Abstract
Objective:
To evaluate sources of 90-day episode spending variation in Medicare patients undergoing bariatric surgery and whether spending variation was related to quality of care.
Summary Background Data:
Medicare’s Bundled Payments for Care Improvement-Advanced (BPCI-A) program includes the first large-scale episodic bundling program for bariatric surgery. This voluntary program will pay bariatric programs a bonus if 90-day spending following surgery falls below a pre-determined target. It is unclear what share of bariatric episode spending may be due to unnecessary variation and thus modifiable through care improvement.
Methods:
Retrospective analysis of fee-for-service Medicare claims data from 761 acute care hospitals providing inpatient bariatric surgery between January 1, 2011 and September 30, 2016. We measured associations between patient and hospital factors, clinical outcomes, and total Medicare spending for the 90-day bariatric surgery episode using multivariable regression models.
Results:
Of 64,537 patients, 46% underwent sleeve gastrectomy, 22% revisited the ED within 90 days, and 12.5% were readmitted. Average 90-day episode payments were $14,124, ranging from $12,220 at the lowest-spending quintile of hospitals to $16,887 at the highest-spending quintile. After risk adjustment, 90-day episode spending was $11,447 at the lowest quintile vs $15,380 at the highest quintile (difference $3932, p<.001). The largest components of spending variation were readmissions (44% of variation, or $2,043 per episode), post-acute care (19%, or $871), and index professional fees (15% or $450). The lowest spending hospitals had the lowest complication, ED visit, post-acute utilization, and readmission rates (p<0.001).
Conclusions and Relevance:
In this retrospective analysis of Medicare patients undergoing bariatric surgery, the largest components of 90-day episode spending variation are readmissions, inpatient professional fees, and post-acute care utilization. Hospitals with lower spending were associated with lower rates of complications, ED visits, post-acute utilization, and readmissions. Incentives for improving outcomes and reducing spending appear to be well-aligned in Medicare’s bundled payment initiative for bariatric surgery.
MINI-ABSTRACT
Medicare’s largest-scale bundled payment program expanded to cover bariatric surgery in 2020. We analyzed the relationship of variation in 90-day episode spending to readmissions and complications. We found that the largest components of spending variation were readmissions (44% of variation), post-acute care (19%), and index professional fees (15%). The lowest-spending hospitals had the lowest readmission and complication rates.
Introduction
In the effort to contain the rising costs of surgical care, bundled payment programs have emerged as a promising alternative payment model. These programs set a target payment for all services in the 30 to 90 days following surgery, aligning the financial incentives for involved providers such as acute care hospitals, physicians, and post-acute care providers. These providers are allowed to share the money that they save but are penalized when total episode spending exceeds the target—encouraging better care coordination and discouraging wasteful spending.
Medicare’s Bundled Payments for Care Improvement Advanced (BPCI-Advanced) is the most recent federal bundled payment effort and includes the first large-scale bundling program for bariatric surgery. Prior evaluations of bundled payment programs have suggested savings of nearly $800 per episode for orthopedic procedures such as lower extremity joint replacement, with the majority of savings resulting from reductions in post-acute care utilization.1,2 However, there are two areas of uncertainty in the expansion of bundled payments to bariatric surgery. First, the sources of spending variation from which to achieve savings are unknown. Second, it is unknown if the overall patterns of spending variation for bariatric surgery are related to discretionary utilization or due to complications, each of which would imply different strategies for cost savings: for instance, utilization management versus quality improvement. Identifying the sources of variation and whether they are modifiable would therefore be critical for achieving savings under bundled payments and improving care for Medicare patients undergoing bariatric surgery.
Prior research on episode spending variation in Medicare bariatric surgery patients preceded the era of sleeve gastrectomy3—now the most commonly performed bariatric procedure nationwide.4,5 We build on this work by studying a contemporary population of Medicare bariatric surgery patients who would be potentially subject to the BPCI-Advanced program. We sought to answer three key questions. First, what are the patient factors associated with variations in 90-day bariatric surgery episode spending? Second, what are the hospital factors associated with variations in 90-day bariatric surgery episode spending? Lastly, we assessed whether variations in 90-day bariatric surgery episode spending was related to differences in utilizations or complication rates after surgery.
Methods
Data Source and Study Cohort
We used 100% claims from the Medicare Provider Analysis and Review (MedPAR) file from January 2011 to September 2016 at nonfederal acute care hospitals. This dataset contains claims of all fee-for-service Medicare patients with Part A and Part B coverage. We analyzed data from inpatient, outpatient, carrier, home health, skilled nursing facility, and long stay hospitals.
We included patients aged 19–85 with continuous coverage for 3 months before and 3 months after the surgical procedure. We included only patients with Diagnosis-Related Group (DRG) codes 619, 620, or 621 reflecting bariatric surgery as well as CPT codes 43775 (for sleeve gastrectomy), 43644, and 43645 (for gastric bypass) with matching ICD9/10 diagnosis codes for morbid obesity (see Appendix). We used the Elixhauser comorbidity coding system to capture all comorbidities documented in the 3 months prior to surgery.6 To capture surgical complications related to bariatric surgery, we used ICD-9 and ICD-10 codes previously described to have high sensitivity and specificity.7 Patients undergoing laparoscopic gastric banding were not included in the cohort, since they represent a minimal proportion of Medicare patients undergoing bariatric surgery.
Hospitals were identified by provider number in the MEDPAR file, and additional hospital information was obtained from the American Hospital Association Annual Survey. We excluded hospitals that performed less than 10 bariatric operations on Medicare beneficiaries during the 7-year study period. Bariatric Center of Excellence (COE) status was obtained by reviewing publicly-available lists of ASMBS and MBSAQIP accredited centers; we assigned COE status to all hospitals that were ever accredited by the ASMBS or MBSAQIP to perform bariatric surgery during the study period.
Outcomes
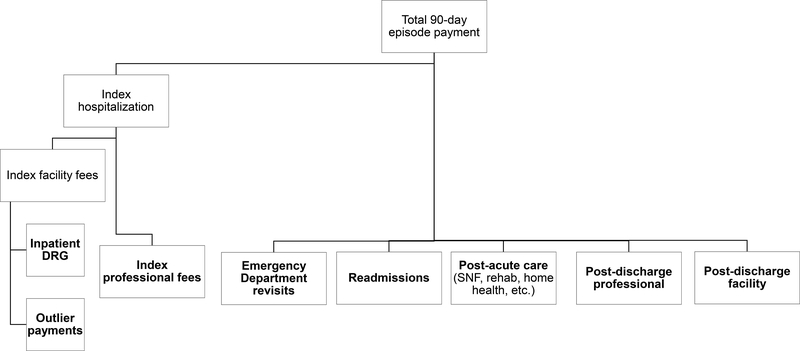
Our primary outcome was 90-day episode payments, defined as the sum of all payments from the day of surgery to 90 days afterward. These included inpatient and outpatient facility fees, professional (physician) fees, and fees from observation stays. We used price-standardization methods previously described to adjust for intended differences in Medicare payment rates (by year, wage index, and graduate medical education expenses; see eAppendix for details).8–11 We broke down episode payments as shown in Figure 1 based on revenue center codes. Emergency department stays were identified using an algorithm previously described by the Research Data Assistance Center (RESDAC).12 If a patient was initially seen in the emergency department after surgery but then readmitted as an inpatient, all spending from that encounter fell into the readmissions category.
Figure 1: Categories of Medicare Spending on Bariatric Surgery.
NOTES: Boldface categories were analyzed as mutually exclusive categories of episode spending after price-standardization and inflation-adjustment. Spending within each category was risk-adjusted using the approach described in the Methods: Analysis subsection.
Analysis
We built regression models to risk-adjust Medicare payments at the 90-day episode level. The outcome variable was the price-standardized 90-day episode payment and the explanatory variables were: year, age, sex, procedure, Elixhauser comorbidities, and hospital characteristics.
We then calculated the percentage of the unadjusted episode payment attributable to each spending category at the patient level. Ninety-day total episode payments were risk-adjusted by using generalized linear models with log link and gamma distribution to calculate predicted 90-day episode payments, calculating the observed/predicted ratio and multiplying this by the population mean episode payment. After risk-adjusting the total 90-day payment, to break the total down into risk-adjusted categories, we then multiplied the risk-adjusted total by the percent attributable to each category. Risk-adjusted payment components were then aggregated at the hospital level. Finally, we generated quintiles of average episode payments at the hospital level based on the risk-adjusted averages. All significance testing was conducted at the 5% level with two-sided tests accounting for hospital-level clustering. All analyses were performed in Stata 15 (College Station, TX). The study was deemed exempt by the University of Michigan institutional review board as it represented a secondary analysis of de-identified data.
Results
We identified 64,537 Medicare patients undergoing bariatric surgery at 761 hospitals between 2011–2016. The average age was 56; 74% were female, and 46% underwent sleeve gastrectomy, with the remaining 54% undergoing gastric bypass (Table 1). At the lowest-spending hospitals (Quintile 1), episodes were more often assigned DRG 621, representing bariatric surgery without major comorbidities or complications (81.2% in Quintile 1 vs, 67.5% in Quintile 5, difference 13.7%, 95% CI for difference 12.7%−14.7%, p <.001). Episodes in the highest-spending hospitals (Quintile 5) were more likely to involve outlier payments (2.8% vs. 0.3%, difference 2.5%, 95% CI for difference 2.2%−2.8%, p<.001), more likely to involve readmissions within 90 days (16.8% vs. 8.3%, difference 8.4%, 95% CI for difference 7.6%−9.2%, p<.001), and more likely to involve ED visits (23.7% vs. 19.5%, difference 4.2%, 95% CI for difference 3.2%−5.2% p<.001).
Table 1:
Patient Characteristics, by Quintile of Hospital Average Episode Spending
| Overall | 1 | 2 | 3 | 4 | 5 | p | |
|---|---|---|---|---|---|---|---|
| N=64,537 | N=12,947 | N=12,877 | N=12,908 | N=12,902 | N=12,903 | ||
| Age, mean (SD) | 55.73 (11.87) | 56.32 (11.71) | 55.83 (11.76) | 55.44 (11.86) | 55.70 (11.95) | 55.34 (12.04) | <0.001 |
| White race | 49,480 (76.7%) | 10,607 (81.9%) | 10,024 (77.8%) | 10,066 (78.0%) | 9,842 (76.3%) | 8,941 (69.3%) | <0.001 |
| Female gender | 47,767 (74.0%) | 9,587 (74.1%) | 9,623 (74.7%) | 9,526 (73.8%) | 9,485 (73.5%) | 9,546 (74.0%) | 0.24 |
| Sleeve gastrectomy | 29,801 (46.2%) | 6,177 (47.7%) | 5,788 (44.9%) | 5,769 (44.7%) | 5,616 (43.5%) | 6,451 (50.0%) | <0.001 |
| Gastric bypass | 34,736 (53.8%) | 6,770 (52.3%) | 7,089 (55.1%) | 7,139 (55.3%) | 7,286 (56.5%) | 6,452 (50.0%) | <0.001 |
| DRG | |||||||
| 619 (Major Complication or Comorbidity) | 3,289 (5.1%) | 311 (2.4%) | 484 (3.8%) | 634 (4.9%) | 751 (5.8%) | 1,109 (8.6%) | <0.001 |
| 620 (Complication or Comorbidity) | 13,197 (20.4%) | 2,125 (16.4%) | 2,462 (19.1%) | 2,529 (19.6%) | 2,992 (23.2%) | 3,089 (23.9%) | |
| 621 (No Complication or Comorbidity) | 48,051 (74.5%) | 10,511 (81.2%) | 9,931 (77.1%) | 9,745 (75.5%) | 9,159 (71.0%) | 8,705 (67.5%) | |
| Original Reason for Medicare Entitlement | |||||||
| Age >65 | 16,718 (25.9%) | 3,524 (27.2%) | 3,295 (25.6%) | 3,211 (24.9%) | 3,405 (26.4%) | 3,283 (25.4%) | <0.001 |
| Disability | 46,477 (72.0%) | 9,306 (71.9%) | 9,410 (73.1%) | 9,439 (73.1%) | 9,189 (71.2%) | 9,133 (70.8%) | |
| ESRD | 518 (0.8%) | 36 (0.3%) | 73 (0.6%) | 105 (0.8%) | 94 (0.7%) | 210 (1.6%) | |
| Disability+ESRD | 821 (1.3%) | 79 (0.6%) | 98 (0.8%) | 153 (1.2%) | 214 (1.7%) | 277 (2.1%) | |
| Outlier payment | 746 (1.2%) | 39 (0.3%) | 56 (0.4%) | 178 (1.4%) | 108 (0.8%) | 365 (2.8%) | <0.001 |
| ED revisit | 14,263 (22.1%) | 2,523 (19.5%) | 2,771 (21.5%) | 2,895 (22.4%) | 3,015 (23.4%) | 3,059 (23.7%) | <0.001 |
| Readmission | 8,062 (12.5%) | 1,076 (8.3%) | 1,371 (10.6%) | 1,576 (12.2%) | 1,877 (14.5%) | 2,162 (16.8%) | <0.001 |
| Any Complication | 7,248 (11.2%) | 965 (7.5%) | 1,192 (9.3%) | 1,355 (10.5%) | 1,704 (13.2%) | 2,032 (15.7%) | <0.001 |
| Comorbidity Count (Elixhauser), mean (SD) | 2.78 (1.57) | 2.60 (1.43) | 2.69 (1.55) | 2.84 (1.57) | 2.85 (1.60) | 2.93 (1.67) | <0.001 |
| Congestive heart failure | 4,062 (6.3%) | 610 (4.7%) | 710 (5.5%) | 843 (6.5%) | 964 (7.5%) | 935 (7.2%) | <0.001 |
| Chronic pulmonary disease | 18,288 (28.3%) | 3,386 (26.2%) | 3,557 (27.6%) | 3,722 (28.8%) | 3,741 (29.0%) | 3,882 (30.1%) | <0.001 |
| Diabetes w/o chronic complications | 29,304 (45.4%) | 5,965 (46.1%) | 5,792 (45.0%) | 5,892 (45.6%) | 5,893 (45.7%) | 5,762 (44.7%) | 0.14 |
| Diabetes w/ chronic complications | 4,676 (7.2%) | 709 (5.5%) | 851 (6.6%) | 962 (7.5%) | 1,074 (8.3%) | 1,080 (8.4%) | <0.001 |
| Renal failure | 4,983 (7.7%) | 672 (5.2%) | 812 (6.3%) | 991 (7.7%) | 1,150 (8.9%) | 1,358 (10.5%) | <0.001 |
| Depression | 19,686 (30.5%) | 4,092 (31.6%) | 4,068 (31.6%) | 4,119 (31.9%) | 3,818 (29.6%) | 3,589 (27.8%) | <0.001 |
| Hypertension | 50,379 (78.1%) | 9,851 (76.1%) | 9,865 (76.6%) | 10,181 (78.9%) | 10,255 (79.5%) | 10,227 (79.3%) | <0.001 |
NOTES: All continuous variables and counts (age, comorbidity count) are presented as mean (SD). All other variables are binary and presented as n, %. Quintiles are based on hospital average episode spending, adjusted for inflation and year only.
Characteristics of the identified hospitals are listed in Table 2. After adjusting for patient characteristics, average episode spending at the hospital level was not monotonically associated with any of the observable characteristics selected for study, including the share of sleeve gastrectomies, bed size, for-profit status, Medicaid share, urban vs. rural setting, geographic region, or bariatric center of excellence accreditation.
Table 2:
Hospital Characteristics, by Quintile of Hospital Average Episode Spending
| Hospital Quintile | |||||||
|---|---|---|---|---|---|---|---|
| Overall | 1 | 2 | 3 | 4 | 5 | p | |
| N=761 | N=202 | N=131 | N=111 | N=130 | N=187 | ||
| Hospital % Sleeve, mean (SD) | 53.5 (29.4) | 53.2 (31.1) | 50.8 (28.5) | 54.2 (28.4) | 51.3 (28.1) | 57.0 (29.3) | 0.34 |
| Beds, mean (SD) | 370 (256.8) | 328 (223.8) | 396 (242.6) | 398 (243.4) | 409 (289.8) | 353 (277.0) | 0.018 |
| Teaching Hospital | 165 (21.7%) | 22 (10.9%) | 36 (27.5%) | 34 (30.6%) | 37 (28.5%) | 36 (19.3%) | <0.001 |
| For Profit | 139 (18.3%) | 37 (18.3%) | 17 (13.0%) | 16 (14.4%) | 24 (18.5%) | 45 (24.1%) | 0.10 |
| Bariatric Center of Excellence | 593 (77.9%) | 144 (71.3%) | 101 (77.1%) | 98 (88.3%) | 108 (83.1%) | 142 (75.9%) | 0.006 |
| Urban | 729 (95.8%) | 188 (93.1%) | 122 (93.1%) | 110 (99.1%) | 129 (99.2%) | 180 (96.3%) | 0.012 |
| Proportion of Medicaid Days, mean (SD) | 0.2 (0.10) | 0.2 (0.11) | 0.2 (0.10) | 0.2 (0.09) | 0.2 (0.10) | 0.2 (0.10) | 0.62 |
| RN/Bed Ratio, mean (SD) | 1.8 (0.61) | 1.7 (0.58) | 1.9 (0.58) | 2.0 (0.66) | 1.8 (0.62) | 1.8 (0.62) | 0.015 |
| Region | 0.92 | ||||||
| Mid-west | 167 (21.9%) | 45 (22.3%) | 31 (23.7%) | 28 (25.2%) | 30 (23.1%) | 33 (17.6%) | |
| North-East | 181 (23.8%) | 46 (22.8%) | 33 (25.2%) | 22 (19.8%) | 35 (26.9%) | 45 (24.1%) | |
| South | 298 (39.2%) | 83 (41.1%) | 46 (35.1%) | 43 (38.7%) | 47 (36.2%) | 79 (42.2%) | |
| West | 115 (15.1%) | 28 (13.9%) | 21 (16.0%) | 18 (16.2%) | 18 (13.8%) | 30 (16.0%) | |
NOTES: All continuous variables (% sleeve, bed size, Medicaid proportion, RN/Bed ratio) are presented as mean (SD). All other variables are binary and presented as n, %. Quintiles are based on hospital average episode spending, adjusted for case mix, inflation, and year.
However, at the patient level, sleeve gastrectomy was associated with $2,011 lower total episode spending relative to gastric bypass (p<0.001, see eTable 1). Over time, episode spending for gastric bypass decreased from 2011–2016, but spending for sleeve gastrectomy did not. Although the majority of patients were entitled to Medicare due to disability (72%) rather than age (26%, Table 1), episode spending in disabled beneficiaries was $596 higher than in patients receiving Medicare due to age (p<.001, eTable 1).
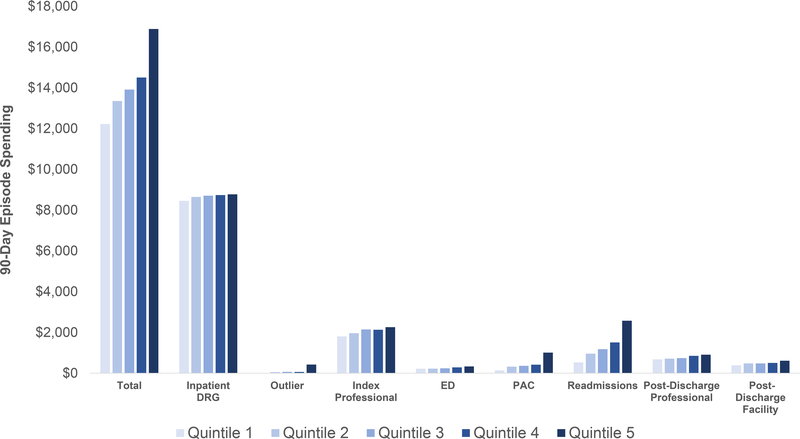
Total 90-day episode payments averaged $14,124 overall (95% CI $13,998-$14,251), from $12,220 at the lowest-spending quintile of hospitals to $16,887 at the highest-spending quintile of hospitals (Figure 2). The largest components of 90-day episode payments across all hospitals were: inpatient DRG payments (61%), professional fees during the index hospitalization (15%) and readmission spending (10%). The components that contributed most to spending variation between the 1st and 5th quintile of hospital were readmission spending (44% of variation, or a difference of $2,043 between quintile 5 and quintile 1), post-acute care (19%, or $871), and professional fees during the index hospitalization (15%, or $450) of variation (Table 3). Together, these three categories accounted for 72% of variation, or $3,364 of the spending difference between the highest and lowest-spending quintiles of hospitals.
Figure 2: Categories of Hospital-Level Medicare Spending Variation in Bariatric Surgery.
NOTES: Quintiles were assigned at the hospital level and based on average spending adjusting for patient severity, demographics, year of surgery, procedure, and hospital characteristics. Differences between Quintile 1 and Quintile 5 spending within all categories were statistically significant with P<0.01 (see Table 3). DRG = Diagnosis-related group; ED = emergency department; PAC = post-acute care
Table 3:
Components of Hospital-Level Spending Variation Across Bariatric Surgery Episodes
| Hospital Quintile | ||||||
|---|---|---|---|---|---|---|
| Spending Category | 1 | 5 | % of Total | % of Q5-Q1 Variation |
Q5-Q1 Difference | p for Q5-Q1 difference |
| Total | $12,220 | $16,887 | $4,667 | <.001 | ||
| Inpatient DRG | $8,452 | $8,772 | 59% | 7% | $320 | <.001 |
| Outlier | $8 | $422 | 1% | 9% | $414 | 0.01 |
| Index Professional | $1,807 | $2,257 | 14% | 10% | $450 | <.001 |
| Emergency Department | $217 | $329 | 2% | 2% | $112 | <.001 |
| Post-Acute Care | $140 | $1,011 | 4% | 19% | $871 | <.001 |
| Readmissions | $532 | $2,575 | 11% | 44% | $2,043 | <.001 |
| Post-Discharge Professional | $676 | $909 | 5% | 5% | $233 | <.001 |
| Post-Discharge Facility | $386 | $612 | 3% | 5% | $226 | 0.00 |
NOTES: Quintiles are based on hospital average episode spending, adjusted for case mix, inflation, year, procedure choice, and hospital characteristics. % of total is calculated as an average across the lowest- and highest-spending quintiles. % of variation is calculated as the percent of the difference in total episode spending between Q5 and Q1 attributable to each category.
At the hospital level, 90-day readmissions rates varied from 7% at the lowest-spending hospitals to 17% at the highest-spending hospitals (p<.001), after accounting for hospital characteristics, procedure choice, and patient characteristics (Table 4). Hospital-level utilization of post-acute care also varied widely, from 5% to 17% (p<.001). ED revisit rates and complication rates also varied with the quintile of hospital spending, but less widely than readmissions rates and institutional PAC utilization.
Table 4:
Utilization and Clinical Outcomes Across Quintiles of Hospital Average Episode Spending
| Hospital Quintile | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | p for Q1-Q5 difference | |
| Total Episode Spending | $12,220 | $13,350 | $13,914 | $14,505 | $16,887 | <.001 |
| Readmissions Rate | 7% | 10% | 12% | 14% | 17% | <.001 |
| ED Revisit Rate | 20% | 20% | 21% | 23% | 24% | <.001 |
| PAC Utilization Rate | 5% | 11% | 10% | 12% | 17% | <.001 |
| Complication Rate | 6% | 10% | 11% | 12% | 14% | <.001 |
NOTES: Quintiles are based on hospital average episode spending, adjusted for case mix, inflation, year, procedure choice, and hospital characteristics. All outcomes (readmissions, ED revisits, PAC utilization, and complications) are measured within 90 days of surgery and averaged at the hospital level. Complications reflect those documented in the inpatient setting within 90 days of surgery. ED = emergency department; PAC = post-acute care
Discussion
In this study of Medicare patients undergoing bariatric surgery, we found significant variation in 90-day episode spending, with payments averaging $12,383 at the lowest-spending quintile of hospitals and $16,705 in the highest-spending quintile. Nearly 80% of variation was driven by just three categories of spending: readmissions, post-acute care utilization, and physician fees from the index hospitalization. Emergency department utilization, DRG payments, outlier payments, and other professional/facility fees incurred in the 90-day episode contributed comparatively little to total episode spending. The lowest-spending hospitals had the lowest complication, ED visit, post-acute utilization, and readmission rates. Given the association between complication rates with ED visit and readmission rates, decreasing the rate of complications represents a promising target for bariatric programs aiming to achieve financial savings through BPCI-Advanced.
Though readmission rates after bariatric surgery have previously been estimated at 5–8% within 30 days,3,13–15 our study found that 90-day readmission rates vary from 7% to 17% between the lowest- and highest-spending hospitals. This differs from prior estimates for several reasons. First, as in BPCI Advanced, we used a 90-day episode definition, which captures more readmissions than the 30-day definition used in prior research. Second, we studied the Medicare population, which has a higher comorbidity burden and readmissions rate than the commercially insured population. Variation in readmissions rates explained 44% of the difference between the lowest- and highest-spending quintiles of hospitals, suggesting that readmissions will be a key target for bariatric surgery programs participating in BPCI-Advanced.
Our data also suggest that some readmissions may be avoidable at the highest spending-programs. For instance, at the lowest-spending hospitals, complication rates and readmissions rates at 90 days are similar (6% for complications, vs. 7% for readmissions). At these hospitals, the readmissions rate is approximately one-third of the ED revisit rate (20%). At the highest-spending hospitals, the readmissions rate exceeds the complication rate (17% vs. 14%, respectively), and the readmissions rate is approximately two-thirds the ED revisit rate (24%). This suggests that the highest-spending hospitals may be readmitting some patients who do not have severe complications and may have a lower threshold to admit patients who could have been treated in the emergency department.
Supporting the notion that a substantial share of readmissions and spending may be avoidable, we noted that complication rates varied more than twofold across quintiles of hospital spending, ranging from 6% of patients at the lowest-spending hospitals to 14% of patients at the highest-spending hospitals. This suggests that aggressive quality improvement efforts have the potential to reduce bariatric surgery episode spending. However, programs may also be able to reduce readmissions rates without reducing complications. According to prior research, the most common symptoms prompting ED utilization after bariatric surgery are abdominal pain, postoperative infection, dehydration, and vomiting.13 Some of these represent true surgical complications (e.g. postoperative infection), but others may simply represent inadequate symptom control and/or poor communication with one’s surgical team. Frequent communication between surgical programs and patients may avert unnecessary readmissions, by monitoring pain and hydration and intervening before readmission becomes necessary. Going forward, programs may also consider developing lower-cost models of care, such as outpatient infusion services or urgent care center bariatric treatment protocols, which are being introduced in the state of Michigan to reduce readmissions and ED utilization.16–18
The second leading component of episode spending variation in our study was post-acute care utilization, contributing to 4% of total spending but 19% of variation between the lowest- and highest-spending quintiles of hospitals. Though most hospitals spent under $500 per patient on post-acute care, the highest-spending quintile of hospitals spent $1,011 per patient on post-acute care. Given that our episode spending estimates are adjusted for patient severity, this suggests that post-acute care utilization is often independent of patient factors. In orthopedic surgery, the utilization of post-acute care (e.g. skilled nursing facilities, rehabilitation, etc.) has been shown to be highly institution-specific and preference-sensitive. Our data suggest that ensuring the appropriateness of post-acute care may be a safe way to reduce episode spending in bariatric surgery as well.
The third leading component of episode spending variation identified was professional fees during the index hospitalization, contributing 15% to total episode spending and 10% to variation between the lowest- and highest-spending quintiles of hospitals. The study design accounted for differences in procedure choice, patient severity, and geographic or contractual differences in Medicare reimbursements for the same services. Thus, payments to surgeons and anesthesiologists are unlikely to be the source of variation, since these will vary minimally across most facilities given the above adjustments. This variation is more likely to represent differences in the use of inpatient physician consultations, such as cardiology and endocrinology, which are routine in some programs but not others.19 Programs seeking to minimize unnecessary index professional payments might limit their reliance on new inpatient consultations, instead establishing medical management plans (e.g. cardiovascular and endocrine care) on an outpatient basis prior to the surgical admission.
BPCI-Advanced participants aiming to earn reconciliation payments will need to keep 90-day episode spending below their program-specific target price, which is based on a complex prediction model that incorporates historical spending, case mix, and peer group characteristics and spending trends. The 90-day spending prediction is discounted by 3%, so participants must spend 97% or less of their predicted amount in order to earn reconciliation payments.20 A 3% discount from the total 90-day payment would range from $350 to $500 for most hospitals in this analysis. As shown in Table 4, spending varies between the lowest- and highest-spending hospitals by over $500 within the categories of readmissions and post-acute care. This suggests that a promising strategy for high-spending hospitals to reduce 3% (or more) of their episode spending would be to focus on these two categories.
Though this analysis suggests that incentives in BPCI-Advanced are well-aligned with the clinical goal of providing efficient, high-quality care, we cannot be certain that the policy will avoid unintended consequences. Like other pay-for-performance efforts, BPCI-Advanced could encourage “cherry picking” healthier patients, or discourage riskier operations like gastric bypass or revisional surgery. The program’s target pricing methods account for patients’ comorbidities, recent healthcare utilization, and DRG assignment—which address these concerns to some degree. The program also compares hospitals to a “peer group” with similar teaching, safety net, and urban/rural status. Bundled payment programs for other conditions such as lower extremity joint replacement have not been associated with significant cherry picking, suggesting that this is unlikely in bariatric bundles as well.2,21 Regarding procedure choice, though incentives to minimize complications may potentially bias hospitals against higher-risk procedures such as revisional surgery or gastric bypass compared to sleeve gastrectomy, existing data suggest that procedure choice is still largely driven by surgeons.22 As the surgeon-level RVU assignments and reimbursements for riskier procedures are not affected, it is unlikely that BPCI-A would result in a large shift towards sleeve gastrectomy above and beyond current secular trends. Finally, the use of observation status has risen in recent years and may have artificially decreased readmissions rates at some facilities more than others.23,24 However, Medicare spending on observation stays is still counted within the 90-day episode payment, so this is unlikely to be a promising strategy for gaming programs such as BPCI-A.
This study has several limitations. First, since the list of participants in BPCI-Advanced bariatric surgery bundles has not yet been released, we are unable to comment specifically on spending variation at those programs. Rather, our findings generalize to all acute care hospitals performing bariatric surgery with 10 or more Medicare cases over a seven-year period. Future studies should examine whether patterns of spending variation are different at BPCI-Advanced participating programs, and whether program participation is associated with subsequent reductions in episode spending. Second, we studied administrative data: episode spending may be confounded by patient severity, practice patterns, or complications in a way that we could not observe using administrative claims. However, we used largely the same administrative data that will be used to determine target prices and reconciliation payments for BPCI-Advanced participants. Third, and related, our observational design does not allow causal inferences: increases in spending may only be associated with readmissions rates and other factors, without a true causal relationship.
Conclusions
In this study of Medicare patients undergoing bariatric surgery, we found wide variation in hospital 90-day episode spending patterns, particularly on readmissions, post-acute care, and professional fees during the index hospitalization. Given the relationship between spending and complication rates, BPCI-Advanced may potentially incentivize a reduction in complications and readmissions.
Supplementary Material
Acknowledgements:
This study was supported by NIA grant 5R01AG039434-06 and NIDDK grant 5R01DK115408-02. We thank Dr. Grace F. Chao for assistance with data preparation and Sarah Clark for research assistance. Dr Chhabra had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding sources: KRC (Institute for Healthcare Policy and Innovation Clinician Scholars Program, NIH Division of Loan Repayment), AAG (AHRQ 5K08HS02362 and P30HS024403, PCORI CE-1304-6596) JBD (NIH/NIA R01AG039434, AHRQ R01HS023597)
Footnotes
Conflict of Interest Disclosures: Dr. Chhabra served as a paid consultant to Blue Cross Blue Shield of Massachusetts for work outside of the present study. Dr. Ghaferi receives salary support from Blue Cross Blue Shield of Michigan as the Director of the Michigan Bariatric Surgery Collaborative. Dr Dimick is a cofounder of ArborMetrix, Inc, a company that makes software for profiling hospital quality and efficiency. Dr Tsai is an advisory board member of SeamlessMD and a consultant for Sigilon Therapeutics. The authors have no conflicts of interest pertaining to the work herein.
References
- 1.Dummit LA, Kahvecioglu D, Marrufo G, et al. Association between hospital participation in a Medicare bundled payment initiative and payments and quality outcomes for lower extremity joint replacement episodes. Jama. 2016;316(12):1267–1278. [DOI] [PubMed] [Google Scholar]
- 2.Barnett ML, Wilcock A, McWilliams JM, et al. Two-Year Evaluation of Mandatory Bundled Payments for Joint Replacement. New England Journal of Medicine.0(0):null. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grenda TR, Pradarelli JC, Thumma JR, Dimick JB. Variation in Hospital Episode Costs With Bariatric Surgery. JAMA Surg. 2015;150(12):1109–1115. [DOI] [PubMed] [Google Scholar]
- 4.Reames BN, Finks JF, Bacal D, Carlin AM, Dimick JB. Changes in bariatric surgery procedure use in Michigan, 2006–2013. JAMA. 2014;312(9):959–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campos GM, Khoraki J, Browning MG, Pessoa BM, Mazzini GS, Wolfe L. Changes in Utilization of Bariatric Surgery in the United States From 1993 to 2016. Annals of Surgery. 2019:1. [DOI] [PubMed] [Google Scholar]
- 6.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Medical care. 1998:8–27. [DOI] [PubMed] [Google Scholar]
- 7.Dimick JB, Nicholas LH, Ryan AM, Thumma JR, Birkmeyer JD. Bariatric surgery complications before vs after implementation of a national policy restricting coverage to centers of excellence. JAMA. 2013;309(8):792–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birkmeyer JD, Gust C, Dimick JB, Birkmeyer NJO, Skinner JS. Hospital quality and the cost of inpatient surgery in the United States. Annals of surgery. 2012;255(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gottlieb DJ, Zhou W, Song Y, Andrews KG, Skinner JS, Sutherland JM. Prices don’t drive regional Medicare spending variations. Health Affairs. 2010;29(3):537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pradarelli JC, Scally CP, Nathan H, Thumma JR, Dimick JB. Hospital Teaching Status and Medicare Expenditures for Complex Surgery. Annals of surgery. 2017;265(3):502–513. [DOI] [PubMed] [Google Scholar]
- 11.Birkmeyer JD, Gust C, Baser O, Dimick JB, Sutherland JM, Skinner JS. Medicare payments for common inpatient procedures: Implications for episode-based payment bundling. Health services research. 2010;45(6p1):1783–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munnich EL, Parente ST. Procedures Take Less Time At Ambulatory Surgery Centers, Keeping Costs Down And Ability To Meet Demand Up. Health Affairs. 2014;33(5):764–769. [DOI] [PubMed] [Google Scholar]
- 13.Telem DA, Yang J, Altieri M, et al. Rates and risk factors for unplanned emergency department utilization and hospital readmission following bariatric surgery. Annals of surgery. 2016;263(5):956–960. [DOI] [PubMed] [Google Scholar]
- 14.Macht R, George J, Ameli O, Hess D, Cabral H, Kazis L. Factors associated with bariatric postoperative emergency department visits. Surgery for Obesity and Related Diseases. 2016;12(10):1826–1831. [DOI] [PubMed] [Google Scholar]
- 15.Hutter MM, Schirmer BD, Jones DB, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Annals of surgery. 2011;254(3):410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khalik HA, Stevens H, Carlin AM, et al. Site-specific approach to reducing emergency department visits following surgery. Annals of surgery. 2018;267(4):721–726. [DOI] [PubMed] [Google Scholar]
- 17.Jaffe TA, Kocher KE, Ghaferi AA. Potentially Avoidable Emergency Department Use: When Policy Expects Patients to be Physicians. Annals of Emergency Medicine. 2018;72(3):256–258. [DOI] [PubMed] [Google Scholar]
- 18.Smith ME, Bonham AJ, Varban OA, Finks JF, Carlin AM, Ghaferi AA. Financial impact of improving patient care setting selection after bariatric surgery. Surg Obes Relat Dis. 2019;15(11):1994–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen LM, Wilk AS, Thumma JR, Birkmeyer JD, Banerjee M. Use of Medical Consultants for Hospitalized Surgical Patients. JAMA Internal Medicine. 2014;174(9):1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chhabra KR, Sheetz KH, Regenbogen SE, Dimick JB, Nathan H. Wide Variation in Surgical Spending Within Hospital Systems: A Missed Opportunity for Bundled Payment Success. Annals of Surgery. 2019. [DOI] [PubMed] [Google Scholar]
- 21.Haas DA, Zhang X, Kaplan RS, Song Z. Evaluation of Economic and Clinical Outcomes Under Centers for Medicare & Medicaid Services Mandatory Bundled Payments for Joint Replacements. JAMA Internal Medicine. 2019;179(7):924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Udelsman B, Jin G, Chang DC, Hutter M, Witkowski ER. Who really decides? Surgeon preference trumps patient factors in predicting whether patients receive a sleeve or bypass. Surgery for Obesity and Related Diseases. 2018;14(11):S40. [Google Scholar]
- 23.Noel-Miller C, Lind K. Is observation status substituting for hospital readmission? Health Affairs Blog. 2015. https://www.healthaffairs.org/do/10.1377/hblog20151028.051459/full/. Accessed 12/18/18.
- 24.Wright S Memorandum Report: Hospitals’ Use of Observation Stays and Short Inpatient Stays for Medicare Beneficiaries, OEI-02–12-00040. Department of Health and Human Services, Office of Inspector General;2013. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.