Summary
Cardiovascular diseases are the most important cause of morbidity and mortality in the civilized world. Stenosis or occlusion of blood vessels leads not only to events that are directly life-threatening, such as myocardial infarction or stroke, but also to a significant reduction in quality of life, for example in lower limb ischemia as a consequence of metabolic diseases. The first synthetic polymeric vascular replacements were used clinically in the early 1950s. However, they proved to be suitable only for larger-diameter vessels, where the blood flow prevents the attachment of platelets, pro-inflammatory cells and smooth muscle cells on their inner surface, whereas in smaller-diameter grafts (6 mm or less), these phenomena lead to stenosis and failure of the graft. Moreover, these polymeric vascular replacements, like biological grafts (decellularized or devitalized), are cell-free, i.e. there are no reconstructed physiological layers of the blood vessel wall, i.e. an inner layer of endothelial cells to prevent thrombosis, a middle layer of smooth muscle cells to perform the contractile function, and an outer layer to provide innervation and vascularization of the vessel wall. Vascular substitutes with these cellular components can be constructed by tissue engineering methods. However, it has to be admitted that even about 70 years after the first polymeric vascular prostheses were implanted into human patients, there are still no functional small-diameter vascular grafts on the market. The damage to small-diameter blood vessels has to be addressed by endovascular approaches or by autologous vascular substitutes, which leads to some skepticism about the potential of tissue engineering. However, new possibilities of this approach lie in the use of modern technologies such as 3D bioprinting and/or electrospinning in combination with stem cells and pre-vascularization of tissue-engineered vascular grafts. In this endeavor, sex-related differences in the removal of degradable biomaterials by the cells and in the behavior of stem cells and pre-differentiated vascular cells need to be taken into account.
Keywords: Blood vessel prosthesis, Regenerative medicine, Stem cells, Footprint-free iPSCs, sr-RNA, Dynamic bioreactor, Sex-related differences
Introduction
Cardiovascular diseases are the leading cause of illness and death in the civilized world [1]. According to the World Health Organization (WHO), more than 30 % of deaths worldwide are caused by these diseases [2]. This is also true for the Czech Republic, where circulatory diseases accounted for over 40 % of all deaths in 2019, while cancer accounted for 25 % [3].
Ischemia of tissues supplied by damaged blood vessels, i.e. vessels with a significantly narrowed or closed lumen, manifests itself not only in serious life-threatening disorders such as heart attack or stroke, but also in a significant reduction in quality of life, e.g. the relatively frequent necrosis of lower limb tissues in diabetes. In the Czech Republic, there are more than 10 000 sudden deaths due to heart attacks every year, and already in 2010 the number of patients with diabetes was around 600 000 - with at least 5 000 of them developing the 'diabetic foot' syndrome, leading to amputation of a limb - and these figures are rising.
An interesting observation from clinical practice is that men are more susceptible to cardiovascular diseases than women, or at least more susceptible than women of childbearing age - after menopause, the susceptibility of women is equal to the susceptibility of men (for a review, see [4]). One of the reasons for the increased propensity of men to cardiovascular diseases is the increased readiness of vascular smooth muscle cells (VSMCs) to migrate and proliferate. In our earlier studies, VSMCs in cultures from male rats migrated and proliferated faster than VSMCs from female rats, even without the current presence of sex hormones in the culture medium (e.g. in a serum-free medium), or even in the case of VSMCs isolated from newborn rats. This difference also increased with the time of cell cultivation, i.e. with the number of passages in the culture (for a review, see [5]). However, some studies suggest that this VSMC behavior is androgen-dependent after all - i.e. it is established prenatally by the action of these hormones, which apparently induce irreversible changes in the expression of some genes. For example, it has been described that in spontaneously hypertensive rats (SHR), the blood pressure reaches significantly higher values in males than in females [6] and depends on prenatal androgen synthesis due to gene expression in the SRY locus on the Y chromosome [7]. Androgens, among other things, increase the sensitivity of VSMCs to adrenergic hormones, and this persists throughout the whole life of the animals, even without the current presence of physiological levels of androgens, e.g. after castration of rats [8]. In addition, VSMCs from the aorta of spontaneously hypertensive male rats were significantly more sensitive to proliferation stimulation by angiotensin II in vitro than female VSMCs [6]. In contrast, estrogens have a rather antiproliferative effect on VSMCs [9–11].
In healthy blood vessels under physiological circumstances, vascular smooth muscle cells (VSMCs) are in a quiescent, non-proliferative, differentiated contractile state. An important factor that keeps them in this state is the endothelial barrier. However, this barrier can be damaged – both mechanically, e.g. by hypertension, and also biochemically – e.g. due to metabolic disorders such as elevated levels of glucose, cholesterol or homocysteine, the presence of oxygen radicals, nicotine, etc. Substances that are not present in the vascular wall under physiological conditions then reach the VSMCs, such as growth factors from the blood, especially platelet-derived growth factor (PDGF), and also vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), nerve growth factor (NGF), and insulin-like growth factor-1 (IGF-1) (for a review, see [1,5]). The blood vessel is further infiltrated by other proteins from the blood, such as albumin, immunoglobulins, and lipidic substances, and also by pro-inflammatory cells such as leukocytes, lymphocytes, monocytes, macrophages and mast cells. These cells synthesize chemokines and cytokines, e.g. interleukins (e.g. interleukin-1), tumor necrosis factors (TNFs, e.g. TNF-α) and monocyte chemoattractant protein-1 (MCP-1), as well as metalloproteases (MMPs, e.g. MMP-2, MMP-9 and MMP-13), degrading the extracellular matrix (ECM) of the vascular wall. All these factors then stimulate the so-called phenotypic modulation of VSMCs, manifested by the transition from the contractile phenotype to the synthetic phenotype and by activation of the migratory and proliferative activity of VSMCs (for a review, see [1,5]).
The migratory and proliferative activity of VSMCs can be so massive that, together with the synthesis of ECM molecules, their calcification and the deposition of lipidic substances (mainly cholesterol), it leads not only to narrowing of the lumen of the damaged vessel, but also to its complete closure. It is not without interest that for this reason some researchers, especially in the 1970s, equated VSMC proliferation with tumor cell growth and viewed atherosclerotic lesions as "benign VSMC tumors" [12,13]. The finding of a connection between viral infection (which is the cause of some tumors) and the subsequent proliferation of VSMCs contributed to this view [14]. Even one of our own earlier studies [15] indicated a connection between the increased proliferative activity of VSMCs and the presence of pathogens in the organism. VSMCs in cultures derived from the aorta of specific pathogen-free (SPF) rats migrated later from explants of the tunica media and intima complex and proliferated more slowly than VSMCs from rats reared under standard conditions in the presence of microorganisms in the surrounding environment and in the rat organism. Nevertheless, the increased growth activity of VSMCs, rather than tumor growth, is a manifestation of high regenerative capacity of these cells, which exceeds the current need to repair the damaged vessel and becomes counterproductive, i.e. leading to irreversible damage and obstruction of the vessel and the need for its replacement. Furthermore, VSMCs are not the only proliferating cell type in the vessel wall – macrophages also proliferate there. Their proliferation is induced by the macrophage colony-stimulating factor (M-CSF), and some authors have attributed even greater importance to it in the pathology of the vascular wall (especially for the formation of atherosclerotic lesions) than to the proliferation of VSMCs themselves [16,17]; for a review, see [5]. There are also sex-related differences not only in the propensity to cardiovascular diseases but also in the manner of their manifestation. For example, an increase in vascular stiffness, which is associated with a higher risk of cardiovascular morbidity and mortality, is likely sex-specific. In Dahl salt-sensitive rats, the ultrastructure of mesenteric arteries showed significant increases in collagen and smooth muscle cell areas, which altered the mechanics and hemodynamics of these arteries and led to higher blood pressure in male rats than in female rats [18]. In coronary artery disease, men are more prone to lipid-rich and inflammatory plaques, while women are more likely to develop fibrous plaques, based on endothelial dysfunction and phenotypic modulation of VSMC [19]. It is widely accepted that during coronary artery disease (CAD), men develop less stable atherosclerosis plaques than women. In CAD, recent studies have revealed that genes more active in men were associated with the immune system, while genes more active in women were associated with mesenchymal cells and endothelial cells [20]. However, in mice with metabolic syndrome, VSMCs from male donors were more prone to dedifferentiation, i.e. loss of contractile markers (leiomodin 1 and calponin) than the cells from female donors. This loss was induced by thrombospondin-1, the expression of which depended on the level of testosterone [1]. In aortic valve stenosis, which is driven by fibrosis and calcification of the aortic valve leaflets, annexin A2 and cystatin C increased the osteoblast-like differentiation of valve interstitial cells (VICs) from male pigs, while in female VICs, these inflammatory proteins promoted activation to myofibroblasts, manifested by increased expression of genes for α-actin and type 1 collagen [21].
This review summarizes the possibilities of surgical treatment of blood vessel obstruction, especially of small and medium-diameter vessels, mainly by replacing them or bypassing them with synthetic and biological vascular substitutes. The review takes into account the experience of our group in this field, in the context of the worldwide trends in the design of vascular substitutes, especially in recent years (2022–2023), including different approaches to patients according to their sex.
Surgical treatment of blood vessel obstruction and vascular substitutes
Vessels affected by stenosis and obliteration can be treated with endovascular (catheterization) approaches, such as balloon angioplasty and stenting, or with surgical approaches such as endarterectomy of short arterial segments. However, if these approaches fail, it is necessary to replace the damaged vessel - either directly, i.e. by resection and interposition grafting with end-to-end anastomoses, or by bridging the damaged section of the vessel by a so-called by-pass with end-to-side anastomoses, i.e. another patent vascular structure. Important vascular replacements often used in current clinical practice are based on synthetic polymers - mainly on expanded polytetrafluoroethylene (ePTFE) and poly(ethylene terephthalate) (PET), or segmented polyurethane (PU), used mainly for the production of hemodialysis access grafts (for a review, see [22–24]). Synthetic polymeric vascular replacements have been used since 1954 when they were prepared from Vinyon N cloth; chemically poly(vinyl chloride). Vinyon N tubes were first tested on dogs in the form of tubular constructs bridging defects of the abdominal aorta [25], and then they were also used in human patients for replacements of resected aneurysms of the abdominal aorta [26].
However, it should be noted that past and present synthetic vascular replacements have been commonly clinically applied in a cell-free form, i.e. without reconstructed physiological layers of the blood vessel wall. These layers include the tunica intima, i.e. the inner layer containing endothelial cells; the tunica media, i.e. the middle layer formed by smooth muscle cells, and the tunica adventitia, i.e. the outer layer containing fibroblasts, adjacent nerve fibers, and also smaller vessels and capillaries, referred to as vasa vasorum. Synthetic polymeric vascular grafts therefore behave more or less passively – at least they lack an endothelial layer, which in its mature confluent state is considered the best way to prevent graft thrombosis, and they also lack a layer of smooth muscle cells, ensuring the contractile and relaxing function of the vessels.
Expanded PTFE, PET and PU, used for the manufacture of currently clinically used vascular prostheses, are highly hydrophobic polymers, and are therefore primarily unsuitable for the adhesion and growth of cells that require moderately hydrophilic surfaces. However, the blood proteins mediating cell adhesion (e.g. fibronectin, vitronectin) are adsorbed on the inner surface of the prostheses, which is followed by the adhesion of platelets, inflammatory cells and VSMCs. VSMCs reach the vascular substitute from the margins of its anastomosis with the original vessel, from the tunica adventitia, and also arise from VSMC precursors present in the blood (for a review, see [24]). In larger-diameter replacements, the adhesion of platelets, immune cells and VSMCs is made difficult or impossible by the fast and strong blood flow, and such replacements may therefore remain patent for a long time. However, in small-diameter vascular grafts, usually 6 mm or less, these processes result in thrombosis, inflammation, and excessive proliferation of VSMCs in an attempt to regenerate the damage, and finally stenosis and failure of the vascular replacement.
Clinically-used small-diameter vascular replacements mostly include an aortocoronary bypass [27,28], then bypasses of the lower limb, especially below-knee bypasses [29], as well as extracranial-intracranial bypasses, e.g. between the middle cerebral artery and the superficial temporal artery or the vertebral artery [30]. Small-diameter vascular replacements are burdened with the highest incidence of complications, leading to stenosis or complete closure of the replacement. In principle, it can be said that there are currently practically no long-term functional replacements for vessels of small diameter (6 mm and less) on the market. Thus, even approximately 70 years since the first vascular replacements were implanted (1952–1954), the design of functional small-diameter vascular replacements remains literally a “search for the Holy Grail” in the field of vascular tissue engineering [31,32].
The “dead end” of tissue engineering
Some renowned authors believe that vascular tissue engineering has now come to a "dead end" or needs to cross a “death valley” [24]. This is explained by the fact that the leading force in this field is no longer exerted by vascular surgeons. Leading roles have been taken over by basic research scientists who are detached from clinical practice, and have been unaware that the construction small-diameter vascular replacements is no longer the "Holy Grail" of vascular tissue engineering! Endovascular treatments, such as percutaneous transluminal angioplasty and the introduction of an endovascular graft (i.e. a special fabric tube device framed with stainless steel self-expanding stents), are now considered to be sufficient, together with the replacement of damaged blood vessels by autologous arteries, which the patient's organism is considered able to provide in sufficient quantities. There may also be some frustration and disappointment, due to the inability, even after 70 years of designing vascular replacements, to construct functional and durable small-diameter vascular grafts. But should we not still try to construct these replacements - at least to have some backup in cases where endovascular treatment fails (e.g. due to fibrosis and calcifications), or where autologous vessels are not available (in elderly patients with comorbidities), or even just to avoid burdening the patient with additional surgery? Moreover, as Zilla et al. (2020) [24] point out, there is also a need for medium-diameter vascular grafts for dialysis patients, who are rapidly increasing in numbers with the development of medicine in modern times – and constructing these grafts would be another important task for vascular tissue engineering. In addition, vascular replacements are needed not only for the arterial system but also for the venous system – for example, lower limb vein diseases alone are very common, affecting approx. 25 % of adults in westernized societies (for a review, see [33]). Venous grafts are also needed to reconstruct the missing portal system in pediatric patients. For this purpose, human decellularized allogeneous veins recellularized using autologous blood have been used [33].
Autologous vascular replacements
What options do we have for the design of small- or medium-diameter vascular replacements? Autologous vessels are considered the gold standard in this respect. These replacements contain physiological layers of the vessel wall - primarily a continuous layer of mature endothelial cells, which is considered the best way to prevent thrombosis, immune cell activation, and excessive VSMC migration and proliferation. Autologous vessels also contain VSMCs of mature contractile phenotype, providing the contractile and relaxing function of the vascular replacement. Furthermore, autologous replacements are not burdened by the risk of immune reaction and subsequent graft rejection, nor by the risk of pathogen transmission, especially viruses and prions, as is the case with allogeneic or even xenogeneic replacements (for a review, see [22,23,34]). On the other hand, autologous transplanted tissue generally has some serious disadvantages. Firstly, it is usually only available in limited quantities, and secondly, obtaining it places an additional burden on the patient due to the additional surgery and damage to another site in the body. Moreover, certain tissues, when transferred to a new site in the body, have mechanical, biochemical and other properties that do not fully meet the requirements for their new function. A typical example is when venous grafts are used as arterial replacements, e.g. saphenous veins in aortocoronary bypass surgery [28]. These grafts are suddenly exposed to much higher blood pressures than they have been permanently adapted to, leading to endothelial damage, vascular distension, mechanical and biochemical damage to VSMCs and their subsequent efforts to regenerate, manifested by excessive migration, proliferation and proteosynthesis in these cells. All this is accompanied by thrombosis and adhesion of immune cells. The consequence is obvious - again the risk of graft stenosis. Although stenosis is treated in clinical practice by the insertion of a stent inside the vessel, this stent, usually metallic, further aggravates the mechanical damage to the endothelium, and there is also a risk of loosening and migration of the stent through the vessel. If the stent releases a drug that inhibits the unwanted proliferation of VSMCs, such as sirolimus (rapamycin), the endothelium, where proliferation and rapid regeneration of the damage would be desirable, is the first to be affected [35]. More recent approaches, in which our group has also participated, have attempted to replace intravascular stents with perivascular drug-eluting systems that simultaneously "wrap" the venous graft, giving it mechanical support and limiting its undesirable initial distension. In our earlier studies, we constructed a synthetic polymer-based outer vessel coating that released sirolimus. This system significantly limited the proliferation of VSMCs even without the presence of sirolimus - only on the basis of mechanical support - while preserving the viability of VSMCs and not damaging the endothelial layer [36–38]. Nevertheless, even this situation is not ideal, and calls for a search for other new options to replace irreversibly damaged vessels.
History and present of tissue-engineered vascular replacements
New options for replacing irreversibly damaged vessels consist in the creation of small- or medium-diameter vascular grafts by tissue engineering methods, which will reconstruct at least the tunica intima and tunica media of the vascular wall (and optimally also the tunica adventitia). Standard tissue engineering methods include (1) the preparation of a suitable cell carrier or scaffold to act as an ECM analogue to anchor the cells, (2) the cellular component of the newly constructed tissue, and (3) appropriate signals (biochemical and mechanical) to induce the desired cell behavior. Researchers have been attempting to create vascular replacements using tissue engineering methods since the 1980s. The work of Weinberg and Bell (1986) [39], who created an in vitro model of a blood vessel using a tubular matrix of bovine collagen, reinforced with a Dacron (PET) mesh, and populated with bovine adventitial fibroblasts, smooth muscle cells, and endothelial cells, may be considered the most prominent in this regard. To date, however, these efforts have not been crowned with success in achieving routine clinical application of constructs of this type, i.e. with all three layers of the physiological vascular wall reconstructed. Usually, only one layer has been reconstructed on these substitutes - initially, most often the endothelial layer, although attempts have also been made to reconstruct other layers, namely using autologous stem cells and skin fibroblasts.
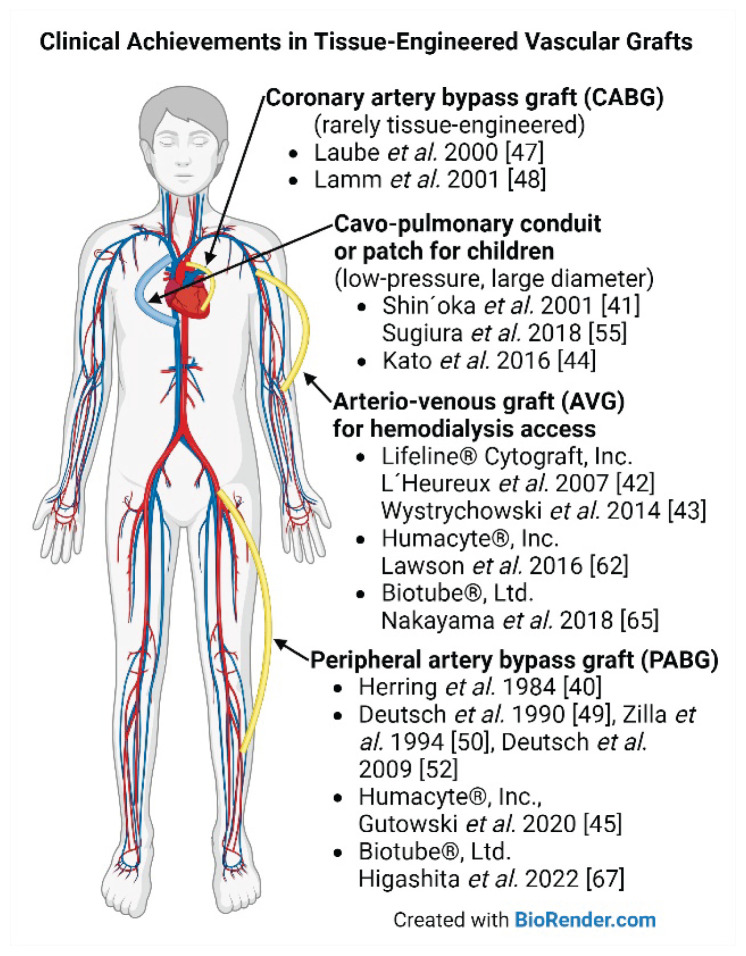
The first clinical use of tissue-engineered vascular grafts was reported by Herring et al. in 1984 [40]. They implanted synthetic vascular prostheses that were in vitro endothelialized in the lumen. The first human implantation of a totally engineered blood vessel (TEBV) without any permanent synthetic support was carried out by Shin’oka et al. (2001) [41] as a pediatric pulmonary artery replacement, i.e., in low-pressure circulation. The first human use of TEBV in high-pressure circulation was performed by L’Heureux et al. (2007) [42] as an arterio-venous (AV) hemodialysis access graft (Lifeline©, Cytograft, Inc.), and Wystrychowski et al. (2014) [43] reported the first implantation of an allogeneic nonliving AV graft within the Lifeline© study. Kato et al. (2016) [44] reported the first clinical application of an in vivo tissue-engineered graft composed of autologous subcutaneous encapsulation tissue (Biotube) used for patch repair of the pulmonary artery in a child. Gutowski et al. (2020; a group of Laura Niklason) [45] first conducted a human trial for peripheral arterial occlusive disease (PAD) with totally bioengineered human acellular vessels (HAV; Humacyte©). As of 2023, HAV is being evaluated in seven trials to treat PAD and vascular trauma, and as a hemodialysis access conduit [46]. The implantation site for clinical evaluation of TEBVs has changed over the years from lower extremity bypass procedures to AV access grafts, for safety reasons and better surveillance opportunities. In the past, only a few trials were conducted on coronary artery bypass grafting [47,48]. Major clinical achievements in tissue engineering of vascular grafts together with landmark publications are summarized in Table 1 and are depicted in Fig. 1. It should be noted that these relatively successful implants have been used within limited clinical trials and are not widely available.
Table 1.
Clinical achievements and landmark publications regarding tissue-engineered vascular grafts. Abbreviations: arterio-venous (AV), bone marrow (BM), endothelial cells (EC), expanded polytetrafluoroethylene (ePTFE), months (M), not applicable (N/A), number of implants (No, n), polycaprolactone (PCL), poly(glycolic acid) (PGA), poly(lactic acid) (PLA), reference(s) (ref.), and vascular smooth muscle cells (VSMC).
| Author, year | Clinical use | Scaffold | Cell type | No | Country | Outcome | Ref. |
|---|---|---|---|---|---|---|---|
| Herring et al. 1984 | Lower extremity bypass | Dacron | Autologous vein EC, one-stage seeding | n = 18 | USA | Improved patency in non-smokers | [40] |
| Deutsch et al. 1990 Zilla et al 1994 Meinhart et al. 1997 Deutsch et al. 2009 |
Lower extremity bypass | ePTFE | Autologous vein EC, two-stage seeding, fibrin-glue | n = 341 | Austria | Improved patency, closing the gap between prosthetic and vein grafts | [49–52] |
| Laube et al. 2000 | Coronary bypass | ePTFE | Autologous EC | n=14 | Germany | Improved patency | [47] |
| Lamm et al. 2001 | Coronary bypass | Allogeneic saphenous vein | Autologous EC | n = 15 | Germany | Patency 80 % at 3M | [48] |
| Shin’oka et al. 2001, 2005 Hibino et al. 2010 Sugiura et al. 2018 |
Cavo-pulmonary conduits in congenital heart disease | Bioabsorbable synthetic polymer: PCL-PLA, PGA | Initially autologous EC; then: BM cells; two-stage seeding | n = 25 grafts n = 19 patches |
Japan | Patent grafts, no aneurysm, rupture, infection, or calcification, reconstituted by host cells, some growth, 7/25 (28 %) stenosis treated with angioplasty | [41,53–55] |
| Bockeria el al. 2017, 2020 | Cavo-pulmonary conduits, in congenital heart disease (Xeltis© Switzerland) | Bioabsorbable synthetic polymer: PCL-2-U-4[1H]-pyrimidinone | N/A | n = 5 | Russia | No graft-related adverse events, endogenous tissue restoration | [56,57] |
| Teebken et al. 2009 | Replacement of iliac vein affected by a tumor | Allogeneic decellularized vena cava | Autologous EC | n = 1 | Germany | Thrombosis at 4M due to recurrent disease and discontinuation of anticoagulation | [58] |
| Olausson et al. 2012, 2014 | Pediatric portal vein Meso-Rex bypass | Allogeneic decellularized iliac vein | Autologous BM or blood cells, two-stage seeding | n = 3 | Sweden | Patent; second graft needed twice | [33,59] |
| L’Heureux et al. 2007 McAllister et al. 2009 Peck et al. 2011 |
AV access for hemodialysis, Lifeline©, Cytograft, USA | N/A | Autologous skin fibroblasts, cell sheets, seeded with vein EC | n = 9 | Argentina, Poland | Primary patency 78 % at 1M, 60 % at 6M, 108 patient-months of use | [42,60,61] |
| Wystrychowski et al. 2014 | AV access for hemodialysis, Lifeline©, Cytograft, USA | N/A | Allogeneic fibroblasts, cell sheets, devitalized | n = 3 | Poland | Primary patency 5M, 7 days (unrelated death), and 3M | [43] |
| Lawson et al. 2016 Kirkton et al. 2019 Jakimowicz et al. 2022 |
AV access for hemodialysis, human acellular vessel, HAV, Humacyte© USA | PGA | Allogeneic VSMC, decellularized | n = 60 | USA, Poland | Secondary patency 89 % at 12M and 58 % at 60M, no infection or aneurysm | [62–64] |
| Gutowski et al. 2020, 2023 | Lower extremity bypass, HAV, Humacyte© USA | PGA | Allogeneic VSMC, decellularized | n = 20 | Poland | Secondary patency 74 % at 48M and 60 % at 72M, no infection or aneurysm | [45,46] |
| Kato et al. 2016 | Pulmonary artery patch | Autologous encapsulation tissue, Biotube | N/A | n = 1 | Japan | Patent graft at 7M | [44] |
| Nakayama et al. 2018, 2020 | AV access for hemodialysis extension, Biotube Co, Ltd | Subcutaneous mandrel implant, in-body tissue architecture | N/A | n = 2 | Japan | Stenosis at 24M and 7M, both treated with angioplasty | [65,66] |
| Higashita et al. 2022 | Lower extremity bypass extension, Biotube Co, Ltd | Subcutaneous mandrel implant, in-body tissue architecture | N/A | n = 1 | Japan | Patent at 12M | [67] |
Fig. 1.
Clinical achievements in tissue engineering of vascular grafts. Created with BioRender.com
However, commonly clinically used constructs that are declared on the market as "tissue-engineered vascular grafts" (TEVG) and that have been approved for current clinical practice include virtually only cell-free products based on decellularized allogeneic (i.e. human) matrices, such as Synergraft® or Cryovein® (CryoLife, Inc, Kennesaw, GA, USA) or on xenogeneic matrices, such as bovine Artegraft® or Procol® (both LeMaitre Vascular, Inc., Burlington, MA, USA; for a review, see [68]).
Several chemically stabilized and/or decellularized matrices are also used to produce vascular patches, i.e., needed to repair vascular defects caused by surgical procedures such as endarterectomy, thrombectomy or embolectomy, after vascular injuries or even after damage to blood vessels due to trauma or cancer. Currently, chemically stabilized matrices derived from bovine pericardium (XenoSure®), porcine pericardium (BioIntegral® NoReact®), or decellularized porcine small intestinal submucosa (SIS, CorMatrix®, Aziyo®) are used for vascular patches, and also synthetic matrices, namely ePTFE (Propaten® Gore®) and PET (Vascutek®), and matrices from autologous vessels, similar to those used for blood vessel replacement. However, synthetic and biological matrices are used in a cell-free form, i.e. in a form that does not fully correspond to the physiological tissue. In addition, biological matrices are often stabilized with glutaraldehyde, making them potentially cytotoxic (for a review, see [23,34]).
Newly-developed vascular grafts based on decellularized matrices
The idea of using decellularized matrices in an acellular form continues to be developed even today. There are at least two important reasons for this. First, the use of decellularized matrices is a kind of "renaissance" of the older idea of using allogeneic or even xenogeneic transplantation of blood vessels and other organs in general. Unlike synthetic substitutes, these biological substitutes have similar mechanical properties, architecture and biochemical composition. However, the main immunogenic factor is the presence of a cellular component. It has been found that by removing this cellular component, i.e. by decellularization, the tissue then loses a substantial part of its immunogenicity - up to 90 % has been reported in the literature [69]. Second, acellular matrices are more easily approved for clinical use than cell-seeded matrices. Seeding matrices with cells usually requires processing the patient tissue outside the operating room, which is tightened prohibitively by new regulations [24]. EU legislation has become so strict that it is virtually impossible to implant constructs colonized with cells, not only allogeneic but also autologous cells from the patient. This is because it is usually necessary to remove the patient's cells from the operating room in order to seed, expand and differentiate them on the tissue-engineered constructs in vitro. Another problem is the cultivation and cryopreservation of cells in media containing components of xenogenic or allogenic origin (e.g. fetal bovine serum, human platelet lysate). EU legislation includes the following standards and regulations: ASTM F3225-17, ISO 10993-1:2018, ISO 7198:2016, EU 2017/745, EU 1394/2007 [70]. Therefore, in clinical practice, cell-free constructs are preferred, which could be colonized by cells only after their implantation into the patient's body. However, we believe that this situation is only temporary and that in the future, after the development of new technologies for the cultivation, preservation and further manipulation of cells, these rules will be adjusted to a more favorable form, tissue engineering will again find a place and all the efforts made for several decades will not be lost and will be crowned with success.
In recent years, decellularized tubular matrices have been prepared e.g. from rat aorta [71], porcine thoracic aorta [72], porcine carotid arteries [73], bovine internal mammary arteries [74], human umbilical artery [75,76], human saphenous vein [77], submillimeter vessels of the human placenta [78], or from rolled human amniotic membrane [79]. Decellularized plant leaves, namely of Viburnum rhytidophyllum, combined with gelatin were also an interesting non-traditional material [80]. The mechanical properties of decellularized matrices have been further improved by crosslinking with genipin, which proved to be better than the traditionally used glutaraldehyde in terms of matrix biocompatibility [81]. In our earlier study, we also achieved similar results with genipin-crosslinked pericardium, which proved to be more advantageous not only in comparison with glutaraldehyde but also in comparison with tannic acid and nordihydroguaiaretic acid [82]. Crosslinking also significantly reduces the residual immunogenicity of the decellularized matrices (for a review, see [24]). To strengthen the mechanical properties of decellularized matrices, they have been used in combination with synthetic polymers, especially degradable polymers such as polycaprolactone (PCL) [71] or poly(L-lactide-co-ɛ-caprolactone) (PLCL) [72]. Attempts have even been made to construct small-diameter blood vessels from synthetic polymers (e.g., PCL) seeded with cells (e.g., dermal fibroblasts) and secondarily decellularized, after the cells have deposited natural ECM proteins onto these polymers [83].
Decellularized matrices are important not only for the construction of small-diameter vascular grafts, but also in the context of Ross surgery. This is a cardiac surgical procedure in which the diseased aortic valve is replaced with the patient's own pulmonary valve, followed by the replacement of the pulmonary valve with a pulmonary homograft (i.e., allograft in more recent terminology). The valved homograft conduit was developed in the late 1960s, but its widespread use was limited by the lack of effective sterilization and preservation methods [84]. Modern cryopreservation methods have extended the shelf life of this conduit, but it is still at risk for degeneration and calcification, which is considered to be a result of immune rejection of this allogeneic implant [85]. In addition, conventional pulmonary homografts lack the additional growth capacity needed in pediatric patients. Therefore, a new and promising alternative approach has been to decellularize these homografts, which was expected to significantly reduce their immunogenicity and provide the possibility for spontaneous recellularization of these grafts with autologous host cells and their further growth [84,85].
In our studies, we also considered direct reconstruction of the aortic valve using decellularized autologous pericardium from the patients [82] or xenogenic (porcine) pericardium [86]. However, pericardium proved to have poor mechanical properties for these purposes - rather it could be used for vascular patches. However, some promising results were obtained in experiments in which autologous pericardium was "trained" in a dynamic bioreactor that stimulated the proliferation of its cellular component as well as the production and maturation of its ECM [87,88].
The biological activity of decellularized scaffolds, including those combined with synthetic polymers, has been further improved by endowing them with various biomolecules, such as heparin [77] or salidroside [72] to increase their antithrombogenic activity, and hepatocyte growth factor [73] or VEGF [74] to increase their spontaneous endothelialization after implantation in experimental animals in vivo, such as rats [71,72,78,79] or rabbits [73]. In such improved vascular replacements, not only spontaneous endothelialization but also regeneration of the smooth muscle layer has often occurred [72,77,83]. However, it is important to note that although these beneficial effects occur in animal models, including large animal models (pigs), they usually do not occur in human patients - especially when the vast majority of patients requiring vascular replacement are already elderly and also suffer from a number of concurrent diseases. In other words, spontaneous endothelialization of vascular replacements cannot be relied upon in human patients, especially in terms of trans-anastomotic endothelial outgrowth or retention of the endothelial cell precursors from the blood. Some hope is placed in the endothelialization of vascular substitutes by a transmural ingrowth of capillaries from the outside into the vascular substitute, facilitated by its porous structure or degradability. However, it should be taken into account that there will not always be enough of these capillaries or small vessels around the implant [24]. In addition, in the case of VSMCs, problems such as their hyperplasia, leading to stenosis of the vascular graft, must be anticipated. This problem has often been addressed by the release of agents inhibiting migration and proliferation of VSMCs, e.g. rapamycin [71], but it would be more physiological to bring the VSMC behavior under control by the presence of a confluent endothelial cell layer and appropriate biochemical and mechanical signals. In other words, to create tissue-engineered vascular grafts for human patients, it is necessary to incorporate the cellular component into the scaffold before it is implanted, i.e. not to wait for secondary spontaneous cell colonization after implantation into the organism.
Recellularization of decellularized matrices, reconstruction of the tunica media
In our studies, we have therefore tried to further develop the idea of using decellularized xenogeneic and allogeneic matrices for creating small-diameter vascular replacements and vascular patches after their preliminary in vitro recellularization. The decellularized tissue can be recellularized with the patient's own cells - especially stem cells. We have investigated the recellularization of decellularized tissues mainly with mesenchymal stem cells (MSCs). These cells can be obtained from patients from their subcutaneous adipose tissue in autologous form, in reasonable quantities and by a relatively minimally invasive method, namely liposuction (for a review, see [89]). This method is less demanding and less painful than, for example, bone marrow aspiration, by which MSCs can also be obtained - many patients undergo liposuction quite voluntarily for aesthetic reasons. The use of adipose tissue-derived stem cells (ADSCs) is generally preferable to the use of already differentiated VSMCs, which may have a lower proliferative capacity, may undergo senescence earlier, and, in particular, would require subcutaneous veins or other vessels to be harvested, which would burden the patient with another demanding surgical procedure. Another source of MSCs for our studies was Wharton's jelly of the umbilical cord. Wharton's jelly stem cells (WJSCs) are usually used in allogeneic form, but they can be obtained in relatively large quantities without any surgery on the patient, and de facto from “biological waste”. Moreover, WJSCs are considered less mature than adult MSCs, i.e. standing on the borderline between pluripotency and multipotency. At least some markers of pluripotency have been detected in them (for a review, see [90]), which offers the possibility to differentiate them into all cell types present in the vascular wall, including endothelial cells. Last but not least, MSCs are generally considered to be poorly immunogenic or even immunomodulatory (for a review, [89]). In our recent study, where decellularized allogeneic or xenogeneic pericardium was recellularized with allogeneic WJSCs and was implanted as vascular patches in laboratory pigs, these WJSCs reduced neo-adventitial inflammatory reaction, patch resorption, as well as neo-intimal hyperplasia on xenografts, suggesting immunomodulatory properties of WJSCs [23].
In our studies, we have used small-diameter porcine arteries for decellularization to create vascular substitutes (Fig. 2) and porcine pericardium to create vascular patches [23,86,91]. The attractiveness of these matrices for stem cell adhesion and growth was enhanced by modifying the matrices with fibrin, further endowed with heparin and VEGF. Differentiation of stem cells towards VSMCs was induced by the appropriate composition of the culture medium, in particular by the presence of transforming growth factor-β1 (TGF-β1) and bone morphogenetic protein (BMP-4), and was further enhanced by mechanical stimulation in a lab-made dynamic bioreactor providing pulsatile pressure stress. The degree of stem cell differentiation towards VSMCs was assessed by the expression of α-actin, calponin-1 and myosin heavy chain at both mRNA and protein levels [23,86,91,92].
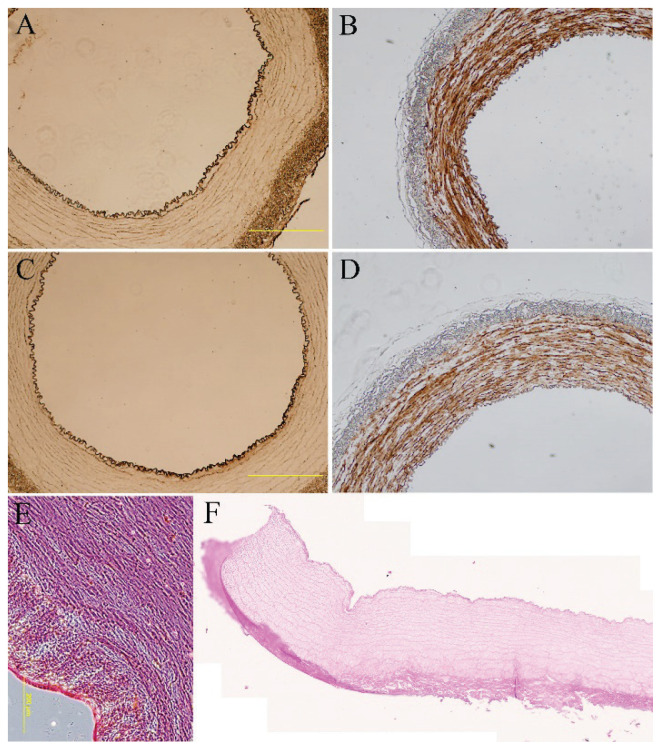
Fig. 2.
Immunohistological staining of von Willebrand factor (A, C) and calponin (B, D) of endothelialized porcine carotid artery that had been decellularized and seeded with human adipose tissue-derived stem cells (A, B) or human bone marrow-derived stem cells (C, D) for 14 days and human umbilical vein endothelial cells for two days of in vitro culture. Hematoxylin-eosin staining of control native (E) and decellularized arteries, tile scan (F). A–E: Olympus IX 71 epifluorescence microscope, DP80 digital camera; A–D: obj.×4, scale bar = 500 μm; E: obj.×10, scale bar = 200 μm; F: ZEISS Axio Scan. Z1 Slide Scanner, obj.×20.
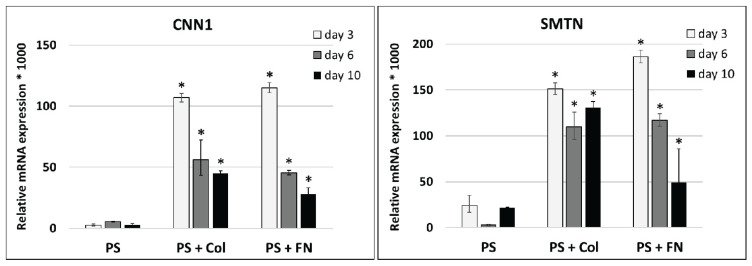
Differentiation of stem cells towards VSMCs can be induced not only by the composition of the culture medium and mechanical stimulation in a dynamic bioreactor, but also by the composition of the cultivation substrate. An interesting finding of our earlier experiments was that coating standard tissue culture polystyrene (PS) with collagen and fibronectin significantly increased the expression of calponin-1 and smoothelin, i.e. an intermediate and a late marker of VSMC differentiation, respectively, in ADSCs in comparison with the pure PS. The differentiation of ADSCs towards VSMCs was induced by a medium with TGF-β1 and BMP-4 (both in a concentration of 2.5 ng/mL). However, the expression of both markers showed a tendency to decrease with culture time (from 3 to 10 days), probably due to negative feedback from the synthesized protein (Fig. 3).
Fig. 3.
The influence of the cultivation substrate on the differentiation of ADSCs towards VSMCs. The correlation of relative mRNA expression of genes for calponin 1 (CNN1) and smoothelin (SMTN) in human adipose-derived stem cells cultivated in differentiation medium on standard tissue culture polystyrene (PS), PS coated with type 1 collagen (PS + Col) or with fibronectin (PS + FN). Measured by real-time PCR. Arithmetic mean ± SD from 2–3 samples for each experimental group and time interval. ANOVA, Student-Newman-Keuls method. Statistical significance: * p≤0.05 in comparison with corresponding control PS samples.
New options for endothelialization of vascular replacements
Pre-seeding decellularized matrices with stem cells facilitated proper endothelialization of these matrices. For this endothelialization under experimental conditions, we used already differentiated endothelial cells such as human umbilical vein endothelial cells (HUVECs). However, for clinical practice, it would be preferable to avoid the direct collection of differentiated endothelial cells from patients, which would again involve surgical removal of a blood vessel, e.g. a subcutaneous vein, followed by relatively demanding isolation of cells, lengthy propagation of the cells, and purification from other cell types. Even after the successful isolation and purification of endothelial cells, the proliferation of these cells takes a relatively long time, especially in elderly patients who are usually burdened by a number of comorbidities. When the endothelial cells are finally sufficiently proliferated, a special dynamic culture system is required to seed them onto the vascular prosthesis, often tens of centimetres long, where they are then cultured until they reach a confluent layer. Indeed, immature, non-confluent and proliferating endothelium can be thrombogenic and immunogenic. The whole process is quite time consuming and, as with any in vitro culture, there is a risk of microbial contamination or pathogen transmission due to the xenogenic components of conventional culture media. For these reasons, this technology referred to as two-stage endothelialization of vascular grafts is not routinely used in clinical practice, although it was developed years ago (for a review, see [22]), and even though Austria was able to comply with the existing strict European regulations and implement the two-stage endothelialization method of a vascular replacement at the Floridsdorf Clinic (see the newspaper article published on October 25, 2020; [93]). Previously, two-stage endothelialization of synthetic vascular prostheses has been clinically tested in infrainguinal prostheses in Vienna, Austria (Hospital Hietzing, [52]), but also at the Université Victor Segalen, Bordeaux, France [94], in Berlin (Charité Hospital, Provitro GmbH; [95]).
In the new era, however, as in the case of VSMCs, we have the opportunity to differentiate endothelial cells from MSCs, which can be isolated from the patient using less invasive methods (e.g., liposuction of subcutaneous fat, from blood or urine, or from the umbilical cord). However, such differentiation is difficult, incomplete or even impossible in the case of MSCs. MSCs are multipotent cells, i.e. (unlike totipotent and pluripotent cells), they are capable of differentiating into only a limited spectrum of cell types. They differentiate reluctantly towards endothelial cells, as common physiological biochemical and mechanical signals in vitro are usually not sufficient to induce polarization, i.e. functional specialization of the cytoplasmic cell membrane, typical for endothelial and epithelial cells (for a review, see [89]).
Direct differentiation of MSCs into endothelial cells by physiological biochemical and mechanical signals could perhaps be possible in WJSCs. Although these cells are counted among the multipotent MSCs, due to their origin from (extra)fetal tissue they are phenotypically more immature and express some markers of pluripotency, such as OCT4, SOX2, NANOG and SSEA4 (for a review, see [90]). Appropriate composition of the culture medium, e.g., the presence of VEGF, EGF, IGF-1, FGF-2, hydrocortisone, heparin and vitamin C, combined with laminar shear stress in a bioreactor, could induce differentiation of WJSCs to endothelial cells. The potential of WJSCs to differentiate into endothelial cells was already shown in a study by Alaminos et al. [96]. Also, in our recent study [23], after implantation of decellularized matrices seeded with WJSCs into a laboratory pig, cells positive for CD31, one of the markers of endothelial cells, were found on these matrices. However, it cannot be excluded that CD31-positive cells migrated to the matrices secondarily from the pig organism, e.g. from the adjacent vascular wall, or were captured from the blood.
For the purposes of vascular tissue engineering, endothelial cells can of course be generated from induced pluripotent stem cells (iPSCs) [97,98]. The iPSCs were first generated in 2006 from mouse embryonic or adult fibroblasts by introducing the genes for OCT4, SOX2, KLF4 and cMyc into these cells using a retroviral system [99]. Later, human dermal fibroblast-derived iPSCs were also generated in this way [100]. In 2007, human iPSCs were also generated from human fibroblasts by introducing genes for OCT4, SOX2, NANOG and LIN28 using a lentiviral system [101]. However, iPSCs generated in this way are burdened with many disadvantages such as low efficiency of the conversion to iPSCs (0.01–0.1 %), incomplete reprogramming, genomic insertion associated with a risk of mutations, tumorigenicity and also immunogenicity of iPSCs, which also hinder their potential introduction into clinical practice. For this reason, modern iPSCs of a new type are currently entering the scene. They are created not by DNA manipulation but by novel RNA technologies - specifically by inserting mRNA encoding transcription factors of pluripotent cells, such as OCT4, KLF4, c-Myc and SOX2, into cells. Moreover, this mRNA can be in the synthetic and self-replicating form (sr-RNA), which eliminates the need for repeated transfection of cells. The sr-RNA encoding transcription factors OCT4, KLF4, SOX2, and cMyc, together with internal ribosome entry site (IRES) and the reporter green fluorescent protein (GFP), were used to generate so-called footprint-free iPSC-derived cardiomyocytes [102,103] or vascular endothelial cells from renal epithelial cells [104]. These cells can be isolated from patients' urine, and thus in an autologous form and by a completely non-invasive method. It could even be assumed that endothelial cells might be generated directly from WJSCs expressing at least some pluripotency markers by introducing sr-RNA encoding directly endothelial markers such as CD31, VEGF receptor 2 (KDR), VE-cadherin or von Willebrand factor. Recently, mRNAs encoding VEGF or runt-related transcription factor 2 (RUNX2), prepared via in vitro transcription, were administered into primary osteoblast-like cells in vitro, derived from cranial bones of neonatal mice, or into rat mandible defects in vivo in order to promote osteogenic cell differentiation, vascularization and healing of the bone defect [105].
Creating vasa vasorum, and spontaneous endothelialization of vascular grafts through these structures
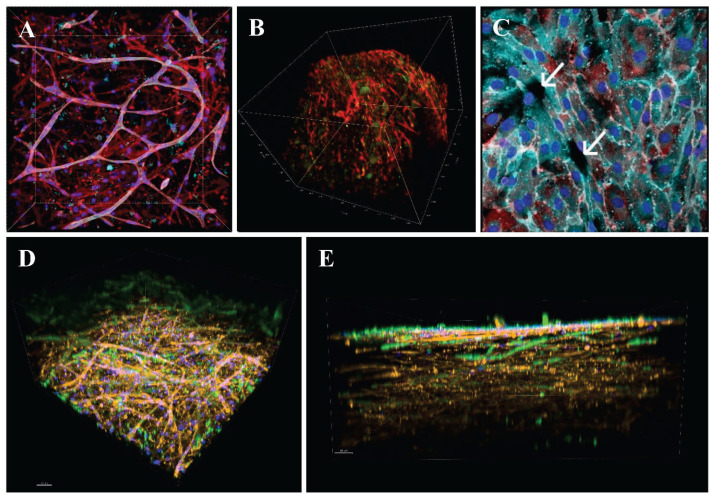
From the preceding text, it follows that from relatively readily available MSCs, such as ADSCs, WJSCs, and also bone marrow MSCs, VSMCs can be directly differentiated by biochemical stimulation with growth factors and other biomolecules and mechanical stimulation in a dynamic bioreactor. In addition, from these MSCs, endothelial cells could be differentiated indirectly, i.e. using sr-RNA or mRNA for endothelial markers. Moreover, we can take advantage of another phenomenon that we have observed in MSCs (specifically ADSCs) and endothelial cells in a three-dimensional collagen hydrogel, namely that these two cell types self-assembled into tubular capillary-like structures in which endothelial cells form an inner layer, and ADSCs attach externally in a pericyte-like manner (Fig. 4A). Interestingly, even the ADSCs themselves were able to form elongated capillary-like structures in the collagen hydrogel environment (Fig. 4B, D). As soon as the capillary-like structures in our experiments reached the surface of the construct, they emerged on it, i.e. they created openings, from which endothelial cells subsequently grew (spread) over the entire surface of the construct and formed a continuous layer on it (Fig. 4C, E). In other words, it would be possible in this way to achieve not only an endothelialized, but also a pre-vascularized blood vessel replacement or patch, i.e., with a pre-formed "vasa vasorum". Thus, we do not have to rely only on transmural vascularization and endothelialization of the vascular substitute after it has been implanted in vivo from its surroundings. Instead, we can move towards these phenomena by pre-vascularization of the construct in vitro. Moreover, spontaneous outflow of newly-formed capillaries can be expected not only on the luminal surface of the vascular substitute or vascular patch, but also on the lateral and external sides. A phenomenon called inosculation, i.e. spontaneous connection of pre-capillaries to capillaries of adjacent tissues and thus to the systemic blood circulation, can then be expected at the sites of pre-capillary outflow [106,107]; for a review, see [24]. If ADSCs are in excess over endothelial cells in the matrix, only a fraction of them will be used for pericyte function, and the remaining fraction may be differentiated towards VSMCs - indeed, pericytes and VSMCs are similar incertain features, e.g. they express α-actin (for a review, see [89,92]). Similar phenomena were observed already in 1962 in the form of the penetration of the full wall thickness of knitted Dacron tubes, implanted into the abdominal aorta of baboons, by sprouting adventitial capillaries, which coalesced with the graft lumen and gave rise to expanding endothelial islands [108]. In another study, endothelial tubes formed in an agarose assay system were followed by multipotent murine embryonic cells, which then differentiated into VSMCs [109]; for a review, see [24].
Fig. 4.
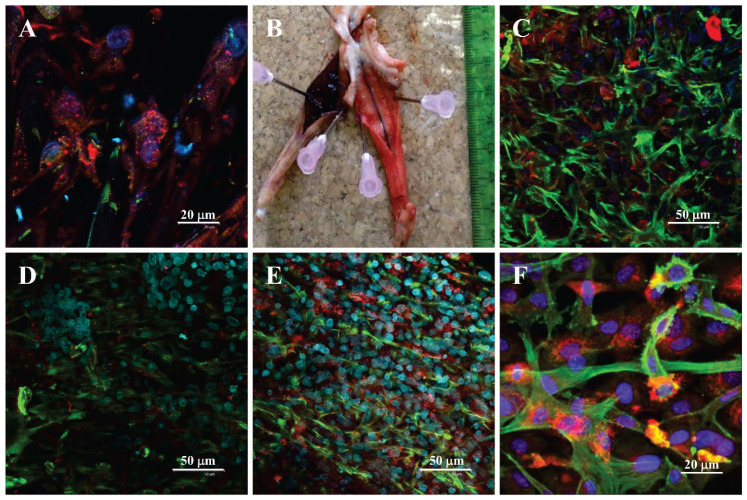
A: Capillary-like network formation in a collagen hydrogel with embedded HUVECs and ADSCs immigrating from an underlying fibrin-modified electrospun nanofibrous polylactide membrane after 14 days in culture. B: Immunofluorescence staining of β-actin (red) and calponin (green) in human ADSCs in collagen gels on day 14 of culture in EGM-2 medium. C: A monolayer of HUVECs originating from the openings (arrows) of capillary-like structures on the surface of the collagen hydrogel. A, C: Both cell types were stained with phalloidin for cytoskeletal F-actin (conjugated with Atto 488; all images; red), and with DAPI for cell nuclei (all images; blue). The CD31 membrane marker of HUVECs was visualized by immunofluorescence (Alexa 633; turquoise). D, E: bottom and side view of the construct, where the collagen hydrogel was enriched with fibronectin (10 μg/ml). Both HUVECs and ADSCs were stained with phalloidin for the cytoskeletal F-actin (red), and with DAPI for the cell nuclei (blue). Von Willebrand factor, a marker of HUVECs, was visualized by immunofluorescence (green). Scale bar 50 μm. A, C, D, E: Dragonfly 503 spinning disk confocal microscope, obj. ×20 (A, D, E), obj. ×40 (C). B: Zeiss Z.1 light-sheet microscope, obj. ×10 for excitation, obj. ×20 for detection, zoom ×0.4 tile scans.
The perspective of hydrogels for creating small-diameter vascular grafts
From the point of view of both pre-vascularization and transmural ingrowth of capillaries, hydrogels appear to be promising for the design of vascular replacements. However, they have rather weak mechanical properties, and are also prone to shrinkage by cells growing inside them. They therefore need to be strengthened and reinforced in some way - for example, with nanoparticles prepared by lyophilization and homogenization of collagen nanofibers created by electrospinning, as we have shown in our recent study [110]. In this study, we also took into account the appropriate composition of the media that would promote the endothelialization and also the differentiation of ADSCs towards VSMCs. In this respect, the so-called external drug delivery system, based on electrospun nanofibrous poly(vinyl alcohol) (PVA) nanofibers, releasing the platelet lysate continuously and for a long period of time, was the best option. Although the differentiation of ADSCs toward VSMCs was slower than in the “classical” differentiation medium for VSMCs containing TGF-β1 and BMP-4, the endothelialization of the construct was more homogeneous and continuous, which was accompanied by a minimum amount of gel shrinkage [110].
Synthetic hydrogels are also promising for the design of small-diameter vascular prostheses. In particular, vascular substitutes made of synthetic hydrogels, such as poly(N-acryloyl glycinamide) with an inner layer of zwitterionic fluorinated hydrogel [111], degradable poly(2-hydroxyethyl methacrylate) [112], and crosslinked PVA [113,114] have recently been experimentally tested. However, these substitutes were often acellular and bioinert in order to prevent the adhesion of platelets, pro-inflammatory cells and bacteria [111,112], or even to limit VSMC hyperplasia [113]. For incorporating a cellular component into these substitutes, such as endothelial cells [113] or VSMCs [115], a synthetic polymer was combined with a natural polymer, such as fucoidan, i.e. a sulfated polysaccharide with a similar structure to that of heparin [114], gelatin, e.g. in a gelatin-methacryloyl hydrogel (GelMa) [115], or a hydrogel prepared from vascular ECM and deposited on PCL scaffolds [116]. The mechanical properties of the hydrogels were further improved with various additives, e.g. graphene [112]. Hydrogel vascular substitutes have also been prepared by a modern method of 3D bioprinting the matrix together with cells [115].
Modern technology I – 3D (bio)printing in creating small-diameter vascular grafts
The modern technology of 3D bioprinting, i.e. simultaneous printing of a matrix, i.e. bioink, together with cells, is currently very popular for the construction of small-diameter vascular grafts, and provides hope for the commercialization of these products. For example, collagen, gelatin, hyaluronic acid, alginate, chitosan, GelMa, and poly(ethylene glycol) diacrylate (PEGDA), have been used as bioinks. Usually, only VSMCs have been printed together with the bioink, whereas endothelial cells have been seeded into the lumen of the tubular structure only secondarily. However, techniques capable of printing endothelial cells into the vascular substitute have already been developed (for a review, see [117]). In a recent study by some members of our group, a porcine collagen hydrogel was printed together with ADSCs [118]. Such constructs could then be used as vascular patches for pre-clinical studies on large animals (pigs), because, as known from other studies by our group, these cells can be differentiated towards VSMCs by an appropriate composition of the culture medium and mechanical training in a bioreactor [23,91].
Even the technology of simple 3D printing of various synthetic and nature-derived polymers is currently used to create small-diameter vascular replacements. For example, these replacements have been created from negatively charged PVA and alginate interpenetrating networks, immersed in a positively-charged chitosan solution. Moreover, these replacements also had antibacterial and angiogenic effects [119]. Antibacterial and angiogenic activity and endothelial regeneration were also promoted in 3D-printed vascular substitutes made of PCL, poly(glycerol sebacate) (PGS) with the addition of bioactive glass [120]. 3D printing technology has often been combined with another advanced technology, namely electrospinning, in the design of vascular prostheses [121,122], including prostheses triple-layered with inner, intermediate and outer layers, simulating the tunica intima, media and adventitia [123]. However, the resulting constructs of both technologies are usually cell-free, and are populated with cells only after they have been implanted into the body of experimental animals, such as sheep [121], dogs [122], or subcutaneously into mice [120], or seeded with HUVECs in vitro [123]. 3D prints of organs and their vasculature are also performed to assist surgeons in imaging and in decision-making in the preparation of live donor organ transplantation [34].
Modern technology II – electrospinning in creating small-diameter vascular grafts
Electrospinning itself is also a very widespread and promising technique for the preparation of small-diameter vascular substitutes. Similarly as for 3D printing, various synthetic and nature-derived polymers and combinations of polymers have been employed for this technology. The synthetic polymers included e.g. PCL [124,125], polylactide [111], poly(1,4-butylene succinate) (PBS) [126], PLCL [127], poly(lactide-co-glycolide) (PLGA) [128], poly-ɛ-caprolactone/polydioxanone (PCL/PDO) [129], poly(dimethylsiloxane) (PDMS) [130] and PU [131], including thermoplastic polyurethane (TPU) and a new self-reinforcing thermoplastic poly(urethane-urea) (TPUU) [132]. The main nature-derived polymers include collagen [133], gelatin [134], tropoelastin [135], PGS [136], chitosan [137] and silk fibroin [138]. The combinations include e.g. PCL with PU [139], PDMS with poly(methyl methacrylate) and TPU [130], PLCL with recombinant humanized collagen [81], and tropoelastin with PGS [135]. Bi-layered scaffolds with an inner electrospun PCL layer promoting the axial alignment of endothelial cells, and a GelMA cast outer layer promoting the circumferential alignment of VSMCs have also been constructed [140]. Another hybrid bi-layered vascular graft comprised the inner PCL/poly (ethylene glycol) methyl ether loaded with VEGF, promoting the adhesion and growth of endothelial cells, and the outer PCL/chitosan layer loaded with PDGF, promoting the differentiation of bone marrow MSCs towards VSMCs [137]. A tri-layered electrospun PCL-collagen/PCL/PCL-gelatin graft imitated the tri-layered architecture of the natural vascular wall and promoted the adhesion of endothelial cells on its inner surface [134].
The endothelialization of electrospun vascular grafts has been further improved by various physical and biochemical modifications. The physical factors include e.g. appropriate hydrophilicity [134], nanofibrous architecture [131] or microgroove patterning [127] of the luminal surface. Biochemical modifications include e.g. the presence of platelet-rich plasma [141], heparin, REDV-containing peptides and VEGF [142], endothelial progenitor cell-binding TPSLEQRTVYAK peptide [125], polydopamine-copper ion complexes, polylysine and Cys-Ala-Gly peptides [127], an adenosine monophosphate-activated protein kinase (AMPK) activator, i.e. 5-aminoimidazole-4-carboxamide ribonu-cleotide (AICAR) [143], and epsin-mimetic endothelium-targeting chimeric (UPI) peptide [144]. Vascular scaffolds based on collagen nanofibers modified with hyaluronic acid oligosaccharides and loaded with apyrase and 5′-nucleotidase enzymes were successfully tested for antithrombosis and in situ endothelialization, although only in a rabbit model [133]. The adhesion, migration and growth of endothelial cells are also improved by loading vascular grafts with nitric oxide, which also prevents the overgrowth of VSMCs and bacterial infection [145]. Electrospun vascular scaffolds can also be loaded with antimicrobial substances, such as cobalt [124] or antibiotics, e.g. tobramycin [128], or anti-inflammatory substances, e.g. those modulating the phenotype of macrophages from pro-inflammatory to anti-inflammatory, such as AICAR mentioned above [143] or MCP-1 [146].
The experimental small-diameter vascular substitutes prepared by electrospinning in the above studies were usually prepared by directly depositing nanofibers around a rotating mandrel, usually metallic, e.g. stainless steel. However, it should be noted that the incorporation of cellular components into tubular structures is relatively difficult and usually requires a dynamic culture system, e.g., a flow-through bioreactor [83]. Therefore, small-diameter vascular substitutes can also be prepared by rolling planar sheets of nanofibers (e.g. PCL) [147] or even other materials (e.g. polymer films fabricated by dropping a polymer solution, such as PU, on water surface [148], after colonizing them with cells. Other important techniques include the self-assembly of elastin-like polypeptides into nanofibrous structures [149]) and so-called scaffold-free tissue engineering, based on cell-sheet technology, i.e. layer-by-layer assembly of continuous cell sheets, containing cells and their ECM, into tubular structures [61,150].
Even classical materials are not completely lost for creating small-diameter vascular grafts
Finally, it is even worth considering materials such as ePTFE and PET for the design of small-diameter vascular replacements. Although these materials are unsuitable for this purpose in their pristine unmodified state, they have long been established for the design of vascular substitutes, and further innovation and modification may have a beneficial effect on their endothelialization and on the reconstruction of the contractile VSMC layer. Recently, the inner surface of small-diameter ePTFE grafts with an inner diameter of 1 mm was modified with heparin and epigallocatechin gallate, while the outer surface was modified with polyethyleneimine and rapamycin. The inner surface then promoted the adhesion and growth of HUVECs, while the outer surface inhibited the proliferation of VSMCs [151]. In another study, the endothelialization of ePTFE vascular prostheses (either in vitro by pre-seeding with endothelial cells or in vivo by capture of endothelial progenitor cells from the blood) was improved by immobilization of antibodies against CD34 on the inner surface of these grafts [152].
In PET grafts, tannic acid-assisted immobilization of Cu2+, carboxybetaine and argatroban improved the blood compatibility, endothelialization and also antimicrobial activity, while the migration and proliferation of VSMCs were attenuated [153]. In this context, it is also necessary to mention the pioneering work of Czech authors (namely Assoc. Prof. Milan Krajíček and his co-workers and followers), who also tried to innovate a material based on polyester (e.g. PET) so that it could be used for the construction of vascular substitutes of small diameter up to 6 mm. When designing small-diameter vascular grafts, these authors tried to mimic the physiological structure of the saphenous vein, which is often used for these grafts. These authors chose a knitted tubular scaffold made of non-resorbable polyester as the basis, which actually represented the tunica media. This scaffold was further enriched with a biologically resorbable component, namely with an inner and outer layer of collagen, which reconstituted the tunica intima and tunica adventitia, respectively. The resulting low-flow, small-diameter vascular grafts were then successfully tested in a large animal model, i.e. a sheep model [154]. These vascular grafts were developed not only on the basis of the commonly used bovine collagen [155], but also on the basis of fish collagen, specifically purified (delipidated) collagen from Czech carp, which was characterized by lower immunogenicity compared to bovine collagen [156] and promoted spontaneous endothelialization of the graft after its implantation in sheep [157]. These novel low-flow, small-diameter composite vascular grafts have also been patented [158].
However, it would also be advisable to introduce physiological cellular components of the vessel wall into these types of vascular substitutes. This task was at least partially undertaken in our work. In our experiments, the inner surface of PET prostheses (VUP Joint-Stock Co., Brno, Czech Republic) was modified with fibrin and fibronectin [159] and was seeded with autologous porcine endothelial cells isolated from the jugular vein, which were expanded in vitro and purified from other cell types. Endothelialization of the grafts was performed in a tubular chamber of a commercially available dynamic culture system (Provitro GmbH, Berlin, Germany), and the grafts were implanted into pigs as iliac-femoral bypasses. On day 22 after implantation, the endothelialized graft was patent, while the unmodified graft was occluded with thrombosis. Immunofluorescence of the grafts after explantation revealed some “contamination” of the endothelial cell layer with VSMCs (Fig. 5). However, the presence of VSMCs gives hope that a neoarterial structure with mature, contractile VSMCs underneath the endothelium will be developed (for a review, see [24]).
Fig. 5.
Performance of a PET vascular prosthesis modified with fibrin and fibronectin, seeded with autologous endothelial cells and implanted into pigs. A: the inner surface pre-seeded with endothelial cells in vitro prior to the implantation; B: the gross morphology of explanted prostheses on day 22 after implantation: left – non-modified, right – pre-endothelialized; C, D, E: proximal, middle and distal parts of the prosthesis, respectively; F: control co-culture of endothelial cells and VSMCs on a glass coverslip. Immunofluorescence stain of von Willebrand factor, a marker of endothelial cells (red) and α-actin, a marker of VSMCs (green). The cell nuclei are counterstained with Hoechst 33342 (blue). Confocal microscope Leica SP2.
Last but not least, even ePTFE and PET (Dacron) are promising for spontaneous endothelialization through vasa vasorum, including capillaries ingrowing transmurally into the vascular replacement from its surroundings after its implantation in vivo. A pre-requisite is that these implants must be thin-walled and of porous structure, with pores of an internal diameter of 60–90 μm, optimally as large as 5000–6000 μm2 (for a review, see [24].
Taking into consideration sex-related differences in vascular tissue engineering
Not only when investigating the causes, the course and the clinical picture of cardiovascular diseases, but also when designing vascular replacements, it is necessary to take into account possible sex-related differences in the response of the organism to the implanted biomaterial and in the behavior of the cellular component of the vascular replacement. For example, in a study performed on PLGA vascular grafts with PLA and PCL sealant, implanted in mice, monocytes and macrophages of male and female recipients responded differently to the biomaterial. This has to be important for further evaluating various biomaterials as well as their translation to the clinic. Males, despite having similar levels of macrophages to females, degrade the implanted biomaterial much more rapidly, while females create more foreign body giant cells and produce more collagen [160]. Also, human pluripotent stem cells (hPSCs), whether embryonic stem cells or induced pluripotent stem cells (iPSCs), behaved differently when derived from male or female donors. Although hPSCs have a similar ability to differentiate towards VSMCs in males and in females, estrogens increase this ability only in females, not in males. Estrogens also promote the synthesis of ECM (collagen type 1 and 3) by hPSCs, and prevent its degradation [161].
Regarding the differentiation of hPSCs towards endothelial cells, male hPSCs efficiently differentiate into CD34+ CD31+ endothelial progenitors using the standard glycogen synthase kinase 3 (GSK3) inhibition protocol, while female hPSCs need the addition of VEGF to this protocol [162]. Another positive effect on endothelial cells is due to estrogens. These hormones mobilize endothelial progenitor cells from the bone marrow, which then have the opportunity to colonize the lumen of blood vessels and differentiate into endothelial cells (for a review, see [163]. Estrogens also stimulate the migration and proliferation of endothelial cells and have numerous other protective effects on these cells and on blood vessels in general, such as activating the synthesis of vasodilators (nitric oxide, hydrogen sulfide, prostacyclin), antioxidant, anti-inflammatory and antithrombotic effects, and reducing the transport of low-density lipoproteins through endothelial cells (for a review, see [4]). However, it should be taken into account that while estrogen can accelerate the endothelialization of denudated arteries or tissue-engineered vascular grafts, tamoxifen does not have this effect [160].
Conclusion and further perspectives
According to some authors, especially clinicians, tissue engineering of small-diameter vascular replacements has reached some kind of "dead end", because even 70 years since the first vascular prostheses were implanted in human patients, there are still no functional small-diameter vascular prostheses on the market. Stenosis and occlusion of small-diameter vessels have to be addressed by endovascular therapies or by the use of autologous vascular grafts. Moreover, neither polymeric nor biological vascular substitutes (decellularized or devitalized) contain a cellular component, especially the inner layer of endothelial cells. Unlike animal models, cell-free vascular substitutes do not spontaneously endothelialize in human patients. Some hope for their endothelialization is seen in the transmural vascularization of porous vascular substitutes by the ingrowth of blood vessels from the surrounding environment. We believe that this vascularization could be aided by pre-vascularization of tissue-engineered vascular constructs already under in vitro conditions by mixing mesenchymal stem cells (MSCs), e.g. derived from adipose tissue, and endothelial cells that are able to self-assemble into pre-capillaries in a 3D environment. Moreover, MSCs are able to differentiate into smooth muscle cells relatively easily by physiological biochemical and mechanical stimulation, and it would be also possible to generate endothelial cells from MSCs using so-called footprint-free iPSCs, obtained by introducing synthetic self-replicating RNA into these cells. Small-diameter vascular replacements could therefore be created by combining new modern tissue engineering techniques such as 3D bioprinting, electrospinning, differentiation of readily available stem cell types and RNA technologies, as well as through personalized medicine that would take into account potential sex and other differences in behavior and in the acceptance of vascular constructs. Even if tissue-engineered blood vessel constructs are not ultimately used as implants in the human body, they can serve as in vitro tissue models for a variety of developmental, physiological, pathophysiological, and pharmacological studies to replace the expensive and less ethical use of laboratory animals in modern 21st-century science according to the 3R principle.
Acknowledgements
This review article, summarizing results from several studies, has been supported by the National Institute for Research of Metabolic and Cardiovascular Diseases project (EXCELES Programme, ID Project No. LX22NPO5104) - funded by the European Union - Next Generation EU. Further support was provided by the Czech Academy of Sciences, Praemium Academiae grant No. AP2202, by the Czech Health Research Council, Ministry of Health of the Czech Republic (grant No. NV19-02-00068), and also by OP JAC Project No. CZ.02.01.01/00/22_008/0004562, of the Ministry of Education, Youth and Sports (MEYS) of the Czech Republic, co-funded by the European Union. We also acknowledge the Light Microscopy Core Facility, Institute of Molecular Genetics, Prague, Czech Republic, supported by MEYS – LM2023050 and RVO – 68378050-KAV-NPUI, for their support with the confocal/widefield/super-resolution imaging/image analysis presented herein. Robin Healey (Czech Technical University in Prague, Czech Republic) is gratefully acknowledged for his language revision of the manuscript.
Abbreviations
- ADSCs
adipose tissue-derived stem cells
- AICAR
5-aminoimidazole-4-carboxamide ribonucleotide
- AMPK
adenosine monophosphate-activated protein kinase
- ANOVA
analysis of variance
- Atto 488
green-fluorescent dye from ATTO-TEC GmbH, Germany, with excitation suited to the 488 nm laser line
- AV
arterio-venous
- BM
bone marrow
- BMP-4
bone morphogenetic protein-4
- CAD
coronary artery disease
- CD
cluster of differentiation
- cMyc
cellular myelocytomatosis oncogene
- CNN1
calponin 1 gene
- Col
collagen
- Cys-Ala-Gly
cysteine-alanine-glycine
- DAPI
4′,6-diamidine-2′-phenylindole dihydrochloride
- DNA
deoxyribonucleic acid
- ECM
extracellular matrix
- EGF
epidermal growth factor
- EGM-2
endothelial cell growth medium-2
- ePTFE
expanded polytetrafluoroethylene
- F-actin
filamentous actin
- FGF
fibroblast growth factor
- FN
fibronectin
- GelMa
gelatin-methacryloyl hydrogel
- GFP
green fluorescent protein
- GSK3
glycogen synthase kinase 3
- HAV
human acellular vessel
- hPSCs
human pluripotent stem cells
- HUVECs
human umbilical vein endothelial cells
- IGF-1
insulin-like growth factor-1
- iPSCs
induced pluripotent stem cells
- IRES
internal ribosome entry site
- KDR
kinase insert domain receptor
- KLF4
Krüppel-like factor 4
- LIN28
Lin-28 homolog A (an RNA-binding protein that binds to and enhances the translation of the insulin-like growth factor 2 mRNA)
- M
months
- M-CSF
macrophage colony-stimulating factor
- MCP-1
monocyte chemoattractant protein-1
- MEYS
Ministry of Education, Youth and Sports
- MMPs
metalloproteases
- mRNA
messenger ribonucleic acid
- MSCs
mesenchymal stem cells
- N/A
not applicable
- NANOG
Tír na nÓg (Irish for "Land of the Young")
- NGF
nerve growth factor
- OCT4
octamer-binding transcription factor 4
- PAD
peripheral arterial occlusive disease
- PBS
poly(1,4-butylene succinate)
- PCL
polycaprolactone
- PCL/PDO
poly-ε-caprolactone/ polydioxanone
- PCR
polymerase chain reaction
- PDGF
platelet-derived growth factor
- PDMS
poly(dimethyl-siloxane)
- PEGDA
poly(ethylene glycol) diacrylate
- PET
poly(ethylene terephthalate)
- PGA
poly(glycolic acid)
- PLA
poly(lactic acid)
- PLCL
poly(L-lactide-co-caprolactone)
- PLGA. poly(lactide-co-glycolide); PS
polystyrene
- PU
polyurethane
- PVA
poly(vinyl alcohol)
- REDV
Arg-Glu-Asp-Val (arginine-glutamic acid-aspartic acid-valine)
- RNA
ribonucleic acid
- RUNX2
runt-related transcription factor 2
- SD
standard deviation
- SHR
spontaneously hypertensive rats
- SMTN
smoothelin gene
- SOX2
(sex determining region Y)-box 2
- SPF
specific pathogen-free
- sr-RNA
synthetic self-replicating ribonucleic acid
- SRY
sex-determining region Y
- SSEA4
stage-specific embryonic antigen-4
- TEBV
totally engineered blood vessel
- TEVG
tissue-engineered vascular grafts
- TGF-β1
transforming growth factor-beta1
- TNFs
tumor necrosis factors
- TPSLEQRTVYAK
Thr-Pro-Ser-Leu-Glu-Gln-Arg-Thr-Val-Tyr-Ala-Lys (threonine-proline-serine-leucine-glutamic acid-gluta-mine-arginine-threonine-valine-tyrosine-alanine-lysine)
- TPU
thermoplastic polyurethane
- TPUU
thermoplastic poly(urethane urea)
- UPI
epsin-mimetic endothelium-targeting chimeric peptide (containing an ubiquitin-interacting motif, a plasma membrane targeting sequence from the Lyn kinase H4 domain, and a tumor homing sequence iRGD)
- VE-cadherin
vascular endothelial cadherin
- VEGF
vascular endothelial growth factor
- VICs
valve interstitial cells
- VSMCs
vascular smooth muscle cells
- WHO
World Health Organization
- WJSCs
Wharton's jelly stem cells
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Gupta S, Khanal S, Bhavnani N, Mathias A, Lallo J, Kiriakou A, Ferrell J, Raman P. Sex-specific differences in atherosclerosis, thrombospondin-1, and smooth muscle cell differentiation in metabolic syndrome versus non-metabolic syndrome mice. Front Cardiovasc Med. 2022;9:1020006. doi: 10.3389/fcvm.2022.1020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Cardiovascular diseases (CVDs) Jun 11, 2021. [accessed December 10, 2023]. https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- 3.OECD. European Observatory on Health Systems and Policies. Czechia: Country Health Profile 2021 OECD; 2021. [accessed December 10, 2023]. https://health.ec.europa.eu/system/files/2021-12/2021_chp_cs_english.pdf . [Google Scholar]
- 4.Robert J. Sex differences in vascular endothelial cells. Atherosclerosis. 2023;384:117278. doi: 10.1016/j.atherosclerosis.2023.117278. [DOI] [PubMed] [Google Scholar]
- 5.Bacakova L, Travnickova M, Filova E, Matějka R, Stepanovska J, Musilkova J, Zarubova J, Molitor M. The role of vascular smooth muscle cells in the physiology and pathophysiology of blood vessels. In: Sakuma K, editor. Muscle Cell and Tissue - Current Status of Research Field. IntechOpen; London, United Kingdom: 2018. pp. 229–256. [DOI] [Google Scholar]
- 6.Loukotová J, Bacáková L, Zicha J, Kunes J. The influence of angiotensin II on sex-dependent proliferation of aortic VSMC isolated from SHR. Physiol Res. 1998;47:501–505. [PubMed] [Google Scholar]
- 7.Ely DL, Falvo J, Dunphy G, Caplea A, Salisbury R, Turner M. The spontaneously hypertensive rat Y chromosome produces an early testosterone rise in normotensive rats. J Hypertens. 1994;12:769–774. doi: 10.1097/00004872-199407000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Ely D, Caplea A, Dunphy G, Daneshvar H, Turner M, Milsted A, Takiyyuddin M. Spontaneously hypertensive rat Y chromosome increases indexes of sympathetic nervous system activity. Hypertension. 1997;29:613–618. doi: 10.1161/01.HYP.29.2.613. [DOI] [PubMed] [Google Scholar]
- 9.Ueda K, Lu Q, Baur W, Aronovitz MJ, Karas RH. Rapid estrogen receptor signaling mediates estrogen-induced inhibition of vascular smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol. 2013;33:1837–1843. doi: 10.1161/ATVBAHA.112.300752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hogg ME, Vavra AK, Banerjee MN, Martinez J, Jiang Q, Keefer LK, Chambon P, Kibbe MR. The role of estrogen receptor α and β in regulating vascular smooth muscle cell proliferation is based on sex. J Surg Res. 2012;173:e1–e10. doi: 10.1016/j.jss.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sivritas D, Becher MU, Ebrahimian T, Arfa O, Rapp S, Bohner A, Mueller CF, Umemura T, Wassmann S, Nickenig G, Wassmann K. Antiproliferative effect of estrogen in vascular smooth muscle cells is mediated by Kruppel-like factor-4 and manganese superoxide dismutase. Basic Res Cardiol. 2011;106:563–575. doi: 10.1007/s00395-011-0174-z. [DOI] [PubMed] [Google Scholar]
- 12.Benditt EP, Benditt JM. Evidence for a monoclonal origin of human atherosclerotic plaques. Proc Natl Acad Sci USA. 1973;70:1753–1756. doi: 10.1073/pnas.70.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearson TA, Kramer EC, Solez K, Heptinstall RH. The human atherosclerotic plaque. Am J Pathol. 1977;86:657–664. [PMC free article] [PubMed] [Google Scholar]
- 14.Benditt EP, Barrett T, McDougall JK. Viruses in the etiology of atherosclerosis. Proc Natl Acad Sci USA. 1983;80:6386–6389. doi: 10.1073/pnas.80.20.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bacakova L, Mares V. Cell kinetics of aortic smooth muscle cells in long-term cultures prepared from rats raised under conventional and spf conditions. Physiol Res. 1995;44:389–398. [PubMed] [Google Scholar]
- 16.Schwartz CJ, Valente AJ, Sprague EA, Kelley JL, Suenram CA, Rozek MM. Atherosclerosis as an inflammatory process: The roles of the monocyte-macrophage a. Ann N Y Acad Sci. 1985;454:115–120. doi: 10.1111/j.1749-6632.1985.tb11849.x. [DOI] [PubMed] [Google Scholar]
- 17.Takahashi K, Takeya M, Sakashita N. Multifunctional roles of macrophages in the development and progression of atherosclerosis in humans and experimental animals. Med Electron Microsc. 2002;35:179–203. doi: 10.1007/s007950200023. [DOI] [PubMed] [Google Scholar]
- 18.Mensah EA, Daneshtalab N, Tabrizchi R. Differential biomechanics in resistance arteries of male compared with female Dahl hypertensive rats. J Hypertens. 2022;40:596–605. doi: 10.1097/HJH.0000000000003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakkers TR, Mokry M, Civelek M, Erdmann J, Pasterkamp G, Diez Benavente E, den Ruijter HM. Sex differences in the genetic and molecular mechanisms of coronary artery disease. Atherosclerosis. 2023;384:117279. doi: 10.1016/j.atherosclerosis.2023.117279. [DOI] [PubMed] [Google Scholar]
- 20.Hartman RJG, Owsiany K, Ma L, Koplev S, Hao K, Slenders L, Civelek M, Mokry M, Kovacic JC, Pasterkamp G, Owens G, Björkegren JLM, den Ruijter HM. Sex-stratified gene regulatory networks reveal female key driver genes of atherosclerosis involved in smooth muscle cell phenotype switching. Circulation. 2021;143:713–726. doi: 10.1161/CIRCULATIONAHA.120.051231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogt BJ, Peters DK, Anseth KS, Aguado BA. Inflammatory serum factors from aortic valve stenosis patients modulate sex differences in valvular myofibroblast activation and osteoblast-like differentiation. Biomater Sci. 2022;10:6341–6353. doi: 10.1039/d2bm00844k. https://doi.org/10.1039/D2BM00844K, https://doi.org/10.1039/D2BM90082C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chlupáč J, Filová E, Bačáková L. Blood vessel replacement: 50 years of development and tissue engineering paradigms in vascular surgery. Physiol Res. 2009:S119–S140. doi: 10.33549/physiolres.931918. [DOI] [PubMed] [Google Scholar]
- 23.Chlupac J, Matejka R, Konarik M, Novotny R, Simunkova Z, Mrazova I, Fabian O, Zapletal M, Pulda Z, Lipensky JF, Stepanovska J, Hanzalek K, Broz A, Novak T, Lodererova A, Voska L, Adla T, Fronek J, Rozkot M, Forostyak S, Kneppo P, Bacakova L, Pirk J. Vascular remodeling of clinically used patches and decellularized pericardial matrices recellularized with autologous or allogeneic cells in a porcine carotid artery model. Int J Mol Sci. 2022;23:3310. doi: 10.3390/ijms23063310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zilla P, Deutsch M, Bezuidenhout D, Davies NH, Pennel T. Progressive reinvention or destination lost? Half a century of cardiovascular tissue engineering. Front Cardiovasc Med. 2020;7:159. doi: 10.3389/fcvm.2020.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Voorhees AB, Jaretzki A, Blakemore AH. The use of tubes constructed from vinyon "n" cloth in bridging arterial defects a preliminary report. Ann Surg. 1952;135:332–336. doi: 10.1097/00000658-195203000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blakemore AH, Voorhers AB. The use of tubes constructed from vinyon "N" cloth in bridging arterial defects-experimental and clinical. Ann Surg. 1954;140:324–334. doi: 10.1097/00000658-195409000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gaudino M, Bakaeen FG, Benedetto U, Di Franco A, Fremes S, Glineur D, Girardi LN, Grau J, Puskas JD, Ruel M, Tam DY, Taggart DP, Antoniades C, Patrono C, Schwann TA, Tatoulis J, Tranbaugh RF. Arterial grafts for coronary bypass: a critical review after the publication of art and radial. Circulation. 2019;140:1273–1284. doi: 10.1161/CIRCULATIONAHA.119.041096. [DOI] [PubMed] [Google Scholar]
- 28.Ghandakly EC, Tipton AE, Bakaeen FG. Pathophysiology and management of saphenous vein graft disease. Expert Rev Cardiovasc. 2023;21:565–572. doi: 10.1080/14779072.2023.2233420. [DOI] [PubMed] [Google Scholar]
- 29.Kim Y, Jung JH, Hwang D, Yun W-S, Huh S, Kim H-K. Below-knee prosthetic bypass is a viable option for limb salvage in patients with extensive femoropopliteal occlusive disease. Vasc Specialist Int. 2023;39:16. doi: 10.5758/vsi.230028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bram R, Almadidy Z, Souter J, Roskova I, Charbel FT. Vertebral artery to middle cerebral artery bypass for flow augmentation. Oper Neurosurg. 2023 doi: 10.1227/ons.0000000000000942. [DOI] [PubMed] [Google Scholar]
- 31.Kakisis JD, Liapis CD, Breuer C, Sumpio BE. Artificial blood vessel: the holy grail of peripheral vascular surgery. J Vasc Surg. 2005;41:349–354. doi: 10.1016/j.jvs.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 32.Eghbalzadeh K, Guschlbauer M, Weber C, Wacker MT, Reinhardt S, Djordjevic I, Sabashnikov A, Maul A, Sterner-Kock A, Wahlers TCW, Scherner M, Wippermann J. Experimental studies for small diameter grafts in an in vivo sheep model-techniques and pitfalls. Thorac Cardiovasc Surg. 2021;69:649–659. doi: 10.1055/s-0039-1687887. [DOI] [PubMed] [Google Scholar]
- 33.Olausson M, Kuna VK, Travnikova G, Bäckdahl H, Patil PB, Saalman R, Borg H, Jeppsson A, Sumitran-Holgersson S. In vivo application of tissue-engineered veins using autologous peripheral whole blood: a proof of concept study. EBioMedicine. 2014;1:72–79. doi: 10.1016/j.ebiom.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fronek J, Chlupac J. Transplantation surgery department at IKEM hospital, Prague, Czech Republic: increasing volume and improving outcomes through innovative clinical strategies and technical approaches. Transplantation. 2023;107:2285–2289. doi: 10.1097/TP.0000000000004633. [DOI] [PubMed] [Google Scholar]
- 35.Chen S, Wang J, Jia F, Shen Z, Zhang W, Wang Y, Ren K, Fu G, Ji J. Bioinspired NO release coating enhances endothelial cells and inhibits smooth muscle cells. J Mater Chem B. 2022;10:2454–2462. doi: 10.1039/D1TB01828K. [DOI] [PubMed] [Google Scholar]
- 36.Filova E, Parizek M, Olsovska J, Kamenik Z, Brynda E, Riedel T, Vandrovcova M, Lisa V, Machova L, Skalsky I, Szarszoi O, Suchy T, Bacakova L. Perivascular sirolimus-delivery system. Int J Pharm Sci. 2011;404:94–101. doi: 10.1016/j.ijpharm.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Skalský I, Filová E, Szárszoi O, Pařízek M, Lytvynets A, Malušková J, Lodererová A, Brynda E, Lisá V, Burdíková Z, Čapek M, Pirk J, Bačáková L. A periadventitial sirolimus-releasing mesh decreased intimal hyperplasia in a rabbit model. Physiol Res. 2011;60:585–588. doi: 10.33549/physiolres.932106. [DOI] [PubMed] [Google Scholar]
- 38.Skalský I, Szárszoi O, Filová E, Pařízek M, Lytvynets A, Malušková J, Lodererová A, Brynda E, Lisá V, Burdíková Z, Čapek M, Pirk J, Bačáková L. A perivascular system releasing sirolimus prevented intimal hyperplasia in a rabbit model in a medium-term study. Int J Pharm Sci. 2012;427:311–319. doi: 10.1016/j.ijpharm.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 39.Weinberg CB, Bell E. A blood vessel model constructed from collagen and cultured vascular cells. Science. 1986;231:397–400. doi: 10.1126/science.2934816. [DOI] [PubMed] [Google Scholar]
- 40.Herring M, Gardner A, Glover J. Seeding human arterial prostheses with mechanically derived endothelium. J Vasc Surg. 1984;1:279–289. https://doi.org/10.1016/0741-5214(84)90059-4, https://doi.org/10.1067/mva.1984.avs0010279. [PubMed] [Google Scholar]
- 41.Shin'oka T, Imai Y, Ikada Y. Transplantation of a tissue-engineered pulmonary artery. N Engl J Med. 2001;344:532–533. doi: 10.1056/NEJM200102153440717. [DOI] [PubMed] [Google Scholar]
- 42.L'Heureux N, McAllister TN, de la Fuente LM. Tissue-engineered blood vessel for adult arterial revascularization. N Engl J Med. 2007;357:1451–1453. doi: 10.1056/NEJMc071536. [DOI] [PubMed] [Google Scholar]
- 43.Wystrychowski W, McAllister TN, Zagalski K, Dusserre N, Cierpka L, L' Heureux N. First human use of an allogeneic tissue-engineered vascular graft for hemodialysis access. J Vasc Surg. 2014;60:1353–1357. doi: 10.1016/j.jvs.2013.08.018. [DOI] [PubMed] [Google Scholar]
- 44.Kato N, Yamagishi M, Kanda K, Miyazaki T, Maeda Y, Yamanami M, Watanabe T, Yaku H. First successful clinical application of the in vivo tissue-engineered autologous vascular graft. Ann Thorac Surg. 2016;102:1387–1390. doi: 10.1016/j.athoracsur.2016.06.095. [DOI] [PubMed] [Google Scholar]
- 45.Gutowski P, Gage SM, Guziewicz M, Ilzecki M, Kazimierczak A, Kirkton RD, Niklason LE, Pilgrim A, Prichard HL, Przywara S, Samad R, Tente B, Turek J, Witkiewicz W, Zapotoczny N, Zubilewicz T, Lawson JH. Arterial reconstruction with human bioengineered acellular blood vessels in patients with peripheral arterial disease. J Vasc Surg. 2020;72:1247–1258. doi: 10.1016/j.jvs.2019.11.056. [DOI] [PubMed] [Google Scholar]
- 46.Gutowski P, Guziewicz M, Ilzecki M, Kazimierczak A, Lawson JH, Prichard HL, Przywara S, Samad R, Tente W, Turek J, Witkiewicz W, Zapotoczny N, Zubilewicz T, Niklason LE. Six-year outcomes of a phase II study of human-tissue engineered blood vessels for peripheral arterial bypass. JVS-Vascular Science. 2023;4:100092. doi: 10.1016/j.jvssci.2022.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laube HR, Duwe J, Rutsch W, Konertz W. Clinical experience with autologous endothelial cell-seeded polytetrafluoroethylene coronary artery bypass grafts. J Thorac Cardiovasc Surg. 2000;120:134–141. doi: 10.1067/mtc.2000.106327. [DOI] [PubMed] [Google Scholar]
- 48.Lamm P, Juchem G, Milz S, Schuffenhauer M, Reichart B. Autologous endothelialized vein allograft: a solution in the search for small-caliber grafts in coronary artery bypass graft operations. Circulation. 2001;104:I108–I114. doi: 10.1161/hc37t1.094527. [DOI] [PubMed] [Google Scholar]
- 49.Deutsch M, Eberl T, Fischlein T, Meinhart J, Minar E, Puschmann R, Schmid P, Zilla P. In vitro endothelialization of ePTFE vascular prostheses in clinical use: preliminary results] Vasa Suppl. 1990;30:219–220. [PubMed] [Google Scholar]
- 50.Zilla P, Deutsch M, Meinhart J, Puschmann R, Eberl T, Minar E, Dudczak R, Lugmaier H, Schmidt P, Noszian I, Fischlein T. Clinical in vitro endothelialization of femoropopliteal bypass grafts: An actuarial follow-up over three years. J Vasc Surg. 1994;19:540–548. doi: 10.1016/S0741-5214(94)70083-4. [DOI] [PubMed] [Google Scholar]
- 51.Meinhart J, Deutsch M, Zilla P. Eight years of clinical endothelial cell transplantation. Closing the gap between prosthetic grafts and vein grafts. ASAIO J. 1997;43:M515–521. doi: 10.1097/00002480-199709000-00034. [DOI] [PubMed] [Google Scholar]
- 52.Deutsch M, Meinhart J, Zilla P, Howanietz N, Gorlitzer M, Froeschl A, Stuempflen A, Bezuidenhout D, Grabenwoeger M. Long-term experience in autologous in vitro endothelialization of infrainguinal ePTFE grafts. J Vasc Surg. 2009;49:352–362. doi: 10.1016/j.jvs.2008.08.101. [DOI] [PubMed] [Google Scholar]
- 53.Shin'oka T, Matsumura G, Hibino N, Naito Y, Watanabe M, Konuma T, Sakamoto T, Nagatsu M, Kurosawa H. Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J Thorac Cardiovasc Surg. 2005;129:1330–1338. doi: 10.1016/j.jtcvs.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 54.Hibino N, McGillicuddy E, Matsumura G, Ichihara Y, Naito Y, Breuer C, Shinoka T. Late-term results of tissue-engineered vascular grafts in humans. J Thorac Cardiovasc Sur. 2010;139:431–436.e1. doi: 10.1016/j.jtcvs.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 55.Sugiura T, Matsumura G, Miyamoto S, Miyachi H, Breuer CK, Shinoka T. Tissue-engineered vascular grafts in children with congenital heart disease: Intermediate term follow-up. J Thorac Cardiovasc Surg. 2018;30:175–179. doi: 10.1053/j.semtcvs.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bockeria LA, Svanidze O, Kim A, Shatalov K, Makarenko V, Cox M, Carrel T. Total cavopulmonary connection with a new bioabsorbable vascular graft: First clinical experience. J Thorac Cardiovasc Surg. 2017;153:1542–1550. doi: 10.1016/j.jtcvs.2016.11.071. [DOI] [PubMed] [Google Scholar]
- 57.Bockeria L, Carrel T, Lemaire A, Makarenko V, Kim A, Shatalov K, Cox M, Svanidze O. Total cavopulmonary connection with a new restorative vascular graft: results at 2 years. J Thorac Dis. 2020;12:4168–4173. doi: 10.21037/jtd-19-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Teebken Puschmann, Rohde Burgwitz, Winkler Pichlmaier, Weidemann Haverich. Human iliac vein replacement with a tissue-engineered graft. Vasa. 2009;38:60–65. doi: 10.1024/0301-1526.38.1.60. [DOI] [PubMed] [Google Scholar]
- 59.Olausson M, Patil PB, Kuna VK, Chougule P, Hernandez N, Methe K, Kullberg-Lindh C, Borg H, Ejnell H, Sumitran-Holgersson S. Transplantation of an allogeneic vein bioengineered with autologous stem cells: a proof-of-concept study. The Lancet. 2012;380:230–237. doi: 10.1016/S0140-6736(12)60633-3. [DOI] [PubMed] [Google Scholar]
- 60.McAllister TN, Maruszewski M, Garrido SA, Wystrychowski W, Dusserre N, Marini A, Zagalski K, Fiorillo A, Avila H, Manglano X, Antonelli J, Kocher A, Zembala M, Cierpka L, de la Fuente LM, L' Heureux N. Effectiveness of haemodialysis access with an autologous tissue-engineered vascular graft: a multicentre cohort study. The Lancet. 2009;373:1440–1446. doi: 10.1016/S0140-6736(09)60248-8. [DOI] [PubMed] [Google Scholar]
- 61.Peck M, Dusserre N, McAllister TN, L' Heureux N. Tissue engineering by self-assembly. Materials Today. 2011;14:218–224. doi: 10.1016/S1369-7021(11)70117-1. [DOI] [Google Scholar]
- 62.Lawson JH, Glickman MH, Ilzecki M, Jakimowicz T, Jaroszynski A, Peden EK, Pilgrim AJ, Prichard HL, Guziewicz M, Przywara S, Szmidt J, Turek J, Witkiewicz W, Zapotoczny N, Zubilewicz T, Niklason LE. Bioengineered human acellular vessels for dialysis access in patients with end-stage renal disease: two phase 2 single-arm trials. The Lancet. 2016;387:2026–2034. doi: 10.1016/S0140-6736(16)00557-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kirkton RD, Santiago-Maysonet M, Lawson JH, Tente WE, Dahl SLM, Niklason LE, Prichard HL. Bioengineered human acellular vessels recellularize and evolve into living blood vessels after human implantation. Sci Transl Med. 2019;11:eaau6934. doi: 10.1126/scitranslmed.aau6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jakimowicz T, Przywara S, Turek J, Pilgrim A, Macech M, Zapotoczny N, Zubilewicz T, Lawson JH, Niklason LE. Five year outcomes in patients with end stage renal disease who received a bioengineered human acellular vessel for dialysis access. EJVES Vascular Forum. 2022;54:58–63. doi: 10.1016/j.ejvsvf.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nakayama Y, Kaneko Y, Okumura N, Tatsumi E. Long-term follow up of first-in-human study in bypass of stenosis av shunt by an autologous in-body-tissue-engineered (biotube) vascular graft. Eur Heart J. 2018:39. doi: 10.1093/eurheartj/ehy565.P2664. [DOI] [Google Scholar]
- 66.Nakayama Y, Kaneko Y, Okumura N, Terazawa T. Initial 3-year results of first human use of an in-body tissue-engineered autologous "Biotube" vascular graft for hemodialysis. J Vasc Access. 2020;21:110–115. doi: 10.1177/1129729819852550. [DOI] [PubMed] [Google Scholar]
- 67.Higashita R, Miyazaki M, Oi M, Ishikawa N. First-in-human results of an in-body tissue architecture-induced tissue-engineered vascular graft "Biotube" for application in distal bypass for chronic limb-threatening ischemia. J Vasc Surg Cases. 2022;8:488–493. doi: 10.1016/j.jvscit.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carrabba M, Madeddu P. Current strategies for the manufacture of small size tissue engineering vascular grafts. Front Bioeng Biotechnol. 2018;6:41. doi: 10.3389/fbioe.2018.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boccafoschi F, Botta M, Fusaro L, Copes F, Ramella M, Cannas M. Decellularized biological matrices: an interesting approach for cardiovascular tissue repair and regeneration. J Tissue Eng Regen Med. 2017;11:1648–1657. doi: 10.1002/term.2103. [DOI] [PubMed] [Google Scholar]
- 70.Bergmeister H, Podesser BK. Preclinical in vivo assessment of tissue engineered vascular grafts and selection of appropriate animal models. In: Walpoth BH, Bergmeister H, Bowlin GL, Kong D, Rotmans JI, Zilla P, editors. Tissue-Engineered Vascular Grafts. Springer International Publishing; Cham: 2020. pp. 63–93. [DOI] [Google Scholar]
- 71.Yang Y, Lei D, Zou H, Huang S, Yang Q, Li S, Qing F-L, Ye X, You Z, Zhao Q. Hybrid electrospun rapamycin-loaded small-diameter decellularized vascular grafts effectively inhibit intimal hyperplasia. Acta Biomater. 2019;97:321–332. doi: 10.1016/j.actbio.2019.06.037. [DOI] [PubMed] [Google Scholar]
- 72.Shi J, Teng Y, Li D, He J, Midgley AC, Guo X, Wang X, Yang X, Wang S, Feng Y, Lv Q, Hou S. Biomimetic tri-layered small-diameter vascular grafts with decellularized extracellular matrix promoting vascular regeneration and inhibiting thrombosis with the salidroside. Materials Today Bio. 2023;21:100709. doi: 10.1016/j.mtbio.2023.100709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cai Z, Tan Z, Tian R, Chen X, Miao P, Yao C, Wang C, Yu Z, Gu Y. Acellular vascular scaffolds preloaded with heparin and hepatocyte growth factor for small-diameter vascular grafts might inhibit intimal hyperplasia. Cell Transplant. 2022;31:096368972211345. doi: 10.1177/09636897221134541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xie X, Wu Q, Liu Y, Chen C, Chen Z, Xie C, Song M, Jiang Z, Qi X, Liu S, Tang Z, Wu Z. Vascular endothelial growth factor attenuates neointimal hyperplasia of decellularized small-diameter vascular grafts by modulating the local inflammatory response. Front Bioeng Biotechnol. 2022;10:1066266. doi: 10.3389/fbioe.2022.1066266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gou K, Hu J-J, Baek S. Mechanical characterization of human umbilical arteries by thick-walled models: Enhanced vascular compliance by removing an abluminal lining. J Mech Behav Biomed Mater. 2023;142:105811. doi: 10.1016/j.jmbbm.2023.105811. [DOI] [PubMed] [Google Scholar]
- 76.Wong V, Gada S, Singh M, Merna N. The development of small-caliber vascular grafts using human umbilical artery: an evaluation of methods. Tissue Eng Part C Methods. 2023;29:1–10. doi: 10.1089/ten.tec.2022.0144. [DOI] [PubMed] [Google Scholar]
- 77.Tardalkar KR, Marsale TB, Bhamare NC, Kshersagar JR, Patil JK, Adnaik A, Joshi MG. Heparin coated decellularized xenogeneic small diameter vascular conduit for vascular repair with early luminal reendothelialization. Cell Tissue Bank. 2023;24:449–469. doi: 10.1007/s10561-022-10046-0. [DOI] [PubMed] [Google Scholar]
- 78.Falkner F, Mayer SA, Thomas B, Zimmermann SO, Walter S, Heimel P, Thiele W, Sleeman JP, Bigdeli AK, Kiss H, Podesser BK, Kneser U, Bergmeister H, Schneider KH. Acellular human placenta small-diameter vessels as a favorable source of super-microsurgical vascular replacements: a proof of concept. Bioengineering. 2023;10:337. doi: 10.3390/bioengineering10030337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang B, Wang X, Kenneth A, Drena A, Pacheco A, Kalvin L, Ibrahim E-S, Rossi PJ, Thatcher K, Lincoln J. Developing small-diameter vascular grafts with human amniotic membrane: long-term evaluation of transplantation outcomes in a small animal model. Biofabrication. 2023;15:025004. doi: 10.1088/1758-5090/acb1da. [DOI] [PubMed] [Google Scholar]
- 80.Gorbenko N, Rinaldi G, Sanchez A, Merna N. Small-caliber vascular grafts engineered from decellularized leaves and cross-linked gelatin. Tissue Eng Part A. 2023;29:397–409. doi: 10.1089/ten.tea.2022.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang H, Xiao Y, Fang Z, Zhang Y, Yang L, Zhao C, Meng Z, Liu Y, Li C, Han Q, Feng Z. Fabrication and performance evaluation of PLCL-hCOLIII small-diameter vascular grafts crosslinked with procyanidins. Int J Biol Macromol. 2023;251:126293. doi: 10.1016/j.ijbiomac.2023.126293. [DOI] [PubMed] [Google Scholar]
- 82.Filová E, Staňková L, Eckhardt A, Svobodová J, Musílková J, Pala J, Hadraba D, Brynda E, Koňařík M, Pirk J, Bačáková L. Modification of human pericardium by chemical crosslinking. Physiol Res. 2020;69:49–59. doi: 10.33549/physiolres.934335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guo J, Huang J, Lei S, Wan D, Liang B, Yan H, Liu Y, Feng Y, Yang S, He J, Kong D, Shi J, Wang S. Construction of rapid extracellular matrix-deposited small-diameter vascular grafts induced by hypoxia in a bioreactor. ACS Biomater Sci Eng. 2023;9:844–855. doi: 10.1021/acsbiomaterials.2c00809. [DOI] [PubMed] [Google Scholar]
- 84.Huyan Y, Chang Y, Song J. Application of homograft valved conduit in cardiac surgery. Front Cardiovasc Med. 2021;8:740871. doi: 10.3389/fcvm.2021.740871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sievers H-H, Stierle U, Schmidtke C, Bechtel M. Decellularized pulmonary homograft (SynerGraft) for reconstruction of the right ventricular outflow tract: first clinical experience. Zeitschrift für Kardiologie. 2003;92:53–59. doi: 10.1007/s00392-003-0883-x. [DOI] [PubMed] [Google Scholar]
- 86.Filova E, Steinerova M, Travnickova M, Knitlova J, Musilkova J, Eckhardt A, Hadraba D, Matejka R, Prazak S, Stepanovska J, Kucerova J, Riedel T, Brynda E, Lodererova A, Honsova E, Pirk J, Konarik M, Bacakova L. Accelerated in vitro recellularization of decellularized porcine pericardium for cardiovascular grafts. Biomed Mater. 2021;16:025024. doi: 10.1088/1748-605X/abbdbd. [DOI] [PubMed] [Google Scholar]
- 87.Straka F, Schornik D, Masin J, Filova E, Mirejovsky T, Burdikova Z, Svindrych Z, Chlup H, Horny L, Vesely J, Pirk J, Bacakova L. A new approach to heart valve tissue engineering based on modifying autologous human pericardium by 3D cellular mechanotransduction. J Biomater Tissue Eng. 2017;7:527–543. doi: 10.1166/jbt.2017.1598. [DOI] [Google Scholar]
- 88.Straka F, Schornik D, Masin J, Filova E, Mirejovsky T, Burdikova Z, Svindrych Z, Chlup H, Horny L, Daniel M, Machac J, Skibová J, Pirk J, Bacakova L. A human pericardium biopolymeric scaffold for autologous heart valve tissue engineering: cellular and extracellular matrix structure and biomechanical properties in comparison with a normal aortic heart valve. J Biomat Sci-Polym E. 2018;29:599–634. doi: 10.1080/09205063.2018.1429732. [DOI] [PubMed] [Google Scholar]
- 89.Bacakova L, Zarubova J, Travnickova M, Musilkova J, Pajorova J, Slepicka P, Kasalkova NS, Svorcik V, Kolska Z, Motarjemi H, Molitor M. Stem cells: their source, potency and use in regenerative therapies with focus on adipose-derived stem cells - a review. Biotechnol Adv. 2018;36:1111–1126. doi: 10.1016/j.biotechadv.2018.03.011. [DOI] [PubMed] [Google Scholar]
- 90.Garzon I, Chato-Astrain J, Campos F, Fernandez-Valades R, Sanchez-Montesinos I, Campos A, Alaminos M, D' Souza RN, Martin-Piedra MA. Expanded differentiation capability of human Wharton's jelly stem cells toward pluripotency: a systematic review. Tissue Eng Part B Rev. 2020;26:301–312. doi: 10.1089/ten.teb.2019.0257. [DOI] [PubMed] [Google Scholar]
- 91.Matějka R, Koňařík M, Štěpanovská J, Lipenský J, Chlupáč J, Turek D, Pražák Š, Brož A, Šimůnková Z, Mrázová I, Forostyak S, Kneppo P, Rosina J, Bačáková L, Pirk J. Bioreactor processed stromal cell seeding and cultivation on decellularized pericardium patches for cardiovascular use. Appl Sci. 2020;10:5473. doi: 10.3390/app10165473. [DOI] [Google Scholar]
- 92.Bacakova L, Travnickova M, Filova E, Matejka R, Stepanovska J, Musilkova J, Zarubova J, Molitor M. Vascular smooth muscle cells (VSMCs) in blood vessel tissue engineering: the use of differentiated cells or stem cells as VSMC precursors. In: Sakuma K, editor. Muscle Cell and Tissue - Current Status of Research Field. IntechOpen; 2018. [DOI] [Google Scholar]
- 93.MedMedia. Relatus Med. Erster Bypass mit gezüchteten Zellen von Wiener Ärzten implantiert. Oct 25, 2020. [accessed April 19, 2024]. https://www.medmedia.at/relatus-med/erster-bypass-mit-gezuechteten-zellen-von-wiener-aerzten-implantiert/
- 94.Bordenave L, Fernandez P, Rémy-Zolghadri M, Villars S, Daculsi R, Midy D. In vitro endothelialized ePTFE prostheses: clinical update 20 years after the first realization. Clin Hemorheol Microcirc. 2005;33:227–234. [PubMed] [Google Scholar]
- 95.Rademacher A, Paulitschke M, Meyer R, Hetzer R. Endothelialization of PTFE vascular grafts under flow induces significant cell changes. Int J Artif Organs. 2001;24:235–242. doi: 10.1177/039139880102400412. [DOI] [PubMed] [Google Scholar]
- 96.Alaminos M, Pérez-Köhler B, Garzón I, García-Honduvilla N, Romero B, Campos A, Buján J. Transdifferentiation potentiality of human wharton's jelly stem cells towards vascular endothelial cells. J Cell Physiol. 2010;223:640–647. doi: 10.1002/jcp.22062. [DOI] [PubMed] [Google Scholar]
- 97.Boonkaew B, Suwanpitak S, Pattanapanyasat K, Sermsathanasawadi N, Wattanapanitch M. Efficient generation of endothelial cells from induced pluripotent stem cells derived from a patient with peripheral arterial disease. Cell Tissue Res. 2022;388:89–104. doi: 10.1007/s00441-022-03576-2. [DOI] [PubMed] [Google Scholar]
- 98.Fan X, Cyganek L, Nitschke K, Uhlig S, Nuhn P, Bieback K, Duerschmied D, El-Battrawy I, Zhou X, Akin I. Functional characterization of human induced pluripotent stem cell-derived endothelial cells. Int J Mol Sci. 2022;23:8507. doi: 10.3390/ijms23158507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 100.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 101.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 102.Steinle H, Weber M, Behring A, Mau-Holzmann U, von Ohle C, Popov A-F, Schlensak C, Wendel HP, Avci-Adali M. Reprogramming of urine-derived renal epithelial cells into ipscs using srrna and consecutive differentiation into beating cardiomyocytes. Mol Ther Nucleic Acids. 2019;17:907–921. doi: 10.1016/j.omtn.2019.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Weber M, Fech A, Jäger L, Steinle H, Bühler L, Perl RM, Martirosian P, Mehling R, Sonanini D, Aicher WK, Nikolaou K, Schlensak C, Enderle MD, Wendel HP, Linzenbold W, Avci-Adali M. Hydrojet-based delivery of footprint-free iPSC-derived cardiomyocytes into porcine myocardium. Sci Rep. 2020;10:16787. doi: 10.1038/s41598-020-73693-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Weber J, Weber M, Feile A, Schlensak C, Avci-Adali M. Development of an in vitro blood vessel model using autologous endothelial cells generated from footprint-free hipscs to analyze interactions of the endothelium with blood cell components and vascular implants. Cells. 2023;12:1217. doi: 10.3390/cells12091217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang M, Fukushima Y, Nozaki K, Nakanishi H, Deng J, Wakabayashi N, Itaka K. Enhancement of bone regeneration by coadministration of angiogenic and osteogenic factors using messenger RNA. Inflamm Regener. 2023;43:32. doi: 10.1186/s41232-023-00285-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tremblay P-L, Hudon V, Berthod F, Germain L, Auger FA. Inosculation of tissue-engineered capillaries with the host's vasculature in a reconstructed skin transplanted on mice. Am J Transplant. 2005;5:1002–1010. doi: 10.1111/j.1600-6143.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 107.Phua QH, Han HA, Soh B-S. Translational stem cell therapy: vascularized skin grafts in skin repair and regeneration. J Transl Med. 2021;19:83. doi: 10.1186/s12967-021-02752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Florey HW, Greer SJ, Kiser J, Poole JC, Telander R, Werthessen NT. The development of the pseudointima lining fabric grafts of the aorta. Br J Exp Pathol. 1962;43:655–660. [PMC free article] [PubMed] [Google Scholar]
- 109.Hirschi KK, Rohovsky SA, D'Amore PA. Cell-cell interactions in vessel assembly: a model for the fundamentals of vascular remodelling. Transpl Immunol. 1997;5:177–178. doi: 10.1016/S0966-3274(97)80034-2. [DOI] [PubMed] [Google Scholar]
- 110.Filova E, Supova M, Eckhardt A, Vrbacky M, Blanquer A, Travnickova M, Knitlova J, Suchy T, Ryglova S, Braun M, Burdikova Z, Schätz M, Jencova V, Lisnenko M, Behalek L, Prochazkova R, Sedlacek R, Kubasova K, Bacakova L. Adipose-derived stem cells in reinforced collagen gel: a comparison between two approaches to differentiation towards smooth muscle cells. Int J Mol Sci. 2023;24:5692. doi: 10.3390/ijms24065692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li S, Zhao F, Tang Y, Zhang Y, Rong H, Liu L, Gao R, Liu X, Huangfu Y, Bai Y, Feng Z, Guo Z, Dong A, Wang W, Kong D, Huang P. Bioinspired, anticoagulative, 19 f mri-visualizable bilayer hydrogel tubes as high patency small-diameter vascular grafts. Small. 2023;19:2302621. doi: 10.1002/smll.202302621. [DOI] [PubMed] [Google Scholar]
- 112.Moura D, Pereira AT, Ferreira HP, Barrias CC, Magalhães FD, Bergmeister H, Gonçalves IC. Poly(2-hydroxyethyl methacrylate) hydrogels containing graphene-based materials for blood-contacting applications: From soft inert to strong degradable material. Acta Biomater. 2023;164:253–268. doi: 10.1016/j.actbio.2023.04.031. [DOI] [PubMed] [Google Scholar]
- 113.Fallon ME, Le HH, Bates NM, Yao Y, Yim EKF, Hinds MT, Anderson DEJ. Hemocompatibility of micropatterned biomaterial surfaces is dependent on topographical feature size. Front Physiol. 2022;13:983187. doi: 10.3389/fphys.2022.983187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yao Y, Zaw AM, Anderson DEJ, Jeong Y, Kunihiro J, Hinds MT, Yim EKF. Fucoidan and topography modification improved in situ endothelialization on acellular synthetic vascular grafts. Bioact Mater. 2023;22:535–550. doi: 10.1016/j.bioactmat.2022.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jin Q, Yu C, Xu L, Zhang G, Ju J, Hou R. Combined light-cured and sacrificial hydrogels for fabrication of small-diameter bionic vessels by 3D bioprinting. Technol Health Care. 2023;31:1203–1213. doi: 10.3233/THC-220393. [DOI] [PubMed] [Google Scholar]
- 116.Tu C, Zhang Y, Xiao Y, Xing Y, Jiao Y, Geng X, Zhang A, Ye L, Gu Y, Feng Z. Hydrogel-complexed small-diameter vascular graft loaded with tissue-specific vascular extracellular matrix components used for tissue engineering. Biomater Adv. 2022;142:213138. doi: 10.1016/j.bioadv.2022.213138. [DOI] [PubMed] [Google Scholar]
- 117.Yang GH, Kang D, An S, Ryu JY, Lee K, Kim JS, Song M-Y, Kim Y-S, Kwon S-M, Jung W-K, Jeong W, Jeon H. Advances in the development of tubular structures using extrusion-based 3D cell-printing technology for vascular tissue regenerative applications. Biomater Res. 2022;26:73. doi: 10.1186/s40824-022-00321-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stepanovska J, Supova M, Hanzalek K, Broz A, Matejka R. Collagen bioinks for bioprinting: a systematic review of hydrogel properties, bioprinting parameters, protocols, and bioprinted structure characteristics. Biomedicines. 2021;9:1137. doi: 10.3390/biomedicines9091137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu H, Liu Z, Wei Y, Hu Y, Zhao L, Wang L, Liang Z, Lian X, Chen W, Wang J, Yu Z, Ma X, Huang D. Complexation-induced resolution enhancement pleiotropic small diameter vascular constructs with superior antibacterial and angiogenesis properties. Adv Healthcare Materials. 2023;12:2301809. doi: 10.1002/adhm.202301809. [DOI] [PubMed] [Google Scholar]
- 120.Alasvand N, Behnamghader A, Milan PB, Simorgh S, Mobasheri A, Mozafari M. Tissue-engineered small-diameter vascular grafts containing novel copper-doped bioactive glass biomaterials to promote angiogenic activity and endothelial regeneration. Materials Today Bio. 2023;20:100647. doi: 10.1016/j.mtbio.2023.100647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fukunishi T, Best CA, Sugiura T, Opfermann J, Ong CS, Shinoka T, Breuer CK, Krieger A, Johnson J, Hibino N. Preclinical study of patient-specific cell-free nanofiber tissue-engineered vascular grafts using 3-dimensional printing in a sheep model. J Thorac Cardiovasc Surg. 2017;153:924–932. doi: 10.1016/j.jtcvs.2016.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Atari M, Saroukhani A, Manshaei M, Bateni P, Zargar Kharazi A, Vatankhah E, Haghjooy Javanmard S. Preclinical in vivo assessment of a cell-free multi-layered scaffold prepared by 3D printing and electrospinning for small-diameter blood vessel tissue engineering in a canine model. Biomater Sci. 2023;11:6871–6880. doi: 10.1039/D3BM00642E. [DOI] [PubMed] [Google Scholar]
- 123.Hu Q, Shen Z, Zhang H, Liu S, Feng R, Feng J, Ramalingam M. Designed and fabrication of triple-layered vascular scaffold with microchannels. J Biomat Sci-Polym E. 2021;32:714–734. doi: 10.1080/09205063.2020.1864083. [DOI] [PubMed] [Google Scholar]
- 124.Huang Z, Zhang Y, Liu R, Li Y, Rafique M, Midgley AC, Wan Y, Yan H, Si J, Wang T, Chen C, Wang P, Shafiq M, Li J, Zhao L, Kong D, Wang K. Cobalt loaded electrospun poly(ε-caprolactone) grafts promote antibacterial activity and vascular regeneration in a diabetic rat model. Biomaterials. 2022;291:121901. doi: 10.1016/j.biomaterials.2022.121901. [DOI] [PubMed] [Google Scholar]
- 125.Yan H, Cheng Q, Si J, Wang S, Wan Y, Kong X, Wang T, Zheng W, Rafique M, Li X, He J, Midgley AC, Zhu Y, Wang K, Kong D. Functionalization of in vivo tissue-engineered living biotubes enhance patency and endothelization without the requirement of systemic anticoagulant administration. Bioact Mater. 2023;26:292–305. doi: 10.1016/j.bioactmat.2023.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Miceli GC, Palumbo FS, Bonomo FP, Zingales M, Licciardi M. Polybutylene succinate processing and evaluation as a micro fibrous graft for tissue engineering applications. Polymers. 2022;14:4486. doi: 10.3390/polym14214486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yi B, Zhou B, Song Z, Yu L, Wang W, Liu W. Step-wise CAG@PLys@PDA-Cu2+ modification on micropatterned nanofibers for programmed endothelial healing. Bioact Mater. 2023;25:657–676. doi: 10.1016/j.bioactmat.2022.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rosalia M, Grisoli P, Dorati R, Chiesa E, Pisani S, Bruni G, Genta I, Conti B. Influence of electrospun fibre secondary morphology on antibiotic release kinetic and its impact on antimicrobic efficacy. Int J Mol Sci. 2023;24:12108. doi: 10.3390/ijms241512108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Obiweluozor FO, Kayumov M, Kwak Y, Cho H-J, Park C-H, Park J, Jeong Y-J, Lee D-W, Kim D-W, Jeong I-S. Rapid remodeling observed at mid-term in-vivo study of a smart reinforced acellular vascular graft implanted on a rat model. J Biol Eng. 2023;17:1. doi: 10.1186/s13036-022-00313-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zizhou R, Khoshmanesh K, Wang X, Houshyar S. Fabrication of nanocomposites with high elasticity and strength for the load-bearing layer of small-diameter vascular grafts. ACS Appl Mater Interfaces. 2023;15:35411–35421. doi: 10.1021/acsami.3c02397. [DOI] [PubMed] [Google Scholar]
- 131.Łopianiak I, Rzempołuch W, Civelek M, Cicha I, Ciach T, Butruk-Raszeja BA. Multilayered blow-spun vascular prostheses with luminal surfaces in Nano/Micro range: the influence on endothelial cell and platelet adhesion. J Biol Eng. 2023;17:20. doi: 10.1186/s13036-023-00337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rohringer S, Grasl C, Ehrmann K, Hager P, Hahn C, Specht SJ, Walter I, Schneider KH, Zopf LM, Baudis S, Liska R, Schima H, Podesser BK, Bergmeister H. Biodegradable, self-reinforcing vascular grafts for in situ tissue engineering approaches. Adv Healthcare Materials. 2023;12:2300520. doi: 10.1002/adhm.202300520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jia W, Liu L, Li M, Zhou Y, Zhou H, Weng H, Gu G, Xiao M, Chen Z. Construction of enzyme-laden vascular scaffolds based on hyaluronic acid oligosaccharides-modified collagen nanofibers for antithrombosis and in-situ endothelialization of tissue-engineered blood vessels. Acta Biomater. 2022;153:287–298. doi: 10.1016/j.actbio.2022.09.041. [DOI] [PubMed] [Google Scholar]
- 134.Lu X, Zou H, Liao X, Xiong Y, Hu X, Cao J, Pan J, Li C, Zheng Y. Construction of PCL-collagen@PCL@PCL-gelatin three-layer small diameter artificial vascular grafts by electrospinning. Biomed Mater. 2023;18:015008. doi: 10.1088/1748-605X/aca269. [DOI] [PubMed] [Google Scholar]
- 135.Wang Z, Mithieux SM, Vindin H, Wang Y, Zhang M, Liu L, Zbinden J, Blum KM, Yi T, Matsuzaki Y, Oveissi F, Akdemir R, Lockley KM, Zhang L, Ma K, Guan J, Waterhouse A, Pham NTH, Hawkett BS, Shinoka T, Breuer CK, Weiss AS. Rapid regeneration of a neoartery with elastic lamellae. Advanced Materials. 2022;34:2205614. doi: 10.1002/adma.202205614. https://doi.org/10.1002/adma.202270323, https://doi.org/10.1002/adma.202205614. [DOI] [PubMed] [Google Scholar]
- 136.Wang Z, Zhang M, Liu L, Mithieux SM, Weiss AS. Polyglycerol sebacate-based elastomeric materials for arterial regeneration. J Biomedical Materials Res. 2023:jbm.a.37583. doi: 10.1002/jbm.a.37583. [DOI] [PubMed] [Google Scholar]
- 137.Sultana T, Fahad MAA, Park M, Kwon SH, Lee B-T. Physicochemical, in vitro and in vivo evaluation of VEGF loaded PCL-mpeg and PDGF loaded PCL-chitosan dual layered vascular grafts. J Biomed Mater Res B Appl Biomater. 2024;112:e35325. doi: 10.1002/jbm.b.35325. [DOI] [PubMed] [Google Scholar]
- 138.Settembrini A, Buongiovanni G, Settembrini P, Alessandrino A, Freddi G, Vettor G, Martelli E. In-vivo evaluation of silk fibroin small-diameter vascular grafts: state of art of preclinical studies and animal models. Front Surg. 2023;10:1090565. doi: 10.3389/fsurg.2023.1090565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou S-Y, Li L, Xie E, Li M-X, Cao J-H, Yang X-B, Wu D-Y. Small-diameter PCL/PU vascular graft modified with heparin-aspirin compound for preventing the occurrence of acute thrombosis. Int J Biol Macromol. 2023;249:126058. doi: 10.1016/j.ijbiomac.2023.126058. [DOI] [PubMed] [Google Scholar]
- 140.Alkazemi H, Huang T, Mail M, Lokmic-Tomkins Z, Heath DE, O'Connor AJ. Spontaneous orthogonal alignment of smooth muscle cells and endothelial cells captures native blood vessel morphology in tissue-engineered vascular grafts. ACS Appl Mater Interfaces. 2023;15:34631–34641. doi: 10.1021/acsami.3c08511. [DOI] [PubMed] [Google Scholar]
- 141.Li G, Yang T, Liu Y, Su H, Liu W, Fang D, Jin L, Jin F, Xu T, Duan C. The proteins derived from platelet-rich plasma improve the endothelialization and vascularization of small diameter vascular grafts. Int J Biol Macromol. 2023;225:574–587. doi: 10.1016/j.ijbiomac.2022.11.116. [DOI] [PubMed] [Google Scholar]
- 142.Tang Y, Yin L, Gao S, Long X, Du Z, Zhou Y, Zhao S, Cao Y, Pan S. A small-diameter vascular graft immobilized peptides for capturing endothelial colony-forming cells. Front Bioeng Biotechnol. 2023;11:1154986. doi: 10.3389/fbioe.2023.1154986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Su H, Liu W, Li X, Li G, Guo S, Liu C, Yang T, Ou C, Liu J, Li Y, Wei C, Huang Q, Xu T, Duan C. Cellular energy supply for promoting vascular remodeling of small-diameter vascular grafts: a preliminary study of a new strategy for vascular graft development. Biomater Sci. 2023;11:3197–3213. doi: 10.1039/D2BM01338J. [DOI] [PubMed] [Google Scholar]
- 144.Changizi S, Sameti M, Bazemore GL, Chen H, Bashur CA. Epsin mimetic upi peptide delivery strategies to improve endothelization of vascular grafts. Macromol Biosci. 2023;23:2300073. doi: 10.1002/mabi.202300073. [DOI] [PubMed] [Google Scholar]
- 145.Kabirian F, Baatsen P, Smet M, Shavandi A, Mela P, Heying R. Carbon nanotubes as a nitric oxide nano-reservoir improved the controlled release profile in 3D printed biodegradable vascular grafts. Sci Rep. 2023;13:4662. doi: 10.1038/s41598-023-31619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cheng C, Li H, Liu J, Wu L, Fang Z, Xu G. Mcp-1-loaded poly( L -lactide-co-caprolactone) fibrous films modulate macrophage polarization toward an anti-inflammatory phenotype and improve angiogenesis. ACS Biomater Sci Eng. 2023;9:4356–4367. doi: 10.1021/acsbiomaterials.3c00476. [DOI] [PubMed] [Google Scholar]
- 147.Zhang C, Cha R, Wang C, Chen X, Li Z, Xie Q, Jia L, Sun Y, Hu Z, Zhang L, Zhou F, Zhang Y, Jiang X. Red blood cell membrane-functionalized Nanofibrous tubes for small-diameter vascular grafts. Biomaterials. 2023;297:122124. doi: 10.1016/j.biomaterials.2023.122124. [DOI] [PubMed] [Google Scholar]
- 148.Li S, Yang L, Zhao Z, Wang J, Lv H, Yang X. Fabrication of mechanical skeleton of small-diameter vascular grafts via rolling on water surface. Biomed Mater. 2023;18:035002. doi: 10.1088/1748-605X/acb89a. [DOI] [PubMed] [Google Scholar]
- 149.Natsume K, Nakamura J, Sato K, Ohtsuki C, Sugawara-Narutaki A. Biological properties of self-assembled nanofibers of elastin-like block polypeptides for tissue-engineered vascular grafts: platelet inhibition, endothelial cell activation and smooth muscle cell maintenance. Regen Biomater. 2023;10:rbac111. doi: 10.1093/rb/rbac111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu W, Yao M, He M, Chen S, Lu Q. Precise preparation of a multilayer tubular cell sheet with well-aligned cells in different layers to simulate native arteries. ACS Appl Mater Interfaces. 2023;15:19966–19975. doi: 10.1021/acsami.3c00471. [DOI] [PubMed] [Google Scholar]
- 151.Ding K, Yu X, Wang D, Wang X, Li Q. Small diameter expanded polytetrafluoroethylene vascular graft with differentiated inner and outer biomacromolecules for collaborative endothelialization, anti-thrombogenicity and anti-inflammation. Colloids Surf B Biointerfaces. 2023;229:113449. doi: 10.1016/j.colsurfb.2023.113449. [DOI] [PubMed] [Google Scholar]
- 152.Zhang Q, Duncan S, Szulc DA, De Mestral C, Kutryk MJ. Development of a universal, oriented antibody immobilization method to functionalize vascular prostheses for enhanced endothelialization for potential clinical application. J Biol Eng. 2023;17:37. doi: 10.1186/s13036-023-00356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Miao C, Wang L, Shang Y, Du M, Yang J, Yuan J. Tannic acid-assisted immobilization of copper(ii), carboxybetaine, and argatroban on poly(ethylene terephthalate) mats for synergistic improvement of blood compatibility and endothelialization. Langmuir. 2022;38:15683–15693. doi: 10.1021/acs.langmuir.2c02508. [DOI] [PubMed] [Google Scholar]
- 154.Špaček M, Chlup H, Mitáš P, Veselý J, Lambert L, Mlček M, Krajíček M, Lindner J, Grus T. Three-layer collagen-based vascular graft designed for low-flow peripheral vascular reconstructions. J Appl Biomed. 2019;17:52–52. doi: 10.32725/jab.2019.002. [DOI] [PubMed] [Google Scholar]
- 155.Grus T, Lambert L, Mlcek M, Chlup H, Honsova E, Spacek M, Burgetova A, Lindner J. In vivo evaluation of short-term performance of new three-layer collagen-based vascular graft designed for low-flow peripheral vascular reconstructions. Biomed Res Int. 2018;2018:1–7. doi: 10.1155/2018/3519596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lambert L, Novakova M, Lukac P, Cechova D, Sukenikova L, Hrdy J, Mlcek M, Chlup H, Suchy T, Grus T. Evaluation of the immunogenicity of a vascular graft covered with collagen derived from the european carp (cyprinus carpio) and bovine collagen. Biomed Res Int. 2019;2019:1–8. doi: 10.1155/2019/5301405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mitas P, Grus T, Lambert L, Mlcek M, Chlup H, Honsova E, Dohnalova M, Suchy T, Burgetova A, Lindner J, Spacek M. The influence of purification of carp collagen used in a novel composite graft with sandwich construction of the wall on its biological properties and graft patency rates. Physiol Res. 2019;68:603–610. doi: 10.33549/physiolres.934117. [DOI] [PubMed] [Google Scholar]
- 158.Grus T, Chlup H, Mlček M, Beran M. Czech patent. Industrial Property Office of the Czech Republic, Registration Number: CZ308556B6. Application number: CZ2017427A, Registennred: 26.7.2017, Granted: 19.10.2020; Kompozitní cévní náhrada a způsob její výroby (Composite vascular replacement and the method of its production) [Google Scholar]
- 159.Chlupáč J, Filová E, Riedel T, Houska M, Brynda E, Remy-Zolghadri M, Bareille R, Fernandez P, Daculsi R, Bourget C, Bordenave L, Bačáková L. Attachment of human endothelial cells to polyester vascular grafts: pre-coating with adhesive protein assemblies and resistance to short-term shear stress. Physiol Res. 2014;63:167–177. doi: 10.33549/physiolres.932577. [DOI] [PubMed] [Google Scholar]
- 160.Blum KM, Roby LC, Zbinden JC, Chang Y-C, Mirhaidari GJM, Reinhardt JW, Yi T, Barker JC, Breuer CK. Sex and Tamoxifen confound murine experimental studies in cardiovascular tissue engineering. Sci Rep. 2021;11:8037. doi: 10.1038/s41598-021-87006-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Li Y, Wen Y, Green M, Cabral EK, Wani P, Zhang F, Wei Y, Baer TM, Chen B. Cell sex affects extracellular matrix protein expression and proliferation of smooth muscle progenitor cells derived from human pluripotent stem cells. Stem Cell Res Ther. 2017;8:156. doi: 10.1186/s13287-017-0606-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Randolph LN, Bao X, Oddo M, Lian XL. Sex-dependent VEGF expression underlies variations in human pluripotent stem cell to endothelial progenitor differentiation. Sci Rep. 2019;9:16696. doi: 10.1038/s41598-019-53054-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gammill HS, Lin C, Hubel CA. Endothelial progenitor cells and preeclampsia. Front Biosci. 2007;12:2383. doi: 10.2741/2240. [DOI] [PubMed] [Google Scholar]