Abstract
The electrophysiological properties of the hearts of women and men are different. These differences are at least partly mediated by the actions of circulating estrogens and androgens on the cardiomyocytes. Experimentally, much of our understanding in this field is based on studies focusing on ventricular tissue, with considerably less known in the context of atrial electrophysiology. The aim of this investigation was to compare the electrophysiological properties of male and female atria and assess responses to acute sex steroid exposure. Age-matched adult male and female C57BL/6 mice were anesthetized (4 % isoflurane) and left atria isolated. Atria were loaded with Di-4-ANEPPS voltage sensitive dye and optical mapping performed to assess action potential duration (APD; at 10 %, 20 %, 30 %, 50 %, and 70 % repolarization) and conduction velocity in the presence of 1 nM and 100 nM 17β-estradiol or testosterone. Male and female left atria demonstrated similar baseline action potential duration and conduction velocity, with significantly greater APD70 spatial heterogeneity evident in females. 17β-estradiol prolonged action potential duration in both sexes – an effect that was augmented in females. Atrial conduction was slowed in the presence of 100 nM 17β-estradiol in both males and females. Testosterone prolonged action potential duration in males only and did not modulate conduction velocity in either sex. This study provides novel insights into male and female atrial electrophysiology and its regulation by sex steroids. As systemic sex steroid levels change and intra-cardiac estrogen synthesis capacity increases with aging, these actions may have an increasingly important role in determining atrial arrhythmia vulnerability.
Keywords: Optical mapping, Atrial electrophysiology, Action potential duration, Conduction velocity, Sex differences, Sex steroids
Graphical abstract
1. Introduction
The electrophysiological properties of the hearts of women and men are different. Women exhibit faster heart rates, longer rate-corrected Q-T intervals and steeper electrical restitution than men [1,2]. These differences are at least partly mediated by the actions of circulating estrogens and androgens that bind to functional receptors localized on/within the cardiomyocytes [3]. Expression of androgen and estrogen receptors (multiple subtypes including estrogen receptors α and β, and G protein-coupled estrogen receptor) within the heart can elicit genomic transcriptional changes and/or more rapid acute actions through activation of numerous signaling pathways [4]. The extent to which sex steroids exert these genomic/non-genomic actions are likely dependent on many factors including the concentration and exposure time to the sex steroids as well as relative receptor expression/localisation and post-translational modification status [5,6].
Experimentally, much of our understanding of sex steroid influence on cardiomyocyte electrophysiology is based on studies focusing on ventricular tissue. Estrogens and androgens have been shown to exert contrasting actions on repolarization currents – estradiol decreases rapid and slowly activating potassium currents (IKr and IKs respectively) and testosterone increases IKr and IKs [7]. There is a lack of consensus in relation to sex steroid influence on the L-type Ca current (ICa,L), with evidence suggesting sex steroids can decrease or increase ICa,L [7]. We and others have shown that ventricular cardiomyocyte Ca2+ transients differ in males and females, at least partly due to contrasting actions of sex steroids on sarcoplasmic reticulum Ca2+ release [4,8,9]. The actions of estrogens and androgens on ventricular cardiomyocyte Na+ entry are unclear.
Androgen and estrogen receptors have been shown in mice to be expressed in atria [10,11], indicating capacity to elicit both genomic and acute sex steroids actions. How sex steroids influence atrial electrophysiology is less clear [12]. This represents an important knowledge gap, especially in the context of atrial fibrillation – the most common sustained arrhythmia in both women and men. Sex differences are evident in the epidemiology and clinical presentation of atrial fibrillation [12,13], and the underlying mechanisms are poorly understood. At the cellular level, atrial fibrillation can occur in response to heterogenous prolongation/shortening of the cardiac action potential duration (APD) and/or conduction slowing [14]. Changes in these properties in response to sex steroids may therefore be important in determining vulnerability to atrial arrhythmias.
To better understand the etiology and optimal therapeutic treatment of atrial arrhythmias in women and men, a clearer understanding of the fundamental influence of sex and sex steroids on atrial electrophysiology is required. In this study, high spatio-temporal optical mapping technologies were utilized to assess action potential and conduction properties of isolated left atria from female and male mice. The acute actions of two different concentrations of either estradiol or testosterone on atrial electrophysiology were then investigated. Initially, atrial electrophysiological responses to a lower concentration of each sex steroid corresponding to approximate ‘physiological’ levels within the circulation were assessed [15,16]. Subsequently, atria were exposed to higher concentrations modelling context-specific settings – specifically, high testosterone levels following exogenous androgen supplementation and high estradiol levels associated with intra-cardiac estrogen synthesis/release. We have previously shown that atria express the aromatase enzyme [17]. This capacity to synthesize estrogens from testosterone would be predicted to culminate in much higher local estrogen concentrations within the extracellular milieu of neighboring cardiomyocytes relative to circulating levels.
2. Materials and methods
2.1. Animal details
Mouse experiments were conducted under the U.K. Animals (Scientific Procedures) Act 1986 and approved by the Home Office (PPL 30/2967) and the institutional review board at the University of Birmingham. C57BL/6 male and female mice were housed in individually ventilated cages, under 12 h light/dark cycles (22 °C, 55 % humidity). Chow and drinking water were available ad libitum.
2.2. Isolated left atrial optical mapping
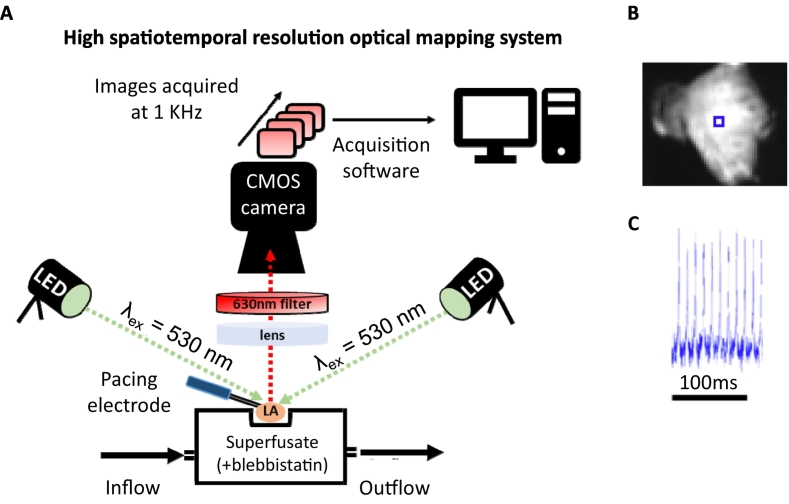
Optical mapping experiments were performed (Fig. 1) as previously reported [18,19]. At approximately 28 weeks of age, male and female mice were anesthetized (4 % isoflurane in O2, 3 l/min) and hearts retrogradely perfused on a Langendorff apparatus (4 ml/min) with 37 °C Krebs-Henseleit buffer (in mM: NaCl, 118.0; KCl, 3.52; MgSO47H2O, 0.83; KH2PO4, 1.18; NaHCO3, 24.90; glucose, 11.0; CaCl2, 1.80; 95 % O2/5% CO2). Hearts were loaded with the voltage-sensitive Di-4-ANEPPS dye (5 μM; Cambridge Biosciences, Cambridge, UK) via bolus injection into the perfusate (5 min). The left atrium was then resected, pinned flat onto the silicone base of an organ bath and continuously superfused with Krebs-Henseleit buffer supplemented with blebbistatin (42.75 μM; 37 °C; 95 % O2/5% CO2) to reduce motion artefacts [20].
Fig. 1.
Utilising optical mapping for imaging potentiometric dye loaded mouse left atria. (A) High spatiotemporal resolution optical mapping system. (B) Exemplar fluorescent image of Di-4-ANEPPS-loaded mouse left atrium. (C) Exemplar trace from marked blue square box collected from Di-4-ANEPPS-loaded mouse left atrium stimulated with paired electrodes where indicates. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Left atria were paced from the posterior wall using a 2 ms bipolar pulse via platinum electrodes at twice the capture threshold. Di-4-ANEPPS was excited at 530 nm by two twin LEDs (Cairn Research, Kent, UK). Emitted fluorescence (630 nm) was captured at 1KHz using a high spatial resolution ORCA flash 4.0 camera (Hamamatsu Photonics, Japan). Effective pixel size was 71.4 μm/pixel. Wide field macroscopic images of tissue sequentially paced at 120 ms, 100 ms and 80 ms cycle lengths (100 pulses/cycle length) were acquired using WinFluor V3.4.9 at various recording timepoints.
2.3. Treatment of left atria with sex steroids
After 10 min of equilibration, a baseline recording of action potentials and conduction properties were made at all cycle lengths. Atria were then superfused with two sequential doses of either 17β-estradiol (1 nM for 10mins, then 100 nM for 10mins), testosterone (1 nM for 10mins, then 100 nM for 10mins), or ethanol vehicle (0.0001 % for 10mins, then 0.01 % for 10mins). At the end of each 10 min superfusion period, action potential and conduction property recordings were made at all cycle lengths. Estradiol/testosterone concentrations and timing of administration were based on previous ex vivo/in vitro studies assessing acute influence of sex steroids on cardiac tissues [[21], [22], [23], [24]].
2.4. Optical mapping analysis
Optical mapping data were analyzed using ElectroMap [18,25]. Ensemble averaging of the last 10 beats of each cycle length was used to assess conduction velocity and APD at 10, 20, 30, 50 and 70 % repolarization (APD30, APD50, APD70) across the whole left atrium. APD70 data are shown in the Figs. of the main text, and APD30/APD50 data shown in the Supplementary Figs. Images were spatially and temporally filtered with a 4 × 4 Gaussian filter and a 3rd order Savitzky-Golay filter, respectively. Non-physiological baseline deviations were corrected for using a Top-Hat filter (100 ms). Conduction and APD70 spatial heterogeneity were defined as the standard deviation of the mean conduction velocity or APD70 measured across a whole left atrium respectively. APD70 alternans, defined as the electrical discordant in duration or amplitude, were analyzed at all pacing cycle lengths.
2.5. Statistical analysis
All data are presented as mean ± standard error and were analyzed blinded. Statistical analyses were performed with one-way, one-way repeated measures and two-way ANOVA as appropriate (GraphPad Prism 8.4) and specifically indicated in the Figure legends throughout. Data were tested for normality using Shapiro-Wilk testing. P < 0.05 was deemed significant and n denotes the number of mice.
3. Results
3.1. Comparable baseline electrophysiological properties in female and male left atria
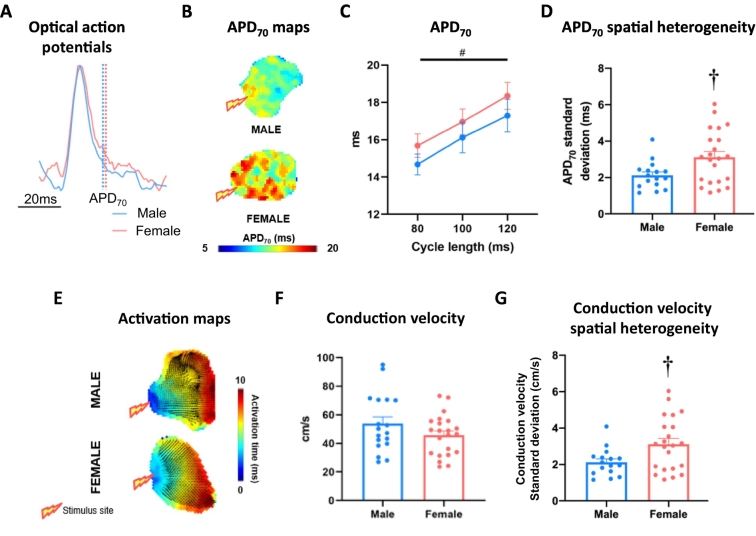
Left atrial APD did not differ between female and male atria and both sexes exhibited APD-rate dependency (Fig. 2C, Supplementary Fig. 1). Females demonstrated more variability in APD70 across the left atrium than males (100 ms cycle length, male vs female: 2.1 ± 0.2 ms vs 3.1 ± 0.3 ms, P < 0.05; Fig. 2D). Conduction velocity was consistent between sexes (100 ms cycle length, male vs female:53.8 ± 4.8 cm/s vs 45.7 ± 3.0 cm/s, P = ns; Fig. 2E-F).
Fig. 2.
Basal electrophysiology in male and female mouse left atria. (A) Exemplar ensemble averaged optical action potential traces, indicating APD70 measurement point in male and female left atria. (B) APD70 maps of tissue paced at 100 ms cycle length. (C) Mean APD70 across 80, 100 and 120 ms paced cycle lengths. (D) APD70 spatial heterogeneity at 100 ms cycle length. (E) Exemplar activation maps from male and female left atria at 100 ms cycle length. (F) Mean conduction velocity of tissue paced at 100 ms cycle length. (G) Male and female conduction velocity spatial heterogeneity (100 ms cycle length). Statistical analysis was conducted using two-way repeated measures ANOVA across different cycle lengths and unpaired t-tests at single 100 ms cycle length. †P < 0.05 between sexes and #P < 0.05 indicates cycle length effect; n = 18 and n = 20 for male and female, respectively.
3.2. Acute estradiol prolongs repolarization in the left atria of both males and females
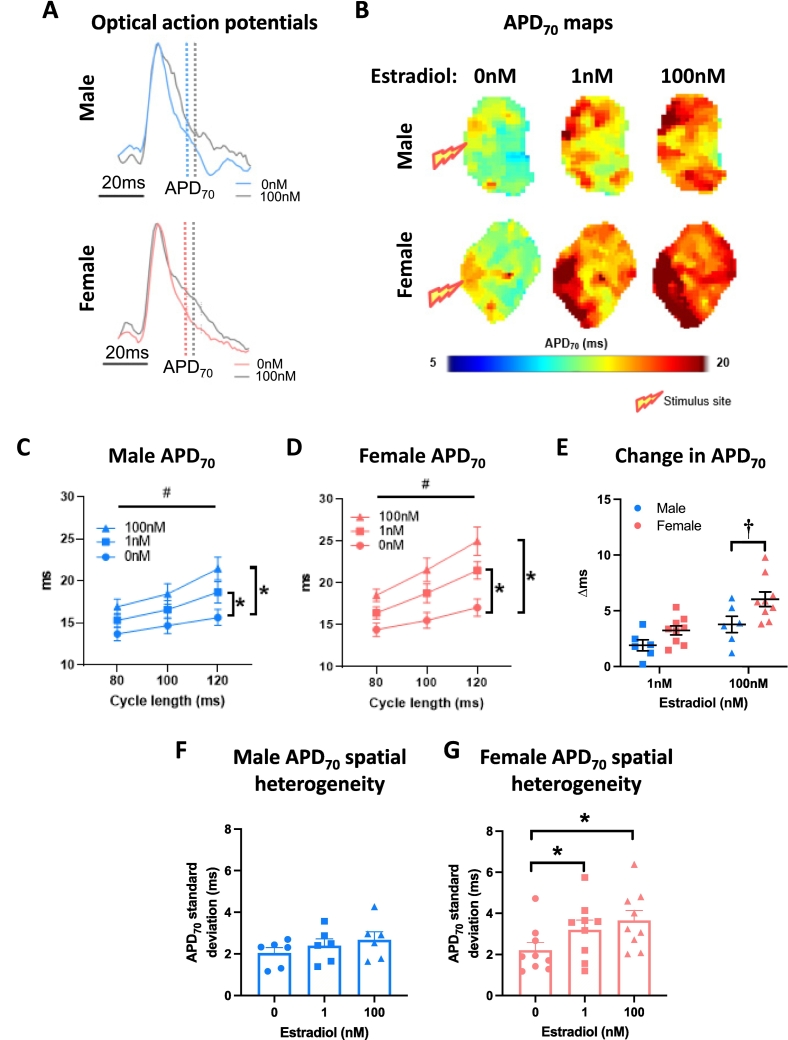
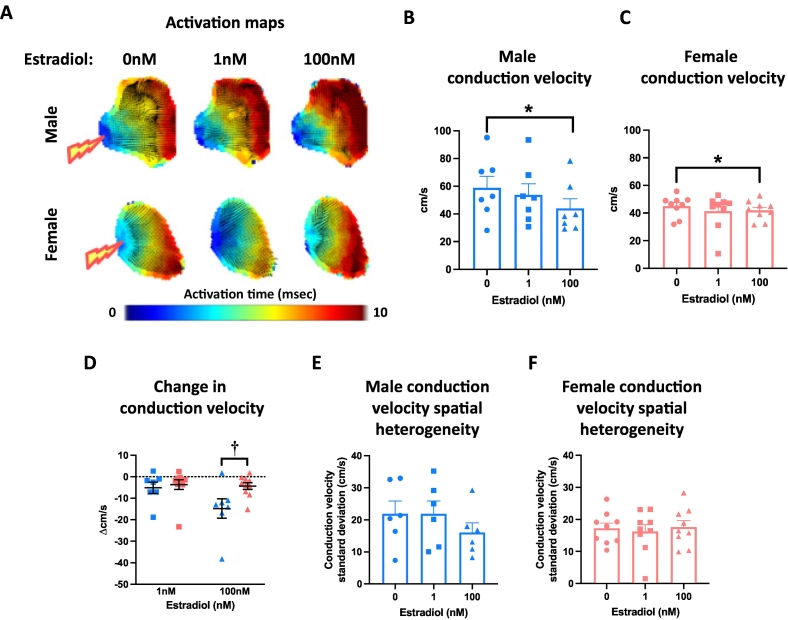
Left atria were superfused with either 17β-estradiol (1 nM, 100 nM) or ethanol vehicle (0.0001 %, 0.01 % respectively). Both concentrations of estradiol prolonged left atrial APD in males and females (Fig. 3A-D). Onset of estradiol influence was apparent at APD30 in both females and males, with evidence for the earlier onset at APD20 in females only (Supplementary Fig. 2). Left atrial APD70 spatial heterogeneity was significantly increased in response to 1 nM and 100 nM estradiol in female atria, but not males (Fig. 3F-G). This was associated with increased APD70 alternans in both female and male atria treated with estradiol (Supplementary Fig. 3). Conduction velocity decreased in response to 100 nM estradiol (but not 1 nM estradiol) in the atria of both males and females (Fig. 4A-C). The extent of this conduction slowing was greater in male than female left atria (Fig. 4D). Conduction spatial heterogeneity was unaffected by estradiol in males and females (Fig. 4E-F).
Fig. 3.
Estradiol prolongs left atrial repolarisation to a greater extent in females than males. (A) Exemplar left atrial optical action potentials treated with 0 nM or 100 nM estradiol. (B) APD70 maps at 100 ms cycle length. (C—D) Male and female mean left atrial APD70 with 0 nM, 1 nM and 100 nM estradiol treatment at 80 ms, 100 ms and 120 ms cycle lengths. (E) Estradiol-induced change in APD70 in male vs female left atria. (F-G) Male and female APD70 spatial heterogeneity with increasing estradiol concentration (100 ms cycle length). Statistical analysis was conducted using two-way across different cycle lengths or one-way repeated measures ANOVA with Sidak's multiple comparison tests across different sex steroid concentrations. #, * and † indicate P < 0.05 cycle length effect, treatment effect (vs 0 nM at all cycle lengths) and sex difference, respectively; n = 6–9.
Fig. 4.
Estradiol slows left atrial conduction velocity to a greater extent in males than females. (A) Exemplar male and female left atrial activation maps treated with 0 nM, 1 nM or 100 nM estradiol. (B—C) Male and female mean conduction velocity with 0 nM, 1 nM and 100 nM estradiol treatment. (D) Estradiol-induced change in conduction velocity in male vs female left atria. (E-F) Male and female conduction velocity spatial heterogeneity with increasing estradiol concentration (100 ms cycle length). Statistical analysis was conducted using one-way (B—C) or two-way (D) repeated measures ANOVA with Sidak's multiple comparisons. * and † indicate P < 0.05, treatment effect and sex difference, respectively; n = 7–9.
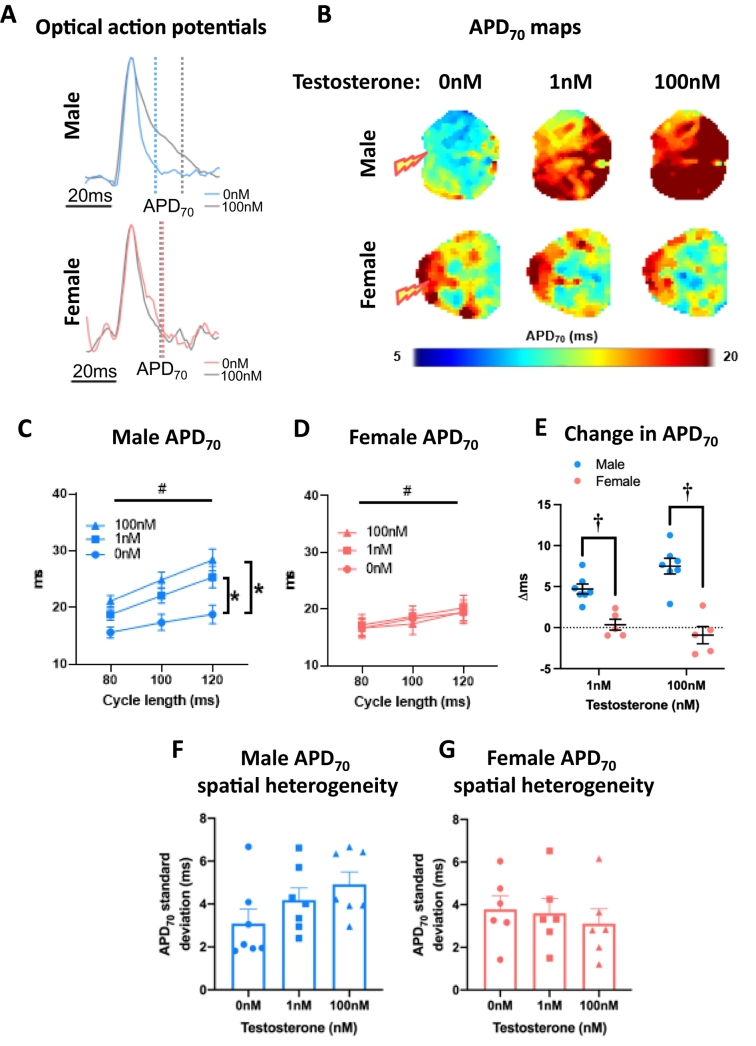
3.3. Acute testosterone prolongs repolarization in the left atria of males but not females
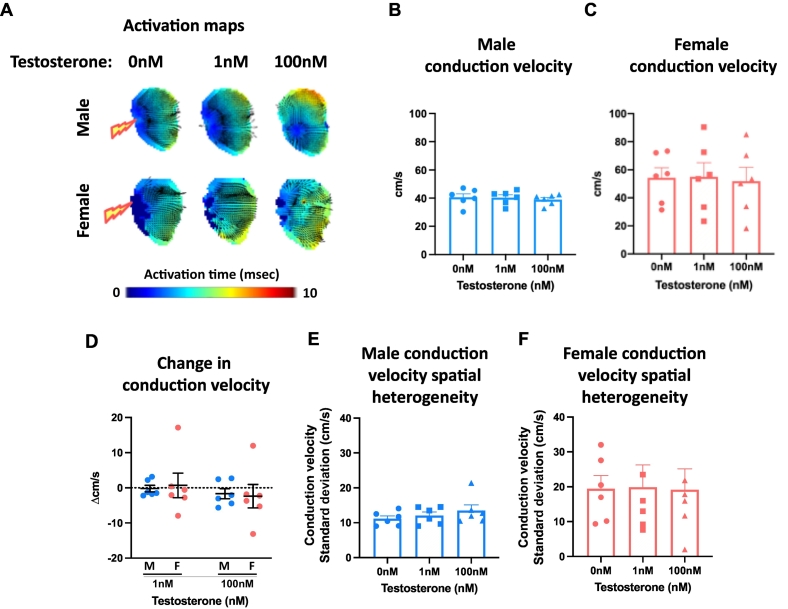
Left atria were superfused with either testosterone (1 nM, 100 nM) or ethanol vehicle (0.0001 %, 0.01 % respectively). Acute exposure to 1 nM and 100 nM testosterone prolonged APD at all paced cycle lengths in males, but not females (Fig. 5A-D, Supplementary Fig. 4). The effect in males was evident as early as APD10. The change in APD was hence significantly greater in male atria compared with female atria (Fig. 5E, Supplementary Fig. 4C,F,I&L). Testosterone had no effect on left atrial APD70 spatial heterogeneity (Fig. 5F-G), conduction velocity (Fig. 6A-C), or conduction velocity spatial heterogeneity (Fig. 6E-F) in either sex. APD70 alternans was increased in male atria only, when treated with 100 nM estradiol (Supplementary Fig. 5).
Fig. 5.
Testosterone prolongs male, but not female left atrial repolarisation. (A) Exemplar male and female ensemble averaged left atrial optical action potentials treated with 0 nM or 100 nM testosterone. (B) APD70 maps at 100 ms cycle length. (C—D) Male and female mean whole left atrial APD70 with 0 nM, 1 nM and 100 nM testosterone treatment at 80 ms, 100 ms and 120 ms cycle lengths. (E) Testosterone-induced change in APD70 in male vs female left atria. (F-G) Male and female APD70 spatial heterogeneity with increasing testosterone concentration (100 ms cycle length). Statistical analysis was conducted using two-way (C—D) or one-way (F-G) repeated measures ANOVA with Sidak's multiple comparison tests. # and * indicate P < 0.05 cycle length effect and treatment effect (vs 0 nM at all cycle lengths), respectively; n = 6–7.
Fig. 6.
Testosterone does not modulate left atrial conduction velocity. (A) Exemplar male and female left atrial activation maps treated with 0 nM, 1 nM or 100 nM testosterone. (B—C) Male and female mean conduction velocity with 0 nM, 1 nM and 100 nM testosterone treatment. (D) Testosterone-induced change in conduction velocity in male vs female left atria. (E-F) Male and female conduction velocity spatial heterogeneity with increasing testosterone concentration (100 ms cycle length). Statistical analysis was conducted using repeated measures one-way ANOVA. P = ns in all cases; n = 6–7. M, male; F, female.
4. Discussion
This novel study demonstrates a rapid modulation of male and female atrial conduction and repolarization properties in response to acute administration of sex steroids. We showed that conduction and action potential properties were similar in isolated left atria from female and male mice, though greater repolarization heterogeneity was evident in female atria (vs males). Sex differences were evident in the electrophysiological responses to acute sex steroid exposure. Estradiol prolonged atrial repolarization to a greater extent in females and augmented conduction slowing in males. The effects of testosterone on atrial electrophysiology were less pronounced. Prolongation of action potentials in the presence of testosterone was evident only in male atria, and conduction velocity did not change in either sex. These findings indicate that sex steroids could have a role in regulating atrial electrophysiology and may contribute to determining atrial arrhythmia vulnerability.
4.1. Male and female left atria demonstrate similar basal electrophysiology
Studies assessing APD in male and female atria have reported conflicting results. Patch-clamp studies with male and female rabbit right atrial cardiomyocytes paced at 1 Hz indicated no sex difference in APD [26]. A subsequent study by the same group reported longer APD in male intact rabbit left atria (vs females) paced at more physiological cycle lengths [27]. Our data demonstrate male and female mouse left atria exhibit similar repolarization times at physiological pacing frequencies. This is consistent with our previous findings in male and female Langendorff-perfused mouse hearts [28]. An advantage of using an optical mapping approach is that action potential characteristics are assessed across the whole atrium, overcoming potential artefacts attributable to selected microelectrode positioning. Indeed, this has revealed the novel finding that female epicardial repolarization is more variable across the atrium than in males. The cellular mechanisms and physiological relevance of this observation is not clear.
4.2. Estradiol prolongs repolarization in both male and female left atria
Atria were superfused with two concentrations of estradiol, initially at 1 nM and subsequently at 100 nM (10mins each). 1 nM estradiol has regularly been used as a ‘physiological’ concentration that approximately models systemic estrogen levels in women [15]. Treatment of atria with 100 nM estradiol was undertaken to model the higher concentrations of estrogens we predict would occur in a setting of intra-cardiac estrogen synthesis/release. We have previously shown that the heart has capacity to synthesize estrogens [17]. Both human atrial appendage and rodent myocardium express aromatase – the enzyme that catalyzes the conversion of testosterone to estrogen [17]. Current methodologies for quantifying tissue-specific estrogen levels are limited by sensitivity and variability. Hence, we have yet to measure the extent of estrogen synthesis within the heart. We expect intra-cardiac estrogen synthesis capacity to be relatively low compared with ovarian output, though predict the localized inter-cellular estrogen levels to be many fold higher than in the circulation. We therefore treated atria with 100 nM estradiol to model these relatively higher localized concentrations expected in a setting of intra-cardiac estrogen synthesis/release.
Both concentrations of estradiol prolonged APD in males and females – an effect that was significantly greater in females. In ventricular tissue, there is a lack of consensus as to how estrogens influence action potential morphology, with estradiol reported to cause APD shortening [24,29], prolongation [24,30,31] or no effect [32]. In addition to the implications of variation in study design and animal/tissue model, the conflicting findings in ventricular preps may be indicative of the reported biphasic influence of estrogen as concentrations increase [24]. 1 nM estradiol have been shown to prolong male and female guinea pig ventricular APD through direct inhibition of the KV11.1 channel that carries IKr [24], though it is unlikely that this explains our findings as IKr contributes minimally to atrial repolarization in mice [33,34]. Prolongation of action potential duration in male adult rat ventricular cardiomyocytes treated with estradiol for 10mins has been shown to be associated with significantly reduced Ito,s and ICa,L [24]. Both of these currents are integral to repolarization in mice atria [35]. Considering the temporal profile of estrogenic influence on APD shown in our study, we speculate that acute estradiol treatment may be modulating Ito,s and ICa,L to cause the action potential prolongation observed in isolated mouse atria.
4.3. Testosterone prolongs left atrial repolarization in males only
Similar to those studies with estradiol, atria were superfused with two concentrations of testosterone – initially at 1 nM and subsequently at 100 nM (10mins each). 1 nM testosterone more closely represents typical serum levels in women. In men, normal serum levels are approximately 10-20 nM. The higher testosterone concentration hence models that expected with testosterone supplementation [36]. Both male and female atria express functional androgen receptors with capacity to respond to testosterone treatment [10,37]. We report a sex-specific action of testosterone on atrial action potential morphology – prolonging APD in males but not females. This effect was evident as early as APD10, indicating potential influence of testosterone on INa during depolarisation and/or Ito in phase 1 of the action potential. Studies assessing the effects of testosterone on atrial electrophysiology are lacking. Our findings contrast with studies of acute testosterone administration in other cell types. Treatment of guinea-pig (combined male/female) ventricular cardiomyocytes with increasing concentrations of testosterone caused a dose-dependent decrease in APD. This was associated with a nitric oxide-dependent decrease in the ICa,L and increase in the IKs [38]. That the actions of testosterone were evident as early as APD10, indicates testosterone may be influencing INa during depolarisation and/or Ito in phase 1 of the action potential. Again, the actions of testosterone on cardiomyocyte Na+ entry are not well understood. Chronic testosterone deficiency has been shown to increase late inward Na+ current in ventricular cardiomyocytes in aged male mice [39]. The extent to which testosterone modulates fast Na+ currents in atrial cardiomyocytes is not known and further studies are required.
4.4. Estradiol but not testosterone slows conduction velocity in male and female left atria
Studies conducted in post-menopausal women indicate that acute estradiol administration slows atrial conduction [40]. We showed that higher concentrations of estradiol slowed atrial conduction in both sexes and that this was accentuated in males. These findings are intriguing as they indicate that, in some settings, estradiol may contribute to the arrhythmogenic atrial substrate. We have previously shown that acute estradiol treatment increases atrial arrhythmia incidence in isolated mouse hearts [17], challenging the pre-conception that estrogenic influence is always beneficial in the heart. This may be especially important in relation to intra-cardiac estrogen synthesis/release within atrial myocardium and epicardial adipose [17]. We expect this could predispose the heart to localized regions of estrogenic influence, with potential to exacerbate conduction heterogeneities across the atria and increase arrhythmia vulnerability.
5. Study limitations
This study represents an initial investigative step towards our understanding of sex steroidal influence on the atria, and numerous aspects remain unresolved. Experimentally, the potential effects of mechanical uncoupling in the presence of blebbistatin (42.75 μM) in this study should be considered [41]. Excitation-contraction uncouplers are required to ensure effective recording of action potential morphology in the isolated atria. Blebbistatin has been reported to modulate cardiac electrical activity [42], though several studies reports findings to the contrary [43,44].
Considering the relatively brief exposure of atria to sex steroids in this study, our findings presumably reflect non-genomic events associated with activation of intracellular signaling pathways. Further studies incorporating longer-term exposure to sex steroids, likely in vivo or in cultured atrial cardiomyocyte/tissue preparations, will reveal genomic responses of atria to sex steroids. It will be necessary to then identify the sex steroid receptor sub-types and ion channels mediating these actions and how the combination of acute and chronic sex steroid actions interact to determine action potential morphology, conduction properties, and atrial arrhythmia vulnerability. We did not address this in this study, partly due to the limitations previously reported in relation to assessing sustained atrial arrhythmias in mice [45]. Our findings of selected action potential prolongation and APD70 alternans in response to acute sex steroid exposure are intriguing and indicate a potential capacity for acute sex steroid exposure to increase atrial arrhythmias. Clearly, a more detailed investigation is required to establish the role of acute/chronic sex steroids in determining atrial arrhythmia vulnerability, in a bid to improve efficacy of anti-arrhythmic treatments in men and women.
6. Summary
This study provides novel insights into the acute regulation of atrial electrophysiology by sex steroids. The findings indicate that estrogens rapidly influence atrial function in both sexes, and that responsiveness to testosterone is more prominent in males.
CRediT authorship contribution statement
Simon P. Wells: Writing – review & editing, Writing – original draft, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. Christopher O'Shea: Writing – review & editing, Software, Methodology, Investigation, Formal analysis. Sarah Hayes: Writing – review & editing, Writing – original draft. Kate L. Weeks: Writing – review & editing, Writing – original draft. Paulus Kirchhof: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology, Investigation, Conceptualization. Lea M.D. Delbridge: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Conceptualization. Davor Pavlovic: Writing – review & editing, Writing – original draft, Supervision, Resources, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization. James R. Bell: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Investigation, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
James Bell reports financial support was provided by the National Health and Medical Research Council. Lea Delbridge reports financial support was provided by the National Health and Medical Research Council. Lea Delbridge reports financial support was provided by the Australian Research Council. Davor Pavlovic reports financial support was provided by the British Heart Foundation. Christopher O’Shea reports financial support was provided by the Wellcome Trust. Kate Weeks reports financial support was provided by the National Heart Foundation of Australia. James Bell (co-corresponding author) serves on the Editorial Board of the Journal of Molecular and Cellular Cardiology Plus. Davor Pavlovic (co-corresponding author) serves as the Editor-in-Chief of the Journal of Molecular and Cellular Cardiology Plus. Christopher O’Shea (co-author) serves as a Guest Editor for the relevant Call for Papers of the Journal of Molecular and Cellular Cardiology Plus. Lea Delbridge (co-corresponding author) serves on the Editorial Board of the Journal of Molecular and Cellular Cardiology (sister journal to JMCC Plus). Davor Pavlovic (co-corresponding author) and Kate Weeks (co-author) serve as Social Media Editors of the Journal of Molecular and Cellular Cardiology (sister journal to JMCC Plus). If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
Assistance with animal cohort management and experimental blinding from Clara Apicella and Syeeda Nashitha Kabir is gratefully acknowledged.
Funding support
This research was supported by the National Health and Medical Research Council (#1099352 and #1125453; L. M. D. Delbridge, J. R. Bell), the Australian Research Council (#DP160102404; L. M. D. Delbridge) and the British Heart Foundation (PG/17/55/33087; RG/17/15/33106; FS/19/12/34204; FS/19/16/34169; FS/PhD/22/29309; D. Pavlovic). C. O'Shea is supported by a Sir Henry Wellcome Fellowship, Wellcome Trust, 221650/Z/20/Z. K.L. Weeks is supported by a Future Leader Fellowship from the National Heart Foundation of Australia (102539).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jmccpl.2024.100079.
Contributor Information
Lea M.D. Delbridge, Email: lmd@unimelb.edu.au.
Davor Pavlovic, Email: d.pavlovic@bham.ac.uk.
James R. Bell, Email: j.bell@latrobe.edu.au.
Appendix A. Supplementary data
Supplementary figures
References
- 1.Rautaharju P.M., Zhou S.H., Wong S., Calhoun H.P., Berenson G.S., Prineas R., et al. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. 1992;8(7):690–695. [PubMed] [Google Scholar]
- 2.Malik M., Hnatkova K., Kowalski D., Keirns J.J., van Gelderen E.M. QT/RR curvatures in healthy subjects: sex differences and covariates. Am J Physiol Heart Circ Physiol. 2013;305(12):H1798–H1806. doi: 10.1152/ajpheart.00577.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menazza S., Murphy E. The expanding complexity of estrogen receptor signaling in the cardiovascular system. Circ Res. 2016;118(6):994–1007. doi: 10.1161/CIRCRESAHA.115.305376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell J.R., Bernasochi G.B., Varma U., Raaijmakers A.J., Delbridge L.M. Sex and sex hormones in cardiac stress—mechanistic insights. J Steroid Biochem Mol Biol. 2013;137:124–135. doi: 10.1016/j.jsbmb.2013.05.015. [DOI] [PubMed] [Google Scholar]
- 5.Arnal J.F., Lenfant F., Metivier R., Flouriot G., Henrion D., Adlanmerini M., et al. Membrane and nuclear estrogen receptor alpha actions: from tissue specificity to medical implications. Physiol Rev. 2017;97(3):1045–1087. doi: 10.1152/physrev.00024.2016. [DOI] [PubMed] [Google Scholar]
- 6.Björnström L., Sjöberg M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol. 2005;19(4):833–842. doi: 10.1210/me.2004-0486. [DOI] [PubMed] [Google Scholar]
- 7.Costa S., Saguner A.M., Gasperetti A., Akdis D., Brunckhorst C., Duru F. The link between sex hormones and susceptibility to cardiac arrhythmias: from molecular basis to clinical implications. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.644279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curl C.L., Wendt I.R., Kotsanas G. Effects of gender on intracellular [Ca2+] in rat cardiac myocytes. Pflugers Arch. 2001;441(5):709–716. doi: 10.1007/s004240000473. [DOI] [PubMed] [Google Scholar]
- 9.Farrell S.R., Ross J.L., Howlett S.E. Sex differences in mechanisms of cardiac excitation-contraction coupling in rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2010;299(1):H36–H45. doi: 10.1152/ajpheart.00299.2010. [DOI] [PubMed] [Google Scholar]
- 10.Lizotte E., Grandy S.A., Tremblay A., Allen B.G., Fiset C. Expression, distribution and regulation of sex steroid hormone receptors in mouse heart. Cell Physiol Biochem. 2009;23(1–3):75–86. doi: 10.1159/000204096. [DOI] [PubMed] [Google Scholar]
- 11.Patel V.H., Chen J., Ramanjaneya M., Karteris E., Zachariades E., Thomas P., et al. G-protein coupled estrogen receptor 1 expression in rat and human heart: protective role during ischaemic stress. Int J Mol Med. 2010;26(2):193–199. doi: 10.3892/ijmm_00000452. [DOI] [PubMed] [Google Scholar]
- 12.Odening K.E., Deiß S., Dilling-Boer D., Didenko M., Eriksson U., Nedios S., et al. Mechanisms of sex differences in atrial fibrillation: role of hormones and differences in electrophysiology, structure, function, and remodelling. Europace. 2019;21(3):366–376. doi: 10.1093/europace/euy215. [DOI] [PubMed] [Google Scholar]
- 13.Westerman S., Wenger N. Gender differences in atrial fibrillation: a review of epidemiology, management, and outcomes. Curr Cardiol Rev. 2019;15(2):136–144. doi: 10.2174/1573403X15666181205110624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heijman J., Guichard J.-B., Dobrev D., Nattel S. Translational challenges in atrial fibrillation. Circ Res. 2018;122(5):752–773. doi: 10.1161/CIRCRESAHA.117.311081. [DOI] [PubMed] [Google Scholar]
- 15.Frederiksen H., Johannsen T.H., Andersen S.E., Albrethsen J., Landersoe S.K., Petersen J.H., et al. Sex-specific estrogen levels and reference intervals from infancy to late adulthood determined by LC-MS/MS. J Clin Endocrinol Metab. 2020;105(3):754–768. doi: 10.1210/clinem/dgz196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Travison T.G., Vesper H.W., Orwoll E., Wu F., Kaufman J.M., Wang Y., et al. Harmonized reference ranges for circulating testosterone levels in men of four cohort studies in the United States and Europe. J Clin Endocrinol Metab. 2017;102(4):1161–1173. doi: 10.1210/jc.2016-2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernasochi G.B., Boon W.C., Curl C.L., Varma U., Pepe S., Tare M., et al. Pericardial adipose and aromatase: a new translational target for aging, obesity and arrhythmogenesis? J Mol Cell Cardiol. 2017;111:96–101. doi: 10.1016/j.yjmcc.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Holmes A.P., Yu T.Y., Tull S., Syeda F., Kuhlmann S.M., O’Brien S.-M., et al. A regional reduction in Ito and IKACh in the murine posterior left atrial myocardium is associated with action potential prolongation and increased ectopic activity. Public Libr Sci. 2016;11(5) doi: 10.1371/journal.pone.0154077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Shea C., Holmes A.P., Yu T.Y., Winter J., Wells S.P., Correia J., et al. ElectroMap: high-throughput open-source software for analysis and mapping of cardiac electrophysiology. Sci Rep. 2019;9(1):1389. doi: 10.1038/s41598-018-38263-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Shea C., Winter J., Kabir S.N., O’Reilly M., Wells S.P., Baines O., et al. Publisher correction: high resolution optical mapping of cardiac electrophysiology in pre-clinical models. Sci Data. 2024;11(1):93. doi: 10.1038/s41597-024-02941-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bordallo J., Cantabrana B., Suárez L., Sánchez M. Testosterone inhibits cAMP-phosphodiesterases in heart extracts from rats and increases cAMP levels in isolated left atria. Pharmacology. 2011;87(3–4):155–160. doi: 10.1159/000324172. [DOI] [PubMed] [Google Scholar]
- 22.Ceballos G., Figueroa L., Rubio I., Gallo G., Garcia A., Martinez A., et al. Acute and nongenomic effects of testosterone on isolated and perfused rat heart. J Cardiovasc Pharmacol. 1999;33(5):691–697. doi: 10.1097/00005344-199905000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Duan J., Esberg L.B., Dai S., Aberle N.S., Lopez F.L., Ren J. Comparison of cardiac contractile and intracellular Ca2+ response between estrogen and phytoestrogen alpha-zearalanol in ventricular myocytes. Endocrine. 2004;24(1):33–38. doi: 10.1385/ENDO:24:1:033. [DOI] [PubMed] [Google Scholar]
- 24.Kurokawa J., Tamagawa M., Harada N., Honda S., Bai C.X., Nakaya H., et al. Acute effects of oestrogen on the guinea pig and human IKr channels and drug-induced prolongation of cardiac repolarization. J Physiol. 2008;586(12):2961–2973. doi: 10.1113/jphysiol.2007.150367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Shea C., Holmes A.P., Yu T.Y., Winter J., Wells S.P., Parker B.A., et al. High-throughput analysis of optical mapping data using ElectroMap. J Vis Exp. 2019;148 doi: 10.3791/59663. [DOI] [PubMed] [Google Scholar]
- 26.Tsai W.-C., Chen Y.-C., Lin Y.-K., Chen S.-A., Chen Y.-J. Sex differences in the electrophysiological characteristics of pulmonary veins and left atrium and their clinical implication in atrial fibrillation. Circ Arrhythm Electrophysiol. 2011;4(4):550–559. doi: 10.1161/CIRCEP.111.961995. [DOI] [PubMed] [Google Scholar]
- 27.Tsai W.-C., Chen Y.-C., Kao Y.-H., Lu Y.-Y., Chen S.-A., Chen Y.-J. Distinctive sodium and calcium regulation associated with sex differences in atrial electrophysiology of rabbits. Int J Cardiol. 2013;168(5):4658–4666. doi: 10.1016/j.ijcard.2013.07.183. [DOI] [PubMed] [Google Scholar]
- 28.Obergassel J., O’Reilly M., Sommerfeld L.C., Kabir S.N., O’Shea C., Syeda F., et al. Effects of genetic background, sex, and age on murine atrial electrophysiology. Europace. 2021;23(6):958–969. doi: 10.1093/europace/euaa369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang C., Poole-Wilson P.A., Sarrel P.M., Mochizuki S., Collins P., MacLeod K.T. Effect of 17 beta-oestradiol on contraction, Ca2+ current and intracellular free Ca2+ in guinea-pig isolated cardiac myocytes. Br J Pharmacol. 1992;106(3):739–745. doi: 10.1111/j.1476-5381.1992.tb14403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y., Hao Y.C., Song L.L., Guo S.M., Gu S.Z., Lu S.G. Effects of sex hormones on action potential and contraction of guinea pig papillary muscle. Zhongguo Yao Li Xue Bao. 1998;19(3):248–250. [PubMed] [Google Scholar]
- 31.Berger F., Borchard U., Hafner D., Pütz I., Weis T.M. Effects of 17beta-estradiol on action potentials and ionic currents in male rat ventricular myocytes. Naunyn Schmiedeberg’s Arch Pharmacol. 1997;356(6):788–796. doi: 10.1007/pl00005119. [DOI] [PubMed] [Google Scholar]
- 32.Moller R.A., Datta S., Strichartz G.R. Beta-estradiol acutely potentiates the depression of cardiac excitability by lidocaine and bupivacaine. J Cardiovasc Pharmacol. 1999;34(5):718–727. doi: 10.1097/00005344-199911000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Babij P., Askew G.R., Nieuwenhuijsen B., Su C.M., Bridal T.R., Jow B., et al. Inhibition of cardiac delayed rectifier K+ current by overexpression of the long-QT syndrome HERG G628S mutation in transgenic mice. Circ Res. 1998;83(6):668–678. doi: 10.1161/01.res.83.6.668. [DOI] [PubMed] [Google Scholar]
- 34.Cordeiro J.M., Spitzer K.W., Giles W.R. Repolarizing K+ currents in rabbit heart Purkinje cells. J Physiol. 1998;508(Pt 3):811–823. doi: 10.1111/j.1469-7793.1998.811bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Joukar S. A comparative review on heart ion channels, action potentials and electrocardiogram in rodents and human: extrapolation of experimental insights to clinic. Lab Anim Res. 2021;37(1):25. doi: 10.1186/s42826-021-00102-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gårevik N., Rane A., Björkhem-Bergman L., Ekström L. Effects of different doses of testosterone on gonadotropins, 25-hydroxyvitamin D3, and blood lipids in healthy men. Subst Abus Rehabil. 2014;5:121–127. doi: 10.2147/SAR.S71285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dart D.A., Waxman J., Aboagye E.O., Bevan C.L. Visualising androgen receptor activity in male and female mice. Public Libr Sci. 2013;8(8) doi: 10.1371/journal.pone.0071694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bai C.X., Kurokawa J., Tamagawa M., Nakaya H., Furukawa T. Nontranscriptional regulation of cardiac repolarization currents by testosterone. Circulation. 2005;112(12):1701–1710. doi: 10.1161/CIRCULATIONAHA.104.523217. [DOI] [PubMed] [Google Scholar]
- 39.Banga S., Mishra M., Heinze-Milne S.D., Jansen H.J., Rose R.A., Howlett S.E. Chronic testosterone deficiency increases late inward sodium current and promotes triggered activity in ventricular myocytes from aging male mice. Am J Physiol Heart Circ Physiol. 2023;325(2):H264–h77. doi: 10.1152/ajpheart.00505.2022. [DOI] [PubMed] [Google Scholar]
- 40.Rosano G.M., Leonardo F., Dicandia C., Sheiban I., Pagnotta P., Pappone C., et al. Acute electrophysiologic effect of estradiol 17beta in menopausal women. Am J Cardiol. 2000;86(12):1385–1387. doi: 10.1016/s0002-9149(00)01251-0. a5–6. [DOI] [PubMed] [Google Scholar]
- 41.O’Shea C., Winter J., Kabir S.N., O’Reilly M., Wells S.P., Baines O., et al. High resolution optical mapping of cardiac electrophysiology in pre-clinical models. Sci Data. 2022;9(1):135. doi: 10.1038/s41597-022-01253-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brack K.E., Narang R., Winter J., Ng G.A. The mechanical uncoupler blebbistatin is associated with significant electrophysiological effects in the isolated rabbit heart. Exp Physiol. 2013;98(5):1009–1027. doi: 10.1113/expphysiol.2012.069369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fedorov V.V., Lozinsky I.T., Sosunov E.A., Anyukhovsky E.P., Rosen M.R., Balke C.W., et al. Application of blebbistatin as an excitation-contraction uncoupler for electrophysiologic study of rat and rabbit hearts. Heart Rhythm. 2007;4(5):619–626. doi: 10.1016/j.hrthm.2006.12.047. [DOI] [PubMed] [Google Scholar]
- 44.Kappadan V., Telele S., Uzelac I., Fenton F., Parlitz U., Luther S., et al. High-resolution optical measurement of cardiac restitution, contraction, and fibrillation dynamics in beating vs. blebbistatin-uncoupled isolated rabbit hearts. Front Physiol. 2020;11:464. doi: 10.3389/fphys.2020.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu F., Pietropaolo M., Cui L., Pandit S., Li W., Tarnavski O., et al. Lack of authentic atrial fibrillation in commonly used murine atrial fibrillation models. PLoS One. 2022;17(1) doi: 10.1371/journal.pone.0256512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures