Abstract
Stroke is a common cerebrovascular disease with high morbidity, mortality, and disability worldwide. Post-stroke dysfunction is related to the death of neurons and impairment of synaptic structure, which results from cerebral ischemic damage. Currently, transcranial magnetic stimulation (TMS) techniques are available to provide clinically effective interventions and quantitative diagnostic and prognostic biomarkers. The development of TMS has been 40 years and a range of repetitive TMS (rTMS) protocols are now available to regulate neuronal plasticity in many neurological disorders, such as stroke, Parkinson disease, psychiatric disorders, Alzheimer disease, and so on. Basic studies in an animal model with ischemic stroke are significant for demonstrating potential mechanisms of neural restoration induced by rTMS. In this review, the mechanisms were summarized, involving synaptic plasticity, neural cell death, neurogenesis, immune response, and blood–brain barrier (BBB) disruption in vitro and vivo experiments with ischemic stroke models. Those findings can contribute to the understanding of how rTMS modulated function recovery and the exploration of novel therapeutic targets.
Graphical Abstract
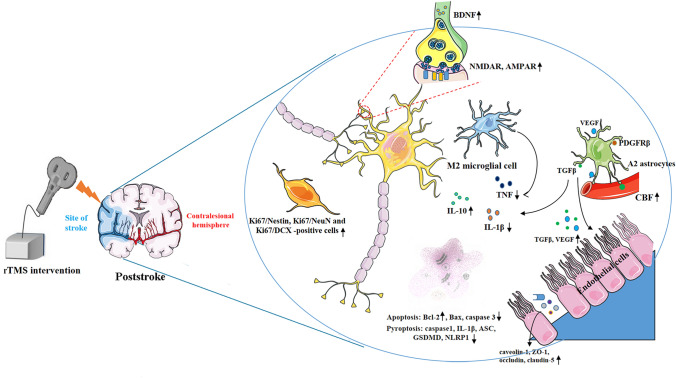
The mechanisms of rTMS in treating ischemic stroke from animal models. rTMS can prompt synaptic plasticity by increasing NMDAR, AMPAR and BDNF expression; rTMS can inhibit pro-inflammatory cytokines TNF and facilitate the expression of anti-inflammatory cytokines IL-10 by shifting astrocytic phenotypes from A1 to A2, and shifting microglial phenotypes from M1 to M2; rTMS facilitated the release of angiogenesis-related factors TGFβ and VEGF in A2 astrocytes, which can contribute to vasculogenesis and angiogenesis; rTMS can suppress apoptosis by increasing Bcl-2 expression and inhibiting Bax, caspase-3 expression; rTMS can also suppress pyroptosis by decreasing caspase-1, IL-1β, ASC, GSDMD and NLRP1 expression. rTMS, repetitive transcranial magnetic stimulation; NMDAR, N-methyl-D-aspartic acid receptors; AMPAR: α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors; BDNF, brain-derived neurotrophic factor; VEGF, vascular endothelial growth factor; GSDMD: cleaved Caspase-1 cleaves Gasdermin D; CBF: cerebral blood flow.
Keywords: rTMS, Synaptic plasticity, Ischemic stroke, Neurogenesis, Blood–brain barrier
Introduction
Stroke is a common cerebrovascular disease with high morbidity, mortality, and disability worldwide. Each year, there were 13.7 million stroke cases worldwide, 5.5 million of whom die and as many suffered permanent loss of function due to debilitation (Collaborators 2019). As reported by the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD), stroke was the second-ranked leading cause of disability-adjusted life-years (DALYs) in both age groups 50–74 years and 75 years (Diseases and Injuries 2020). The clinical characteristics of stroke patients include dysfunction of cognitive and motor, language disorders, pain, as well as negative mood, which finally impacts the daily life and social ability in patients (Aras et al. 2020; Scuteri et al. 2020; Lodha et al. 2021; Sadlonova et al. 2021). Thus, stroke carries a huge financial burden on individuals and society because of the requirement of full-time care and hospitalization for stroke patients with severe symptoms.
In the clinic, the exploration of effective strategies for treating stroke has gained the focus of world attention. Tissue plasminogen activator is the only existing effective therapy for acute ischemic stroke. Its application is restricted because of a narrow time window (National Institute of Neurological and Stroke rt 1995). There are no effective therapies to restore function of patients via reversing the impaired brain tissue during the recovery period of stroke. Currently, the best management of stroke is to lower initial impact, avoid further development of the complication, and facilitate the maximization of functional ability by extensive physiotherapy. Thereby, it is an urgent problem to explore an effective and safe treatment for improving functional dysfunction after stroke.
Recently, non-invasive brain stimulation (NIBS) is of great current interest, including repetitive transcranial magnetic stimulation (rTMS), transcranial direct current stimulation (tDCS), and motor cortex stimulation (MCS). rTMS is an indolent strategy used to stimulate the brain regions through the intact skull using magnetic fields (Hallett 2007). As a beneficial neurorehabilitative strategy, it can increase functional recovery both in the acute and subacute phases of ischemic stroke patients (Kim et al. 2014; Blesneag et al. 2015; Tosun et al. 2017). It is effective to improve locomotor deficiency and daily activities, reduce depression and anxiety, and alleviate dysphagia in stroke patients (Frey et al. 2020; Pitts et al. 2020).
According to the stimulation frequency, the effect caused by TMS or rTMS on concurrent cerebral areas is either exciting or inhibiting (Chervyakov et al. 2018; Kim and Yim 2018; Du et al. 2019). Commonly, high-frequency (HF) stimulation (> 5 Hz) induces facilitatory effects, whereas low-frequency (LF) stimulation (≤ 1 Hz) produces inhibitory effects. Theta burst stimulation (TBS) is the HF TMS protocol and is based on the endogenous neural theta rhythm (5 Hz) (Wang et al. 2019). The TBS paradigm includes continuous TBS (cTBS) and intermittent TBS (iTBS). The cTBS typically causes a depressive effect on cortical excitability and the trains of bursts are delivered continuously for 20–40 s. ITBS generally results in a facilitatory effect and its trains are delivered for only 2–8 s pauses (Cardenas-Morales et al. 2010). Individual motor performance depends on the degree of cortical spinal tract (CST) damage, interhemispheric connectivity, and motor cortex excitability (Volz et al. 2015). Normalization of contralesional intracortical excitability, the integrity of ipsilesional CST, and activation of the ipsilesional hemisphere were significantly related to post-stroke motor recovery by rTMS over different cerebral regions (Ameli et al. 2009; Mello et al. 2015; Li et al. 2016).
The beneficial effect of TMS has been observed in an ischemic animal model, including reduced neurological scores, motor threshold (MT), and infarct areas in rats (Feng et al. 2005). By using resting-state functional magnetic resonance imaging, HF rTMS stimulation over the ipsilesional sensorimotor cortex and affected limb increased regional brain activities in the sensorimotor cortex of the ipsilateral hemisphere, whereas decreased brain activities in the sensorimotor cortex of the contralateral hemisphere (Gao et al. 2020). The effects of HF rTMS over the ipsilesional hemisphere are associated with serval molecular mechanisms, including ischemic tolerance, neurogenesis, angiogenesis, anti-apoptosis, inflammation, injury response, or neuroplasticity (Boonzaier et al. 2018; Caglayan et al. 2019). Summarizing basic research findings contributes to the understanding of how rTMS modulated function recovery and the exploration of novel therapeutic targets.
Search Strategy
In this Narrative Review, the relevant abstracts and articles published from inception to June 2022 were searched from the MEDLINE (PubMed), EMBASE, Web of Science. The search terms were (transcranial magnetic stimulation or theta-burst stimulation) and (stroke or cerebral ischemia or ischemic stroke) and the concerned disease is ischemic stroke. The studies in which objects were patients were excluded.
The Effect of rTMS on Changes of Synaptic Plasticity
Synaptic plasticity plays an important role in TMS-induced function recovery after stroke. As reported by a previous study, the dendritic density, total dendritic length, and the number of dendritic branching points were remarkably increased in the undamaged motor cortex after TMS intervention (Mei et al. 2006). Post-synaptic density and synaptic curvatures also significantly increased, whereas the synapse cleft width was significantly lowered (Mei et al. 2006). In the photothrombotic (PT) rat model, rTMS over the ipsilesional primary motor cortex (M1) significantly alleviated neuronal degeneration and synaptic loss in the cortical region surrounding infarct tissue, of which mechanisms were related to inhibiting overexpression of pro-inflammatory cytokines, reactive micro/astrogliosis, oxidative neuronal damage, and oxidative stress (Hong et al. 2020). Thus, TMS can induce significant changes in the structure, number, and function of synapses after stroke.
The rTMS can induce synaptic plasticity by regulating α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPAR) and N-methyl-d-aspartate-receptor (NMDAR) in an animal model with stroke. The number and subunit composition of those receptors are not static but change in response to synaptic strength, sensory experiences or neuronal activity (Greger et al. 2002; Paoletti et al. 2013). AMPARs are tetrameric assemblies of subunits GluR1-4, and the majority are GluR1/2 and GluR2/3 heteromers (Hanley 2014). Most neuronal AMPARs contain GluR2 subunit, which is impermeable to Ca2+ and determines the critical biophysical properties of the receptor (Isaac et al. 2007; Hanley 2014). Thus, the change in AMPA receptor Ca2+ permeability shows significant influences on synaptic transmission and intracellular signaling events. In stroke model with middle cerebral artery occlusion, rats with rTMS stimulation over ipsilesional sensorimotor cortex exhibited obvious upregulation in post-synaptic density protein 95 (PSD 95), glutamate receptor 2/3 (mGlu 2/3R), and synapsin-1 (SYN) in the ipsilateral sensorimotor cortex (Gao et al. 2020).
The activation of GluN2A-containin NMDARs might contribute to neuronal survival, whereas activation of GluN2B-containing NMDARs can induce neuronal death in the early period of stroke (Ge et al. 2020). Owing to the ischemia-induced reduction of NMDARs, it is critical for neuroprotection to normalize the activities of NMDARs post-stroke (Liu et al. 2010). ITBS intervention can significantly improve the cognitive impairments caused by cerebral small vessel disease in stroke-prone renovascular hypertensive rats. The associated mechanisms were likely related to the increased expression of NMDAR subunits NMDAR1, NMDAR2A and NMDAR2B, and AMPAR subunit GluR1, and p-CaMKIIα in the hippocampus. (Cai et al. 2020). The combination of brain stimulation (transcranial direct current stimulation and rTMS) and minocycline intervention also can increase neuroprotective effect by increasing neuronal and synaptic activity by activation of NMDAR (Alam et al. 2016). Conversely, blockade of NMDARs can weaken iTBS-reduced activation of inhibitory interneuron subtypes expressing GAD67 and PV (fast spiking vs. non-fast spiking subtypes) (Labedi et al. 2014). Thus, the effects of rTMS are deeply related to synaptic plasticity changes by increasing the expression of NMDARs.
Brain-derived neurotrophic factor (BDNF) also participated in mechanisms of rTMS-induced synaptic plasticity after ischemic stroke (Zhang et al. 2007; Cui et al. 2020). It is a neurotrophic factor and is necessary for learning and memory by supporting neuronal growth and driving AMPARs to synapses (Keifer 2022). BDNF can facilitate the regeneration of endothelial progenitor cells by modulating the complex changes in the microRNAs expression (He et al. 2018). A previous study also reported that BDNF exerts neuroprotection by modulating NMDAR/Ca2+ -dependent signaling (Lau et al. 2015). In ischemic rats induced by the two-vessel occlusion, rTMS can improve learning and memory function through facilitating BDNF expression. Subsequently, rTMS restored cholinergic system activity by increasing choline acetyltransferase and acetylcholinesterase activity, and enhancing the density of cholinergic neurons in the hippocampus of rats (Zhang et al. 2018). In the oxygen–glucose deprivation (OGD) model of primary neurons or the cerebral ischemic rat model, the expression of BDNF was upregulated in the cortex after rMS or pairing TMS intervention (Gao et al. 2020; Li et al. 2020). Furthermore, the increased methyl CpG binding protein 2 (MeCP2) phosphorylation can prompt the interaction between BDNF exon IV and receptor for activated C kinase 1 (RACK1), which might be the mechanism of TMS-induced synaptic plasticity (Li et al. 2020).
rTMS can induce the change of synaptic associated genes in an animal model with stroke. By bioinformatics analysis, the major functional category in the hippocampus of rats with rTMS was involved in chemical synaptic transmission regulation. Several differentially expressed genes (DEGs), such as Dlx6, Calb2, Zic1, Crhr2, and Gng4, were enhanced as verified by quantitative Real-Time PCR (qPCR) (Hong et al. 2021). In those associated genes, calretinin (Calb2) is associated with the formation of learning and memory by regulating long-term potentiation. Calb2 also plays an important role in cognitive function in mice with Alzheimer disease (Camp and Wijesinghe 2009; Coronas-Samano et al. 2016). However, the effect of the other genes in the development of ischemic stroke remains to be explored. rTMS-induced change of synaptic associated genes might be associated with the activation of second messenger. A previous study has reported that rTMS can strongly activate the phosphorylation of ribosomal protein S6, which is a hallmark of activating AKT-mTOR pathway and immediate early gene expression (Pirbhoy et al. 2016). rTMS also strongly increased expression level of the immediate early genes Arc and c-fos in cortical neurons (Pirbhoy et al. 2016).
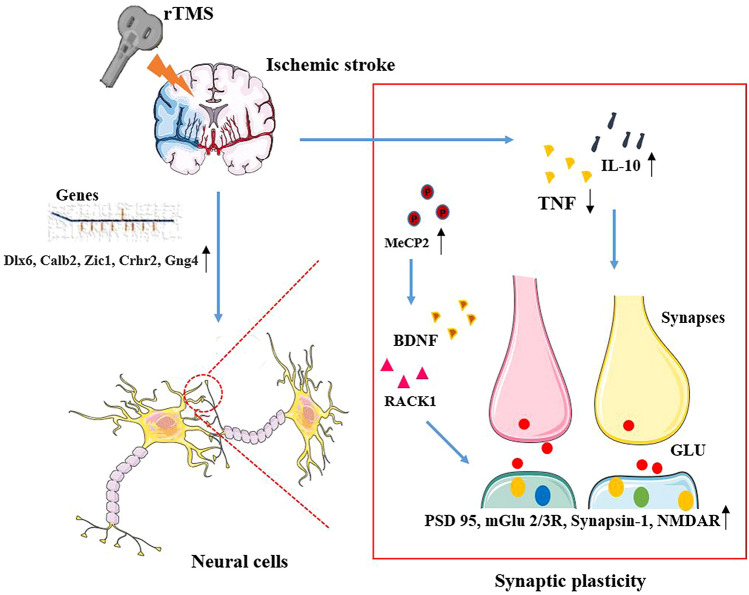
Those alterations of synaptic plasticity, such as the increased number and activity of synapses, contribute to the surviving brain regions undertaking the function of impaired brain tissues (Jones and Adkins 2015; Okabe et al. 2016). However, the effects of rTMS on network reorganization are still unclear. Taken together, rTMS can induce the beneficial changes in the structure, number, and function of the synapses and prompt the potentiation of excitatory synaptic transmission by modulating synaptic plasticity-associated proteins and genes, as shown in Fig. 1.
Fig. 1.
Mechanisms of rTMS in synaptic plasticity after ischemic stroke. Several molecules play important roles in the modulation of synaptic plasticity. After ischemic stroke, rTMS can enhance the levels of synaptic plasticity-associated genes, such as Dlx6, Calb2, Zic1, Crhr2, and Gng4, and Grin3a; rTMS can also inhibit the overexpression of pro-inflammatory cytokines TNF, and facilitate the expression of anti-inflammatory cytokines IL-10 to improve the environment of synaptic growth; In addition, rTMS-induced MeCP2 phosphorylation facilitated the expression of BDNF and the interaction between BDNF exon IV and RACK1. Ultimately, those proteins and genes can improve the structure and function of synapses by up-regulating the levels of PSD 95, mGlu 2/3R, and synapsin-1. rTMS repetitive transcranial magnetic stimulation, NMDAR N-methyl-d-aspartic acid receptors, BDNF brain-derived neurotrophic factor, PSD 95 post-synaptic density protein 95, mGlu2/3R glutamate receptor 2/3, MeCP2 methyl CpG binding protein 2, RACK receptor for activated C kinase 1, GLU glutamate
The Effect of rTMS on Neurogenesis
After ischemic stroke, endogenous neural stem cells (NSCs) often migrate to the damaged area and differentiate into astrocytes to the formation of glial scar instead of neurons (Faiz et al. 2015). In the subventricular zone (SVZ), 10 Hz rTMS over the ipsilateral M1 cortex significantly facilitated the proliferation of adult NSCs via mediating the miR-25/p57 pathway in the infarct cortex following cerebral ischemic injury (Guo et al. 2014). In a recent study, rTMS over the ipsilateral M1 cortex can facilitate the migration of NSCs and lower neuronal loss by increasing the expression of the stromal cell-derived factor-1α (SDF-1α)-1α/CXC chemokine receptor 4 (CXCR4) axis in peri-infarct cortical tissue of rats with ischemic stroke (Deng et al. 2021).
A previous study showed for the first time that the combination of rTMS over the ipsilateral hemisphere and human NSCs exerted effective effects on functional recovery by increasing neurogenesis and the expression of BDNF following ischemic stroke. They also found that rTMS can facilitate the neural differentiation of human embryonic stem cells (ESCs) in the forebrain (Peng et al. 2019). Additionally, LF-magnetic stimulation and iTBS can facilitate the generation of mature neurons from human-induced pluripotent stem cells (iPSCs). HF-magnetic stimulation can facilitate vesicular glutamate transporters 2 (Vglut2) transcription in iPSC-derived neurons (Liu et al. 2020), consisting with the effect of TMS in promoting neurogenesis.
In addition, rTMS over the ipsilateral hemisphere can regulate BDNF/tropomyosin-related kinase B (TrkB) pathway to facilitate neurogenesis in the ipsilateral hippocampus (Guo et al. 2017). Conventional 20 Hz rTMS or iTBS over the ipsilateral M1 cortex significantly decreased the infarct area and improved neurological function. These beneficial effects might be related to facilitating neurogenesis as shown by the upregulation of Ki67/Nestin, Ki67/NeuN, and Ki67/DCX-positive cells in the striatum surrounding the infarct region and activating the BDNF/TrkB signaling pathway in the ipsilateral hemisphere excluding infarct area (Luo et al. 2017). BDNF/TrkB-specific inhibitor ANA12 can abolish the activation of BDNF/TrkB/AKT pathway and the proliferation of neural stem cells (NSCs) in the model of OGD plus reoxygenation (He et al. 2020). Importantly, rTMS also exerted a restorative effect by facilitating the proliferation and neuronal differentiation of NSCs, possibly through the MAPK signaling pathway in mice with intracerebral hemorrhage (ICH) (Cui et al. 2019). Newborn neurons after ischemic stroke can generate functional projections to the other brain regions, which might be important for motor functional recovery (Sun et al. 2012). However, a great deal of evidence remains to be explored in the future. Thus, neurogenesis caused by rTMS exerted an important role in the restoration of neural network.
The Effect of rTMS on Neural Immune Response
There are several studies have shown the benefits of rTMS in improving the damage of ischemic stroke by regulating neural immune response. In the PT model, rTMS over the infarcted hemisphere can modulate complex cytokine response by decreasing peripheral immune cell infiltration in the peri-infarct area (Zong et al. 2020a, 2020b). There is a shift in astrocytic phenotypes from A1 to A2, and a switch in microglial M1/M2 phenotype activation after rTMS intervention (Zong et al. 2020a). In the vessel, the astrocytic A2 phenotypes significantly increased after the application of rTMS, which causes a reduction of excessive astrocyte-vasculature interactions in the peri-infarct zone. The associated mechanism was related to enhanced levels of platelet-derived grow factor receptor beta (PDGFRβ) that mediated the interaction of A2 astrocytes and their neighbor vasculature (Zong et al. 2020b).
In a cell study, rTMS can effectively inhibit neurotoxic astrocytic polarization in the astrocytes culture medium after OGD/R injury. Specifically, the level of pro-inflammatory mediator TNF was downregulated and anti-inflammatory mediator IL-10 was upregulated after OGD injury (Hong et al. 2020). Calcitonin gene-related peptide (CGRP) is a neuropathic pain-related molecule and might be a potential mechanism of anti-inflammation of rTMS over trigeminal ganglion after focal ischemic stroke. rTMS can facilitate the secretion of CGRP by alleviating the levels of TNF and IL-1β, and reducing the number of activated microglia in the cerebral cortex. The inhibitor of CGRP can abolish rTMS-induced the reduction of infarct area and the increase of TNF and IL-1β (Wang et al. 2020).
In addition, rTMS also exerted the neuroprotective effect by regulating superoxide production, NADPH oxidase activity, and oxidative neuronal damage in the peri-infarct regions. After PT stroke, the levels of superoxide anion production and the activity of NADPH oxidase were significantly decreased on days 5 and 21. However, the level of mitochondrial manganese superoxide dismutase (MnSOD) was increased and the release of anti-inflammatory cytokines was reduced in peri-infarct areas after rTMS intervention (Zong et al. 2020a). Thus, rTMS can facilitate neural immune response by regulating A2 astrocyte, M2 microglia, and inhibiting oxidative stress in ischemic animals.
The Effect of rTMS on Blood–Brain Barrier Disruption (BBB) and Vascular Repair
rTMS can exert a great protective effect by promoting the restoration of the peri-infarct microvasculature and BBB following stroke, as shown in Fig. 2. In PT model, as shown by Zong et al. rTMS over the infarcted hemisphere can improve BBB function by mitigating BBB permeabilization and preserving important BBB components claudin-5, ZO-1, occludin, and caveolin-1 from PT-caused degradation in the peri-infarct zone. Furthermore, the damage to morphology, vascular structure, and perfusion was reversed by rTMS, contributing to the improvement of local tissue oxygenation. These results were accompanied by upregulation of regulatory factors and robust protection of critical vascular components. They also showed that rTMS can decrease the apoptosis of existing vascular and newborn endothelial cells in the peri-infarct zone, which plays a crucial role in a long-term angiogenesis effect (Zong et al. 2020b).
Fig. 2.
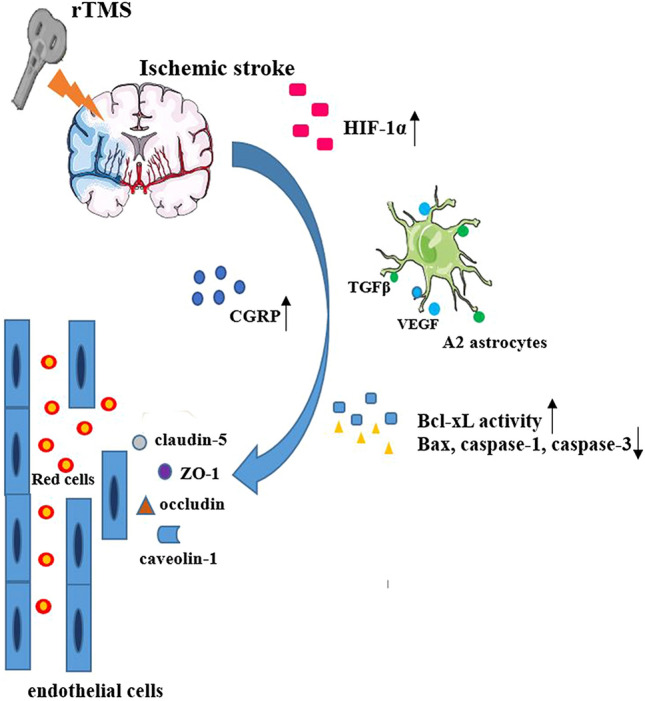
Mechanisms of rTMS in BBB after ischemic stroke. rTMS can facilitate the ischemia-induced increase of HIF-1α and A1 shift to A2 in vessel-associated astrocytes. The increased HIF-1α can promote the release of angiogenesis-related factors TGFβ and VEGF in A2 astrocytes. In addition, TMS can also facilitate the secretion of CGRP, increase the activity of Bcl-xL, and decrease the activation of Bax, caspase-1, caspase-3. Ultimately, those molecules can improve BBB function by mitigating BBB permeabilization by enhancing the expression of claudin-5, ZO-1, occludin, and caveolin-1. BBB blood–brain barrier disruption, HIF-1α hypoxia-inducible factor-1α, CGRP calcitonin gene-related peptide, VEGF vascular endothelial growth factor
Moreover, angiogenesis-related factors TGFβ and vascular endothelial growth factor (VEGF) play important roles in vasculogenesis and angiogenesis (Patel-Hett and D’Amore 2011). There were obvious amplifications of TGFβ and VEGF in A2 astrocytes of the peri-infarct zone, indicating that A2 astrocytes might secrete cluster VEGF onto the vasculature. In addition, rTMS treatment markedly promoted PT-induced increase of hypoxia-inducible factor-1α (HIF-1α) which was reported to be upstream of VEGF (Zong et al. 2020b).
rTMS over the ipsilesional M1 cortex can improve cerebral blood flow (CBF), potentially by enhancing Bcl-xL activity and decreasing Bax, caspase-1, and caspase-3 activations in the striatum of animals with ischemic stroke (Caglayan et al. 2019). In addition, rTMS over trigeminal ganglion can also improve CBF in the middle cerebral artery (MCA) dominated area by promoting the secretion of calcitonin gene-related peptide (CGRP) in serum and cerebral cortex, which was reversed by CGRP8-37, an antagonist of CGRP (Wang et al. 2020). CGRP, as reported by a previous study, can decrease BBB disruption by improving ultrastructural damage in capillary endothelium cells of rats with ischemic stroke (Liu et al. 2011). Thus, the associated mechanism of rTMS-induced BBB and CBF recovery is related to regulating HIF-1α signaling, and prompting A1 to shift to A2 in vessel-associated astrocytes, inhibiting apoptosis and increasing the secretion of CGRP in serum and cerebral cortex.
The Effect of rTMS on Neural Cell Death
Cell apoptosis is an important pathophysiology process of ischemic stroke and participated in the mechanisms of rTMS treating ischemic stroke. The pro-apoptotic protein, such as caspase-1, caspase-3, Bcl-2-associated protein X (Bax), and the anti-apoptotic protein, such as Bcl-2, and Bcl-xL, play key roles in regulating the progress of apoptosis (Khoshnam et al. 2017). The cleavage of caspase-3 can initiate apoptosis, but the anti-apoptotic proteins have special significance in that they can determine if the neural cells commit to apoptosis or abort the process (Elmore 2007). Previous studies indicated that rTMS over the ipsilesional hemisphere decreased infarct volume, and improved cognitive and motor function, which is related to the inhibition of apoptosis by increasing the Bcl-2 level and decreasing the caspase-3 and Bax level following ischemic stroke (Yoon et al. 2011; Guo et al. 2017; Caglayan et al. 2019). rTMS can also effectively inhibit the intrinsic mitochondrial membrane caspase-3/9 apoptotic pathway and preserve mitochondrial membrane integrity in the cortex surrounding the infarct region (Zong et al. 2020a). The percentage of TUNEL-positive cells was significantly decreased after rTMS intervention in the ipsilateral hippocampus or cortex after stroke (Guo et al. 2017; Zong et al. 2020a). In addition, TMS combined with electroacupuncture can improve neurological function, which was associated with the upregulation of Bcl-2 mRNA level and downregulation of caspase-3 expression in infarcted areas (Li et al. 2012).
In addition, iTBS could alleviate ischemic injury-induced locomotor dysfunction and neuronal pyroptosis by modulating innate immune and inflammatory responses. There were observed significant decreases in IL-1β, IL-17A, TNF, IFN-γ, an increase in IL-10, and reductions in neuronal pyroptosis-associated proteins, such as Caspase-1, IL-1β, IL-18, ASC, NLRP1, and cleaved Caspase-1 cleaves Gasdermin D (GSDMD), in the peri-ischemic area rather than in the border of ischemic core. Moreover, iTBS shifted the balance of microglial M1/M2 phenotype by suppressing pro-inflammatory M1 activation and elevating the anti-inflammatory M2 activation in the peri-ischemic area via suppressing TLR4/NFκB/NLRP3 signaling pathway (Luo et al. 2022). Taken together, the rTMS can improve cognitive and motor function by suppressing apoptosis and pyroptosis.
Future Directions for Animal rTMS
The Effect of rTMS in Glial Cells
Glial cells play a critical role in synaptic remodeling owing to they are associated with synaptic plasticity and neuronal connectivity. Neuronal circuits undergo ongoing structural alteration by synaptic interconnecting in response to experience. Synapses are constantly formed and eliminated, which are associated with astrocyte and microglial interaction and regulation. After ischemic stroke, synapse remodeling is crucial to change the structure of neuronal circuits to produce functional compensation (Tennant et al. 2017; Wahl et al. 2017). Exploring remodeling processes is essential for understanding how experiences modulated plasticity and promoting functional rehabilitation. According to a report by Phi T. Nguyen et al., the molecular interaction between microglia and neurons can drive experience-dependent synapse remodeling (Nguyen et al. 2020). Thus, we speculated that rTMS might remodel impaired synapses by regulating molecular interaction between microglia and neurons. Therefore, extending experiments to explore glial cells such as astrocytes and microglia may progress our cognition of the synaptic plasticity and find new therapeutic targets for rTMS.
The Exploration of Dual-Site Stimulation
There have been many dual-site stimulations in the post-stroke, especially in the ipsilesional and contralesional hemispheres. However, the brain stimulation combined with the peripheral nerve stimulation might exert benefits for post-stroke dysfunction by prompting the synaptic connection between the center neural system and peripheral nerve. Paired associative stimulation (PAS) is a protocol to produce synaptic plasticity non-invasively in healthy individuals and patients with stroke or other disorders (Dixon et al. 2016; Kumar et al. 2017; Palmer et al. 2018). It aims to synchronize the activity of presynaptic neurons to elicit spike-timing-dependent plasticity (Feldman 2012). The combination of non-invasive electrical stimulation over the spinal cord or peripheral nerves and TMS over the motor cortex was the most common PAS for motor function recovery. However, the associated basic mechanisms remain unclear. In the future, basic researches are essential to explore the associated mechanism of dual-site stimulation targeting synaptic plasticity. Those mechanisms can contribute to progressing our understanding of changes in the synaptic connection between the center neural system and peripheral nerve.
Others
In addition, several key issues involving the role of TMS in the treatment of stroke still require to be explored. In this review, the majority of studies were related to the application of HF TMS over the ipsilesional hemisphere despite no obvious difference between the effect of HF and LF TMS. The cerebellum also participates in the regulation of cortical excitability, and rTMS or TMS over the cerebellum is also a treatment option for stroke. In a randomized clinical trial, cerebellar iTBS showed an enhancement of gait and balance recovery and an increase in neural activity over the posterior parietal cortex by affecting cerebello-cortical plasticity in patients with hemiparetic stroke (Koch et al. 2019). However, the mechanisms in molecular biology remain to need to be explored in animal experiments.
Single-pulse TMS is often used as a sensitive strategy to quantitatively assess the functional state of the motor system (Escudero et al. 1998; Bolay et al. 2000; Lyseniuk et al. 2014). It is always applied to the cortex but not the deep brain areas. A recent study showed that deep magnetic induction tomography (MIT) combined with improved TMS coils enables the detection of hemorrhagic stroke in humans, providing a crucial base for a deeper research of deep strokes (Lv and Luo 2021). Thereby, the application of TMS to deep stroke detection needs to be studied in the future. Moreover, the duration, initiation time, intensity, and frequency of stimulation also need to be explored in the future.
Conclusion
In this review, we investigate the mechanisms of TMS for treating ischemic stroke. Those mechanisms are essential to progress our understanding of rTMS-induced improvement of the dysfunction after ischemic stroke. rTMS can contribute to the potentiation of excitatory synaptic transmission by modulating synaptic plasticity-associated proteins and genes. In addition, rTMS can significantly inhibit neural cell death, regulate immune response, promote neurogenesis and improve BBB and vascular repair. In the future, more molecular mechanisms will be explored to understand the scope and capacity of rTMS for treating different neurological diseases.
Author Contributions
YX, YZ and CL drafted the manuscript; YH and JH prepared figures; YX, LL and YB edited and revised the manuscript; YB approved the final version of the manuscript.
Funding
This study was funded by National Natural Science Foundation of China (Grant no. 82072540 and Grant no. 82002391).
Data Availability
Not applicable.
Declarations
Conflict of interest
The authors declare no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alam MA, Subramanyam Rallabandi VP, Roy PK (2016) Systems biology of immunomodulation for post-stroke neuroplasticity: multimodal implications of pharmacotherapy and neurorehabilitation. Front Neurol 7:94. 10.3389/fneur.2016.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameli M, Grefkes C, Kemper F, Riegg FP, Rehme AK, Karbe H et al (2009) Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol 66(3):298–309. 10.1002/ana.21725 [DOI] [PubMed] [Google Scholar]
- Aras B, Inal O, Kesikburun S, Yasar E (2020) Response to speech and language therapy according to artery involvement and lesion location in post-stroke aphasia. J Stroke Cerebrovasc Dis 29(10):105132. 10.1016/j.jstrokecerebrovasdis.2020.105132 [DOI] [PubMed] [Google Scholar]
- Blesneag AV, Slavoaca DF, Popa L, Stan AD, Jemna N, Isai Moldovan F et al (2015) Low-frequency rTMS in patients with subacute ischemic stroke: clinical evaluation of short and long-term outcomes and neurophysiological assessment of cortical excitability. J Med Life 8(3):378–387 [PMC free article] [PubMed] [Google Scholar]
- Bolay H, Gursoy-Ozdemir Y, Unal I, Dalkara T (2000) Altered mechanisms of motor-evoked potential generation after transient focal cerebral ischemia in the rat: implications for transcranial magnetic stimulation. Brain Res 873(1):26–33. 10.1016/s0006-8993(00)02466-5 [DOI] [PubMed] [Google Scholar]
- Boonzaier J, van Tilborg GAF, Neggers SFW, Dijkhuizen RM (2018) Noninvasive brain stimulation to enhance functional recovery after stroke: studies in animal models. Neurorehabil Neural Repair 32(11):927–940. 10.1177/1545968318804425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caglayan AB, Beker MC, Caglayan B, Yalcin E, Caglayan A, Yulug B et al (2019) Acute and post-acute neuromodulation induces stroke recovery by promoting survival signaling, neurogenesis, and pyramidal tract plasticity. Front Cell Neurosci 13:144. 10.3389/fncel.2019.00144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Qiu B, Liao M, Liu X, Lin J, Lan L et al (2020) Intermittent theta burst stimulation improves the spatial cognitive function of rats with chronic hypertension-induced cerebral small vessel disease. Neuroscience 437:98–106. 10.1016/j.neuroscience.2020.04.029 [DOI] [PubMed] [Google Scholar]
- Camp AJ, Wijesinghe R (2009) Calretinin: modulator of neuronal excitability. Int J Biochem Cell Biol 41(11):2118–2121. 10.1016/j.biocel.2009.05.007 [DOI] [PubMed] [Google Scholar]
- Cardenas-Morales L, Nowak DA, Kammer T, Wolf RC, Schonfeldt-Lecuona C (2010) Mechanisms and applications of theta-burst rTMS on the human motor cortex. Brain Topogr 22(4):294–306. 10.1007/s10548-009-0084-7 [DOI] [PubMed] [Google Scholar]
- Chervyakov AV, Poydasheva AG, Lyukmanov RH, Suponeva NA, Chernikova LA, Piradov MA et al (2018) Effects of navigated repetitive transcranial magnetic stimulation after stroke. J Clin Neurophysiol 35(2):166–172. 10.1097/WNP.0000000000000456 [DOI] [PubMed] [Google Scholar]
- Collaborators GBDS (2019) Global, regional, and national burden of stroke, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 18(5):439–458. 10.1016/S1474-4422(19)30034-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coronas-Samano G, Baker KL, Tan WJ, Ivanova AV, Verhagen JV (2016) Fus1 KO mouse as a model of oxidative stress-mediated sporadic Alzheimer’s disease: circadian disruption and long-term spatial and olfactory memory impairments. Front Aging Neurosci 8:268. 10.3389/fnagi.2016.00268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui M, Ge H, Zeng H, Yan H, Zhang L, Feng H et al (2019) Repetitive transcranial magnetic stimulation promotes neural stem cell proliferation and differentiation after intracerebral hemorrhage in mice. Cell Transplant 28(5):568–584. 10.1177/0963689719834870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Kim CS, Kim Y, Sohn MK, Jee S (2020) Effects of Repetitive Transcranial Magnetic Stimulation (rTMS) combined with aerobic exercise on the recovery of motor function in ischemic stroke rat model. Brain Sci 10(3):186. 10.3390/brainsci10030186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Guo F, Han X, Huang X (2021) Repetitive transcranial magnetic stimulation increases neurological function and endogenous neural stem cell migration via the SDF-1alpha/CXCR4 axis after cerebral infarction in rats. Exp Ther Med 22(3):1037. 10.3892/etm.2021.10469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diseases GBD, Injuries C (2020) Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet 396(10258):1204–1222. 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon L, Ibrahim MM, Santora D, Knikou M (2016) Paired associative transspinal and transcortical stimulation produces plasticity in human cortical and spinal neuronal circuits. J Neurophysiol 116(2):904–916. 10.1152/jn.00259.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Yang F, Hu J, Hu J, Xu Q, Cong N et al (2019) Effects of high- and low-frequency repetitive transcranial magnetic stimulation on motor recovery in early stroke patients: evidence from a randomized controlled trial with clinical, neurophysiological and functional imaging assessments. Neuroimage Clin 21:101620. 10.1016/j.nicl.2018.101620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516. 10.1080/01926230701320337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudero JV, Sancho J, Bautista D, Escudero M, Lopez-Trigo J (1998) Prognostic value of motor evoked potential obtained by transcranial magnetic brain stimulation in motor function recovery in patients with acute ischemic stroke. Stroke 29(9):1854–1859. 10.1161/01.str.29.9.1854 [DOI] [PubMed] [Google Scholar]
- Faiz M, Sachewsky N, Gascon S, Bang KW, Morshead CM, Nagy A (2015) Adult neural stem cells from the subventricular zone give rise to reactive astrocytes in the cortex after stroke. Cell Stem Cell 17(5):624–634. 10.1016/j.stem.2015.08.002 [DOI] [PubMed] [Google Scholar]
- Feldman DE (2012) The spike-timing dependence of plasticity. Neuron 75(4):556–571. 10.1016/j.neuron.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng HL, Yan L, Guan YZ, Cui LY (2005) Effects of transcranial magnetic stimulation on motor cortical excitability and neurofunction after cerebral ischemia-reperfusion injury in rats. Chin Med Sci J 20(4):226–230 [PubMed] [Google Scholar]
- Frey J, Najib U, Lilly C, Adcock A (2020) Novel TMS for Stroke and Depression (NoTSAD): accelerated repetitive transcranial magnetic stimulation as a safe and effective treatment for post-stroke depression. Front Neurol 11:788. 10.3389/fneur.2020.00788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao BY, Sun CC, Xia GH, Zhou ST, Zhang Y, Mao YR et al (2020) Paired associated magnetic stimulation promotes neural repair in the rat middle cerebral artery occlusion model of stroke. Neural Regen Res 15(11):2047–2056. 10.4103/1673-5374.282266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Chen W, Axerio-Cilies P, Wang YT (2020) NMDARs in cell survival and death: implications in stroke pathogenesis and treatment. Trends Mol Med 26(6):533–551. 10.1016/j.molmed.2020.03.001 [DOI] [PubMed] [Google Scholar]
- Greger IH, Khatri L, Ziff EB (2002) RNA editing at arg607 controls AMPA receptor exit from the endoplasmic reticulum. Neuron 34(5):759–772. 10.1016/s0896-6273(02)00693-1 [DOI] [PubMed] [Google Scholar]
- Guo F, Han X, Zhang J, Zhao X, Lou J, Chen H et al (2014) Repetitive transcranial magnetic stimulation promotes neural stem cell proliferation via the regulation of MiR-25 in a rat model of focal cerebral ischemia. PLoS ONE 9(10):e109267. 10.1371/journal.pone.0109267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Lou J, Han X, Deng Y, Huang X (2017) Repetitive transcranial magnetic stimulation ameliorates cognitive impairment by enhancing neurogenesis and suppressing apoptosis in the hippocampus in rats with ischemic stroke. Front Physiol 8:559. 10.3389/fphys.2017.00559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M (2007) Transcranial magnetic stimulation: a primer. Neuron 55(2):187–199. 10.1016/j.neuron.2007.06.026 [DOI] [PubMed] [Google Scholar]
- Hanley JG (2014) Subunit-specific trafficking mechanisms regulating the synaptic expression of Ca(2+)-permeable AMPA receptors. Semin Cell Dev Biol 27:14–22. 10.1016/j.semcdb.2013.12.002 [DOI] [PubMed] [Google Scholar]
- He T, Sun R, Li Y, Katusic ZS (2018) Effects of brain-derived neurotrophic factor on microrna expression profile in human endothelial progenitor cells. Cell Transplant 27(6):1005–1009. 10.1177/0963689718761658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Chen S, Tsoi B, Qi S, Gu B, Wang Z et al (2020) Alpinia oxyphylla Miq. and its active compound p-coumaric acid promote brain-derived neurotrophic factor signaling for inducing hippocampal neurogenesis and improving post-cerebral ischemic spatial cognitive functions. Front Cell Dev Biol 8:577790. 10.3389/fcell.2020.577790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Liu Q, Peng M, Bai M, Li J, Sun R et al (2020) High-frequency repetitive transcranial magnetic stimulation improves functional recovery by inhibiting neurotoxic polarization of astrocytes in ischemic rats. J Neuroinflamm 17(1):150. 10.1186/s12974-020-01747-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Chen J, Li C, An D, Tang Z, Wen H (2021) High-frequency rTMS improves cognitive function by regulating synaptic plasticity in cerebral ischemic rats. Neurochem Res 46(2):276–286. 10.1007/s11064-020-03161-5 [DOI] [PubMed] [Google Scholar]
- Isaac JT, Ashby MC, McBain CJ (2007) The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron 54(6):859–871. 10.1016/j.neuron.2007.06.001 [DOI] [PubMed] [Google Scholar]
- Jones TA, Adkins DL (2015) Motor system reorganization after stroke: stimulating and training toward perfection. Physiology 30(5):358–370. 10.1152/physiol.00014.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer J (2022) Regulation of AMPAR trafficking in synaptic plasticity by BDNF and the impact of neurodegenerative disease. J Neurosci Res 100(4):979–991. 10.1002/jnr.25022 [DOI] [PubMed] [Google Scholar]
- Khoshnam SE, Winlow W, Farzaneh M, Farbood Y, Moghaddam HF (2017) Pathogenic mechanisms following ischemic stroke. Neurol Sci 38(7):1167–1186. 10.1007/s10072-017-2938-1 [DOI] [PubMed] [Google Scholar]
- Kim J, Yim J (2018) Effects of high-frequency repetitive transcranial magnetic stimulation combined with task-oriented mirror therapy training on hand rehabilitation of acute stroke patients. Med Sci Monit 24:743–750. 10.12659/msm.905636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WS, Jung SH, Oh MK, Min YS, Lim JY, Paik NJ (2014) Effect of repetitive transcranial magnetic stimulation over the cerebellum on patients with ataxia after posterior circulation stroke: a pilot study. J Rehabil Med 46(5):418–423. 10.2340/16501977-1802 [DOI] [PubMed] [Google Scholar]
- Koch G, Bonni S, Casula EP, Iosa M, Paolucci S, Pellicciari MC et al (2019) Effect of cerebellar stimulation on gait and balance recovery in patients with hemiparetic stroke: a randomized clinical trial. JAMA Neurol 76(2):170–178. 10.1001/jamaneurol.2018.3639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Zomorrodi R, Ghazala Z, Goodman MS, Blumberger DM, Cheam A et al (2017) Extent of dorsolateral prefrontal cortex plasticity and its association with working memory in patients with alzheimer disease. JAMA Psychiat 74(12):1266–1274. 10.1001/jamapsychiatry.2017.3292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labedi A, Benali A, Mix A, Neubacher U, Funke K (2014) Modulation of inhibitory activity markers by intermittent theta-burst stimulation in rat cortex is NMDA-receptor dependent. Brain Stimul 7(3):394–400. 10.1016/j.brs.2014.02.010 [DOI] [PubMed] [Google Scholar]
- Lau D, Bengtson CP, Buchthal B, Bading H (2015) BDNF reduces toxic extrasynaptic NMDA receptor signaling via synaptic NMDA receptors and nuclear-calcium-induced transcription of inhba/Activin A. Cell Rep 12(8):1353–1366. 10.1016/j.celrep.2015.07.038 [DOI] [PubMed] [Google Scholar]
- Li M, Peng J, Song Y, Liang H, Mei Y, Fang Y (2012) Electro-acupuncture combined with transcranial magnetic stimulation improves learning and memory function of rats with cerebral infarction by inhibiting neuron cell apoptosis. J Huazhong Univ Sci Technolog Med Sci 32(5):746–749. 10.1007/s11596-012-1028-0 [DOI] [PubMed] [Google Scholar]
- Li J, Zhang XW, Zuo ZT, Lu J, Meng CL, Fang HY et al (2016) Cerebral functional reorganization in ischemic stroke after repetitive transcranial magnetic stimulation: an fMRI study. CNS Neurosci Ther 22(12):952–960. 10.1111/cns.12593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Shang J, Zhang C, Lu R, Chen J, Zhou X (2020) Repetitive transcranial magnetic stimulation alleviates neurological deficits after cerebral ischemia through interaction between RACK1 and BDNF exon IV by the phosphorylation-dependent factor MeCP2. Neurotherapeutics 17(2):651–663. 10.1007/s13311-019-00771-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Zhao W, Xu T, Pei D, Peng Y (2010) Alterations of NMDA receptor subunits NR1, NR2A and NR2B mRNA expression and their relationship to apoptosis following transient forebrain ischemia. Brain Res 1361:133–139. 10.1016/j.brainres.2010.09.035 [DOI] [PubMed] [Google Scholar]
- Liu Z, Liu Q, Cai H, Xu C, Liu G, Li Z (2011) Calcitonin gene-related peptide prevents blood-brain barrier injury and brain edema induced by focal cerebral ischemia reperfusion. Regul Pept 171(1–3):19–25. 10.1016/j.regpep.2011.05.014 [DOI] [PubMed] [Google Scholar]
- Liu G, Li XM, Tian S, Lu RR, Chen Y, Xie HY et al (2020) The effect of magnetic stimulation on differentiation of human induced pluripotent stem cells into neuron. J Cell Biochem. 10.1002/jcb.29647 [DOI] [PubMed] [Google Scholar]
- Lodha N, Patel P, Shad JM, Casamento-Moran A, Christou EA (2021) Cognitive and motor deficits contribute to longer braking time in stroke. J Neuroeng Rehabil 18(1):7. 10.1186/s12984-020-00802-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Zheng H, Zhang L, Zhang Q, Li L, Pei Z et al (2017) High-frequency Repetitive Transcranial Magnetic Stimulation (rTMS) improves functional recovery by enhancing neurogenesis and activating BDNF/TrkB signaling in ischemic rats. Int J Mol Sci 18(2):455. 10.3390/ijms18020455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Liu M, Fan Y, Zhang J, Liu L, Li Y et al (2022) Intermittent theta-burst stimulation improves motor function by inhibiting neuronal pyroptosis and regulating microglial polarization via TLR4/NFkappaB/NLRP3 signaling pathway in cerebral ischemic mice. J Neuroinflamm 19(1):141. 10.1186/s12974-022-02501-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y, Luo H (2021) A new method of haemorrhagic stroke detection via deep magnetic induction tomography. Front Neurosci 15:659095. 10.3389/fnins.2021.659095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyseniuk VP, Balitskii AP, Samosiuk NI (2014) The application of transcranial magnetic stimulation for the functional diagnostics of motor disturbances in the patients presenting with ischemic stroke. Vopr Kurortol Fizioter Lech Fiz Kult 1:9–14 [PubMed] [Google Scholar]
- Mei YW, Liu CY, Zhang XQ (2006) Effects of transcranial magnetic stimulation on recovery of neural functions and changes of synaptic interface and dendritic structure in the contralateral brain area after cerebral infarction: experiment with rats. Zhonghua Yi Xue Za Zhi 86(37):2639–2642 [PubMed] [Google Scholar]
- Mello EA, Cohen LG, Monteiro Dos Anjos S, Conti J, Andrade KN, Tovar Moll F et al (2015) Increase in short-interval intracortical facilitation of the motor cortex after low-frequency repetitive magnetic stimulation of the unaffected hemisphere in the subacute phase after stroke. Neural Plast 2015:407320. 10.1155/2015/407320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute of Neurological, D., and Stroke rt, P.A.S.S.G. (1995) Tissue plasminogen activator for acute ischemic stroke. N Engl J Med 333(24):1581–1587. 10.1056/NEJM199512143332401 [DOI] [PubMed] [Google Scholar]
- Nguyen PT, Dorman LC, Pan S, Vainchtein ID, Han RT, Nakao-Inoue H et al (2020) Microglial remodeling of the extracellular matrix promotes synapse plasticity. Cell 182(2):388–403. 10.1016/j.cell.2020.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okabe N, Shiromoto T, Himi N, Lu F, Maruyama-Nakamura E, Narita K et al (2016) Neural network remodeling underlying motor map reorganization induced by rehabilitative training after ischemic stroke. Neuroscience 339:338–362. 10.1016/j.neuroscience.2016.10.008 [DOI] [PubMed] [Google Scholar]
- Palmer JA, Wolf SL, Borich MR (2018) Paired associative stimulation modulates corticomotor excitability in chronic stroke: a preliminary investigation. Restor Neurol Neurosci 36(2):183–194. 10.3233/RNN-170785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Bellone C, Zhou Q (2013) NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci 14(6):383–400. 10.1038/nrn3504 [DOI] [PubMed] [Google Scholar]
- Patel-Hett S, D’Amore PA (2011) Signal transduction in vasculogenesis and developmental angiogenesis. Int J Dev Biol 55(4–5):353–363. 10.1387/ijdb.103213sp [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng JJ, Sha R, Li MX, Chen LT, Han XH, Guo F et al (2019) Repetitive transcranial magnetic stimulation promotes functional recovery and differentiation of human neural stem cells in rats after ischemic stroke. Exp Neurol 313:1–9. 10.1016/j.expneurol.2018.12.002 [DOI] [PubMed] [Google Scholar]
- Pirbhoy PS, Farris S, Steward O (2016) Synaptic activation of ribosomal protein S6 phosphorylation occurs locally in activated dendritic domains. Learn Mem 23(6):255–269. 10.1101/lm.041947.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts LL, Rogers L, Wang X, Bahia MM, Cherney LR (2020) Functionally navigated transcranial magnetic stimulation to evoke lingual pressure in stroke survivors with dysphagia and healthy adults: a proof of concept trial. Top Stroke Rehabil 27(4):241–250. 10.1080/10749357.2019.1701175 [DOI] [PubMed] [Google Scholar]
- Sadlonova M, Wasser K, Nagel J, Weber-Kruger M, Groschel S, Uphaus T et al (2021) Health-related quality of life, anxiety and depression up to 12 months post-stroke: influence of sex, age, stroke severity and atrial fibrillation—a longitudinal subanalysis of the find-AFRANDOMISED trial. J Psychosom Res 142:110353. 10.1016/j.jpsychores.2020.110353 [DOI] [PubMed] [Google Scholar]
- Scuteri D, Mantovani E, Tamburin S, Sandrini G, Corasaniti MT, Bagetta G et al (2020) Opioids in post-stroke pain: a systematic review and meta-analysis. Front Pharmacol 11:587050. 10.3389/fphar.2020.587050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhang QW, Xu M, Guo JJ, Shen SW, Wang YQ et al (2012) New striatal neurons form projections to substantia nigra in adult rat brain after stroke. Neurobiol Dis 45(1):601–609. 10.1016/j.nbd.2011.09.018 [DOI] [PubMed] [Google Scholar]
- Tennant KA, Taylor SL, White ER, Brown CE (2017) Optogenetic rewiring of thalamocortical circuits to restore function in the stroke injured brain. Nat Commun 8:15879. 10.1038/ncomms15879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosun A, Ture S, Askin A, Yardimci EU, Demirdal SU, Kurt Incesu T et al (2017) Effects of low-frequency repetitive transcranial magnetic stimulation and neuromuscular electrical stimulation on upper extremity motor recovery in the early period after stroke: a preliminary study. Top Stroke Rehabil 24(5):361–367. 10.1080/10749357.2017.1305644 [DOI] [PubMed] [Google Scholar]
- Volz LJ, Sarfeld AS, Diekhoff S, Rehme AK, Pool EM, Eickhoff SB et al (2015) Motor cortex excitability and connectivity in chronic stroke: a multimodal model of functional reorganization. Brain Struct Funct 220(2):1093–1107. 10.1007/s00429-013-0702-8 [DOI] [PubMed] [Google Scholar]
- Wahl AS, Buchler U, Brandli A, Brattoli B, Musall S, Kasper H et al (2017) Optogenetically stimulating intact rat corticospinal tract post-stroke restores motor control through regionalized functional circuit formation. Nat Commun 8(1):1187. 10.1038/s41467-017-01090-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Han J, Higashimori H, Wang J, Liu J, Tong L et al (2019) Long-term depression induced by endogenous cannabinoids produces neuroprotection via astroglial CB1R after stroke in rodents. J Cereb Blood Flow Metab 39(6):1122–1137. 10.1177/0271678X18755661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Liu Z, Ge X, Hu X, Cao X, Li L et al (2020) Neuropathic pain releasing calcitonin gene related peptide protects against stroke in rats. Am J Transl Res 12(1):54–69 [PMC free article] [PubMed] [Google Scholar]
- Yoon KJ, Lee YT, Han TR (2011) Mechanism of functional recovery after repetitive transcranial magnetic stimulation (rTMS) in the subacute cerebral ischemic rat model: neural plasticity or anti-apoptosis? Exp Brain Res 214(4):549–556. 10.1007/s00221-011-2853-2 [DOI] [PubMed] [Google Scholar]
- Zhang X, Mei Y, Liu C, Yu S (2007) Effect of transcranial magnetic stimulation on the expression of c-Fos and brain-derived neurotrophic factor of the cerebral cortex in rats with cerebral infarct. J Huazhong Univ Sci Technolog Med Sci 27(4):415–418. 10.1007/s11596-007-0416-3 [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Li L, Huo JT, Cheng M, Li LH (2018) Effects of repetitive transcranial magnetic stimulation on cognitive function and cholinergic activity in the rat hippocampus after vascular dementia. Neural Regen Res 13(8):1384–1389. 10.4103/1673-5374.235251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong X, Dong Y, Li Y, Yang L, Li Y, Yang B et al (2020a) Beneficial effects of theta-burst transcranial magnetic stimulation on stroke injury via improving neuronal microenvironment and mitochondrial integrity. Transl Stroke Res 11(3):450–467. 10.1007/s12975-019-00731-w [DOI] [PubMed] [Google Scholar]
- Zong X, Li Y, Liu C, Qi W, Han D, Tucker L et al (2020b) Theta-burst transcranial magnetic stimulation promotes stroke recovery by vascular protection and neovascularization. Theranostics 10(26):12090–12110. 10.7150/thno.51573 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.