Summary
Background
Uganda has had seven Ebola disease outbreaks, between 2000 and 2022. On Sept 20, 2022, the Ministry of Health declared a Sudan virus disease outbreak in Mubende District, Central Uganda. We describe the epidemiological characteristics and transmission dynamics.
Methods
For this descriptive study, cases were classified as suspected, probable, or confirmed using Ministry of Health case definitions. We investigated all reported cases to obtain data on case-patient demographics, exposures, and signs and symptoms, and identified transmission chains. We conducted a descriptive epidemiological study and also calculated basic reproduction number (Ro) estimates.
Findings
Between Aug 8 and Nov 27, 2022, 164 cases (142 confirmed, 22 probable) were identified from nine (6%) of 146 districts. The median age was 29 years (IQR 20–38), 95 (58%) of 164 patients were male, and 77 (47%) patients died. Symptom onsets ranged from Aug 8 to Nov 27, 2022. The case fatality rate was highest in children younger than 10 years (17 [74%] of 23 patients). Fever (135 [84%] of 160 patients), vomiting (93 [58%] patients), weakness (89 [56%] patients), and diarrhoea (81 [51%] patients) were the most common symptoms; bleeding was uncommon (21 [13%] patients). Before outbreak identification, most case-patients (26 [60%] of 43 patients) sought care at private health facilities. The median incubation was 6 days (IQR 5–8), and median time from onset to death was 10 days (7–23). Most early cases represented health-care-associated transmission (43 [26%] of 164 patients); most later cases represented household transmission (109 [66%]). Overall Ro was 1·25.
Interpretation
Despite delayed detection, the 2022 Sudan virus disease outbreak was rapidly controlled, possibly thanks to a low Ro. Children (aged <10 years) were at the highest risk of death, highlighting the need for targeted interventions to improve their outcomes during Ebola disease outbreaks. Initial care-seeking occurred at facilities outside the government system, showing a need to ensure that private and public facilities receive training to identify possible Ebola disease cases during an outbreak. Health-care-associated transmission in private health facilities drove the early outbreak, suggesting gaps in infection prevention and control.
Funding
None.
Introduction
Ebola disease comprises a set of diseases caused by viruses within the genus Ebolavirus in the family Filoviridae.1 Of the six known species of Ebolavirus, only four are pathogenic for humans (Sudan virus, Zaire Ebola virus, Tai Forest virus, and Bundibugyo virus). The case fatality rate (CFR) for Ebola disease ranges from 25% to 90%, depending on the viral species and supportive care available.2 There are no approved vaccines against Sudan virus, but candidate therapeutics and vaccines are available.3
On Sept 19, 2022, a blood sample from a 26-year-old male from Ngabano Village in Madudu Subcounty, Mubende District, central Uganda tested positive for Sudan virus at the Uganda Virus Research Institute. The Ministry of Health declared an outbreak of Sudan virus disease on Sept 20, 2022, representing the eighth recognised outbreak of Sudan virus disease globally. Before this outbreak, Uganda had reported six Ebola disease outbreaks, and four Sudan virus disease outbreaks since 2000 (figure 1).4
Figure 1.
Previous Ebola disease outbreaks in Uganda, 2000–19
Years of the outbreak are indicated. Numbers indicate cases and numbers in parentheses indicate deaths. Note that the Ebola virus cases (2019) represented spillover from the Democratic Republic of the Congo in 2018, and that 2012 a and 2012 b are two separate outbreaks that occurred in the same year.
Research in context.
Evidence before this study
This study presents epidemiological evidence from the Sudan virus disease outbreak in Uganda between Aug 8 and Nov 27, 2022. Although the genus Ebolavirus includes four ebolaviruses that are associated with human disease, most epidemiological data around ebolaviruses are specific to Zaire Ebola virus disease. As the 2022 outbreak was only the eighth recognised Sudan virus disease outbreak globally, this study represents an important opportunity to strengthen the scientific knowledge regarding Sudan virus. We considered the results of our analysis in the context of published literature from past outbreaks. We searched PubMed on April 30, 2023, using the search terms “Ebola” AND “virus” AND “disease”; “Sudan” AND “virus” AND “disease”; “Ebola” AND “Sudan”; “Ebola” AND “Africa”. We searched for peer-reviewed studies published between Aug 1, 1976 and April 30, 2023, with English language restrictions. We selected 24 relevant publications based on applicability to context, strength of information presented, and generalisability to the 2022 Uganda outbreak.
Added value of this study
We document the detailed epidemiological characteristics of the 2022 Sudan virus disease outbreak in Uganda, underscoring differences in the characteristics of Ebola disease outbreaks, mainly between Sudan virus disease and Ebola virus disease. We have shown the challenges with identifying Ebola disease outbreaks early in settings where a myriad of diseases, such as malaria, typhoid, or other viral haemorrhagic fevers share non-specific presenting signs and symptoms with Ebola disease. We suggest that viral haemorrhagic fever outbreaks could occur undetected more often than is known.
Implications of all the available evidence
Sudan virus has the potential to cause a devastating pandemic but could be less likely to do so than other ebolaviruses. Documenting its epidemiological and transmission dynamics provides an opportunity to inform needed changes to reduce the time to detection of similar outbreaks.
Preliminary investigations following the death of the index case revealed suspicious deaths in Mubende District as far back as August, 2022. We aimed to conduct a descriptive study to describe the epidemiological characteristics of the Sudan virus disease cases and Sudan virus transmission in the 2022 outbreak in Uganda.
Methods
Case definitions
Cases were classified as suspected, probable, or confirmed using Ministry of Health case definitions.5 Cases were labelled with a leading “c” and sequential numbers to indicate the order of diagnosis; some probable cases were labelled with random identifier numbers.
Laboratory testing
Blood specimens were tested using RT-quantitative PCR or ELISA (Centers for Disease Control and Prevention [CDC], Atlanta, GA, USA) at the Uganda Virus Research Institute Viral Haemorrhagic Fever laboratory and the mobile laboratory in Mubende. The Uganda Virus Research Institute used the MagMax Kit (Applied Biosystems, Lithuania) to extract RNA, followed by RT-PCR on Applied Biosystems QuantStudio 5 or 7500 Real-Time PCR System instruments (Applied Biosystems, Lithuania) using the CDC custom primers and probes. The mobile laboratory used RealStar Filovirus Kits (Altona Diagnostics, Germany). Samples were tested by PCR for laboratory detection of the six species that cause Ebola disease, Marburg disease, Crimean–Congo haemorrhagic fever, and Rift Valley fever viruses. We conducted genetic sequencing and obtained complete sequences from 104 (73%) of 142 confirmed cases, which was used in the transmission analysis.
Data collection
Field investigations were conducted from Sept 20, 2022, to Jan 10, 2023. We investigated all reported cases to obtain data on demographics, clinical characteristics, exposures, and outcomes. Data were collected by trained field epidemiologists using the Ministry of Health Viral Haemorrhagic Fever case investigation form and were entered into Go.data (version 2.0; Geneva, Switzerland) and the Uganda electronic Results Dispatch System (version 2022, Kampala, Uganda). For case-patients who recovered or died before Sept 20, 2022, we conducted a medical records review at health facilities in affected areas and interviews with health-care workers and family members to retrospectively classify cases. For case patients who were alive or reported their onset after Sept 20, 2022, data were collected prospectively during case investigations. Contacts (family members) living in the same household were interviewed on behalf of the deceased. Reported suspected cases were first investigated and sampled for laboratory analysis and then reclassified into confirmed or a non-case. Therefore, the study included only probable and confirmed cases. Missing data were excluded. Data on race and ethnicity were not collected because it had no relevance to our study objectives. Data on sex was self-reported by study participants (male and female). Additionally, Uganda mainly comprises people from more than 50 indigenous ethnic groups, all of whom share similar racial characteristics. Given this homogeneity, the distinction between race and ethnicity is less pronounced than in more diverse populations. Participants provided oral informed consent.
This study was in response to a national public health emergency; accordingly, in reference to a memorandum of understanding and an umbrella protocol (project identification number 0900f3eb81f96394), the study was waived the process of the full Institutional Review Board. The Ministry of Health, which gave the directive to investigate, provided the mandate for the Public Health Fellowship Program to conduct the emergency response, and collect, analyse, and disseminate data through scientific publications. Informed verbal consent for study publication was sought (yes or no) and was documented on every record. Data was stored in a password-protected computer, and an anonymised version of the dataset was shared only with permission from the data team lead. This study activity was reviewed by the CDC and conducted consistent with applicable federal law and CDC policy (eg, 45 Code of Federal Regulations part 46, 21 Code of Federal Regulations part 56; 42 United States Code (USC) 241 (d); 5 USC 552a; and 44 USC 3501 et seq).
Statistical analysis
We used Excel (version 2019) and Epi Info (version 7.2.5.0) to analyse the data. The overall basic reproduction number (Ro) and 95% credible interval (CrI) were estimated using the EpiEstim package in R (version 4.2.1). We calculated frequencies and proportions and compared proportions using χ2. A p value of less than 0·05 was considered significant. We calculated attack rates using the Uganda Bureau of Statistics district population projections. District attack rates were plotted on a map using QGIS (version 3.16.16). We calculated the overall CFR, stratified by age, sex, and district. We calculated the median time from exposure to onset (incubation period), onset to isolation, isolation to discharge, and onset to death for cases with available data. We plotted an epidemic curve by illness onset and case classification to illustrate trends. We used data on confirmed cases to calculate daily and weekly incidence rates and estimated the serial interval of Sudan virus (time between onset in a primary case and subsequent cases). We calculated the median and mean of a discretized gamma distribution of this serial interval by examining epidemiologically linked pairs of cases, considering their onset and confirmation dates. Using estimates of the mean and standard deviation of the fitted serial interval obtained earlier, we calculated the Ro. We estimated Ro through the branching process model, which derives transmission dynamics by examining how infections propagate through a population. The EpiEstim package implements a Bayesian estimation of the reproduction number using dates of symptom onset and information on the serial interval distribution. The package uses a simple Poisson distributed model describing incidence on a given day, with a mean incidence determined by the total force of infection on that day. The incidence based on symptom onset date and the discrete serial interval distribution are used to estimate the likelihood of observing the data given the model and parameters defined as a function of the reproduction number.
Role of the funding source
There was no funding source for this study.
Results
In total, 164 case-patients (142 confirmed, 22 probable) were identified in nine (6%) of 146 districts: Mubende, Kassanda, Kampala, Kyegegwa, Wakiso, Jinja, Bunyangabu, Kagadi, and Masaka (table 1; appendix 1 p 1). Symptom onsets ranged from Aug 8 to Nov 27, 2022 (figure 2). 77 (47%, 95% CI 39–55) of 164 case-patients died (table 1). Among 142 confirmed case-patients, 55 died (39%, 31–47). Ten (7%) of 142 confirmed cases were dead at sample collection. Among 6212 samples tested, 142 (2·3%) were positive for Sudan virus, eight (0·1%) for Crimean–Congo haemorrhagic fever, and six (0·1%) for Rift Valley fever. Crimean–Congo haemorrhagic fever and Rift Valley fever were responded to as separate outbreaks (appendix 1 p 2).
Table 1.
Baseline characteristics of cases
| All cases (N=164) | Confirmed cases (n=142) | Probable cases (n=22) | p value (confirmed vs probable) | ||
|---|---|---|---|---|---|
| Sex | .. | .. | .. | 0·20 | |
| Male | 95/164 (58%) | 85/142 (60%) | 10/22 (45%) | .. | |
| Female | 69/164 (42%) | 57/142 (40%) | 12/22 (55%) | .. | |
| Age group, years | 164/164 (100%); 29 (20–38) | 142/164 (87%); 29 (22–38) | 22/164 (13%); 22 (5–39) | .. | |
| <10 | 23/164 (14%) | 14/142 (10%) | 9/22 (41%) | 0·0001 | |
| 10–19 | 18/164 (11%) | 16/142 (11%) | 2/22 (9%) | 0·78 | |
| 20–29 | 46/164 (28%) | 43/142 (30%) | 3/22 (14%) | 0·12 | |
| 30–39 | 42/164 (26%) | 39/142 (27%) | 3/22 (14%) | 0·19 | |
| 40–49 | 22/164 (13%) | 21/142 (15%) | 1/22 (4%) | 0·16 | |
| ≥50 | 13/164 (8%) | 9/142 (6%) | 4/22 (18%) | 0·05 | |
| District | |||||
| Mubende | 83/164 (51%) | 64/142 (45%) | 19/22 (86%) | 0·0004 | |
| Kassanda | 51/164 (31%) | 49/142 (35%) | 2/22 (5%) | 0·0048 | |
| Kampala | 19/164 (12%) | 18/142 (13%) | 1/22 (3%) | 0·18 | |
| Kyegegwa | 4/164 (2%) | 3/142 (2%) | 0/22 (0) | 0·50 | |
| Wakiso | 3/164 (2%) | 3/142 (2%) | 0/22 (0) | 0·50 | |
| Jinja | 1/164 (1%) | 2/142 (1%) | 0/22 (0) | 0·64 | |
| Bunyangabu | 1/164 (1%) | 1/142 (1%) | 0/22 (0) | 0·64 | |
| Kagadi | 1/164 (1%) | 1/142 (1%) | 0/22 (0) | 0·64 | |
| Masaka | 1/164 (1%) | 1/142 (1%) | 0/22 (0) | 0·64 | |
| Died | 77/164 (47%) | 55/142 (39%) | 22/22 (100%) | <0·0001 | |
| Symptoms* | |||||
| Fever | 135/160 (84%) | 116/138 (84%) | 19/22 (86%) | 0·81 | |
| Vomiting or nausea | 93/160 (58%) | 78/138 (57%) | 15/22 (68%) | 0·33 | |
| General weakness | 89/160 (56%) | 78/138 (57%) | 11/22 (50%) | 0·54 | |
| Diarrhoea | 81/160 (51%) | 65/138 (47%) | 16/22 (73%) | 0·02 | |
| Headache | 74/160 (46%) | 72/138 (52%) | 2/22 (9%) | 0·0002 | |
| Loss of appetite | 62/160 (39%) | 58/138 (42%) | 4/22 (18%) | 0·03 | |
| Abdominal pain | 64/160 (40%) | 54/138 (39%) | 10/22 (45%) | 0·59 | |
| Muscle pain | 49/160 (31%) | 47/138 (34%) | 2/22 (9%) | 0·02 | |
| Joint pain | 49/160 (31%) | 47/138 (34%) | 2/22 (9%) | 0·02 | |
| Chest pain | 47/160 (29%) | 43/138 (31%) | 4/22 (18%) | 0·21 | |
| Cough | 32/160 (20%) | 26/138 (19%) | 6/22 (27%) | 0·39 | |
| Difficulty breathing | 26/160 (16%) | 22/138 (16%) | 4/22 (18%) | 0·81 | |
| Unexplained bleeding | 21/160 (13%) | 13/138 (9%) | 8/22 (36%) | 0·0004 | |
| Sore throat | 15/160 (9%) | 15/138 (11%) | 0/22 (0) | 0·10 | |
| Jaundice | 12/160 (8%) | 9/138 (7%) | 3/22 (14%) | 0·26 | |
| Difficulty swallowing | 10/160 (6%) | 10/138 (7%) | 0/22 (0) | 0·20 | |
| Skin rash | 9/160 (6%) | 8/138 (6%) | 1/22 (5%) | 0·85 | |
| Hiccups | 9/160 (6%) | 7/138 (5%) | 2/22 (9%) | 0·45 | |
| Coma | 9/160 (6%) | 8/138 (6%) | 1/22 (5%) | 0·85 | |
| Disoriented | 8/160 (5%) | 8/138 (6%) | 0/22 (0) | 0·24 | |
| Sensitivity to light | 7/160 (4%) | 7/138 (5%) | 0/22 (0) | 0·29 | |
Data are n/N (%) or n/N (%); median (IQR).
Data only available for 160 of the 164 cases, 138 of 160 cases of whom were confirmed and 22 were probable cases.
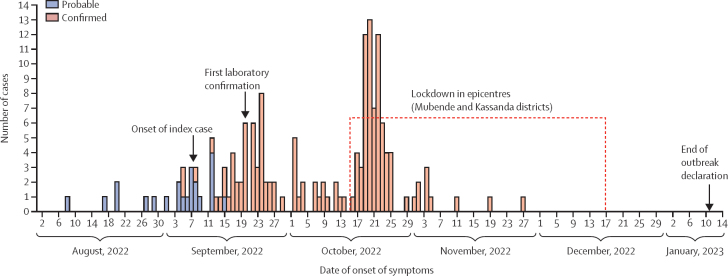
Figure 2.
Cases by illness onset and case classification (n=164) in Uganda, Aug 8–Nov 27, 2022
Four cases without onset dates have been included by their date of laboratory confirmation and one case, which was a stillbirth, has been included by its date of birth (Nov 27).
Overall, the median age was 29 years (IQR 20–38), 95 (58%) of 164 patients were male, and 37 (23%) of 164 patients were children (age range 0–17 years). Symptom data were missing for four patients (three patients who had been isolated as a precaution reported no symptoms at the time of their positive PCR test but developed symptoms shortly thereafter, and one patient without symptom data was a stillbirth from a confirmed case-patient) and were available for 160 (98%) of 164 patients, of whom 157 (98%) of 160 reported symptoms at diagnosis. The most common symptoms were fever (135 [84%] of 160 patients), vomiting (93 [58%]), general weakness (89 [56%]), diarrhoea (81 [51%]), and headache (74 [46%]). Unexplained bleeding (haemorrhagic signs) was present in 21 (13%) of 160 (table 1), 81% (17 of 21 patients) of whom died.
Age, sex, and symptoms differed between confirmed and probable patients (table 1). Confirmed cases were older in median age compared with probable cases (median 29 years [IQR 22–38] vs 22 years [5–39]; p=0·15; appendix 1 p 1), and less likely to be from Mubende (45% [64 of 142 patients] vs 86% [19 of 22 patients]; p=0·0004). 85 (60%) of 142 confirmed cases and ten (45%) of 22 probable cases were male (p=0·20).
Before the outbreak was declared and the first Ebola Treatment Units activated at Mubende and Fort Portal Regional Referral Hospitals on Sept 20, 2022, 26 (60%) of 43 patients with data on care-seeking first sought care at private facilities, whereas 17 (40%) first sought care at public facilities (all at Mubende Regional Referral Hospital). After the outbreak was confirmed, most known contacts who developed symptoms were evacuated directly to Ebola Treatment Units for testing. Activation for Entebbe Regional Referral Hospital Ebola Treatment Unit in Wakiso District was on Oct 6, 2022, and was Oct 15, 2022 for Mulago National Referral Hospital Ebola Treatment Unit in Kampala District.
Most patients seeking care outside of Ebola Treatment Units were initially treated for malaria, peptic ulcers, or typhoid. Among 63 cases with clear exposure and onset dates, the median incubation period was 6 days (IQR 5–8). Among 74 fatal cases with clear onset and death dates, the median time from onset to death was 10 days (7–23). Among 118 cases with clear onset and isolation dates, the median was 5 days (3–8), which reduced as the outbreak progressed, with a median of 6 days (3–8) in September, 2022, 5 (2–7) in October, and 4 (3–11) in November (appendix 1 p 3). 27 case-patients (22 patients probable [retrospectively identified] and five patients confirmed) spent nearly their entire illness outside of an Ebola Treatment Unit; the five confirmed case-patients were almost fully recovered when they tested positive. Among 71 case-patients with clear isolation and discharge dates, median duration of isolation was 11 days (7–16; appendix 1 p 3).
The CFR was not significantly different between females (37 [54%] of 69 patients) and males (40 [42%] of 95 patients; p=0·13; table 2). Case fatality was highest in children younger than 10 years (17 [74%] of 23 patients) and lowest among individuals aged 20–29 years (16 [35%] of 46 patients; table 2). The CFR was significantly lower in Kampala (three [16%] of 19 patients) than Kassanda (23 [45%] of 51 patients), or Mubende districts (47 [57%] of 83 patients; p<0·0001).
Table 2.
Death by age, sex, and district of residence
| All cases | Died | p value | ||
|---|---|---|---|---|
| Sex | .. | .. | 0·13 | |
| Male | 95/164 (58%) | 40/95 (42%) | .. | |
| Female | 69/164 (42%) | 37/69 (54%) | .. | |
| Age group, years | .. | .. | 0·29 | |
| <10 | 23/164 (14%) | 17/23 (74%) | .. | |
| 10–19 | 18/164 (11%) | 8/18 (44%) | .. | |
| 20–29 | 46/164 (28%) | 16/46 (35%) | .. | |
| 30–39 | 42/164 (26%) | 15/42 (36%) | .. | |
| 40–49 | 22/164 (13%) | 13/22 (59%) | .. | |
| ≥50 | 13/164 (8%) | 8/13 (62%) | .. | |
| District | .. | .. | <0·0001 | |
| Mubende | 83/164 (51%) | 47/83 (57%) | .. | |
| Kassanda | 51/164 (31%) | 23/51 (45%) | .. | |
| Kampala | 19/164 (12%) | 3/19 (16%) | .. | |
| Kyegegwa | 4/164 (2%) | 1/4 (25%) | .. | |
| Wakiso | 3/164 (2%) | 0/3 (0) | .. | |
| Jinja | 1/164 (1%) | 1/1 (25%) | .. | |
| Bunyangabu | 1/164 (1%) | 0/1 (0) | .. | |
| Kagadi | 1/164 (1%) | 1/1 (25%) | .. | |
| Masaka | 1/164 (1%) | 1/1 (25%) | .. | |
Data are n/N (%).
Kassanda (attack rates 16 per 100 000 population) and Mubende districts (14 per 100 000 population) were the most affected, followed by Kampala, Kyegegwa, and Bunyagabu districts (one per 100 000 population; figure 3). 19 (13%) of 143 patients with data on occupation were health-care workers of which seven (37%) died.
Figure 3.
Attack rates by district of residence in Uganda, August–November, 2022
Overall, most early cases represented health-care-associated transmission (43 [26%] of 164 patients), and most later cases represented household transmission (109 [66%]). Among the confirmed cases, 99 (70%) of 142 patients resulted from household transmission, 32 (23%) patients from health-care-associated exposures, eight (6%) patients from participation in burial preparations (without other community exposures), two (1%) patients from vertical transmission, and one (1%) patient likely resulted from sexual transmission (table 3). Among the probable cases, 11 (50%) of 22 patients were health-care-associated, ten (45%) patients from household transmission, and one (5%) patient probably resulted from burial preparations.
Table 3.
Transmission modes
| All cases | Confirmed cases | Probable cases | |
|---|---|---|---|
| Household | 109/164 (66%) | 99/142 (70%) | 10/22 (45%) |
| Health-care-associated | 43/164 (26%) | 32/142 (23%) | 11/22 (50%) |
| Burial | 9/164 (5%) | 8/142 (6%) | 1/22 (5%) |
| Vertical transmission | 2/164 (1%) | 2/142 (1%) | 0/22 (0) |
| Sexual transmission | 1/164 (1%) | 1/142 (0) | 0/22 (0) |
Data are n/N (%).
Between Aug 8 and Nov 27, 2022, 43 health-care-associated infections occurred, 18 (42%) among patients who initially presented to Facility A, which is a private clinic in Ngabano village, Madudu Subcounty, Mudende District, for different conditions and returned approximately one week after discharge with signs and symptoms of Sudan virus disease. Of these 18 patients, at least ten (56%) returned for treatment at Facility A while symptomatic with Sudan virus disease and probably spread the infection to others at the facility. Ten health-care-associated infections have been also thought to have occurred at Mubende Regional Referral Hospital.
The outbreak was limited to Mubende District until Sept 23, 2022, when the first confirmed case-patient in neighbouring Kassanda (c031) was identified. C031 was likely exposed by a probable case-patient (c037) who was a next-door neighbour. On Oct 17, 2022, c083 was confirmed. Exposure to this case-patient led to confirmed infection in 33 people in Kassanda, which became the second epicentre.
Sudan virus probably spread into Kyegegwa through exposures during the care and burial of a probable case-patient who was also exposed at Facility A. The only patient identified in Bunyangabu was a health-care worker who attended to at least one confirmed case-patient before the outbreak was identified. On Sept 28, 2022, Kagadi confirmed its first case in a health-care worker who had previously been working in Mubende and was likely to be exposed while treating one of the earliest confirmed case-patients.
A high number of transmission events spread across districts were linked to two case-patients (c083, to whom 33 transmission events were linked, and c081, to whom at least 18 transmission events were linked). 25 (76%) of 33 patients infected through exposure to c083 were male. C081 and c083 acquired Sudan virus after exposure in a private clinic in Kassanda. Sudan virus in Wakiso was probably imported from someone visiting or working in this private clinic. Following onset, c081 travelled to Kampala for treatment at a private clinic, resulting in infection of a patient being treated there the same day (c153). c153 subsequently travelled to Masaka for treatment and became ill. C161 lived near the clinics where c081 and c153 received treatment, and became infected through an undetermined mechanism. c161 travelled to Jinja while ill. Although the specific interactions between c081, c153, and c161 are unknown, genetic sequencing data has showed the viruses from these patients to be closely related.
There was one case of suspected sexual transmission and two cases of vertical transmission. C163 in Mubende probably acquired Sudan virus through sexual intercourse with c040 after the recovery of c040. Genetic sequencing showed the viruses from the two patients to be closely linked. C163 was diagnosed 31 days after the discharge of c040 from the Ebola Treatment Unit with a negative PCR test. Four patients were known to be pregnant at diagnosis; two (50%) of four had spontaneous abortions, and both died. One of the four pregnant women delivered a live infant and survived; the infant survived a week before dying with confirmed Sudan virus infection. The last confirmed case-patient was a antepartum stillbirth delivered from a Sudan virus survivor.
The mean serial interval was 13·8 days (SD 7·6) and the median was 13 days (IQR 10–17). The median and 95% CrI for the Ro was 1·25 (IQR 1·18–1·35, 95% CrI 1·03–1·51; appendix 1 p 6).
Discussion
The 2022 Sudan virus disease outbreak in Uganda lasted at least 4 months. Despite delays in detection, it was controlled approximately 2 months after identification. The epidemiology of infections was generally consistent with previously described Sudan virus disease outbreaks of comparable magnitude.6, 7 Health-care-associated transmission drove the early part of the outbreak, whereas household transmission drove the later phases. The Ro was low, which was consistent with the limited existing data on Sudan virus disease outbreaks.8 Approximately one in eight patients was a health-care worker. Children and females had the highest risk of death. Small private health facilities were sources of outbreak amplification.
Detection of this outbreak was delayed by approximately 6 weeks. Uganda has a robust surveillance system that has previously rapidly identified high-consequence pathogens, including the pneumonic plague in 2019 and Ebola virus disease in 2018.9 However, during both of these outbreaks, ongoing outbreaks in neighbouring Democratic Republic of the Congo might have resulted in heightened vigilance among clinicians. Additionally, the non-specific nature of Sudan virus disease symptoms, the rarity with which it appears in human populations, and the endemic nature of typhoid and malaria in Uganda, make the detection of viral haemorrhagic fever difficult. Although frank haemorrhage is commonly considered a hallmark of Ebola disease, few patients during this outbreak were bleeding at diagnosis, similar to other Ebola disease outbreaks.10, 11
Many case-patients who initially presented at Facility A returned there with signs and symptoms of Sudan virus disease before dying. Given its small size and the age range of patients who presented repeatedly and died, it is surprising that Facility A did not report these recurring visits, which could be associated in part with it being a private facility. Public and private facilities are mandated to report all suspected cases of priority diseases as per the Integrated Disease Surveillance and Response framework;3 however, in practice, private facilities often do not report as reliably as public facilities.12 It is also surprising that the family clusters with multiple fatal cases in August, 2022, went unreported by community health workers engaged in the event-based surveillance system. The incidental identification of patients with other viral haemorrhagic fever among suspected Sudan virus disease cases suggests that patients with viral haemorrhagic fever could at times go undetected. Identifying viral haemorrhagic fever is dependent on increased vigilance and a clinical surveillance network that includes laboratory diagnostics for several viral haemorrhagic fevers.13 With a low Ro for many viral haemorrhagic fevers, it is possible that some outbreaks emerge and end before they are recognised. Since 2001, the Ministry of Health has been implementing Integrated Disease Surveillance and Response training to improve early detection and reporting of priority diseases. New versions of Integrated Disease Surveillance and Response are periodically released and re-training is conducted among subnational units.14 The third version of Integrated Disease Surveillance and Response began roll-out in 2021, but Mubende had not yet been trained at the time of the outbreak. Accelerating new training that specifically includes private health facilities, strengthening of community-based surveillance, and continuous clinician education with emphasis on infection prevention and control could reduce delays to viral haemorrhagic fever outbreak detection.
Specific therapeutics for Ebola disease could lower the CFR. In October, 2022, the Ministry of Health approved two investigational therapeutics (MBP134 and remdesivir) for compassionate use in consenting patients. Three vaccine candidates were also approved for a clinical trial, but the first doses (Sabin Vaccine Institute's ChAd3-Sudan virus (Arezzo, Italy) arrived on Dec 8, 2022, when the outbreak was largely over, and only six contacts were still under the 21-day follow-up. As such, management of cases during this outbreak was still largely supportive. The overall CFR was 47%, consistent with previous Sudan virus disease outbreaks in which more than a single case was reported.6, 15 Sudan virus disease is associated with a lower CFR than Ebola virus disease but higher than Bundibugyo virus disease.15, 16 Before the outbreak was identified, most patients first sought care at private facilities, which comprise 55% of health-care facilities in Uganda.17 Although rehydration with intravenous fluids in these facilities might have prolonged their lives, it also was likely to have facilitated the transmission of Sudan virus by exposing other patients and staff there. The duration of onset to discharge and recovery and onset to death was consistent with previous Ebola disease outbreaks, as was the duration from onset to death.6, 11
Cases were more frequent in males than females, which probably reflects the 33 transmission events within the male-dominated social network of a single case-patient. CFRs were higher in females than males, and highest among children. Literature is mixed on sex-specific risk of death; some studies have found that females are less likely to die from Ebola disease than men, whereas others have found men to be at greater risk.18, 19 The variation in age-specific CFR is consistent with previous studies,20, 21 and has been attributed to differences in immunity and physiological reserve between children and young adults, and the increased likelihood of comorbidities in older people.20, 22 CFRs were also higher in Mubende than in the other two districts with high numbers of cases. However, Mubende, which was the first district affected, also had a much higher proportion of probable cases than the other two districts, which contributed to the higher CFR. It is likely that other cases occurred in Mubende among people who survived but were not identified before the outbreak was detected. This theory is also potentially supported by the different age distributions of probable and confirmed cases. Children, who are more likely to die of Ebola disease than young adults,21 represented the highest proportion of probable but not confirmed cases. This finding could suggest that at least some adults were infected, received supportive care, and survived before the outbreak was recognised.
Health-care-associated infections in health facilities comprised nearly one in three cases. This frequency is on the higher end of health-care-associated transmission in previous outbreaks.23, 24 Transmission early during the outbreak appeared to be driven primarily by patients who visited a private facility, resulting in health-care-associated transmission being the predominant mode of transmission for probable case-patients. Household transmission drove the later phases of the outbreak.
Unsupervised burials of Ebola disease patients can pose a high risk for spread among contacts who touch the body or fluids during burial preparations.25 During this outbreak, burials comprised only 5% of transmission events, which is much lower than has been reported in other Ebola disease outbreaks in Uganda and elsewhere.11, 16, 26 Following the declaration of the outbreak, safe and dignified burial teams were composed, and supervised burials were conducted for most cases. This rapid implementation of the intervention, combined perhaps with religious or cultural differences in the frequency of high-risk burial behaviours between Uganda and west Africa, could have contributed to the lower contribution of burial-related exposures in Uganda.
There was documented vertical transmission from two cases, and two of four pregnant women had spontaneous abortions without the fetuses being tested. Among the recovered pregnant patients with Ebola disease, the virus could persist in placental and amniotic fluids following recovery and can pose a risk of infection to others during labour and delivery.27 Additionally, one case-patient could have had a sexually acquired infection. Survivor follow-up programmes have detected Ebola virus fragments in vaginal secretions for up to 33 days after symptom onset, and in semen for at least 1 year after recovery; the live virus has been isolated from semen several months after onset.28 Such prolonged viral shedding could be associated with sexual transmission, which, although documented previously among Ebola virus disease cases, has not been confirmed among Sudan virus disease cases.29 Safer sex practices are encouraged for survivors for at least 1 year after recovery,29 and survivors who contract Ebola disease during their pregnancy need monitoring to minimise the risk of spread to people involved in the delivery.27 In November, 2022, Uganda launched an Ebola disease survivor programme that offers psychosocial support, conducting eye, semen, and breastmilk screening for the virus, and provides monthly follow-up reviews for 18 months.
Our investigation had limitations. The primary case-patient was not identified; records at the health facility where the earliest identified case-patient was suspected to have acquired infection were incomplete. Patients who survived early in the outbreak, and some who died, were likely missed, resulting in an underestimation of the outbreak size. Additionally, we did not have detailed clinical data once patients entered the Ebola Treatment Units apart from discharge, death, and haemorrhagic signs. Patients could have developed other symptoms in the Ebola Treatment Unit that were not documented in our study. Lastly, some data were collected retrospectively and could be a source of recall bias.
The epidemiological features of this outbreak were generally similar to previous outbreaks. Enhanced surveillance, health-care worker training in both public and private facilities, and community engagement are vital for early detection and effective response to future outbreaks.
Uganda Ebola Response Team
Contributors
Equitable partnership declaration
Data sharing
The datasets upon which our findings have been based belong to the Ministry of Health of Uganda. An anonymised, de-identified version of the dataset can be availed upon reasonable request to the corresponding author, with permission from the Ministry of Health.
Declaration of interests
We declare no competing interests.
Acknowledgments
All organisations and agencies that were involved in the outbreak response including but not limited to: the Ministry of Health, District Local Governments countrywide, Uganda Virus Research Institute, Uganda National Health Laboratory and Diagnostics Services, Infectious Disease Institute, Kampala City Council Authority, US Centers for Disease Control and Prevention, WHO, Baylor College of Medicine Children's Foundation, African Field Epidemiology Network, Makerere University School of Public Health, United Nations Children's Emergency Fund, World Alliance for Lung and Intensive Care Medicine in Uganda, United States Agency for International Development, Médecins Sans Frontiers, Joint Mobile Emerging Disease Intervention Clinical Capability, Jhpiego, International Federation of Red Cross and Red Crescent Societies, Management Sciences for Health, The African Medical and Research Foundation, Medical Teams International, World Food Program, International Organization for Migration, United Kingdom Aid, European Union, Save the Children, Africa Centers for Disease Control and Prevention, and African Union.
Editorial note: The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Acknowledgments
Allan Komakech (Clarke International University, Kampala, Uganda); Allan Komakech (Africa Centers for Disease Control and Prevention, Kinshasa, Democratic Republic of the Congo); Doreen Nsiimire Gonahasa, Lilian Bulage, Joshua Kayiwa (National Public Health Emergency Operations Center, Kampala, Uganda); Denis Okethwangu (Baylor College of Medicine Children's Foundation Uganda, Kampala, Uganda); Lisa J Nelson, Amy L Boore, Sandra Nabatanzi, Thomas Nsibambi, Jonathan Ntale, Christina Mwangi, Arthur G Fitzmaurice, Claire Biribawa, Enos Sande, Joseph Ojwang, Mary Choi, Terrence Lo, Jason Malefant, Trevor Shoemaker, Joel M Montgomery, Caitlin M Cossaboom, Carrie Eggers, John D Klena, Shannon Whitmer, Modupe O Osinubi, Katrin S Sadigh, Waverly Vosburgh, Mary-Claire Worrell, James A Fuller (Centers for Disease Control and Prevention, Kampala, Uganda); Henry Kyobe Bosa, Bernard Lubwama, Paul Mbaka, Godfrey Bwire, Rony Reginald Bahatungire, Sarah Byakika, Deborah Aujo, Mudarshir Bbuye, Jovan Baryamujura, John- Baptist Waniaye, Michael Mwanga, Jackson Amone, Paska Apiyo, Richard Kabanda (Ministry of Health, Kampala, Uganda); Immaculate Nabukenya, Judith Nanyondo Semanda, Grace Akello, Herbert Kiirya Isabirye (Infectious Disease Institute, Kampala, Uganda); Paul Edward Okello, Elizabeth Babirye Katana, Job Morukileng, Hildah Tendo Nansikombi, Immaculate Atuhaire, Alice Asio, Sarah Elayeete, Edirisa Junior Nsubuga, Veronica Masanja, Stella Martha Migamba, Patience Mwine, Petranilla Nakamya, Rose Nampeera, Andrew Kwiringira, Mariam Komugisha, Brian Kibwika, Innocent Ssemanda, Yasiini Nuwamanya, Adams Kamukama, Dorothy Aanyu, Dominic Kizza, Shaban Senyange, Irene Byakatonda Kyamwine, Allan Komakech (Uganda National Institute of Public Health, Kampala, Uganda); Andrew Niwagaba Bakainaga, Bernadette Basuta Mirembe, Esther Namukose Muwanguzi, Emmanuel Ochien, Innocent Komakech, Muzafalu Senyonga, Solome Okware, Ivan Kimuli, Moses Rubangakene, Richardson Mafigiri, Charles Njuguna, Alex Chimbaru, Bongomin Bodo, Tonny Akera, Philimon Kabagambe, Nasan Natseri, Charles Okot Lukoya, Annet Alenyo Ngabirano (WHO, Kampala, Uganda); Felix Ocom, Milton Makoba Wetaka, Simon Kyazze, Anita Kisakye (National Public Health Emergency Operations Center, Kampala, Uganda); Mohammed Lamorde, Daniel Bulwadda, Lydia Nakiire, Peter Babigumira Ahabwe (Infectious Disease Institute, Kampala, Uganda); Annet Martha Nankya (Uganda Virus Research Institute, Entebbe, Uganda); Daniel Ayen Okello, Sarah K Zalwango (Kampala City Council Authority, Kampala, Uganda); Herbert Kiirya Isabirye (Mbale Regional Public Emergency Operations Centre, Mbale, Uganda); John-Baptist Kibanga (Baylor College of Medicine Children's Foundation, Kampala, Uganda); Stephen Balinandi, Sophia Mulei, Luke Nyakarahuka, Jimmy Baluku, Jackson Kyondo, Alex Tumusiime, Julius Julian Lutwama, Pontiano Kaleebu (Uganda Virus Research Institute, Entebbe, Uganda); Pontiano Kaleebu (The Medical Research Council and Uganda Virus Research Institute, Entebbe, Uganda); Pontiano Kaleebu (London School of Hygiene & Tropical Medicine Uganda Research Unit, Entebbe, Uganda); Ben Masiira (African Field Epidemiology Network, Kampala, Uganda); Dativa Aliddeki (Africa Centers for Disease Control and Prevention, Nairobi, Kenya); Remmy Buhuguru (Mubende Regional Referral Hospital, Mubende, Uganda); Hakeem Kasumba, Bosco Vito Sendikadiwa, Joseph Kabanda (Kassanda District Health Office, Kassanda, Uganda); Shevin T Jacob (World Alliance for Lung and Intensive Care Medicine in Uganda, Kampala, Uganda); Shevin T Jacob, Tom E Fletcher (Liverpool School of Tropical Medicine, Liverpool, UK); William A Fischer II (The University of North Carolina at Chapel, Chapel Hill, NC, USA); Daniel Youkee, Marta Lado (Partners in Health, Freetown, Sierra Leone); Daniel Youkee, Marta Lado (King's College London, London, UK); Hans-Joerg Lang (Witten/Herdecke University, Witten, Germany); Nathan Kenya Mugisha, Bernard Opar Toliva, Savio Mwaka (World Alliance for Lung and Intensive Care Medicine in Uganda); Olivia Namusisi (African Field Epidemiology Network, Kampala, Uganda); Christopher Nsereko (Entebbe Regional Referral Hospital); Ibrahim Mugerwa, Susan Nabadda (Uganda National Health Laboratory Services, Kampala, Uganda); Isaac Ssewanyana (Uganda National Health Laboratory Services, Kampala, Uganda).
ZK led the conceptualisation and drafting of the manuscript, and ARA, JRH, and MNi were involved in conceptualisation. ZK, JRH, MNi, and SRA participated in the field response, in which ZK, MNi, SRA, ARA, and DE accessed and verified the raw data. ZK, MNi, and SRA collected data in the field, conducted data validation and data analysis, and edited and reviewed the manuscript. ARA and JRH reviewed the analyses and manuscript, and had final oversight. JRAO, DA, HGM, DJK, ANM, AK, MNa, IM, PJE, and AN were involved in the response coordination, and reviewed the analyses and manuscript. SNK, DK, BK, SG, RM, DE, HNN, JFZ, BA, PCK, MGZ, PK, BNS, RA, MWW, RZ, and TK participated in the response, data collection, and data validation in addition to editing the manuscript.
The authors of this paper have submitted an equitable partnership declaration (appendix 2). This statement allows researchers to describe how their work engages with researchers, communities, and environments in the countries of study. This statement is part of The Lancet Global Health's broader goal to decolonise global health.
Contributor Information
Zainah Kabami, Email: zkabami@uniph.go.ug.
Uganda Ebola Response Team:
Henry Kyobe Bosa, Allan Komakech, Doreen Nsiimire Gonahasa, Lilian Bulage, Irene Byakatonda Kyamwine, Denis Okethwangu, Joshua Kayiwa, Lisa J Nelson, Amy L Boore, Sandra Nabatanzi, Thomas Nsibambi, Jonathan Ntale, Christina Mwangi, Arthur G Fitzmaurice, Claire Biribawa, Enos Sande, Joseph Ojwang, Mary Choi, Terrence Lo, Jason Malefant, Trevor Shoemaker, Joel M Montgomery, Caitlin M Cossaboom, Carrie Eggers, John D Klena, Shannon Whitmer, Modupe O Osinubi, Katrin S Sadigh, Waverly Vosburgh, Mary-Claire Worrell, James A Fuller, Bernard Lubwama, Immaculate Nabukenya, Paul Edward Okello, Elizabeth Babirye Katana, Job Morukileng, Hildah Tendo Nansikombi, Paul Mbaka, Immaculate Atuhaire, Alice Asio, Sarah Elayeete, Edirisa Junior Nsubuga, Veronica Masanja, Stella Martha Migamba, Patience Mwine, Petranilla Nakamya, Rose Nampeera, Andrew Kwiringira, Mariam Komugisha, Brian Kibwika, Innocent Ssemanda, Yasiini Nuwamanya, Adams Kamukama, Dorothy Aanyu, Dominic Kizza, Shaban Senyange, Andrew Niwagaba Bakainaga, Godfrey Bwire, Felix Ocom, Milton Makoba Wetaka, Simon Kyazze, Anita Kisakye, Mohammed Lamorde, Lydia Nakiire, Peter Babigumira Ahabwe, Bernadette Basuta Mirembe, Esther Namukose Muwanguzi, Emmanuel Ochien, Innocent Komakech, Annet Martha Nankya, Sarah Byakika, Anne Nakinsinge, Daniel Ayen Okello, Sarah K Zalwango, Muzafalu Senyonga, Solome Okware, Rony Reginald Bahatungire, Ivan Kimuli, Moses Rubangakene, Richardson Mafigiri, Daniel Bulwadda, Deborah Aujo, Herbert Kiirya Isabirye, Judith Nanyondo Semanda, John-Baptist Kibanga, Mudarshir Bbuye, Jovan Baryamujura, Grace Akello, Stephen Balinandi, Charles Njuguna, Alex Chimbaru, Bongomin Bodo, Tonny Akera, Philimon Kabagambe, Nasan Natseri, Sophia Mulei, Luke Nyakarahuka, Jimmy Baluku, Jackson Kyondo, Alex Tumusiime, Julius Julian Lutwama, Pontiano Kaleebu, Ben Masiira, Dativa Aliddeki, Remmy Buhuguru, Hakeem Kasumba, Bosco Vito Sendikadiwa, Joseph Kabanda, Shevin T Jacob, Tom E Fletcher, William A Fischer II, Daniel Youkee, Marta Lado, Hans-Joerg Lang, Nathan Kenya Mugisha, Olivia Namusisi, Bernard Opar Toliva, Savio Mwaka, Christopher Nsereko, Charles Okot Lukoya, Annet Alenyo Ngabirano, John- Baptist Waniaye, Michael Mwanga, Jackson Amone, Paska Apiyo, Richard Kabanda, Ibrahim Mugerwa, Isaac Ssewanyana, and Susan Nabadda
Supplementary Materials
References
- 1.Kuhn JH, Adachi T, Adhikari NKJ, et al. New filovirus disease classification and nomenclature. Nat Rev Microbiol. 2019;17:261–263. doi: 10.1038/s41579-019-0187-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO Ebola disease. 2024. https://www.afro.who.int/health-topics/ebola-disease
- 3.WHO Ebola disease caused by Sudan ebolavirus—Uganda. 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON428
- 4.Centers for Disease Control and Prevention Ebola. Outbreak history. 2024. https://www.cdc.gov/vhf/ebola/history/chronology.html
- 5.Ministry of Health Republic of Uganda. Case definitions for Ebola virus disease. 2024. https://www.health.go.ug/2022/09/22/case-definitions-for-ebola-virus-disease
- 6.Okware SI, Omaswa FG, Zaramba S, et al. An outbreak of Ebola in Uganda. Trop Med Int Health. 2002;7:1068–1075. doi: 10.1046/j.1365-3156.2002.00944.x. [DOI] [PubMed] [Google Scholar]
- 7.Deng IM, Duku O, Gillo AL, et al. Ebola haemorrhagic fever in Sudan, 1976. Report of a WHO/International study team. Bull World Health Organ. 1978;56:247–270. [PMC free article] [PubMed] [Google Scholar]
- 8.Chowell G, Hengartner NW, Castillo-Chavez C, Fenimore PW, Hyman JM. The basic reproductive number of Ebola and the effects of public health measures: the cases of Congo and Uganda. J Theor Biol. 2004;229:119–126. doi: 10.1016/j.jtbi.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Gonahasa DN, Basuta BM, Nabatanzi S, Kwesiga B, Bulage L, Ario AR. Prompt response to a cross-border plague outbreak in Zombo District, minimized spread, Uganda, March 2019. Journal of Intervational Epidemiology and Public Health. 2022;5:6. [Google Scholar]
- 10.Nsio J, Ardiet D-L, Coulborn RM, et al. Differential symptomology of possible and confirmed Ebola virus disease infection in the Democratic Republic of the Congo: a retrospective cohort study. Lancet Infect Dis. 2023;23:91–102. doi: 10.1016/S1473-3099(22)00584-9. [DOI] [PubMed] [Google Scholar]
- 11.Wamala JF, Lukwago L, Malimbo M, et al. Ebola hemorrhagic fever associated with novel virus strain, Uganda, 2007–2008. Emerg Infect Dis. 2010;16:1087–1092. doi: 10.3201/eid1607.091525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nansikombi HT, Kwesiga B, Aceng FL, Ario AR, Bulage L, Arinaitwe ES. Timeliness and completeness of weekly surveillance data reporting on epidemic prone diseases in Uganda, 2020–2021. BMC Public Health. 2023;23:647. doi: 10.1186/s12889-023-15534-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nyakarahuka L, Mulei S, Whitmer S, et al. First laboratory confirmation and sequencing of Zaire ebolavirus in Uganda following two independent introductions of cases from the 10th Ebola outbreak in the Democratic Republic of the Congo, June 2019. PLoS Negl Trop Dis. 2022;16 doi: 10.1371/journal.pntd.0010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masiira B, Nakiire L, Kihembo C, et al. Evaluation of integrated disease surveillance and response (IDSR) core and support functions after the revitalisation of IDSR in Uganda from 2012 to 2016. BMC Public Health. 2019;19:46. doi: 10.1186/s12889-018-6336-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lefebvre A, Fiet C, Belpois-Duchamp C, Tiv M, Astruc K, Aho Glélé LS. Case fatality rates of Ebola virus diseases: a meta-analysis of World Health Organization data. Med Mal Infect. 2014;44:412–416. doi: 10.1016/j.medmal.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Nyakarahuka L, Kankya C, Krontveit R, et al. How severe and prevalent are Ebola and Marburg viruses? A systematic review and meta-analysis of the case fatality rates and seroprevalence. BMC Infect Dis. 2016;16:708. doi: 10.1186/s12879-016-2045-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ministry of Health Republic of Uganda. Hospitals. https://www.health.go.ug/hospitals/#:~:text=45%C2%B716%25%20(3%2C133)%20of%20health,7)%20community%2Downed%20facilities
- 18.Agua-Agum J, Ariyarajah A, Blake IM, et al. Ebola virus disease among male and female persons in west Africa. N Engl J Med. 2016;374:96–98. doi: 10.1056/NEJMc1510305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nkangu MN, Olatunde OA, Yaya S. The perspective of gender on the Ebola virus using a risk management and population health framework: a scoping review. Infect Dis Poverty. 2017;6:135. doi: 10.1186/s40249-017-0346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forna A, Nouvellet P, Dorigatti I, Donnelly CA. Case fatality ratio estimates for the 2013–2016 west African Ebola epidemic: application of boosted regression trees for imputation. Clin Infect Dis. 2020;70:2476–2483. doi: 10.1093/cid/ciz678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garske T, Cori A, Ariyarajah A, et al. Heterogeneities in the case fatality ratio in the west African Ebola outbreak 2013–2016. Philos Trans R Soc Lond B Biol Sci. 2017;372 doi: 10.1098/rstb.2016.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smit MA, Michelow IC, Glavis-Bloom J, Wolfman V, Levine AC. Characteristics and outcomes of pediatric patients with Ebola virus disease admitted to treatment units in Liberia and Sierra Leone: a retrospective cohort study. Clin Infect Dis. 2017;64:243–249. doi: 10.1093/cid/ciw725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selvaraj SA, Lee KE, Harrell M, Ivanov I, Allegranzi B. Infection rates and risk factors for infection among health workers during Ebola and Marburg virus outbreaks: a systematic review. J Infect Dis. 2018;218:S679–S689. doi: 10.1093/infdis/jiy435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brainard J, Hooper L, Pond K, Edmunds K, Hunter PR. Risk factors for transmission of Ebola or Marburg virus disease: a systematic review and meta-analysis. Int J Epidemiol. 2016;45:102–116. doi: 10.1093/ije/dyv307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faye O, Boëlle P-Y, Heleze E, et al. Chains of transmission and control of Ebola virus disease in Conakry, Guinea, in 2014: an observational study. Lancet Infect Dis. 2015;15:320–326. doi: 10.1016/S1473-3099(14)71075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barbarossa MV, Dénes A, Kiss G, Nakata Y, Röst G, Vizi Z. Transmission dynamics and final epidemic size of Ebola virus disease outbreaks with varying interventions. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bebell LM. In: Pregnant in the time of Ebola. Schwartz DA, Anoko JN, Abramowitz SA, editors. Springer; Switzerland: 2019. Ebola virus disease and pregnancy: perinatal transmission and epidemiology; pp. 53–65. [Google Scholar]
- 28.Bausch DG, Crozier I. The Liberia men's health screening program for Ebola virus: win–win–win for survivor, scientist, and public health. Lancet Glob Health. 2016;4:e672–e673. doi: 10.1016/S2214-109X(16)30207-8. [DOI] [PubMed] [Google Scholar]
- 29.Whitmer SLM, Ladner JT, Wiley MR, et al. Active Ebola virus replication and heterogeneous evolutionary rates in EVD survivors. Cell Rep. 2018;22:1159–1168. doi: 10.1016/j.celrep.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets upon which our findings have been based belong to the Ministry of Health of Uganda. An anonymised, de-identified version of the dataset can be availed upon reasonable request to the corresponding author, with permission from the Ministry of Health.