Abstract
Spinal cord involvement is a rare complication of the schistosomiasis manifesting as myeloradiculopathy, medullary or conus–cauda equina syndrome which can lead to potentially serious long-term disability. Computed tomography and magnetic resonance imaging coupled with biochemical parameters have become the mainstay of diagnosis. Biopsy which is the gold standard of diagnosis demonstrating the organism is usually reserved for cases of diagnostic challenge. We report a rare case of upper thoracic spinal cord schistosomiasis diagnosed by biopsy in an 18-year-old male migrant presenting to a spine and orthopaedic centre in Ghana with complaints of upper back pain and associated myeloradiculopathy symptoms. Initial suspicion of intramedullary cord tumour was made based on magnetic resonance imaging findings warranting biopsy which revealed schistosoma spp. He was treated with anthelminthics and corticosteroids with a resolution of symptoms.
Keywords: Anthelminthics, back, corticosteroids, myeloradiculopathy, pain, schistosomiasis, spinal cord, thoracic
Introduction
Spinal cord manifestation is an uncommon complication of schistosomiasis,[1,2] a parasitic infestation caused by adult blood-borne trematode helminths.[3] The burden of this infection lies within sub-Saharan Africa, South America, the middle-east, and Asia as well as other parts of Africa affecting over 200 million people.[4,5]
This spinal cord manifestation also referred to as neuroschistosomiasis affects 1%–4% of people with systemic disease.[4] The manifestation of this disease includes myeloradiculopathy, medullary or conus–cauda equina syndrome, causing serious and long-term disability.[6]
We report a rare case of upper thoracic spinal cord schistosomiasis diagnosed by biopsy in an 18-year-old male migrant who presented with myeloradiculopathy symptoms with the initial suspicion of intramedullary cord tumour based on MRI findings.
Case Report
Case history
An 18-year-old male from Sierra Leone who had been living in a suburb of Accra for the last 3 years presented with progressive back pain and burning sensation in both lower limbs of 2 months duration. The pain was associated with numbness and paraesthesia along both lower limbs as well as heaviness in both lower limbs. He had also noticed a change in his gait with bowel and bladder dysfunction with inability to feel his legs occasionally. He had been experiencing constipation (passage of stools once a week) for the past two months and recurrent urinary retention and takes almost about 3 h to void. He had no history of PTB, no trauma, no unintentional weight loss, or fever. Upon enquiry after biopsy results, he alluded to fetching water from ponds in his native country and swimming in freshwater as well. However, there was no history of terminal haematuria.
Physical examination results
On examination, was a young male fully conscious with an unsteady gait. There was no deformity or tenderness along the spine.
Apart from the extensor hallucis longus which had a power of 4/5 on the left, all the myotomes had a power of 5/5. The reflexes in both right and left lower limbs were exaggerated with bilateral ankle clonus. There was an altered sensation up to the level of T5. In the upper limbs, the powers and reflexes were normal.
Results of pathological test and other investigations
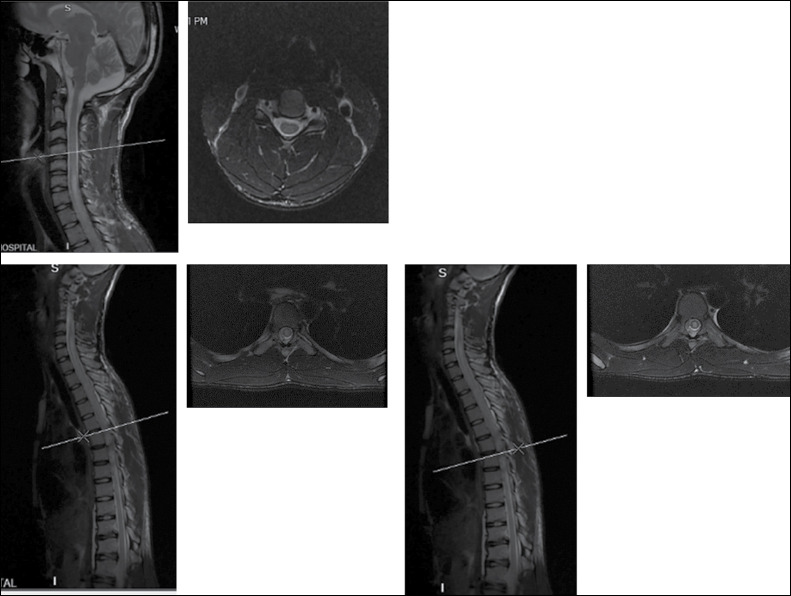
A review of cervicothoracic MRI [Figure 1] showed signal cord changes at C5–C7 level, and an intramedullary mass at T2–T5 with an associated syrinx in the thoracic region. An impression of thoracic myelopathy secondary to intramedullary tumour was made. Blood workup done was normal. The patient and caregiver were counselled for laminectomy, durotomy and biopsy.
Figure 1.
Magnetic resonance imaging showing signal changes at C5–C7 and an intramedullary mass at T2–T5
Approximately 7 weeks after presentation, the patient underwent T3–T4 laminectomy with durotomy and biopsy of the mass [Figure 2]. Intraoperatively, grossly the cord looked normal in size with greyish colour. It was devoid of nodular appearance or gritty texture. The syrinx was drained and a biopsy was undertaken.
Figure 2.
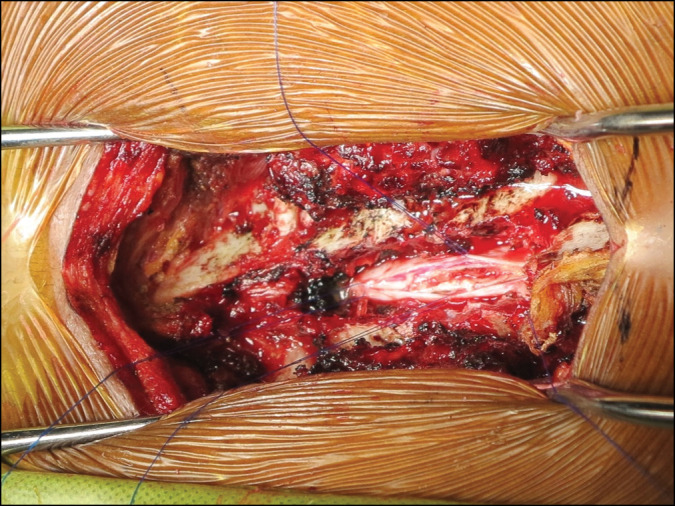
Intraoperative photo showing durotomy
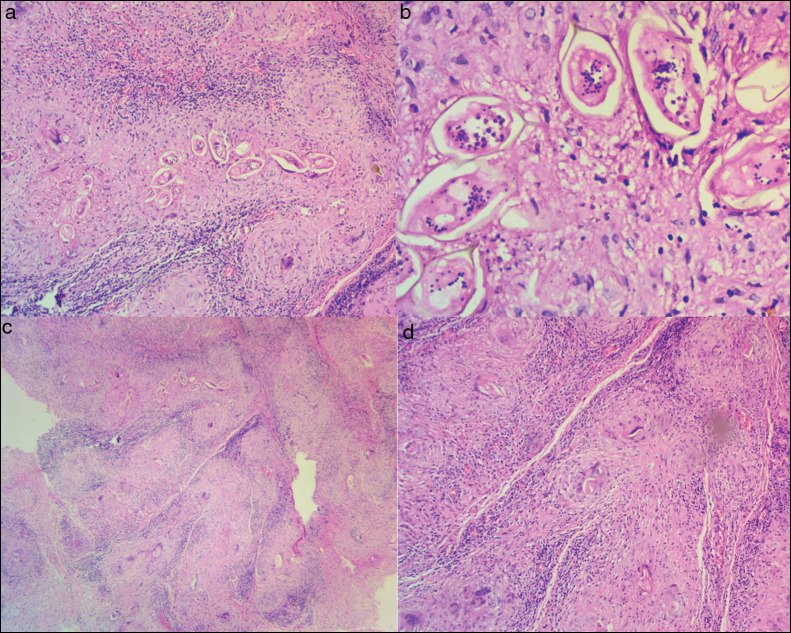
Postoperatively patient was managed on analgesics and steroids awaiting the biopsy results. Macroscopic features at histopathology were multiple irregular soft brown tissue fragments measuring 0.3 cm on average. Microscopic examination showed schistosoma ova (with terminal spine) [Figure 3a and b], inciting granulomatous inflammation [Figure 3c] with background lymphoplasmacytosis [Figure 3d].
Figure 3.
(a and b) Schistosoma ova (with terminal spine) at 40× and 400× magnification respectively (haematoxylin and eosin staining). (c) Ova inciting granulomatous inflammation (magnification—40×). (d) background of lymphoplasmacytosis (magnification—200×) (haematoxylin and eosin staining)
Treatment plan
Upon consultation with a neurologist and infectious disease specialist, the patient was started on praziquental and the steroids continued as well as pregabalin.
Actual outcome
At review 6 weeks after the biopsy, the burning sensation had significantly improved and the heaviness in the lower limbs had resolved. The power in all myotomes of the lower limbs was 5/5.
Discussion
It is estimated that about 120 million people globally have symptomatic presentation of schistosomiasis with about 20 million people suffering severe disease. Over 90% of patients with neuroschistosomiasis are in sub-Saharan Africa.[7,8,9] The index case is a native of Sierra Leone within the endemic region of sub-Saharan Africa. The most common variants that affect the spinal cord are Schistosoma mansoni and S. haematobium.[10]
The clinical course of this infection can take three forms; myelitis, pseudotumoral or granulomatous and myeloradiculopathy.[11,12] With respect to the spinal cord, the presentation varies from cord (medullary), cord and nerve roots (myeloradicular) to conus and cauda equina manifestation.[5] The index case had features suggestive of the myeloradicular form.
It has been postulated that the organism gains access to neural elements by two mechanisms. Firstly, an increase in intraabdominal pressure causes retrograde flow from the abdominal veins through the Batson plexus which serves as a connection to the spinal venous system. This leads to seeding of the spinal venous plexus with eggs of the organism. The second mechanism is by anomalous migration of the adult worms which are resident in the portal venous system into the spinal venous plexus by the same connection and subsequent deposition of eggs within the neural tissue.[4,13,14]
The deposition of the eggs in the neural tissue evokes an inflammatory response leading to myelitis or on some occasions a granuloma formation which destroys the cord producing a mass effect.[13,14]
The anatomic location of cord involvement has been shown to vary in the literature. Ferrari reported the lower thoracic as the commonest location followed by the lumbar region in a retrospective review of 214 cases.[15] Paz et al. reported lower thoracic/lumbosacral, conus medullaris, and cauda equina as being the commonly affected sites.[16] The presence of the lesion in the upper thoracic further highlights the uncommon presentation of this case.
Neuroschistosomiasis poses a diagnostic challenge, especially in non-endemic regions.[6] Even within endemic regions, the diagnosis can be difficult. Haribhai et al. proposed diagnostic criteria based on clinical, biochemical and radiological markers in the south African population.[17] Similarly, most reports have combined clinical and radiological markers for diagnosis.[6,16] Biopsy of neural tissue and identification of the ova (which may be calcified) or worm within a granulomatous inflammatory process with or without marked fibrosis demonstrable in the index case is the gold standard.[3,10,18,19] However the risks associated with biopsy limits its utilisation.[3]
In this case, the suspicion of an intramedullary tumour of the cord which warranted a biopsy for definitive diagnosis helped clinch the diagnosis. Silver and Martins similarly reported the use of biopsy in the diagnosis based on preliminary suspicion of a cervical cord tumour in a patient in Brazil.[20]
The evolvement of radiological investigations from computed tomography (CT) scans to magnetic resonance imaging (MRI) have become an integral part of the diagnosis demonstrating characteristic features of neuroschistosomiasis aiding the diagnosis. Conventional and computed myelography commonly shows a non-specific intramedullary enlargement in the caudal aspect of the cord.[17,21] MRI findings augmented by contrast show certain features suggestive of neuroschistosomiasis including expansion of cord typically located in the lower cord and conus medullaris), thickening of spinal nerve roots (especially cauda equina), intramedullary oedema, syrinx and heterogeneous multinodular involvement of the cord. These features are as a result of reaction to or the direct presence of the eggs of the organism.[3,4,5,16,18] In the index case the cervicothoracic MRI with contrast showed signal cord changes at C5–C7 and an intramedullary mass at T2–T5 with associated syrinx.
With respect to intra-op findings, Saleem et al. reported a nodular appearance of the cord or gritty feeling of the cord.[5] None of these features were present during the biopsy of this case.
The microscopic pathological assessment of the biopsy specimen shows either the eggs or organism surrounding the inflammatory cells or granuloma within the grey or white matter. Meningeal involvement has also been established in pathological specimens.[5,20] These features were present in this case consistent with what prevails in the literature.
The management options for SCS vary from the use of antihelminthics, corticosteroids to surgery either alone or in combinations, within a background of paucity of consensus guidelines or randomised control trials in the management of the pathology.[6,18] Several reports indicate the combination of steroids and antihelminthics as the mainstay of treatment.[6,16,20,22]
Surgery involving laminectomy and debulking is indicated in patients with pseudotumoral forms or in the form of biopsy when the diagnosis is questionable.[16] Saleem et al. reported an attempt at total resection of the masses in all subjects in addition to combination medical therapy.[5] The role of surgery in this case was the suspicion of intramedullary tumour warranting biopsy for definitive diagnosis.
Reported complications as a result of neuroschistosomiasis include bowel and bladder dysfunction, reduced sensation, the persistence of lower limb weakness and complications associated with chronic steroid use.[6] Ferrari[15] review of neuroschistosomiasis indicated about a third of patients suffered sequelae of the pathology limiting walking, with a mortality of 9.9%.
Conclusion
Spinal cord involvement is a rare complication of schistosomiasis which requires a high index of suspicion and a detailed history for exposure especially in endemic regions. Biopsy of the cord in the phase of diagnostic challenge is an acceptable means of diagnosis. Medical therapy is effective in the treatment of the disease.
Author contribution
KPY, RAW, and DNO designed the study; DNO wrote the introduction; DNO, VN, and FA wrote the methodology, and performed data analysis and interpretation. KPY and RAW drafted the manuscript. All the authors read and approved the final manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
The authors declare no conflicts of interest.
Ethical approval
Institutional review board approval was obtained from the Noguchi Memorial Institute for Medical Research prior to initiation of the study (Certified Protocol Number: 015/14-15revised. 2022). All patients who agreed to partake in this study were given a written consent.
Data availability
Data used for this study is readily available upon request.
Acknowledgements
We would like to express our profound gratitude to FOCOS management for their support throughout the study.
References
- 1.Luyendijk W, Lindeman J. Schistosomiasis (bilharziasis) mansoni of the spinal cord stimulating an intramedullary tumor. Surg Neurol. 1975;4:457–60. [PubMed] [Google Scholar]
- 2.Dar J, Zimmerman RR. Schistosomiasis of the spinal cord. Surg Neurol. 1977;8:416–8. [PubMed] [Google Scholar]
- 3.Ferrari TCA, Moreira PRR. Neuroschistosomiasis: Clinical symptoms and pathogenesis. Lancet Neurol. 2011;10:853–64. doi: 10.1016/S1474-4422(11)70170-3. [DOI] [PubMed] [Google Scholar]
- 4.Vale TC, de Sousa-Pereira SR, Ribas JGR, Lambertucci JR. Neuroschistosomiasis mansoni: Literature review and guidelines. Neurologist. 2012;18:333–42. doi: 10.1097/NRL.0b013e3182704d1e. [DOI] [PubMed] [Google Scholar]
- 5.Saleem S, Belal AI, El-Ghandour NM. Spinal cord schistosomiasis: MR imaging appearance with surgical and pathologic correlation. Am J Neuroradiol. 2005;26:1646–54. [PMC free article] [PubMed] [Google Scholar]
- 6.Wilton A de, Aggarwal D, Jäger HR, Manji H, Chiodini PL. Delayed diagnosis of spinal cord schistosomiasis in a non-endemic country: A tertiary referral centre experience. PLoS NeglTrop Dis. 2021;15:e0009161. doi: 10.1371/journal.pntd.0009161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engels D, Chitsulo L, Montresor A, Savioli L. The global epidemiological situation of schistosomiasis and new approaches to control and research. Acta Trop. 2002;82:139–46. doi: 10.1016/s0001-706x(02)00045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ross AGP, Bartley PB, Sleigh AC, Olds GR, Li Y, Williams GM, et al. Schistosomiasis. N Engl J Med. 2002;346:1212–20. doi: 10.1056/NEJMra012396. [DOI] [PubMed] [Google Scholar]
- 9.Joshi TN, Yamazaki MK, Zhao H, Becker D. Spinal schistosomiasis: Differential diagnosis for acute paraparesis in a U.S. resident. J Spinal Cord Med. 2010;33:256–60. doi: 10.1080/10790268.2010.11689703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carod-Artal FJ. Neurological complications of Schistosoma infection. Trans R Soc Trop Med Hyg. 2008;102:107–16. doi: 10.1016/j.trstmh.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Faust EC. An inquiry into the ectopic lesions in schistosomiasis. Am J Trop Med Hyg. 1948;s1-28:175–99. doi: 10.4269/ajtmh.1948.s1-28.175. [DOI] [PubMed] [Google Scholar]
- 12.Bird AV. Spinal cord complications of Bilharziasis. S Afr Med J. 1965;39:158–62. [PubMed] [Google Scholar]
- 13.Pittella JEH. Neuroschistosomiasis. Brain Pathol. 1997;7:649–62. doi: 10.1111/j.1750-3639.1997.tb01080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scrimgeour EM, Gajdusek DC. Involvement of the central nervous system in Schistosoma mansoni and S. haematobium infection. A review. Brain. 1985;108:1023–38. doi: 10.1093/brain/108.4.1023. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari TCA. Spinal cord schistosomiasis: A report of 2 cases and review emphasizing clinical aspects. Medicine (Baltimore) 1999;78:179–90. doi: 10.1097/00005792-199905000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Paz J, Valente M, Casella E, Marques Dias MJ. Spinal cord schistosomiasis in children: Analysis of seven cases. Arq Neuropsiquiatr. 2002;60:224–30. doi: 10.1590/s0004-282x2002000200007. [DOI] [PubMed] [Google Scholar]
- 17.Haribhai HC, Bhigjee AI, Bill PL, Cosnett JE. Schistosoma in the spinal cord. J Neurol Neurosurg Psychiatry. 1988;51:158. doi: 10.1136/jnnp.51.1.158-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adeel AA. Spinal cord schistosomiasis. Sudan J Paediatr. 2015;15:23–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Goldblum J, Lamps L, McKenney J, Myers J. Rosai and Ackerman’s Surgical Pathology. 11th ed. Vol. 2. Cambridge, MA: Elsevier; 2017. pp. 626–7. [Google Scholar]
- 20.Luciana CS, Silver C, Martins K, José RL. Cervical spinal cord schistosomiasis. Rev Soc Bras Med Trop. 2002;35:35–40. doi: 10.1590/s0037-86822002000500023. [DOI] [PubMed] [Google Scholar]
- 21.Herskowitz A. Spinal cord involvement with Schistosoma mansoni: Case report. J Neurosurg. 1972;36:494–8. doi: 10.3171/jns.1972.36.4.0494. [DOI] [PubMed] [Google Scholar]
- 22.Haribhai HC, Bhigjee AI, Bill PLA, Pammenter MD, MODI G, Hoffmann M, et al. Spinal cord schistosomiasis: A clinical, laboratory and radiological study, with a note on therapeutic aspects. Brain. 1991;114:709–26. doi: 10.1093/brain/114.2.709. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used for this study is readily available upon request.