Abstract
Human cytomegalovirus (HCMV) evades healthy immune responses during infection, and this evasion may allow HCMV to establish latency in the host. The human vasculature has been recognized as a site of HCMV infection and may also be a site of latent HCMV infection. As the interface between circulating cells and underlying parenchymal cells, the vascular endothelium provides signals for local reaction of inflammatory cells. We propose that HCMV down-regulates expression of the proinflammatory chemokine RANTES from the infected endothelium, which may result in reduced recruitment of mononuclear cells to the site of infection. Abortive HCMV infection of primary endothelial cells with the clinical isolate HCMV 4010, under conditions in which viral gene expression could not occur, induced high levels of RANTES expression. Replicative HCMV infection, however, induced cells in parallel cultures to express significantly lower levels of RANTES. Expression of the chemokines interleukin 8 and MCP-1 by endothelial cells was found to be unaffected by replicative HCMV infection and thus may not play an important role during early HCMV infection of the endothelium. HCMV may regulate RANTES expression from endothelial cells as a mechanism to evade the local immune response to infection.
The competent immune system typically limits human cytomegalovirus (HCMV) infection from developing into symptomatic disease but does not eliminate HCMV from the host. HCMV quietly persists until the host becomes immunocompromised, by such conditions as immunosuppressive therapy or human immunodeficiency virus (HIV) infection, and then HCMV may subsequently develop into symptomatic disease (reviewed in reference 2). In order to maintain its anonymity during infection, HCMV has developed strategies to evade immune responses (reviewed in reference 13). The human vasculature has been recognized as a site of HCMV infection and may also be a site of virus latency (16, 27, 29). As the interface between circulating leukocytes and underlying parenchymal cells, the vascular endothelium provides signals for local response of inflammatory cells. During cell injury, such as viral infection, chemokines are among the first line of effector signals that attract circulating leukocytes to the site of injury (17). HCMV-associated diseases, such as atherosclerotic lesions, pneumonitis, and retinitis, are characterized by inflammatory responses that might be orchestrated by chemokines expressed at the site of infection.
Early stages of virus infection that include particle binding and internalization activate contrasting responses in the host cell. On one hand, virus infection activates a series of cellular responses that establish an environment for virus replication (reviewed in reference 11), whereas on the other hand, virus infection triggers the cell to broadcast foreign invasion and injury to circulating immune cells (17). HCMV particles that have been inactivated by UV irradiation are only capable of binding and internalization into the host cells, because viral gene expression and subsequent steps of virus replication do not occur (11). Thus, UV-irradiated HCMV serves as a model of infection to study the cellular responses that occur at early stages of infection.
Chemokines are small chemoattractant cytokines expressed and secreted as an inflammatory response and function to attract specific immune cells during foreign invasion (i.e., virus infection or tissue wounding). There are four subfamilies of chemokines that are characterized by the position of the first cysteine residues (C, CC, CXC, and CX3C). The largest families (CC and CXC chemokines) may also be distinguished by the cells they attract: CC chemokines mobilize mononuclear cells (monocytes, lymphocytes, etc.), and CXC chemokines typically attract neutrophils. In certain inflammatory reactions, the proinflammatory cytokines tumor necrosis factor alpha (TNF-α) and gamma interferon (IFN-γ) stimulate endothelial cells to express the CC chemokine RANTES, which leads to selective recruitment of circulating cells (18). Similarly, the early stages of virus infection might activate endothelial cells to express and secrete chemokines as an initial inflammatory response to infection.
HCMV infection activates fibroblasts to express and secrete CC and CXC chemokines (10, 15, 19). Michelson and colleagues demonstrated that activation of chemokine expression occurred before viral gene expression (19), thereby suggesting that the response might be a cellular response to announce foreign invasion and to stimulate an inflammatory response. Hirsch and Shenk further demonstrated that a soluble factor in the medium of infected cells activated expression of the chemokine MCP-1, but expression was inhibited during virus replication (15), and first suggested the notion that transcription of chemokines could be down-regulated during HCMV infection.
HCMV has developed several mechanisms to evade the immune response during infection. Although HCMV infection activates chemokine expression in fibroblasts, it is unclear whether this pattern occurs in endothelial cells. Our infection model of a clinical isolate of HCMV adapted to endothelial cells may provide insights into differences between abortive and replicative infection of endothelial cells. In this paper, we have tested the hypothesis that HCMV replication regulates the immediate response of endothelial cells to express the proinflammatory chemokine RANTES.
MATERIALS AND METHODS
Reagents.
Recombinant RANTES, interleukin 8 (IL-8), and Quantikine kits for immunoassay of chemokine protein were obtained from R&D Systems (Minneapolis, Minn.). Actinomycin D was obtained from Sigma Chemical Corp. (St. Louis, Mo.).
Cells.
Human umbilical vein endothelial cells (HUVECs) were harvested from umbilical veins by procedures described elsewhere (12), which were further modified for HCMV infection (5).
Virus.
Human HCMV strain 4010 has been described previously (5). Cell-free virus was prepared from supernatants of 4010-infected HUVEC cultures that were spun by low-speed centrifugation to remove cells and debris, followed by ultracentrifugation at 72,000 × g at 4°C in an SW28 rotor (Beckman Instruments L5–50 ultracentrifuge). The pellet containing concentrated virus particles was resuspended in medium and stored at −80°C.
UV-irradiated HCMV (UV-HCMV) was prepared by irradiating cell-free virus stock in a 1-ml dilution of medium at an intensity of 7,000 μW/cm2 (Foto/UV 300 Transilluminator) for 15 min at 4°C.
Immunofluorescence of IE1.
HUVECs were seeded onto coverslips and infected with either wild-type HCMV (WT-HCMV) or UV-HCMV at 1 PFU/cell. Following 72 h of infection, the cells were rinsed, fixed in paraformaldehyde, permeabilized with 0.1% Triton–phosphate-buffered saline (PBS), and incubated with monoclonal antibody to the immediate-early protein of HCMV (IE72) (Autogen Bioclear). For visualization, the slides were incubated with rabbit anti-mouse immunoglobulin congugated to tetramethyl rhodamine isocyanate (TRITC) (Dako Corporation, Carpenteria, Calif.) and inspected by fluorescent confocal microscopy.
Filtration of WT-HCMV and UV-HCMV through 100-kDa membrane.
Inocula of WT-HCMV and UV-HCMV were divided equally, and one portion of each was filtered through a sterile Ultrafree 100-kDa membrane (Millipore Corp., Bedford, Mass.) according to the manufacturer's instructions. Briefly, inoculum was spun through the Ultrafree filter at 12,000 rpm for 10 min. The filtrate was collected and added to subconfluent monolayers in parallel with unfiltered inoculum.
Quantification of chemokine mRNA.
Quantification of relative amounts of mRNA was performed by RNase protection assay (RPA). RNA was isolated from HCMV- and UV-HCMV-infected, as well as uninfected, HUVECs by using Trizol reagent obtained from Gibco-BRL Life Sciences (Gaithersburg, Md.) as recommended by the manufacturer, further purified by precipitation with LiCl, and quantified by spectrophotometry. The RPA was carried out with a RiboQuant multiprobe kit obtained from Pharmingen (San Diego, Calif.). Briefly, a multiprobe cDNA template set (hCK-5) including RANTES was transcribed with T7 polymerase and [32P]UTP (Amersham, Arlington Heights, Ill.) to generate a 32P-labeled antisense RNA probe. The probe was hybridized with 5 μg of RNA for 16 h at 56°C; excess free probe was then digested with RNase for 45 min at 30°C, followed by proteinase K digestion for 15 min at 37°C and ethanol precipitation. The RNase-protected duplexes were resolved on denaturing polyacrylamide gels and analyzed by phosphor screen autoradiography, with quantification of activity by ImageQuant (Storm Optical Scanner; Molecular Dynamics, Sunnyvale, Calif.).
RESULTS
Abortive HCMV infection induces high-level expression of RANTES.
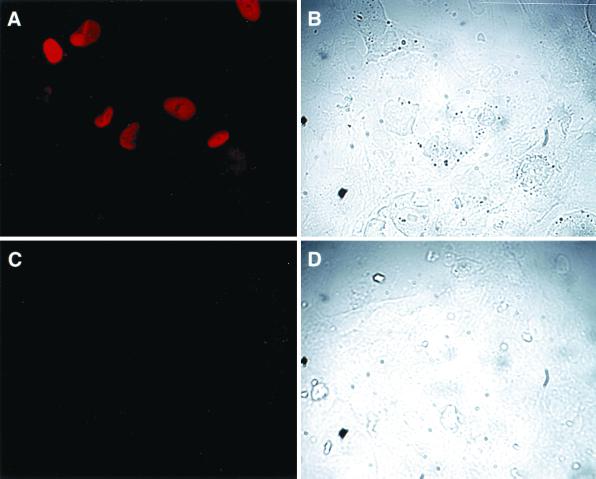
In order to determine whether endothelial cells express chemokines during early stages of HCMV infection, primary endothelial cells (HUVECs) were exposed to partially purified cell-free HCMV under conditions in which replicative infection could not occur. Viral gene expression was disabled by UV irradiation of the HCMV inoculum prior to infection, and the absence of replicative HCMV infection was confirmed by analysis of immunostaining for IE72 at 96 h postinfection (Fig. 1). Endothelial cells infected with WT-HCMV demonstrated staining for IE72 (Fig. 1A), whereas cells infected with PFU equivalents (units of UV-HCMV infection designated as PFU equivalents because UV-HCMV does not form plaques) of UV-HCMV did not demonstrate any IE72 staining (Fig. 1C). For each image, a bright-field image was captured that displays the field of cells that were stained with antibody to IE72 (Fig. 1B and D). In addition, inactivation of virus infectivity of UV-HCMV was confirmed by the absence of viral cytopathology at 96 h postinfection.
FIG. 1.
Expression of HCMV IE protein in WT-HCMV-infected endothelial cells but not UV-irradiated HCMV-infected endothelial cells. Subconfluent monolayers of HUVECs were infected with either WT-HCMV (A and B) or UV-HCMV (C and D) for 96 h and then fixed and stained for HCMV IE72 protein (A and C). The bright-field images of the stained WT-HCMV-infected cells (B) and the UV-HCMV-infected cells (D) are presented to confirm cell presence.
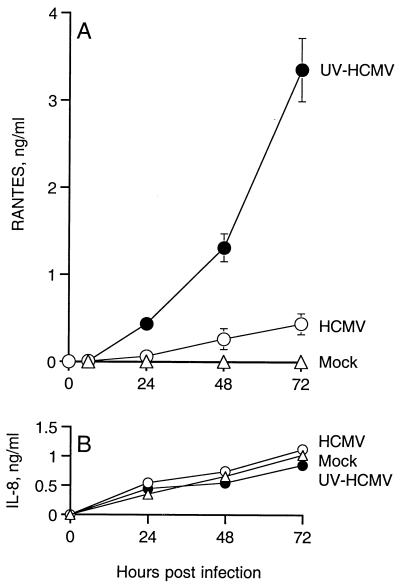
During abortive infection, UV-HCMV activated endothelial cells to express high levels of RANTES (4 ng/ml) in the absence of viral gene expression (Fig. 2A). In contrast, replicative HCMV infection (WT-HCMV), at the same multiplicity of infection, induced nearly 20-fold less expression of RANTES. As seen in Fig. 2A, the rate of RANTES expression over the time period of 0 to 72 h was significantly greater from cells infected with UV-HCMV than from those infected with WT-HCMV. In a separate assay, collection of culture supernatants at 24-h intervals indicated that RANTES expression peaks at 24 to 48 h from UV-HCMV-infected and WT-HCMV-infected endothelial cells (data not shown). UV-irradiated culture medium did not stimulate HUVECs to express RANTES (data not shown), thereby indicating that RANTES expression is specific to UV irradiation of the virus particles rather than irradiation of the medium of the inoculum. Heat inactivation of HCMV particle infectivity (56°C for 30 min) abrogated the ability of HCMV particles to stimulate endothelial cells to express RANTES (data not shown). Unlike the denaturing effect of heat, UV irradiation of HCMV does not affect the efficiency of binding or internalization of virus particles (14), although the UV-irradiated virus particle might have altered properties that induce cell signaling. In any case, the early events of virus entry into the cell likely play an important role in the induction of cellular expression of RANTES during HCMV infection.
FIG. 2.
Expression of chemokines during infection of HUVECs with HCMV. HUVECs were infected with 0.01 PFU of WT-HCMV per cell (○) or 0.01 PFU equivalents of HCMV irradiated by UV light per cell (●). Mock-infected HUVECs (▵) were also assayed for chemokine expression. Expression of RANTES represents cumulative amounts of secreted chemokine over 72 h. Supernatants were collected and assayed for RANTES (A) and IL-8 (B) protein. Error bars represent the standard error of the mean of three experiments.
Endothelial cells constitutively express IL-8, and this expression was not significantly affected by infection with either UV-irradiated or WT-HCMV (Fig. 2B). Quiescent or uninfected HUVECs in culture did not express RANTES but expressed constitutively high levels of IL-8 (Fig. 2). Taken together, these data indicate that RANTES but not IL-8 expression in HUVECs is regulated by HCMV infection.
HCMV particles are necessary to induce RANTES expression from endothelial cells.
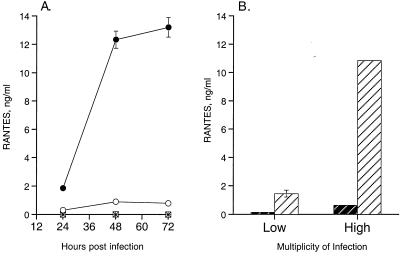
Since viral infection may produce cytokines, such as TNF-α and IFN-γ, that are capable of stimulating RANTES expression from HUVECs (18), and since a soluble factor in infected-cell extracts has been shown to induce HCMV-infected fibroblasts to express the chemokine MCP-1 (15), it was possible that cytokines in our inoculation medium might be responsible for RANTES expression from UV-HCMV-infected endothelial cells. We tested this hypothesis by infection of HUVECs with virus-particle-free filtrates of either WT-HCMV or UV-HCMV (Fig. 3A). Removal of virus particles from the inoculum by a 100-kDa-cutoff membrane was confirmed by the absence of HCMV cytopathology in the treated cell cultures. HUVECs incubated with filtrate from either the WT-HCMV or UV-HCMV inoculum did not express RANTES (Fig. 3A). Furthermore, neither TNF-α nor IFN-γ was expressed by HUVECs during infection with either WT-HCMV or UV-HCMV (data not shown). Thus, these results suggest that during either abortive or replicative HCMV infection, expression of RANTES from HUVECS is dependent on the interaction of HCMV particles with the host cell. Moreover, RANTES expression during HCMV infection is not due to exposure to residual cytokines or low-molecular-weight stimulatory factors that may be present in the partially purified virus stock.
FIG. 3.
RANTES expression is dependent on particle interaction with the cells. (A) HCMV particle-free inoculum does not induce RANTES expression. Subconfluent monolayers of HUVECs were infected with HCMV inoculum (○), UV-HCMV inoculum (●), and filtrate from HCMV inoculum (□) and filtrate from UV-HCMV inoculum (∗), in which inoculum was passed through a 100-kDa-cutoff membrane to remove virus particles. Supernatants of the cultures were collected at designated time points and assayed for RANTES protein. This assay represents three identical experiments in which the fold differences in RANTES expression between UV-HCMV and WT-HCMV did not differ by more than 10%. (B) RANTES expression is related to PFU equivalents of UV-HCMV. Expression of RANTES was assayed following 72 h of infection at a high multiplicity of infection (1 PFU/cell) and low multiplicity of infection (0.02 PFU/cell). Open hatched bars represent WT-HCMV, and solid hatched bars represent UV-HCMV. Error bars represent the standard error of duplicates within the experiment, and this experiment is representative of three replicative assays.
Could uninfected bystander cells be contributing to RANTES expression?
The level of infection used in our model was approximately 1 cell infected per 20 uninfected cells. We chose this level of infection in order to model the relatively low level of HCMV infection that is typically demonstrated in immunohistochemically stained tissue samples of in vivo HCMV infection (3, 28, 30). If uninfected bystander cells were responsible for high-level RANTES expression during low-level infection, then a similar assay at a high level of infection, in which every cell demonstrated HCMV cytopathology, would demonstrate lower levels of RANTES due to the absence of uninfected bystander cells. Interestingly, we found that high PFU equivalents of UV-HCMV stimulated proportionately high levels of RANTES (Fig. 3B). At 48 h postinfection, HUVECs infected with a low multiplicity of infection (PFU equivalents) of UV-HCMV or WT-HCMV expressed approximately a 7.5-fold-lower level of RANTES than that expressed at a high multiplicity of infection of UV-HCMV or WT-HCMV (Fig. 3B). Moreover, at both low and high multiplicities of infection, the PFU equivalents of UV-HCMV stimulated an approximately 12-fold-greater level of RANTES than WT-HCMV infection of HUVECs. Thus, these data suggest that it is the cells infected with HCMV particles that express RANTES, rather than the neighboring uninfected cells.
Replicative HCMV infection controls abundance of RANTES mRNA in endothelial cells.
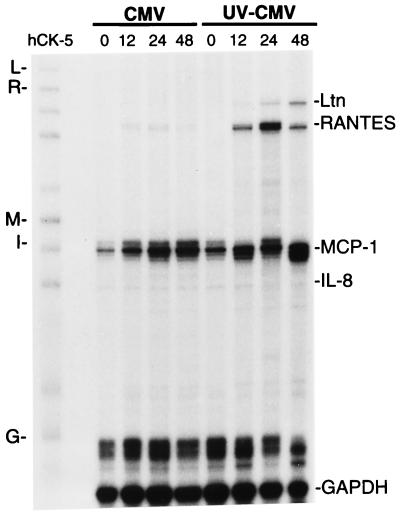
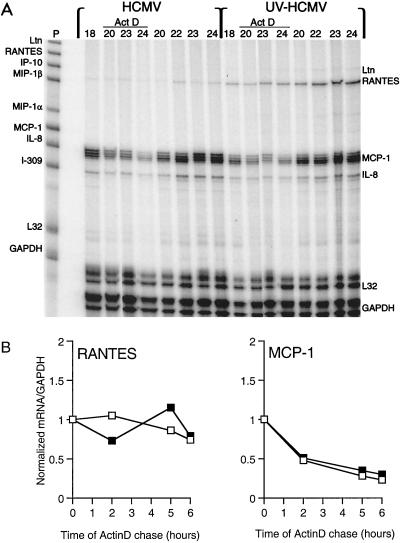
In order to determine whether HCMV replication might regulate RANTES mRNA, we assayed the relative levels of steady-state mRNA of RANTES and other chemokines in WT-HCMV- and UV-HCMV-infected HUVECs by RPA (Fig. 4). Total RNA was isolated from HUVEC cultures infected with WT-HCMV and UV-HCMV during 3 days of culture, and specific chemokine RNA was quantified. During infection with UV-HCMV, RANTES mRNA was detected at 12 h, peaked at 24 h, and decreased at 48 h postinfection. In contrast, infection with WT-HCMV induced significantly lower levels of RANTES mRNA at 12 and 24 h. Expression of RANTES mRNA in both WT-HCMV and UV-HCMV cultures was not detected at 6 h or earlier (data not shown). Message for the C chemokine lymphotactin was also expressed at 12 and 24 h postinfection in the UV-HCMV-infected HUVECs but was undetectable in the WT-HCMV-infected HUVEC cultures (Fig. 4). There was no difference in expression of MCP-1 and IL-8 in the WT-HCMV and UV-HCMV cultures (Fig. 4), as well as mock-infected HUVECs (data not shown). Message of CC chemokines MIP-1α and MIP-1β was not detected in HUVECs. These results demonstrate that the levels of steady-state RANTES mRNA are consistent with the respective protein levels in abortive and replicative HCMV infection of HUVECs. Moreover, these results suggest that replicative HCMV infection may partially inhibit expression of RANTES and lymphotactin mRNA.
FIG. 4.
Time course of chemokine mRNA expression during infection of HUVECs with WT-HCMV and UV-HCMV. Total RNA was isolated from infected HUVEC cultures at designated time points (0, 12, 24, and 48 h) postinfection and assayed for specific mRNA expression by RPA with a 32P-labeled multi-RNA probe of human CC chemokines. Unhybridized probe hCK-5 (far left lane) with mRNA of lymphotactin (Ltn [L in the left margin]), RANTES (R), MCP-1 (M), IL-8 (I), and GAPDH (G) is identified. The method is described in detail in Materials and Methods.
RANTES mRNA is not rapidly degraded during infection of endothelial cells with WT-HCMV.
The stability of mRNA in eukaryotic cells can vary according to cell type or extracellular stress (25). In order to determine whether the lower steady-state levels of RANTES mRNA from the WT-HCMV-infected HUVECs represented differential RNA stability during replicative HCMV infection, we analyzed the stability of the transcripts in the presence of RNA synthesis inhibitor actinomycin D. Cultures of HUVECs were infected with either WT-HCMV or UV-HCMV for 18 h to initiate RANTES mRNA synthesis, and then the medium was supplemented with actinomycin D. At specific times during the actinomycin D chase, the cells were harvested for total RNA and assayed by RPA for levels of chemokine mRNA (Fig. 5A). Although UV-HCMV-infected cultures express significantly more steady-state RANTES mRNA than WT-HCMV-infected cultures, the rate of RANTES message degradation during the actinomycin D chase did not differ significantly between the two cultures (Fig. 5B). The levels of MCP-1 and IL-8 mRNA did not significantly differ between the two infection cultures, thereby underscoring the specific inhibitory effect of HCMV replicative infection on RANTES expression. These results indicate that the newly transcribed RANTES mRNA is relatively stable in endothelial cells infected with WT-HCMV or UV-HCMV, and hence, alterations in steady-state RANTES mRNA during replicative infection may represent effects on transcription.
FIG. 5.
Stability of mRNA following transcription in HCMV- and UV-HCMV-infected HUVECs. (A) HCMV- and UV-HCMV-infected HUVECs were incubated with actinomycin D (Act D)-supplemented medium following 18 h of infection. At specific time points prior to (18 h) and during the actinomycin D chase (20, 22, 23, and 24 h), the cells were harvested and assayed for steady-state mRNA by RPA (Materials and Methods). Specific chemokine mRNA was detected with a 32P-labeled multi-RNA probe (P), hCK-5 (Pharmingen). Ltn, lymphotactin. (B) Relative degradation of RANTES and MCP-1 mRNA during the actinomycin D (5 μg/ml) chase. The levels of mRNA of RANTES, MCP-1, and GAPDH during the actinomycin D chase depicted on the autoradiogram were quantified by phosphor screen autoradiography. Each value represents the ratio of RANTES or MCP-1 mRNA to levels of control mRNA of GAPDH. □, HCMV-infected HUVECs; ▪, UV-HCMV-infected HUVECs.
Replicative HCMV infection inhibits UV-HCMV-induced RANTES expression.
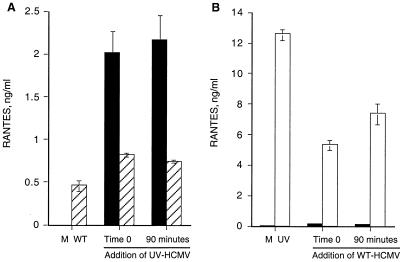
Our results thus far involved infection in parallel cultures, so we next investigated coinfection of cultures with WT-HCMV and UV-HCMV to determine which mechanism of RANTES expression is dominant. The assay was designed to determine whether the mechanism of WT-HCMV regulation of RANTES expression would predominate during coinfection with UV-HCMV and block UV-HCMV-induced high-level RANTES expression. Endothelial cells were coinfected with WT-HCMV at a high level of infection (multiplicity of infection of 1 PFU/cell) and with UV-HCMV at a lower level of infection (multiplicity of infection of 0.2 PFU/cell) (Fig. 6A). Mock-infected endothelial cells were infected with UV-HCMV in the same manner. As expected, mock infection did not stimulate any RANTES expression, and WT-HCMV infection stimulated moderate levels of RANTES (Fig. 6A). Addition of UV-HCMV to the cultures stimulated significantly less RANTES in the WT-HCMV-infected cells (0.8 ng) than in mock-infected cells (2.1 ng). The level of RANTES expression was unchanged whether UV-HCMV was added simultaneously (time zero) with WT-HCMV infection or 90 min following WT-HCMV infection. Thus, the inhibitory effect not only occurred early but was sustained for the first 90 min of infection. These data suggest that WT-HCMV regulation of RANTES expression predominates in the infected cells and at least partially inhibits the responsiveness of the cells to coinfection by UV-HCMV.
FIG. 6.
WT-HCMV inhibits UV-HCMV induction of RANTES expression from endothelial cells. (A) Subconfluent monolayers of HUVECs were mock infected or infected with WT-HCMV at 1.0 PFU/cell. In parallel cultures, 0.2 PFU equivalent of UV-HCMV was added either simultaneously (time zero) or following 90 min of infection with WT-HCMV, at which point the inoculum of WT-HCMV was removed from the cells prior to addition of UV-HCMV. Hatched bars represent cultures infected with WT-HCMV, and solid bars represent mock-infected cultures. (B) HUVECs were mock infected or infected with 1 PFU equivalent of UV-HCMV per cell. In parallel, cultures were infected with 0.2 PFU of WT-HCMV per cell either simultaneously (time zero) or following 90 min of treatment with UV-HCMV, at which point the UV-HCMV inoculum was removed prior to addition of WT-HCMV. Open bars represent cultures infected with UV-HCMV, and solid bars represent mock-infected cultures. Supernatants from all cultures were collected at 72 h postinfection and assayed for RANTES protein. Error bars represent standard errors of duplicates within the experiment, and this experiment is representative of three replicative assays.
Alternatively, we set up the converse assay in order to determine whether high-level infection with UV-HCMV would dominate and overwhelm the regulatory effect of WT-HCMV on RANTES expression. Endothelial cells were coinfected with 1 PFU equivalent of UV-HCMV/cell and with fivefold less WT-HCMV (0.2 PFU/cell), which was added either simultaneously (time zero) or after 90 min of UV-HCMV treatment (Fig. 6B). As expected, UV-HCMV stimulated high levels of RANTES (13 ng/ml), but in the presence of WT-HCMV infection, RANTES expression from the coinfected cultures was significantly reduced (6 ng/ml) (Fig. 6B). Infection of mock-infected HUVECs with WT-HCMV (0.2 PFU/cell) resulted in very low levels of RANTES expression (0.5 ng/ml). Simultaneous addition of WT-HCMV was slightly more effective than addition following a 90-min delay in reducing the effect of UV-HCMV. These data support the hypothesis that infection of endothelial cells with WT-HCMV down-regulates expression of RANTES induced by infection with UV-HCMV.
The high efficiency of low-level WT-HCMV infection to inhibit high-level UV-mediated RANTES expression during coinfection suggests that additional factors may be involved in concert with infection. The supernatants from WT-HCMV and UV-HCMV cultures at 24-h intervals following infection were assayed for secretion of cytokines with immunosuppressive properties: IL-10, IL-4, and TGF-β. In replicate assays, IL-10 or IL-4 could not be detected, and TGF-β was detected but in comparable amounts in the supernatants from WT-HCMV and UV-HCMV cultures (data not shown). In addition, supernatants from HCMV-infected HUVECs were transferred to UV-HCMV-infected cultures at various times postinfection (3, 18, and 24 h) to determine whether suppressive factors that might inhibit RANTES expression are secreted during WT-HCMV infection. The WT-HCMV supernatants did not significantly alter the level of RANTES expression from the UV-HCMV-treated cells (data not shown). Thus, it seems unlikely that a viral or cell protein that suppresses RANTES expression from neighboring cells is secreted during viral replication.
DISCUSSION
In this study, we report that abortive infection of primary endothelial cells (HUVECs) by UV-irradiated HCMV (which allows virus entry and uncoating but blocks viral gene expression) stimulates robust expression of RANTES from infected endothelial cells. Replicative HCMV infection, on the other hand, results in significantly less, although still physiologically relevant, RANTES expression. We propose that endothelial cells respond to inactive HCMV by expressing high levels of RANTES but this response is partially inhibited during active HCMV infection of endothelial cells.
We confirmed that RANTES expression during HCMV infection was independent of the presence of uninfected bystander cells or proinflammatory low-molecular-weight proteins in the inoculum. In assays in which every cell was infected with HCMV, there was still a >10-fold decrease in RANTES expression during replicative HCMV infection compared to the level of RANTES expressed during abortive HCMV infection. Furthermore, in assays in which virus particles were removed from the inoculum by filtration, neither the filtrate of the WT-HCMV inoculum nor that of the UV-HCMV inoculum stimulated expression of RANTES from HUVECs. This finding was interesting, since Hirsch and Shenk reported that a filterable factor present in the infection inoculum was responsible for the high-level MCP-1 expression during infection of fibroblasts with an attenuated strain of HCMV (15). Our results also raised the possibility that proinflammatory mediators might be expressed during abortive infection, which in turn might stimulate RANTES expression. We found that abortive HCMV infection (by WT-HCMV) does not induce HUVECs to express TNF-α or IFN-γ, two cytokines that stimulate HUVECs to express RANTES (18), although we note that other, as-yet-undetermined mediators might be expressed. In this report, our data provide evidence that the interaction between the HCMV particles, either noninfectious (UV-HCMV) or infectious (WT-HCMV), and endothelial cells is required for high-level RANTES expression. We propose that when HCMV particles associate with endothelial cells, signals are relayed by the cell to broadcast foreign invasion. Thus, since there may be a significant population of noninfectious particles to perpetuate the cell's response to express RANTES, we suggest that during replication, HCMV dampens this particular cellular response to viral infection.
Our next experiments were designed to determine whether regulation of RANTES expression during replicative HCMV infection occurred at the level of transcription. By analysis of chemokine mRNA with a multiplex RPA directed to identify several chemokine RNA species, we demonstrated that during abortive HCMV infection of HUVECs (infection with UV-HCMV), mRNA of both lymphotactin and RANTES is up-regulated at 12 h and peaks at 24 h postinfection. The level of RANTES message was significantly lower during replicative HCMV infection than during abortive infection, thereby indicating that alterations in steady-state mRNA are reflected in the alterations in protein in these cultures. Interestingly, WT-HCMV specifically down-regulated RANTES and lymphotactin message. The levels of MCP-1 and IL-8 message were unaffected by WT-HCMV infection, thus indicating that WT-HCMV infection does not have a global inhibitory effect on the expression of chemokines.
We determined that RANTES mRNA is stable following transcription in HCMV-infected HUVECs by analysis of steady-state mRNA during actinomycin D chase assays. These results demonstrated that the diminution of steady-state RANTES mRNA during replicative HCMV infection of endothelial cells does not reflect message degradation following transcription. The relative stability of message in both WT- and UV-HCMV-infected endothelial cells suggests that the steady-state levels determined by RPA may reflect transcriptional regulation in our system. Hence, we conclude that HCMV replication down-regulates cellular expression of RANTES at the level of transcription in endothelial cells.
The coinfection studies addressed whether inhibition of RANTES expression might be regulated by successful competition of cell surface receptors by WT-HCMV particles. We determined that a greater population of WT particles than UV-irradiated particles overrides the stimulatory effect of UV-HCMV. While this result suggests that WT particles are successfully competing with UV-irradiated particles for receptor sites, the effect was also demonstrated when the cells were infected with WT-HCMV 90 min prior to infection with UV-HCMV (Fig. 6A). Thus, either receptor desensitization persists for greater than 90 min, or additional factors besides receptor competition might be involved. In the converse assay, a greater population of UV-irradiated particles than WT particles did not completely override the inhibitory effect of WT-HCMV infection (Fig. 6B). These results, therefore, suggest that WT-HCMV particles are able to infect HUVECs despite the high-level competition from noninfectious particles and that this infection efficiently regulates RANTES expression. Given that many of the well-documented immunosuppressive cytokines are not expressed by endothelial cells during HCMV infection and that supernatants from WT-HCMV cultures do not affect UV-HCMV-induced RANTES expression, we propose that a viral mechanism within the cell regulates the expression of RANTES.
Unlike most other proinflammatory signals expressed during cell activation, RANTES is expressed by T cells and endothelial cells at significantly later times—as late as 48 h to 5 days following cell activation (18, 26). Therefore, the expression timetable in our system is typical for RANTES: RANTES message was not detected at 6 h, was detected in low levels at 12 h, and reached maximum expression at 24 h postinfection with either UV-HCMV or WT-HCMV (Fig. 4). This delayed period between activation and expression of RNA message may allow the virus to establish inhibitory mechanisms to regulate transcription.
Nelson and colleagues characterized the transcriptional regulation of RANTES expression and determined that the region immediately upstream of the RANTES gene contains an unusually large number of potential binding sites for transcription factors (20). They concluded that the RANTES gene has the potential to be regulated by a wide range of transcriptional controls in different tissues (20). We have begun studies to determine whether RANTES promoter activity is regulated during HCMV infection. In our transfection system in HUVECs, the RANTES promoter linked to a luciferase reporter was very sensitive to and rapidly activated by WT-HCMV infection, whereas UV-HCMV infection did not significantly affect RANTES promoter activity (data not shown). These results are opposite from the transcription levels of RANTES that we report in expression assays with the endogenous promoter (Fig. 4 and 5). Therefore, we believe that either we have an incomplete RANTES promoter that lacks a suppressor site that is activated during HCMV infection or that the mechanism of transfected promoter activity does not use machinery similar to endogenous promoter activity in the same cell. Thus, we speculate that binding of the virus particle to the cell activates a specific transcription factor that initiates RANTES transcription in endothelial cells but that during HCMV replication, a virally induced suppressor protein is activated that down-regulates transcription of this proinflammatory chemokine.
Increased levels of RANTES have been reported for several diseases that are associated with HCMV infection. Compared to controls, patients with atherosclerosis demonstrate higher levels of RANTES RNA in coronary arteries, and patients with chronic renal failure or chronic renal transplant rejection have significantly higher levels of RANTES protein in their plasma (9, 23). Interestingly, a study of patients with chronic renal transplant rejection indicated that those who develop HCMV infection have significantly lower levels of RANTES in their plasma than those without HCMV infection (9). This finding supports our hypothesis that HCMV may down-regulate expression of RANTES during active viral infection.
The lung is also a prominent site of RANTES expression during disease. RANTES is expressed during HCMV pneumonitis, but the levels (100 to 200 pg/ml) may represent lower levels than those in non-HCMV pneumonitis. RANTES has been detected in lung tissues of other diseases—for example, interstitial lung disease (24) and non-infection-related pneumonitis (21)—and in some instances, RANTES is expressed at significantly high levels during disease, for example, allergic asthma (1) and respiratory syncytial virus infection (4). Cross-comparison of these studies is difficult because of the different methods of detection (RNA versus protein). Thus, further studies must be done that examine the levels of RANTES from patients with non-HCMV pneumonitis compared to those from patients with HCMV pneumonitis. These studies will provide insight to whether active HCMV infection down-regulates RANTES expression in patients with pneumonitis.
In addition to regulating expression, HCMV has evolved other mechanisms to modulate extracellular chemokine levels. Recent data from our and other laboratories indicate that HCMV encodes a receptor for RANTES, US28, that binds and internalizes extracellular RANTES (6, 7) and in doing so effectively depletes RANTES from the surrounding medium. Although it might be attractive to suggest that US28 may account for reduced RANTES expression during replicative infection, as presented in this paper, we reason that US28 is not a likely candidate. We have shown that expression not only of RANTES protein but also of RANTES mRNA is reduced. In addition, reduced RANTES mRNA is detected as early as 12 h postinfection, whereas US28 is not functionally active on the infected cell surface until late in infection (approximately 72 h postinfection) (5). Thus, HCMV may have developed two mechanisms to down-regulate RANTES expression. At early times of infection, HCMV partially inhibits RANTES mRNA transcription, and at late times of infection, HCMV-infected cells express a RANTES receptor, US28, that depletes accumulating extracellular concentrations of RANTES (6).
The results presented here suggest that HCMV may down-regulate expression of chemokines that not only recruit monocytes/macrophages (RANTES) and lymphocytes (lymphotactin or RANTES) but also promote transmigration of lymphocytes across the endothelium. Recent reports suggest that RANTES and lymphotactin enhance movement of lymphocytes across HUVECs (8), while other chemokines, such as MIP-1α, MIP-1β, MCP-1, and IL-8, did not demonstrate a selective ability to induce lymphocyte migration across HUVECs (8) and were not regulated in our system (Fig. 4). Taken together, our results suggest that during HCMV infection of HUVECs, the virus down-regulates expression of chemokines such as RANTES and lymphotactin that may be involved in defense against virus infection.
Thus, we speculate that during HCMV infection—for example, during HCMV retinitis, in which mononuclear cells are typically absent from the inflammatory response to infection (22)—RANTES expression is down-regulated to limit monocyte and/or lymphocyte recruitment to the site of infection. These results may underscore the importance of RANTES as an innate cellular response to viral infection. Diminution of RANTES expression during infection may mask the infected cells from circulating mononuclear cells and thus reduce recruitment of these cells to the infected endothelium.
ACKNOWLEDGMENTS
We thank Lisa Lehman for technical assistance and Natalie Avdi, Jerry Nick, Ken Malcolm, and Peter Henson for helpful discussions.
This work was supported by the American Heart Association (Scientist Development grant 9730253N and Beginning grant-in-aid CWGB-17–98) and the National Institutes of Health (grants HL-57960 and M01 RR00069, General Clinical Research Centers Program, National Centers for Research Resources).
REFERENCES
- 1.Alam R, York J, Boyars M, Stafford S, Grant J, Lee J, Forsythe P, Sim T, Ida N. Increased MCP-1, RANTES, and MIP-1α in bronchoalveolar lavage fluid of allergic asthmatic patients. Am J Respir Crit Care Med. 1996;153:1398–1404. doi: 10.1164/ajrccm.153.4.8616572. [DOI] [PubMed] [Google Scholar]
- 2.Alford C, Britt W. Cytomegalovirus. In: Knipe D, editor. Virology. 2nd ed. New York, N.Y: Raven Press, Ltd.; 1990. pp. 1981–2010. [Google Scholar]
- 3.Arbustini E, Grasso M, Diegoli M, Percivalle E, Grossi P, Bramerio M, Campana C, Goggi C, Gavazzi A, Vigano M. Histophathologic and molecular profile of HCMV infections in patients with heart transplants. Am J Clin Pathol. 1992;98:205–213. [PubMed] [Google Scholar]
- 4.Becker S, Reed W, Henderson F, Noah T. RSV infection of human airway epithelial cells causes production of the β-chemokine RANTES. Am J Physiol. 1997;272:L512–L520. doi: 10.1152/ajplung.1997.272.3.L512. [DOI] [PubMed] [Google Scholar]
- 5.Billstrom M A, Johnson G L, Avdi N J, Worthen G S. Intracellular signaling by the chemokine receptor US28 during human cytomegalovirus infection. J Virol. 1998;72:5535–5544. doi: 10.1128/jvi.72.7.5535-5544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Billstrom M, Lehman L, Worthen G. Depletion of extracellular chemokines during HCMV infection of endothelial cells Am. J Respir Cell Mol Biol. 1999;21:163–167. doi: 10.1165/ajrcmb.21.2.3673. [DOI] [PubMed] [Google Scholar]
- 7.Bodaghi B, Jones T, Zipeto D, Vita C, Sun L, Laurent L, Arenzana-Seisdedos F, Virelizier J-L, Michelson S. Chemokine sequestration by viral chemoreceptors as a novel viral escape strategy: withdrawal of chemokines from the environment of CMV-infected cells J. Exp Med. 1998;188:855–866. doi: 10.1084/jem.188.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borthwick N J, Akbar A N, MacCormac L P, Lowdell M, Craigen J L, Hassan I, Grundy J E, Salmon M, Yong K L. Selective migration of highly differentiated primed T cells, defined by low expression of CD45RB, across human umbilical vein endothelial cells: effects of viral infection on transmigration. Immunology. 1997;90:272–280. doi: 10.1046/j.1365-2567.1997.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corsi M, Leone G, Fulgenzi A, Wasserman K, Leone F, Ferrero M. RANTES and MCP-1 chemokine plasma levels in chronic renal transplant dysfunction and chronic renal failure. Clin Biochem. 1999;32:455–460. doi: 10.1016/s0009-9120(99)00038-7. [DOI] [PubMed] [Google Scholar]
- 10.Craigen J, Yong K, Jordan N, MacCormac L, Westwick J, Akbar A, Grundy J. HCMV infection up-regulates IL-8 gene expression and stimulates neutrophil transendothelial migration. Immunology. 1997;92:138–145. doi: 10.1046/j.1365-2567.1997.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fortunato E, McElroy A, Sanchez V, Spector D. Exploitation of cellular signaling and regulatory pathways by human cytomegalovirus. Trends Microbiol. 2000;8:111–119. doi: 10.1016/s0966-842x(00)01699-1. [DOI] [PubMed] [Google Scholar]
- 12.Gimbrone M. Culture of vascular endothelium. Prog Hemostasis Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- 13.Hengel H, Brune W, Koszinowski U. Immune evasion by cytomegalovirus—survival strategies of a highly adapted opportunist. Trends Microbiol. 1998;6:190–197. doi: 10.1016/s0966-842x(98)01255-4. [DOI] [PubMed] [Google Scholar]
- 14.Hirai K, Maeda F, Watanabe Y. Expression of early virus functions in HCMV infected HEL cells: effect of UV light-irradiation of the virus. J Gen Virol. 1978;38:121–133. doi: 10.1099/0022-1317-38-1-121. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch A, Shenk T. Human cytomegalovirus inhibits transcription of the CC chemokine MCP-1 gene. J Virol. 1999;73:404–410. doi: 10.1128/jvi.73.1.404-410.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koskinen P, Krogerus L, Nieminen M, Mattila S, Hayry P, Lautenschlager I. Quantitation of CMV infection-associated histologic findings in endomyocardial biopsies of heart allografts. J Heart Lung Transplant. 1993;12:343–354. [PubMed] [Google Scholar]
- 17.Luster A. Chemokines: chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 18.Marfaing-Koka A, Devergne O, Gorgone G, Portier A, Schall T, Galanaud P, Emilie D. Regulation of the production of the RANTES chemokine by endothelial cells. J Immunol. 1995;154:1870–1878. [PubMed] [Google Scholar]
- 19.Michelson S, Dal Monte P, Zipeto D, Bodaghi B, Laurent L, Oberlin E, Arenzana-Seisdedos F, Virelizier J-L, Landini M. Modulation of RANTES production by human cytomegalovirus infection of fibroblasts. J Virol. 1997;71:6495–6500. doi: 10.1128/jvi.71.9.6495-6500.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson P, Kim H, Manning W, Goralski T, Krensky A. Genomic organization and transcriptional regulation of the RANTES chemokine gene. J Immunol. 1993;151:2601–2612. [PubMed] [Google Scholar]
- 21.Oshima M, Maeda A, Ishioka S, Hiyama K, Yamakido M. Expression of CC chemokines in bronchoalveolar lavage cells from patients with granulomatous lung disease. Lung. 1999;177:229–240. doi: 10.1007/pl00007643. [DOI] [PubMed] [Google Scholar]
- 22.Palestine A. Clinical aspects of CMV retinitis. Rev Infect Dis. 1988;10:S515–S521. doi: 10.1093/clinids/10.supplement_3.s515. [DOI] [PubMed] [Google Scholar]
- 23.Pattison J, Nelson P, Huie P, Sibley R, Krensky A. RANTES chemokine expression in transplant-associated accelerated atherosclerosis. J Heart Lung Transplant. 1996;15:1194–1199. [PubMed] [Google Scholar]
- 24.Petrek M, Pantelidis P, Southcott A, Lympany P, Safranek P, Black C, Kolek V, Weigl E, duBois R. The source and role of RANTES in interstitial lung disease. Eur Respir J. 1997;10:1207–1216. doi: 10.1183/09031936.97.10061207. [DOI] [PubMed] [Google Scholar]
- 25.Sachs A. Messenger RNA degradation in eukaryotes. Cell. 1993;74:413–421. doi: 10.1016/0092-8674(93)80043-e. [DOI] [PubMed] [Google Scholar]
- 26.Schall T, Jongstra J, Dryer B, Jorgensen J, Clayberger C, Davis M, Krensky A. A human T cell-specific molecule is a member of a new gene family. J Immunol. 1988;141:1018–1025. [PubMed] [Google Scholar]
- 27.Skowronski E, Mendoza A, Smith S, Jaski B. Detection of CMV in paraffin-embedded postmortem coronary artery specimens of heart transplant recipients by the PCR: implications of CMV association with grafter atherosclerosis. J Heart Lung Transplant. 1993;12:717–723. [PubMed] [Google Scholar]
- 28.Toorkey C, Carrigan D. Immunohistochemical detection of an immediate early antigen of human cytomegalovirus in normal tissues. J Infect Dis. 1989;160:741–751. doi: 10.1093/infdis/160.5.741. [DOI] [PubMed] [Google Scholar]
- 29.Wu T, Hruban R, Ambinder R, Pizzorno M, Cameron D, Baumgartner W, Reitz B, Hayward G, Hutchins G. Demonstration of CMV nucleic acids in the coronary arteries of transplanted hearts. Am J Pathol. 1992;140:739–747. [PMC free article] [PubMed] [Google Scholar]
- 30.Yamashiroya H, Ghosh L, Yang R, Robertson A. Herpesviridae in the coronary arteries and aorta of young trauma victims. Am J Pathol. 1988;130:71–79. [PMC free article] [PubMed] [Google Scholar]