Abstract
Objectives:
Ultra-short heart rate variability (HRV) metrics represent autonomic tone parameters derived using small epochs of interbeat interval data. These measures have risen in popularity with the advent of wearable devices that can capture interbeat interval data using electrocardiography (ECG) or photoplethysmography. Autonomic neuropathy in diabetes mellitus (DM) is well established, wherein 5-min HRV is conventionally used. Ultra-short measures have the potential to serve as markers of reduced autonomic tone in this patient population.
Methods:
Data of patients with Type I and Type II DM who had presented to our laboratory for autonomic neuropathy assessment were chosen for analysis. One-minute and 2-min epochs were chosen from 5 min of ECG data using standard software. Time domain, frequency domain, and nonlinear measures were computed from 1 to 2 min epochs, and reliability was compared with measures derived from 5-min HRV using intraclass correlation coefficients (ICCs).
Results:
Data of 131 subjects (79 males, 52 females; mean age = 53.3 ± 12.16 years) were analyzed. All ultra-short HRV measures derived from 1 min to 2 min data showed good to excellent reliability (median ICC values ranging from 0.83 to 0.94) when compared with 5-min metrics. The notable exception was very low frequency (VLF) power, which showed poor reliability (median ICC = 0.43).
Conclusions:
Ultra-short HRV metrics derived from 1 to 2 min epochs of ECG data can be reliably used as predictors of autonomic tone in patients with DM. VLF power is poorly reproducible in these small epochs, probably due to variability in respiratory rates. Our findings have implications for ultra-short HRV estimation using short epochs of ECG data.
Keywords: Autonomic nervous system, autonomic neuropathy, diabetes complications, diabetes mellitus, electrocardiography, heart rate
Introduction
There is a growing body of literature that recognizes the importance of heart rate variability (HRV) as a marker for autonomic nervous system integrity. HRV analysis involves the computation of the time domain, frequency domain, and nonlinear metrics using interbeat interval data. The duration of data acquisition usually varies from 5 min (short-term HRV) to 24 h (long-term HRV). Debate continues regarding the optimal duration of HRV analysis. While short-term HRV is routinely used across laboratories worldwide, it does not provide optimum information about physiological influences acting on a slow time scale, such as hormonal influences and circadian rhythm. Long-term HRV recording requires Holter-based or similar technology and may not be feasible for large-volume data acquisition regarding the time and equipment costs involved.[1,2,3]
Ultra-short HRV metrics are being explored which analyze small epochs of interbeat interval data ranging from 30 s to a few minutes. These metrics are of considerable interest in recent times since the rise of wearable technology and consumer-based heart rate monitoring products have revolutionized domains of heart rate acquisition and analysis. It is very convenient and practical to acquire interbeat interval data using electrocardiography (ECG) or photoplethysmography (PPG) based technology omnipresent in wearables. Therefore, the utility of ultra-short HRV indices is being explored in different clinical disorders as a marker of morbidity and disease progression.[4,5,6,7]
Diabetes mellitus (DM) continues to be a disorder of global magnitude, with its incidence and prevalence rising steadily over the years.[8,9] Autonomic neuropathy is the hallmark of DM and evaluation of HRV can provide valuable information on this disease. However, there are few, if any, reports of autonomic tone assessment using ultra-short time epochs in this disorder.[10] Since there is immense potential for ultra-short HRV indices in screening for autonomic neuropathy in this disorder, we undertook the present work to look at the reliability of these indices with respect to standard short-term HRV (5 min) indices.
Methods
The study was approved by the institute ethics committee of our institution. Data of clinically diagnosed patients of DM who were referred to our department were chosen for the study. These patients were referred to the autonomic function laboratory of our department for assessment of autonomic neuropathy from the department of endocrinology and metabolism of our hospital.
All the patients had Lead II ECG data acquired for 5 min using a digital data acquisition system at our laboratory (Biopac MP 150® system, Biopac Systems Inc. USA). The data were acquired in the supine position after a supine rest of 10 min. All the patients were requested to refrain from tea/coffee on the day of the test and report to the laboratory 2 h after a light meal. Lead II ECG was recorded in the supine position in a noise-free, temperature-controlled environment for 5 min using Acqknowledge® software version 4.4 (Biopac Systems Inc. USA).
HRV was assessed using LabChart Pro® software version 8 (AD Instruments, Australia). Data were analyzed for 5 min using the built in HRV analysis toolbox of the software. Data were visually inspected carefully to exclude any dataset with ectopics or movement artifacts. Time domain indices (standard deviation of normal-to-normal intervals [SDNN], root mean square of the standard deviation of interbeat intervals [RMSSD], percentage of interbeat intervals varying by more than 50 ms [pNN50]), frequency domain indices (total power, very low frequency [VLF] power, low frequency [LF] power, and high frequency [HF] power), and nonlinear measures (standard deviation [SD1], SD2, and SD1/SD2) were computed and tabulated. These indices have been described in detail previously.[11,12,13,14]
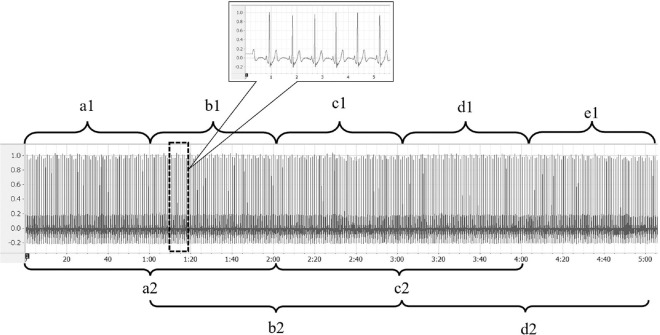
For ultra-short HRV analysis, ECG data were divided into epochs of 1 min and 2 min. Five-minute ECG was divided into five epochs of 0–1st min, 1st–2nd min, 2nd–3rd min, 3rd–4th min, and 4th–5th min each for 1 min epoch HRV analysis. Similarly, 5-min ECG data were divided into four 2-min epochs of 0–2nd min, 1st–3rd min, 2nd–4th min, and 3rd–5th min each for 2-min epoch HRV analysis. The epochs were labeled as a1, b1, c1, d1, and e1 for 1-min epochs and a2, b2, c2, and d2 for 2-min epochs, respectively. The epochs were randomly chosen for each subject using validated Random Allocation Software by Saghaei.[15] The epochs were fed into the software as a1, b1, c1, d1, and e1 for 1-min epochs and a2, b2, c2, and d2 for 2-min epochs, and a random list was generated. This list was used for choosing the epoch according to participant number. The same is elaborated in Figure 1.
Figure 1.
Division of electrocardiography (ECG) signal into epochs for analysis. Representative record for 5-min Lead II ECG signal used for epoch-based heart rate variability (HRV) analysis. X axis represents time and Y axis represents signal amplitude. The ECG signal was divided into 5 epochs of 1 min each (a1–e1, ranging from 0 to 1 min, 1 to 2 min, 2 to 3 min, 3 to 4 min, and 4 to 5 min, respectively; shown in upper panel). For the analysis of 2-min epochs, the ECG signal was divided into epochs a2–d2 (ranging from 0 to 2 min, 1 to 3 min, 2 to 4 min, and 3 to 5 min, respectively; shown in lower panel). The dotted rectangle depicts a representative segment of ECG signal whose magnified view is shown in the dialogue box. These segment notations (a1–e1 and a2–d2) were put into validated software for randomization and a series was generated for HRV analysis of the study subjects
HRV indices were tabulated using a spreadsheet program (Microsoft Excel® 2021, Microsoft Corp., Redmond, USA). Statistical analysis was done using MedCalc® Statistical Software version 19.2.6 (MedCalc Software BV, Ostend, 127 Belgium). Intraclass correlation coefficient (ICC) was used to calculate the reliability of the measures derived from 1 to 2 min epochs when compared with 5-min epochs. A two-way random effects model was used to compute the ICC. Grading of reliability using ICC was done using criteria described previously.[16]
Results
Data of 169 subjects were assessed for eligibility. Since HRV metrics derived from 1 min data can be drastically affected by even a few ectopics, datasets with ectopics/artifacts were excluded from the analysis. Finally, data of 131 subjects (79 males, 52 females; mean age = 53.3 ± 12.16 years) were included in the present study. Other descriptive statistics of the patients are shown in Table 1.
Table 1.
Characteristics of the patient population
| Parameter | Males (n=79) | Females (n=52) |
|---|---|---|
| Age (years) | 53.94±12.14 | 52.33±12.22 |
| Height (cm) | 168.04±6.65 | 155.37±7.27 |
| Weight (kg) | 72.98±13.14 | 66.79±11.43 |
| BMI (kg/m2) | 25.80±4.17 | 27.67±4.44 |
BMI: Body mass index
SDNN, RMSSD, and pNN50 were computed for the time domain and total power, VLF power, LF power, HF power, and LF/HF ratio were computed for the frequency domain. Nonlinear measures computed were SD1 and SD2. The median values of the parameters are summarized in Table 2. The repeatability of HRV metrics was assessed using ICC, using a two-way random effects model. All the metrics demonstrated good to excellent repeatability as assessed using ICC values (median values ranging from 0.83 to 0.95), except for poor repeatability shown by VLF power, since it had a median ICC value of 0.43 only. The ICC values are summarized in Table 3.
Table 2.
Heart rate variability measures derived using 1-min, 2-min, and 5-min epochs
| Domain | Metric | 1 min | 2 min | 5 min |
|---|---|---|---|---|
| Time domain | SDNN | 16.34 (9.87–24.20) | 17.16 (11.72–28.09) | 20.27 (13.29–29.86) |
| RMSSD | 12.45 (6.90–20.41) | 12.64 (6.51–22.71) | 13.44 (7.02–22.38) | |
| pNN50 | 0.00 (0.00–1.58) | 0.00 (0.00–1.94) | 0.00 (0.00–2.38) | |
| Frequency domain | Total power | 181.50 (55.14–466.33) | 269.10 (93.73–665.93) | 341.00 (140.600–743.90) |
| VLF power | 38.37 (17.04–103.13) | 108.9 (39.45–237.68) | 150.60 (73.93–314.80) | |
| LF power | 47.78 (15.43–143.73) | 66.04 (19.75–197.48) | 69.17 (25.39–195.28) | |
| HF power | 49.50 (15.62–171.13) | 61.94 (14.53–165.88) | 60.69 (16.49–180.50) | |
| Nonlinear | SD1 | 8.86 (4.91–14.53) | 8.96 (4.62–15.83) | 9.51 (4.97–15.85) |
| SD2 | 19.87 (12.0–30.28) | 22.42 (14.73–36.49) | 26.65 (17.93–38.62) |
Values expressed as median (IQR). SD1 and SD2 represent SD of Poincare plots along and perpendicular to the line of identity. SD: Standard deviation; SDNN: SD of normal-to-normal intervals; RMSSD: Root mean square of SD of interbeat intervals; pNN50: Percentage of interbeat intervals varying by more than 50 ms; LF: Low frequency; VLF: Very LF; HF: High frequency, IQR: Interquartile range
Table 3.
Intraclass correlation coefficients for heart rate variability measures
| Domain | Metric | Intraclass correlation coefficient |
|---|---|---|
| Time domain | SDNN | 0.88 (0.85–0.91) |
| RMSSD | 0.94 (0.92–0.96) | |
| pNN50 | 0.93 (0.91–0.95) | |
| Frequency domain | Total power | 0.83 (0.78–0.87) |
| VLF power | 0.43 (0.32–0.53) | |
| LF power | 0.93 (0.91–0.95) | |
| HF power | 0.93 (0.91–0.95) | |
| Nonlinear | SD1 | 0.94 (0.93–0.96) |
| SD2 | 0.85 (0.81–0.89) |
Intraclass correlation coefficient expressed as ICC (95% CI) for a two-way random effect model. SD1 and SD2 represent SD of Poincare plots along and perpendicular to the line of identity. All HRV measures demonstrated good to excellent repeatability except for VLF power that demonstrated poor repeatability. SD: Standard deviation; SDNN: SD of normal-to-normal intervals; RMSSD: Root mean square of SD of interbeat intervals; pNN50: Percentage of interbeat intervals varying by more than 50 ms; LF: Low frequency; VLF: Very LF; HF: High frequency; HRV: Heart rate variability; CI: Confidence interval, ICC: Intraclass correlation coefficient
Discussion
HRV provides insight into the autonomic neuromodulation of the heart. It has been shown to be a predictor of morbidity and mortality in a wide spectrum of clinical conditions such as arrhythmias, atherosclerosis, heart failure, epilepsy, spinocerebellar ataxia, and DM.[17,18,19,20] Indices derived using interbeat intervals are classified into the time domain, frequency domain, and nonlinear metrics. While time domain indices represent the variability of the interbeat interval series, frequency domain indices represent the energy/power distribution across different frequency bands such as VLF, LF, and HF.[12] Additional bands may be observed with long-term (24 h) HRV data such as ultra-LF. Long-term HRV provides additional information regarding the influences of circadian rhythm, thermoregulatory influences, and the renin–angiotensin–aldosterone system.[21]
Short-term HRV requires a minimum duration of 5 min of noise- and artifact-free data acquisition. ECG and PPG signals have been explored to compute the indices associated with short-term HRV.[22] While short-term HRV is widely used in autonomic function laboratories across the globe, the assessment requires the acquisition of 5 min of ECG data, which requires the subject to be reasonably still. The presence of artifacts makes it difficult to reliably detect interbeat intervals using R peaks. Even advanced software tools find it computationally challenging to correctly identify the fiducial points in the noisy ECG signal. Attempts are being made to circumvent this time cost of 5 min for signal acquisition and analysis.
The rise of wearable technology and smartphone-based heart rate monitoring has opened up new dimensions for HRV analysis. Smartwatches have risen in popularity as consumer-grade devices. Many smartwatches have ECG and PPG acquisition capabilities. In addition, multiple smartphone-based applications are available to measure heart rate using inbuilt camera and flash.[23] Therefore, there is renewed interest in the exploration of ultra-short HRV indices using such devices. Before the application of ultra-short metrics in clinical use, the metrics need to be validated across the patient population.
Ultra-short HRV measures have been explored in depth in the context of sports physiology. This is relevant because almost all outdoor sports require the participant to be in motion and therefore it is practical to extract short-term segments of beat-to-beat interval data. Furthermore, HRV has shown immense potential as a predictor of performance in different sports. Therefore ultra-short metrics have been explored in different sports such as cycling, futsal, basketball, soccer, rugby, and recovery from exercise and training.[24] Ultra-short metrics are also being explored in different diseased states such as epilepsy,[5] obstructive sleep apnea,[6] risk stratification for myocardial infarction,[25] and myocarditis.[26]
However, we found only a single study wherein ultra-short measures were evaluated in patients with DM.[10] Nussinovitch et al.[10] evaluated ultra-short HRV measures (10 s and 1 min) in 48 patients with DM. They reported 10 s recordings to be insufficient for commenting upon the reliability of SDNN, pNN50, and total power and recommended 1 min or longer for this assessment. The limitations of the report were the small sample size and lack of comments on geometric measures of HRV.
In the present work, we had a reasonably larger sample size (n = 131) and evaluated HRV metrics derived from 1-min as well as 2-min epochs chosen randomly. All the HRV metrics showed good to excellent reliability for the subjects except for VLF power (ICC = 0.43). This is consistent with the previous literature. The poor repeatability of VLF power can be attributed to the differences in respiratory rates across different visits.
VLF power is primarily driven by the respiratory rhythm.[27] The respiratory rate of the patients in the present work was spontaneous and therefore may be the likely mechanism for the variation in VLF power across different small epochs of 1 and 2 min. In addition, since VLF frequency is on the lower side, small epochs may not capture an adequate number of cycles for spectral analysis.[28] Metronomic/paced breathing may have produced consistent responses in VLF power with an improvement in reliability. However, there are conflicting reports regarding the use of metronomic or paced breathing for HRV assessment. Different groups have used spontaneous breathing for HRV assessment and have concluded that spontaneous breathing protocol leads to better reliability of blood pressure values.[29] However, some reports differ in this context and recommend used of paced breathing for better results.[30] All data in the present study were recorded under spontaneous breathing protocol. Based on our data, we can conclude that VLF may not be a reliable index for ultra-short HRV metrics.
Conclusions
Ultra-short HRV indices derived using 1 min and 2 min epochs reveal good to excellent reliability in patients with DM, except for VLF power. These indices can be used as a screening tool for the presence of autonomic neuropathy in addition to standard indices derived from 5-min ECG data. The poor reliability of VLF power is probably due to the variation in respiratory rate across different epochs. Furthermore, the number of cycles of respiration captured in small epochs may be too small for spectral analysis purposes. This is an avenue that can be explored in future work.
Ethical statement
The study was approved by the Institute Ethics Committee, All India Institute of Medical Sciences, Jodhpur, vide letter number AIIMS/IEC/3156 dated July 9, 2020.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Pham T, Lau ZJ, Chen SH, Makowski D. Heart rate variability in psychology: A review of HRV indices and an analysis tutorial. Sensors (Basel) 2021;21:3998. doi: 10.3390/s21123998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar SM, Vaishali K, Maiya GA, Shivashankar KN, Shashikiran U. Analysis of time-domain indices, frequency domain measures of heart rate variability derived from ECG waveform and pulse-wave-related HRV among overweight individuals: An observational study. F1000Res. 2023;12:1229. doi: 10.12688/f1000research.139283.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agelink MW, Malessa R, Baumann B, Majewski T, Akila F, Zeit T, et al. Standardized tests of heart rate variability: Normal ranges obtained from 309 healthy humans, and effects of age, gender, and heart rate. Clin Auton Res. 2001;11:99–108. doi: 10.1007/BF02322053. [DOI] [PubMed] [Google Scholar]
- 4.Hietakoste S, Korkalainen H, Kainulainen S, Sillanmäki S, Nikkonen S, Myllymaa S, et al. Longer apneas and hypopneas are associated with greater ultra-short-term HRV in obstructive sleep apnea. Sci Rep. 2020;10:21556. doi: 10.1038/s41598-020-77780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.You SM, Jo HJ, Cho BH, Song JY, Kim DY, Hwang YH, et al. Comparing ictal cardiac autonomic changes in patients with frontal lobe epilepsy and temporal lobe epilepsy by ultra-short-term heart rate variability analysis. Medicina (Kaunas) 2021;57:666. doi: 10.3390/medicina57070666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ha SS, Kim DK. Diagnostic efficacy of ultra-short term HRV analysis in obstructive sleep apnea. J Pers Med. 2022;12:1494. doi: 10.3390/jpm12091494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orini M, van Duijvenboden S, Young WJ, Ramírez J, Jones AR, Hughes AD, et al. Long-term association of ultra-short heart rate variability with cardiovascular events. Sci Rep. 2023;13:18966. doi: 10.1038/s41598-023-45988-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anjana RM, Deepa M, Pradeepa R, Mahanta J, Narain K, Das HK, et al. Prevalence of diabetes and prediabetes in 15 states of India: Results from the ICMR-INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5:585–96. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- 9.Anjana RM, Unnikrishnan R, Deepa M, Pradeepa R, Tandon N, Das AK, et al. Metabolic non-communicable disease health report of India: The ICMR-INDIAB national cross-sectional study (ICMR-INDIAB-17) Lancet Diabetes Endocrinol. 2023;11:474–89. doi: 10.1016/S2213-8587(23)00119-5. [DOI] [PubMed] [Google Scholar]
- 10.Nussinovitch U, Cohen O, Kaminer K, Ilani J, Nussinovitch N. Evaluating reliability of ultra-short ECG indices of heart rate variability in diabetes mellitus patients. J Diabetes Complications. 2012;26:450–3. doi: 10.1016/j.jdiacomp.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 11.Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Task force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 12.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017;5:258. doi: 10.3389/fpubh.2017.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ravichandran S, Srivastav S, Kamble PH, Chambial S, Shukla R, Sharma P, et al. VEGF-A and cardiac autonomic function in newly diagnosed type 2 diabetes mellitus: A cross-sectional study at a tertiary care center. J Family Med Prim Care. 2019;8:3185–90. doi: 10.4103/jfmpc.jfmpc_537_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunikullaya UK, Kunnavil R, Vijayadas, Goturu J, Prakash VS, Murthy NS. Normative data and gender differences in heart rate variability in the healthy young individuals aged 18-30 years, a South Indian cross-sectional study. Indian Pacing Electrophysiol J. 2021;21:112–9. doi: 10.1016/j.ipej.2021.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saghaei M. Random allocation software for parallel group randomized trials. BMC Med Res Methodol. 2004;4:26. doi: 10.1186/1471-2288-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–63. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamuli D, Kaur M, Sethi T, Singh A, Faruq M, Jaryal AK, et al. Cortical and subcortical brain area atrophy in SCA1 and SCA2 patients in India: The structural MRI underpinnings and correlative insight among the atrophy and disease attributes. Neurol India. 2021;69:1318–25. doi: 10.4103/0028-3886.329596. [DOI] [PubMed] [Google Scholar]
- 18.Stein PK, Pu Y. Heart rate variability in congestive heart failure. In: Kamath MV, Watanabe MA, Upton ARM, editors. Heart rate variability (HRV) signal analysis clinical applications. Florida (USA): CRC Press; 2013. pp. 303–24. [Google Scholar]
- 19.Gerasimova-Meigal L, Meigal A, Sireneva N, Saenko I. Autonomic function in Parkinson's disease subjects across repeated short-term dry immersion: Evidence from linear and non-linear HRV parameters. Front Physiol. 2021;12:712365. doi: 10.3389/fphys.2021.712365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saroha D, Panda S, Deora S, Mohammed S. Cardiac abnormalities in refractory status epilepticus-an exploratory study. J Epilepsy Res. 2023;13:42–50. doi: 10.14581/jer.23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burr RL, Motzer SA, Chen W, Cowan MJ, Shulman RJ, Heitkemper MM. Heart rate variability and 24-hour minimum heart rate. Biol Res Nurs. 2006;7:256–67. doi: 10.1177/1099800405285268. [DOI] [PubMed] [Google Scholar]
- 22.Ambarish V, Barde P, Vyas A, Deepak KK. Comparison between pre-prandial and post-prandial heart rate variability (HRV) Indian J Physiol Pharmacol. 2005;49:436–42. [PubMed] [Google Scholar]
- 23.Moya-Ramon M, Mateo-March M, Peña-González I, Zabala M, Javaloyes A. Validity and reliability of different smartphones applications to measure HRV during short and ultra-short measurements in elite athletes. Comput Methods Programs Biomed. 2022;217:106696. doi: 10.1016/j.cmpb.2022.106696. [DOI] [PubMed] [Google Scholar]
- 24.Canino MC, Dunn-Lewis C, Proessl F, LaGoy AD, Hougland JR, Beck AL, et al. Finding a rhythm: Relating ultra-short-term heart rate variability measures in healthy young adults during rest, exercise, and recovery. Auton Neurosci. 2022;239:102953. doi: 10.1016/j.autneu.2022.102953. [DOI] [PubMed] [Google Scholar]
- 25.Karp E, Shiyovich A, Zahger D, Gilutz H, Grosbard A, Katz A. Ultra-short-term heart rate variability for early risk stratification following acute ST-elevation myocardial infarction. Cardiology. 2009;114:275–83. doi: 10.1159/000235568. [DOI] [PubMed] [Google Scholar]
- 26.Perek S, Nussinovitch U, Cohen R, Gidron Y, Raz-Pasteur A. Ultra short heart rate variability predicts clinical outcomes in patients with a clinical presentation consistent with myocarditis: A derivation cohort analysis. J Clin Med. 2022;12:89. doi: 10.3390/jcm12010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartikainen JE, Tahvanainen KU, Kuusela TA. Short-term measurement of heart rate variability. In: Malik M, editor. Clinical guide to cardiac autonomic tests. Dordrecht: Springer Netherlands; 1998. pp. 149–76. [Google Scholar]
- 28.Rajendra Acharya U, Paul Joseph K, Kannathal N, Lim CM, Suri JS. Heart rate variability: A review. Med Biol Eng Comput. 2006;44:1031–51. doi: 10.1007/s11517-006-0119-0. [DOI] [PubMed] [Google Scholar]
- 29.Kowalewski MA, Urban M. Short-and long-term reproducibility of autonomic measures in supine and standing positions. Clin Sci (Lond) 2004;106:61–6. doi: 10.1042/CS20030119. [DOI] [PubMed] [Google Scholar]
- 30.Gisselman AS, D’Amico M, Smoliga JM. Optimizing intersession reliability of heart rate variability-the effects of artifact correction and breathing type. J Strength Cond Res. 2020;34:3199–207. doi: 10.1519/JSC.0000000000002258. [DOI] [PubMed] [Google Scholar]