Abstract
Objective
Percent glycated albumin (%GAlb) is a marker of glycemia over the past 2 to 3 weeks in nonpregnant individuals. Longitudinal changes in %GAlb extending throughout pregnancy and postpartum (PP) have not been described. We aimed to describe levels of %GAlb throughout pregnancy and PP and relationships with glycemia.
Study Design
Fifty women among those in the Study of Pregnancy Regulation of INsulin and Glucose cohort underwent 75-g oral glucose tolerance tests (OGTTs) at a mean of 13 weeks (V1) and 26 weeks (V2) of gestation and 11 weeks’ PP. %GAlb was measured on frozen plasma samples.
Results
Total albumin decreased from V1 to V2 and increased PP to levels higher than at V1. %GAlb declined between V1 and V2 (β = −0.63% 95% CI [−0.8, −0.6] p < 0.001) and remained stable between V2 and PP (β = −0.04% [−0.3, 0.2] p = 0.78). Body mass index (BMI) was inversely related to %GAlb in pregnancy (V1: rho = −0.5, p = 0.0001; V2 rho = −0.4, p = 0.006), but not PP (rho = −0.15, p = 0.31). The longitudinal changes in %GAlb persisted after adjusting for BMI. Neither glycemia measurements nor hemoglobin A1c were associated with %GAlb at any time point, and adjustments for BMI did not reveal additional associations.
Conclusion
%GAlb decreases between early and late gestation and remains decreased PP, despite a PP increase in total albumin above early pregnancy values. Given the lack of correlation with OGTT values or A1c, %GAlb is unlikely to be useful in assessing glycemia in pregnant or PP women.
Keywords: gestational diabetes, gestational glycemia, glycated albumin, pregnancy biomarkers, postpartum diabetes, postpartum screening
Gestational diabetes mellitus (GDM) is a prevalent complication of pregnancy, affecting more than 1 in 20 pregnancies in the United States.1–3 Universal screening for GDM using oral glucose tolerance tests (OGTTs) is standard in prenatal care, with the goal of identifying and treating cases to reduce the risk of GDM-associated adverse pregnancy outcomes. Although the disease is in part related to the hormonally mediated changes in the placenta, GDM is associated with an up to 70% risk of progression to diabetes in the decades following delivery.4–6 Given the risk of progression to DM, women typically undergo OGTT screening at 4 to 12 weeks’ postpartum (PP) to assess for the persistence of dysglycemia.4,7
As OGTT testing is cumbersome and associated with low testing adherence,5,8–13 other biomarkers for glycemia have been studied. Despite its promise, hemoglobin A1c (A1c) has not been shown to correlate with glycemia beyond the first trimester given the alterations of pregnancy on red cell mechanics and anemia of pregnancy.14 And with respect to PP screening, while some studies have shown it may be useful in conjunction with fasting blood glucose, A1c has not demonstrated sufficient evidence to supplant the OGTT in the screening of PP women following pregnancies affected by GDM.14–18 Outside of pregnancy, percent glycated albumin (%GAlb) correlates well with glycemia in the preceding 2 to 3 weeks and is unaffected by red cell turnover, anemia, or iron deficiency.19–22 Offsetting these advantages, %GAlb is inversely correlated with body mass index (BMI), which diminishes its usefulness as a glycemic marker when BMI is changing.20,23 Prior reports of %GAlb as a marker of glycemia during pregnancy18,20,22–29 have been limited by inclusion of pregnancies affected by GDM only, lack of an established reference range for %GAlb in pregnancy, or exclusion of the PP period.
We sought to characterize changes in the levels of %GAlb throughout gestation and PP as part of a pilot study to better examine the relationship between this biomarker and glycemia during these critical time periods.
Materials and Methods
In this pilot study, we studied a randomly selected subset of women in the Study of Pregnancy Regulation of INsulin and Glucose (SPRING, NCT02763267) cohort in Boston, MA. We recruited women directly through our academic medical center and through advertisements in the Boston area. We enrolled pregnant people in the first trimester and followed them longitudinally throughout pregnancy and PP. We enrolled nonpregnant women for a cross-sectional study. Women were eligible if they had at least one risk factor for GDM (i.e., history of GDM in a prior pregnancy without regard to BMI, first-degree family history of DM or GDM without regard to BMI, or BMI ≥25 kg/m2 plus one additional risk factor).30 We excluded women if they had preexisting DM or were using medications known to affect glucose tolerance (i.e., metformin or systemic corticosteroids). The Mass General Brigham Institutional Review Board approved the study, and all participants provided written informed consent.
We studied participants three times across pregnancy; at 7 to 15 weeks’ gestation (V1), in mid-late pregnancy 24 to 30 weeks’ gestation (V2), and at 6 to 24 weeks’ PP (V3). This analysis included a randomly selected subset of 50 women who attended all three study visits. These women were chosen out of a total of 55 women in the SPRING study at the time with complete data available. Baseline demographic information was obtained by a survey, which included self-reported age, gravidity, parity, income, race, family history of DM, and personal history of GDM. Pregnancy outcome data were also collected. Pregnant women completed an OGTT at all three study visits. We tested nonpregnant women with a single OGTT. Participants fasted for at least 8 hours prior to the OGTT. After the fasting blood sample, participants consumed a 75-g standard OGTT beverage within 5 minutes with blood then drawn at 30 minutes, 1 hour, and 2 hours after the glucose load.
We used the International Association of the Diabetes in Pregnancy Study Groups’ 2010 criteria to define GDM (fasting glucose ≥ 92 mg/dL, 1-hour glucose ≥ 180 mg/dL, 2-hour glucose ≥ 153 mg/dL)31 in the first and second trimesters of pregnancy. The 50 pregnant participants with complete data samples and the 50 nonpregnant controls were collected between 2016 and 2020.
We shipped frozen plasma samples from the SPRING cohort to the Advanced Research and Diagnostics Laboratory at the University of Minnesota for analysis. We measured total serum albumin and glycated albumin on the Roche Cobas c502 module using the Lucica Glycated Albumin-L diagnostic reagents. %GAlb was calculated per the manufacturer’s instructions.32
Statistical Analysis
We compared baseline demographics of the pregnant cohort to the nonpregnant cohort with chi-square analyses or Student’s t-test where appropriate. Given the previously described negative correlation between %GAlb and BMI, we first performed a longitudinal analysis of BMI to describe its behavior throughout pregnancy and PP. We then conducted longitudinal analyses using generalized estimating equations with an unstructured correlation structure, allowing for varying correlation between timepoints, to determinethebehavior of total serum albumin and %GAlb throughout gestation and PP. We accounted for the relationship of BMI to %GAlb with further adjustments.
We then performed an analysis of %GAlb in pregnant compared with nonpregnant controls at each time point with linear regression. Initial analyses were unadjusted, followed by adjustments for BMI alone. We then created a fully adjusted model including age, BMI, marital status, parity, race/ethnicity, and family history of DM. We used these regression models to examine the differences in total albumin between pregnant and nonpregnant participants.
Using Spearman’s correlations coefficients and linear regression models with adjustment for BMI and weeks’ PP (where appropriate), we explored the relationship between %GAlb throughout the study time points to the fasting, 1-hour, 2-hour, and mean OGTT values. Mean OGTT was calculated based on mean glucose level during the OGTT by averaging the fasting, 1-hour, and 2-hour values. We also performed an analysis to assess the relationship to hemoglobin A1c using Spearman’s correlations. We generated glycemia correlation coefficients to assess the relationship between the changes in the %GAlb between time points (V1 to V2 and V2 to PP) and the various results from the OGTT.
The longitudinal analyses and analyses comparing pregnant and nonpregnant participants described above were also conducted restricting to participants with and without GDM.
Statistical analyses were conducted in STATA IC version 16 (College Station, TX).
Results
Characteristics of participants in this analysis are presented in Table 1 with comparisons to nonpregnant controls. Pregnant participants (N = 50) were older, were less likely to be nulliparous and to have a family history of DM, and were more likely to be married. Among those with prior pregnancies, pregnant participants were more likely to have a history of GDM in the past. Among the pregnant participants studied, 10 (20%) were diagnosed with GDM in the current pregnancy and 3 (6%) were diagnosed with preeclampsia. In the PP period, 2 (4%) were diagnosed with DM and 1 (2%) was diagnosed with prediabetes. The average gestational age at the V1 visit was 12.9 (standard deviation [SD] = 1.6) weeks’ gestation, at V2 was 26.2 (SD = 1.4) weeks’ gestation, and at V3 was 11 (SD = 4.9) weeks’ PP. As expected, BMI was significantly higher as pregnancy progressed from V1 to V2 (β = 2.05 kg/m2 [1.65, 2.45] p < 0.001) and remained higher PP (V3) compared with the start of pregnancy (V1 β = 0.65 kg/m2 [0.11, 1.19] p = 0.019). PP women (V3) had lower BMIs compared with pregnant women in V2 (β = −1.4 kg/m2 [−1.97, −0.83] p < 0.001). When compared with nonpregnant controls, pregnant women at V2 and PP women (V3) had higher BMIs (V2 β = 2.86 kg/m2 [0.96, 4.76] p = 0.003, V3 β = 2.61 kg/m2 [0.34, 4.89] p = 0.003). Pregnant women at V1 had BMIs that trended higher than nonpregnant controls, although without reaching statistical significance (β = 1.96 kg/m2 [−0.31, 4.24] p = 0.09).
Table 1.
Patient demographics
| Nonpregnant women (n = 50) Mean (SD) or N (%) | Pregnant/postpartum women (n = 50) Mean (SD) or N (%) | p-Value | |
|---|---|---|---|
| Age (y) | 27.5 (6.5) | 32.3 (4.5) | <0.001 |
| Nulliparous | 42 (84%) | 21 (42%) | <0.001 |
| History of GDMa | 1 (12.5%) | 6 (21%) | 0.06 |
| BMI at V1 | 27.2 (5.4) | 29.2 (6.1) | 0.09 |
| Family history of DM | 26 (52%) | 13 (26%) | 0.008 |
| Race/ethnicity | |||
| Hispanic | 9 (18%) | 10 (20%) | 0.07 |
| Non-Hispanic | |||
| White | 21 (42%) | 31 (62%) | |
| Black/African American | 7 (14%) | 6 (12%) | |
| Asian | 11 (22%) | 2 (4%) | |
| Other | 2 (4%) | 1 (2%) | |
| College graduate | 38 (76%) | 42 (84%) | 0.32 |
| Smoker | 5 (10%) | 8 (16%) | 0.31 |
| Married | 6 (12%) | 37 (74%) | <0.001 |
Abbreviations: DM, diabetes mellitus; GDM, gestational diabetes mellitus; SD, standard deviation.
Note: Total N = complete cases.
% calculated only out of parous people.
The mean total albumin was 3.78 g/dL (SD = 0.29) at V1, 3.27 g/dL (SD = 0.25) at V2, and 4.35 g/dL (SD = 0.30) at V3. The mean %GAlb at V1 was 12.6% (SD = 1.1), at V2 11.9% (SD = 0.96), and PP 11.9% (SD = 0.84). Longitudinal analyses demonstrated that total serum albumin was decreased at V2 compared with V1 (β = −0.51 g/dL [−0.59, −0.43] p < 0.001) and was increased PP compared with V1 (β = 0.56 g/dL [0.49, 0.63] p < 0.001; Fig. 1). In contrast, percent GAlb was decreased at V2 compared with V1 and remained lower than V1 in the PP period (V2 β = −0.63% [−0.73, −0.55] p < 0.001, V3 β = −0.67% [−0.90, −0.45] p < 0.001). BMI and %GAlb were negatively correlated in early pregnancy (V1) and mid-late pregnancy (V2), but not PP (Fig. 2). The observed longitudinal changes in %GAlb did not change after adjustment for BMI (compared with V1, V2 β = −0.57% [−0.70, −0.43] p < 0.001, V3 β = −0.65% [−0.88, −0.42] p < 0.001).
Fig. 1.
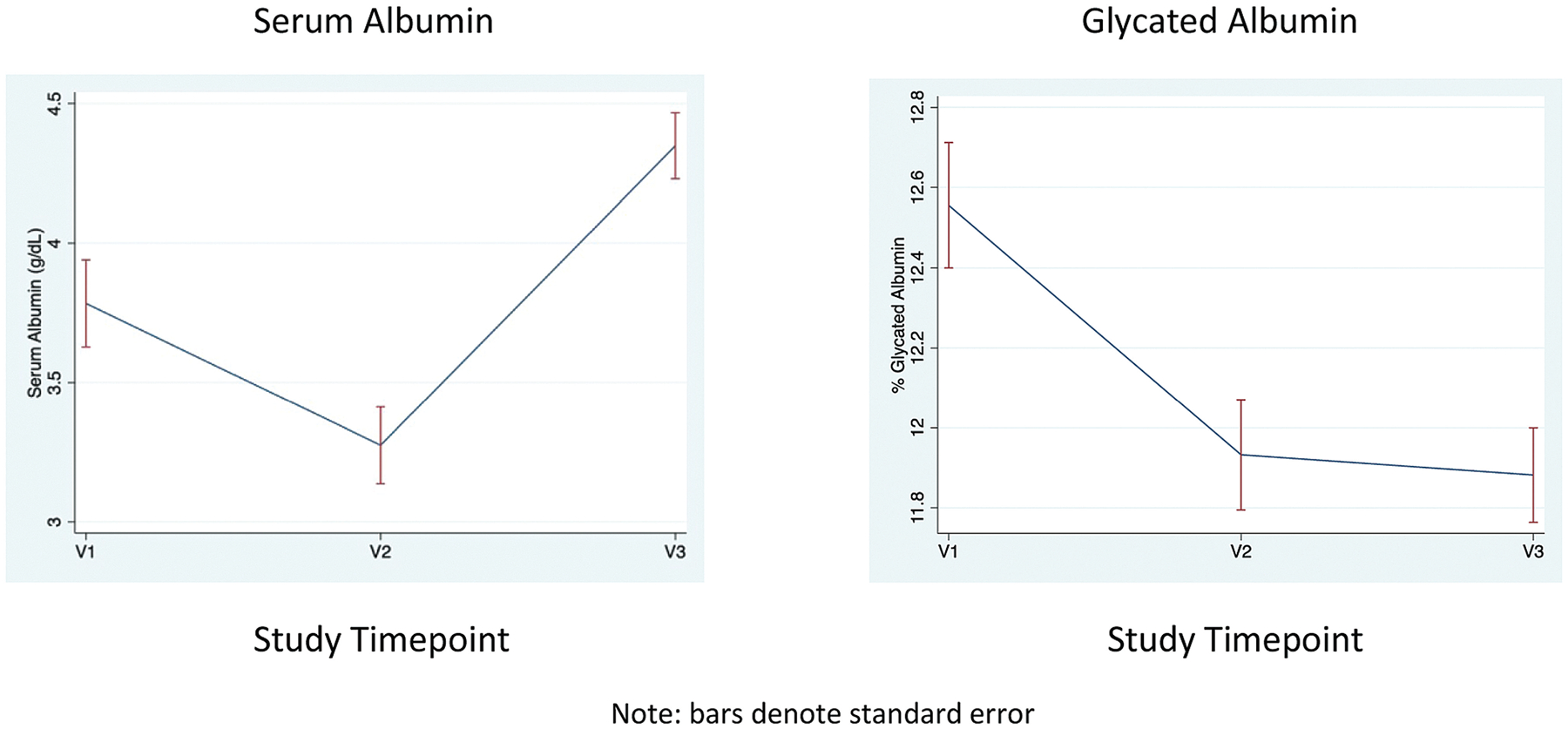
Longitudinal trends in total serum albumin and %glycated albumin.
Fig. 2.
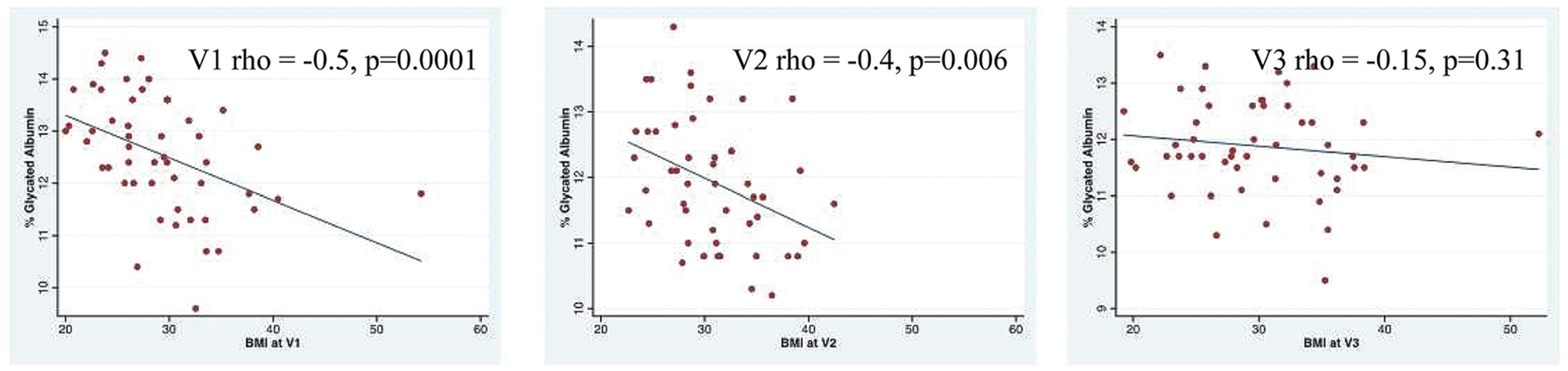
Correlations between body mass index (BMI) and %glycated albumin.
Pregnant participants were compared with nonpregnant controls. Nonpregnant participants had a mean total albumin of 4.38 g/dL (SD = 0.37). Unadjusted comparisons demonstrated that total albumin among pregnant participants at V1 and V2 were significantly lower than nonpregnant controls (V1 β = −0.60 g/dL [−0.73, −0.47] p < 0.001, V2 β = −1.11 g/dL [−1.23, −0.98] p < 0.001) and remained significantly lower when adjusting for BMI alone and fully adjusting for baseline differences. PP women (V3) had total albumin levels similar to nonpregnant controls (β = −0.03 g/dL [−0.17, 0.10] p = 0.63). Adjustments for BMI and full adjustments did not reveal significant associations between PP women and total albumin levels.
The mean %GAlb among nonpregnant participants was 12.5% (SD = 1.4). Unadjusted comparisons demonstrated that %GAlb was similar at V1 in pregnant women to nonpregnant controls (β = 0.06% [−0.44, 0.56] p = 0.81); however, % GAlb at V2 and V3 was lower than in nonpregnant controls (V2 β = −0.56% [−1.04, −0.08] p = 0.02, V3 β = −0.61% [−1.07, −0.16] p = 0.01). Adjustments for BMI did not alter the significant difference between %GAlb in PP women at V3 and nonpregnant controls (β = −0.45% [0.9, 0.01] p = 0.047), although the difference between pregnant women at V2 and nonpregnant controls was attenuated after BMI adjustment (β = −0.22% [−0.68, 0.25] p = 0.36). In the fully adjusted model including adjustments for BMI, age, marital status, parity, race/ethnicity, and family history of DM, there were no significant differences between the nonpregnant controls and pregnant/PP participants noted at any time point.
We found no significant correlations between %GAlb and glycemia at the fasting, 1-hour postload, or 2-hour postload, nor did we identify associations between %GAlb and mean OGTT or A1c (Table 2). Adjustments for BMI revealed no additional associations with glycemia at any OGTT time point or with A1c (Table 3). Further adjustments for weeks PP at V3 also did not reveal significant associations.
Table 2.
Associations between glycemia and % glycated albumin
| % Glycated Alb nonpregnant (n = 50) |
% Glycated Alb pregnant V1 (n = 50) |
% Glycated Alb pregnant V2 (n = 49) |
% Glycated Alb pregnant V3 (n = 50) |
|
|---|---|---|---|---|
| Fasting | Rho: 0.002 p-Value: 0.99 |
Rho: −0.06 p-Value: 0.68 |
Rho: −0.14 p-Value: 0.35 |
Rho: 0.11 p-Value: 0.46 |
| 1-h post-load | Rho: 0.07 p-Value: 0.62 |
Rho: −0.15 P-value: 0.28 |
Rho: 0.06 P-value: 0.67 |
Rho: 0.16 p-Value: 0.26 |
| 2-h post-load | Rho: 0.10 p-Value: 0.47 |
Rho: −0.13 p-Value: 0.35 |
Rho: 0.06 P-value: 0.70 |
Rho: −0.006 p-Value: 0.97 |
| Mean OGTT | Rho: 0.08 p-Value: 0.58 |
Rho: −0.12 p-Value: 0.39 |
Rho: 0.04 p-Value: 0.80 |
Rho: 0.12 p-Value: 0.42 |
| A1c | Rho: −0.05 p-Value: 0.74 |
Rho: −0.12 p-Value: 0.40 |
Rho: 0.20 p-Value: 0.16 |
Rho: 0.11 p-Value: 0.45 |
Abbreviations: Alb, albumin; OGTT, oral glucose tolerance test.
Table 3.
Body mass index adjusted associations of glycemia with % glycated albumin
| Beta (95% CI) for %GAlb predictor | p-Value | |
|---|---|---|
| V1 glycemic outcomes | ||
| Fasting glucose | −0.25 (−2.16, 1.66) | 0.79 |
| 1-h glucose | −1.19 (−10.0, 7.65) | 0.79 |
| 2-h glucose | −0.25 (−8.11, 7.61) | 0.95 |
| Mean OGTT | −0.56 (−5.99, 4.87) | 0.84 |
| A1c | −0.02 (−0.07, 0.04) | 0.59 |
| V2 glycemic outcomes | ||
| Fasting glucose | −0.78 (−2.70, 1.13) | 0.41 |
| 1-h glucose | 6.29 (−3.84, 16.42) | 0.22 |
| 2-h glucose | 3.79 (−6.38, 13.98) | 0.46 |
| Mean OGTT | 3.10 (−3.37, 9.58) | 0.34 |
| A1c | 0.06 (−0.02, 0.14) | 0.14 |
| V3 glycemic outcomes | ||
| Fasting glucose | 1.45 (−1.19, 4.09) | 0.27 |
| 1-h glucose | 7.43 (−4.51, 19.4) | 0.22 |
| 2-h glucose | 5.54 (−4.35, 15.4) | 0.27 |
| Mean OGTT | 4.81 (−2.24, 11.9) | 0.18 |
| A1c | 0.01 (−0.07, 0.10) | 0.72 |
Abbreviations: CI, confidence interval; GAlb, glycated albumin; OGTT, oral glucose tolerance test
Stratified analyses were performed to determine whether longitudinal changes in %GAlb differed among women with pregnancies with and without GDM. Student’s t-test did not reveal significant differences in %GAlb between those with and without GDM at any of the study time points (V1 12.7 vs. 12.5% [p = 0.58], V2 12.3 vs. 11.8% [p = 0.18], V3 12.2 vs. 11.8% [p = 0.19]).
Unadjusted longitudinal analyses among the 10 participants with GDM showed that %GAlb was decreased at V2 compared with V1 and remained decreased PP (V2 β = −0.45% [−0.87, −0.04] p = 0.03, V3 β = −0.54% [−1.03, −0.05] p = 0.030), similar to the overall cohort. Unadjusted longitudinal analyses among the 40 participants without GDM showed similar results (V2 β = −0.67% [−0.79, −0.54] p < 0.001, V3 β = −0.71% [−0.96, −0.45] p < 0.001). When comparing pregnant and nonpregnant participants, the fully adjusted model comparing 10 women with GDM to nonpregnant controls revealed a higher %GAlb at V1 in pregnant women with GDM (β = 1.56% [0.32, 2.80] p = 0.02); however, there were no significant differences between %GAlb of pregnant participants with GDM and nonpregnant controls at V2 or V3 (V2 β = 0.9% [−0.36, 2.15] p = 0.16, V3 β = 1.02% [−0.15, 2.19] p = 0.09).
Conclusion
In this pilot study, we found that %GAlb is not reflective of glycemia throughout gestation or PP. Although %GAlb is not dependent on stable red blood cell kinetics like hemoglobin A1c and avoids the difficulties of an OGTT, our findings do not support its use over the OGTT in pregnancy or the PP period.
The study demonstrated that though total serum albumin progressively decreases throughout gestation and returns to a nonpregnant state PP, %GAlb does not behave in this manner. Albumin turnover time is 25 days assuming normal gastrointestinal and renal function, and its synthesis is based on colloid oncotic pressure.33 Serum albumin is thought to decline in pregnancy not because of an increase in catabolism, but rather as a result of hemodilution from plasma volume expansion.34,35 In the PP state, plasma volume returns to its prepregnancy state, leading us to hypothesize that %GAlb would be similar in the PP and nonpregnant state. Unexpectedly, we found that despite the similarities between total serum albumin in the nonpregnant and PP states, %GAlb remained lower PP compared with the first trimester and to nonpregnant controls. Our findings suggest that the physiology of albumin glycation is unique in the PP period and does not immediately return to its nonpregnant behavior. This may explain the lack of usefulness of this biomarker in the PP period, especially when the average weeks PP at the V3 time point was 11 weeks.
Others have examined the behavior of this biomarker during gestation to examine its role in gestational diabetes diagnosis, monitoring, and predicting neonatal outcomes20,26,36; however, the PP period was not included in their analyses. While some investigators have proposed that % GAlb is useful in correlation with self-monitored postprandial glycemia among pregnancies affected by GDM,23,37 these studies were restricted to those with GDM compared with controls and did not control for the effect of BMI on %GAlb. Additionally, the study by Li et al23 assessed over 3,000 pregnancies and thus their observed effects while statistically significant may not be clinically significant and applicable at the individual patient level. Our study is in line with others who have demonstrated the lack of correlation between %GAlb and OGTT, the lack of defined parameters for measurement during pregnancy, and the inability to utilize the biomarker for GDM diagnosis.20,26,38 Our analysis not only adds to the body of literature suggesting that %GAlb is not predictive of glycemia throughout gestation, but also adds novel data regarding its lack of utility in the PP period.
Our study reaffirmed the findings of other investigators that BMI has an inverse relationship with %GAlb throughout gestation.20,23 BMI did not decline PP to its early pregnancy state, reflecting weight retention in the PP period. The lack of PP weight loss may be partially responsible for the lack of return of %GAlb to nonpregnant levels, as higher BMI may have been correlated with lower %GAlb levels, but BMI did not completely explain the findings in adjusted models.
The study demonstrated that %GAlb did not correlate with glycemia during early or midpregnancy assessments or PP. However, it was notable that %GAlb was higher among women with GDM than nonpregnant controls in early pregnancy. Therefore, it is possible that there is an association between glycemia and %GAlb in early pregnancy that our study was too small to detect; the physiology of pregnancy and the PP period may have changes that disrupt this relationship as gestation progresses. The physiology of PP women with respect to glycemia and biomarkers denoting glycemic status and control deserve further research attention.
Strengths
Strengths of our study include its longitudinal design, the utilization of a cohort at high risk for the development of GDM, and the inclusion of A1c measurements in the analysis. We also note several limitations. First, OGTT glucose levels capture glycemia at a moment in time in response to a glucose load and do not necessarily correlate with glycemia during daily life. Second, our sample size was limited; however, if an association between %GAlb and glycemia was not identified with 50 participants, it is unlikely to be meaningful for an individual patient. Finally, our study population was at high risk for dysglycemia and may not be generalizable across the entire population. However, the given the high-risk population in this cohort, our findings may be most relevant to a population of women more likely to undergo early pregnancy screening, to be diagnosed with GDM, and therefore to require PP glycemic assessment.
Conclusion
In conclusion, we have demonstrated that %GAlb fails to correlate with glycemia throughout gestation or in the PP period. Further research should be directed to identify other biomarkers that could yield reliable results with respect to pregnant and PP glycemic status without the burden of an OGTT. Additionally, future efforts should aim to better understand PP physiology so that PP glycemia can be accurately assessed in a patient-friendly manner.
Key Points.
Changes in %GAlb extending to the postpartum period have not been described.
%GAlb decreases in pregnancy and remains decreased postpartum, despite a postpartum increase in total albumin above early pregnancy values.
Glycemia measurements nor A1c were associated with %GAlb at any time point, therefore, %GAlb is unlikely to be useful in assessing glycemia in pregnant or postpartum women.
Funding
Research reported in this manuscript was supported by the National Institutes of Health under award number NIDDK K23DK113218 and the Robert Wood Johnson Foundation’s Harold Amos Medical Faculty Development Program. Data collection was also supported by the Clinical Research Center at Massachusetts General Hospital under grant number 1UL1TR002541-01. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the National Center for Advancing Translational Science, or the National Institutes of Health. Additional funding support was provided by the Department of Obstetrics, Gynecology, and Reproductive Biology at Massachusetts General Hospital.
Footnotes
This work was presented at the American Diabetes Association (ADA) Annual Meeting, held virtually June 2021.
Conflict of Interest
None declared.
References
- 1.Waters TP, Kim SY, Werner E, et al. Should women with gestational diabetes be screened at delivery hospitalization for type 2 diabetes? Am J Obstet Gynecol 2020;222(01):73.e1–73.e11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correa A, Bardenheier B, Elixhauser A, Geiss LS, Gregg E. Trends in prevalence of diabetes among delivery hospitalizations, United States, 1993–2009. Matern Child Health J 2015;19(03):635–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deputy NP, Kim SY, Conrey EJ, Bullard KM. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth - United States, 2012–2016. MMWR Morb Mortal Wkly Rep 2018;67(43):1201–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ACOG Practice Bulletin No. ACOG practice bulletin no. 190: gestational diabetes mellitus. Obstet Gynecol 2018;131(02): e49–e64 [DOI] [PubMed] [Google Scholar]
- 5.England LJ, Dietz PM, Njoroge T, et al. Preventing type 2 diabetes: public health implications for women with a history of gestational diabetes mellitus. Am J Obstet Gynecol 2009;200(04):365.e1–365.e8 [DOI] [PubMed] [Google Scholar]
- 6.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 2002;25(10):1862–1868 [DOI] [PubMed] [Google Scholar]
- 7.Metzger BE, Buchanan TA, Coustan DR, et al. Summary and recommendations of the Fifth International Workshop-Conference on Gestational Diabetes Mellitus. Diabetes Care 2007;30 (Suppl 2):S251–S260 [DOI] [PubMed] [Google Scholar]
- 8.Nielsen KK, Kapur A, Damm P, de Courten M, Bygbjerg IC. From screening to postpartum follow-up - the determinants and barriers for gestational diabetes mellitus (GDM) services, a systematic review. BMC Pregnancy Childbirth 2014;14:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hunt KJ, Logan SL, Conway DL, Korte JE. Postpartum screening following GDM: how well are we doing? Curr Diab Rep 2010;10 (03):235–241 [DOI] [PubMed] [Google Scholar]
- 10.Hale NL, Probst JC, Liu J, Martin AB, Bennett KJ, Glover S. Postpartum screening for diabetes among Medicaid-eligible South Carolina women with gestational diabetes. Womens Health Issues 2012;22(02):e163–e169 [DOI] [PubMed] [Google Scholar]
- 11.Oza-Frank R Postpartum diabetes testing among women with recent gestational diabetes mellitus: PRAMS 2009–2010. Matern Child Health J 2014;18(03):729–736 [DOI] [PubMed] [Google Scholar]
- 12.Van Ryswyk E, Middleton P, Hague W, Crowther C. Clinician views and knowledge regarding healthcare provision in the postpartum period for women with recent gestational diabetes: a systematic review of qualitative/survey studies. Diabetes Res Clin Pract 2014; 106(03):401–411 [DOI] [PubMed] [Google Scholar]
- 13.Vesco KK, Dietz PM, Bulkley J, et al. A system-based intervention to improve postpartum diabetes screening among women with gestational diabetes. Am J Obstet Gynecol 2012;207(04):283.e1–283.e6 [DOI] [PubMed] [Google Scholar]
- 14.Edelson PK, James KE, Leong A, et al. Longitudinal changes in the relationship between hemoglobin a1c and glucose tolerance across pregnancy and postpartum. J Clin Endocrinol Metab 2020;105(05):e1999–e2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claesson R, Ekelund M, Ignell C, Berntorp K. Role of HbA1c in postpartum screening of women with gestational diabetes mellitus. J Clin Transl Endocrinol 2014;2(01):21–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Picón MJ, Murri M, Muñoz A, Fernández-García JC, Gomez-Huelgas R, Tinahones FJ. Hemoglobin A1c versus oral glucose tolerance test in postpartum diabetes screening. Diabetes Care 2012;35 (08):1648–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katreddy MV, Pappachan JM, Taylor SE, Nevill AM, Indusekhar R, Nayak AU. Hemoglobin A1c in early postpartum screening of women with gestational diabetes. World J Diabetes 2013;4(03): 76–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu X, Wu N, Al-Mureish A. A review on research progress in the application of glycosylated hemoglobin and glycated albumin in the screening and monitoring of gestational diabetes. Int J Gen Med 2021;14:1155–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zendjabil M Glycated albumin. Clin Chim Acta 2020;502: 240–244 [DOI] [PubMed] [Google Scholar]
- 20.Dong Y, Zhai Y, Wang J, et al. Glycated albumin in pregnancy: reference intervals establishment and its predictive value in adverse pregnancy outcomes. BMC Pregnancy Childbirth 2020; 20(01):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kohzuma T, Koga M. Lucica GA-L glycated albumin assay kit: a new diagnostic test for diabetes mellitus. Mol Diagn Ther 2010;14 (01):49–51 [DOI] [PubMed] [Google Scholar]
- 22.Freitas PAC, Ehlert LR, Camargo JL, Freitas PAC, Ehlert LR, Camargo JL. Glycated albumin: a potential biomarker in diabetes. Arch Endocrinol Metab 2017;61(03):296–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li HP, Wang FH, Tao MF, Huang YJ, Jia WP. Association between glycemic control and birthweight with glycated albumin in Chinese women with gestational diabetes mellitus. J Diabetes Investig 2016;7(01):48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hashimoto K, Osugi T, Noguchi S, et al. A1C but not serum glycated albumin is elevated because of iron deficiency in late pregnancy in diabetic women. Diabetes Care 2010;33(03):509–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koga M, Kasayama S. Clinical impact of glycated albumin as another glycemic control marker. Endocr J 2010;57(09):751–762 [DOI] [PubMed] [Google Scholar]
- 26.Saglam B, Uysal S, Sozdinler S, Dogan OE, Onvural B. Diagnostic value of glycemic markers HbA1c, 1,5-anhydroglucitol and glycated albumin in evaluating gestational diabetes mellitus. Ther Adv Endocrinol Metab 2017;8(12):161–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kurishita M, Nakashima K, Kozu H. Glycated hemoglobin of fractionated erythrocytes, glycated albumin, and plasma fructosamine during pregnancy. Am J Obstet Gynecol 1992;167(05):1372–1378 [DOI] [PubMed] [Google Scholar]
- 28.Nathan DM, McGee P, Steffes MW, Lachin JMDCCT/EDIC Research Group. Relationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes 2014;63(01):282–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu J, Chen Y, Li C, Tao M, Teng Y. The diagnostic value of glycated albumin in gestational diabetes mellitus. J Endocrinol Invest 2018;41(01):121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Diabetes Association Professional Practice Committee. 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022;45(Suppl 1):S17–S38 [DOI] [PubMed] [Google Scholar]
- 31.Diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33(03):676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sato A, Yada S, Hosoba E, Kanno H, Miura H. Establishment of glycated albumin unit conversion equation from the standardized value (mmol/mol) to the routinely used value (%). Ann Clin Biochem 2019;56(02):204–209 [DOI] [PubMed] [Google Scholar]
- 33.Levitt DG, Levitt MD. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gen Med 2016;9:229–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bacq Y, Zarka O, Bréchot JF, et al. Liver function tests in normal pregnancy: a prospective study of 103 pregnant women and 103 matched controls. Hepatology 1996;23(05):1030–1034 [DOI] [PubMed] [Google Scholar]
- 35.Honger PE. Albumin metabolism in normal pregnancy. Scand J Clin Lab Invest 1968;21(01):3–9 [DOI] [PubMed] [Google Scholar]
- 36.Sugawara D, Sato H, Ichihashi K, Nagai K, Kawano A. Glycated albumin level during late pregnancy as a predictive factor for neonatal outcomes of women with diabetes. J Matern Fetal Neonatal Med 2018;31(15):2007–2012 [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto K, Koga M. Indicators of glycemic control in patients with gestational diabetes mellitus and pregnant women with diabetes mellitus. World J Diabetes 2015;6(08):1045–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mendes N, Tavares Ribeiro R, Serrano F. Beyond self-monitored plasma glucose and HbA1c: the role of non-traditional glycaemic markers in gestational diabetes mellitus. J Obstet Gynaecol 2018; 38(06):762–769 [DOI] [PubMed] [Google Scholar]


