Abstract
Herpes simplex virus (HSV) ICP0 can effectively activate gene expression from otherwise silent promoters contained on persisting viral genomes. However, the expression of high levels of ICP0, as from ICP4− HSV type 1 (HSV-1) vectors, results in marked toxicity. We have analyzed the results of ICP0 expressed from an E1− E4− adenovirus vector (AdS.11E4ICP0) in which ICP0 expression is controlled from the endogenous adenoviral E4 promoter. In this system, the expression level of ICP0 was reduced more than 1,000-fold relative to the level of expression from HSV-1 vectors. This low level of ICP0 did not affect cellular division or greatly perturb cellular metabolism as assessed by gene expression array analysis comparing the effects of HSV and adenovirus vector strains. However, this amount of ICP0 was sufficient to quantitatively destroy ND10 structures as measured by promyelocytic leukemia immunofluorescence. The levels of adenovirus-expressed ICP0 were sufficient to activate quiescent viral genomes in trans and promote persistent transgene expression in cis. Moreover, infection of complementing cells with AdS.11E4ICP0 promoted viral growth and resulted in a 20-fold increase in the plaquing efficiency of d109, a virus defective for all five immediate-early genes. Thus, the low level expression of ICP0 from the E1− E4− adenovirus vector may increase the utility of adenovirus vectors and also provides a means to efficiently quantify and possibly propagate HSV vectors defective in ICP0. Importantly, the results demonstrate that the activation function of ICP0 may not result from changes in cellular gene expression, but possibly as a direct consequence of an enzymatic function inherent to the protein that may involve its action at ND10 resulting in the preferential activation of viral genomes.
During productive herpes simplex virus type 1 (HSV-1) infection, three classes of viral genes are temporally expressed in the following order: immediate-early (IE), early (E), and late (L) genes (24, 25). Four of the five IE genes (ICP0, ICP4, ICP22, and ICP27) encode the main regulatory functions for virus gene expression (42, 45, 56). One of these proteins, ICP0, is not essential for virus growth in vitro; however, ICP0-defective viruses grow very slowly and are considerably impaired at low multiplicities of infection (47, 54). ICP0 mutants also reactivate very poorly from latency in mice and rabbit models (3, 7, 11, 21, 34, 46), suggesting a role for ICP0 in reactivation from latency.
The mechanism by which ICP0 facilitates viral growth and the reactivation from latency is not well understood. Several groups have shown that ICP0 will nonspecifically transactivate reporter genes in transient assays (12, 19, 39, 44). Mutants of ICP0 that are defective for this activation function are also defective for promoting viral growth and reactivation from latency (3), suggesting that the ability to activate gene expression reflects the requirement for ICP0 in viral infection. It has been shown that HSV gene expression becomes repressed in cells in the absence of viral IE gene expression (43, 48) and that ICP0 is sufficient to reactivate gene expression from quiescently persisting HSV genomes in cells (22, 48, 59). Therefore, it is believed that repression is counteracted by the action of ICP0. ICP0 may counteract repression by stimulating the degradation of a number of cellular proteins via the ubiquitin-proteasome pathway (15, 17). However, ICP0 has been proposed to interact with a number of cellular factors representative of a variety of cellular pathways that are potentially capable of contributing to its properties. These include components of cellular transcriptional (33), translational (29), protein degradation (13, 14, 15, 16), and cell cycle control (14, 23, 30, 35) pathways.
Given the plethora of potential interactions between ICP0 and critical cellular functions it is reasonable to assume that ICP0 would have effects on host cell metabolism and survival. Indeed, several studies have documented the deleterious effects of ICP0 on cellular metabolism, particularly in dividing cells (14, 23, 50, 58). The observed effects of ICP0 on aspects of cellular metabolism may be a direct reflection of the mechanism by which ICP0 functions in viral infection, or they may be a simple by product of ICP0 action, having little to do with the mechanism by which ICP0 activates gene expression. Alternatively, such effects may be the consequence of overproduction of ICP0.
In the present study we investigated the quantitative requirement for ICP0 with respect to the activation of quiescent genomes, the activation of viral gene expression, and the ability to complement ICP0 mutants. We have found it possible to maintain ICP0 gene activation function while reducing or eliminating its toxic properties by expressing ICP0 from an E1− E4− adenovirus in which ICP0 expression is controlled from the endogenous adenovirus E4 promoter. The reduced amount of ICP0 expressed from AdS.11E4(ICP0) was sufficient to activate quiescent viral genomes in trans and promote persistent transgene expression in cis. Gene expression array experiments demonstrated that expression of low levels of ICP0 from AdS.11E4(ICP0) did not greatly affect the expression of cellular genes relative to an HSV mutant where IE gene expression is limited to ICP0. However, the ICP0 expressed from the adenoviral backbone was sufficient to disrupt ND10 structures, which are punctate nuclear domains of unknown function that are modified during HSV infection (37). Moreover, infection with AdS.11E4(ICP0) complemented growth of ICP0-deficient HSV-1, suggesting that the low level of ICP0 expression from the E1− E4− adenovirus vector may provide a means to efficiently quantify and possibly propagate such HSV-1 IE mutants. Importantly, the results demonstrate that the activation function of ICP0 may result not from changes in cellular gene expression but possibly as a direct consequence of an enzymatic function inherent to the protein.
MATERIALS AND METHODS
Cells and viruses.
Human embryonic lung (HEL) fibroblasts and Vero cells were obtained from the American Type Culture Collection and maintained in Dulbecco's modified Eagle medium (DMEM)–10% fetal bovine serum (FBS) as previously described. HSV-1 IE complementing cell line FO6 is a Vero-derived cell line previously described (50). Adenovirus mutants AdS.10, AdS.11D, and AdS.11E4(ICP0) are diagrammed in Fig. 1 and were constructed at GenVec, Inc., Gaithersburg, Md. Details of their construction will be published elsewhere. The wild-type (wt) strain of HSV-1 used in these experiments is strain KOS. HSV-1 IE mutant viruses d99, d105, d106, and d109 have been previously described (48), and their genome structures are also shown in Fig. 1.
FIG. 1.
Adenovirus and HSV-1 mutant virus structures. (A) Adenovirus constructs compared to wild-type Ad5 structure (top) with respect to E1, E3, and E4 regions. All mutants contain the HCMVIEp-SEAP transgene inserted in the E1 locus and are also deficient for E3 function. Shown also is the insertion of a nonsense spacer or HSV-1 ICP0 in the E4 locus of viruses AdS.11D and AdS.11E4(ICP0), respectively. (B) The HSV-1 viral genome (top) demonstrating the locus of the 5 IE genes (arrows) relative to the unique long (UL), unique short (US), and repeat sequences (boxes). IE deletion mutants have been previously reported (31).
Microscopy and immunofluorescence.
Confluent monolayers of HEL cells were infected with d109 (multiplicity of infection [MOI] = 10) and then superinfected 24 h later with either d105 or Ad11S.E4(ICP0) at the indicated MOI. Cells were visualized by fluorescence microscopy using a Nikon Diaphot 300 with appropriate filters for green fluorescent protein (GFP) detection.
For immunofluorescence studies, infected and uninfected cells were prepared on circular coverslips. The cells were infected with d109 (MOI = 10) and superinfected 24 h later with either d105, AdS.10, AdS.11D, or AdS.11E4(ICP0). At 24 h postsuperinfection, cells were fixed in 4% paraformaldehyde and permeabilized with 0.2% Triton X-100 and stained for promyelocytic leukemia (PML) detection as previously described (48). Anti-PML antibody (Santa Cruz Biotechnology) was used at a dilution of 1:30. The stained antigens were visualized with the appropriate cubes for fluorescence imaging in conjunction with a Nikon FXA photomicroscope.
Western blot analysis.
HEL cells were infected (10 PFU of HSV-1 or 1,000 focus-forming units (FFU) of adenovirus per cell) with the indicated virus. At the indicated times postinfection, cells were harvested in a Triton X-100-containing lysis buffer as previously described, and equal amounts of total protein were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as previously described (23). Proteins were transferred to polyvinylidene difluoride membranes and probed with anti-ICP0 antibody (Goodwin Institute for Cancer Research) or anti-GFP antibody (CLONTECH) and detected as previously described (23).
Cell proliferation assay.
Cellular proliferation following infection was assessed as previously described (23). Briefly, 105 HEL cells were seeded in duplicate on 35-mm-diameter dishes. Cells were then infected or mock infected with 10 PFU/cell (HSV-1) or 1,000 FFU/cell (adenovirus). Cells were harvested by trypsinization at the indicated times postinfection and counted on a hemocytometer.
Microarray analysis.
HEL cells were infected as described above and total RNA was isolated and poly(A)+ mRNA was selected as previously described (23). Microarray analysis was conducted by Incyte Pharmaceuticals on Human UniGEM V and data was analyzed using GEMTools software.
35S metabolic labeling.
HEL cells were infected or mock infected with d109 (MOI = 20) and maintained at 34°C for 7 days in DMEM containing 2% FBS. Cells were then superinfected with d105, AdS.11D, or AdS.11E4(ICP0) at the highest MOI shown in Fig. 2. Cells were pulsed with [35S]met-[35S]cys 12 h postsuperinfection for 12 h, at which time cell lysates were harvested as described above and separated by SDS-PAGE and protein profiles were visualized by autoradiography.
FIG. 2.
Transactivation of quiescent viral genes. The ability of ICP0 expressed from either d105 or from AdS.11E4(ICP0) to transactivate the otherwise silent HCMVIEp-GFP transgene encoded by d109 was assayed at increasing MOIs Confluent monolayers of HEL cells were infected with d109 (MOI = 10) and then superinfected 24 h later with either d105 or Ad11S.E4(ICP0) at the indicated MOI. Cells were visualized by fluorescence microscopy 48 h later. d109-infected cells that were not superinfected are also shown (mock). The single fluorescent cell in this field represents a rare event that was not seen in many other fields of d109-infected HEL cells.
Complementation of virus growth, plaquing efficiency, and virus yield.
HEL cells were infected with d109 (MOI = 20) and maintained at 34°C for 7 days in DMEM supplemented with 2% FBS. Cells were then infected with d99 in combination with either d105, AdS.11D, or Ad.S11E4(ICP0) as indicated. Supernatants were collected at the indicated times postinfection and d99 titer was determined by plating on Vero cells. Plating on FO6 cells and counting only small GFP+ plaques approximated d109 titers. For determination of plaquing efficiency, FO6 cells were infected with AdS.11E4(ICP0) at increasing particle/cell ratios. Twenty-four hours later, the cells were infected with d109 and the numbers of resulting plaques were counted. To determine virus yield, HEL cells were infected with d109 (MOI = 0.001) 24 h postinfection with AdS.11E4(ICP0), and supernatants were harvested on days 1, 2, 3, and 4, and the resulting virus yield determined by titration on AdS.11E4(ICP0) infected FO6 cells.
SEAP expression in HEL cells.
HEL cell monolayers were infected at 1,000 PFU/cell with AdS.10, AdS11.D, or AdS.11E4(ICP0). The levels of transgene expression were monitored every three days for secretory alkaline phosphatase (SEAP) activity in the medium after complete medium change. The medium was aliquoted and stored at −80°C. SEAP expression levels were detected by chemiluminescence using Phospha-Light (Tropix). SEAP activity is expressed as relative light units in 2 μl of medium.
RESULTS
AdS11E4(ICP0) can activate expression from silent promoters on quiescent HSV-1 genomes.
To assess the general activity of ICP0 expressed from an E1− E3− E4− adenovirus vector, we first assessed the ability of AdS.11E4(ICP0) to transactivate gene expression from promoters contained on quiescent HSV-1 genomes. The virus d109 (Fig. 1) is deleted for all 5 HSV-1 IE functions and thus is replication incompetent and transcriptionally silent following infection of noncomplementing cells (48). Samaniego et al. (48) have shown that d109 genomes persist in a quiescent state in the nucleus of infected cells for extended periods of time without any detectable cytotoxic effect. d109 contains an HCMVIEp-GFP transgene cassette that is also efficiently repressed by the host cell following infection (48) such that GFP expression is undetectable in >99% of infected HEL cells. Expression of ICP0 following superinfection by an HSV-1 IE mutant virus such as d105 (Fig. 1), which is defective for the expression of all the IE proteins except ICP0, can activate expression from this otherwise silent HCMVIEp-GFP transgene encoded by d109. Thus, as a marker of ICP0 activity when expressed from AdS.11E4(ICP0), we compared the induction of GFP expression from d109 as a function of d105 or AdS.11E4(ICP0) at different MOI (Fig. 2).
Expression of ICP0 from AdS.11E4(ICP0) was able to induce GFP expression from resident d109 genomes. As with d105, infection with increasing MOI of AdS.11E4(ICP0) resulted in a dose response with respect to the activation of quiescent d109 genomes as shown by the increased number of GFP+ cells at an increasing MOI. The control E1− E3− E4− adenovirus AdS.11D failed to induce GFP expression (data not shown) (see Fig. 6), indicating that the effect was due to ICP0 expression. While infection with d105 was capable of transactivating d109 genomes at a low MOI, a relatively higher number of infectious units (approximately 1,000-fold higher) of AdS.11E4(ICP0) was necessary for a similar response.
FIG. 6.
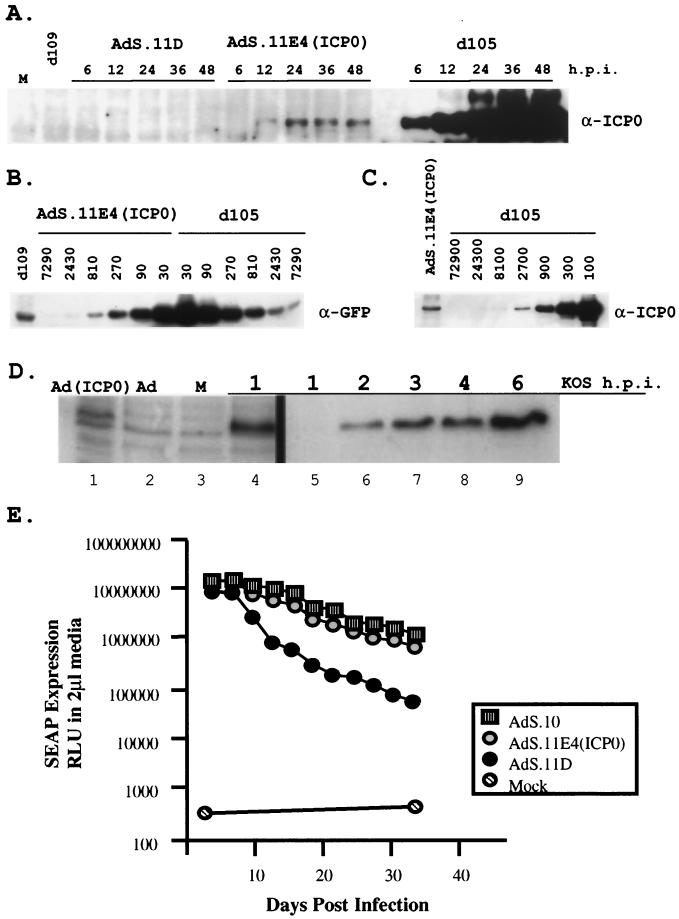
Expression level of ICP0 and transactivation of gene expression. HEL cells were infected with HSV-1 IE mutant virus d109 (MOI = 10) 24 h prior to superinfection with 10 PFU of d105 or 1,000 FFU of either AdS.11D or AdS.11E4(ICP0) per cell. (A) Kinetics of ICP0 expression. Total cellular protein was harvested at the indicated times postsuperinfection and analyzed by Western blot for ICP0. Mock and d109 lanes represent the 24 h.p.i. time point indicated. d105 lanes represent 1/10 of the total protein loaded in all other lanes. (B) Western blot of GFP induction. Samples shown in panel A were analyzed for induction of GFP expression. The labels above the lanes identified as AdS.11E4(ICP0) and d105 represent the fold-dilution of the sample in that lane relative to the d109 sample lane. (C) Western blot of ICP0 expression. Total protein samples obtained at the same 24 h.p.i. defined in panel A and described in part B were analyzed by Western blot for ICP0. The lanes identified as d105 are labeled as fold dilutions of the input protein shown in the AdS.11E4(ICP0) lane. (D) Comparison of the levels of ICP0 synthesized in wt HSV infection and during infection with AdS.11E4(ICP0). HEL were cells infected with AdS.11E4(ICP0) (lane 1) or AdS.11D (lane 2) for 24 h as described above, or with wild-type HSV-1, KOS at an MOI of 10 for the indicated times (hours) postinfection (lanes 4 through 9). For these experiments, wild-type HSV-1 (KOS) was adsorbed to cells at 4°C for 1 h, after which time 37°C medium was added (t = 0 h.p.i.) and incubation was continued at 37°C. At the indicated times (h.p.i.), total cellular protein was harvested and analyzed by Western blot for ICP0. Lanes 5 through 9 represent 1/10 the total protein loaded in all other lanes. Lane 3 represents uninfected cells (M). The images on the blot were directly quantified using a Molecular Dynamics Storm 840 set to fluorescence-chemifluorescence. The method of quantification is referred to in the text. (E) HEL cells infected with 1,000 PFU of AdS.10, AdS11.D or AdS11.E4(ICP0) were monitored every 3 days for SEAP expression.
HSV mutants defective for ICP4 function express dramatically reduced levels of early transcripts and protein; however, they express significant levels of ICP6. ICP6 expression from such mutants is significantly reduced if ICP0 is additionally inactivated, implying that ICP6 expression is enhanced by ICP0 (50). Therefore, we examined the ability of ICP0 expressed from AdS.11E4(ICP0) to activate ICP6 expression from resident d109 genomes, and compared this to the expression of ICP6 from HSV d105 genomes (Fig. 3). As ICP0 has been shown to activate ICP6 expression (20), it is of note that AdS.11E4(ICP0) also induced expression of ICP6 from resident d109 genomes. This indicates that transactivation by ICP0 expressed from AdS.11E4(ICP0) was not limited to transgenes controlled by the HCMVIE promoter. Also visible in Fig. 3 are ICP0 expressed from d105, SEAP from the adenovirus strains and GFP from d109 when superinfected with an ICP0-expressing HSV or adenovirus. The ICP0 expressed from AdS.11E4(ICP0) is not detectable by this analysis.
FIG. 3.
Synthesis of viral and cellular proteins. HEL cells were infected or mock infected with d109 (MOI = 20) and maintained at 34°C for 7 days in DMEM containing 2% FBS. Cells were then superinfected with d105, AdS.11D, or AdS.11E4(ICP0) at the highest MOI shown in Fig. 2. Cells were labeled with [35S]met-[35S]cys 12 h postsuperinfection for 12 h, at which time cell lysates were harvested and separated by SDS-PAGE and protein profiles were visualized by autoradiography. Virally encoded proteins ICP6, ICP0, SEAP, and GFP are indicated (arrows). ICP6 is encoded only by HSV-1 vectors d109 and d105, whereas ICP0 is encoded by both d105 and AdS.11E4(ICP0). SEAP is contained only on the adenoviral vectors while GFP is encoded only by the HSV-1 vector d109.
Complementation of ICP0-deficient virus and reactivation of quiescent HSV-1 genomes.
Given that expression of ICP0 from AdS.11E4(ICP0) activated expression of endogenous promoters on quiescent HSV-1 genomes, it was of interest to investigate whether such delivery of ICP0 was sufficient with respect to supporting HSV-1 replication. HSV-1 mutants deficient for ICP0 function are replication competent but replicate to levels several logs less than those of wt viruses. HEL cells were first infected with d109 and allowed to incubate for 1 week. The cultures were then superinfected with the indicated viruses and at various times postinfection were assayed for virus yield. As expected, d99 grew, albeit poorly, on d109-infected HEL cells (Fig. 4A), but was not able to reactivate quiescent d109 (Fig. 4B). Both d105 and AdS.11E4(ICP0) were able to complement the growth of d99 (Fig. 4A) and reactivate quiescent d109 genomes (Fig. 4B). AdS.11D was not able to complement d99 nor reactivate quiescent d109 genomes.
FIG. 4.
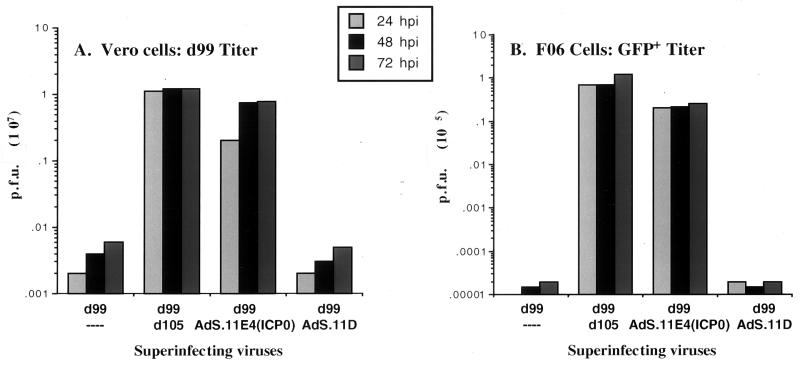
Complementation of ICP0-deficient virus and reactivation of quiescent HSV genomes. HEL cells were infected with d109 (MOI = 20) and maintained at 34°C for 7 days in DMEM supplemented with 2% FBS. Cells were then infected with d99 in combination with either d105, AdS.11D, or Ad.S11E4(ICP0) as indicated. Supernatants were collected at the indicated times postinfection, and the d99 titer was determined by plating on Vero cells (A). d109 titers were approximated by plating harvested supernatants on FO6 cells and counting only small GFP+ plaques (B).
AdS.11E4(ICP0) increases d109 plaquing efficiency and virus yield.
Cell culture systems to efficiently propagate and quantitate HSV-1 IE mutant viruses have been designed to complement deleted viral functions in trans by stably transfecting the complementing viral genes into the cellular genome (8, 9, 49, 50). However, growth of HSV-1 with multiple IE deletions on these complementing cell lines typically produces viral stocks with lower titers than the wild-type virus. Given the role of ICP0 in supporting viral gene expression and replication and the ability of ICP0 expressed from AdS.11E4(ICP0) to complement replication of ICP0 deficient HSV-1, we were interested in whether AdS.11E4(ICP0) may be useful in propagating and/or quantitating HSV-1 IE mutant viruses. Infection of the ICP4, ICP27, and ICP0 complementing cell line FO6 (49) with AdS.11E4(ICP0) enhanced the plaquing efficiency of d109 (Fig. 5). This is illustrated by the increased number of plaques obtained as a function of AdS.11E4(ICP0) (Fig. 5A). The control E1− E4− adenovirus AdS.11D failed to exhibit this effect (data not shown). Consistent with this result, AdS.11E4(ICP0) enhanced d109 virus growth rate on Vero-derived complementing cell line FO6 as shown by the increased rate of production of d109 progeny virus in Fig. 5B.
FIG. 5.
AdS.11E4(ICP0) increases d109 plaquing efficiency and virus yield. (A) FO6 cells were infected with AdS.11E4(ICP0) at increasing particle/cell ratios as indicated. After 24 h, the infected monolayers were used to plaque a stock of d109. Four days later the plaques were counted, and the resulting titer of the stock is represented as a function of AdS.11E4(ICP0). The asterisk denotes that, with more than 5.7 × 102 particles/cell (approximately 11 FFU/cell), FO6 cells failed to survive 24 h and the monolayer was destroyed prior to infection with d109. (B) FO6 cells were infected with 1.9 × 102 particles/cell AdS.11E4(ICP0) or AdS.11D. After 24 h, the cells were infected with d109 (MOI = 0.001) and supernatants were harvested on day 1, 2, 3, and 4, and the resulting virus yield was determined by titering on ÁdS.11E4(ICP0)-infected FO6 cells.
Interestingly, Vero cells exhibited marked sensitivity to adenovirus particles compared to HEL cells. As shown in Fig. 5A, infection with more than 5.7 × 102 particles/cell (approximately 11 FFU/cell) resulted in rapid cell death. This effect was not limited to FO6 cells, as Vero cells were also sensitive to equivalent AdS.11E4(ICP0) particles (data not shown). Infection with the control E1− E4− adenovirus AdS.11D also resulted in death of Vero and FO6 cells (data not shown), supporting the hypothesis that this toxicity arises as a function of the adenovirus particle. This is in marked contrast to the sensitivity of HEL cells shown in Fig. 2, which tolerated approximately 100-fold more virus per cell. The adenoviral penton has been shown to bind integrins αvβ3 and αvβ5 during virion internalization (2, 57). This has the capacity to be toxic to adherent cells by disrupting normal integrin interactions with the extracellular matrix, thereby promoting cell detachment (18, 41). Thus, various cell types most likely exhibit various sensitivities to the adenoviral particle depending on their dependence on αvβ3 and αvβ5 for attachment. It may be expected that differences in the cellular adhesion molecules utilized by different cell types may result in differences in sensitivity to adenoviral particles in vivo as well.
Kinetics of ICP0 expression from AdS.11E4(ICP0) and effect on transgene expression.
In the absence of ICP4 function, the expression of undeleted IE genes from HSV-1 IE mutant viruses is increased relative to wt levels. Thus, the effects and cytotoxic properties of such viruses may be related to effects that arise as a consequence of prolonged exposure to high levels of IE protein activity. It was thus of interest to investigate the level of ICP0 expressed from AdS.11E4(ICP0), where ICP0 expression is controlled from the adenovirus E4 promoter. We analyzed ICP0 expression from d105 or AdS.11E4(ICP0) and induction of d109-encoded HCMVIEp-GFP following infection of d109 infected HEL cells at the highest MOI shown in Fig. 2. ICP0 expression was easily detectable by Western blot analysis within 6 hpi following d105 infection (Fig. 6A). Expression of ICP0 from AdS.11E4(ICP0), however, was not detectable until 12 hpi, and then only minimally so by loading 10-fold excess protein in each lane compared to d105 lanes. While ICP0 expression continued to increase throughout the 48 h assayed following d105 infection, ICP0 expressed from AdS.11E4(ICP0) reached a maximal level by 24 hpi that was maintained through 48 hpi. By serial dilutions of d105-infected cell lysates we determined that AdS.11E4(ICP0)-infected cells expressed >1,000-fold less ICP0 than d105-infected cells (Fig. 6C). Using a similar dilution scheme, we also assessed the relative level of GFP induction by these two routes of ICP0 expression. The expression of ICP0 from AdS.11E4(ICP0) resulted in an approximately 500-fold induction of GFP compared to baseline, which was only approximately 3-fold less induction of GFP compared to the effect of over 1,000-fold more ICP0 expressed from d105 (Fig. 6B). Thus, AdS.11E4(ICP0) can efficiently transactivate gene expression despite the expression of very low levels of ICP0.
d105-infected cells overproduce ICP0 relative to wt virus-infected cells. Thus, we were compelled to ascertain how the levels of ICP0 in AdS.11E4(ICP0)- and wt HSV-infected cells compared and at what time postinfection with wt HSV these levels are similar. By comparison, accumulation of ICP0 during the first 24 h of infection with AdS.11E4(ICP0) was similar to that synthesized within the first hour postinfection with wt HSV (Fig. 6D). Quantification of the images of Fig. 6D revealed that the amount synthesized after 1 h of infection with KOS was 2.5 times that seen during infection with AdS.11E4(ICP0). The maximum level of ICP0 synthesized in KOS infection in the course of this experiment (6 h) was 48 times that synthesized during AdS.11E4(ICP0) infection. Therefore, it is possible that sufficient levels of ICP0 to activate gene expression during wt virus infection accumulate within the first hour postinfection and that these levels are considerably lower than the maximum level of accumulation during infection.
In the absence of IE functions, the host cell very efficiently represses HSV-1 genomes. As ICP0 can effectively relieve this repression and activate expression from such silenced promoters, it is possible that ICP0 activity may also promote prolonged transgene expression by preventing the repression and shutoff of heterologous viral genomes in cis. Both AdS.11E4(ICP0) and AdS.11D contain an HCMVIEp-SEAP transgene cassette in the E1 locus (Fig. 1) which was used to assay transgene expression over time. While AdS.11D retained SEAP expression levels above background, there was a noticeable decline in transgene expression within 10 days, which continued to decline through 36 days (Fig. 6E). In contrast, viruses that expressed either ICP0 from AdS.11E4(ICP0) or the E4 region from AdS.10 retained significantly higher levels of SEAP expression for a prolonged time. Thus, the presence of ICP0 can prolong gene expression such that the kinetics of transgene expression were similar to those seen in the presence of adenovirus E4 region.
Interaction with ND10 structures.
The experiment described above suggests that both ICP0 and the adenoviral E4 region can function to prolong transgene expression in cells. Both ICP0 (16, 36, 37) and E4orf3 protein (4, 26) have been demonstrated to disrupt PML-containing nuclear domains (PODs or ND10), resulting in the dispersal of ND10 components, such as PML, from their usual residence in discrete, nuclear punctate structures of interphase cells. The disruption of ND10 is postulated to be related to the gene activation function of ICP0 as mutations in the ICP0 RING finger domain fail to disrupt ND10 and are also impaired for gene activation function (10, 16, 36). Thus, we were interested in the interrelationship between ND10 disruption by adenovirus- or HSV-1-expressed ICP0 compared to E4orf3 function with respect to activation of gene expression. Expression of ICP0 from d105 resulted in loss of PML punctate staining as well as activation of the HCMVIEp-GFP transgene encoded by the resident d109 genomes (Fig. 7). Expression of ICP0 at low levels from AdS.11E4(ICP0) similarly resulted in destruction of ND10 structures and activation of GFP expression. The control E1− E4− adenovirus AdS.11D failed to alter PML staining and did not activate expression from d109. In the cases where the cells were mock infected or superinfected with AdS.11D and AdS.10, both the high- and low-magnification fields showing GFP-positive cells were selected because they contain a rare GFP-positive d109-infected HEL cell. These presumably would be instances where the d109 genome or the appropriate part of the d109 genome was in a state conducive to transcriptional activity. Interestingly, under these conditions the genomes are active without ND10 destruction in the cases of mock- and AdS.11D-infected cells. As expected, infection with AdS.10, which expresses E4orf3, resulted in PML disruption; however, GFP expression from d109 was not activated. Thus, while PML destruction may be involved in the mechanism of ICP0 activation, it is not a sufficient condition to allow activation of viral gene expression. Additionally, it may not be necessary, since the results of Fig. 7 demonstrate that it is possible to have active human cytomegalovirus promoters on d109 genomes in the absence of ND10 destruction.
FIG. 7.
Disruption of ND10 and activation of gene expression. The ability of ICP0 expressed from AdS.11E4(ICP0) to disrupt ND10 was assayed by immunofluorescent staining of ND10 constituent PML as described in Materials and Methods. Cells in the left column were visualized prior to fixation by fluorescence microscopy for GFP detection (20X objective). Cells in the middle column were visualized after fixation by fluorescence microscopy for GFP detection (100X objective plus oil). Micrographs in the right column represent the same field as that in the middle row visualized for rhodamine isothiocyanate detection (PML staining). Rows are identified as d109 infected plus respective superinfecting virus [top to bottom: mock, AdS.11D, AdS.10, AdS.11E4(ICP0), d105].
Effect of adenovirus vectors on cellular proliferation.
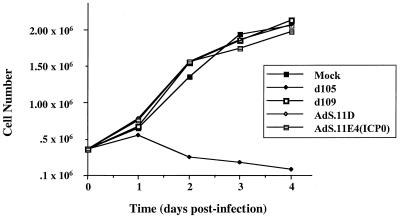
The disruption of ND10 was the only disturbance of cellular metabolism we could observe following infection with AdS.11E4(ICP0). However, this did not lead to obvious alterations in the phenotype of infected cells. In fact, we did not observe any morphologic nor cytotoxic effects of AdS.11E4(ICP0) infection on HEL cells, even at high MOIs (Fig. 1). In contrast, d105-infected cells flatten, become enlarged, fail to proliferate, and often display multiple subnuclei, with cell death apparent within several days. We have previously shown that ICP4− HSV-1 strains which express ICP0 exhibit a failure of proliferation and decreased cell survival (23, 48, 58). As a means to assess cell survival as a function of AdS.11E4(ICP0), we monitored cellular proliferation following infection. As shown in Fig. 8, HEL cells infected with d109 or AdS.11E4(ICP0) proliferated to the same extent as mock-infected cells through 3 days postinfection, at which time cell monolayers reached confluency. This is in contrast to the effect of high-level expression of ICP0 from d105, which resulted in a failure of proliferation and eventual cell death which was apparent by 48 hpi. The control E1− E3− E4− adenovirus, AdS.11D, also did not affect cellular proliferation. Thus, despite disrupting ND10 structures, which have been associated with the proliferative status of the cell (31, 38), AdS.11E4(ICP0) did not affect cellular proliferation.
FIG. 8.
Effect of AdS.11E4(ICP0) on cellular proliferation. HEL cells were infected with each indicated virus, and cell counts were determined 1, 2, 3, and 4 days postinfection as indicated and described in Materials and Methods.
Effect on cellular gene expression.
It is possible that the mechanism of ICP0 activity in activating viral gene expression occurs via manipulation of cellular factors potentially leading to perturbation of cellular metabolic functions. This mechanism might then also be expected to result in changes in the expression of cellular genes as a direct or indirect consequence of ICP0 activity. We have previously shown that the sole expression of ICP0 leads to changes in the expression of a subset of cellular genes following infection (23). We were interested in assessing the effect on cellular gene expression patterns following infection with AdS.11E4(ICP0) as a means of identifying potential toxic effects. In particular, we were interested in comparing the effects of the viral transactivators ICP0 and E4 in a similar background. To assess the effects on cellular gene expression, we employed gene expression microarrays to simultaneously assay the expression of >7,500 cellular genes following infection with an E1− E3− adenovirus (AdS.10), an E1− E3− E4− adenovirus (AdS.11D), an E1− E3− E4− adenovirus expressing ICP0 [AdS.11E4(ICP0)], or an ICP0 expressing HSV-1 IE mutant virus (d106). d106 is the virus that d105 was derived from (48). These viruses differ only in the presence of the GFP expression cassette inserted in the deleted ICP27 locus of d106. d105 and d106 behave identically with respect to the synthesis of ICP0 and their effects on cells. We assayed expression at a time postinfection (48 h postinfection [hpi]) when ICP0 expression and transactivation function is apparent (Fig. 6) following infection with AdS.11E4(ICP0). Figure 9 demonstrates that each of the adenovirus mutants resulted in significantly fewer changes in the expression of cellular genes than d106. The numbers of differentially expressed cellular genes are 10, 18, 23, and 427, for AdS.10, AdS.11D, AdS.11E4(ICP0), and d106, respectively. Differentially expressed genes are those whose expression is increased or decreased by a factor of more than 2 as a consequence of infection. The expression of E4 or ICP0 in the absence of E1 and E3 both resulted in a small number (8 and 13, respectively) of differentially expressed genes compared to the E1− E3− E4− adenovirus, with most of the changes representing induction events. Most of the remaining genes are those that were differentially expressed with all three adenoviruses. Of note is the observation that while d106 infection results in the differential expression of 427 genes following infection, reduction of ICP0 expression >1,000-fold following infection with AdS.11E4(ICP0) reduced this number to 23, which is only 13 more than that seen in the absence of ICP0. This is consistent with the lack of toxicity observed with this virus. Thus, the transactivation of transgenes on viral genomes can be preserved without significantly perturbing cellular gene expression by reducing the expression level of ICP0 as by infection with AdS.11E4(ICP0).
FIG. 9.
Effect on cellular gene expression patterns. The effect of infection by AdS.11D (E1− E3− E4−), AdS.11E4(ICP0) (E1− E3− E4− E4ICP0), and Ads.10 (E1− E3−) adenovirus on cellular gene expression was assayed by gene expression microarrays (A to C). Poly(A)+ mRNA from infected and mock-infected cells was isolated at 48 h.p.i. For comparison, microarray analysis of d106 at 6 hpi is also shown (D). The mock-infected mRNA fraction was labeled with Cy5 while the infected mRNA fraction was independently labeled with Cy3 and analyzed as described in Materials and Methods. The scatter plots shown are in log-log scales of fluorescent intensity read in each channel. Each gene on the chip is represented by a single spot on the graphs. The diagonal lines represent the fold increase or decrease in the infected cell sample relative to the mock sample. The arrows in panels A to C indicate the expression level of the adenovirus-encoded transgene SEAP.
One of the cellular genes represented on the microarrays is alkaline phosphatase, which cohybridizes to the SEAP transgene encoded by the adenovirus mutant genomes shown in Fig. 1. The arrows in Fig. 9A to C identify the expression profile of this gene. Note that the Cy3 signal intensity for this gene is similar for each of the adenovirus vectors, which indicates that transgene expression level is independent of transactivator function at this time postinfection and is at a level comparable to those of the mostly highly expressed genes in the cell. This effect was also observed at the level of protein synthesis in Fig. 3. However, as shown in Fig. 6D, maintenance of adenovirus transgene expression requires transactivator function, which can efficiently be supplied by either E4 or ICP0.
DISCUSSION
The role of HSV-1 ICP0 during infection is considered to be the promotion of lytic cycle events via the ability of ICP0 to activate, or derepress, viral gene expression facilitating the expression of the regulated repertoire of lytic cycle viral genes. Activation of gene expression by ICP0 occurs at the level of mRNA synthesis (27, 50), although ICP0 does not itself bind DNA. Thus, ICP0 activity is likely mediated through complex interactions and manipulations of host cell factors and metabolic pathways. Consequently, the effects of ICP0 on the host may be functionally related to its mechanism of action. Alternatively, such effects may be a reflection of downstream effects of ICP0 action, which may also arise secondary to the overexpression of ICP0 in some situations. In the present study, we investigated the quantitative and functional consequence of ICP0 expressed at low abundance in a heterologous, adenovirus system absent from the HSV-1 particle. We examined the consequences of such ICP0 expression and found it to retain wt function with respect to activation and reactivation of viral gene expression, complementation of virus replication, and reactivation of persistently infected quiescent virus in an in vitro model. The amount of ICP0 synthesized from the adenovirus was similar to that synthesized within the first hour postinfection with wt HSV, suggesting that ICP0 could efficiently function to activate gene expression very early in wt virus infection. Furthermore, we demonstrate that such low-level expression of ICP0 does not significantly perturb cellular metabolic function although ND10 structures were destroyed. The significance of these results has important implications for understanding ICP0's mechanism(s) of action as well as for the utility of gene transfer studies.
We have extended previous studies in which ICP0 has been expressed in adenovirus systems (6, 22, 37, 59, 60) with respect to several important aspects. First, we have assessed the quantitative requirement of ICP0 by expressing ICP0 from the endogenous adenovirus E4 promoter in an E1− E4− adenovirus. Previous studies have utilized E1− adenoviruses in which either the adenovirus major late promoter or the native ICP0 promoter drives ICP0 expression in the presence of endogenous E4 function. In these contexts, ICP0 expression levels were shown to be similar to those of HSV-1 (59, 60) as opposed to the very low levels of ICP0 expressed from AdS.11E4(ICP0). Second, ICP0 encodes activities that are potentially shared by adenovirus E4orf3, and thus we have employed a defective adenovirus deleted for both E1 and E4 coding sequences to more accurately attribute effects to ICP0. In particular, the disruption of ND10 is a shared function of ICP0 and E4orf3; however, in our studies we differentiate the disruption of ND10 by these two viral proteins from activation of gene expression.
We previously demonstrated that expression of ICP0 results in inhibition of cellular S phase and mitosis (23), suggesting a role for cell cycle events in regulating HSV-1 gene expression and/or replication. Indeed, it has been shown that ICP0 interacts with cyclin D3 (30), and that pharmacologic inhibition of cyclin-dependent kinase reduces viral IE and E transcription (28, 51, 52), indicating that cell cycle dysregulation may relate to gene expression. While we did not directly test whether these interactions occur in AdS.11E4(ICP0)-infected cells, the low-level expression of ICP0 retained function with respect to gene activation without affecting cellular proliferation. Thus, the cell cycle arrest that occurs following expression of ICP0 from ICP4− HSV-1 likely represents a dosage effect not functionally related to its activation of gene expression function. Additionally, while low levels of ICP0 expressed from AdS.11E4(ICP0) remained capable of interacting with host cell metabolic functions as demonstrated by disruption of ND10 structures and alteration in the expression of a limited number of cellular genes by expression array analysis, there was no obvious nor morphological toxicity attributed as a result.
The apparent lack of toxicity in the presence of low levels of ICP0, coupled with the retention of transgene expression is potentially useful with respect to gene transfer efforts. The success of most viral vector-based gene transfer strategies depends on absence of vector-mediated toxicity as well as efficient transgene expression. In HSV-1 systems, reducing toxicity involves deleting viral regulatory functions to restrict viral gene expression patterns. Elimination of HSV-1 toxicity requires deletion of all five IE functions (48). Thus, viruses like AdS.11E4(ICP0) may have utility in gene transfer strategies due to the prolongation of gene expression and reduced toxicity. Given this observation, it may also be possible to engineer HSV-based vectors to express similarly low levels of ICP0. However, the ability to reactivate quiescent HSV genomes despite the low-level expression of ICP0 remains a continuing concern. Additionally, we demonstrate that ICP0 expressed from an E1− E3− E4− adenovirus can enhance virus yield and plaquing efficiency of an HSV-1 mutant virus with deletions of all five IE functions in complementing cell line FO6. Thus, AdS.11E4(ICP0) provides a unique reagent to support the production of higher-titer stocks of ICP0-deficient HSV-1 as well as facilitate the quantification of resulting viral stocks. Another observation that is relevant to gene transfer studies is the finding that the expression of the transgene in all the adenoviruses tested (SEAP) is at a level comparable to those of the most highly expressed genes in the cell (Fig. 9). While the expression of transgenes from the HCMV promoter in adenovirus is often used as a paradigm for efficient transgene expression, this high level is at the extreme of cellular gene expression, which may not be necessary or even desirable from the standpoint of gene therapy.
From our results, it is clear that ICP0 can activate gene expression without significantly affecting host cell function. We have previously shown that ICP0 expression can result in the alteration in the expression of a subset of cellular genes (23) under conditions where ICP0 was greatly overexpressed relative to that from AdS.11E4(ICP0). Presumably this overexpression contributed to the observed toxicity. These differentially expressed cellular genes may represent targeted cellular functions required for HSV-1 gene expression and replication or may arise as consequence of nonspecific ICP0 gene activation mechanisms. For example, ICP0 may facilitate derepression of gene expression by altering the higher order structure of chromatin. The effect of ICP0 on cellular metabolic function is similar to the inhibition of histone deacetylase by trichostatin A such as induction of p21, alteration in the expression of a subset of cellular genes, and derepression of cellular and viral gene expression (23). This suggests that ICP0 may alter the acetylation state of core histones, resulting in derepression of gene expression. ICP0 expressed at low levels from AdS.11E4(ICP0) resulted in the differential expression of dramatically fewer cellular genes than when it was expressed at high levels from HSV-1. Thus, if ICP0 possesses activities that affect chromatin packaging, then HSV-1 genomes must be more sensitive than cellular genomes or ICP0 activity must be targeted to viral as opposed to cellular genomes. This possibility is currently being addressed.
The targeting of viral genomes to ND10 and the disruption of PML-containing nuclear structures by viral regulatory proteins has been associated with several viral systems, including HSV (15, 25, 35) cytomegalovirus (1), adenovirus (4, 26), simian virus 40 (26), and Epstein-Barr virus (55). One hypothesis is that ND10 represent the site of deposition of input viral genomes where viral transcription is initiated. Conversely, they could represent a cellular compartment repressive for viral gene expression, as several ND10 protein components are functionally linked to cellular interferon pathways (5, 32, 53). This suggests that disruption of ND10 may be a virus encoded mechanism to escape a cellular antiviral response and would therefore be a necessary early event in the replication cycle of many viruses by allowing efficient expression of viral genes. Studies to assess the intranuclear location of persisting d109 genomes should help address this issue. However, our results indicate that disruption of ND10 is not sufficient to turn on viral gene expression, as an adenovirus which expresses E4 proteins disrupts ND10 but fails to activate expression from quiescent HSV-1 genomes. This suggests that ICP0 possesses additional activities necessary for its role in gene activation that E4 does not. It is unknown at present whether ICP0 expressed at low levels in this system is capable of mediating other previously identified interactions with cellular proteins or functions such as DNA-dependent protein kinase (33, 40), translation factor EF1δ (30), or ubiquitin/protein modification pathways (13, 14, 15, 17). Since ICP0 specifically and efficiently activated viral gene expression, it is reasonable to propose that ICP0 possesses an enzyme-like function such as that involving the proteasome-ubiquitin pathway proposed by Everett and colleagues (13, 14) that results in the destruction of ND10 and the modification of chromatin structure at specific sites in the nucleus such as ND10. Studies to address this hypothesis are currently under way.
ACKNOWLEDGMENTS
This work was supported by NIH grants AI44812 and DK44935.
REFERENCES
- 1.Ahn J H, Hayward G S. The major immediate-early proteins IE1 and IE2 of human cytomegalovirus colocalize with and disrupt PML-associated nuclear bodies at very early times in infected permissive cells. J Virol. 1997;71:4599–4613. doi: 10.1128/jvi.71.6.4599-4613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bai M, Campisi L, Freimuth P. Vitronectin receptor antibodies inhibit infection of HeLa and A549 cells by adenovirus type 12 but not by adenovirus type 2. J Virol. 1994;68:5925–5932. doi: 10.1128/jvi.68.9.5925-5932.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cai W, Astor T L, Liptak L M, Cho C, Coen D M, Schaffer P A. The herpes simplex virus type 1 regulatory protein ICP0 enhances virus replication during acute infection and reactivation from latency. J Virol. 1993;67:7501–7512. doi: 10.1128/jvi.67.12.7501-7512.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvalho T, Seeler J S, Ohman K, Jordan P, Pettersson U, Akusjarvi G, Carmo-Fonseca M, Dejean A. Targeting of adenovirus E1A and E4-ORF3 proteins to nuclear matrix-associated PML bodies. J Cell Biol. 1995;131:45–56. doi: 10.1083/jcb.131.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chelbi-Alix M K, Pelicano L, Quignon F, Koken M H, Venturini L, Stadler M, Pavlovic J, Degos L, de The H. Induction of the PML protein by interferons in normal and APL cells. Leukemia. 1995;9:2027–2033. [PubMed] [Google Scholar]
- 6.Chen J, Silverstein S. Herpes simplex viruses with mutations in the gene encoding ICP0 are defective in gene expression. J Virol. 1992;66:2916–2927. doi: 10.1128/jvi.66.5.2916-2927.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clements G B, Stow N D. A herpes simplex virus type 1 mutant containing a deletion within immediate early gene 1 is latency-competent in mice. J Gen Virol. 1989;70:2501–2506. doi: 10.1099/0022-1317-70-9-2501. [DOI] [PubMed] [Google Scholar]
- 8.DeLuca N A, Schaffer P A. Activities of herpes simplex virus type I (HSV-1) ICP4 genes specifying nonsense peptides. Nucleic Acids Res. 1987;15:4491–4511. doi: 10.1093/nar/15.11.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeLuca N A, McCarthy A M, Schaffer P A. Isolation and characterization of deletion mutants of herpes simplex virus type 1 in the gene encoding immediate-early regulatory protein ICP4. J Virol. 1985;56:558–570. doi: 10.1128/jvi.56.2.558-570.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Everett R, O'Hare P, O'Rourke D, Barlow P, Orr A. Point mutations in the herpes simplex virus type 1 Vmw110 RING finger helix affect activation of gene expression, viral growth, and interaction with PML-containing nuclear structures. J Virol. 1995;69:7339–7344. doi: 10.1128/jvi.69.11.7339-7344.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Everett R D. Construction and characterization of herpes simplex virus type 1 mutants with defined lesions in immediate early gene 1. J Gen Virol. 1989;70:1185–1202. doi: 10.1099/0022-1317-70-5-1185. [DOI] [PubMed] [Google Scholar]
- 12.Everett R D. Trans activation of transcription by herpes virus products: requirement for two HSV-1 immediate-early polypeptides for maximum activity. EMBO J. 1984;3:3135–3141. doi: 10.1002/j.1460-2075.1984.tb02270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Everett R D. ICP0 induces the accumulation of colocalizing conjugated ubiquitin. J Virol. 2000;74:9994–10005. doi: 10.1128/jvi.74.21.9994-10005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everett R D, Earnshaw W C, Findlay J, Lomonte P. Specific destruction of kinetochore protein CENP-C and disruption of cell division by herpes simplex virus immediate-early protein Vmw110. EMBO J. 1999;18:1526–1538. doi: 10.1093/emboj/18.6.1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Everett R D, Freemont P, Saitoh H, Dasso M, Orr A, Kathoria M, Parkinson J. The disruption of ND10 during herpes simplex virus infection correlates with the Vmw110- and proteasome-dependent loss of several PML isoforms. J Virol. 1998;72:6581–6591. doi: 10.1128/jvi.72.8.6581-6591.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everett R D, Maul G G. HSV-1 IE protein Vmw110 causes redistribution of PML. EMBO J. 1994;13:5062–5069. doi: 10.1002/j.1460-2075.1994.tb06835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Everett R D, Meredith M, Orr A, Cross A, Kathoria M, Parkinson J. A novel ubiquitin-specific protease is dynamically associated with the PML nuclear domain and binds to a herpesvirus regulatory protein. EMBO J. 1997;16:556–577. doi: 10.1093/emboj/16.7.1519. . (Erratum, 16:1519–1530.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everett S F, Ginsberg H S. A toxin-like material separable from type 5 adenovirus particles. Virology. 1958;6:770–771. doi: 10.1016/0042-6822(58)90123-5. [DOI] [PubMed] [Google Scholar]
- 19.Gelman I H, Silverstein S. Identification of immediate early genes from herpes simplex virus that transactivate the virus thymidine kinase gene. Proc Natl Acad Sci USA. 1985;82:5265–5269. doi: 10.1073/pnas.82.16.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein D J, Weller S K. Herpes simplex virus type 1-induced ribonucleotide reductase activity is dispensable for virus growth and DNA synthesis: isolation and characterization of an ICP6 lacZ insertion mutant. J Virol. 1988;62:196–205. doi: 10.1128/jvi.62.1.196-205.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon Y J, McKnight J L, Ostrove J M, Romanowski E, Araullo-Cruz T. Host species and strain differences affect the ability of an HSV-1 ICP0 deletion mutant to establish latency and spontaneously reactivate in vivo. Virology. 1990;178:469–477. doi: 10.1016/0042-6822(90)90344-q. [DOI] [PubMed] [Google Scholar]
- 22.Harris R A, Everett R D, Zhu X X, Silverstein S, Preston C M. Herpes simplex virus type 1 immediate-early protein Vmw110 reactivates latent herpes simplex virus type 2 in an in vitro latency system. J Virol. 1989;63:3513–3515. doi: 10.1128/jvi.63.8.3513-3515.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobbs W E, II, DeLuca N A. Perturbation of cell cycle progression and cellular gene expression as a function of herpes simplex virus ICP0. J Virol. 1999;73:8245–8255. doi: 10.1128/jvi.73.10.8245-8255.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974;14:8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honess R W, Roizman B. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc Natl Acad Sci USA. 1975;72:1276–1280. doi: 10.1073/pnas.72.4.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishov A M, Maul G G. The periphery of nuclear domain 10 (ND10) as site of DNA virus deposition. J Cell Biol. 1996;134:815–826. doi: 10.1083/jcb.134.4.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jordan R, Schaffer P A. Activation of gene expression by herpes simplex virus type 1 ICP0 occurs at the level of mRNA synthesis. J Virol. 1997;71:6850–6862. doi: 10.1128/jvi.71.9.6850-6862.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan R, Schang L, Schaffer P A. Transactivation of herpes simplex virus type 1 immediate-early gene expression by virion-associated factors is blocked by an inhibitor of cyclin-dependent protein kinases. J Virol. 1999;73:8843–8847. doi: 10.1128/jvi.73.10.8843-8847.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawaguchi Y, Bruni R, Roizman B. Interaction of herpes simplex virus 1 α regulatory protein ICP0 with elongation factor 1δ: ICP0 affects translational machinery. J Virol. 1997;71:1019–1024. doi: 10.1128/jvi.71.2.1019-1024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawaguchi Y, Van Sant C, Roizman B. Herpes simplex virus 1 α regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J Virol. 1997;71:7328–7336. doi: 10.1128/jvi.71.10.7328-7336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koken M H, Linares-Cruz G, Quignon F, Viron A, Chelbi-Alix M K, Sobczak-Thepot J, Juhlin L, Degos L, Calvo F, de The H. The PML growth-suppressor has an altered expression in human oncogenesis. Oncogene. 1995;10:1315–1324. [PubMed] [Google Scholar]
- 32.Lavau C, Marchio A, Fagioli M, Jansen J, Falini B, Lebon P, Grosveld F, Pandolfi P P, Pelicci P G, Dejean A. The acute promyelocytic leukaemia-associated PML gene is induced by interferon. Oncogene. 1995;11:871–876. [PubMed] [Google Scholar]
- 33.Lees-Miller S P, Long M C, Kilvert M A, Lam V, Rice S A, Spencer C A. Attenuation of DNA-dependent protein kinase activity and its catalytic subunit by the herpes simplex virus type 1 transactivator ICP0. J Virol. 1996;70:7471–7477. doi: 10.1128/jvi.70.11.7471-7477.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leib D A, Coen D M, Bogard C L, Hicks K A, Yager D R, Knipe D M, Tyler K L, Schaffer P A. Immediate-early regulatory gene mutants define different stages in the establishment and reactivation of herpes simplex virus latency. J Virol. 1989;63:759–768. doi: 10.1128/jvi.63.2.759-768.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lomonte P, Everett R D. Herpes simplex virus type 1 immediate-early protein Vmw110 inhibits progression of cells through mitosis and from G1 into S phase of the cell cycle. J Virol. 1999;73:9456–9467. doi: 10.1128/jvi.73.11.9456-9467.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maul G G, Everett R D. The nuclear location of PML, a cellular member of the C3HC4 zinc-binding domain protein family, is rearranged during herpes simplex virus infection by the C3HC4 viral protein ICP0. J Gen Virol. 1994;75:1223–1233. doi: 10.1099/0022-1317-75-6-1223. [DOI] [PubMed] [Google Scholar]
- 37.Maul G G, Guldner H H, Spivack J G. Modification of discrete nuclear domains induced by herpes simplex virus type 1 immediate early gene 1 product (ICP0) J Gen Virol. 1993;74:2679–2690. doi: 10.1099/0022-1317-74-12-2679. [DOI] [PubMed] [Google Scholar]
- 38.Mu Z M, Chin K V, Liu J H, Lozano G, Chang K S. PML, a growth suppressor disrupted in acute promyelocytic leukemia. Mol Cell Biol. 1994;14:6858–6867. doi: 10.1128/mcb.14.10.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Hare P, Hayward G S. Evidence for a direct role for both the 175,000- and 110,000-molecular-weight immediate-early proteins of herpes simplex virus in the transactivation of delayed-early promoters. J Virol. 1985;53:751–760. doi: 10.1128/jvi.53.3.751-760.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parkinson J, Lees-Miller S P, Everett R D. Herpes simplex virus type 1 immediate-early protein Vmw110 induces the proteasome-dependent degradation of the catalytic subunit of DNA-dependent protein kinase. J Virol. 1999;73:650–657. doi: 10.1128/jvi.73.1.650-657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira H G. A protein factor responsible for the early cytopathic effect of adenovirus. Virology. 1958;6:601–611. doi: 10.1016/0042-6822(58)90109-0. [DOI] [PubMed] [Google Scholar]
- 42.Pereira L, Wolff M H, Fenwick M, Roizman B. Regulation of herpesvirus macromolecular synthesis. V. Properties of alpha polypeptides made in HSV-1 and HSV-2 infected cells. Virology. 1977;77:733–749. doi: 10.1016/0042-6822(77)90495-0. [DOI] [PubMed] [Google Scholar]
- 43.Preston C M, Nicholl M J. Repression of gene expression upon infection of cells with herpes simplex virus type 1 mutants impaired for immediate-early protein synthesis. J Virol. 1997;71:7807–7813. doi: 10.1128/jvi.71.10.7807-7813.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quinlan M P, Knipe D M. Stimulation of expression of a herpes simplex virus DNA-binding protein by two viral functions. Mol Cell Biol. 1985;5:957–963. doi: 10.1128/mcb.5.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields' Virology. Philadelphia, Pa: Lippincott-Raven Publishers; 1996. pp. 2231–2295. [Google Scholar]
- 46.Russell J, Stow N D, Stow E C, Preston C M. Herpes simplex virus genes involved in latency in vitro. J Gen Virol. 1987;68:3009–3018. doi: 10.1099/0022-1317-68-12-3009. [DOI] [PubMed] [Google Scholar]
- 47.Sacks W R, Schaffer P A. Deletion mutants in the gene encoding the herpes simplex virus type 1 immediate-early protein ICP0 exhibit impaired growth in cell culture. J Virol. 1987;61:829–839. doi: 10.1128/jvi.61.3.829-839.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Samaniego L A, Neiderhiser L, DeLuca N A. Persistence and expression of the herpes simplex virus genome in the absence of immediate-early proteins. J Virol. 1998;72:3307–3320. doi: 10.1128/jvi.72.4.3307-3320.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samaniego L A, Webb A L, DeLuca N A. Functional interactions between herpes simplex virus immediate-early proteins during infection: gene expression as a consequence of ICP27 and different domains of ICP4. J Virol. 1995;69:5705–5715. doi: 10.1128/jvi.69.9.5705-5715.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samaniego L A, Wu N, DeLuca N A. The herpes simplex virus immediate-early protein ICP0 affects transcription from the viral genome and infected-cell survival in the absence of ICP4 and ICP27. J Virol. 1997;71:4614–4625. doi: 10.1128/jvi.71.6.4614-4625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schang L M, Phillips J, Schaffer P A. Requirement for cellular cyclin-dependent kinases in herpes simplex virus replication and transcription. J Virol. 1998;72:5626–5637. doi: 10.1128/jvi.72.7.5626-5637.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schang L M, Rosenberg A, Schaffer P A. Transcription of herpes simplex virus immediate-early and early genes is inhibited by roscovitine, an inhibitor specific for cellular cyclin-dependent kinases. J Virol. 1999;73:2161–2172. doi: 10.1128/jvi.73.3.2161-2172.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stadler M, Chelbi-Alix M K, Koken M H, Venturini L, Lee C, Saib A, Quignon F, Pelicano L, Guillemin M C, Schindler C, et al. Transcriptional induction of the PML growth suppressor gene by interferons is mediated through an ISRE and a GAS element. Oncogene. 1995;11:2565–2573. [PubMed] [Google Scholar]
- 54.Stow N D, Stow E C. Isolation and characterization of a herpes simplex virus type 1 mutant containing a deletion within the gene encoding the immediate early polypeptide Vmw110. J Gen Virol. 1986;67:2571–2585. doi: 10.1099/0022-1317-67-12-2571. [DOI] [PubMed] [Google Scholar]
- 55.Szekely L, Pokrovskaja K, Jiang W Q, de The H, Ringertz N, Klein G. The Epstein-Barr virus-encoded nuclear antigen EBNA-5 accumulates in PML-containing bodies. J Virol. 1996;70:2562–2568. doi: 10.1128/jvi.70.4.2562-2568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watson R J, Preston C M, Clements J B. Separation and characterization of herpes simplex virus type 1 immediate-early mRNAs. J Virol. 1979;31:42–52. doi: 10.1128/jvi.31.1.42-52.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wickham T J, Mathias P, Cheresh D A, Nemerow G R. Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 58.Wu N, Watkins S C, Schaffer P A, DeLuca N A. Prolonged gene expression and cell survival after infection by a herpes simplex virus mutant defective in the immediate-early genes encoding ICP4, ICP27, and ICP22. J Virol. 1996;70:6358–6369. doi: 10.1128/jvi.70.9.6358-6369.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu X X, Chen J X, Young C S, Silverstein S. Reactivation of latent herpes simplex virus by adenovirus recombinants encoding mutant IE-0 gene products. J Virol. 1990;64:4489–4498. doi: 10.1128/jvi.64.9.4489-4498.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu X X, Young C S, Silverstein S. Adenovirus vector expressing functional herpes simplex virus ICP0. J Virol. 1988;62:4544–4553. doi: 10.1128/jvi.62.12.4544-4553.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]