Abstract
Following brain infection, the Challenge Virus Standard strain of rabies virus infects the retina. Rabies virus ocular infection induces the infiltration of neutrophils and predominantly T cells into the eye. The role of tumor necrosis factor alpha (TNF-α)-lymphotoxin signaling in the control of rabies virus ocular infection and inflammatory cell infiltration was assessed using mice lacking the p55 TNF-α receptor (p55TNFR−/− mice). The incidence of ocular disease and the intensity of retinal infection were greater in p55TNFR−/− mice than in C57BL/6 mice: the aggravation correlated with less neutrophil and T-cell infiltration. This indicates that cellular infiltration is under the control of the p55 TNF-α receptor and suggests that inflammatory cells may protect the eye against rabies virus ocular infection. The role of T cells following rabies virus ocular disease was assessed by comparison of rabies virus infection in nude mice with their normal counterparts. Indeed, the incidence and severity of the rabies virus ocular disease were higher in athymic nude mice than in BALB/c mice, indicating that T lymphocytes are protective during rabies virus ocular infection. Moreover, few T cells and neutrophils underwent apoptosis in rabies virus-infected retina. Altogether, these data suggest that T lymphocytes and neutrophils are able to enter the eye, escape the immune privilege status, and limit rabies virus ocular disease. In conclusion, rabies virus-mediated eye disease provides a new model for studying mechanisms regulating immune privilege during viral infection.
The eye has been described as an immunologically privileged site because it can accept grafts from allogeneic hosts. This immune privilege (29, 30) results from a combination of sequestration of autoantigen behind the blood-ocular barrier, induction of immune deviation phenomena (19, 20), presence of anti-inflammatory molecules in the eye (1, 7, 31–35, 39), and restriction of T-cell infiltration by the expression of Fas ligand (FasL) by ocular cells (8, 11–13). However, ocular viral infections induce an inflammatory response, including the recruitment and activation of T lymphocytes. This immune response, aimed at the clearance of the pathogen, may lead to either the protection or the destruction of the eye structure. This dual role is well illustrated by ocular diseases induced by infection with herpes simplex virus (HSV). Neutrophils and CD4+ T lymphocytes are key mediators of the immunopathological keratitis induced by corneal infection with HSV type 1 (HSV-1) (6, 36), whereas T lymphocytes protect the retina of the eye during acute retinal necrosis following HSV-1 injection (2). Thus, the mechanisms modulating an inflammatory reaction in the eye may depend on the site of viral replication, and they remain poorly defined.
Rabies virus induces an ocular disease in mice and humans (14; S. Camelo et al., submitted for publication). After infection in the hind limbs, the neurotropic Challenge Virus Standard (CVS) strain of rabies virus travels along the spinal cord and spreads to the brain and then to the eye. Viral invasion through the optic nerve leads to infection of the retinal ganglion cell (RGC) from day 6 postinfection (p.i.), but not of the photoreceptors. Rabies virus also infects the trigeminal ganglia, resulting in infection of the cornea by 12 days p.i. in mice (Camelo et al., submitted) and also in humans (17, 40). Morphological study of humans (14) and terminal deoxynucleotidyltransferase-mediated dUTP-tetramethylrhodamine-conjugated nick end labeling (TUNEL) staining of mice (Camelo et al., submitted) indicate that rabies virus ocular infection induces little apoptosis of the RGC despite morphological alteration and apoptosis of uninfected photoreceptors.
Tumor necrosis factor alpha (TNF-α) is involved in the immune response during CVS acute encephalitis (5). In this article, we address the role of the immune response and of TNF-α in the control of rabies virus infection in the eye. We used immunocytochemistry to describe the infiltration of inflammatory cells in the retinas of rabies virus-infected mice. Using mice lacking p55TNFR, the p55 TNF-α receptor (p55TNFR−/− mice), athymic nude mice, and wild-type BALB/c and C57BL/6 mice, we assessed the role of signaling through p55TNFR and of infiltrating T lymphocytes in the outcome of rabies virus ocular disease. Finally, the sensitivity of inflammatory cells to undergo apoptosis was assessed by a combination of immunocytochemistry and TUNEL assays on frozen eye sections.
Susceptibility to rabies ocular pathology and cellular infiltration are under the control of p55TNFR. Rabies virus-induced ocular disease was more severe in nude mice than in BALB/c mice, and only rare T lymphocytes in the eyes of CVS-infected mice were apoptotic. Therefore, despite the privileged immune status of the eye, T lymphocytes are able to enter the eye and are required to limit rabies virus ocular disease. Rabies virus-mediated eye disease is a promising model for elucidation of the mechanisms regulating the antiviral response in an immunologically privileged site.
MATERIALS AND METHODS
Virus.
The rabies laboratory strain Challenge Virus Standard (CVS), which was obtained from the American Type Culture Collection, Manassas, Va. (stock no. Vr959), was propagated in BSR cells, a baby hamster kidney (BHK-21)-derived cell line. The cell culture supernatant was used as the inoculum.
Mice, infection, and assessment of clinical symptoms.
Experiments were performed with 6-week-old female BALB/c mice (H-2d, I-E+), athymic nude mice, and C57BL/6 mice (H-2b, I-E−) from Janvier (St. Berthevin, France). TNF-α receptor p55-deficient mice (p55TNFR−/−) (H-2b, I-E) (27) were kindly provided by Werner Lesslauer of Hoffman-Laroche Ltd. and were backcrossed to a C57BL/6 genetic background. Mice were bred at the Pasteur Institute under specific-pathogen-free conditions in filter top cages. Mice were kept on a 12-h light-dark cycle. Mice were injected (100 μl) (day 0) intramuscularly (i.m.) in both hind legs with 107 infectious particles of rabies virus diluted in RPMI 1640 medium without l-glutamine and with 1% gentamicin. Control mock-infected mice were injected by the same route with the same volume of RPMI 1640 medium and 1% gentamicin but without infectious particles. The ocular disease was characterized by signs of periocular inflammation, and a damage score was defined as follows: 0 = both eyes open, 0.5 = one eye closed, 1 = both eyes closed, 1.5 = inflammation over the eyelid of one eye, and 2 = inflammation over the eyelids of both eyes. Cumulative average disease scores reported in Table 1 correspond to the sum of the mean score for all mice in a group from day 1 p.i. through the end of the infection divided by the number of mice in each group.
TABLE 1.
Comparison of ocular disease in C57BL/6, p55TNFR−/−, BALB/c, and nude mice
| Mice | n | Day of onset (mean ± SD)a | Day of relapse (mean ± SD) | Cumulative average disease scorec |
|---|---|---|---|---|
| C57BL/6 | 20 | 7.4 ± 0.8 | 9.35 ± 1.34 | 7.55 |
| p55 TNFR−/− | 9 | 6.77 ± 0.78 | —b | 14 |
| BALB/c | 19 | 5.92 ± 1.16 | 10 ± 1.16 | 6.94 |
| Nu/Nu | 8 | 7.125 ± 0.6 | — | 13.375 |
The day of onset was the first day that clinical signs appeared.
—, absence of remission.
Cumulative average disease scores were calculated as follows: the sum of clinical scores from day 1 p.i. through the end of the infection was divided by the number of mice in each group (n).
Reagents.
Fluorescein isothiocyanate (FITC)-conjugated anti-rabies virus monoclonal antibody (MAb) nucleocapsid was purchased from Sanofi Diagnostics Pasteur (Marnes-la-Coquette, France). FITC-conjugated MAbs directed against TNP, CD3, CD11b, and Ly-6G (Gr-1) and purified MAbs directed against CD16/CD32 FcIIγ/III receptors (Fc block) that were used for immunocytochemistry were all obtained from PharMingen-Becton-Dickinson. PVA3 is an ammonium sulfate-purified MAb produced in the laboratory (21). Streptavidin-conjugated horseradish peroxidase was obtained from Amersham. Hypnorm was purchased from Janssen (Oxford, United Kingdom). RPMI 1640 medium was obtained from Gibco BRL, Life Technologies (Cergy-Pontoise, France). Phenylmethylsulfonyl fluoride and aprotinin were purchased from Sigma Chemical Co. (St. Louis, Mo.). ABTS (2,2′-azino-di-[3-ethylbenzthiazoline sulfonate]) and TUNEL reagents were purchased from Boehringer Biochemicals (Mannheim, Germany).
Preparation of nervous system samples for enzyme-linked immunosorbent assay (ELISA).
Mice were perfused with 50 ml of phosphate-buffered saline (PBS) under terminal anesthesia with Hypnorm. Eyes were dissected free of the cranium, homogenized in 0.5 ml of ice-cold RPMI 1640 supplemented with 1% bovine serum albumin (BSA)–1% gentamicin–1 mM phenylmethylsulfonyl fluoride–1 mM aprotinin, and centrifuged at 11,000 × g for 10 min at 5°C. Pellets and supernatants were collected separately and stored at −80°C until use.
Determination of rabies virus N protein by immunocapture ELISA.
Microtiter plates were coated by incubation overnight with 0.4 μg of the specific mouse MAb anti-N protein, PVA3, per ml in 0.05 M carbonate buffer, pH 9.6, at 4°C. Plates were washed with PBS-Tween and were blocked with 10% goat serum in PBS-Tween. Dilutions (1:10 and 1:30) of homogenized eye samples from infected and uninfected mice and serial dilutions of a recombinant N protein (gift from André Aubert, Virbac, France), used as a standard, were incubated for 2 h at 37°C. Plates were thoroughly washed with PBS-Tween and incubated with biotinylated anti-N protein PVA3 MAb, and bound antibody was then detected with streptavidin-conjugated horseradish peroxidase and ABTS. Concentrations were determined using the linear portion of the curve obtained with recombinant N protein standard and expressed in nanograms/milliliter after subtracting the background optical density of uninfected mouse brains.
Preparation of eye sections.
Eye tissue sections were prepared for immunostaining to detect apoptosis and cell infiltration. To prevent contamination with red blood cells, mice were perfused with PBS and then with 50 ml of 4% paraformaldehyde. Eyes were removed and incubated overnight in 4% phosphonoformic acid. Tissues were incubated for a further 24 h in 15% sucrose in PBS and were then snap frozen in nitrogen-cooled isopentane. Tissues were embedded in Tissue Tek O.C.T. compound. Cryostat sections (10 μm thick) were cut and mounted on slides ready for staining.
Immunofluorescence labeling.
For immunofluorescence labeling, 10-μm-thick cryostat sections of OCT-embedded samples were incubated with 10% fetal calf serum (FCS) in PBS overnight and then permeabilized with 3% FCS and 0.5% Triton-100 in PBS. The slides were incubated for 5 min at 4°C with Fc block to prevent nonspecific binding and then for 30 min at 37°C with FITC-coupled MAbs. The sections were washed by incubation overnight in 1% FCS in PBS and were then examined by fluorescence microscopy.
Detection of apoptosis by the TUNEL method.
DNA fragmentation was detected in eye sections by labeling the 3′ OH DNA terminus (TUNEL technique) as previously described (9). Eye sections mounted on slides were fixed in pure ethanol for 30 min at −20°C and dried. The sections were rehydrated and permeabilized by incubation with 10 μg of proteinase K/ml in PBS for 15 min at room temperature and were then washed twice in PBS. Eye sections were incubated for 30 min at 37°C with 25 μl per slot of the labeling preparation containing 12.5 U of terminal deoxynucleotidyltransferase, 2.5 mM CoCl2, 0.2 M potassium cacodylate, 25 mM Tris-HCl, and 0.05 nmol of tetramethylrhodamine-conjugated 6-dUTP. They were incubated for 15 min at room temperature in 4× saline sodium citrate buffer (30 mM trisodium citrate and 0.3 M NaCl) and were then processed for double immunostaining by following the protocol described above.
Image analysis.
Slides were examined using appropriate fluorescence filters on a Leica microscope. Images were processed using Adobe Photoshop software and were printed on a color printer (Epson Stylus Color 800).
Statistical analysis.
Data were analyzed by Student's t test. Results are expressed as means ± standard deviations (SD). A P value of <0.05 was considered significant.
RESULTS
p55TNFR modulates rabies virus ocular disease.
Rabies virus injected i.m. into the hind legs travels through the spinal cord, infects the brain, and induces ocular disease. Using p55TNFR−/− mice, we assessed the role of signaling through p55TNFR in the outcome of rabies virus ocular disease. The incidence of rabies virus ocular disease (Fig. 1A) and level of CVS infection in brains (Fig. 1B) and eyes of mock-infected mice (Fig. 1C), CVS-infected C57BL/6 mice (Fig. 1D), and CVS-infected p55TNFR−/− mice (Fig. 1E) were compared. Despite a similar invasion of brains in both types of mice (Fig. 1B), the incidence of rabies virus ocular disease was higher in p55TNFR−/− mice than in C57BL/6 mice (Fig. 1A and Table 1). Thus, the absence of p55TNFR accelerated the onset of ocular symptoms (mean day of 50% incidence was day 6 p.i. for p55TNFR−/− mice versus day 8 p.i. for C57BL/6 mice) and increased the percentage of mice with ocular disease between days 6 and 10 p.i. In contrast to C57BL/6 mice, no transient phase of remission around days 8 and 9 p.i. was observed for p55TNFR−/− mice. The ocular disease was more severe in p55TNFR−/− mice than in C57BL/6 mice, with cumulative mean disease scores (see Materials and Methods) of 14 for p55TNFR−/− mice and 7.55 for C57BL/6 mice (Table 1). Moreover, rabies virus antigens were distributed in the ganglion cell layer (GCL) and dendrites of RGC in the inner plexiform layer (IPL) of p55TNFR−/− mice (Fig. 1E), whereas in C57BL/6 mice (Fig. 1D), infection was strictly restricted to the GCL until day 10 p.i. This observation suggests that rabies virus spreads over the GCL to the inner nuclear layer (INL) in the retinas of p55TNFR−/− mice but not in C57BL/6 mice and that virus infection is less controlled in the absence of p55TNFR. These data indicate that expression of p55TNFR and the subsequent TNF-α signaling delay the onset of ocular symptoms and reduce the severity of rabies virus ocular infection.
FIG. 1.
Rabies virus ocular disease is more severe in p55TNFR−/− than in C57BL/6 mice. (A) Kinetics of noncumulative incidence of ocular disease from days 3 to 15 was compared for p55TNFR−/− mice (n = 10) and C57BL/6 mice (n = 12). (B) Rabies virus N-protein concentrations in homogenates of brains from p55TNFR−/− mice and C57BL/6 mice determined by ELISA between 3 and 8 days p.i. Each bar is the mean of duplicate determinations for two mice per time point. Error bars show the SD. Shown is the dissemination of rabies virus antigen detected by immunocytochemistry with PVA3 FITC-coupled MAb on retina slides from mock-infected mice (C), C57BL/6 CVS-infected mice (D), and p55TNFR−/− mice infected with CVS (E). Bars, 50 μm.
Leukocyte infiltration in eyes of CVS-infected mice.
To test whether rabies virus ocular infection is associated with the recruitment of cells of the immune system, frozen eye sections from mice infected with CVS or mock infected were stained with Hoechst blue. Nuclei of infiltrating cells were present in the eyes of CVS-infected mice (Fig. 2B) but not in those of mock-infected mice (Fig. 2A). To determine the nature of the infiltrating cells, we used MAbs specific for Gr-1 (neutrophils) and CD3 (T lymphocytes) to stain frozen slides of eye sections from CVS-infected mice (Fig. 1D, F, H, and J) and mock- infected controls (Fig. 2C, E, G, and I). Neutrophils were observed, mostly in the vitreous cavity (data not shown), the nerve fiber, the IPLs, INLs, and even the outer plexiform layers (OPLs) of the retina (Fig. 1D). T lymphocytes were also present in the retina (data not shown) and in the vitreous cavity (Fig. 2F), in the ciliary body (Fig. 2H), and in the cornea (Fig. 2J). Thus, 8 days after rabies virus infection, inflammatory cells including neutrophils and T lymphocytes were present not only in the GCL that was infected but also in the INL and OPL, which were not infected, and in the cornea and ciliary bodies, in which N protein was not detected at that time.
FIG. 2.
T-lymphocyte and neutrophil infiltration of the eyes of CVS-infected mice. The nature of infiltrating cells was determined in the vitreous humor, retina, and cornea of mock-infected and CVS-infected mice 10 days p.i. Inflammatory cells were detected by Hoechst blue staining in the IPL of the eyes from CVS-infected C57BL/6 mice (B) but not in those from mock-infected mice (A). Frozen eye sections obtained from mock-infected mice (C, E, G, and I) and CVS-infected mice (D, F, H, and J) were incubated in the presence of FITC-conjugated MAb specific for Gr-1 (C and D) or FITC-conjugated MAb specific for CD3 (E to J). Infiltrating neutrophils and T lymphocytes were located mainly in the GCLs, IPLs, and INLs of the retinas of CVS-infected mice (D), but T lymphocytes were also present in the vitreous humor (F), the ciliary body (H), and the cornea (J). VITR, vitreous cavity; OPL, outer plexiform layer; ONL, outer nuclear layer. Bars, 40 μm (A and B), 20 μm (C to F), and 100 μm (G to J).
Absence of p55TNFR reduces the number of neutrophils and T lymphocytes in the eyes of rabies virus-infected mice.
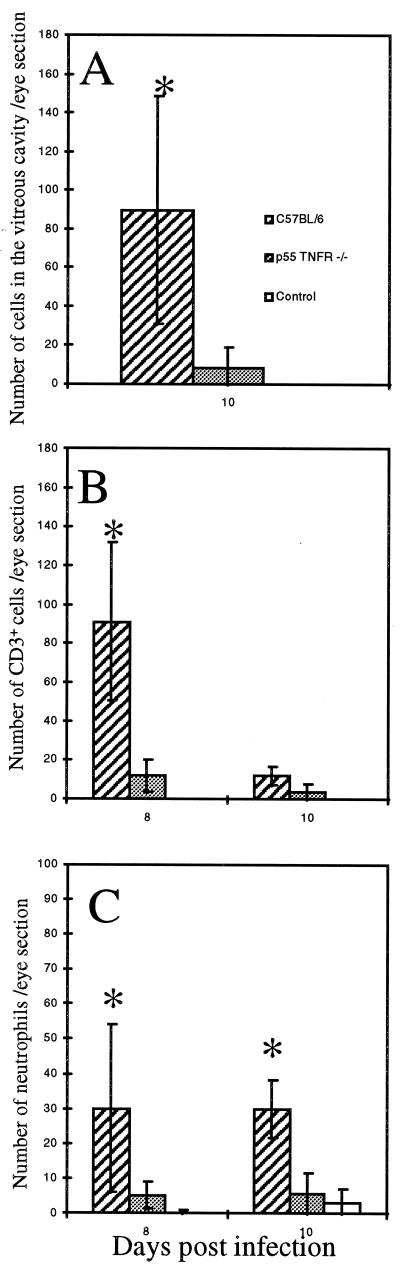
Since we previously observed that p55TNFR is involved in inflammatory-cell recruitment in the brains of CVS-infected mice (5), we compared the numbers of cells infiltrating the eyes of rabies virus-infected C57BL/6 and p55TNFR−/− mice. There were far fewer inflammatory cells in the vitreous cavities (including CD3+ T lymphocytes and Gr-1+ neutrophils; data not shown) of p55TNFR−/− mice than in those of C57BL/6 mice on day 10 p.i. (Fig. 3A; 89.7 ± 67.5 cells for C57BL/6 mice versus 8.2 ± 12.5 cells for p55TNFR−/− mice, P < 0.05). The number of CD3+ T lymphocytes per eye section, determined by immunofluorescence, was also lower for the eyes of p55TNFR−/− mice (Fig. 3B; 91 ± 49 cells for C57BL/6 mice versus 11.9 ± 8.7 cells for p55TNFR−/− mice on day 8 p.i. [P < 0.05] and 11.9 ± 6.2 cells for C57BL/6 mice versus 3.4 ± 4.8 cells for p55TNFR−/− mice on day 10 p.i. [P < 0.05]). There were also fewer neutrophils per eye section (Fig. 3C; 30 ± 27 cells for C57BL/6 mice versus 5.1 ± 4.9 cells for p55TNFR−/− mice on day 8 p.i. [P < 0.05] and 29.9 ± 11.3 cells for C57BL/6 mice versus 5.8 ± 8.9 cells for p55TNFR−/− mice on day 10 p.i. [P < 0.05]). Thus, there is less infiltration by inflammatory cells in the retinas, the ciliary bodies, the corneas, and the vitreous cavities of p55TNFR−/− mice than in those of C57BL/6 mice. Moreover, the number of infiltrating cells in the eyes of p55TNFR−/− mice was similar to that in the eyes of uninfected mice. These results indicate that p55TNFR is a major factor involved in the recruitment of inflammatory cells in the eyes. It also appears that neutrophils and T lymphocytes present in the eyes may protect against the induction of rabies virus-induced ocular disease.
FIG. 3.
There is less cellular infiltration of the eyes in p55TNFR−/− mice. The number and nature of infiltrating cells in frozen slides of eye tissue were compared between C57BL/6 and p55TNFR−/− mice. (A) Mean cell number in the vitreous humor of C57BL/6 and p55TNFR−/− mice 10 days p.i. and uninfected mice (n = 3 per day and per group). (B and C) Number of neutrophils (B) and of CD3-positive T lymphocytes (C) detected on frozen eye sections (n = 8 or 9) at 8 or 10 days p.i. for C57BL/6, p55TNFR−/−, or uninfected mice (n = 3 per day and per group). Data are the mean numbers of cells per eye section plus or minus SD. ∗, statistical significance of Student's t test at a P value of <0.05 between C57BL/6 and p55TNFR−/− mice 10 days p.i.
Rabies virus-induced ocular disease is more severe in nude mice than in BALB/c mice.
To determine the role of T lymphocytes during rabies ocular disease, we compared the incidence and severity of CVS-induced ocular disease in nude mice and BALB/c mice infected with 107 PFU of the CVS strain of rabies virus (Fig. 4 and Table 1). The incidence of ocular disease, the level of infection (data not shown), and the severity of ocular disease as assessed by the cumulative mean disease score (Table 1) were similar for C57BL/6 mice (7.5) and BALB/c mice (6.9). We could therefore use nude and BALB/c mice to assess the role of T cells in rabies virus-induced ocular disease. From day 7 p.i. to the end of the infection, a higher percentage of nude mice than BALB/c mice developed rabies virus ocular disease: on day 8 p.i., 100% of nude mice and none of the infected BALB/c mice displayed symptoms of CVS-induced ocular disease (Fig. 4). Symptoms of rabies virus-induced ocular disease were also more severe in nude mice than in BALB/c mice (Table 1). This indicates that T cells are involved in the control of both the time course and severity of the ocular disease induced by CVS infection. Thus, the recruitment of protective T cells in the eyes of infected mice may account for the protective role of p55TNFR during rabies virus ocular disease.
FIG. 4.
Comparison of rabies virus-induced ocular disease in BALB/c and nude (Nu/Nu) mice. Kinetics of noncumulative incidence of ocular disease from days 3 to 12 was compared between BALB/c mice (n = 8) and nude mice (n = 8).
The majority of CD3+ T lymphocytes in eyes of rabies virus-infected mice are not apoptotic.
Constitutive expression of FasL in the eye is believed to induce apoptosis of infiltrating activated CD3+ T lymphocytes expressing Fas at their surface. To determine whether T lymphocytes and/or neutrophils undergo apoptosis in the eyes of CVS-infected mice, we performed double immunostaining for TUNEL and either neutrophils (Gr-1) (Fig. 5A) or T lymphocytes (CD3) (Fig. 5B and C). In C57BL/6 mice, the vast majority of infiltrating T lymphocytes were not apoptotic: only 1.45% ± 0.9% of the T lymphocytes were double positive for TUNEL and CD3 staining and only 18.3% ± 12.7% of all apoptotic cells 8 days p.i. were T lymphocytes when the T-cell infiltration was at its maximum. Similarly, only 4.3% ± 4.4% of the T cells were apoptotic when T-cell infiltration was severely reduced, and these cells constituted 1.5% ± 1.5% of the maximum number of TUNEL-positive cells 10 days p.i. Similar findings were obtained with p55TNFR−/− mice, indicating that apoptosis of infiltrating T cells did not differ between C57BL/6 and p55TNFR−/− mice. Altogether, our results indicate that T cells infiltrate the eye in response to CVS ocular infection and limit the spread of CVS from neuron to neuron.
FIG. 5.
Most of the infiltrated CD3+ T cells are not apoptotic in the eyes of CVS-infected mice. Apoptosis was detected by the TUNEL technique in the retinas of mock-infected (A) and CVS-infected (B to D) mice. No TUNEL-positive cells were detected in mock-infected mice. Also shown is codetection of apoptosis (TUNEL red) and Gr-1-positive cells (B) or CD3-positive cells (green) in the retinas (C) and vitreous cavities (D) of CVS-infected mice 10 days p.i. (B and C) TUNEL-positive cells (white arrows) are mainly photoreceptors and do not correspond to inflammatory cells (open arrows). (D) Both apoptotic (white arrow) and nonapoptotic (open arrows) CD3-positive cells were observed. OPL, outer plexiform layer; ONL, outer nuclear layer; RPE, retinal pigmented epithelium. Bars, 50 μm (A and B), 25 μm (C), and 10 μm (D).
DISCUSSION
We studied the role of immune factors and of p55TNFR in particular in the control of mouse susceptibility to ocular disease induced by rabies virus infection. Although viral spread was similar in the brain and in the GCL of the retina, rabies virus antigens were more widely distributed in the IPL and INL of p55TNFR−/− mice than in those of C57BL/6 mice. Moreover, the incidence and severity of rabies ocular disease were greater in p55TNFR−/− mice than in C57BL/6 mice. This indicated that p55TNFR is involved in the control of the rabies virus-induced ocular disease. A protective role for TNF-α has been already described for acquired ocular toxoplasmosis (10). TNF-α signaling through p55TNFR may induce the production of antiviral molecules, such as chemokines and/or interferons, which may contribute to the control of rabies virus spread in the retina. Indeed, we have detected by ELISA the presence of interleukin 6, gamma interferon, and monocyte chemoattractant protein 1 in homogenized eyeball supernatant (data not shown). Moreover, TNF-α signaling may also induce expression of nitric oxide synthase and recruitment of T lymphocytes, which have been proposed to be the mechanisms responsible for protection against ocular toxoplasmosis (10, 15).
The numbers of T cells and neutrophils present in the eyes of p55TNFR−/− mice were smaller than those in C57BL/6 mice during CVS-induced ocular disease. This suggests that p55TNFR is involved in inflammatory-cell recruitment in the eyes. This observation is in agreement with our previous finding that during rabies virus-induced acute encephalitis, inflammatory-cell recruitment in the brain and spinal cord is controlled by expression of p55TNFR (5). Moreover, it has been shown that lymphotoxin–TNF-α signaling via p55TNFR promotes leukocyte recruitment in other models of inflammation (26).
The aggravation of the CVS-induced ocular disease in p55TNFR−/− mice despite less infiltration of both neutrophils and T lymphocytes suggested a protective role for these inflammatory cells in the eyes of CVS-infected mice. Indeed, the incidence and severity of ocular disease were higher in athymic nude mice than in BALB/c littermates, which were used as controls, indicating that T lymphocytes protected mice against CVS-induced ocular disease. Similar observations have been reported for the first steps of the acute HSV ocular infection in which both neutrophils and T cells have an antiviral effect (2, 22, 23, 28, 37). The mechanism of the limitation of CVS-induced ocular disease by T cells remains to be determined. Possibly, T lymphocytes limit the spread of rabies virus in the retina. However, since only rare infected RGC were apoptotic (Camelo et al., submitted), it is more likely that the protection by T lymphocytes is independent of the destruction of infected RGC. Altogether, these data indicate that protection against CVS-induced ocular disease is provided by infiltration of T cells under the control of p55TNFR.
Involvement of T cells in protection against rabies virus-induced ocular disease occurs in an immune privileged site where activated T lymphocytes expressing Fas might be in close contact with ocular cells expressing FasL and thus should die of apoptosis. In contrast, 8 days p.i., few CD3-TUNEL double-positive cells were present in the retinas and in the vitreous cavities of CVS-infected mice and the majority of the infiltrated T cells were not apoptotic. Similarly, the absence of T lymphocyte apoptosis has been observed in murine cytomegalovirus-infected retina (4), where these cells also play a protective role (3). Moreover, this is in agreement with involvement of the T cells in the limitation of the spread of rabies virus despite the ocular immune privilege. However, at 10 days p.i., the number of infiltrated CD3+ T cells in the CVS-infected retinas had considerably decreased, suggesting that T-cell-mediated inflammation had been resolved. The reduction in the number of T cells in the retinas correlated with the aggravation of the CVS-induced ocular disease in BALB/c mice, confirming that when T cells were present they were protective. The reduction of the number of infiltrating T cells 10 days p.i. suggests that regulatory mechanisms are able to limit the duration of the T-cell infiltration in the eyes and that the immune privilege is only partially abrogated. In contrast, Gr-1+ neutrophils were present in similar numbers at both 8 and 10 days p.i., suggesting that these cells were not as susceptible as T cells to Fas- or FasL-induced apoptosis. Indeed, it has been shown that soluble FasL attracts neutrophils (25), suggesting that the immunoregulatory mechanisms affecting the recruitment of T cells and neutrophils might differ. Like the responses to ocular infections by HSV (38), murine cytomegalovirus (16), Listeria monocytogenes (24), and retinal allograft (18), our work shows that there is an inflammatory reaction mediated by neutrophils and T cells in the eyes during rabies virus-induced ocular disease. Thus, ocular immune privilege is not absolute and protective T cells are able to enter the eye to limit the rabies virus infection.
In conclusion, we demonstrate that p55TNFR limits ocular disease via the recruitment of T lymphocytes in the eye, indicating that infiltration by protective immune cells occurs in this site of immune privilege. Rabies virus ocular disease in mice may be a valuable model for studying antiviral immune responses in a privileged immune site.
ACKNOWLEDGMENTS
This work was supported by an institutional grant from the Institut Pasteur.
We are grateful to Werner Lesslauer, Hoffman Laroche Ltd., for the gift of p55TNFR-deficient mice and to Yvonne de Kozak and Marie-Christine Naud for critical advice and gifts of material for eye section preparation. We also thank Harris Ripps for helpful discussions. We thank Viviane Calaora for help with photography and image analysis and Nam Tam To for assistance with immunocytochemistry.
REFERENCES
- 1.Apte R S, Sinha D, Mayhew E, Wistow G J, Niederkorn J Y. Role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J Immunol. 1998;160:5693–5696. [PubMed] [Google Scholar]
- 2.Azumi A, Atherton S S. Sparing of the ipsilateral retina after anterior chamber inoculation of HSV-1: requirement for either CD4+ or CD8+ T cells. Investig Ophthalmol Vis Sci. 1994;35:3251–3259. [PubMed] [Google Scholar]
- 3.Bigger J E, Tanigawa M, Thomas III C A, Atherton S S. Protection against murine cytomegalovirus retinitis by adoptive transfer of virus-specific CD8+ T cells. Investig Ophthalmol Vis Sci. 1999;40:2608–2613. [PubMed] [Google Scholar]
- 4.Bigger J E, Tanigawa M, Zhang M, Atherton S S. Murine cytomegalovirus infection causes apoptosis of uninfected retinal cells. Investig Ophthalmol Vis Sci. 2000;41:2248–2254. [PubMed] [Google Scholar]
- 5.Camelo S, Lafage M, Lafon M. Absence of the p55 Kd TNF-α receptor promotes survival in rabies virus acute encephalitis. J Neurovirol. 2000;6:507–518. doi: 10.3109/13550280009091951. [DOI] [PubMed] [Google Scholar]
- 6.Doymaz M Z, Rouse B T. Herpetic stromal keratitis: an immunopathologic disease mediated by CD4+ T lymphocytes. Investig Ophthalmol Vis Sci. 1992;33:2165–2173. [PubMed] [Google Scholar]
- 7.Ferguson T A, Fletcher S, Herndon J, Griffith T S. Neuropeptides modulate immune deviation induced via the anterior chamber of the eye. J Immunol. 1995;155:1746–1756. [PubMed] [Google Scholar]
- 8.Ferguson T A, Griffith T S. A vision of cell death: insights into immune privilege. Immunol Rev. 1997;156:167–184. doi: 10.1111/j.1600-065x.1997.tb00967.x. [DOI] [PubMed] [Google Scholar]
- 9.Gavrieli Y, Sherman Y, Ben-Sasson S A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992;119:493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gazzinelli R T, Brezin A, Li Q, Nussenblatt R B, Chan C C. Toxoplasma gondii: acquired ocular toxoplasmosis in the murine model, protective role of TNF-alpha and IFN-gamma. Exp Parasitol. 1994;78:217–229. doi: 10.1006/expr.1994.1022. [DOI] [PubMed] [Google Scholar]
- 11.Griffith T S, Brunner T, Fletcher S M, Green D R, Ferguson T A. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270:1189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 12.Griffith T S, Ferguson T A. The role of FasL-induced apoptosis in immune privilege. Immunol Today. 1997;18:240–244. doi: 10.1016/s0167-5699(97)81663-5. [DOI] [PubMed] [Google Scholar]
- 13.Griffith T S, Yu X, Herndon J M, Green D R, Ferguson T A. CD95-induced apoptosis of lymphocytes in an immune privileged site induces immunological tolerance. Immunity. 1996;5:7–16. doi: 10.1016/s1074-7613(00)80305-2. [DOI] [PubMed] [Google Scholar]
- 14.Haltia M, Tarkkanen A, Kivela T. Rabies: ocular pathology. Br J Ophthalmol. 1989;73:61–67. doi: 10.1136/bjo.73.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayashi S, Chan C C, Gazzinelli R T, Pham N T, Cheung M K, Roberge F G. Protective role of nitric oxide in ocular toxoplasmosis. Br J Ophthalmol. 1996;80:644–648. doi: 10.1136/bjo.80.7.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holland G N, Fang E N, Glasgow B J, Zaragoza A M, Siegel L M, Graves M C, Saxton E H, Foos R Y. Necrotizing retinopathy after intraocular inoculation of murine cytomegalovirus in immunosuppressed adult mice. Investig Ophthalmol Vis Sci. 1990;31:2326–2334. [PubMed] [Google Scholar]
- 17.Houff S A, Burton R C, Wilson R W, Henson T E, London W T, Baer G M, Anderson L J, Winkler W G, Madden D L, Sever J L. Human-to-human transmission of rabies virus by corneal transplant. N Engl J Med. 1979;300:603–604. doi: 10.1056/NEJM197903153001105. [DOI] [PubMed] [Google Scholar]
- 18.Jiang L Q, Jorquera M, Streilein J W, Ishioka M. Unconventional rejection of neural retinal allografts implanted into the immunologically privileged site of the eye. Transplantation. 1995;59:1201–1207. [PubMed] [Google Scholar]
- 19.Kaplan H J, Streilein J W. Analysis of immunologic privilege within the anterior chamber of the eye. Transplant Proc. 1977;9:1193–1195. [PubMed] [Google Scholar]
- 20.Kaplan H J, Streilein J W. Immune response to immunization via the anterior chamber of the eye. I. F. lymphocyte-induced immune deviation. J Immunol. 1977;118:809–814. [PubMed] [Google Scholar]
- 21.Lafon M, Wiktor T J. Antigenic sites on the ERA rabies virus nucleoprotein and non-structural protein. J Gen Virol. 1985;66:2125–2133. doi: 10.1099/0022-1317-66-10-2125. [DOI] [PubMed] [Google Scholar]
- 22.Larsen H S, Feng M F, Horohov D W, Moore R N, Rouse B T. Role of T-lymphocyte subsets in recovery from herpes simplex virus infection. J Virol. 1984;50:56–59. doi: 10.1128/jvi.50.1.56-59.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Larsen H S, Russell R G, Rouse B T. Recovery from lethal herpes simplex virus type 1 infection is mediated by cytotoxic T lymphocytes. Infect Immun. 1983;41:197–204. doi: 10.1128/iai.41.1.197-204.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li X Y, Niederkorn J Y. Immune privilege in the anterior chamber of the eye is not extended to intraocular Listeria monocytogenes. Ocul Immunol Inflamm. 1997;5:245–257. doi: 10.3109/09273949709085065. [DOI] [PubMed] [Google Scholar]
- 25.Ottonello L, Tortolina G, Amelotti M, Dallegri F. Soluble Fas ligand is chemotactic for human neutrophilic polymorphonuclear leukocytes. J Immunol. 1999;162:3601–3606. [PubMed] [Google Scholar]
- 26.Peschon J J, Torrance D S, Stocking K L, Glaccum M B, Otten C, Willis C R, Charrier K, Morrissey P J, Ware C B, Mohler K M. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J Immunol. 1998;160:943–952. [PubMed] [Google Scholar]
- 27.Rothe J, Lesslauer W, Lotscher H, Lang Y, Koebel P, Kontgen F, Althage A, Zinkernagel R, Steinmetz M, Bluethmann H. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 28.Rouse B T, Wardley R C, Babiuk L A, Mukkur T K. The role of neutrophils in antiviral defense—in vitro studies on the mechanism of antiviral inhibition. J Immunol. 1977;118:1957–1961. [PubMed] [Google Scholar]
- 29.Streilein J W. Immunologic privilege of the eye. Springer Semin Immunopathol. 1999;21:95–111. doi: 10.1007/BF00810243. [DOI] [PubMed] [Google Scholar]
- 30.Streilein J W, Ksander B R, Taylor A W. Immune deviation in relation to ocular immune privilege. J Immunol. 1997;158:3557–3560. [PubMed] [Google Scholar]
- 31.Takeuchi M, Alard P, Streilein J W. TGF-beta promotes immune deviation by altering accessory signals of antigen-presenting cells. J Immunol. 1998;160:1589–1597. [PubMed] [Google Scholar]
- 32.Taylor A W, Alard P, Yee D G, Streilein J W. Aqueous humor induces transforming growth factor-beta (TGF-beta)-producing regulatory T-cells. Curr Eye Res. 1997;16:900–908. doi: 10.1076/ceyr.16.9.900.5043. [DOI] [PubMed] [Google Scholar]
- 33.Taylor A W, Streilein J W. Inhibition of antigen-stimulated effector T cells by human cerebrospinal fluid. Neuroimmunomodulation. 1996;3:112–118. doi: 10.1159/000097235. [DOI] [PubMed] [Google Scholar]
- 34.Taylor A W, Streilein J W, Cousins S W. Alpha-melanocyte-stimulating hormone suppresses antigen-stimulated T cell production of gamma-interferon. Neuroimmunomodulation. 1994;1:188–194. doi: 10.1159/000097167. [DOI] [PubMed] [Google Scholar]
- 35.Taylor A W, Streilein J W, Cousins S W. Immunoreactive vasoactive intestinal peptide contributes to the immunosuppressive activity of normal aqueous humor. J Immunol. 1994;153:1080–1086. [PubMed] [Google Scholar]
- 36.Thomas J, Gangappa S, Kanangat S, Rouse B T. On the essential involvement of neutrophils in the immunopathologic disease: herpetic stromal keratitis. J Immunol. 1997;158:1383–1391. [PubMed] [Google Scholar]
- 37.Tumpey T M, Chen S H, Oakes J E, Lausch R N. Neutrophil-mediated suppression of virus replication after herpes simplex virus type 1 infection of the murine cornea. J Virol. 1996;70:898–904. doi: 10.1128/jvi.70.2.898-904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whittum J A, McCulley J P, Niederkorn J Y, Streilein J W. Ocular disease induced in mice by anterior chamber inoculation of herpes simplex virus. Investig Ophthalmol Vis Sci. 1984;25:1065–1073. [PubMed] [Google Scholar]
- 39.Yoshitoshi T, Shichi H. Immunosuppressive factors in porcine vitreous body. Curr Eye Res. 1991;10:1141–1149. doi: 10.3109/02713689109024132. [DOI] [PubMed] [Google Scholar]
- 40.Zaidman G W, Billingsley A. Corneal impression test for the diagnosis of acute rabies encephalitis. Ophthalmology. 1998;105:249–251. doi: 10.1016/s0161-6420(98)92860-3. [DOI] [PubMed] [Google Scholar]