Abstract
Background:
In Japan, oral propranolol (PPL) and pulsed dye laser are available for infantile hemangioma (IH) treatment without patient cost-sharing. However, no standardized algorithm exists to guide treatment selection that balances efficacy, potential side effects, and aesthetic risks. This study presents a comprehensive approach utilizing a treatment algorithm and aesthetic risk scoring system.
Methods:
This retrospective study analyzed outcomes from 156 patients with IHs. Oral PPL was used in IH patients with functional issues, whereas the rest underwent an aesthetic risk assessment that categorized them into low-, moderate-, or high-risk groups to guide treatment choices. Final treatment decisions depended on parental preference. The outcomes of algorithm-compliant and noncompliant patients were compared statistically.
Results:
The risk score's interrater reliability was 0.973 (95% confidence interval 0.933–0.992), with a mean intrarater reliability of 0.968 ± 0.027 and a mean evaluation time of 14.1 ± 5.0 seconds per case. Among the 156 patients, 88% pursued the algorithm-recommended treatment, whereas 12% opted for different approaches. Algorithm-compliant patients experienced significantly fewer sequelae than did noncompliant patients (5% versus 33%, P < 0.001). Compared with noncompliant patients, algorithm-compliant patients tended to require shorter treatment durations (17.9 versus 25.4 mo, P = 0.08) and fewer laser sessions (5.8 versus 7.2, P = 0.30), with a younger age at resolution (21.3 versus 29.0 mo, P = 0.08).
Conclusions:
Aesthetic concerns can be crucial for patients with IHs. This study introduces a comprehensive IH management algorithm to reduce the sequelae requiring surgical interventions and improve patients’ quality of life.
Takeaways
Question: Can our novel algorithm address the challenges of managing infantile hemangiomas (IHs) to reduce sequelae and improve outcomes?
Findings: Our study demonstrates the effectiveness of an algorithm-based approach to managing IHs, leading to fewer sequelae and better quality-of-life outcomes for patients. The algorithm, incorporating original aesthetic risk assessment and treatment selection, including oral propranolol and pulsed laser treatment, significantly reduced sequelae in compliant patients compared with noncompliant ones.
Meaning: Our study introduces a comprehensive algorithm for managing IHs, emphasizing the importance of addressing aesthetic concerns to minimize surgical interventions and improve patient quality of life.
INTRODUCTION
Infantile hemangiomas (IHs) are benign vascular tumors that develop in infants, with a reported incidence of 4%–10% in Western countries1–3 and approximately 1% in Asia.4 Although the majority of IHs are considered nonlife-threatening and are often clinically observed without intervention,1 oral propranolol (PPL) has been the preferred treatment option for higher-risk patients since 2008,5 replacing the traditionally used steroids. In Japan, all IH treatment approaches, except topical timolol, are easily accessible and covered by health insurance, facilitating early specialist consultations without significant financial barriers, particularly before a patient reaches 1 year of age.6,7
Despite the availability of diverse treatment options in countries like Japan, no standardized algorithm exists to guide treatment selection that balances efficacy, potential side effects, and aesthetic risks. Traditionally, dermatologists and pediatricians have primarily managed IHs by focusing on functional disturbances and have often overlooked aesthetic aspects, such as sequelae after involution. Plastic surgeons often see patients with IHs only after involution. However, plastic surgeons note that early and appropriate treatment may prevent the need for more extensive procedures and the potential for unsatisfactory scarring outcomes.8–11 Furthermore, many parents express concerns about the aesthetic outcomes of their children’s IHs, even if the child is not experiencing functional issues.
At our university hospital facility, various treatments, including PPL, pulsed dye laser (PDL), and surgery, are offered to patients with IHs ranging from mild to severe. Additionally, plastic surgeons are involved in patients’ initial consultations. Since 2018, we have been developing an original algorithm for IH treatment selection. This algorithm prioritizes PPL treatment for patients with functional issues, whereas, for those primarily seeking aesthetic improvements, treatment decisions are guided by a balance of efficacy and potential side effects, as informed by the literature and the expertise of plastic surgeons. This study aimed to assess the outcomes of algorithm-compliant versus noncompliant groups to evaluate the utility of our algorithm.
MATERIALS AND METHODS
Algorithm and Risk Scoring System Development
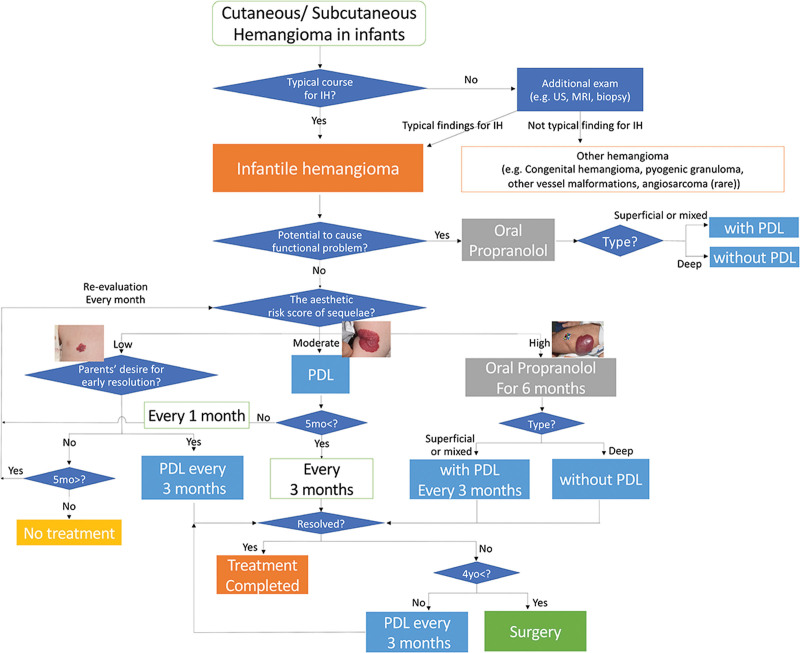
The algorithm and risk scoring system were developed based on previous studies and clinical experience1,9,12 (Fig. 1). The diagnosis of IHs typically relies on clinical appearance and history. Clinical guidelines outline criteria for patients with high-risk IHs, including those with potential airway obstruction or functional impairments.1 PPL is recommended as a first-line treatment approach for these patients, with medical interventions prioritized over parental preference.
Fig. 1.
Overview of our novel algorithm for treatment selection.
Cosmetic considerations also guide treatment decisions for IHs, with factors such as lesion location, size, type and the presence of the ledge effect influencing the risk of sequelae. These sequelae involve various tissue changes, categorized into those requiring surgical interventions, such as redundant skin, and those that can be managed more conservatively, such as hyperpigmentation.13 The former mainly occur with thick mixed IHs, particularly those with a ledge effect, whereas the latter mainly occur with superficial IHs. Horizontal size is also important when predicting the difficulty of surgery if sequelae occur. Finally, location is also important, especially in exposed areas such as the head and neck. Consequently, these factors were incorporated into a novel risk scoring system we developed to guide treatment selection based on cosmetic necessity. (See figure, Supplemental Digital Content 1, which shows the original aesthetic risk scoring system for assessing aesthetic concerns in patients with IHs. http://links.lww.com/PRSGO/D521.)
Our scoring system is based on the anatomical location (3, 2, 1, and 0 points for the face, scalp with hair, exposed areas excluding the head, and nonexposed areas like the trunk, respectively), horizontal size (3, 2, 1, and 0 points for a total body surface area of >1%, 0.5%–1%, <0.5% with an area of 1 cm2 or more, and less than 1 cm2, respectively), type (3, 1, and 0 points for mixed, deep, superficial types, respectively), and the ledge effect (an additional one point for mixed type with the ledge effect), for a maximum of 10 points. The palm method (considering the size of the entire surface of patient’s hand as 1% of the total body surface area) was used.14 The risk of developing sequelae associated with cosmetic problems is classified as either high (6–10 points), moderate (3–5 points), or low (0–2 points).
Treatment recommendations are then determined using an algorithm based on the assessed risk level. Patients with low-risk IHs can receive no treatment or PDL therapy for early resolution, whereas patients with moderate-risk IHs are advised to receive PDL therapy. High-risk patients are recommended to receive oral PPL, with adjunctive PDL therapy if needed12,15 (Fig. 1).
Oral PPL is typically continued for 6 months for patients with high-risk or functionally problematic IHs only4,16 to minimize the risk of life-threatening side effects.17,18 On the contrary, PDL therapy is continued until lesion resolution or until the patient reaches the age of 4 years.9 Persistent lesions at the age of 4 years require surgical excision and are diagnosed as sequelae. Other kinds of sequelae that do not necessitate surgical intervention, such as hypo/hyperpigmentation, are classified as “treatment complete.” Patients with low-risk IHs who forego treatment are advised to monitor for symptom persistence and return for follow-up if symptoms persist for 2 years.
Pilot Study on the Reliability of the Risk Assessment System
To assess the reliability and usability of our scoring system, five plastic surgeons who do not specialize in IH treatment (listed in the acknowledgments section) independently evaluated photographs of 10 IH cases at their initial consultations. Each surgeon conducted two scoring evaluations 1 week apart for the same case series and recorded the time required for the second evaluation. The interclass correlation coefficient (ICC) was calculated for each surgeon’s two assessments, indicating the intrarater reliability. Additionally, the ICC was computed as the average of the two assessments by all five surgeons, indicating the inter-rater reliability.
Evaluation of Treatment Effectiveness Using the Algorithm
We identified consecutive patients who met the following criteria through a comprehensive review of hospital records: (1) aged under 2 years at their first visit between April 2018 and March 2020; (2) clinically diagnosed with IHs by board-certified plastic surgeons; and (3) initiated treatment or opted for observation until natural regression. Patient details, including the age at initial consultation; treatment dates; sex; lesion location, size, and type; and the selected treatment, were collected and anonymized. Treatment recommendations were guided by our novel risk scoring system. Parents received explanations of treatment side effects, and, when oral PPL was prescribed (3 mg/kg/day), pediatric collaboration ensured proper education and inpatient monitoring for 1 week followed by outpatient treatment for 6 months.4,16 The final treatment decisions were made by parents, who provided written informed consent.
An evaluation of the results was performed in March 2023, ensuring a minimum of 4 years of follow-up for all patients. The patients were categorized into algorithm-compliant or noncompliant groups, and statistical comparisons were conducted. Fisher exact test was used to assess the incidence of sequelae, whereas the Student t test was used to analyze the treatment duration, number of PDL sessions, and IH resolution age.
This retrospective cohort study was conducted at the Nippon Medical School Musashi-Kosugi Hospital (Kanagawa, Japan), adhered to the principles of the Declaration of Helsinki, and was approved by the hospital’s ethics committee (approval no. 737-5-48).
Statistical Analysis
IBM SPSS Statistics (version 29.0.2.0) was used for all statistical analyses. Statistical significance was set at a P value of less than 0.05. ICC values were categorized as follows: less than 0.5, poor; 0.5–0.75, moderate; 0.75–0.9, good; and greater than 0.90, excellent reliability.19
RESULTS
Reliability of the Risk Assessment System
The risk score’s reliability was tested by five plastic surgeons. The interrater reliability was 0.973 (95% confidence interval 0.933–0.992), and the mean intrarater reliability was 0.968 ± 0.03. The mean time for evaluations was 14.1 ± 5.0 seconds per case.
Treatment Effectiveness Based on the Algorithm
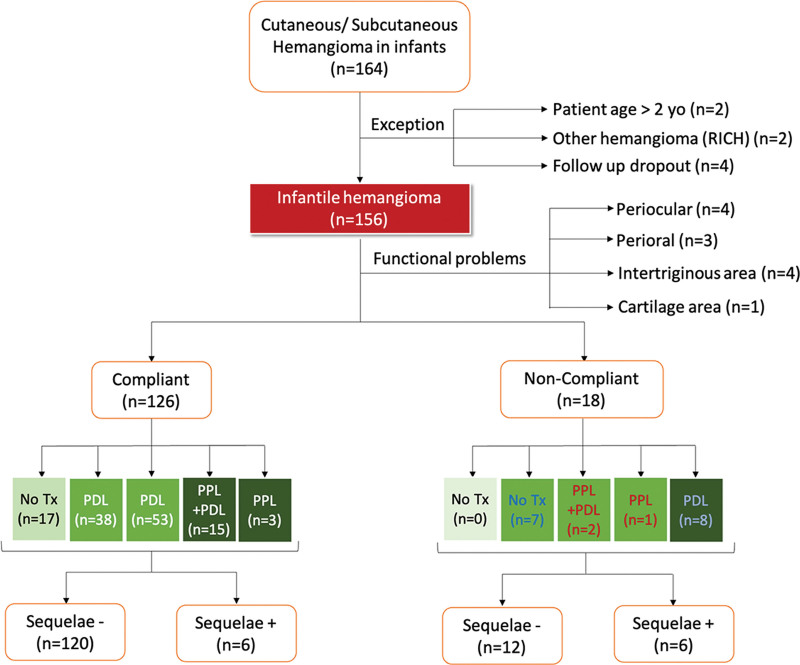
Of the 164 patients with IHs who presented at our hospital during the study period, 156 met the inclusion criteria after excluding eight patients (Fig. 2). Table 1 summarizes the backgrounds of these 156 patients, all of whom were of Japanese origin. The average age at the first hospital visit was 3.9 months, and 63.5% of the patients were female children. The mean horizontal size of the IHs was 2.6 cm in diameter, with 42% located in the craniofacial region and 28% in unexposed trunk areas.
Fig. 2.
Breakdown of the 156 enrolled patients based on algorithm compliance, treatment selection, and outcomes.
Table 1.
Patient Background Information (n = 156)
| Age at First Presentation (Months Old, Mean ± SD) |
3.9 ± 3.1 |
|---|---|
| Sex (%) | |
| Male | 57 (36.5) |
| Female | 99 (63.5) |
| IH size (cm, mean ± SD) | 2.6 ± 2.3 |
| IH Location (n, %) | |
| Head | 58 (37) |
| Neck | 8 (5) |
| Chest | 16 (10) |
| Abdomen | 15 (10) |
| Genitalia | 2 (1) |
| Back | 9 (6) |
| Buttocks | 2 (1) |
| Upper extremities | 30 (20) |
| Lower extremities | 16 (10) |
Based on the algorithm, 12 patients with IHs were treated with oral PPL for functional problems, whereas 144 patients with IHs without functional issues were assessed for aesthetic risk. Supplemental Digital Content 2 shows that 38%, 44%, and 18% of the 144 patients were classified as being at low, moderate, and high risk, respectively. Ultimately, 126 of the 144 patients (88%) complied with the algorithm, whereas 18 patients (12%) did not, either due to social circumstances (distance to the hospital or difficulty attending frequent hospital visits due to work or other siblings) or fear of side effects. (See table, Supplemental Digital Content 2, which shows treatment outcomes. http://links.lww.com/PRSGO/D522.)
Among the algorithm-compliant patients, the treatment choices varied based on the assessed risk level. In algorithm-compliant patients, 17 (13%) low-risk patients chose no treatment, 38 (30%) low-risk patients chose PDL, 53 (42%) moderate-risk patients chose PDL, 15 (12%) high-risk patients chose oral PPL with PDL, and three (2%) high-risk patients chose only oral PPL treatment.
Among the noncompliant patients, none of the patients classified as having a low aesthetic risk underwent oral PPL (0%), seven (39%) patients with moderate-risk IHs underwent no treatment due to a fear of PDL-related pain, and three (17%) patients with moderate risk IHs took oral PPL because it was believed to be the most effective approach (two of these patients also received PDL treatment). Eight patients with high-risk IHs did not take oral PPL even though it was recommended. Of note, patients in both the algorithm-compliant and noncompliant groups who initially did not receive treatment did not later undergo any treatments during the observation period.
In the algorithm-compliant group, sequelae requiring surgical interventions were observed in two patients (5%) in the PDL-treated, low-risk group; two patients (4%) in the PDL-treated, moderate-risk group; one patient (7%) in the oral PPL and PDL-treated, high-risk group; and one patient (33%) in the oral PPL-treated, high-risk group. In contrast, in the noncompliant group, all three patients (100%) in the moderate -risk group who received the treatment for higher-risk patients (namely, oral PPL) had sequelae. In the high-risk group, of those who received the treatment for the lower-risk group (namely PDL), three patients (38%) had sequelae.
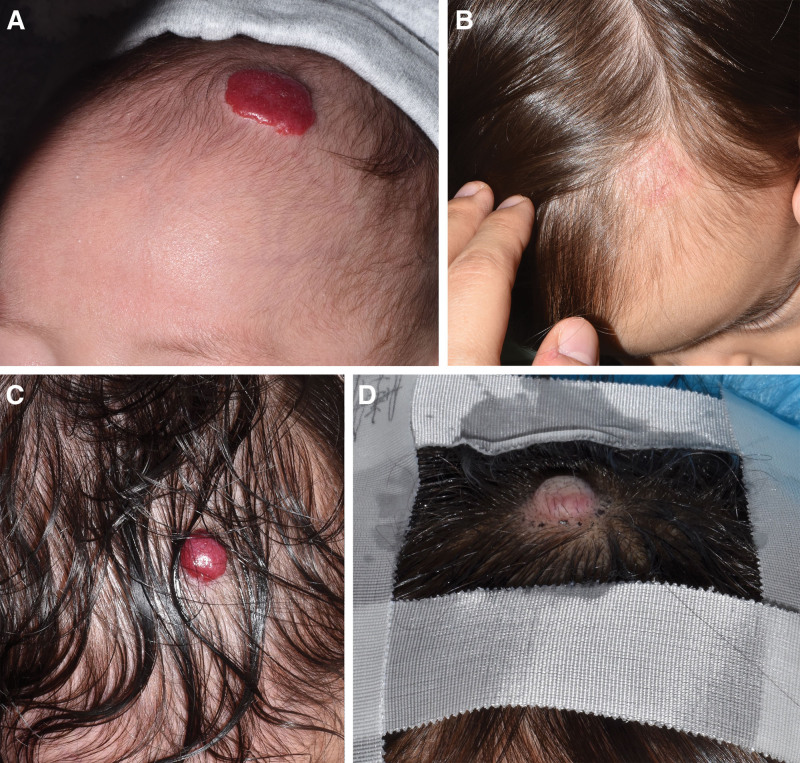
Representative high-risk cases in the compliant and noncompliant groups are shown in Figure 3. Both cases had IHs on hair-bearing areas of the head (2 points), with a size of >1 cm2 (one point), a thick mixed type (3 points), and the ledge effect (additional 1 point). In total, both had risk scores of 7 points and were classified having a high aesthetic risk. The compliant case (a) received oral PPL with PDL starting at 4 months of age, with almost complete lesion resolution by the age of 1 year (b). In contrast, the noncompliant patient (c) refused oral PPL treatment due to a fear of side effects and only received PDL treatment, with the lesion persisting even at 4 years of age. This patient eventually underwent an excisional surgery.
Fig. 3.
Comparison between algorithm-compliant and noncompliant patients with similar severity. The compliant case (A) received oral PPL with PDL starting at the age of 4 months, with almost complete lesion resolution by the age of 1 year. (B). In contrast, the noncompliant patient (C) refused oral PPL treatment due to a fear of side effects and only received PDL treatment, with the lesion persisting even at the age of 4 years. This patient eventually underwent an excisional surgery.
Of the patients prescribed PPL, none were unable to take it owing to contraindications. None of the patients experienced any side effects of PDL or PPL treatment, including bradycardia, hypoglycemia, cold intolerance for oral PPL, or burn or ulcer formation for PDL treatment.
A statistical comparison of the outcomes of patients with IHs who opted for treatment between the algorithm-compliant (n = 109) and noncompliant (n = 11) groups was conducted for the incidence of sequelae (Table 2). The incidence of sequelae was significantly lower in the algorithm-compliant group than in the noncompliant group (P < 0.001).
Table 2.
Statistical Comparison of Outcomes between Algorithm-compliant and Noncompliant Groups
| Compliant (n = 109) | Noncompliant (n = 11) | P | |
|---|---|---|---|
| Sequelae (n, %) | 6 (5%) | 6 (33%) | 0.00096 |
| Mean treatment duration (months, ±SD) | 17.9 ± 9.3 | 25.4 ± 12.5 | 0.08 |
| No. PDL sessions (n, ±SD) | 5.8 ± 3.0 | 7.2 ± 2.2 | 0.30 |
| Age at resolution (months old, ±SD) | 21.3 ± 9.4 | 29.0 ± 14.7 | 0.08 |
Among patients with IHs who healed without sequelae, the mean treatment duration (months), number of PDL sessions (n), and age at which IH was determined to be resolved (months) were also statistically evaluated (Table 2 and Supplemental Digital Content 2). Among PDL-treated patients in the algorithm-compliant group, the mean treatment duration tended to become longer as the aesthetic risk score became higher. The average ages at resolution of IHs in the compliant group without sequelae was less than 2 years (low-risk patients, 21.8 months; moderate-risk patients, 20.0 months; high-risk patients treated with PDL, 22.3 months, and high-risk patients treated without PDL, 13.8 months). The number of PDL sessions for the algorithm-compliant group was approximately five to six times (low-risk, 5.7 sessions; moderate-risk, 6.2 sessions; and high-risk, 5.1 sessions). Patients with high-risk IHs who received oral PPL with PDL treatment had a lower mean number of laser treatment sessions than did patients with moderate-risk IHs who only received PDL (6.2 versus 5.1). Patients treated only with oral PPL, mainly those with deep-type IHs, had a shorter treatment duration (mean 9.8 months, age at resolution 13.8 months). On the contrary, in the noncompliant group, even patients in whom IHs were cured without sequelae had longer treatment durations (25.4 months), more PDL sessions (7.2 sessions), and an older age at resolution (29.0 months). These results were compared between the algorithm-compliant and noncompliant groups; however, no significant differences were observed (Table 2).
DISCUSSION
Historical Evaluation of IH Treatment
The emergence of oral PPL use for IHs has sparked numerous attempts to evaluate the severity of IHs. Notable scoring systems, such as the Hemangioma Activity Score,20 Hemangioma Severity Scale, Hemangioma Dynamic Complication Scale,21 Hemangioma Activity and Severity Index,22 and Infantile Hemangioma Referral Score23 have been developed with varying objectives (Table 3). Although useful individually, none of these systems fully capture the complexity of IHs and are applicable only to specific circumstances.
Table 3.
Previously Reported Scoring Systems for IHs
| Abbreviation | Full Name | Year | Developer | Overview | Strengths | Limitations |
|---|---|---|---|---|---|---|
| HAS | The Hemangioma Activity Score | 2011 | Janmohamed20 | The HAS is designed to assess the proliferative activity of IHs, utilizing swelling, color, and ulceration as objective measures | –Does not require measuring the size of the lesion –Suitable for objectively assessing a worsening or improvement of lesions –Appropriate for a retrospective analysis of photography |
–Not suitable for comparing severity between different patients –Does not consider the anatomical location or size for assessments |
| HSS and HDCS | The Hemangioma Severity Scale and the Hemangioma Dynamic Complication Scale | 2012 | Haggstrom21 | HSS offers a severity score for IHs by considering factors like size, location, and systemic anomaly risk factors, aiming to standardize a clinical severity measurement. HDCS, used in conjunction with HSS, evaluates complications of IH longitudinally | –Demonstrates high inter- and intrarater reliabilities (99%) –Incorporates subjective factors, such as pain, into the assessment –Evaluated by multiple review studies, serves as both a triage tool and treatment predictor |
–Time consuming to apply –Not suitable for retrospective photograph analysis |
| HASI | Hemangioma Activity and Severity Index | 2015 | Semkova22 | The HASI is comprised of two sections: one evaluates disease activity based on changing features, whereas the other assesses the severity, including complication risks | –Combines the advantages of both the HAS and HSS | –Not well reviewed in terms of treatment outcomes or selection |
| IHReS | The Infantile Hemangioma Referral Score | 2020 | Léauté-Labrèze et al23 | A 2-part algorithm consisting of 12 questions, The IHReS aids nonexpert primary care physicians in making timely referrals to specialists | –An easy-to-use scoring system –Effective in reflecting a specialist’s diagnosis |
–Not suitable for specialists’ use in selecting treatments – Not well reviewed in terms of treatment outcomes or selection |
This study’s risk scoring system is unique because it excludes patients with functional issues who are uniformly treated with PPL. Instead, it focuses on patients with more mild disease who are traditionally untreated but may still have aesthetic concerns evaluated by plastic surgeons. By targeting this population, we can address potential long-lasting aesthetic sequelae and the surgical difficulties that may arise when these sequelae persist. In modern times, especially in the field of plastic surgery, the range of conditions treated has expanded and the number of treatment options has increased. Therefore, the development of a systematic approach for appropriate treatment decision-making is urgently needed.24–26
Considering Aesthetic Factors: Insights from Plastic Surgery
During initial consultations, many pediatricians and dermatologists inform families that IHs will resolve naturally. Consequently, families often expect complete tumor disappearance, anticipating surgical outcomes akin to “nothing happened at all.” However, in more challenging cases, reconstructive surgery may entail complex procedures that can pose challenges for achieving aesthetically satisfactory results despite meticulous attention to color and texture matching. Hence, it is crucial to consider the long-term outcomes of these patients, particularly the ease of conducting future surgical interventions and any potential difficulties managing sequelae. A plastic surgeon’s perspective is essential for these decisions.
Our system offers specific and objective indicators that are particularly beneficial for less-experienced physicians in regions where a broad spectrum of treatments is available without financial constraints, such as in Japan. The previously referenced scoring systems were primarily designed to identify severe cases in Western contexts and often lack clear indicators of early regression, which are desired by parents for more mild cases. Consequently, these conventional scoring systems may not align with medical practices in the Asia-Pacific region, where IH occurrence is typically low and the severity is mild. Our scoring system and treatment algorithm offer valuable insights into treatment decisions in this region.
Reliability and Practicality of the Scoring System
Our system offers simplicity and efficiency compared with previous scoring systems, with high inter- and intrarater reliabilities of approximately 97%. This indicates user-friendliness, particularly for physicians who are less experienced with diagnosing and treating IHs. Moreover, the quick assessment time of our system, of 14 seconds per case, underscores its practicality in busy clinical settings.
Utility of the Treatment Algorithm
The success of our algorithm-based treatment approach was evident in the low incidence of sequelae in compliant versus noncompliant cases (5% versus 33%, P < 0.05; Table 2). However, due to the small sample size of noncompliant patients, our statistical comparisons lacked precision. It is also difficult to understand why sequelae persisted in three patients at the moderate-risk level, as they were expected to respond well to oral PPL treatment. These three cases are detailed in the Supplemental Digital Content 3. The perceived lower effectiveness of PPL treatment in this moderate-risk group may be due to the small sample size, as the success rate of PPL is not necessarily 100%.27 Further investigations with larger sample sizes may yield more accurate results. [See table, Supplemental Digital Content 3, which shows analysis of three cases: Middle aesthetic risk, treated with oral propranolol, yet developed sequelae (nonalgorithm compliant). http://links.lww.com/PRSGO/D523.]
A comparative analysis of the treatment outcomes between the algorithm-compliant and noncompliant groups showed a trend favoring the algorithm-compliant group, although statistical significance was not achieved, likely due to sample size limitations. Moreover, our algorithm’s emphasis on avoiding the side effects of PPL and PDL therapy proved successful, with no adverse effects observed.17,18 This finding highlights the importance of judicious treatment selection, particularly for PPL, to mitigate potential risks.
LIMITATIONS
This study has limitations. First, tracking untreated patients became challenging as clinic visits decreased, hindering accurate assessment of IH lesion persistence. Second, our relatively small sample size, particularly of noncompliant patients, limited our ability to verify statistically significant differences between the compliant and noncompliant groups. Third, IH diagnosis relied solely on clinical findings without pathological confirmation. However, the study results are likely minimally affected by these limitations because clinical assessments generally provide reliable IH diagnoses.
CONCLUSIONS
In conclusion, our algorithm presents two significant advantages: minimal residual sequelae and a low incidence of adverse effects associated with oral PPL and PDL treatment. Despite its limitations, the algorithm consistently yielded favorable outcomes, establishing it as a viable therapeutic strategy for various IH cases. Because this scale is user-friendly in clinical settings, we anticipate the further examination and validation of this scale, leading to further elucidation of its usefulness.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
ACKNOWLEDGMENTS
We are grateful to Dr. Jun Hayawaka for caring for patients with IH who were prescribed propranolol. We also express our gratitude to Dr. Hiroaki Kuwahara, Dr. Mami Shoji, and Dr. Takafumi Ito for their assistance in the pilot study of our scoring system.
Supplementary Material
Footnotes
Published online 19 September 2024.
Presented at PRS Korea 2023.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Krowchuk DP, Frieden IJ, Mancini AJ, et al. ; Subcommittee on the Management of Infantile Hemangiomas. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143:e20183475. [DOI] [PubMed] [Google Scholar]
- 2.Rodríguez Bandera AI, Sebaratnam DF, Wargon O, et al. Infantile hemangioma. Part 1: epidemiology, pathogenesis, clinical presentation and assessment. J Am Acad Dermatol. 2021;85:1379–1392. [DOI] [PubMed] [Google Scholar]
- 3.Sandru F, Turenschi A, Constantin AT, et al. Infantile hemangioma: a cross-sectional observational study. Life (Basel). 2023;13:1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaneko T, Sasaki S, Baba N, et al. Efficacy and safety of oral propranolol for infantile hemangioma in Japan. Pediatr Int. 2017;59:869–877. [DOI] [PubMed] [Google Scholar]
- 5.Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, et al. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–2651. [DOI] [PubMed] [Google Scholar]
- 6.Mimura H, Akita S, Fujino A, et al. Japanese clinical practice guidelines for vascular anomalies 2017. J Dermatol. 2020;47:e138–e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Research and Development Agency (RIKEN). Subsidy for infant/child medical expenses. Available at: https://wiss.riken.jp/onarrival_subsidy.html. Accessed April 18, 2024. [Google Scholar]
- 8.Giugliano C, Reculé F, Guler K, et al. Persistent nasal infantile hemangioma: a surgical treatment algorithm. J Craniofac Surg. 2018;29:1509–1513. [DOI] [PubMed] [Google Scholar]
- 9.Couto RA, Maclellan RA, Zurakowski D, et al. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg. 2012;130:619–624. [DOI] [PubMed] [Google Scholar]
- 10.Gao W, Jin Y, Lin X. Nasolabial flap based on the upper lateral lip subunit for large involuted infantile hemangiomas of the upper lip. Ann Plast Surg. 2020;84:545–549. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Z, Yang X, Zhao Y, et al. Early surgical management of large scalp infantile hemangioma using the TopClosure tension-relief system. Medicine (Baltim). 2015;94:e2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugimoto A, Aoki R, Toyohara E, et al. Infantile hemangiomas cleared by combined therapy with pulsed dye laser and propranolol. Dermatol Surg. 2021;47:1052–1057. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Kang J, Bai S, et al. Skin sequelae in patients with infantile hemangioma: a systematic review. Eur J Pediatr. 2023;182:479–488. [DOI] [PubMed] [Google Scholar]
- 14.Agarwal P, Sahu S. Determination of hand and palm area as a ratio of body surface area in Indian population. Indian J Plast Surg. 2010;43:49–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy KK, Blei F, Brauer JA, et al. Retrospective study of the treatment of infantile hemangiomas using a combination of propranolol and pulsed dye laser. Dermatol Surg. 2013;39:923–933. [DOI] [PubMed] [Google Scholar]
- 16.Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet. 2017;390:85–94. [DOI] [PubMed] [Google Scholar]
- 17.Léaute-Labrèze C, Boccara O, Degrugillier-Chopinet C, et al. Safety of oral propranolol for the treatment of infantile hemangioma: a systematic review. Pediatrics. 2016;138:e20160353. [DOI] [PubMed] [Google Scholar]
- 18.Pandey V, Tiwari P, Imran M, et al. Adverse drug reactions following propranolol in infantile hemangioma. Indian Pediatr. 2021;58:753–755. [PubMed] [Google Scholar]
- 19.Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15:155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janmohamed SR, de Waard-van der Spek FB, Madern GC, et al. Scoring the proliferative activity of haemangioma of infancy: the haemangioma activity score (HAS). Clin Exp Dermatol. 2011;36:715–723. [DOI] [PubMed] [Google Scholar]
- 21.Haggstrom AN, Beaumont JL, Lai JS, et al. Measuring the severity of infantile hemangiomas: instrument development and reliability. Arch Dermatol. 2012;148:197–202. [DOI] [PubMed] [Google Scholar]
- 22.Semkova K, Kazandjieva J, Kadurina M, et al. Hemangioma activity and severity index (HASI), an instrument for evaluating infantile hemangioma: development and preliminary validation. Int J Dermatol. 2015;54:494–498. [DOI] [PubMed] [Google Scholar]
- 23.Léauté-Labrèze C, Baselga Torres E, Weibel L, et al. The infantile hemangioma referral score: a validated tool for physicians. Pediatrics. 2020;145:e20191628. [DOI] [PubMed] [Google Scholar]
- 24.Guarro G, Cozzani F, Rossini M, et al. Wounds morphologic assessment: application and reproducibility of a virtual measuring system, pilot study. Acta Biomed. 2021;92:e2021227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winter E, Glauser G, Caplan IF, et al. The LACE+ index as a predictor of 30-day patient outcomes in a plastic surgery population: a coarsened exact match study. Plast Reconstr Surg. 2020;146:296e–305e. [DOI] [PubMed] [Google Scholar]
- 26.Guarro G, Cozzani F, Rossini M, et al. The modified TIME-H scoring system, a versatile tool in wound management practice: a preliminary report. Acta Biomed. 2021;92:e2021226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ji Y, Chen S, Yang K, et al. Efficacy and safety of propranolol vs atenolol in infants with problematic infantile hemangiomas: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2021;147:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.