Abstract
Aim:
This study aimed to evaluate the expression of tcdA, tcdB, and binary toxin genes (cdtA and cdtB) by Real-Time PCR and molecular typing of Clostridioides difficile isolated from patient diarrhea samples from Hamadan Hospitals, west of Iran.
Background:
The concentration of C. difficile toxins (CDTs) is associated with the severity of the disease and the mortality rate. Measuring CDT levels could provide a reliable and objective means of determining the severity of C. difficile infection (CDI).
Methods:
From November 2018 to September 2019, 130 diarrhea samples were collected from hospitalized patients in three hospitals in Hamadan. C. difficle isolates were detected by culture and PCR. The presence of the genes encoding the toxin was identified by PCR, whereas the measurement of toxin expression was conducted using a relative Real-Time PCR technique. Genetic linkage of the isolates was also assessed by Ribotyping and Repetitive Extragenic Palindromic (rep-PCR) methods.
Results:
Among 130 diarrhea samples, 16 (12.3%) were positive for C. difficile. Genes encoding cdtA and tcdB were detected in all isolates, and 8 (50%) and 6 (37.5%) isolates were positive for the cdtA and cdtB genes. Real-time PCR results showed different expression levels of the toxin genes. A significant increase in the expression of the tcdA gene was observed compared with the control strain (P<0.05). Besides, more expression of cdtA gene was observed in the strains compared with cdtB gene. Ribotyping and rep-PCR results showed high genetic diversity of C. difficile among hospitals investigated.
Conclusion:
We encountered toxigenic C. difficile strains with various toxin expression levels, ribotypes, and rep types based on the findings of this study. This indicated that various clones from various sources circulate in the hospitals and among patients.
Key Words: Clostridioides difficile, Gene expression, Real-Time PCR, Molecular typing
Introduction
Clostridioides difficile is a Gram-positive, obligate anaerobic spore-forming bacterium that was identified as an important human pathogen, especially in hospitalized patients. C. difficle infection (CDI) is primarily associated with severe diarrhea followed by antibiotic use (1, 2). Toxigenic C. difficile strains produce two major exotoxins called toxin A (enterotoxin) and toxin B (cytotoxin). They are principally involved in the pathophysiology of CDI (2). The main symptoms of CDI are diarrhea, inflammation, and tissue necrosis, which are caused by the toxins' complicated cascade of cellular reactions in the host. It is difficult to comprehend the factors that contributed to the epidemic of some C. difficile strains. Debilitating recurrent infections are common. For accurate epidemiological research, as well as the most effective strategies for management and prevention, toxins should be detected for diagnosis (3, 4). The level of C. difficile toxins (CDTs) in feces is linked to disease severity and mortality rate. It may be possible to accurately and objectively define the severity of CDI by measuring CDT levels (5). Consequently, it is possible to predict the pathogenicity of C. difficile toxins by measuring their gene expression levels using a molecular technique like Real-Time PCR. Although Real-time PCR was used in the majority of studies to directly detect C. difficile from stool samples (6-8). Molecular typing methods are highly beneficial for epidemiological studies and bacterial genetic associations due to the paramount significance of regulating bacterial infections, particularly nosocomial infections (9). Various molecular typing methods were used in epidemiological studies to study genetic diversity and genetic relatedness and find the origin of CDI (10-12). Among the molecular methods of bacterial typing, PCR ribotyping is considered the standard method for molecular typing of C. difficile (10, 11, and 13). Most studies in Iran have focused on PCR-based ribotyping (13-15). Rep-PCR is a PCR-based typing technology that generates fingerprints directly, without the need for the endonuclease enzyme. Since rep-PCR is an affordable and rapid method; it is cost-effective and can be used to molecular typing of C. difficile strains (16-17). As one of the main goals of this study was the molecular typing of C. difficile strains, it was necessary to compare different genotypes based on antibiotic resistance patterns and toxin profiles; therefore, we used the results of our previous study, which determined antibiotic resistance patterns and toxin profiles of C. difficile strains (18). This study aimed to assess the gene expression levels of tcdA and tcdB genes, as well as the binary toxin genes of C. difficile, using Real-time PCR assay. Furthermore, the study aimed to analyze the genetic diversity of C. difficile strains via rep-PCR and Ribotyping.
Methods
Identification of C. difficile isolates
In a previous cross-sectional study, we isolated 16 strains of C. difficile from 130 diarrheal samples collected from patients hospitalized in Hamadan hospitals in western Iran between November 2018 and September 2019 (18). C. difficile strains were identified and confirmed by microbiological tests and PCR technique. C. difficile colonies were maintained in cooked meat broth at 4 ⁰C (18). The antimicrobial susceptibility to vancomoycin, metronidazole, and clindamycin was assessed using the agar dilution method in accordance with the CLSI and EUCAST guidelines in the preceding study. The frequency of tcdA, tcdB, cdtA and cdtB genes was detected by PCR technique in our previous study (18). All data for identification, antimicrobial susceptibility and toxin genes frequencies of were presented in ref. 18 in this study (18).
DNA extraction and PCR
Genomic DNAs were extracted from C. difficile colonies on CCFA plates using a commercial DNA extraction kit (Qiagen, Hilden, Germany). All C. difficile isolates were subjected to the rep-PCR typing and Ribotyping.
RNA Extraction and cDNA synthesis
All C. difficile strains were subjected to Real-time PCR (RT-PCR) to determine toxin genes expression levels. Total RNA was extracted using an RNA extraction kit (SinaClon, Iran), and cDNA was subsequently created using a cDNA synthesis kit (Yekta Tajhiz Azma, Iran), following the manufacturer's instructions.
Real-Time PCR reaction
Real-time quantification of cDNA was performed with the detection system (Roche, Germany) using the SYBR Green PCR Master Mix. The optimized reaction consisted of a Master Mix (10X), 0.4 µl of each primer of toxin genes (10 pmol) as previously described (19, 20), 2 µl of cDNA (100 μg/ml), and 7.2 µl of DEPC-water in a total volume of 20 µl. Toxin genes expression levels (fold changes) of all genes were calculated using the 2−ΔΔCt method (21). The expression levels of toxin-encoding genes were compared to the expression levels of these genes in standard positive control strains of C. difficile. C. difficile strain VPI 10463 was used as a positive control for the tcdA and tcdB genes and C. difficile strain RIGLD 141 was used as a positive control for the cdtA and cdtB genes. The real-time PCR procedure was programmed as follows. Initial denaturation at (95°C for 15 min), followed by 40 cycles at (95°C for 5 sec), (60°C for 34 sec), (72°C for 30 sec), and Melting curve at (60°C for 1 min) and (95°C for 15 sec).
rep-PCR typing
Diversity and genetic linkage among C. difficile isolates were assessed by rep-PCR using the primer REP-F: 5’-ICGICTTATCIGGCCTAC-3’ and REP-R: 5’-IIIICGICGICATCIGGC -3’ (16), according to the following protocol: Initial denaturation (95°C for 2 min) followed by 45 cycles of denaturation (95°C for 30 sec), annealing (38°C for 1 min), extension (72°C for 2 min), and a final cycle of extension at 72°C for 16 min. The PCR products were electrophoresed on a 1% agarose gel at a voltage of 70 V for duration of 1 hour. The resulting band patterns were captured using a gel documentation system (16). The observed band patterns were analyzed and grouped together using BioNumeric software version 7.1 (Applied Maths, Belgium).
Ribotyping
To determine the ribotypes of C. difficile isolates, PCR was performed using specific primers (P3: 5ʹ-CTGGGGTGAAGT CGTAACAAG-3ʹ and P5: 5ʹ-GCGCCCTTTGTAGCTTGACC-3ʹ) according to the following program: Initial denaturation (95°C for 2 min) followed by 35 cycles of denaturation (94°C for 60 s), annealing (55°C for 30 sec), extension (72 °C for 90 s), and a final cycle of extension at 72°C for 10 min (22). Different profiles or ribotypes of C. difficile strains were analyzed using BioNumeric software version 7.1 (Applied Maths).
Statistical analysis
Statistical analysis was conducted using SPSS software, version 21 for Windows (SPSS Inc., Chicago, IL, USA). A p-value of <0.05 was deemed statistically significant.
Results
Our study results showed that out of 130 stool samples, 16 samples (12.3.6%) were positive for C. difficile. All C. difficile isolates (100%) carried both tcdA and tcdB genes (tcdA+/tcdB+) and were considered toxin producing strains. Binary toxin genes (cdtA and cdtB) were detected in 6 (37.5%) and 8 (50%) isolates, respectively. Co-presence of the cdtA and cdtB genes was not observed in any isolates.
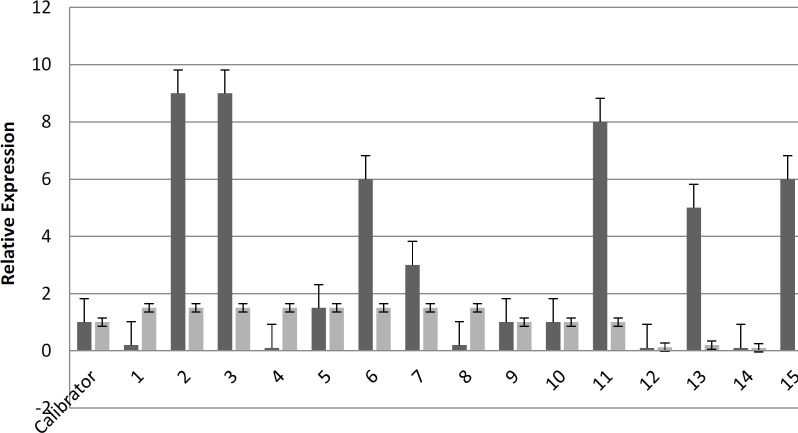
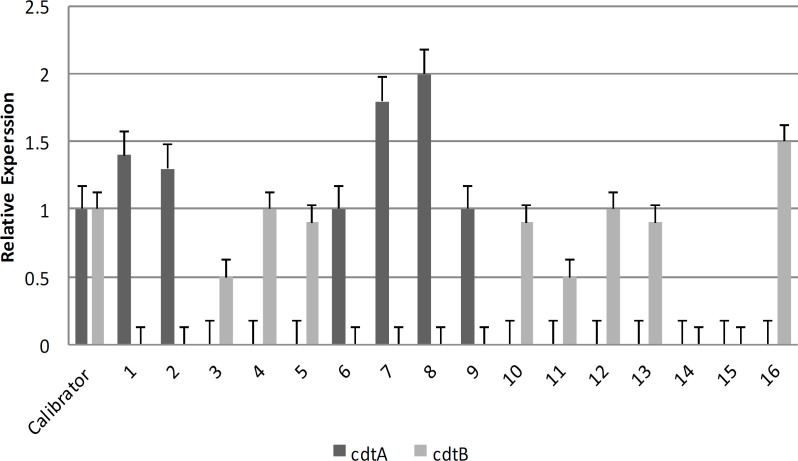
The results of Real-Time PCR showed that various gene expression levels were observed. Based on real-time PCR, increased expression was mainly observed in the tcdA gene. Out of the eight isolates, 43.7% exhibited elevated levels of tcdA gene expression compared to the control strain. Five isolates exhibited reduced tcdB gene expression compared to the reference strain, but there was no indication of an upregulation in tcdB gene expression (Figure 1). Therefore, based on Real-time PCR results of the binary toxin genes (Figure 2), an increased cdtA gene expression was observed in 6 (37.5 %) strains. Only one strain had higher expression of the cdtB gene compared to the control strain.
Figure 1.
Comparison of tcdA and tcdB gene expression levels in C. difficile isolated from diarrhea samples of patients in Hamadan hospitals. (Calibrator: strain VPI 10463 as a positive control strain for the tcdA and tcdB genes)
Figure 2.
Comparison of cdtA and cdtB gene expression levels in C. difficile isolated from diarrhea samples of patients in Hamadan hospitals (Calibrator: strain RIGLD 141 as a positive control strain for the cdtA and cdtB genes)
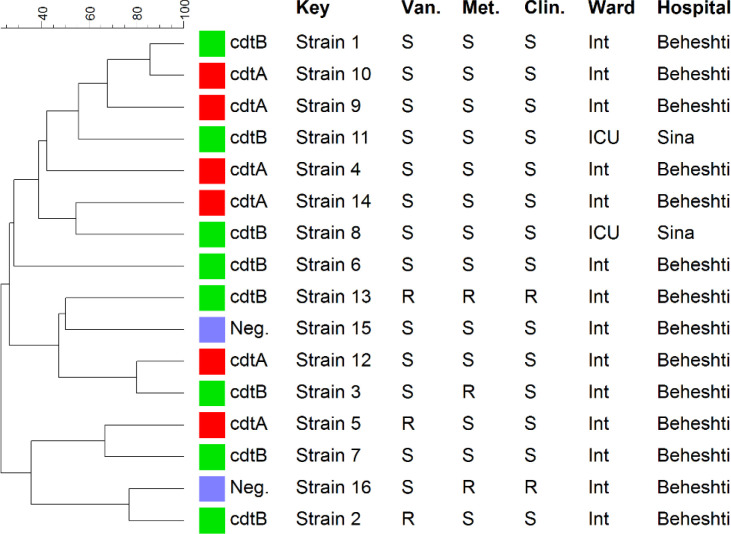
In every strain of C. difficilis, ribotyping analysis showed distinct banding patterns. Figure 3 depicts the sixteen distinct profiles that were observed. However, some of these strains were isolated from the same hospital and wards and shared the same resistance pattern and toxins. Toxin profiles and patterns of resistance in ribotypes did not significantly correlate. This study's molecular typing analysis reveals that ribotyping is more effective at distinguishing C. difficile strains. The ribotyping method had a higher discriminatory power than rep-PCR method when online formula for calculating discriminatory power was used (http://insilico.ehu.es/mini_tools/discriminatory_power/index.php). The discriminatory power of rep-PCR was 0.97, while that of ribotyping was 1.0.
Figure 3.
Characterization of 16 C. difficile strains isolated from diarrhea samples of patients in Hamadan hospitals by ribotype, binary toxin pattern, antibiotic resistance pattern, hospital and ward (all strains are tcdA+/tcdB+)
Neg: negative, Int: internal, ICU: Intensive care unit, Van: vancomycin, Met: Metronidazole, Clin: clindamycin, S: sensitive, R: resistant
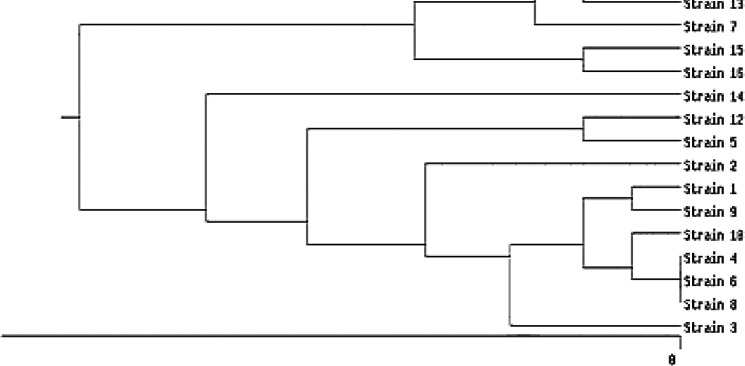
Based on band samples obtained by the electrophoresis of PCR products of REP regions in different C. difficile isolates, the size of the fragments varied from 500 bp to 2000 bp. It was found that there was high genetic diversity in C. difficile strains (Figure 4), so more than 80% of the isolates were classified into different types or categories. Based on rep-PCR analysis, only three isolates were categorized into a shared class, whereas 14 distinct kinds were identified using REP sample profiles. In fact, three isolates could not be differentiated by rep-PCR and to better identify them, more powerful molecular or sequencing-based methods are needed. All 16 isolates were similar in their tcdA and tcdB toxin gene profiles but differed in their binary toxin gene profiles and antibiotic resistance patterns (Figure 3). There was no significant correlation between REP types with resistance profile and toxin profile (P>0.05).
Figure 4.
Dendrogram of rep-PCR patterns of 16 C. difficile strains isolated from diarrhea samples of patients at Hamadan hospitals. The numbers of isolates, binary toxin patterns, antibiotic resistance patterns, hospitals, and wards are the same in Figures 3 and 4. No rep-PCR products were detected in isolates number 4, 6, and 8, thus clustering them into a common type.
Discussion
This study found that 12% of hospitalized patients in Hamadan hospitals were at risk for CDI. The fact that all C. difficile strains were toxin-producing or toxinogenic is a key finding. All C. difficile isolates tested were positive for the tcdA and tcdB. Some C. difficile strains contained genes encoding the binary toxins cdtA and cdtB. Real-Time PCR findings indicated varying expression levels of the toxin genes. A notable rise in tcdA gene expression was noted compared to the control strain (P<0.05). Furthermore, the strains exhibited a greater level of expression of the cdtA gene compared to the cdtB gene. The ribotyping and rep-PCR data demonstrated a significant genetic variability of C. difficile across the hospitals examined. Multiple prevalence reports of C. difficile infection (CDI) have been recorded in different geographic areas (23-27). Our study's CDI prevalence was lower than that of other Iranian studies (28-30). Variation in CDI prevalence may be affected by the subject population, C. difficile detection methods, and hospital infection control strategies (18).
C. difficile strains were more resistant to vancomycin and metronidazole in our study, but less to clindamycin. In Iran and other regions, numerous reports of C. difficile antibiotic resistance were published (15, 24- 32). More than 50% and 30% of C. difficile strains isolated from Tehran hospitals were found to be resistant to metronidazole in a recently published Iranian study (31).
The results of our study demonstrate the distribution of different ribotypes of C. difficile strains in hospitals in Hamadan. C. difficile ribotype diversity was high, but no predominant ribotype was found (Figure 2). This study's findings are consistent with those of previous studies carried out in Iran and other countries (13-15, 33- 35). In this study, we were compelled to conduct ribotyping for C. difficile strains using P3 and P3 primers and standard gel agarose electrophoresis due to restrictions and a lack of facilities to transport ribotyping-PCR products abroad for capillary electrophoresis. Unfortunately, it was not possible to compare the ribotype patterns with known reference strains. However, based on the results of ribotyping by capillary electrophoresis, distinct ribotypes were identified in hospitals in Tehran and Isfahan (13, 36). From 2004 to 2018, ribotypes 001, 0126, and 084 were found to be the most prevalent in Tehran in a study by Azimirad et al (13). Kuhsari et al.'s study from Tehran found that in three tertiary care hospitals in Tehran, ribotypes 039, AI-12, and AI-21 were found to be the predominant ribotypes among clinical and non-clinical C. diff isolates (36). According to a research conducted at one of Isfahan's teaching hospitals, the most prevalent ribotype among C. difficile strains recovered from stool samples of patients who experienced diarrhea was ribptype 078. (37). One case of the highly virulent ribotype (027) in children younger than five years old was reported from Iran (38). The tcdA/tcdB toxin profiles of all ribotypes in our study were comparable, but their patterns of antibiotic resistance and cdtA/cdtB profiles were distinct. Moreover, it is possible that the environment provided distinct conditions for the relevant C. difficile strains such as endogenous infection and spread of strains from outside and inside the hospital. Relatively effective infection control strategies appear to be responsible for the lack of spread of the dominant clones of C. difficile among patients in different hospital wards. C. difficile strains isolated from hospitalized patients in the wards did not come from the same source because they had distinct ribotypes in the studied hospitals.
The research used the rep-PCR approach in addition to ribotyping to identify the strains of C. difficile. Both of these methods rely on PCR. There are currently no reports from Iran about the molecular typing of C. difficile using the rep-PCR approach. More than 80% of the isolates in this study were classified as belonging to one of several types or categories, and only three were classified as belonging to a single type, indicating a high genetic diversity among C. difficile isolates.
Our findings showed that rep-PCR methods were less effective at discrimination than ribotyping. Ribotyping identified 16 different ribotypes and rep-PCR identified 14 different types. Some epidemiological studies have used rep-PCR for molecular typing of C. difficile strains. Russello et al. studied the molecular characterization of C. difficile-associated diarrhea using rep-PCR in Italy. Their results indicated that rep-PCR could be used as a reliable method for molecular typing of C. difficile and as a useful method for better infection control in hospitals (39). Rahmati et al. conducted a research in which they used three REP-PCR-based approaches, including rep-PCR, ERIC-PCR, and the BOX method, to discriminate fifty C. difficile strains with ribotype 001 from the UK. Analysis of the results showed that all methods exhibited satisfactory levels of performance and reproducibility. However, rep-PCR is more specific and provides more information about the epidemiology of C. difficile disease in UK hospitals (40).
For the purpose of typing 205 C. difficile isolates, the researchers in Finland compared an automated rep-PCR method, PCR ribotyping, and pulsed-field gel electrophoresis (PFGE). In the local clinical microbiology labs, the automated rep-PCR-based typing method is an option for first-line molecular typing. Because this method was faster and easier to use than PCR ribotyping or PFGE typing, it required less hands-on time. However, there are differences between their rep-PCR method and ours. In their investigation, the researchers used microfluidics lab-on-a-chip technology to detect the rep-PCR products and then separate the amplicons. Hence, it is essential to enhance and adapt fundamental molecular typing techniques to enhance results (41).There were differences in the expression levels of toxin genes according to the Real-Time PCR results in this study. Some toxin genes were either not expressed at all in some strains or were expressed differently in others. The highest expression levels were observed in the expression of the tcdA gene. In 43.7% of isolates, tcdA gene expression was higher than control strains or calibrators. It is suggested that the mechanisms affecting the expression of these genes to be further investigated in terms of the difference in the expression of various toxin genes in C. difficile strains.
In Iran, there haven't been any Real-Time PCR studies on the levels of tcdA, tcdB, cdtA, and cdtB gene expression. Real-time PCR method, which is mostly used for diagnostic purposes, was used in numerous studies to directly detect C. difficile from stool samples (7, 8, 42). Following the confirmation of the samples by PCR and culture, Real-Time PCR was used in our investigation to examine the expression levels of toxin genes in different strains of C. difficile. Real-Time PCR confirmed the presence of C. difficile colonies in our investigation, and various strains displayed varying levels of expression. The studies that have utilized the Real-Time PCR method in Iran and other nations are the subject of the discussion that follows.
Luna et al. used Real-Time PCR at Texas Children's Hospital to diagnose CDI. Real-Time PCR, conducted directly on feces samples, demonstrated an ideal performance with a sensitivity of 95%, specificity of 100%, positive predictive value of 100%, and negative predictive value of 99% (7). Kim et al. conducted a study in which a colony of toxigenic C. difficile strains (two distinct strains with tcdA+/tcdB-, tcdA+/tcdB- profiles) was used as a standard for the diagnosis of CDI in stools in South Korea using Real-Time PCR method. The device-defined cut-offs were used to automatically interpret the results (43). Real-time PCR was used to test how quickly C. difficile could be found in feces, based on Belanger et al. They came to the conclusion that because measuring the cytotoxin in tissue cultures is the gold standard for identifying C. difficile and detecting toxicogenic C. difficile, it takes a long time and requires 24 hours of incubation. Consequently, they chose Real-Time PCR because it is fast, specific, and sensitive. Real-Time PCR can also directly identify C. difficile in feces (19).
Song et al. used Real-Time PCR as a quick diagnostic method for C. difficile infection. Out of 207 samples, 117 cases were found to have CDI. Real-Time PCR, toxin assay, and tissue culture were all 87.2%, 48.7%, and 65% sensitive, respectively. Their findings indicate that Real-Time PCR is a very effective method for properly and rapidly diagnosing CDI. Furthermore, their study demonstrates that Real-Time PCR is the optimal approach for accurately and swiftly diagnosing CDI and identifying toxin-producing genes (44). Because this was the first study in the west of Iran to compare the level of toxin gene expression and molecular typing of C. difficile isolates, we encountered a number of challenges and limitations, including low hospital cooperation in providing samples and financial constraints brought on by economic sanctions. Antimicrobial susceptibility selective culture media, supplements, and antibiotic powders were required for the isolation and identification of C. difficile strains. In most cases, we were unable to obtain these materials, which presented numerous challenges and prevented us from collecting additional samples, and examining additional strains. Even sending ribotyping results to capillary electrophoresis was impossible.
Conclusion
Based on the results of molecular typing of C. difficile strains, different C. difficile clones circulating in Hamadan hospital and CDI may be in terms of the acquisition of different endogenous or environmental pathogenic strains. Besides, strains differed from each other based on the expression of toxin genes. Further studies are recommended for factors or mechanisms that affect the expression level of C. difficile toxins. In order to obtain more precise and cohesive results, it is advised to employ a variety of molecular typing methodologies and to examine additional strains of C. difficile that have been collected from a variety of regions.
Acknowledgements
We extend our gratitude to all the individuals at the Foodborne and Waterborne Disease Research Center, Research Institute for Gastroenterology and Liver Disease, Shahid Beheshti University of Medical Sciences, Tehran, IRAN, for facilitating the subculture of our samples and confirming the C. difficile isolates. We also acknowledge the staff of the microbiology laboratory at Sina and Beheshti Hospitals in Hamadan.
Ethical approval
The present study was ethically approved by the Institutional Review Board of Hamadan University of Medical Sciences (IR.UMSHA.REC.1397.510).
Funding
This research has been supported by Vice Chancellor for Research & Technology of Hamadan University of Medical Sciences, Hamadan, IRAN (Grant no: 9708295113).
Conflict of interests
The authors declare that they have no competing interests.
References
- 1.Murray PR, Rosenthal KS, Pfaller MA. Medical microbiology E-book. Elsevier Health Sciences; 2020. [Google Scholar]
- 2.Mullish BH, Williams HR. Clostridium difficile infection and antibiotic-associated diarrhoea. Clin Med. 2018;18:237. doi: 10.7861/clinmedicine.18-3-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orrell KE, Melnyk RA. Large clostridial toxins: mechanisms and roles in disease. Microbiol Mol Biol Rev. 2021;85:00064–21. doi: 10.1128/MMBR.00064-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372:1539–48. doi: 10.1056/NEJMra1403772. [DOI] [PubMed] [Google Scholar]
- 5.Cohen NA, Miller T, Na’aminh W, Hod K, Adler A, Cohen D, et al. Clostridium difficile fecal toxin level is associated with disease severity and prognosis. United Eur Gastroenterol J. 2018;6:773–80. doi: 10.1177/2050640617750809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paitan Y, Miller-Roll T, Adler A. Comparative performance study of six commercial molecular assays for rapid detection of toxigenic Clostridium difficile. Clin Microbiol Infect. 2017;23:567–72. doi: 10.1016/j.cmi.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Luna RA, Boyanton Jr BL, Mehta S, Courtney EM, Webb CR, Revell PA, et al. Rapid stool-based diagnosis of Clostridium difficile infection by real-time PCR in a children's hospital. J Clin microbiol. 2011;49:851–7. doi: 10.1128/JCM.01983-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Jong E, De Jong AS, Bartels CJ, Van Der Rijt-Van Den Biggelaar C, Melchers WJ, Sturm PD. Clinical and laboratory evaluation of a real-time PCR for Clostridium difficile toxin A and B genes. Eur J Clin Microbiol Infect Dis. 2012;31:2219–25. doi: 10.1007/s10096-012-1558-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabat AJ, Budimir A, Nashev D, Sa-Leao R, van Dijl J, Laurent F, et al. Overview of molecular typing methods for outbreak detection and epidemiological surveillance. Euro Surveill. 2013;18:20380. doi: 10.2807/ese.18.04.20380-en. [DOI] [PubMed] [Google Scholar]
- 10.Liu XS, Li WG, Zhang WZ, Wu Y, Lu JX. Molecular Characterization of Clostridium difficile Isolates in China from 2010 to 2015. Front Microbiol. 2018;9:845. doi: 10.3389/fmicb.2018.00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen GL, Li SH, Qin Z, Yang YJ, Bai LX, Ge WB, Liu XW, Li JY. Isolation, molecular typing and antimicrobial resistance of Clostridium difficile in dogs and cats in Lanzhou city of Northwest China. Frot Vet Sci. 2022;9:1032945. doi: 10.3389/fvets.2022.1032945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei HL, Wei SH, Huang CW, Shih CH, Huang YW, Lu MC, et al. Molecular typing and epidemiology of Clostridium difficile in respiratory care wards of central Taiwan. J Microbiol Immunol Infect. 2015;48:65–71. doi: 10.1016/j.jmii.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 13.Azimirad M, Alebouyeh M, Rashidan M, Aslani MM, Zali MR. Comparison of common molecular typing methods for differentiation of Clostridium difficile strains in the study of hospital acquired diarrhea. Arch Clin Infect Dis. 2018:13. [Google Scholar]
- 14.Azimirad M, Krutova M, Yadegar A, Shahrokh S, Olfatifar M, Aghdaei HA, et al. Clostridioides difficile ribotypes 001 and 126 were predominant in Tehran healthcare settings from 2004 to 2018: a 14-year-long cross-sectional study. Emerg Microbes Infect. 2020;9:1432–1443. doi: 10.1080/22221751.2020.1780949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baghani A, Mesdaghinia A, Kuijper EJ, Aliramezani A, Talebi M, Douraghi M. High prevalence of Clostridiodes diffiicle PCR ribotypes 001 and 126 in Iran. Sci Rep. 2020;10:1–9. doi: 10.1038/s41598-020-61604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorkh MAG, Shokoohizadeh L, Rashidi N, Tajbakhsh E. Molecular analysis of Pseudomonas aeruginosastrains isolated from burn patients by REPetitive extragenic palindromic-PCR (REP-PCR) Iran Red Crescent Med J. 2017;19:43508. [Google Scholar]
- 17.Lin T, Lin L, Zhang F. Review on molecular typing methods of pathogens. Open J Med Microbiol. 2014;4:147. [Google Scholar]
- 18.Shokoohizadeh L, Alvandi F, Yadegar A, Azimirad M, Hashemi SH, Alikhani MY. Frequency of toxin genes and antibiotic resistance pattern of Clostridioides difficile isolates in diarrheal samples among hospitalized patients in Hamadan, Iran. Gastroenterol Hepatol Bed Bench. 2021;14:165. [PMC free article] [PubMed] [Google Scholar]
- 19.Bélanger SD, Boissinot M, Clairoux N, Picard FJ, Bergeron MG. Rapid detection of Clostridium difficile in feces by real-time PCR. J Clin Microbiol. 2003;41:730–4. doi: 10.1128/JCM.41.2.730-734.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Persson S, Torpdahl M, Olsen KE. New multiplex PCR method for the detection of Clostridium difficile toxin A (tcdA) and toxin B (tcdB) and the binary toxin (cdtA/cdtB) genes applied to a Danish strain collection. Clin Microbiol Infect. 2008;14:1057–64. doi: 10.1111/j.1469-0691.2008.02092.x. [DOI] [PubMed] [Google Scholar]
- 21.Pfaffl MW. Quantification strategies in real-time PCR. AZ of quantitative PCR. 2004;1:89–113. [Google Scholar]
- 22.Sisto F, Maraschini A, Fabio G, Serafino S, Zago M, Scaltrito MM, et al. Isolation and characterization of a new Clostridium difficile ribotype during a prospective study in a hospital in Italy. Curr Microbiology. 2015;70:151–3. doi: 10.1007/s00284-014-0697-2. [DOI] [PubMed] [Google Scholar]
- 23.Curcio D, Cané A, Fernández FA, Correa J. Clostridium difficile-associated diarrhea in developing countries: A systematic review and meta-analysis. Infect Dis Ther. 2019;8:87–103. doi: 10.1007/s40121-019-0231-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forrester JD, Cai LZ, Mbanje C, Rinderknecht TN, Wren SM. Clostridium difficile infection in low and middle human development index countries: a systematic review. Trop Med Int Health. 2017;22:1223–32. doi: 10.1111/tmi.12937. [DOI] [PubMed] [Google Scholar]
- 25.Borren NZ, Ghadermarzi S, Hutfless S, Ananthakrishnan AN. The emergence of Clostridium difficile infection in Asia: A systematic review and meta-analysis of incidence and impact. PloS One. 2017;12:0176797. doi: 10.1371/journal.pone.0176797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malekzadegan Y, Halaji M, Hasannejad-Bibalan M, Jalalifar S, Fathi J, Ebrahim-Saraie HS. Burden of clostridium (clostridioides) difficile infection among patients in western asia: a systematic review and meta-analysis. Iran J Public Health. 2019;48:1589. [PMC free article] [PubMed] [Google Scholar]
- 27.Ghasemi A, Mohabati Mobarez A, Mostafavi E. Antibiotic susceptibility profile of Clostridium Difficile bacteria isolated from older residents of a nursing home in Iran. Iran J Ageing. 2021;15:496–505. [Google Scholar]
- 28.Shoaei P, Shojaei H, Khorvash F, Hosseini SM, Ataei B, Tavakoli H, et al. Molecular epidemiology of Clostridium difficile infection in Iranian hospitals. Antimicrob Resist Infect Control. 2019;8:1–7. doi: 10.1186/s13756-018-0454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azimirad M, Krutova M, Balaii H, Kodori M, Shahrokh S, Azizi O, et al. Coexistence of Clostridioides difficile and Staphylococcus aureus in gut of Iranian outpatients with underlying inflammatory bowel disease. Anaerobe. 2020;61:102113. doi: 10.1016/j.anaerobe.2019.102113. [DOI] [PubMed] [Google Scholar]
- 30.Alimolaei M, Rahimi HR, Ezatkhah M, Bafti MS, Afzali S. Prevalence, characteristics and antimicrobial susceptibility patterns of Clostridioides difficile isolated from hospitals in Iran. J Glob Antimicrob Resist. 2019;19:22–7. doi: 10.1016/j.jgar.2019.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Mohammadbeigi M, Safayi Delouyi Z, Mohammadzadeh N, Alaalmohadesin A, Taheri K, Edalati E, et al. Prevalence and antimicrobial susceptibility pattern of toxigenic Clostridium difficile strains isolated in Iran. Turk J Med Sci. 2019;49:384–91. doi: 10.3906/sag-1808-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spigaglia P. Recent advances in the understanding of antibiotic resistance in Clostridium difficile infection. Ther Adv Infect Dis. 2016;3:23–42. doi: 10.1177/2049936115622891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin D, Luo Y, Huang C, Cai J, Ye J, Zheng Y, et al. Molecular Epidemiology of Clostridium difficile Infection in Hospitalized Patients in Eastern China. J Clin Microbiol. 2017;55:801–10. doi: 10.1128/JCM.01898-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Rossen TM, van Prehn J, Koek A, Jonges M, van Houdt R, van Mansfeld R, et al. Simultaneous detection and ribotyping of Clostridioides difficile, and toxin gene detection directly on fecal samples. Antimicrob Resist Infect Control. 2021;10:1–9. doi: 10.1186/s13756-020-00881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Lee K, Rajyaguru U, Jones CH, Janezic S, Rupnik M, et al. Ribotype classification of Clostridioides difficile isolates is not predictive of the amino acid sequence diversity of the toxin virulence factors TcdA and TcdB. Front Microbiol. 2020;11:1310. doi: 10.3389/fmicb.2020.01310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kouhsari E, Douraghi M, Fakhre Yaseri H, Talebi M, Ahmadi A, Sholeh M, et al. Molecular typing of Clostridioides difficile isolates from clinical and non-clinical samples in Iran. APMIS. 2019;127:222–227. doi: 10.1111/apm.12937. [DOI] [PubMed] [Google Scholar]
- 37.Jalali M, Khorvash F, Warriner K, Weese JS. Clostridium difficile infection in an Iranian hospital. BMC Res Notes. 2012;5:159. doi: 10.1186/1756-0500-5-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khoshdel A, Habibian R, Parvin N, Doosti A, Famouri F, Eshraghi A, et al. Molecular characterization of nosocomial Clostridium difficile infection in pediatric ward in Iran. Springerplus. 2015;4:627. doi: 10.1186/s40064-015-1268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russello G, Russo A, Sisto F, Scaltrito MM, Farina C. Laboratory diagnosis of Clostridium difficile associated diarrhoea and molecular characterization of clinical isolates. New Microbiologica. 2012;35:307–16. [PubMed] [Google Scholar]
- 40.Rahmati A, Gal M, Northey G, Brazier J. Subtyping of Clostridium difficile polymerase chain reaction (PCR) ribotype 001 by REPetitive extragenic palindromic PCR genomic fingerprinting. J Hosp Infect. 2005;60:56–60. doi: 10.1016/j.jhin.2004.09.034. [DOI] [PubMed] [Google Scholar]
- 41.Pasanen T, Kotila SM, Horsma J, Virolainen A, Jalava J, Ibrahem S, et al. Comparison of repetitive extragenic palindromic sequence based PCR with PCR ribotyping and pulsed field gel electrophoresis in studying the clonality of Clostridium difficile. Clin Microbiol Infect. 2011;17:166–75. doi: 10.1111/j.1469-0691.2010.03221.x. [DOI] [PubMed] [Google Scholar]
- 42.Song PH, Min JH, Kim YS, Jo SY, Kim EJ, Lee KJ, et al. Rapid and accurate diagnosis of Clostridium difficile infection by real-time polymerase chain reaction. Intest Rese. 2018;16:109–15. doi: 10.5217/ir.2018.16.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim H, Jeong SH, Kim M, Lee Y, Lee K. Detection of Clostridium difficile toxin A/B genes by multiplex real-time PCR for the diagnosis of C. difficile infection. J Med Microbiol. 2012;61:274–7. doi: 10.1099/jmm.0.035618-0. [DOI] [PubMed] [Google Scholar]
- 44.Song PH, Min JH, Kim YS, Jo SY, Kim EJ, Lee KJ, et al. Rapid and accurate diagnosis of Clostridium difficile infection by real-time polymerase chain reaction. Intest Res. 2018;16:109–15. doi: 10.5217/ir.2018.16.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]