Abstract
To identify improved adenovirus vectors for cardiovascular gene therapy, a library of adenovirus vectors based on adenovirus serotype 5 (Ad5) but carrying fiber molecules of other human serotypes, was generated. This library was tested for efficiency of infection of human primary vascular endothelial cells (ECs) and smooth muscle cells (SMCs). Based on luciferase, LacZ, or green fluorescent protein (GFP) marker gene expression, several fiber chimeric vectors were identified that displayed improved infection of these cell types. One of the viruses that performed particularly well is an Ad5 carrying the fiber of Ad16 (Ad5.Fib16), a subgroup B virus. This virus showed, on average, 8- and 64-fold-increased luciferase activities on umbilical vein ECs and SMCs, respectively, compared to the parent vector. GFP and lacZ markers showed that approximately 3-fold (ECs) and 10-fold (SMCs) more cells were transduced. Experiments performed with both cultured SMCs and organ cultures derived from different vascular origins (saphenous vein, iliac artery, left interior mammary artery, and aorta) and from different species demonstrated that Ad5.Fib16 consistently displays improved infection in primates (humans and rhesus monkeys). SMCs of the same vessels of rodents and pigs were less infectable with Ad5.Fib16 than with Ad5. This suggests that either the receptor for human Ad16 is not conserved between different species or that differences in the expression levels of the putative receptor exist. In conclusion, our results show that an Ad5-based virus carrying the fiber of Ad16 is a potent vector for the transduction of primate cardiovascular cells and tissues.
Delivery of transgenes to the vessel wall in vivo is one of the major challenges for those who are developing gene therapy for treatment of cardiovascular disease, in particular for preventing restenosis after angioplasty or bypass surgery. The potency of several gene therapy approaches using genes such as those encoding p21 (33, 34), ATF-BPTI (22), and nitric oxide synthase (16, 28, 29) is now being tested in various in vitro and in vivo models, using different gene delivery vehicles (16, 28). However, progress with this gene therapy approach has been seriously hampered due to the inefficient delivery of genes to the vessel wall. The delivery vehicle of choice is a replication-deficient adenoviral vector, which in most cases is based on adenovirus 5 (Ad5). This adenovirus was chosen because of its broad host range, because of its high and transient levels of transgene expression, and because adenoviruses are not complement inactivated such that in vivo delivery is feasible (reviewed in reference 5). Unfortunately, subgroup C adenoviruses, to which Ad5 belongs, transduce smooth muscle cells (SMCs) very poorly, most likely because they require the coxsackie adenovirus receptor (CAR) (14, 19), which is not detectable on these cells using a flow cytometer. Experiments performed by us and others have shown that SMCs can only be transduced using high multiplicities of infection (MOIs) of adenovirus per cell.
Thus, adenovirus as a gene delivery vehicle for treatment of cardiovascular disease holds great promise but should be improved in terms of gene transfer to SMCs. Several groups have reported on improved adenoviral vectors for cardiovascular tissues, showing approximately three- to eightfold improvement in the transduction of either endothelial cells (ECs) or SMCs (10, 30). At present, 51 different human adenovirus serotypes have been identified. Since the fiber molecule of an adenovirus serotype determines its host range, we generated a library of Ad5-based vectors carrying the fibers of alternative serotypes. This strategy was chosen to maximize the chance that a recombinant vector can be generated and propagated reproducibly to high titers. From this library, several fiber chimeric adenoviruses were tested on human SMCs taking Ad5 as a reference. An Ad5 that carries the fiber of Ad16 (Ad5.Fib16) showed an improved transduction rate on human SMCs. Furthermore, we tested the transduction capacity of Ad5.Fib16 on organ cultures of blood vessels of various species, including humans, pigs, rhesus monkeys, and rats. This was done in order to identify suitable animal models for the use of Ad5.Fib16-based cardiovascular gene transfer. In summary, the present report shows that we have identified an adenovirus vector, Ad5.Fib16, with improved infection characteristics on SMCs and ECs compared to the parent vector. This virus is expected to improve the therapeutic window for the development of gene therapy for the treatment of cardiovascular disease.
MATERIALS AND METHODS
Construction of pBr/Ad.BamRΔFIB.
Plasmid pBr/Ad.Bam-rITR contains the Ad5 sequence from the BamHI site (nucleotide [nt] 21562) until the 3′ end. This plasmid was used as a template for PCR to delete fiber sequences (nt 31042 to 32787). For this purpose, a PCR was performed using the oligonucleotides NY-up (5′-CGACATATGTAG ATGCATTAGTTTG TGTT ATGTTTCAACGTG-3′), which contains both an NdeI site and an NsiI site, and NY-down (5′-GGAGACCACTGCCATGTTG-3′). The expected 2,200-bp DNA fragment was digested with SbfI (present just upstream of NY-down) and NdeI and was subsequently cloned into an SbfI- and NdeI-digested plasmid, pBr/Ad.Bam-rITR. The resulting plasmid, pBr/Ad.BamRΔFIB, thus lacks part of the fiber starting from the NdeI site until the stop codon but instead contains a unique NsiI site directly after the fiber stop codon. The restriction sites NdeI and NsiI were introduced into the tail of degenerate oligonucleotides used to amplify fiber sequences from alternative adenovirus serotypes and for subsequent cloning into pBr/Ad.BamRΔFIB. All human wild-type adenoviruses (from Ad1 to Ad51), except for serotypes 40 and 41, were propagated on PER.C6 (11), after which virus DNA was isolated from crude lysate as described previously (35). The serotypes Ad40 and Ad41 were propagated to low titers on PER.C6 cells. Although low titers were obtained, sufficient DNA could be isolated for PCR amplification of fiber molecules.
The plasmid library generated was coded pBr/Ad.BamRΔFIBXX (where “XX” represents the serotype number from which the fiber was amplified). The amplified sequences inserted in the pBr/Ad.BamRΔFIBXX constructs were sequenced to confirm the existence of an open reading frame. Protein sequences were aligned and putative fiber domains, such as the trimerization domain (TLWT), were localized. To maximize the chance of generating fiber chimeric recombinant adenoviruses, an additional cloning step was performed. All pBr/Ad.BamRFIBXX constructs, as well as plasmid pBr/Ad.AflII-BamHI, were digested with BamHI and PacI, separated on a gel, and isolated using agarase enzyme (Roche, Almere, The Netherlands) according to the instructions supplied by the manufacturer. Contruct pBR/Ad.AflII-BamHI contains the Ad5 viral genome sequence from AflII to BamHI (nt 3534 to 21566). Just upstream of AflII, a PacI site was generated to allow PacI-BamHI release of the Ad5 genome sequence. This fragment, together with a BamHI-PacI fragment isolated from Pbr/Ad.BamHIRFibXX and a PacI-digested pWE cosmid vector, results in the formation of pWE/Ad.AflII-rITRFibXX construct. The fragments obtained were subsequently cloned (three-point ligation) into a PacI-digested pWE.pac cosmid using a packaging kit according to the instructions provided by the manufacturer (Stratagene).
Cosmid pWE.pac was generated from pWE15 (Clontech) by inserting into the EcoRI sites a synthetic DNA fragment containing a PacI restriction site. Cosmids obtained were verified with restriction enzyme analysis. The cosmid library generated was coded pWE/Ad.AflII-rITR.pac/FibXX. To generate recombinant adenovirus, two DNA molecules were cotransfected on PER.C6 cells: (i) pWE/Ad.AflII-rITR/FibXX linearized with PacI and (ii) a plasmid encoding the Ad5 sequence, in which the E1 region is replaced by a marker gene. This plasmid, either coded pClip or pAdapt, contains the Ad5 sequence from nt 1 to 454 (left ITR and packaging signal), a cassette for transgene expression containing the cytomegalovirus (CMV) promoter (nt −601 to −14 [pClip] or nt −735 to +95 [pAdapt]), a polylinker, Simian virus 40 (SV40) intron-poly (A) from pcDNA1 (HhaI-AvrII fragment; Invitrogen), and a second Ad5 sequence ranging from nt 3511 to 6095. pAdapt lacks the SV40 intron sequences. The Ad5 sequence (nt 3511 to 6095) enables the generation of recombinant adenovirus through homologous recombination with pWE/Ad.AflII-rITR/FibXX on PER.C6 cells.
Generation and purification of fiber chimeric adenovirus.
To generate recombinant virus, both plasmids described above were transfected in PER.C6 using Lipofectamine according to the instructions provided by the manufacturer (Life Technologies). At 24 h prior to transfection, PER.C6 cells were seeded at a cell density of 3.5 × 106 cells in poly-l-lysine-coated T25 flasks and cultured overnight at 37°C. Six days after transfection, cells were harvested, freeze-thawed, centrifuged for 5 min at 3,000 rpm, and stored at −20°C. Of the crude lysate, 3 to 5 ml was used to inoculate 4×T175 three-layer flasks containing 70% confluent layers of PER.C6 cells. Usually a full cytopathic effect is obtained within 2 to 4 days. The virus is purified using a two-step CsCl purification method. Finally, the virus was stored in aliquots at −85°C. The number of virus particles per ml was determined by high-pressure liquid chromatography as described elsewhere (26). The production yields in virus particles per milliliter of batches of fiber chimeric viruses used in the experiments were as follows: Ad5.Luc (three batches, 2.2 × 1011, 1.6 × 1012, and 4.3 × 1012), Ad5.GFP (two batches, 8.4 × 1011 and 5.1 × 1011), Ad5.LacZ (two batches, 1.3 × 1012 and 5.0 × 1011), Ad5.Fib11.Luc (1.1 × 1012), Ad5.Fib35.Luc (two batches, 1.4 × 1012 and 2.0 × 1012), Ad5.Fib16.Luc (six batches, range of 9.0 × 1011 to 3.6 × 1012), Ad5.Fib51.Luc (three batches, 1.0 × 1012, 3.2 × 1012, and 5.1 × 1012), Ad5.Fib16.GFP (two batches, 4.8 × 1011 and 5.1 × 1011), Ad5.Fib16.LacZ (three batches, 4.6 × 1012, 5.3 × 1011, and 5.2 × 1011), and Ad5.Fib51.LacZ (2.1 × 1012). Luciferase and LacZ are driven by the weak CMV promoter (pClip), whereas green fluorescent protein (GFP) is driven by the normal CMV promoter (pAdapt).
Isolation and transduction of SMCs and ECs.
ECs and SMCs were isolated and cultured as described previously (15, 21, 31). Cultures of primary SMCs and ECs, isolated and used in all experiments described, were tested for purity using SMC–α-actin antibodies, DiI-AcLDL uptake, or antibodies against CD31 or van Willebrand factor (data not shown). Cells were seeded in 24-well plates at 4 × 104 cells per well. At 24 h after seeding, cells were washed with medium containing 0.1% human serum albumin (HSA) and incubated for 2 h in 200 μl of medium containing increasing amounts of virus particles. Cells were subsequently washed with medium containing 0.1% HSA, and fresh medium, without virus particles, supplemented with serum was added. Cells were analyzed for transgene expression after 48 h at 37°C in 5% CO2. LacZ, luciferase, and GFP expression as markers for gene transfer have been reported previously (4, 7–9, 20). Cell staining for expression of the CAR and αv-integrins has been described previously (23).
Organ cultures.
The culture of the vessel wall segments was done as described previously (22). All human vessel specimens were obtained according to the guidelines of the Institutional Review Board. Briefly, segments were collected in sterile RPMI 1640 tissue culture medium supplemented with 20 mmol of HEPES buffer (Life Technologies) per liter, 4 IU of sodium heparin (Leo Pharmaceuticals, Weesp, The Netherlands) per ml, 2.5 μg of gentamicin per ml, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 2 mmol of l-glutamine per liter, and 2.5 μg of amphotericin B per ml. Excess fat and adventitial connective tissue were gently removed. Vessel segments were either cut into 5-mm rings or opened longitudinally along their upper aspects and separated into small pieces of 5 by 5 mm. After infection, the vessel segments were cultured for 48 h at 37°C in a humidified atmosphere of 5% CO2 in air in the culture medium described above without heparin but supplemented with 30% heat-inactivated fetal calf serum (FCS). Infections were performed at 37°C for 1 to 1.5 h. The vessel segments were transferred from the infection medium to the culture medium (RPMI 1640–30% FCS) and cultured for 48 h. Tissue segments were fixed in 2% formaldehyde–0.25% glutaraldehyde in phosphate-buffered saline (PBS) for 15 min, washed with PBS for 15 min, and stained for β-galactosidase activity for 6 h at 37°C. Samples were fixed overnight poststaining and subsequently stored in PBS at 4°C. Macroscopic pictures were taken using digital imaging equipment. Segments were embedded in paraffin, cross-sectioned at 10 μm, and counterstained with hematoxylin-phloxin-saphrane.
RESULTS
Human SMCs and ECs.
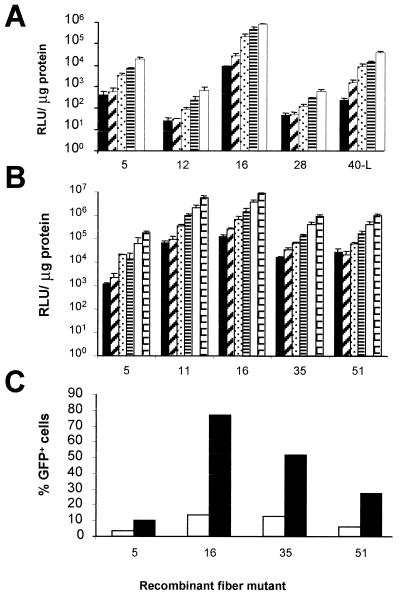
A small panel was initially chosen from the adenovirus fiber library to test whether the infection efficiency of human SMCs could be improved. The panel consisted of the parent vector (Ad5) and the fiber mutants 12, 16, 28, and 40-L, representing subgroups C, A, B, D, and F, respectively. Fiber 40-L represents the “long” fiber of human serotype 40. Based on luciferase transgene expression measured in human umbilical vein SMCs 48 h after infection, the subgroup B fiber 16 chimeric virus, Ad5.Fib16, gave the highest transgene expression, which proved to be approximately 100-fold higher than that with Ad5 (Fig. 1A). Ad5.Fib16.Luc was subsequently compared in several experiments (n = 8) to Ad5.Luc on the SMCs from human umbilical vein. On average, Ad5.Fib16.Luc yielded (64 ± 20)-fold (mean ± the standard error of the mean [SEM])-increased luciferase transgene expression compared to Ad5.Luc.
FIG. 1.
(A) Luciferase activity, expressed in relative light units (RLU) per microgram of total cellular protein, in human umbilical vein SMCs at 48 h after a 2-h exposure to 50, 250, 1,250, 2,500, or 5,000 virus particles of Ad5, Ad5.Fib12, Ad5.Fib16, Ad5.Fib28, or Ad5.Fib40-L per cell. Values represent the mean ± the standard deviation (SD) of three samples. (B) Luciferase activity in human umbilical vein SMCs 48 h after a 2-h exposure to 312, 625, 1,250, 2,500, 5,000, or 10,000 virus particles of Ad5, Ad5.Fib11, Ad5.Fib16, Ad5.Fib35, or Ad5.Fib51 per cell. Values represent the mean ± the SD of three samples. (C) GFP expression measured 48 h after a 2-h exposure to 250 (white bar) or 2,500 (black bar) virus particles of Ad5, Ad5.Fib16, Ad5.Fib35, or Ad5.Fib51 per cell. Cells not exposed to virus were used to set the flow cytometer at a background level of 1%. Values represent the average of two samples.
Next, several other subgroup B members were tested to investigate whether the improved infection of human SMCs could be obtained with any subgroup B fiber. Ad5-based vectors carrying the fibers of serotypes 11, 16, 35, and 51 were all superior to Ad5, although small differences in the levels of transgene expression were observed between the subgroup B fibers (Fig. 1B and C).
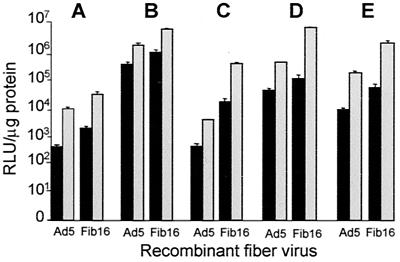
To investigate whether the improved transduction observed with Ad5.Fib16 was consistent for SMCs from different vascular origins, we isolated and transduced SMCs from the iliac artery, the left interior mammary artery (LIMA), aorta, and the saphenous vein. The results (Fig. 2) indicated that in human SMCs, Ad5.Fib16 yields 10- to 100-fold-increased luciferase transgene expression compared to the parent vector. The variability in luciferase activity measured in SMCs of different origins reflect differences in the transduction efficiencies of SMCs, since these cell types were seeded, infected, and analyzed simultaneously.
FIG. 2.
Luciferase transgene expression in human SMCs of various origins measured 48 h after a 2-h exposure to 50 (black bars) or 2,500 (gray bars) virus particles of Ad5 or Ad5.Fib16 per cell. (A) Iliac artery. (B) LIMA. (C) Umbilical vein. (D) Aorta. (E) Saphenous vein. Values represent the mean ± the SD of three samples.
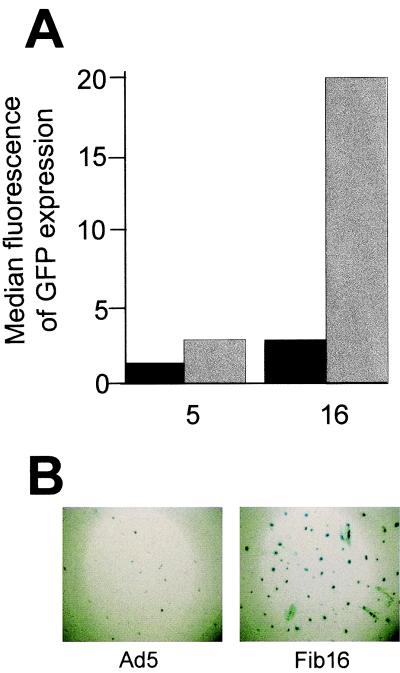
To exclude the possibility that differences in the transgene expression levels observed between fiber chimeric vectors are due to the efficiency of virus production, Ad5 was compared to Ad5.Fib16 in several experiments (n = 6) using either LacZ or GFP as a marker; representative results are shown in Fig. 3. Analysis of GFP expression indicated that a 10-fold-lower dose of Ad5.Fib16 can be used to obtain comparable levels of transgene expression in umbilical vein SMCs (Fig. 3A). Scoring for LacZ-positive cells revealed that approximately 10-fold-more β-galactosidase-positive cells were obtained after transduction with Ad5.Fib16 compared to transduction with Ad5 (Fig. 3B).
FIG. 3.
(A) Human umbilical vein SMCs were exposed for 2 h to 500 (black bars) or 5,000 (gray bars) virus particles of Ad5 or Ad5.Fib16 per cell. Cells not exposed to virus were used to set a background fluorescence level of 3. Shown is the median fluorescence of GFP expression as measured by the flow cytometer 48 h after virus exposure. (B) Human aorta SMCs were exposed for 2 h to 5,000 virus particles of Ad5 or Ad5.Fib16 per cell. The percentage of cells positive for β-galactosidase activity was determined by cell count and proved in this particular experiment to be 4.8-fold higher with Ad5.Fib16 than with Ad5 (n = 3 wells per virus).
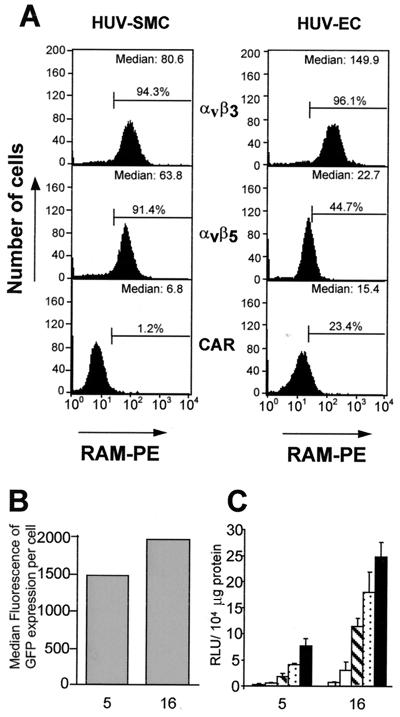
Human ECs express low amounts of the CAR, in contrast to human SMCs, which are negative for CAR (Fig. 4A). As a consequence, ECs can be better transduced with recombinant Ad5 vectors. Ad5 was compared to Ad5.Fib16 in several experiments (n = 9) with luciferase, LacZ, or GFP as a marker. Representative experiments are shown in Fig. 4B and C. Based on the data presented in Fig. 4C, an (8 ± 3)-fold (mean ± the SEM)-better transduction was consistently observed with Ad5.Fib16 compared to Ad5. However, ECs derived from various origins, i.e., microvascular, aortic, or umbilical-vein ECs displayed differences in adenovirus susceptibility both for Ad5 and for Ad5.Fib16, in such a way that although all ECs were better infected by Ad5.Fib16, the fold improvement in transducibility by Ad5.Fib16 differed per type of EC, as did the absolute degree of transduction by either Ad5 of Ad5.Fib16 (data not shown).
FIG. 4.
(A) Flow cytometric detection for expression on human umbilical vein (HUV) SMCs and ECs of the CAR or integrin αvβ3 or αvβ5. Cells stained only with the secondary antibody (RAM-PE) were used to set a background level of 1%. Values represent the percentages of cells scored positive for expression of CAR, αvβ3, or αvβ5. (B) Human ECs derived from the aorta were exposed for 2 h to 500 virus particles of Ad5 or Ad5.Fib16 per cell. Shown is the median fluorescence of GFP expression 48 h after virus exposure. Values represent the average of two samples. (C) Human ECs derived from the aorta were exposed for 2 h to 50, 250, 1,000, 2,500, 5,000, or 10,000 virus particles of Ad5 or Ad5.Fib16 per cell. Luciferase activity was measured 48 h after virus exposure. Values represent the mean ± the SD of three samples.
Human organ culture.
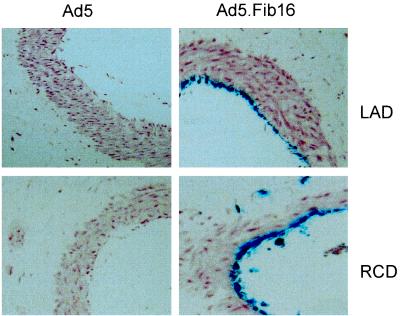
To extend our investigations into the efficiency of transduction of Ad5.Fib16, organ cultures of blood vessel segments were used. As a target tissue for the prevention of restenosis following percutaneous transluminal coronary angioplasty (PTCA), we focused on the left and right coronary arteries descending (LAD and RCD, respectively). For these experiments, Ad5.LacZ and Ad5.Fib16.LacZ were used. Small pieces of both the LAD and the RCD (ca. 3 to 5 mm) derived from a human hypertrophic heart were exposed for 2 h to 1010 virus particles and were stained 48 h later for LacZ expression. Histological analysis on both arteries demonstrated that the transduction of endothelial cells is much more effective when using Ad5.Fib16 than when using Ad5 (Fig. 5). The difference between Ad5 and Ad5.Fib16 in these experiments proved to be much higher than expected based on cell culture experiments. The experiments described above thus indicate that Ad5.Fib16 is superior to Ad5 for the genetic modification of human SMCs and ECs. The data shown demonstrate consistency, not only between cells derived from various origins, but also using different batches of fiber chimeric virus, as well as batches of fiber chimeric viruses carrying different marker genes.
FIG. 5.
Histologic sections of the LAD and the RCD. Arteries were dissected from a hypertrophic human heart and exposed for 2 h to 1010 virus particles of Ad5.LacZ or Ad5.Fib16.LacZ. Cells were fixed and stained for β-galactosidase expression 48 h post-virus exposure. Cells positive for β-galactosidase expression were scored as ECs with an occasional macrophage-like cell. An extensive neointima formation (dark red area) is clearly visible between the EC layer and the SMC layer (light red area).
Nonhuman SMCs.
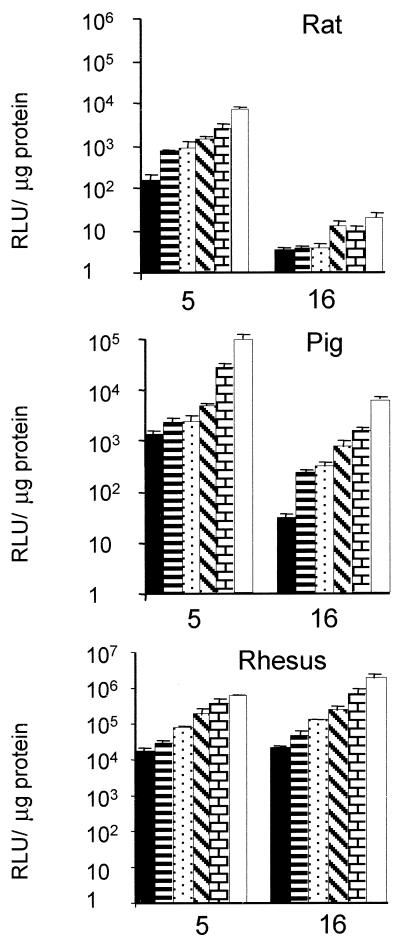
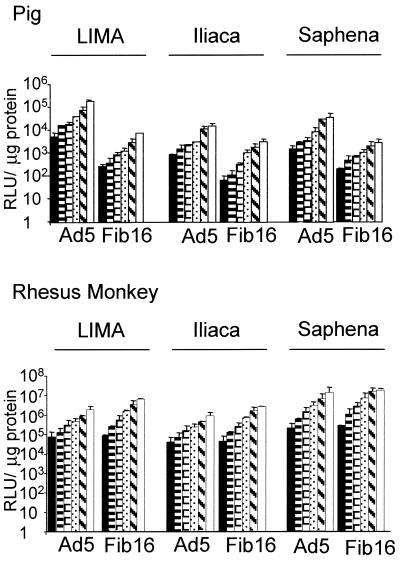
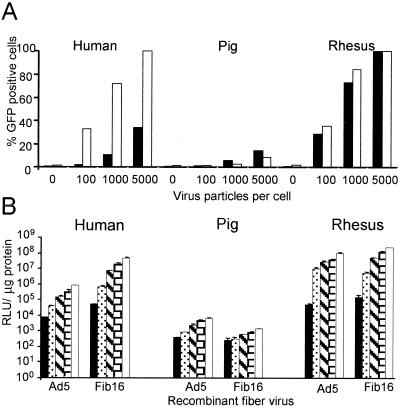
We next turned to SMCs from different species to identify a suitable in vivo animal model for testing Ad5.Fib16. We focused on the pig, the rhesus monkey, and the rat. For these three species, SMCs derived from the aorta were tested. Infection of SMCs from rhesus monkeys with Ad5.Fib16 is superior (ca. fivefold) to Ad5 infection, based on luciferase transgene expression (Fig. 6). In contrast, Ad5.Fib16.Luc gave approximately 5- to 10-fold and 100-fold less luciferase activity in pig and rat SMCs, respectively, compared to Ad5.Luc. To obtain detectable transgene expression in SMCs derived from pig aorta in this experiment, the dose of adenovirus needed to be much higher than the dose needed for the transduction of SMC derived from either the rat or the rhesus monkey. Furthermore, a panel of SMCs derived from different origins of pig or rhesus monkey was infected. The panel consisted of the iliac artery, the LIMA, and the saphenous vein. These experiments confirmed that SMCs from pig are less susceptible to Ad5.Fib16 than to Ad5. The observed transduction was clearly virus dose dependent (Fig. 7). To compare the susceptibility of saphenous vein SMCs derived from different species toward Ad5 and Ad5.Fib16, cells derived from pig, rhesus monkey, and human sources were cultured, infected, and analyzed simultaneously. The virus used either carried GFP or luciferase as a marker gene. This experiment confirms that Ad5.Fib16 does not infect porcine SMCs with a higher efficiency than that of Ad5, whereas it does in primates. Also, the results show that for Ad5 a 5- or 50-fold-higher virus dose is required on porcine SMCs to obtain transgene expression levels similar to those observed in human or rhesus monkey SMCs, respectively. The latter finding indicates that for Ad5 differences also exist in the transduction efficiency of SMCs derived from different species (Fig. 8).
FIG. 6.
Luciferase transgene expression in aortic SMCs derived from various species as determined 48 h post-virus exposure. Rat and rhesus monkey aortic SMCs were exposed for 2 h to 156, 312, 625, 1,250, 2,500, or 5,000 virus particles of Ad5 or Ad5.Fib16 per cell. Porcine aortic SMCs were exposed for 2 h to 780, 1,560, 3,125, 6,250, 12,500, or 25,000 virus particles of Ad5 or Ad5.Fib16 per cell. Values represent the mean ± the SD of three samples.
FIG. 7.
Transgene expression upon infection with Ad5 or Ad5.Fib16 of SMCs derived from porcine or rhesus monkey iliac artery, LIMA, or saphenous vein. Porcine and rhesus monkey SMCs were exposed for 2 h to 780, 1,560, 3,125, 6,250, 12,500, or 25,000 (pig) or 156, 312, 625, 1,250, 2,500 or 5,000 (monkey) virus particles per cell and analyzed 48 h after virus exposure for luciferase activity. Values represent the mean ± the SD of three samples.
FIG. 8.
(A) GFP expression in saphenous vein SMCs of different origins. Cells were exposed for 2 h to 0, 100, 1,000, or 5,000 virus particles of Ad.5 (black bar) or Ad5.Fib16 (white bar) per cell. Shown is the percentage of cells positive for GFP, as determined by flow cytometry, at 48 h after infection. (B) Luciferase transgene expression in saphenous vein SMCs. Cells were exposed for 2 h to 100, 1,000, 5,000, 10,000, or 20,000 virus particles per cell. Luciferase transgene expression, measured 48 h after infection, is expressed in RLU per microgram of total protein.
Saphenous vein organ culture.
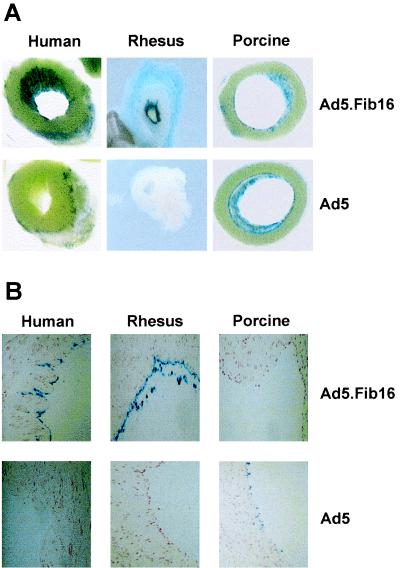
To extend the observations from cell culture experiments, small slices of saphenous vein (ca. 5 mm) derived from human, pig, or rhesus monkey sources were exposed for 2 h to 1010 virus particles and were stained 48 h postinfection to determine β-galactosidase expression. Macroscopic and microscopic histological analysis of the saphenous vein samples (Fig. 9) showed that, in agreement with the cell culture experiments, Ad5.Fib16 is superior to Ad5 for infecting primate vascular tissues. As observed on human coronary arteries, the difference between Ad5 and Ad5.Fib16 on primate saphenous vein samples is more striking than that seen in cell culture experiments. These experiments were performed with an adenoviral vector containing a short CMV promoter (Ad.Clip), known to be less potent than the normal CMV promoter, Ad.Adapt, in order to make the effect of the difference in transfection efficiency more pronounced. In contrast to the cell culture experiments, Ad5 performs better on saphenous vein slices in pig vessels compared to primates, as evidenced by the LacZ-positive cells. The reason for this observation is difficult to understand, but it must be related to differences in the cellular susceptibility to the adenoviruses of cultured cells compared to that of intact tissue.
FIG. 9.
Saphenous vein sections (macroscopic [A] and histological microscopic [B]) stained for LacZ expression 48 h after a 2-h virus exposure (Ad5 or Ad5.Fib16). Sections derived from human or rhesus monkey saphenous veins were found to be negative for LacZ upon transduction with Ad5. With Ad5.Fib16, ECs were stained bright blue. Sections derived from pigs demonstrated a few blue cells with Ad5, whereas no blue cells were detected in sections derived from saphenous vein transduced with Ad5.Fib16.
DISCUSSION
To identify an adenovirus with improved infection characteristics in SMCs and ECs for the treatment of cardiovascular disease, we generated a library of Ad5-based vectors carrying the fiber from alternative human serotypes. An initial screening, using a few fiber chimeric viruses representing different subgroups of human adenoviruses, resulted in the identification of subgroup B fiber chimeric viruses as being superior to Ad5 for infecting human SMCs and ECs. Further analysis identified Ad5.Fib16 as the most potent variant.
The present study, aimed to identify improved adenovirus vectors for cardiovascular tissues, revealed several interesting phenomena that have implications in the search for improved vectors for application in vascular gene therapy.
First, it was observed that the efficiency to infect SMCs derived from various vascular tissues varied, indicating that the expression of the receptor(s) mediating binding and internalization of these viruses was variable in SMCs of different origins. This finding is important for the identification of vectors needed for application in a specific vascular tissue, e.g., coronary artery-specific infection after PTCA or saphenous vein-specific infection during bypass vein graft surgery.
A second interesting observation is the fact that, although all of the subgroup B-derived fiber chimeric vectors showed a better infection of human vascular cells compared to the parent Ad5 vectors, small but consistent differences were found in the levels of transgene expression obtained using different Ad5-based vectors carrying subgroup B fibers. Studies performed by Roelvink et al. (24) have demonstrated that subgroup B adenoviruses do not utilize the CAR. An adenovirus subgroup B receptor has not yet been identified. The differences in transgene expression levels between viruses carrying the fiber of serotypes 11, 35, and 51 might reflect differences in receptor usage of subgroup B adenoviruses or differences in the affinity to bind to a certain receptor. This hypothesis is supported by recent findings of Shayakhmetov et al., who demonstrated that Ad3 and Ad35 might recognize different receptors (27).
It should be realized that batches of fiber chimeric adenoviruses cannot be quantified based on the amounts of infectious units. The amount of infectious units or PFU is usually determined using cell lines such as 293 or HER.911 (12), which are known to express CAR. However, it is not known how many molecules acting as a receptor for a particular fiber chimeric virus are expressed. Therefore, infection experiments conducted to compare different fiber chimeric adenoviruses have to be performed using equal amounts of virus particles per cell. Since virus titrations on the basis of virus particles per cell could be less accurate compared to titrations made on the basis of IU or PFU per cells, a set of criteria had to be fulfilled in order to identify a vector with impoved infection characteristics for human SMCs and ECs. These criteria were defined to exclude that differences in transgene expression are related to differences in virus quality. These criteria included the use of different virus batches of a particular virus, different cell isolations, and different marker genes. We thus tested several batches of Ad5.Fib16 and Ad5 on SMCs originating from different vessels, using GFP, LacZ, or luciferase as a marker. Since the results of all experiments performed were consistent, we conclude that Ad5.Fib16 represents the most potent vector of the panel tested. This vector was further analyzed on the SMCs of different species.
This analysis led to another interesting observation that relates to the fact that the susceptibilities of SMCs derived from different species toward the chimeric Ad5.Fib16 are variable. We have shown that Ad5.Fib16 improves transgene expression, compared to Ad5, in SMCs derived from humans and the rhesus monkey. In contrast, rat or pig cardiovascular tissues and cells were only poorly transduced with Ad.5Fib16. These latter two animals are most frequently used for cardiovascular studies. It should therefore be realized that the evaluation of Ad5.Fib16, as an imported vector for gene therapy treatment of human cardiovascular disease, could not be performed in animal models such as the rat or the pig. This certainly has major implications in identifying relevant in vivo animal models for testing the efficacy, safety, and toxicity of this fiber-modified adenovirus. The preferred animals for testing Ad5.Fib16 should be nonhuman primates, i.e., baboons, rhesus monkeys, or cynomolgus monkeys, to perform preclinical cardiovascular studies. Several groups have reported that coronary artery atherosclerosis and hypercholesterolemia in nonhuman primates is very similar to human disease, thus demonstrating that these nonhuman primates provide a suitable model for studying gene therapy approaches for the treatment of cardiovascular disease (1, 2, 6, 13, 17, 25).
The findings presented here also stress the importance of identifying the route of entry, the attachment molecules employed, and the tissue-specific expression patterns of molecules that can be utilized by adenovirus vectors genetically modified to enter specific cells of human or nonhuman origin. This latter characteristic certainly will help to predict whether results obtained with an adenovirus gene transfer method can be directly extrapolated from animals to human patients and vice versa. Moreover, detailed knowledge concerning the efficiency of an adenovirus to infect a particular tissue from different animals will help to predict the severity of adenovirus-related toxicity, since this feature of an adenovirus is most likely closely correlated with the ability of the virus to infect a tissue.
Another interesting observation is the discrepancy in the observed transduction efficiencies when either cells or tissues were used. The findings indicate that the susceptibilities to adenovirus of cells in their “native” environment or cells in culture dishes differ strikingly. Usually, results obtained in vivo are disappointing. In the experiments presented here Ad5.Fib16 proved even more superior to Ad5 in organ culture experiments than in cell culture experiments, indicating that vector selection based only on cell transduction experiments is insufficient.
In summary, we have developed a library of Ad5-based vectors carrying the fiber molecule of alternative human adenovirus serotypes. Studies performed with SMCs derived from different species demonstrated the superiority of Ad5.Fib16 in human- and rhesus-derived cardiovascular cells and tissues. However, this superiority could not be reproduced in rat and pig cells, indicating that the attachment molecule utilized by Ad5.Fib16 is not conserved between species or that differences in the levels of expression of the putative attachment molecule exist.
The identification of an adenovirus vector with increased transduction efficiency for SMCs combined with tissue-specific promoters, such as SM22α (3, 18, 32), further confined by using local delivery strategies, should increase efficacy and reduce vector-related toxicity and therefore create more favorable conditions for the treatment of cardiovascular disorders.
ACKNOWLEDGMENTS
We thank J. de Bakker (Department of Experimental Cardiology, Amsterdam Medical Center), J. A. de Bruijn (Department of Pathology, Leiden University Medical Center), and E. Kuhn (Biomedical Primate Research Center, Rijswijk, The Netherlands) for porcine, human, and rhesus cardiovascular tissues, respectively. We also thank J. M. Bergelson (Harvard Medical School, Boston, Mass.) for providing the CAR antibody.
REFERENCES
- 1.Armstrong M L. Atherosclerosis in rhesus and cynomolgus monkeys. Primates Med. 1976;9:16–40. [PubMed] [Google Scholar]
- 2.Armstrong M L, Megan M B, Cheng F H, Warner E D. Dietary disaccharides in experimental atherosclerosis in rhesus monkeys. Exp Mol Pathol. 1976;24:302–319. doi: 10.1016/0014-4800(76)90067-8. [DOI] [PubMed] [Google Scholar]
- 3.Bonin L R, Madden K, Shera K, Ihle J, Matthews C, Aziz S, Perez-Reyes N, McDougall J K, Conroy S C. Generation and characterization of human smooth muscle cell lines derived from atherosclerotic plaque. Arterioscler Thromb Vasc Biol. 1999;19:575–587. doi: 10.1161/01.atv.19.3.575. [DOI] [PubMed] [Google Scholar]
- 4.Bonnerot C, Rocancourt D, Briand P, Grimber G, Nicolas J F. A beta-galactosidase hybrid protein targeted to nuclei as a marker for developmental studies. Proc Natl Acad Sci USA. 1987;84:6795–6799. doi: 10.1073/pnas.84.19.6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bout A. Prospects for human gene therapy. Eur J Drug Metab Pharmacokinet. 1996;21:175–179. doi: 10.1007/BF03190267. [DOI] [PubMed] [Google Scholar]
- 6.Cefalu W T, Terry J G, Thomas M J, Morgan T M, Edwards I J, Rudel L L, Kemnitz J W, Weindruch R. In vitro oxidation of low-density lipoprotein in two species of nonhuman primates subjected to caloric restriction. J Gerontol A Biol Sci Med Sci. 2000;55:B355–B361. doi: 10.1093/gerona/55.7.b355. [DOI] [PubMed] [Google Scholar]
- 7.Chalfie M, Tu Y, Euskirchen G, Ward W W, Prasher D C. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- 8.de Wet J R, Wood K V, DeLuca M, Helinski D R, Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987;7:725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Wet J R, Wood K V, Helinski D R, DeLuca M. Cloning of firefly luciferase cDNA and the expression of active luciferase in Escherichia coli. Proc Natl Acad Sci USA. 1985;82:7870–7873. doi: 10.1073/pnas.82.23.7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dmitriev I, Krasnykh V, Miller C R, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel D T. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fallaux F J, Bout A, van der Velde I, van den Wollenberg D J, Hehir K M, Keegan J, Auger C, Cramer S J, van Ormondt H, van der Eb A J, Valerio D, Hoeben R C. New helper cells and matched early region 1-deleted adenovirus vectors prevent generation of replication-competent adenovirus. Hum Gene Ther. 1998;9:1909–1917. doi: 10.1089/hum.1998.9.13-1909. [DOI] [PubMed] [Google Scholar]
- 12.Fallaux F J, Kranenburg O, Cramer S J, Houweling A, van Ormondt H, Hoeben R C, van der Eb A J. Characterization of 911: a new helper cell line for the titration and propagation of early region 1-deleted adenoviral vectors. Hum Gene Ther. 1996;7:215–222. doi: 10.1089/hum.1996.7.2-215. [DOI] [PubMed] [Google Scholar]
- 13.Heistad D D, Armstrong M L, Marcus M L. Hyperemia of the aortic wall in atherosclerotic monkeys. Circ Res. 1981;48:669–675. doi: 10.1161/01.res.48.5.669. [DOI] [PubMed] [Google Scholar]
- 14.Hsu K H, Lonberg-Holm K, Alstein B, Crowell R L. A monoclonal antibody specific for the cellular receptor for the group B coxsackieviruses. J Virol. 1988;62:1647–1652. doi: 10.1128/jvi.62.5.1647-1652.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaffe E A, Nachman R L, Becker C G, Minick C R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Investig. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janssens S, Flaherty D, Nong Z, Varenne O, Van Pelt N, Haustermans C, Zoldhelyi P, Gerard R, Collen D. Human endothelial nitric oxide synthase gene transfer inhibits vascular smooth muscle cell proliferation and neointima formation after balloon injury in rats. Circulation. 1998;97:1274–1281. doi: 10.1161/01.cir.97.13.1274. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan J R, Manuck S B, Adams M R, Williams J K, Register T C, Clarkson T B. Plaque changes and arterial enlargement in atherosclerotic monkeys after manipulation of diet and social environment. Arterioscler Thromb. 1993;13:254–263. doi: 10.1161/01.atv.13.2.254. [DOI] [PubMed] [Google Scholar]
- 18.Kim S, Lin H, Barr E, Chu L, Leiden J M, Parmacek M S. Transcriptional targeting of replication-defective adenovirus transgene expression to smooth muscle cells in vivo. J Clin Investig. 1997;100:1006–1014. doi: 10.1172/JCI119611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mapoles J E, Krah D L, Crowell R L. Purification of a HeLa cell receptor protein for group B coxsackieviruses. J Virol. 1985;55:560–566. doi: 10.1128/jvi.55.3.560-566.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prendergast F G, Mann K G. Chemical and physical properties of aequorin and the green fluorescent protein isolated from Aequorea forskalea. Biochemistry. 1978;17:3448–3453. doi: 10.1021/bi00610a004. [DOI] [PubMed] [Google Scholar]
- 21.Quax P H A, Grimbergen J M, Lansink M, Bakker A H, Blatter M C, Belin D, van Hinsbergh V W M, Verheijen J H. Binding of human urokinase-type plasminogen activator to its receptor: residues involved in species specificity and binding. Arterioscler Thromb Vasc Biol. 1998;18:693–701. doi: 10.1161/01.atv.18.5.693. [DOI] [PubMed] [Google Scholar]
- 22.Quax P H A, Lamfers M L M, Lardenoye J H P, Grimbergen J M, de Vries M R, Slomp J, de Ruiter M C, Kockx M M, Verheijen J H, van Hinsbergh V W M. Adenoviral expression of a urokinase-receptor-targeted protease inhibitor inhibits neointima formation in murine and human blood vessels. Circulation. 2001;103:562–569. doi: 10.1161/01.cir.103.4.562. [DOI] [PubMed] [Google Scholar]
- 23.Rea D, Schagen F H, Hoeben R C, Mehtali M, Havenga M J, Toes R E, Melief C J, Offringa R. Adenoviruses activate human dendritic cells without polarization toward a T-helper type 1-inducing subset. J Virol. 1999;73:10245–10253. doi: 10.1128/jvi.73.12.10245-10253.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roelvink P W, Lizonova A, Lee J G, Li Y, Bergelson J M, Finberg R W, Brough D E, Kovesdi I, Wickham T J. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol. 1998;72:7909–7915. doi: 10.1128/jvi.72.10.7909-7915.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schneider D B, Fly C A, Dichek D A, Geary R L. Adenoviral gene transfer in arteries of hypercholesterolemic nonhuman primates. Hum Gene Ther. 1998;9:815–821. doi: 10.1089/hum.1998.9.6-815. [DOI] [PubMed] [Google Scholar]
- 26.Shabram P W, Giroux D D, Goudreau A M, Gregory R J, Horn M T, Huyghe B G, Liu X, Nunnally M H, Sugarman B J, Sutjipto S. Analytical anion-exchange HPLC of recombinant type-5 adenoviral particles. Hum Gene Ther. 1997;8:453–465. doi: 10.1089/hum.1997.8.4-453. [DOI] [PubMed] [Google Scholar]
- 27.Shayakhmetov D M, Papayannopoulou T, Stamatoyannopoulos G, Lieber A. Efficient gene transfer into human CD34+ cells by a retargeted adenovirus vector. J Virol. 2000;74:2567–2583. doi: 10.1128/jvi.74.6.2567-2583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varenne O, Pislaru S, Gillijns H, Van Pelt N, Gerard R D, Zoldhelyi P, Van de Werf F, Collen D, Janssens S P. Local adenovirus-mediated transfer of human endothelial nitric oxide synthase reduces luminal narrowing after coronary angioplasty in pigs. Circulation. 1998;98:919–926. doi: 10.1161/01.cir.98.9.919. [DOI] [PubMed] [Google Scholar]
- 29.von der Leyen H E, Gibbons G H, Morishita R, Lewis N P, Zhang L, Nakajima M, Kaneda Y, Cooke J P, Dzau V J. Gene therapy inhibiting neointimal vascular lesion: in vivo transfer of endothelial cell nitric oxide synthase gene. Proc Natl Acad Sci USA. 1995;92:1137–1141. doi: 10.1073/pnas.92.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wickham T J, Tzeng E, Shears L L, Roelvink P W, Li Y, Lee G M, Brough D E, Lizonova A, Kovesdi I. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wijnberg M J, Quax P H A, Nieuwenbroek N M, Verheijen J H. The migration of human smooth muscle cells in vitro is mediated by plasminogen activation and can be inhibited by alpha2-macroglobulin receptor associated protein. Thromb Haemost. 1997;78:880–886. [PubMed] [Google Scholar]
- 32.Yamamura H, Masuda H, Ikeda W, Tokuyama T, Takagi M, Shibata N, Tatsuta M, Takahashi K. Structure and expression of the human SM22alpha gene, assignment of the gene to chromosome 11, and repression of the promoter activity by cytosine DNA methylation. J Biochem. 1997;122:157–167. doi: 10.1093/oxfordjournals.jbchem.a021722. [DOI] [PubMed] [Google Scholar]
- 33.Yang Z, Simari R D, Tanner F, Stephen D, Nabel G J, Nabel E G. Gene transfer approaches to the regulation of vascular cell proliferation. Semin Interv Cardiol. 1996;1:181–184. [PubMed] [Google Scholar]
- 34.Yang Z Y, Simari R D, Perkins N D, San H, Gordon D, Nabel G J, Nabel E G. Role of the p21 cyclin-dependent kinase inhibitor in limiting intimal cell proliferation in response to arterial injury. Proc Natl Acad Sci USA. 1996;93:7905–7910. doi: 10.1073/pnas.93.15.7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang W W, Koch P E, Roth J A. Detection of wild-type contamination in a recombinant adenoviral preparation by PCR. BioTechniques. 1995;18:444–447. [PubMed] [Google Scholar]