesis? J Exp Biol. 2015;218:2321–2325. doi: 10.1242/jeb.119164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Periasamy M, Bhupathy P, Babu GJ. Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology. Cardiovasc Res. 2008;77:265–273. doi: 10.1093/cvr/cvm056. [DOI] [PubMed] [Google Scholar]
- 91.Sahoo SK, Shaikh SA, Sopariwala DH, Bal NC, Periasamy M. Sarcolipin protein interaction with sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) is distinct from phospholamban protein, and only sarcolipin can promote uncoupling of the SERCA pump. J Biol Chem. 2013;288:6881–6889. doi: 10.1074/jbc.M112.436915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fajardo VA, Bombardier E, Vigna C, Devji T, Bloemberg D, Gamu D, Gramolini AO, Quadrilatero J, Tupling AR. Co-expression of SERCA isoforms, phospholamban and sarcolipin in human skeletal muscle fibers. PLoS One. 2013;8:e84304. doi: 10.1371/journal.pone.0084304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bhupathy P, Babu GJ, Periasamy M. Sarcolipin and phospholamban as regulators of cardiac sarcoplasmic reticulum Ca2+ ATPase. J Mol Cell Cardiol. 2007;42:903–911. doi: 10.1016/j.yjmcc.2007.03.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bal NC, Maurya SK, Sopariwala DH, Sahoo SK, Gupta SC, Shaikh SA, Pant M, et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med. 2012;18:1575–1579. doi: 10.1038/nm.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.MacLennan DH, Kranias EG. Phospholamban: a crucial regulator of cardiac contractility. Nat Rev Mol Cell Biol. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 96.Babu GJ, Bhupathy P, Timofeyev V, Petrashevskaya NN, Reiser PJ, Chiamvimonvat N, Periasamy M. Ablation of sarcolipin enhances sarcoplasmic reticulum calcium transport and atrial contractility. Proc Natl Acad Sci U S A. 2007;104:17867–17872. doi: 10.1073/pnas.0707722104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shaikh SA, Sahoo SK, Periasamy M. Phospholamban and sarcolipin: Are they functionally redundant or distinct regulators of the Sarco(Endo)Plasmic Reticulum Calcium ATPase? J Mol Cell Cardiol. 2016;91:81–91. doi: 10.1016/j.yjmcc.2015.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meizoso-Huesca A, Pearce L, Barclay CJ, Launikonis BS. Ca(2+) leak through ryanodine receptor 1 regulates thermogenesis in resting skeletal muscle. Proc Natl Acad Sci U S A. 2022;119:e2119203119. doi: 10.1073/pnas.2119203119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rowland LA, Bal NC, Kozak LP, Periasamy M. Uncoupling Protein 1 and Sarcolipin Are Required to Maintain Optimal Thermogenesis, and Loss of Both Systems Compromises Survival of Mice under Cold Stress. J Biol Chem. 2015;290:12282–12289. doi: 10.1074/jbc.M115.637603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bal NC, Maurya SK, Singh S, Wehrens XH, Periasamy M. Increased Reliance on Muscle-based Thermogenesis upon Acute Minimization of Brown Adipose Tissue Function. J Biol Chem. 2016;291:17247–17257. doi: 10.1074/jbc.M116.728188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bal NC, Singh S, Reis FCG, Maurya SK, Pani S, Rowland LA, Periasamy M. Both brown adipose tissue and skeletal muscle thermogenesis processes are activated during mild to severe cold adaptation in mice. J Biol Chem. 2017;292:16616–16625. doi: 10.1074/jbc.M117.790451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Morrissette JM, Franck JP, Block BA. Characterization of ryanodine receptor and Ca2+-ATPase isoforms in the thermogenic heater organ of blue marlin (Makaira nigricans) J Exp Biol. 2003;206:805–812. doi: 10.1242/jeb.00158. [DOI] [PubMed] [Google Scholar]
- 103.Shiuchi T, Haque MS, Okamoto S, Inoue T, Kageyama H, Lee S, Toda C, et al. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 2009;10:466–480. doi: 10.1016/j.cmet.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 104.Bombardier E, Smith IC, Gamu D, Fajardo VA, Vigna C, Sayer RA, Gupta SC, Bal NC, Periasamy M, Tupling AR. Sarcolipin trumps beta-adrenergic receptor signaling as the favored mechanism for muscle-based diet-induced thermogenesis. FASEB J. 2013;27:3871–3878. doi: 10.1096/fj.13-230631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zekri Y, Flamant F, Gauthier K. Central vs. Peripheral Action of Thyroid Hormone in Adaptive Thermogenesis: A Burning Topic. Cells. 2021;10:1327. doi: 10.3390/cells10061327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Solinas G, Summermatter S, Mainieri D, Gubler M, Montani JP, Seydoux J, Smith SR, Dulloo AG. Corticotropin-releasing hormone directly stimulates thermogenesis in skeletal muscle possibly through substrate cycling between de novo lipogenesis and lipid oxidation. Endocrinology. 2006;147:31–38. doi: 10.1210/en.2005-1033. [DOI] [PubMed] [Google Scholar]
- 107.Stewart MA, Franks-Skiba K, Chen S, Cooke R. Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc Natl Acad Sci U S A. 2010;107:430–435. doi: 10.1073/pnas.0909468107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schmid M, Toepfer CN. Cardiac myosin super relaxation (SRX): a perspective on fundamental biology, human disease and therapeutics. Biol Open. 2021;10:bio057646. doi: 10.1242/bio.057646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cooke R. The role of the myosin ATPase activity in adaptive thermogenesis by skeletal muscle. Biophys Rev. 2011;3:33–45. doi: 10.1007/s12551-011-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Maurya SK, Bal NC, Sopariwala DH, Pant M, Rowland LA, Shaikh SA, Periasamy M. Sarcolipin Is a Key Determinant of the Basal Metabolic Rate, and Its Overexpression Enhances Energy Expenditure and Resistance against Diet-induced Obesity. J Biol Chem. 2015;290:10840–10849. doi: 10.1074/jbc.M115.636878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Braun JL, Teng ACT, Geromella MS, Ryan CR, Fenech RK, MacPherson REK, Gramolini AO, Fajardo VA. Neuronatin promotes SERCA uncoupling and its expression is altered in skeletal muscles of high-fat diet-fed mice. FEBS Lett. 2021;595:2756–2767. doi: 10.1002/1873-3468.14213. [DOI] [PubMed] [Google Scholar]
- 112.Hofmann WE, Liu X, Bearden CM, Harper ME, Kozak LP. Effects of genetic background on thermoregulation and fatty acid-induced uncoupling of mitochondria in UCP1-deficient mice. J Biol Chem. 2001;276:12460–12465. doi: 10.1074/jbc.M100466200. [DOI] [PubMed] [Google Scholar]
- 113.Flachs P, Rossmeisl M, Kuda O, Kopecky J. Stimulation of mitochondrial oxidative capacity in white fat independent of UCP1: A key to lean phenotype. Biochim Biophys Acta. 2013;1831:986–1003. doi: 10.1016/j.bbalip.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 114.Rohm M, Zeigerer A, Machado J, Herzig S. Energy metabolism in cachexia. EMBO Rep. 2019;20:e47258. doi: 10.15252/embr.201847258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Keipert S, Lutter D, Schroeder BO, Brandt D, Stahlman M, Schwarzmayr T, Graf E, et al. Endogenous FGF21-signaling controls paradoxical obesity resistance of UCP1-deficient mice. Nat Commun. 2020;11:624. doi: 10.1038/s41467-019-14069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kazak L, Rahbani JF, Samborska B, Lu GZ, Jedrychowski MP, Lajoie M, Zhang S, et al. Ablation of adipocyte creatine transport impairs thermogenesis and causes diet-induced obesity. Nat Metab. 2019;1:360–370. doi: 10.1038/s42255-019-0035-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.West DB, Boozer CN, Moody DL, Atkinson RL. Dietary obesity in nine inbred mouse strains. Am J Physiol. 1992;262:R1025–R1032. doi: 10.1152/ajpregu.1992.262.6.R1025. [DOI] [PubMed] [Google Scholar]
- 118.Goldsmith R, Joanisse DR, Gallagher D, Pavlovich K, Shamoon E, Leibel RL, Rosenbaum M. Effects of experimental weight perturbation on skeletal muscle work efficiency, fuel utilization, and biochemistry in human subjects. Am J Physiol Regul Integr Comp Physiol. 2010;298:R79–R88. doi: 10.1152/ajpregu.00053.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, et al. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kozak LP. Play Up! Play Up! And Play the Game! First Edition. FriesenPress; 2020. p. 156. [Google Scholar]
- 121.Ravussin E, Enerback S, Koza R. Leslie Paul Kozak, PhD (1940–2023) Obesity. 2023;31:2885–2886. doi: 10.1002/oby.23919. [DOI] [Google Scholar]