Abstract
Background:
In recent years, the use of immune checkpoint inhibitors (ICIs) has become a cornerstone in cancer treatment. However, this has also resulted in the emergence of immune-related adverse events, notably ICI hepatitis, posing a significant clinical challenge. While steroids are the primary treatment, there are increasing cases of steroid-refractory ICI hepatitis. Our objective is to investigate the management of ICI hepatitis and its response to steroid treatment.
Methods:
PubMed/MEDLINE, EMBASE, and CENTRAL databases were searched in July 2023 based on keywords including ICIs (anti–Programmed cell death protein 1/Programmed Death-Ligand 1, anti–CTLA–4, and anti-LAG3) and hepatitis.
Results:
A total of 4358 studies were screened, and 44 studies were included in this systematic review. One thousand eight hundred fifty-six patients with ICI hepatitis were included (grade 1-2: 31.7%, grade 3-4: 56.0%, and unknown: 12.3%) with 1184 patients who received corticosteroid treatment. The duration of treatment and dosage varied considerably across the studies. Mycophenolate mofetil was the predominant agent used in 68 out of 82 cases (82.9%), followed by infliximab and azathioprine. A summary estimate of the proportion of steroid-refractory hepatitis in a random effects model was 16% (95% CI: 11%–23%). An estimated 40% (95% CI: 30%–51%) of patients of all patients with ICI hepatitis were rechallenged with an ICI, and of those rechallenged, there was an estimated 22% (95% CI: 15%–30%) recurrence.
Conclusions:
Corticosteroids are the primary treatment for ICI hepatitis, with mycophenolate mofetil used as a secondary option for steroids-refractory cases. Current practices mostly rely on expert consensus, highlighting the need for further research to validate and optimize these treatments, particularly for steroid-resistant cases.
INTRODUCTION
Immune checkpoint inhibitors (ICIs) have become a cornerstone in cancer treatment, demonstrating lasting efficacy even in patients with metastatic cancer, and are increasingly employed in (neo)adjuvant and maintenance therapy.1 However, this has also resulted in the emergence of immune-related adverse events (irAEs), which are strongly associated with but not limited to immune activation associated with antitumor immune responses.2 Long-term implications and management for irAEs are essential in improving survival with ICIs.
The liver is one of the frequently involved organs in irAE, along with the skin, gut, endocrine gland, and lungs.3 Incidence of ICI hepatitis is around 5%–10% of patients treated with ipilimumab, nivolumab, pembrolizumab as single agents but increases as high as 25%–30% in ipilimumab and nivolumab combination therapy.4 Steroids are advised as the initial course of treatment, but there are limitations to the current recommendations as the guidelines are derived largely from expert opinion and case studies.5
In this study, we aim to conduct a comprehensive review of the treatment approaches and responses for ICI hepatitis, primarily to steroids and secondary immunosuppressants as needed. We further explore the response with rechallenge with an ICI and the recurrent rate of ICI hepatitis.
METHODS
Literature search and eligibility
This study was prospectively registered at PROSPERO (registration number: CRD42023450088) and followed the MOOSE reporting guidelines (Supplemental Table S1, http://links.lww.com/HC9/B40). We searched PubMed/MEDLINE, EMBASE, and Cochrane Central Register of Controlled Trials (CENTRAL) databases in July 2023 based on keywords including currently approved “immune checkpoint inhibitors” (anti–Programmed cell death protein 1 (PD-1)/Programmed Death-Ligand 1 (PD-L1), anti–CTLA–4, and anti-LAG3) and “hepatitis” (complete search strategy provided in Supplemental Table S2, http://links.lww.com/HC9/B40) as keywords by investigator (Soo Young Hwang). Two independent researchers (Soo Young Hwang and Pinghsin Hsieh) reviewed the eligibility of the studies independently, and any disagreement was resolved upon discussion between the 2 researchers. Studies that have a description of steroid usage as a treatment for ICI hepatitis or any other treatment for ICI hepatitis were included. Non-English studies, case reports, meeting abstracts, studies on data that were reported in included studies, and studies with insufficient data were excluded.
Data extraction
From the eligible studies, we extracted the name of the first author, publication year, country, study design, number of patients with ICI hepatitis, stage of ICI hepatitis, cancer type and stage, ICIs, steroid dosage and duration of treatment, secondary immunosuppressive agents, number of patients who were rechallenged, peak ALT levels, adverse events of steroids, and other irAE. The Newcastle–Ottawa Scale (NOS) was applied to assess the risk of bias in the observational studies.
ICI hepatitis
In the setting that ICI is the most likely cause of liver injury, Common Terminology Criteria for Adverse Events, Version 5 (CTCAE) defines grade 1 hepatitis as AST/ALT 1–3× the upper limit of normal (ULN) or total bilirubin 1–1.5× ULN, grade 2 hepatitis as AST/ALT >3–5× ULN or total bilirubin >1.5–3× ULN, grade 3 hepatitis as AST/ALT >5–20× ULN or total bilirubin >3–10× ULN, and grade 4 hepatitis as AST/ALT >20× ULN or total bilirubin >10× ULN or hepatic decompensation.6
Statistical analysis
Meta-analysis of proportions was performed based on the number of patients treated with steroids and the number of patients requiring a secondary immunosuppressant as the primary outcome. Secondary outcomes were the proportion of patients with ICI hepatitis who were rechallenged with an ICI and the proportion of ICI hepatitis recurrence. The proportion of each study outcome was calculated using a logit transformation. The random effects model was used to obtain the summary estimates, and the summary results were displayed in forest plots. The Q and Higgins I2 statistics were calculated to evaluate the heterogeneity in the included studies.7 Publication bias was visually assessed by plotting effect size against sample size (ie, funnel plot) (Supplemental Figure S1, http://links.lww.com/HC9/B40). We performed additional analyses to further explore the heterogeneity of the study. These included subgroup analysis on the country of origin and tumor type, with a focus on melanoma (Supplemental Figure S4, http://links.lww.com/HC9/B40).
A meta-regression analysis was performed based on the primary outcome with moderators, including the percentage of patients who received combination treatment and the percentage of patients with advanced hepatitis (grade 3-4). In addition, we conducted a meta-regression analysis based on the primary outcome and the year of publication. In addition, the association between the number of patients with ICI hepatitis who did not receive any intervention and the percentage of grade 1-2 hepatitis was investigated through a meta-regression analysis.
RESULTS
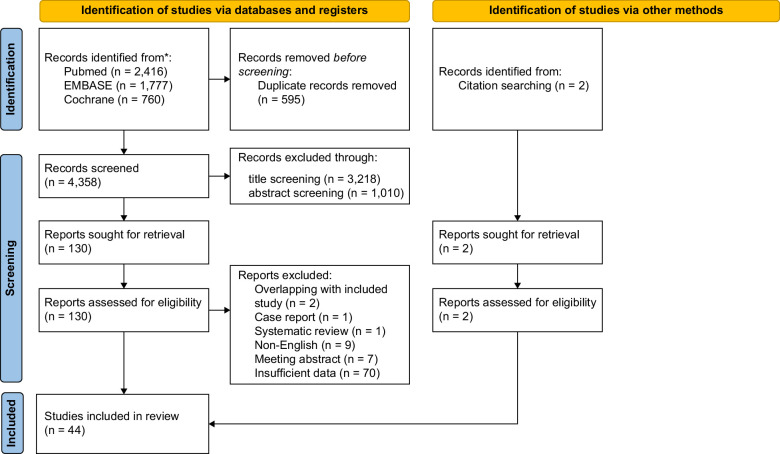
Through a comprehensive search of the 3 databases, 4358 potentially eligible studies were identified and independently screened with an in-depth full-text screening of 130 studies and 44 studies included for final analysis8–51 (Figure 1; Tables 1–3).
FIGURE 1.
Flow diagram of all included studies. *Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers). **If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools. From Page et al.52 For more information, visit: http://www.prisma-statement.org/.
TABLE 1.
Basic characteristics of all included studies
| Cancer | Grade of ICI hepatitis | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Age | Male, n (%) | Type (n) | Stage (%) | ICI | N. received ICI | 1 | 2 | 3 | 4 | Total |
| Leroy et al8 | 82 [80–90] | 14 (60.9) | Melanoma (23) | Stage 4 | Ipilimumab | 23 | 0 | 0 | 2 | 0 | 2 |
| Luo et al9 | Lung | 51 | 6 | ||||||||
| Romanski et al10 | 60 [38–87] | 19 (44.2) | Melanoma | Stage 4 | Ipilimumab (14), pembrolizumab (16), nivolumab (1), ipilimumab + nivolumab (12) | 521 | 179 | 15 | 23 | 5 | 265 |
| Miller et al11 | 60 [IQR: 54–69] | 61 (61) | Melanoma (53), GU (14), lung, head, neck (12), GI (9), other solid (2), hematological (10) | Stage 3 (9) Stage 4 (91) |
CTLA-4 monotherapy (25), PD-1/PD-L1 monotherapy (46), combination (29) | 5762 | 0 | 0 | 85 | 15 | 100 |
| Smith et al12 | 53.8 [IQR: 46.9–60.7] | 22 (69) | Melanoma | Stage 3 (8) Stage 4 (92) |
Ipilimumab + nivolumab | 63 | 11 | 21 | 32 | ||
| Yamamoto et al13 | 70 [30–84] | 14 (66.67) | NSCLC (3), RCC (7), urothelial (1), MM (8), other (2) | Nivolumab (10), pembrolizumab (3), atezolizumab (1), ipilimumab (2), ipilimumab + nivolumab (5) | 245 | 0 | 7 | 9 | 5 | 21 | |
| Takinami et al14 | 55.5 [IQR: 54–68] | 4 (50) | Melanoma (6), renal cell (2) | Pembrolizumab (1), ipilimumab (2), ipilimumab + nivolumab (5) | 530 | 0 | 3 | 5 | 8 | ||
| Owen et al15 | Melanoma | Stage 4 | Anti-PD1, anti-PD1 + anti-CTLA4, anti-PD1 ± anti-CTLA4 | 118 | 0 | 2 | 8 | 2 | 12 | ||
| Li et al16 | 57.8 (13.7) | 47 (54.0) | Melanoma, NSCLC, RCC, breast cancer, urothelial cancer, other | Nivolumab (11), pembrolizumab (43), cemiplimab, ipilimumab (18), ipilimumab + nivolumab (49), anti–PD-L1 (7) | 7046 | 0 | 0 | 60 | 27 | 87 | |
| 61.6 (15.5) | 66 (51.6) | Nivolumab (11), pembrolizumab (14), cemiplimab (2), ipilimumab (9), ipilimumab + nivolumab (45), anti–PD-L1 (6) | 0 | 0 | 106 | 22 | 128 | ||||
| Cunningham et al17 | 47.9 (95% CI: 39.3–58.4) | 9 (52.9) | Head and neck (4), melanoma (8), pancreas (1), colorectal (2), sarcoma (1), RCC (1) | Anti-PD1 (11), anti–PD-L1 (1), anti-CTLA4 (3), combination (1), blinded (1) | 450 | 0 | 4 | 13 | 17 | ||
| Sanz-Segura et al18 | 132 | 2 | 2 | 0 | 0 | 4 | |||||
| da Silva et al19 | 65 | 2 (66.7) | Lung (2), melanoma (1) | Pembrolizumab (1), nivolumab (2) | 151 | 3 | |||||
| Huffman et al20 | 57 [32–82] | 12 (75) | Melanoma | Stage 4 | Ipilimumab (12), pembrolizumab (3), ipilimumab + nivolumab (2) | 218 | 3 | 1 | 8 | 3 | 17 |
| Cheung et al21 | 62 [21–76] | 11 (52) | Melanoma (17), renal cell (1), non–small cell lung (2), epithelial mesothelioma (1) | ipilimumab, nivolumab, pembrolizumab, ipilimumab + nivolumab, Checkmate 238 | 453 | 3 | 4 | 9 | 5 | 21 | |
| Shimomura et al22 | NSCLC | Stage 4 | Anti–PD-1 inhibitors | 375 | 18 | 10 | 6 | 0 | 34 | ||
| Swanson et al (2022) | 70 [54–86] | 1 (50) | cSCC | Cemiplimab (2) | 39 | 2 | |||||
| de la Bruyère et al24 | Melanoma (8), lung (4) | PD(L)-1 inhibitors (6), CTLA-4 inhibitors (6) | 150 | 0 | 0 | 12 | 12 | ||||
| Swanson et al (2022) | 65 [47–70] | 3 (50) | Pancreatic (3), HCC (2), RCC (1) | Stage 4 | Durvalumab combination (6) | 112 | 0 | 3 | 3 | 0 | 6 |
| Sawada et al26 | 64.0 [48–76] | 7 (87.5) | NSCLC (3), MM (1), GC (2), RCC (1), HNSCC (1) | Nivolumab (8), pembrolizumab (5), ipilimumab (4) | 135 | 0 | 3 | 5 | 0 | 8 | |
| Fan et al27 | 60 [IQR: 57–65] | 8 (38) | Bladder (2), breast (4), esophageal (2), GBM (2), gastric (2), liposarcoma (1), melanoma (3), NSCLC (3), ovarian (1), pancreatic (1) | Stage 4 (33) | CTLA-4 (20), CTLA-4 + PD-1/PD-L1 (3), PD-1/PD-L1 (16) | 331 | 6 | 15 | 21 | ||
| Kitagataya et al28 | 67 [25–92] | 9 (52.9) | Melanoma (5), lung (1), lymphoma (1), other (1) | Nivolumab (8), pembrolizumab (5), ipilimumab (4) | 202 | 3 | 6 | 6 | 2 | 17 | |
| Zheng et al29 | Anti–PD-1/PD-L1 inhibitor | 240 | 1 | 0 | 3 | 0 | 4 | ||||
| Daniello et al30 | NSCLC | Stage 4 | Anti-PD(L)1 inhibitors | 894 | 2 | 7 | 20 | 4 | 33 | ||
| Cheng et al31 | 63 [56–69] | 3 (100) | Melanoma | Stage 4 | Ipilimumab | 3 | |||||
| Pollack et al32 | Melanoma | Stage 4 | anti–PD-1 + ipilimumab | 13 | 24 | 37 | |||||
| De Martine et al33 | 63 [33–84] | 7 (44) | Melanoma (12), bronchial (1), renal clear cell (1), bladder (1), cholangiocarcinoma (1) | Stage 4 | Anti–PD-1/PD-L1 (9), anti-CTLA4 (7) | 536 | 0 | 0 | 16 | 16 | |
| Simonaggio et al34 | 159 | 0 | 4 | 8 | 5 | 17 | |||||
| Imoto et al35 | 63 [49–69] | 31 (63.6) | 387 | 45 | 11 | 56 | |||||
| Zen et al36 | 70 [59–74] | 8 (80) | NSCLC (4), urothelial (3), merkel cell (1), melanoma (1), colon (1) | Stage 4 | Pembrolizumab (6), atezolizumab (4) | 10 | |||||
| Riveiro-Barciela et al37 | 62.8 [IQR: 56.6–70.5] | 14 (50) | NSCLC (21.4%), melanoma (17.9%), urothelial (14.3%) | Anti-CTLA4 (10), anti–PD-1/PD-L1 (18) | 414 | 0 | 0 | 28 | 28 | ||
| Gauci et al38 | 52 [IQR: 47–67] | 14 (66.7) | Melanoma | Stage 3 (5), Stage 4 (95) | Ipilimumab (7), nivolumab (3), pembrolizumab (1), ipilimumab + nivolumab (10) | 339 | 0 | 0 | 10 | 11 | 21 |
| Patrinely, Jr. et al39 | 63 | 88 (53.7) | Lung (12), melanoma (138), renal (5), squamous cell (2), other (7) | Stage 4 (86) | Ipilimumab (7), ipilimumab + nivolumab (97), ipilimumab + pembrolizumab (3), nivolumab (19), pembrolizumab (34), other anti–PD-1/PD-L1 (4) | 164 | 16 | 50 | 75 | 23 | 164 |
| Rini et al40 | RCC | Stage 4 | Pembrolizumab + axitinib (429), sunitinib (425) | 861 | 125 | ||||||
| Lin et al41 | 34 (66.67) | Anti-PD1 | 1310 | 37 | 14 | 51 | |||||
| Personeni et al42 | 71 [49–83] | 5 (55.56) | HCC | BFTABLE CLC stage B or C |
Anti–PD-1/PD-L1 ± anti–CTLA–4 antibodies and/or targeted agents (including sorafenib, cabozantinib, and an investigational c-Met inhibitor) | 58 | 0 | 0 | 9 | 0 | 9 |
| Purde et al43 | 61 [41–73] | 6 (54.55) | NSCLC (6), melanoma (5) | Stage 4 | Anti-PD1 (6), CTLA4 (1), anti-PD1 + CTLA4 (3) | 135 | 6 | 4 | 1 | 11 | |
| Ng et al44 | HCC | Stage 4 | 168 | 12 | 12 | 24 | |||||
| Riveiro-Barciela et al45 | 65 [IQR: 56–75] | 11 (47.8) | NSCLC (7), Urinary tract (6), melanoma, (4), endometrial (2), HCC (1), cholangiocarcinoma (1), breast(1) chordoma (1) | Stage 3 (30%) Stage 4 (70%) |
Anti-PD1 or anti–PD-1/PD-L1 (18), anti–CTLA-4 ± anti-PD1 (4), CD40 agonist antibodies (1) | 0 | 0 | 19 | 4 | 23 | |
| Alomari et al46 | Stage 4 | Nivolumab (9), pembrolizumab (7), ipilimumab (1), avelumab (2), nivolumab and ipilimumab (4) | 567 | 8 | 9 | 4 | 2 | 23 | |||
| Miah et al47 | 60 [IQR: 51.9–66.8] | 30 (46.9) | Head and neck (2), melanoma (24), NSCLC + SCLC (9), RCC (7), Other (22) | Stage 4 | PD1 or CTLA monotherapy (46), Combination PD-1 and CTLA-4 (13), other (5) | 1096 | 30 | 34 | 64 | ||
| Hountondji et al48 | 63 [23–89] | 63 (53.8) | Melanoma (49), lung (32), renal (16), urothelial (6), cutaneous and oral SCC (7), GI (3), HCC (2), hematological (1), pancreatic(1) | Stage 1-2 (29%) Stage 3 (16%) Stage 4 (54%) |
Anti–PD-1 (62), anti–PD-L1 (8), anti–CTLA–4 (4), anti–PD-1 + anti–CTLA–4 (42), anti–PD-1 + anti-LAG-3 (1) | 1058 | 4 | 17 | 73 | 23 | 117 |
| Matsukane et al49 | 1008 | 17 | 15 | 33 | 65 | ||||||
| Parlati et al50 | 62 [IQR: 48–73] | 14 (40) | Melanoma (19), lymphoma (1), NSCLC (10), other (5) | Anti–PD-1 monotherapy (26), anti-PD1/anti-CTLA4 (9) | 5 | 7 | 12 | 11 | 35 | ||
| Storm et al51 | 62.1 (16.7) | 55 (56.7) | Head and neck (10), lung (13), skin (42), GI (5), GU (22), sarcoma (4), other (1) | Pembrolizumab (30), nivolumab (13), ipilimumab/nivolumab combination (43), cemiplimab (2), ipilimumab (5), atezolizumab (4) | 2611 | 37 | 46 | 14 | 97 | ||
Age is summarized in median (range), median [IQR Q1-Q3], mean (SD).
Abbreviations: cSCC, cutaneous squamous cell carcinoma; GBM, glioblastoma multiforme; GI, gastrointestinal; GU, genitourinary; HNSCC, head and neck squamous cell carcinoma; ICI, immune checkpoint inhibitor; MM, multiple myeloma; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; RCC, renal cell carcinoma; NSCLC, non-small cell lung cancer.
TABLE 3.
Studies on recurrence of immune checkpoint inhibitor hepatitis
| Study | No. treated with steroids | No. treated with secondary immunosuppressants | No. rechallenged/recurrence |
|---|---|---|---|
| Leroy et al8 | 2 | 1 (MMF) | |
| Luo et al9 | 6 | 5 (MMF) | 1/0 |
| Romanski et al10 | 31 | 2 (MMF) | |
| Miller et al11 | 67 | 3 (MMF) | 31/8 |
| Smith et al12 | 31 | 1 (infliximab) | 17/3 |
| Yamamoto et al13 | 13 | 2 (MMF) | |
| Takinami et al14 | 6 | 2 (MMF) | 3/0 |
| Owen et al15 | 10 | 1 (MMF, azathioprine) | |
| Li et al16 | 87 | 32 | |
| Li et al16 | 128 | 29 | |
| Cunningham et al17 | 15 | 1 (MMF) | 7/1 |
| da Silva et al19 | 3 | 1/0 | |
| Huffman et al20 | 16 | 2 (AZA 1 CsA 1) | |
| Cheung et al21 | 18 | 10 (infliximab 2 MMF 8 tacrolimus 1) | 4/0 |
| de la Bruyère et al24 | 7 | 1 | 3/1 |
| Swanson et al (2022) | 3 | 0 | 1/0 |
| Fan et al27 | 17 | 6 (MMF) | |
| Kitagataya et al28 | 4 | 2 (MMF) | |
| Zheng et al29 | 3 | 1 (MMF, gamma globulin) | |
| Daniello et al30 | 27 | 2 | |
| Cheng et al31 | 3 | 0 | |
| Pollack et al32 | 36 | 3 (MMF) | 29/5 |
| De Martine et al33 | 10 | 1 (MMF) | 3/1 |
| Simonaggio et al34 | 13 | 2 (MMF) | 5/3 |
| Imoto et al35 | 4 | 3 (MMF 2, infliximab 1) | |
| Zen et al36 | 10 | 1 (MMF, AZA) | |
| Riveiro-Barciela et al37 | 28 | 10 | 6/0 |
| Gauci et al38 | 13 | 0 | 8/0 |
| Patrinely, Jr. et al39 | 150 | 37 | 66/17 |
| Rini et al40 | 68 | 100/45 | |
| Personeni et al42 | 3 | 6/0 | |
| Purde et al43 | 6 | 0 | 3/1 |
| Riveiro-Barciela et al45 | 19 | 2 (MMF) | 23/8 |
| Miah et al47 | 46 | 3 (MMF, MMF+infliximab) | 11/0 |
| Hountondji et al48 | 93 | 18 (MMF 17 rituximab 1) | 51/12 |
| Matsukane et al49 | 29 | 33/8 | |
| Parlati et al50 | 20 | 8/0 | |
| Storm et al51 | 78 | 10 (MMF 9, other 1) | 32/13 |
| Cunningham et al17 | 15 | 1 (MMF) | 7/1 |
| da Silva et al19 | 3 | 1/0 | |
| Huffman et al20 | 16 | 2 (AZA 1 CsA 1) | |
| Cheung et al21 | 18 | 10 (infliximab 2 MMF 8 tacrolimus 1) | 4/0 |
| de la Bruyère et al24 | 7 | 1 | 3/1 |
| Swanson et al (2022) | 3 | 0 | 1/0 |
| Fan et al27 | 17 | 6 (MMF) | |
| Kitagataya et al28 | 4 | 2 (MMF) | |
| Zheng et al29 | 3 | 1 (MMF, gamma globulin) | |
| Daniello et al30 | 27 | 2 | |
| Cheng et al31 | 3 | 0 | |
| Pollack et al32 | 36 | 3 (MMF) | 29/5 |
| De Martine et al33 | 10 | 1 (MMF) | 3/1 |
| Simonaggio et al34 | 13 | 2 (MMF) | 5/3 |
| Imoto et al35 | 4 | 3 (MMF 2, infliximab 1) | |
| Zen et al36 | 10 | 1 (MMF, AZA) | |
| Riveiro-Barciela et al37 | 28 | 10 | 6/0 |
| Gauci et al38 | 13 | 0 | 8/0 |
| Patrinely, Jr. et al39 | 150 | 37 | 66/17 |
| Rini et al40 | 68 | 100/45 | |
| Personeni et al42 | 3 | 6/0 | |
| Purde et al43 | 6 | 0 | 3/1 |
| Riveiro-Barciela et al45 | 19 | 2 (MMF) | 23/8 |
| Miah et al47 | 46 | 3 (MMF, MMF + infliximab) | 11/0 |
| Hountondji et al48 | 93 | 18 (MMF 17 rituximab 1) | 51/12 |
| Matsukane et al49 | 29 | 33/8 | |
| Parlati et al50 | 20 | 8/0 | |
| Storm et al51 | 78 | 10 (MMF 9, other 1) | 32/13 |
Abbreviations: AZA, azathioprine; CsA, cyclosporine; MMF, mycophenolate mofetil.
Baseline characteristics
A total of 1856 patients with ICI hepatitis were included. Five hundred ninety (31.7%) of the patients developed grade 1-2 hepatitis, and 1,043 (56.0%) of the patients developed grade 3-4 hepatitis.
The prevalence of ICI hepatitis in our study was 6.38% (1856 cases out of 29,112 patients who received an ICI). The estimated median age of patients with ICI hepatitis was 63 (range: 21–90), with 55.7% (692 out of 1243) of male patients in advanced stages of cancer, stages 3 and 4. Ten studies were conducted in Asia, 13 studies were conducted in North America, 17 studies in Europe, 1 in Australia, and 3 studies were multinational. ICI included in the study were anti-PD1 nivolumab, pembrolizumab, cemiplimab; anti–PD-L1 atezolizumab, durvalumab; and anti-CTLA4 ipilimumab. Combination therapies consist of ipilimumab and nivolumab, ipilimumab and pembrolizumab. 37.52% (454 out of 1218) of patients were treated with combination therapy, and 62.48% (756 out of 1218) of patients were treated with monotherapy. Two hundred eighteen (38.05%) of the patients experienced disease progression regarding ICI, while 355 (61.95%) of the patients experienced stable disease or response from the ICI.
Steroid as a first-line treatment of ICI hepatitis
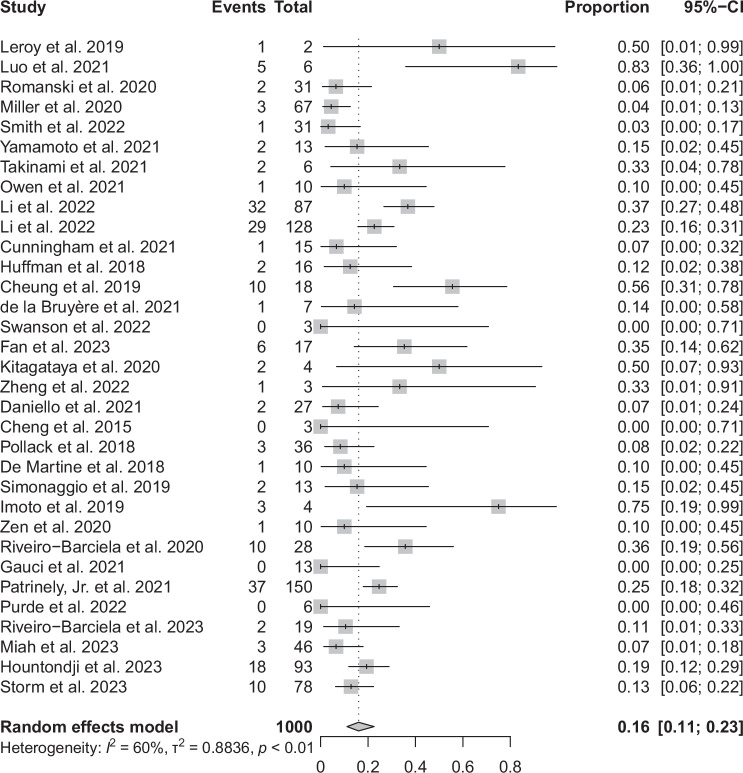
One thousand one hundred eighty-four patients out of a total of 1864 patients received corticosteroid treatment Table 2. The duration of treatment varied considerably across the studies, ranging from 3 to 361 days. Similarly, there was substantial variation in dosage, from oral prednisone at 0.5 mg/kg to i.v. methylprednisolone at 2 mg/kg. In total, 32 studies reported on steroid-refractory cases that necessitated the use of second-line immunosuppressants. Mycophenolate mofetil was the predominant agent used in 68 out of 82 cases (82.9%). Other treatments included infliximab in 5 out of 82 cases (6.1%), azathioprine in 3 out of 82 cases (3.7%), and 1 case each for rituximab, gamma globulin, tacrolimus, and cyclosporine. A summary estimate of the proportion of steroid-refractory hepatitis in a random effects model was 16% (95% CI: 11%–23%) (Figure 2). There was moderate heterogeneity (I 2 = 60%) in the analysis. The funnel plot (Supplemental Figure S1, http://links.lww.com/HC9/B40) showed no visual asymmetry, and statistical analysis showed no evidence of publication bias (p < 0.001). Subgroup analyses based on the country of origin did not demonstrate statistically significant differences in the proportion of patients requiring additional immunosuppressants (chi-square 5.71, df = 3, p = 0.13) (Supplemental Figure S3, http://links.lww.com/HC9/B40) and there was no statistically significant association with the publication year (coefficient = −0.031, p = 0.784) (Supplemental Figure S5, http://links.lww.com/HC9/B40).
TABLE 2.
Studies on steroid-refractory hepatitis (primary outcome: usage of second-line immunosuppressants)
| Study | Total no. ICI hepatitis | No. received steroids | Steroid dose, duration | Side effects of steroid | Peak ALT levels, IU/L | Unit |
|---|---|---|---|---|---|---|
| Romanski et al10 | 265 | 31 | Cumulative dose of prednisolone (mg) grade 2: 737.5 (375–6000) grade 3: 2325 (575–5987.5) grade 4: 4975 (1867.5–6000) |
Median (range) | ||
| Miller et al11 | 100 | 67 | grade 3: 44 (25–71) days grade 4: 90 (43–121) d |
Anti–CTLA–4: 670 (310–2,574), anti–PD-1/PD-L1 482 (297–2946), combination 414 (300–2991) | Median (IQR) | |
| Smith et al12 | 32 | 31 | Induction: mean 69 (23) (mg) prednisone—equivalent/d (adjusted for weight, mean dose of 0.86 mg/kg 0.21 mg/kg) | Mean (SD) | ||
| Yamamoto et al13 | 21 | 13 | CS 1 mg/kg (5), 0.7 mg/kg (2), 0.5 mg/kg (2), pulse (5) 10 mg (1) | |||
| Owen et al15 | 12 | 10 | 1.8 (1.0–11.4) mo | Median (range) | ||
| Li et al16 | 87 | 87 | Initial mPSL ≥1.5 mg/kg maximum CS dose 2.0 (2.0–2.0) i.v. steroids 80 (92.0%) 60 (40–85) d until achieving a prednisone dose ≤10 mg |
Infection 16 (18.4%), GI bleed 2 (2.3%), hyperglycemia requiring Tx 20 (23.3%), peak glucose 195 (154–286) | 391 (248–606) | Median (IQR) |
| Li et al16 | 128 | 128 | Initial mPSL <1.5 mg/kg maximum steroid dose 1.0 (1.0–1.3) i.v. steroids 42 (32.8%) 44 (32–70) d until achieving a prednisone dose ≤10 mg |
infection 9 (7.0%), GI bleed 3 (2.3%), hyperglycemia requiring Tx 10 (7.8%), peak glucose 166 (137–205) | 314 (234–468) | Median (IQR) |
| Cunningham et al17 | 17 | 15 | DXA 4 mg (1), steroid 1.5 mg/kg i.v. (1), PDN 1 mg/kg (7), PDN taper (2), CS 2 mg/kg i.v. (3) NA |
217 (145–324) | Mean (95% CI) | |
| Sanz-Segura et al18 | 4 | 2 | Oral CS 1 mg/kg/d | |||
| Huffman et al20 | 17 | 16 | Prednisone (14), dexamethasone (2), high-dose methylprednisolone (3) 42 (7–78) d |
261 (IQR: 110–615) | Median (range) Median (IQR) |
|
| Cheung et al21 | 21 | 18 | Dexamethasone (1), prednisolone (11), methylprednisolone (7) | 610 (183–1088.5) | Median (IQR) | |
| Shimomura et al22 | 34 | 7 | High-dose (≥0.5 mg/kg of prednisolone) (6), low-dose (<0.5 mg/kg of prednisolone) (1) | |||
| Swanson et al23 | 2 | 1 | 6 wk | |||
| de la Bruyère et al24 | 12 | 7 | CS 1 mg/kg (3), ≥2 mg/kg (4) 42 (30–44) d |
Median (IQR) | ||
| Swanson et al25 | 6 | 3 | CS 1 mg/kg (5), 0.7 mg/kg (2), 0.5 mg/kg (2), pulse (5) 10 mg (1) 28–77 d |
415 [30–946] | Median (range) | |
| Fan et al27 | 21 | 17 | Prednisone >1 mg/kg/d: 9 58 (14–111) d |
Hyperglycemia (14, 82%), leukocytosis (7, 41%), infection (3, 18%), AMS, melena, venous thromboembolism | Median (IQR) | |
| Kitagataya et al28 | 17 | 4 | PSL 2 mg/kg/d (2), 1 mg/kg/d (1), 1000 mg (1) | 185.5 (61–2488) | Median (range) | |
| Zheng et al29 | 4 | 3 | mPSL 2 mg/kg, i.v. 3 d |
|||
| Daniello et al30 | 33 | 27 | Initial dose: 87 (92), average dose: 47 (37) 33 (27) d |
Mean (SD) | ||
| Cheng et al31 | 3 | 3 | mPSL 1 g | 372, 1211, 896 | ||
| De Martine et al33 | 16 | 10 | Corticosteroid 0.2 mg/kg/d (2), 0.5 mg/kg/d (2), 1 mg/kg/d (5), 2.5 mg/kg/d (1) | 460 (266–3137) | Median (range) | |
| Imoto et al35 | 56 | 4 | mPSL 1000 mg/d (1), PSL 0.6 mg/kg/d (2), PSL 1 mg/kg/d (2) | 58 (47–129) | Median (range) | |
| Zen et al36 | 10 | 10 | PSL (50 mg/d) (3), PSL (40 mg/d) (3), predonisone (80 mg/d) (1), steroid mini pulse (mPSL, 500 mg/d, 3 d), followed by PSL (50 mg/d) (1), mPSL (1), PSL (1) | 226 (93–504) | Median (IQR) | |
| Riveiro-Barciela et al37 | 28 | 28 | Initial dose 60 (52–70) mg/d 2.3 (1.3–3.1) mo |
Infection (2) | 351 (208–910) | Median (IQR) |
| Gauci et al38 | 21 | 13 | 1 [IQR: 1; 1] (0.3; 2) mg/kg/d 1.8 [IQR: 1.7; 3.5] (1.2–12.6) mo |
663 [IQR: 422; 1380] (173–3537) | Median [IQR] (range) | |
| Patrinely, Jr. et al39 | 164 | 150 | PDN or mPSL (147), DXA (1), hydrocortisone (2)| Initially required low-dose steroids (<50 mg daily or <1 mg/kg) (20), required high-dose steroids (129) |
Adrenal insuff (2), infection (7), GI (3), hyperglycemia/diabetes (22), insomnia (7), mood changes (7), muscle weakness/myalgias (3), osteoporosis (2), weight gain (3), others (6) | ||
| Rini et al40 | 125 | 68 | High-dose (≥ 40 mg/d of prednisone or equivalent) (61), low-dose (7) | |||
| Lin et al41 | 51 | 8 | Prednisone 0.5–2 mg/kg 3–6 wk |
|||
| Personeni et al42 | 9 | 3 | Prednisone 1–2 mg/kg | Grade 3-4: 88 (13 –147) grade 1-2: 37 (11–146) |
Median (range) | |
| Purde et al43 | 11 | 6 | 80 (13–145) days | NA | Median (IQR) | |
| Riveiro-Barciela et al45 | 23 | 19 | Prednisone (12), methylprednisone (7) recurrence (n = 8) 63 (25) non-recurrence (n = 15) 66 (18) median (range) 8 wk (0.5–51 wk) |
280 (188–438) | Median (IQR) mean (SD) |
|
| Alomari et al46 | 23 | 20 | >4 wk (18) < 4 wk (2) | |||
| Miah et al47 | 64 | 46 | PDN (23), DXA (6), mPSL (4) median 45 d (range: 21–120 d) |
|||
| Matsukane et al49 | 65 | 29 | Low-dose (< 0.5 mg/kg PSL) (n = 93), moderate to high dose (0.5–2.0 mg/kg PSL) (n = 36), i.v. mPSL pulse therapy (500–1000 mg, 3 d) (n = 41) |
Abbreviations: CS, corticosteroid; DXA, dexamethasone; mPSL, methylprednisolone; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1; PDN, prednisone; PSL, prednisolone.
FIGURE 2.
Forest plot of the proportion of steroid-refractory ICI hepatitis. Abbreviation: ICI, immune checkpoint inhibitor.
The proportion of patients requiring additional immunosuppressants was not statistically associated with percentage of combination ICI therapy (coefficient = −0.461, p = 0.546) or percentage of grade 3-4 hepatitis (coefficient = 0.03, p = 0.976).
An estimated 23% (95% CI: 15%–35%) of the patients with ICI hepatitis did not receive any steroids, correlated with the proportion of grade 1-2 hepatitis in the cohort (coefficient = 3.22, p < 0.001) (Supplemental Figure S2, http://links.lww.com/HC9/B40). The most common side effects of steroid treatment were infection (11.6%, 38 out of 329 cases) and hyperglycemia (20.1%, 66 out of 329 cases). Other side effects reported were gastrointestinal bleeding, altered mental status, mood changes, muscle weakness or myalgia, and osteoporosis.
ICI rechallenge
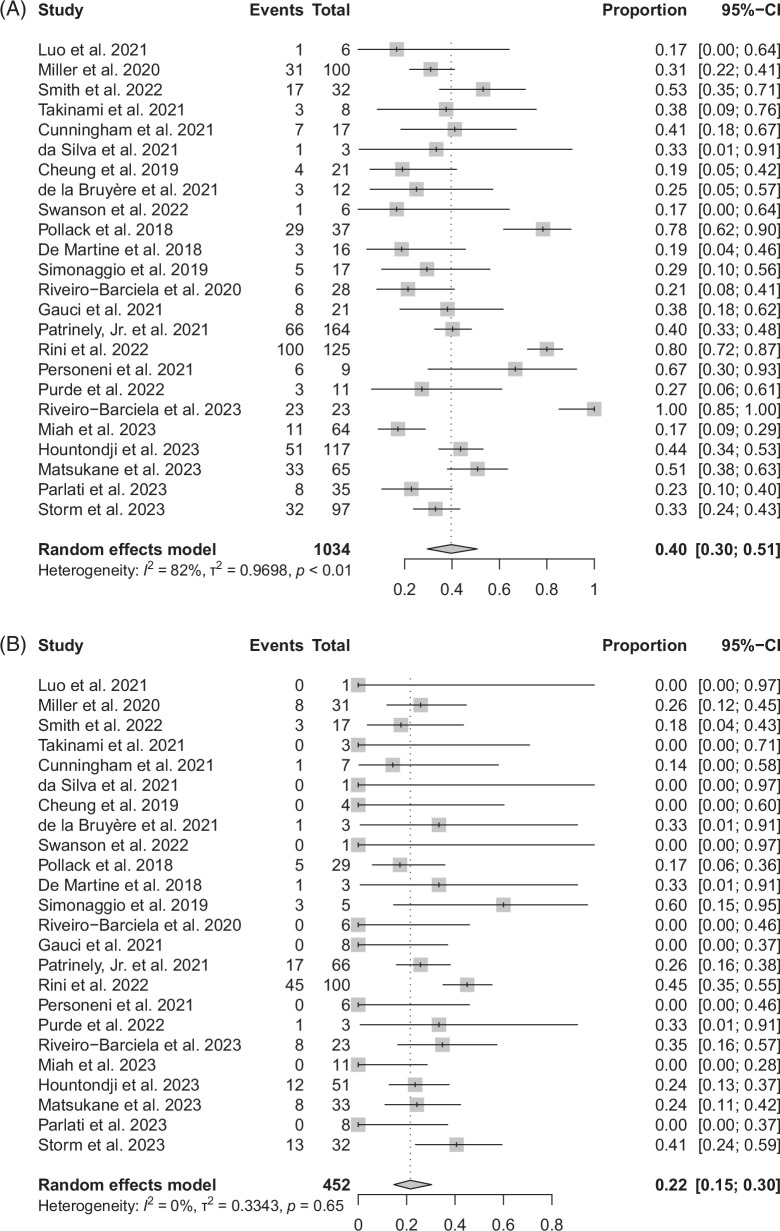
After the resolution of ICI hepatitis or improvement to grade 1 hepatitis, patients were rechallenged with the ICI based on clinical judgment Table 3. An estimated 40% (95% CI: 30%–51%) of patients of all patients with ICI hepatitis were rechallenged with an ICI, and of those rechallenged (Figure 3A), there was an estimated 22% (95% CI: 15%–30%) recurrence (Figure 3B). There was high heterogeneity (I 2 = 81.8%) in the proportion of patients rechallenged out of the total patients with ICI hepatitis. The funnel plot analysis showed no evidence of publication bias (p < 0.001) for this outcome. Previously developing advanced ICI hepatitis (grade 3-4) did not have a significant association with the proportion of patients rechallenged (coefficient = 0.197, p = 0.848) nor the recurrence of ICI hepatitis (coefficient = 0.449, p = 0.553).
FIGURE 3.
(A) Proportion of patients who were rechallenged with an ICI. (B) Proportion of ICI recurrences in patients who were rechallenged. Abbreviation: ICI, immune checkpoint inhibitor.
DISCUSSION
Steroid treatment was the primary intervention in over 75% of patients with ICI hepatitis while 16% of the patients who received steroids required a secondary immunosuppressant in management. An estimated 23% of patients, mostly with grades 1-2 hepatitis, did not require any intervention. Of those who were rechallenged with an ICI, only 22% of the patients experienced a recurrence of ICI hepatitis. Steroids are the treatment of choice given that it is considered that high-dose glucocorticoids do not interfere with the antitumor response of ICIs but there are also controversial studies against this.1,53
Current AGA guidelines suggest liver monitoring for grade 1 hepatitis, ICI discontinuation for grade 2 and higher, and if the patient is symptomatic of liver toxicity, an equivalent of prednisone 0.5–1.0 mg/kg/d should be administered for grade 2 hepatitis. For grade 3 hepatitis, initiation of an equivalent of 1–2 mg/kg of methylprednisone is recommended, and a second-line immunomodulator such as an azathioprine or mycophenolate mofetil can be considered if there is no clinical improvement in 3–5 days. For grade 4 hepatitis, permanent discontinuation of ICI and initiation of an equivalent of 2 mg/kg/d of methylprednisone is recommended.5 Third-line immunosuppressive therapy brought into consideration is anti-thymocyte globulin for ipilimumab-induced hepatitis or tacrolimus, whereas infliximab is not recommended.4
Several studies included in our analysis asserted that there is greater risk than benefit in the use of high-dose steroids compared to low-dose steroids and association with poor survival.9,16,22,27,49 This can be interpreted by 3 hypotheses: (1) patients who are treated with high-dose steroids have more advanced hepatitis; (2) patients with advanced cancer treated with ICIs are at higher risk for side effects of immunosuppression, especially infection; and (3) high-dose steroids compromise the effectiveness of ICIs. Li et al16 compared 87 patients in the ≥1.5 mg/kg methylprednisone equivalent group and 128 patients in the <1.5 mg/kg group with grade 3-4 ICI hepatitis and reported that there was no difference in the development of steroid-refractory hepatitis but longer exposure and higher incidence of infection. However, the high-dose steroid group also had a higher percentage of ipilimumab and nivolumab combination therapy, which can contribute to a higher risk of disease.16 Corticosteroids can inhibit the antitumor immune response of ICIs by suppressing low-affinity memory T cells, particularly in a higher dose and earlier administration timing.54
Anti–CTLA–4 mAbs have been associated with a higher incidence of ICI hepatitis compared to anti-PD1/anti–PD-L1 mAbs, and combination therapy was considered a higher risk than monotherapy, although our study did not demonstrate a statistically significant relationship.10,17,47 Several studies have suggested that specific histopathologic patterns may correlate with the type of ICI used. Furthermore, these studies indicate that treatment responses may vary based on the characteristic histopathologic pattern of ICI hepatitis. De Martin et al33 observed a more prevalent pattern of granulomatous hepatitis with anti–CTLA–4 mAbs and a more heterogeneous pattern, mainly lobular hepatitis in anti–PD-1/PD-L1 mAbs. Different histopathologic patterns were also associated with different treatment responses. A study of 20 biopsied patients reported that patients with an acute granulomatous profile defined by the presence of granulomas or acute hepatitis with a toxic profile defined by the presence of eosinophilic polynuclear cells had a better response to corticosteroids, whereas patients with a cholangitic lesion with recorded bile duct lesions had a worse response.50
As a second-line immunomodulator, mycophenolate mofetil was used in the majority of cases refractory to steroids. Interestingly, infliximab, which was not recommended in the AGA guidelines due to potential idiosyncratic liver injury, was the second-line drug of choice in 5 cases and azathioprine in 3 cases.5 Mycophenolate mofetil is a purine antagonist that inhibits the proliferation and activation of both T and B lymphocytes and has been used as a second-line agent for steroid-refractory autoimmune hepatitis.55–57 Azathioprine, traditionally the first-line steroid-sparing agent for autoimmune hepatitis, is less favored in ICI treatment. This is due to its slow onset of immunosuppressive effect, which can take several months to reach peak efficacy. In addition, azathioprine’s metabolites can potentially cause hepatotoxicity, further complicating its use in patients already experiencing liver inflammation.31,58 While the selection of second-line immunomodulators originates from agents used to manage autoimmune hepatitis, it is worth noting that ICI hepatitis exhibits distinct characteristics compared to autoimmune hepatitis, including analytic factors such as lower levels of gammaglobulins, immunoglobulin G, and ANAs.37
Diagnosis and management of ICI hepatitis are challenging in that it is a distinct etiology that is a DILI but also has components of immunological characteristics. ICI hepatitis is a clinical diagnosis of exclusion, and certain adjunctive parameters, such as the RUCAM score, were used to assist in determining whether hepatitis is a DILI.59 Also, as the majority of studies for ICI hepatitis are conducted on patients with advanced cancer, such as patients with stage 4 melanoma or non–small cell lung cancer, hepatic metastases can be a confounding factor in the evaluation of ICI hepatitis.10,25
ICIs were rechallenged after resolution or improvement to grade 1 hepatitis in an estimated 40% of the cases. Recurrence of ICI hepatitis was present in 22% of all rechallenged cases, mainly in anti–PD-1/PD-L1 agents, and was noted to be not as severe as the initial event.34,45,48 Hountondji et al48 suggested that rechallenge was even possible after grade 3-4 hepatitis. ICI rechallenge is important because patients at advanced cancer stage have limited options for treatment and because irAEs, including ICI hepatitis, have been associated with improved antitumor efficacy and overall survival.44,46,47,60 Our findings suggest that rechallenge of ICIs should be reconsidered more frequently after successful treatment of ICI hepatitis. Two studies compared the outcome between patients who underwent ICI rechallenge and those who did not; Simonaggio et al34 found no significant difference in median progression-free survival time between the rechallenged and non-rechallenged groups, including irAE from other systems. Similarly, Miah et al47 reported no difference in best overall response or time to death between these groups. However, these findings need to be interpreted cautiously due to the potential for substantial selection bias based on the severity and treatment response of ICI hepatitis. It is also critical that rechallenge would often involve a different regimen, such as switching the class from anti-CTLA4 to anti-PD (L)1 therapy or de-escalation from combination therapy to monotherapy.11,14,21,37,38,48,51
Our study is the first meta-analysis to quantify the prognosis and treatment response of ICI hepatitis with steroid treatment as the primary treatment. However, our study also had several limitations. First, the variability in the dosage and duration of steroids were high between studies, and it could have been an overgeneralization in estimating the effect of steroids on whether patients received steroid treatment or not. Second, not all studies reported patient characteristics we considered important. For example, while earlier studies provided the detailed dosage and regimen of ICI therapy, most recent studies only included broad categories of ICI therapy used, potentially introducing greater heterogeneity into the analysis. Lastly, although we determined that this is minimal in our study, there is still a possibility of publication bias.
CONCLUSIONS
Our meta-analysis reveals that corticosteroids remain the primary treatment for ICI hepatitis, with mycophenolate mofetil serving as a secondary option in steroids-refractory cases. ICI rechallenge resulted in recurrence in approximately one-fifth of the cases, typically with less severe presentations. However, current practices largely rely on expert consensus, highlighting the need for prospective studies on key areas. These include establishing standardized steroid treatment protocols, evaluating the efficacy of mycophenolate mofetil in steroid-refractory cases, and assessing the safety and efficacy of ICI rechallenge following ICI hepatitis.
Supplementary Material
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author.
CONFLICTS OF INTEREST
The authors have no conflicts to report.
Footnotes
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events, Version 5; ICI, immune checkpoint inhibitor; irAE, immune-related adverse event; NOS, Newcastle–Ottawa Scale; ULN, upper limit of normal.
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal’s website, www.hepcommjournal.com.
Contributor Information
Soo Young Hwang, Email: sooyoungsarah@gmail.com.
Pinghsin Hsieh, Email: Pinghsin.hsieh@umm.edu.
Wei Zhang, Email: wzhang50@mgh.harvard.edu.
REFERENCES
- 1.Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-checkpoint inhibitors: Long-term implications of toxicity. Nat Rev Clin Oncol. 2022;19:254–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sullivan RJ, Weber JS. Immune-related toxicities of checkpoint inhibitors: Mechanisms and mitigation strategies. Nat Rev Drug Discov. 2022;21:495–508. [DOI] [PubMed] [Google Scholar]
- 3.Romão R, Mendes AS, Ranchor R, Ramos MJ, Coelho J, Pichel RC, et al. Impact of immune-related adverse events on immune checkpoint inhibitors treated cancer patients’ survival: single center experience and literature review. Cancers. 2023;15:888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–iv142. [DOI] [PubMed] [Google Scholar]
- 5.Dougan M, Wang Y, Rubio-Tapia A, Lim JK. AGA clinical practice update on diagnosis and management of immune checkpoint inhibitor colitis and hepatitis: Expert review. Gastroenterology. 2021;160:1384–1393. [DOI] [PubMed] [Google Scholar]
- 6.Institute NC . Common Terminology Criteria for Adverse Events (CTCAE) v5.0. 2017. Accessed May 2024. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5x7.pdf
- 7.Julian PTH, Simon GT, Jonathan JD, Douglas GA. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leroy V, Gerard E, Dutriaux C, Prey S, Gey A, Mertens C, et al. Adverse events need for hospitalization and systemic immunosuppression in very elderly patients (over 80 years) treated with ipilimumab for metastatic melanoma. Cancer Immunol Immunother. 2019;68:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luo J, Beattie JA, Fuentes P, Rizvi H, Egger JV, Kern JA, et al. Beyond steroids: Immunosuppressants in steroid-refractory or resistant immune-related adverse events. J Thoracic Oncol. 2021;16:1759–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Romanski NA, Holmstroem RB, Ellebaek E, Svane IM. Characterization of risk factors and efficacy of medical management of immune-related hepatotoxicity in real-world patients with metastatic melanoma treated with immune checkpoint inhibitors. Eur J Cancer. 2020;130:211–218. [DOI] [PubMed] [Google Scholar]
- 11.Miller ED, Abu-Sbeih H, Styskel B, Nogueras Gonzalez GM, Blechacz B, Naing A, et al. Clinical characteristics and adverse impact of hepatotoxicity due to immune checkpoint inhibitors. Am J Gastroenterol. 2020;115:251–261. [DOI] [PubMed] [Google Scholar]
- 12.Smith MK, Chan Y, Suo AE, Shaheen AA, Congly SE, Tandon P, et al. Clinical course and treatment implications of combination immune checkpoint inhibitor-mediated hepatitis: A multicentre cohort. J Can Assoc Gastroenterol. 2022;5:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamamoto A, Yano Y, Ueda Y, Yasutomi E, Hatazawa Y, Hayashi H, et al. Clinical features of immune-mediated hepatotoxicity induced by immune checkpoint inhibitors in patients with cancers. J Cancer Res Clin Oncol. 2021;147:1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takinami M, Ono A, Kawabata T, Mamesaya N, Kobayashi H, Omori S, et al. Comparison of clinical features between immune-related sclerosing cholangitis and hepatitis. Investig New Drugs. 2021;39:1716–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owen CN, Bai X, Quah T, Lo SN, Allayous C, Callaghan S, et al. Delayed immune-related adverse events with anti-PD-1-based immunotherapy in melanoma. Ann Oncol. 2021;32:917–925. [DOI] [PubMed] [Google Scholar]
- 16.Li M, Wong D, Vogel AS, Sack JS, Rahma OE, Hodi FS, et al. Effect of corticosteroid dosing on outcomes in high-grade immune checkpoint inhibitor hepatitis. Hepatology. 2022;75:531–540. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham M, Iafolla M, Kanjanapan Y, Cerocchi O, Butler M, Siu LL, et al. Evaluation of liver enzyme elevations and hepatotoxicity in patients treated with checkpoint inhibitor immunotherapy. PLoS One. 2021;16:e0253070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanz-Segura P, García-Cámara P, Fernández-Bonilla E, Arbonés-Mainar JM, Bernal Monterde V. Gastrointestinal and liver immune-related adverse effects induced by immune checkpoint inhibitors: A descriptive observational study. Gastroenterol Hepatol. 2021;44:261–268. [DOI] [PubMed] [Google Scholar]
- 19.da Silva JA, Falcão D, Cardoso C, Pires AL, Araújo A, Castro-Poças F. Hepatic immune-mediatedadverseeffects of immune checkpoint inhibitors: Analysis of real-life experience. Ann Hepatol. 2021;26. doi: 10.1016/j.aohep.2021.100561 [DOI] [PubMed] [Google Scholar]
- 20.Huffman BM, Kottschade LA, Kamath PS, Markovic SN. Hepatotoxicity after immune checkpoint inhibitor therapy in melanoma: Natural progression and management. Am J Clin Oncol. 2018;41:760–765. [DOI] [PubMed] [Google Scholar]
- 21.Cheung V, Gupta T, Payne M, Middleton MR, Collier JD, Simmons A, et al. Immunotherapy-related hepatitis: Real-world experience from a tertiary centre. Frontline Gastroenterol. 2019;10:364–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimomura K, Yamaguchi T, Oya Y, Uchida K, Murotani K. Impact of corticosteroids for IrAEs on the clinical outcome of immunotherapy in patients with NSCLC. Anticancer Res. 2022;42:5961–5969. [DOI] [PubMed] [Google Scholar]
- 23.Swanson L, Kassab I, Tsung I, Worden FP, Fontana RJ. Infrequent liver injury from cemiplimab in patients with advanced cutaneous squamous cell carcinoma. Immunotherapy. 2022;14:409–418. [DOI] [PubMed] [Google Scholar]
- 24.Bruyère CL, Souquet PJ, Dalle S, Corbaux P, Boespflug A, Duruisseaux M, et al. Investigating the impact of immune-related adverse events, glucocorticoid use and immunotherapy interruption on long-term survival outcomes. Cancers. 2021;13:2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swanson LA, Kassab I, Tsung I, Schneider BJ, Fontana RJ. Liver injury during durvalumab-based immunotherapy is associated with poorer patient survival: A retrospective analysis. Front Oncol. 2022;12:984940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawada K, Hayashi H, Nakajima S, Hasebe T, Fujiya M, Okumura T. Non-alcoholic fatty liver disease is a potential risk factor for liver injury caused by immune checkpoint inhibitor. J Gastroenterol Hepatol. 2020;35:1042–1048. [DOI] [PubMed] [Google Scholar]
- 27.Fan C, Kim A, Li S, Naidoo J, Cappelli LC, Brahmer JR, et al. Outcomes of immunotherapy-related hepatotoxicity from a multi-disciplinary toxicity team. J Cancer Res Clin Oncol. 2023;149:877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitagataya T, Suda G, Nagashima K, Katsurada T, Yamamoto K, Kimura M, et al. Prevalence, clinical course, and predictive factors of immune checkpoint inhibitor monotherapy-associated hepatitis in Japan. J Gastroenterol Hepatol. 2020;35:1782–1788. [DOI] [PubMed] [Google Scholar]
- 29.Zheng J, Cui T, Gao Y, Li T. Retrospective analysis of immune-related adverse events of the immune checkpoint inhibitors of PD-1/PD-l1 in the Fujian provincial hospital. Eur J Inflamm. 2022;20:1721727X2210915. doi: 10.1177/1721727X221091540 [DOI] [Google Scholar]
- 30.Daniello L, Elshiaty M, Bozorgmehr F, Kuon J, Kazdal D, Schindler H, et al. Therapeutic and prognostic implications of immune-related adverse events in advanced non-small-cell lung cancer. Front Oncol. 2021;11:703893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng R, Cooper A, Kench J, Watson G, Bye W, McNeil C, et al. Ipilimumab-induced toxicities and the gastroenterologist. J Gastroenterol Hepatology. 2015;30:657–666. [DOI] [PubMed] [Google Scholar]
- 32.Pollack MH, Betof A, Dearden H, Rapazzo K, Valentine I, Brohl AS, et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma. Ann Oncol. 2018;29:250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Martin E, Michot JM, Papouin B, Champiat S, Mateus C, Lambotte O, et al. Characterization of liver injury induced by cancer immunotherapy using immune checkpoint inhibitors. J Hepatol. 2018;68:1181–1190. [DOI] [PubMed] [Google Scholar]
- 34.Simonaggio A, Michot JM, Voisin AL, Le Pavec J, Collins M, Lallart A, et al. Evaluation of readministration of immune checkpoint inhibitors after immune-related adverse events in patients with cancer. JAMA Oncol. 2019;5:1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Imoto K, Kohjima M, Hioki T, Kurashige T, Kurokawa M, Tashiro S, et al. Clinical features of liver injury induced by immune checkpoint inhibitors in Japanese patients. Can J Gastroenterol Hepatol. 2019;2019:6391712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zen Y, Chen YY, Jeng YM, Tsai HW, Yeh MM. Immune-related adverse reactions in the hepatobiliary system: Second-generation check-point inhibitors highlight diverse histological changes. Histopathology. 2020;76:470–480. [DOI] [PubMed] [Google Scholar]
- 37.Riveiro‐Barciela M, Barreira‐Díaz A, Vidal‐González J, Muñoz‐Couselo E, Martínez‐Valle F, Viladomiu L, et al. Immune-related hepatitis related to checkpoint inhibitors: Clinical and prognostic factors. Liver Int. 2020;40:1906–1916. [DOI] [PubMed] [Google Scholar]
- 38.Gauci ML, Baroudjian B, Bédérède U, Zeboulon C, Delyon J, Allayous C, et al. Severe immune-related hepatitis induced by immune checkpoint inhibitors: Clinical features and management proposal. Clin Res Hepatol Gastroenterol. 2021;45:101491. [DOI] [PubMed] [Google Scholar]
- 39.Patrinely JR, Jr, McGuigan B, Chandra S, Fenton SE, Chowdhary A, Kennedy LB, et al. A multicenter characterization of hepatitis associated with immune checkpoint inhibitors. Oncoimmunology. 2021;10:1875639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rini BI, Atkins MB, Plimack ER, Soulières D, McDermott RS, Bedke J, et al. Characterization and management of treatment-emergent hepatic toxicity in patients with advanced renal cell carcinoma receiving first-line pembrolizumab plus axitinib. Results from the KEYNOTE-426 Trial. Eur Urol Oncol. 2022;5:225–234. [DOI] [PubMed] [Google Scholar]
- 41.Lin Z, Zhang X, Zhou Y, Chen C, He L, Li H, et al. Hepatotoxicity associated with PD-1 blockade antibodies in cancer patients co-infected with hepatitis B virus. Cancer Immunol Immunother. 2022;71:1247–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Personeni N, Pressiani T, D’Alessio A, Prete MG, Bozzarelli S, Terracciano L, et al. Hepatotoxicity in patients with hepatocellular carcinoma on treatment with immune checkpoint inhibitors. Cancers (Basel). 2021;13:5665. doi: 10.3390/cancers13225665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purde MT, Niederer R, Wagner NB, Diem S, Berner F, Hasan Ali O, et al. Presence of autoantibodies in serum does not impact the occurrence of immune checkpoint inhibitor-induced hepatitis in a prospective cohort of cancer patients. J Cancer Res Clin Oncol. 2022;148:647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ng KYY, Tan SH, Tan JJE, Tay DSH, Lee AWX, Ang AJS, et al. Impact of immune-related adverse events on efficacy of immune checkpoint inhibitors in patients with advanced hepatocellular carcinoma. Liver Cancer. 2022;11:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riveiro-Barciela M, Barreira-Díaz A, Callejo-Pérez A, Muñoz-Couselo E, Díaz-Mejía N, Díaz-González Á, et al. Retreatment with immune checkpoint inhibitors after a severe immune-related hepatitis: Results from a prospective multicenter study. Clin Gastroenterol Hepatol. 2023;21:732–740. [DOI] [PubMed] [Google Scholar]
- 46.Alomari M, Al Ashi S, Chadalavada P, Khazaaleh S, Covut F, Al Momani L, et al. Gastrointestinal toxicities of immune checkpoint inhibitors are associated with enhanced tumor responsiveness and improved survival. Gastroenterology Res. 2022;15:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miah A, Tinoco G, Zhao S, Wei L, Johns A, Patel S, et al. Immune checkpoint inhibitor-induced hepatitis injury: risk factors, outcomes, and impact on survival. J Cancer Res Clin Oncol. 2023;149:2235–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hountondji L, Ferreira De Matos C, Lebossé F, Quantin X, Lesage C, Palassin P, et al. Clinical pattern of checkpoint inhibitor-induced liver injury in a multicentre cohort. JHEP Rep. 2023;5:100719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsukane R, Suetsugu K, Hata K, Matsuda K, Nakao S, Minami H, et al. Systematic surveillance of immune-related adverse events in clinical practice and impact of subsequent steroid medication on survival outcomes. Int J Clin Oncol. 2023;28:860–871. [DOI] [PubMed] [Google Scholar]
- 50.Parlati L, Marcin K, Terris B, Vallet-Pichard A, Corouge M, Hollande C, et al. Histological characteristics and management of hepatitis on immune checkpoint inhibitors: A retrospective descriptive study. J Clin Med. 2023;12:3751. doi: 10.3390/jcm12113751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Storm EM, Makrakis D, Lin GI, Talukder R, Bakaloudi DR, Shah EE, et al. Role of underlying liver pathology in the development of immune-related hepatitis: A case–control study. Targeted Oncology. 2023;18:601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bai X, Hu J, Betof Warner A, Quach HT, Cann CG, Zhang MZ, et al. Early use of high-dose glucocorticoid for the management of irAE is associated with poorer survival in patients with advanced melanoma treated with anti–PD-1 monotherapy. Clin Cancer Res. 2021;27:5993–6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tokunaga A, Sugiyama D, Maeda Y, Warner AB, Panageas KS, Ito S, et al. Selective inhibition of low-affinity memory CD8(+) T cells by corticosteroids. J Exp Med. 2019;216:2701–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mir R, Shaw HM, Nathan PD. Immunosuppressive agents and their role in managing immunotherapy toxicities in melanoma. Clin Skin Cancer. 2017;2:18–23. [Google Scholar]
- 56.Kadokawa Y, Inoue S, Tatsumi A, Uchida M, Fujita K, Takagi M, et al. Efficacy and safety of mycophenolate mofetil in treating immune-related hepatitis induced by immune checkpoint inhibitor use: A retrospective study. JGH Open. 2023;7:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heneghan MA, McFarlane IG. Current and novel immunosuppressive therapy for autoimmune hepatitis. Hepatology. 2002;35:7–13. [DOI] [PubMed] [Google Scholar]
- 58.Remash D, Prince DS, McKenzie C, Strasser SI, Kao S, Liu K. Immune checkpoint inhibitor-related hepatotoxicity: A review. World J Gastroenterol. 2021;27:5376–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012-. Roussel Uclaf Causality Assessment Method (RUCAM) in Drug Induced Liver Injury. Accessed May 4, 2019. https://www.ncbi.nlm.nih.gov/books/NBK548272/
- 60.Okada N, Kawazoe H, Takechi K, Matsudate Y, Utsunomiya R, Zamami Y, et al. Association between immune-related adverse events and clinical efficacy in patients with melanoma treated with nivolumab: A multicenter retrospective study. Clin Ther. 2019;41:59–67. [DOI] [PubMed] [Google Scholar]