Abstract
Lung cancer, highly prevalent and the leading cause of cancer-related death globally, persists as a significant challenge due to the lack of definitive tumor markers for early diagnosis and personalized therapeutic interventions. Recently, extracellular vesicles (EVs), functioning as natural carriers for intercellular communication, have received increasing attention due to their ability to traverse biological barriers and deliver diverse biological cargoes, including cytosolic proteins, cell surface proteins, microRNA, lncRNA, circRNA, DNA, and lipids. EVs are increasingly recognized as a valuable resource for non-invasive liquid biopsy, as well as drug delivery platforms, and anticancer vaccines for precision medicine in lung cancer. Herein, given the diagnostic and therapeutic potential of tumor-associated EVs for lung cancer, we discuss this topic from a translational standpoint. We delve into the specific roles that EVs play in lung cancer carcinogenesis and offer a particular perspective on how advanced engineering technologies can overcome the current challenges and expedite and/or enhance the translation of EVs from laboratory research to clinical settings.
Key words: Lung cancer, Extracellular vesicles, Therapy resistance, Delivery, Diagnostics, Therapeutics, Nanotechnology, Engineering
Graphical abstract
This review discusses lung cancer management challenges and explores the potential of extracellular vesicles (EVs) for personalized treatment and diagnosis, focusing on enhancing clinical translation through advanced engineering technologies. Created with Biorender.com.
1. Introduction
Lung cancer, a formidable malignancy, is one of the most common cancers, with an estimated 2.2 million new cases in 2020. It constitutes 11.4% of all cancer diagnoses, second only to female breast cancer at 11.7%. Simultaneously, it remains the leading cause of cancer-related death globally (estimated 1.8 million deaths per year)1,2. Non-small cell lung carcinomas (NSCLCs), delineated into subtypes including adenocarcinoma (AD), squamous cell carcinoma (SCC), and large cell carcinoma (LCC), collectively account for approximately 85% of all lung cancer3, 4, 5. Unfortunately, more than 50% of patients with NSCLC have metastatic disease at the time of diagnosis, dramatically reducing the 5-year relative survival rate to a mere 6%, much less than that of all stages of lung cancer (21%)6. The global intricacies of lung cancer epidemiology, influenced by dynamic trends in population dynamics, aging, and smoking prevalence, underscore the pressing need for innovative paradigms from early diagnosis to effective treatment aimed at mortality reduction.
Current methods for the spectrum of lung cancer management, from screening and diagnosis to treatment and treatment monitoring, include a variety of approaches, such as chest X-ray (CXR), low-dose computed tomography (LDCT), sputum exfoliative cytology, bronchoscopy, and lung solid biopsy, but these approaches face formidable challenges. CXR examination cannot fully show early pulmonary lesions, with high false negatives and accessible delay of the disease7,8. LDCT for high-risk individuals has been utilized to improve the early diagnosis of lung cancer, although adopting this method for population screening has proven challenging due to its high false-positive rate (1.9%–2.5%) and severe cumulative radiation exposure9, 10, 11. Sputum exfoliative cytology and bronchoscopy are valuable due to their simplicity, speed, and cost-effectiveness, while these assays suffer limited sensitivity and limitation in subtyping12,13. Lung biopsy along with histological and molecular testing, has been recommended by the World Health Organization (WHO) for lung cancer staging and classification, and treatment decisions14,15; however, this approach can produce false-negative results caused by improper surgical technique or failed sampling of tumor tissue limited by spatial and temporal tumor heterogeneity15,16. Lung cancer exhibits not only intratumoral heterogeneity but also intertumoral heterogeneity, and both complicate diagnosis as well as treatment. Despite ongoing efforts to improve lung cancer treatment outcomes, including the development of innovative immuno- and molecularly targeted therapies, more efforts are needed, especially because of unknown drug resistance mechanisms and the complex cellular interactions within the tumor microenvironment (TME)17, 18, 19.
Cells universally discharge tiny vesicles, known as extracellular vesicles (EVs), into the circulation to modulate physiological and pathophysiological processes. These vesicles are increasingly considered to hold great potential as a versatile tool for both the diagnosis and treatment of lung cancer. With distinctive biological pathways and the information EVs offer on individual variations and heterogeneity, pivotal involvement of EVs from lung cancer cells has been identified ranging from tumor progression, metastasis, and drug resistance20. Understanding and interfering with these biological processes is considered crucial for new and innovative strategies like immunotherapy and targeted therapy, and for the traditional strategies of radiotherapy and chemotherapy in lung cancer treatment. This entails screening, targeting, and regulating specific groups of lung cancer associated EVs isolated during critical stages like metastasis or drug resistance. The functional analysis of EVs in lung cancer not only reveals valuable insights into disease mechanisms but also introduces a new focal point in liquid biopsy, which is preferred for early diagnosis or as a population screening approach. EVs carry diverse biomolecules and molecular signatures, reflecting genetic mutations, protein expression alterations, and other molecular changes associated with the clinical characteristics of lung cancer patients21. Thus, EVs can provide indicators for early diagnosis and also prognosis, to guide personalized treatment strategies for improved patient outcomes22. EVs can also be designed for therapeutic drug delivery, which presents a unique strategy for precise and individualized lung cancer treatment. EVs, with their distinct biocompatibility, serve as natural carriers for molecules influencing tumor processes and can encapsulate antitumor drugs, enhancing delivery efficiency and pharmacokinetics.
This review provides a comprehensive overview of the biogenesis and the molecular function of EVs for lung cancer, with a particular emphasis on how this knowledge contributes to the translation of EVs to clinical settings, for early diagnosis, prognosis, and personalized treatment (Fig. 1). Diverging from other reviews that emphasize signaling pathways, our review primarily emphasizes the potential for translation, assessing the proximity of studies to real-world clinical applications. Additionally, we aim to explore how advanced engineering technologies, especially nanotechnologies and nanotools, can enhance the translation of laboratory research into clinical practice.
Figure 1.
A wide range of EV-based studies, spanning from fundamental science to diagnostics and therapeutics, have the potential to enhance the management of lung cancer. Created with Biorender.com.
2. Pivotal role of EVs in lung cancer carcinogenesis and progression
2.1. The definition, origin, and biogenesis of different types of EVs
The terminology associated with EVs has evolved as research on EVs and their subpopulations continues to become more extensive. To avoid confusion, in this review, we adhere to the latest recommendations from MISEV2023 for our subsequent discussion. We term EVs as all particles released from cells enclosed by a lipid bilayer but cannot replicate, which consist of several frequently discussed subsets based on their original and biogenesis: exosomes from the endosomal system, ectosomes or microvesicles origin from the plasma membrane, as well as apoptotic bodies from the cellular degradation. The term “Exosomes”, in this review, would only be used unless the subcellular origin from the endosomal pathway can be demonstrated, to represent the most extensive subtype of EVs with a diameter generally smaller than 200 nm.
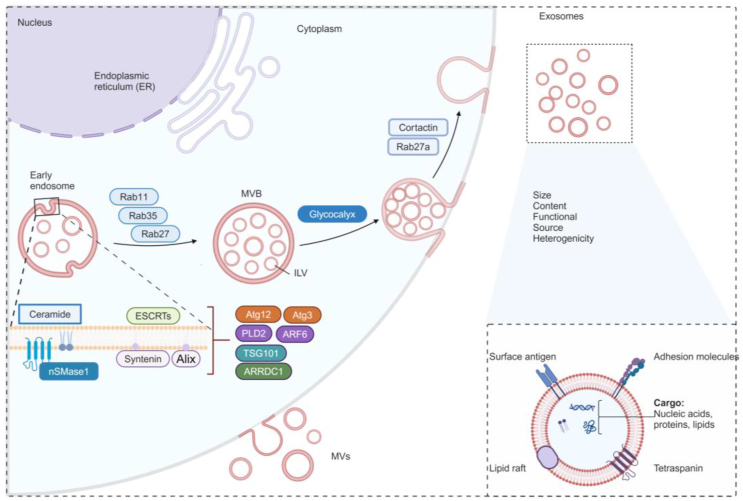
The biogenesis of exosomes starts with the invagination of the plasma membrane, to form early endosomes associated with cell surface proteins and soluble extracellular proteins (Fig. 2). Subsequentially, these endosomes mature into late endosomes, also known as multivesicular bodies (MVBs), along with the formation of intraluminal vesicles (ILVs) within the MVBs23. The endosomal protein sorting complex (ESCRT) ubiquitinated cargoes and mediates their packaging into the ILVs within MVBs24. These MVBs that are not degraded by lysosomes or autophagosomes, would finally regulated by Rab GTPase25, autophagy mediators26, cortactin27, and the composition of the glycocalyx28, fuse with plasma membrane to release ILVs as exosomes into the extracellular fluids. These exosomes can subsequently enter recipient cells to influence their functions.
Figure 2.
The biogenesis of exosomes, the most extensively studied subtype of EVs. Created with Biorender.com.
Recent research has unveiled alternative mechanisms on ESCRT-independent cargo sorting machinery responsible for regulating the biogenesis of EVs. For example, endoplasmic reticulum (ER)-late endosome membrane contact sites regulate the fusion of MVBs with the plasma membrane, leading to the release of EVs29. Alternatively, exosomes can arise at the nuclear envelope through nSMase1-dependent ceramide synthesis30. Ceramide and proteolipid proteins played a prominent role in endosomal sorting in oligodendrocytes in the absence of indispensable ESCRT components were observed31. Most recently, it has been shown that tetraspanins32, which were classically identified as exosomal cargoes, the enzyme neutral sphingomyelinase 2 (nSMase 2)33, and autophagy-related protein LC334, have been demonstrated to serve as a promoter or function as a molecular guide for the exosomal cargoes sorting. Nevertheless, the content and functions of EVs and exosomes are thought to be largely reflective of the cell of origin23. Precisely intricate mechanisms contributing to specific cargo encapsulated into EVs would be further elucidated to provide more insight into understanding the composition and functions of EVs.
The content of EVs and exosomes can be associated with cancer, directly (e.g., tumor cell) or indirectly (e.g., cell involved in angiogenesis). Accordingly, EVs have been reported to be associated with diverse, distinct pathological processes of lung cancer, specifically EVs carrying cargoes including proteins (mutant oncoprotein KRAS-associated protein complex35, cytokine-like secreted protein [FAM3C]36, membrane protein tetraspanin-837, cell migration-inducing and hyaluronan-binding protein [CEMIP]38, angiopoietin-like 439, PKM2, and HDAC4 [LOC85009]40,41,42) and nucleic acids (miR-1246b43, miR-122-5p44, lnc-MMP2-245, circTLCD4-RWDD346, and circZNF45147). In this review, we specifically focus on exosome-enriched EVs implicated in various physiological and pathological processes of lung cancer (Fig. 3), highlighting the distinct functions of proteins and nucleic acids (microRNA, lncRNA, circRNA, and DNA), either encapsulated into or on the surface of EVs, in influencing the progression, metastasis, and therapeutic resistance of lung cancer. We discuss representative studies, or the latest findings, on how EVs utilize this cargo to initiate unique biological processes. We also give perspective on which future studies need to be implemented to translate this knowledge to clinical applications for enhanced lung cancer management.
Figure 3.
EVs transport diverse cargoes to influence different aspects of the progression of lung cancer, including angiogenesis, metabolism, cell proliferation and metastasis, tumor drug resistance, and immune modulation. Created with Biorender.com.
2.2. The impact of EV on metabolism in lung cancer
EV-mediated communication shapes the overall metabolic landscape of recipient cells, which has important implications in the context of lung cancer, including metastasis, therapeutic responses, and microbiome modulation. Studies using the Lewis lung carcinoma model showed that interleukin-6 and parathyroid hormone-related protein derived from EVs induced myotube atrophy and adipocyte lipolysis, highlighting the role of EV-mediated metabolism in cancer-related processes48,49. Another study showed that when lncRNA ROLLCSC from lung cancer stem cell-derived EVs was transferred to non-stem lung cancer cells, lipid metabolism was stimulated to enhance metastasis through competing with miR-5623-3p and miR-217-5p as endogenous RNA50. Furthermore, miR-4466 in neutrophil-derived EVs promoted the acquisition of tumor cell stemness and switched energy metabolism to facilitate lung cancer metastasis51. In addition, metabolomic signatures of 102 urinary EVs samples from lung cancer patients were analyzed with machine learning using a random forest model, which identified a potential metabolome panel consisting of organic acids, lipid molecules, and heterocyclic compounds, suggesting the bidirectional effect between EVs and lung cancer52. Tumor-derived EVs also reprogrammed the glycolytic-dominant metabolism of macrophages within the premetastatic niche, increasing glucose uptake and repressing mitochondrial oxidative phosphorylation, which ultimately primes lymph nodes for lung cancer metastasis53. Like other malignancies, lung cancer has been considered a disease of aging, characterized by a process of cellular senescence54. EVs are capable of reversing aging phenotypes and thus show potential as antiaging agents that could be redirected for lung cancer therapy. While these findings provide valuable insights, a more comprehensive understanding of factors influencing EV function in metabolism and aging, along with proof-of-concept therapeutic models, is essential for maximizing the therapeutic potential of EVs for lung cancer in clinical settings.
2.3. The impact of EV-mediated angiogenesis on lung cancer
Angiogenesis, a hallmark of cancer, facilitates tumor growth and metastasis55, and angiogenesis can be mediated by EVs. For instance, EV-associated neovasculature has been widely demonstrated to facilitate tumor angiogenesis through multitudinous cargoes and serves as a crucial mediator between lung cancer cells and vascular endothelial cells. The transfer of miRNA mediated by EVs has also been widely demonstrated to affect tumor angiogenesis in lung cancer. For example, miR-21056 and miR-14157 act as proangiogenic factors, modulating downstream JAK2/STAT3 and KLF12 pathways, respectively. Angiogenesis was promoted by miR-23a, which enhanced prolyl hydroxylase mediated accumulation of HIF-1 α and tight junction protein ZO-1-associated vascular permeability58. Lung cancer-derived EVs transporting miR-197-3p directly silenced anti-angiogenic TIMP2 and TIMP 3, leading to angiogenesis and migration of human umbilical vein endothelial cells (HUVECs)59. In addition to miRNA, synaptotagmin 7 (SYT7) confers angiogenesis and metastasis by promoting the secretion of EVs containing centrosomal protein of 55 kDa (CEP55) and the transfer of these EVs to HUVECs60. These investigations show the role of EVs in promoting lung cancer progression through the modulation of angiogenesis, offering insights into future research avenues to enhance the diagnosis and treatment of lung cancer. Interventions targeting specific EV cargoes linked to tumor angiogenesis could inhibit or reverse this biological process, holding promise for mitigating lung cancer progression and metastasis.
2.4. The impact of EV on lung cancer progression and metastasis
Metastatic progression in lung cancer is a complex process where cancer cells disseminate from the primary tumor to distant organs. In this process, cancer cells escape from the primary tumor, enter into the circulatory system, extravasate via interactions with adhesion molecules and proteases in the TME at secondary sites, and subjugate the metastatic stroma through extracellular matrix (ECM) remodeling.
Regarding the invasion of cancer, the upregulated secretion of EVs from cancer cells in a localized context, is the initial step in this process, these EVs can further enter the circulatory system to affect far-off organs, discharging factors directly into recipient cells that can induce alterations in gene expression and, via distinct signaling pathways, shape an environment conducive to cancer metastasis. Notably, the upregulated release of EVs also reveals a positive-feedback loop to promote the maturation of invadopodia as a docking site for EVs release61, and also promotes an assembly of proteases and adhesion molecules that enables ECM degradation, an important progress in vascular sprouting and cancer cell extravasation.
The accumulation of EVs also contributes to the formation of premetastatic niches at future metastatic sites in different organs, including the liver, brain, bone, and so forth. For instance, at the liver metastatic site, EVs sorting of miR-122-5p package by lung cancer cells increases the expression of the mesenchymal markers N-cadherin and vimentin while decreasing the expression of the epithelial markers E-cadherin and β-catenin, which promotes the process of epithelial-to-mesenchymal transition (EMT), reprogramming the premetastatic microenvironment to facilitate hepatic metastasis44. Moreover, EVs secreting miR-92a impact the vasculature and recruit of bone-marrow–derived cells (BMDCs) to establish a liver premetastatic niche by targeting Smad7, which enables activation of hepatic stellate cells, accumulation of immunosuppressive cells and deposition of extracellular matrix in distant liver organs62. At a brain metastatic site, EVs carrying LINC00482, a type of long noncoding RNA (lncRNA) associated with NSCLC, can induce the conversion of the microglial cell line HMC3 to an M2 phenotype, promoting the acquisition of malignant properties of NSCLC cells and, eventually, the brain metastasis of NSCLC63. Another study found that chronic nicotine exposure in the brain premetastatic niche promotes the secretion of exosomal miR-4466, reinforcing stemness and metabolic switching of lung cancer cells through the SKI/SOX2/CPTIA axis, facilitating metastasis. Thus, elevated exosomal miR-4466 levels in individuals who smoke may serve as a prognostic indicator for lung cancer brain metastasis51. And a prospective observation study of patients with lung cancer receiving whole-brain radiotherapy for brain metastasis found that higher levels of circulating EV integrin β3 correlated with reduced overall survival and higher cumulative incidence of intracranial failure64. Furthermore, at the bone metastatic site, modulates osteoclast differentiation and stimulates bone metastasis. Distinctly, in a model for bone metastasis of NSCLC, EVs deriving LncRNA-SOX20T can activate a miRNA-194-5p/RAC1 axis and TGF-β/PFHrP/RANKL signaling pathway, to initiate osteoclast differentiation and stimulate bone metastasis65.
Similar to other solid tumors, the lung cancer metastasis site is a nonrandom but specific organ. This is referred to as organotropism, which was initially introduced in 1889 by Stephen Paget in the context of the hypothesis “the seed and soil”66,67. Related to this phenomenon, a recent study examining a large cohort of patients with lung adenocarcinoma (LUAD) showed that site-specific metastasis involved distinctive mutations, including inactivation of TP53, SMARC, CDKN2A, and APOBEC associated with liver metastasis. But there is currently limited documentation on the role of EVs, and their association of these mutations in the organotropism of lung cancer metastasis68,69.
EVs may emerge as a critical aspect of organotropism, mediating bidirectional communication between primary and metastatic sites. We envision that future studies using an intravital, nanolabeling strategy for real-time imaging will reveal that EV entry into metastatic organs precedes the homing of cancer cells, emphasizing the importance of understanding EV fate in cancer progression, metastasis, and lung cancer metastatic organotropism. Although challenges persist, advances in nanotools for EV tracking approaches and in vivo experimental models may provide new insight into understanding the fate of EVs and their impact on the earliest stage of metastatic cancer progression.
2.5. The impact of EV on lung cancer immunity
Treatment options for lung cancer were historically restricted to traditional methods such as surgery, chemoradiotherapy, and targeted drug therapy, but new methods have recently become available, notably, immunotherapy. This transformative approach includes immune checkpoint inhibitors (ICI), chimeric antigen receptor (CAR) T cells, monoclonal antibodies, vaccines, and autologous cellular therapies to modulate the immunosuppressive TME70. Given their pivotal role in mediating the communication and phenotypes between cancer cells and immune cells to reconstruct the TME, EVs have emerged as key contributors to boosting the effectiveness of immunotherapy for current approaches.
EVs from lung cancer cells can impact the malignant phenotype of immune cells in TME, and of all the immune cells, macrophages have received the most attention. EVs released from tumor cells originating from the lungs can cause a polarization shift in macrophages, toward the pro-tumorigenic M2 phenotype71, 72, 73, 74. In addition, tumor-derived EVs containing TRIM59 were reported to activate the NLRP3 inflammasome in macrophages to promote the IL-1β secretion, fostering a proinflammatory TME and thereby enhancing lung cancer development. Although much of the research has focused on macrophages, EVs from tumors have also been observed to impact various immune cells of myeloid and lymphoid origin, including monocytes, neutrophils, myeloid-derived suppressor cells (MDSCs), mast cells, dendritic cells (DCs), NK cells, and T cells75. For example, lung cancer cell-derived EVs containing miR-150-5p, were shown to reduce the expression of CD226 on NK cells to promote an immunosuppressive microenvironment within the TME, favoring the progression of lung cancer76.
Understanding the role of EVs in facilitating bidirectional communication between cellular components within the TME could also establish effective therapeutic strategies. For example, miR-146a released from DC-derived EVs can be transferred to recipient DC cells to inhibit endotoxin-induced inflammation, whereas, in a mouse model of lung cancer, miR-155 can promote this inflammation, suggesting that DC-derived EVs can be engineered to elicit antitumor immunity77. Moreover, lung tumor cell lysate pulse treatment enhanced DC function and, when combined with DC-derived EVs, enhanced allogeneic T-cell proliferation and elicited antitumor immunity with great cytotoxic activity against A549 cells, suggesting DC-derived EVs can be used for vaccinating against lung cancer78. In a mouse model of lung cancer, a combination of anti-PD-L1 and tumor cell-derived EVs induced by methotrexate enhanced T-cell antitumor responses by downregulating the expression of PD-1 in neutrophils79. Similarly, engineered tumor cell-derived exosome-like nanovesicles (eNVs) employed as a tumor vaccine inhibited lung tumor growth. This intervention led to a reprogramming of the immunosuppressive TME, marked by enhanced DC maturation, increased infiltration of effector T cells, and reduced proportions of immunosuppressive cells, including M2 macrophages, MDSCs, and regulatory T cells (Tregs)80. Consequently, leveraging these interactions for therapeutic purposes has been suggested as a strategy to manage the progression of lung cancer75.
2.6. The impact of EV in mediating therapy resistance
EVs play a critical role in the development of resistance to lung cancer therapies, including chemotherapy, radiotherapy, immunotherapy, and targeted therapy. EV-mediated therapy resistance operates through distinct yet interconnected mechanisms. These include facilitating drug export81, transmitting cargo that actively promotes resistance to therapy82,83, influencing the TME84, and functioning as decoys for antibody-based therapeutics85,86. Understanding and mitigating these pathways thus provides opportunities for developing effective therapies against lung cancer.
2.6.1. Facilitating drug export
EVs resist therapy most directly by facilitating drug export. EVs can facilitate drug export directly, by drug transporters, and contribute to intracellular drug reduction and transport through drug efflux pumps contained within EVs from lung cancer cells. For example, exosomal FTO facilitated the expression of membrane transporter protein ABCC10 in recipient cells via an FTO/YTHDF2/ABCC10 axis in an m6A-dependent manner, eventually leading to the promotion of drug efflux and acquired resistance to gefitinib in NSCLC87.
2.6.2. Transmitting cargo to increase tumor cell survival and resistance
In addition to efflux pumps, EVs can also transfer cargo such as non-coding RNA (circRNA, miRNAs, and lncRNA) and protein to confer resistance to lung cancer cells, increasing their survival. For example, researchers using an in vivo NSCLC xenograft tumor model transferred cisplatin-sensitive NSCLC cells into mice and exposed the mice to EVs from cisplatin-resistant NSCLC cells. The EVs from cisplatin-resistant NSCLC cells contained the circRNA vacuole membrane protein 1 (circVAMP1), which induced resistance in the cisplatin-sensitive cells by acting as microRNA-524-5p (miR-524-5p) sponge88. EVs carrying miRNAs can also induce cisplatin resistance89,90, EVs transferring functional miR136-5p to target PPP2R2A can support LUAD cell migration and invasion and mediate anlotinib resistance91. Additionally, EVs can also enhance the autophagic process or sequential pathways to facilitate cell survival in a drug-independent manner, as miR-425-3p packaged into EVs also involves a mechanism of autophagic activation responsible for cisplatin resistance89. While docetaxel resistance can be induced from exosomal transfer lncRNA small nucleolar RNA host gene 7 (SNHG7) to recipient LUAD cells92. Another study found that cells resistant to Osimertinib, containing wild-type Epidermal growth factor receptor (EGFR), encapsulated EVs via clathrin-dependent endocytosis. Then, these cells transferred the wild-type EGFR protein to sensitive cells with mutated EGFR, impacting the transcription of a Rab GTPase (RAB17), which activated downstream signaling pathways PI3K/AKT and MAPK to trigger osimertinib therapy resistance93.
Transient exposure to chemotherapeutic drugs not only significantly increases EV secretion from cells for efflux but also accelerates the circulation of these EVs back into the plasma membrane of sensitive recipient tumor cells, which rapidly acquire resistance to chemotherapeutical drugs94. Such mechanisms have been reported for different drugs, including vincristine, cisplatin, and doxorubicin. For example, vincristine induces resistant cancer cells to increase the secretion of ABCB1-enriched EVs, which can dose-dependently internalize sensitive cancer cells through intercellular EV endocytosis mediated by dynamin 2 and clathrin. Additionally, vincristine prompts the enhanced recycling of EVs back to the plasma membrane of drug-sensitive cells in a manner dependent on the Rab GTPase family (Rab8B and Rab5), by rapid ABCB1 alteration in cells from NSCLC patients receiving platinum-based regimens94.
2.6.3. Influencing the TME to trigger environment-mediated drug resistance
EV signaling can also induce therapy-resistant conditions in the TME to trigger environment-mediated drug resistance. EVs can mediate communication between lung cancer cells and a variety of cells in the TME, including stromal cells, tumor-infiltrating immune cells, tumor-associated macrophages, and tumor-associated endothelial cells. For instance, one study found that PKM2, an enzyme that catalyzes anaerobic glycolysis, packaged in EVs from hypoxic cisplatin-resistant NSCLC cells, was transferred to drug-sensitive cells. Exosomal PKM2 enables NSCLC cells to produce reductive metabolites to neutralize reactive oxygen species, inhibit apoptosis, and reprogram cancer-associated fibroblasts (CAFs), forming an acidic TME that subsequently fostered NSCLC cell proliferation, inhibited apoptosis, and enhanced resistance to cisplatin40.
2.6.4. Functioning as decoys for target therapy
Tumor-derived EVs containing PD-L1 can function as decoys for anti-PD-L1 antibodies, to mediate anti-PD-L1 therapy resistance. Demonstrating this in a mice prostate tumor model, EVs isolated from the serum of lung cancer patients substantially decreased the binding of anti-PD-L1 antibodies to tumor PD-L1. Additionally, lung tumor tissue EVs containing PD-L1 significantly blunted the inhibited tumor progression by Anti-PD-L1 antibody treatment combined with simultaneously intratumorally injected human peripheral blood mononuclear cells95. Similarly, exosomal PD-L1, upregulated by interferon-gamma (IFN-γ) in lung cancer cell lines, has the potential physical interaction with ligands on CD8+ T cells, which could be a mechanism of immunosuppression and resistance to anti-PD-1 therapy96.
Taken together, the studies described in sections 2.2 through 2.6 suggest that EVs play a pivotal role in various facets of lung cancer, including metabolism, angiogenesis, cancer progression, metastasis, immunity, and therapy resistance. In addition to describing the above studies, we have also summarized a list of different cargos from EVs and their functions related to lung cancer carcinogenesis (Supporting Information Table S1). Understanding these intricate pathways provides a foundation for developing effective therapeutic strategies against lung cancer. The complex interplay between EVs and the TME, immune cells, and resistance mechanisms emphasizes the need for continued research to reveal a better understanding of signaling pathways. The integration of advanced technologies, such as nano-labeling strategies and real-time imaging approaches, holds immense potential to enhance our understanding of EV fate in cancer progressions. Already, the fates of endogenous and exogenous EVs have been examined in vivo, in preclinical studies or some invertebrate and vertebrate model organisms, usually using fluorescently or bioluminescently labeled tags in combination with an imaging technology, such as X-ray computed tomography (CT) imaging97, magnetic particle imaging (MPI)98, magnetic resonance imaging (MRI)99, positron emission tomography (PET)100, or single-photon emission computed tomography (SPECT)101, which enable to provide valuable mechanistic insights into EVs biosynthesis and release, pharmacokinetics and pharmacodynamics, biodistribution and function. Most recently, metabolic tagging technology has been used to label EVs with unique chemical tags, enabling the tracking and targeted modulation of EVs in vivo102. Functions such as real-time biodistribution and uptake of EVs103, developmental and behavioral roles of EVs104, and recapitulating some aspects of human physiology and disease processes, tumor growth, and metastasis105,106, have been demonstrated. With continued advancements in technique-specific sensitivity and spatial resolution, alongside the development of novel strategies for specific labeling techniques, we anticipate significant improvement in the ability to understand the fate of EVs. Moreover, distinguishing between exogenous and endogenous EVs in terms of the route and timing of administration, dosage, non-EV constituents of administration, and compositional complexities will become essential for achieving a comprehensive understanding as well as physiological relevance. We envision that continued development in technology and models will help unravel associated mechanisms and further improve our ability to harness the diagnostic and therapeutic potential of EVs for the management of lung cancer, marking a promising avenue for future advancements in the field.
3. Toward clinical applications of EVs for lung cancer with recent technological advances
3.1. Isolation and characterization of EVs for diagnosis and therapeutic applications
The isolation and purification of specific target exosomes are often the first steps in the study of any disease, whether for mechanistic understanding, biomarker selection, or clinical diagnostic and therapeutic purposes. And lung cancer is no exception. Challenges arise from limited cancer-derived EVs and complexities in body fluids, where considerable overlap in size and composition exists among EV subtypes. Conventional technologies including size-based isolation of all EVs ultracentrifugation107,108, density gradient centrifugation35,109, size exclusion chromatography110, immunoaffinity separation methods using antibodies or capture agents, such as receptors, peptides, and aptamers, that target specific subpopulations of EVs are an effective means of capturing highly specific and unsophisticated EVs in plasma or serum samples, have been developed for EVs separation towards lung cancer research. However considerable obstacles persist in advancing specific exosomes for large-scale clinical applications, ranging from the requirement for expensive instruments or reagents, required large sample volume as well as skilled personnel, low yield, and purity for the subtypes of EVs population.
There is a growing emphasis on the exploration of miniaturized systems, particularly lab-on-a-chip platforms, for the rapid, convenient, and on-site separation of EVs, which aim to accelerate both exosome research and clinical translation. The vibrant development of nanotechnology, especially the integration of various nanotechniques into microfluidic chips, has become a research hot spot. Microfluidic platforms with passive micro- and nanostructures can be used alone or in combination with other techniques such as magnetic nanoparticle-based immunoaffinity, DNA assembly, acoustics, and electrokinetics111,112. In one approach, microfluidics designed with passive micro- or nanostructures separated EVs from other particles on the basis of size, and the precise manipulation of EVs was achieved by utilizing the elastic lift force inside a viscoelastic medium, not an external field. Notably, this approach did not require any pretreatment, which simplified the processing step, but there was a potential risk of clogging and a comparably long processing time113, 114, 115. Electrical-based devices employ dielectrophoretic forces to transport dielectric particles (i.e., EVs) through an irregular electric field in a manner depending on the particle size, field frequency, and medium viscosity116, 117, 118. With fast processing steps and a small-volume sample requirement, this approach is portable, scalable, timesaving, and inexpensive, and it produces concentrated and high-purity EV samples119, 120, 121. Hydrodynamic properties-based isolation approaches including viscoelastic122 and electrohydrodynamic123. Acoustic field-based separation approaches have also been integrated into microfluidic channels to remove large particles from a small volume of biological samples can be fast and produce high-purity EVs with label-free isolation methods124,125.
Due to the existing limitations in achieving precise and reproducible isolation and characterization of exosomes, numerous studies exploring the clinical applications of EVs for lung cancer have yet to fully exploit the wealth of information these vesicles offer. The forefront of this transformative landscape lies in the development of novel technologies, particularly microfluidic advancements, poised to play a pivotal role in shaping the future of the EV field. However, beyond the imperative need for user-friendly exosome isolation tools that feature direct capture from samples (e.g., serum, plasma, and body fluids), maintain structural integrity, simplify processing steps, and minimize time, it is essential that these approaches be meticulously tailored for subsequent applications. Seamless collaboration between clinicians and researchers remains indispensable, ensuring the continual evolution and practical implementation of exosome isolation techniques and analyses in real-world scenarios.
3.2. Recent advances in analyzing EVs for lung cancer
In the context of implementing EVs for lung cancer, a comprehensive approach to EV analysis is essential. Firstly, decoding the intricate signaling pathways related to lung cancer treatment is pivotal for identifying relevant targets or biomarkers, as well as forming the basis for targeted therapies. Secondly, rapid analysis of identified EV markers is crucial for swift diagnostics and personalized treatment guidance, emphasizing the need for streamlined processes adaptable to the dynamic nature of lung cancer progression. Thirdly, ensuring the quality control of EVs intended for drug delivery involves meticulous examination of their size, composition, and specific biomarkers to ensure safety and efficacy in therapeutic applications126,127.
EV analysis for lung cancer has been achieved with a diverse range of techniques enhanced by nanotechnology. Examples are colorimetric37, fluorescence128, and plasmon-enhanced methods129 as well as electrical approaches130. Notably, recent advancements include the integration of machine learning with nanoparticle analysis; these cutting-edge analytical techniques offer a more comprehensive understanding of EVs and are also necessary for advancing diagnostics, personalized treatment, and drug delivery (Fig. 4)131, 132, 133, 134.
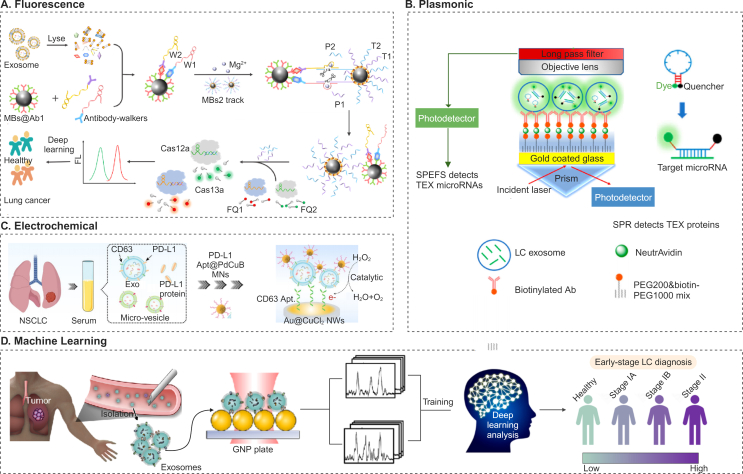
Figure 4.
Different techniques for EV analysis towards cancer diagnosis. (A) DNAzyme walkers-triggered CRISPR-Cas12a/Cas13a bioassay was devised for concurrently detecting proteins (SAA1 and FV) from plasma EVs, the DNAzyme walkers exhibited specific cleavage of tracks, releasing multiple P1 (ssRNA) and P2 (ssDNA) molecules from individual SAA1/FV entities, thereby amplifying the initial signal. Subsequent hybridization of P1 and P2 with crRNA activated Cas13a and Cas12a, prompting the cleavage of reporters (ssRNA FQ1 and ssDNA FQ2) and generating discernible fluorescence signals. Reprinted with the permission from Ref. 132. Copyright © 2023 2024 Elsevier B.V. (B) Surface plasmon enhanced fluorescence spectroscopy (SPEFS) assay for lung cancers. Gold-coated glass slide is treated with PEG and antibodies specific to bind tumor-overexpressed proteins. The binding of tumor-derived EVs (TEVs) to these antibodies alters the local refractive index, facilitating the detection of TEVs proteins through surface plasmon resonance (SPR). Subsequently, molecular beacons are introduced to penetrate TEVs and attach to target microRNAs. The fluorescence signals emitted by the molecular beacons upon binding enable the detection of TEVs microRNAs. Reprinted with the permission from Ref. 133. Copyright © 2023 American Chemical Society. (C) A sandwich electrochemical sensor to detect PD-L1+ exosomes with high sensitivity, distinguishing between micro-vesicles and exosomes based on size and surface markers like CD63. The sensor selectively captures exosomes using CD63 Apt. tethered to Au@CuCl2 NWs, while PdCuB MNs modified by PD-L1 Apt. enhance electrochemical signals for specific recognition of PD-L1+ exosomes, offering a promising approach for early cancer screening. Reprinted with the permission from Ref. 134. Springer Nature, Copyright © 2023, The Author(s). (D) Machine learning is employed for exosome profiling for lung cancer stage classification, followed by the collection of surface-enhanced Raman spectroscopy spectroscopic data of blood EVs captured on a gold nanoparticle (GNP) array substrate. Reprinted with the permission from Ref. 131. Copyright © 2020 American Chemical Society.
The heterogeneity of EV composition in lung cancer poses challenges and opportunities. Despite the challenges, innovative platforms have been developed for multiplex profiling in single-EV detection. The pursuit of single-EV detection is considered the ultimate solution for highly sensitive EV analysis and a potent strategy to address the inherent heterogeneity of EVs. Ongoing advancements in analytical tools hold the promise of unlocking new possibilities for advanced single-EV detection technologies. For instance, platforms enrich and molecular EVs enable multiplex profiling of surface proteins at the single-EV level135,136. Integrating next-generation sequencing (NGS) into single EV detection137, along with innovative approaches like flow cytometry (FCM) with microbeads138, or membrane fusion technologies139, enhances the detection of specific EVs from cancer patients. We have summarized recent progress regarding the use of specific EVs from clinical samples for lung cancer diagnosis and prognosis, along with the corresponding diagnostic performance (Supporting Information Table S2).
EVs containing tumor-specific components correlate with tumor staging and prognosis, and these EVs can be readily isolated from various bodily fluids, holding promise for early-stage lung cancer diagnosis and real-time monitoring of lung cancer progression and metastasis dynamics. Numerous ongoing clinical trials are evaluating the diagnostic, discriminative, and prognostic value of EVs in the context of lung cancer. In one trial (Chinese clinical trial registry number chiCTR1800020223), platelets from a cohort of 80 patients with advanced NSCLC released significantly more EVs into the circulation after treatment with chemotherapy and/or ICI suggesting a predictive model for the progression of advanced NSCLC140. In another clinical study, EVs from metastatic NSCLC patients unresponsive to ICI (anti-PD1) contained higher levels of the platelet activation marker CD62P than EVs from patients with a complete or partial response, indicating that activated platelet-derived EVs may promote anti-PD1 therapy resistance involving a mechanism partially dependent on PD-L1141. Additionally, patients are being recruited for a study investigating the performance of EV EML4-ALK fusion detection in NSCLC diagnosis and the potential prognostic value for dynamic monitoring of EV EML4-ALK fusion (NCT04499794).
Another crucial future direction in research toward EVs analysis is the expedited quality control of EVs for biological applications, focusing on efficient characterization using minimal samples while maintaining consistency. Continued advancement in this area will elevate therapeutic outcomes for lung cancer patients, contributing to the broader landscape of precision medicine.
3.3. Preparation of EVs as therapeutic carriers
The role of EVs in lung cancer therapy extends beyond targeting the signaling pathways of endogenous EVs for lung cancer therapy, as explored in the aforementioned section 2. In addition to that, exogenous EVs have emerged as promising drug delivery vehicles in the targeted therapy of lung cancer, leveraging their inherent advantages such as biocompatibility, low immunogenicity, and natural ability to transport bioactive molecules142,143. Various types of EVs, including exosomes and microvesicles, have been extensively studied and implemented using in vivo models, demonstrating their potential to efficiently deliver therapeutic payloads to target cells. Advanced technologies have been employed to prepare natural EVs (Supporting Information Table S3), including size/density-based methods (ultracentrifugation, density gradient centrifugation, size exclusion chromatography), affinity-based methods (immunocapture using antibodies or capture agents), and microfluidic-based methods (viscoelastic flows or with nano/microstructure, electrical-based device, hydrodynamic, acoustic fluidic), to ensure their optimal characteristics for drug delivery applications. In addition to the preparation of natural EVs, engineered EVs, modified for enhanced targeting and cargo-loading capabilities, have been developed for subsequent therapeutic applications144. EV-based drug carriers offer versatility and efficacy, representing a novel and innovative approach for transforming targeted lung cancer therapy142, 143, 144.
Natural EVs that exhibit intercellular communication roles, good biocompatibility, low toxicity and immunogenicity, a long time circulating in the blood, and permeability to various biological barriers are initially subject to facilitate the therapeutic effects of cells employed in lung cancer therapeutic applications. Numerous preclinical models have demonstrated the advantageous effects, including accelerated apoptosis and reduced tumor growth induced by natural EVs derived from mesenchymal stem/stromal cells (MSC-EVs). Biological cargos carried by EVs play a fundamental role in orchestrating the therapeutic activities of EVs145, 146, 147, 148. For example, miR-193, carried by MSC-EVs, reduced cisplatin resistance and suppressed the proliferation, migration, and invasion of NSCLC cells147, and miR-30b-5p, carried by MSC-EVs, promoted apoptosis and inhibited lung tumorigenesis in nude mice146. A phase II clinical trial worked on the immunotherapeutic effects of DC-derived EVs carrying IFN-γ combined with MHC-I and MHC-II could enhance the antitumor effect of NK cells in NSCLC patients149.
Therapeutic applications based on natural EVs face challenges. Obtaining a quantity of high purity natural EVs on a large scale is complex, and potential contamination with unwanted components may occur, affecting the safety and efficacy of therapeutic interventions. Moreover, the lack of specificity in targeting cells or tissues and the biological barriers faced by EVs, such as immune system clearance, pose challenges for their effective use. Additional complexities associated with harnessing the therapeutic potential of natural EVs are loading therapeutic cargo or targeting anchors with high specificity and efficiency, standardizing isolation and production protocols, and ensuring storage stability. Addressing these challenges necessitates further research and technological advancements to optimize natural EVs for practical and effective therapeutic applications.
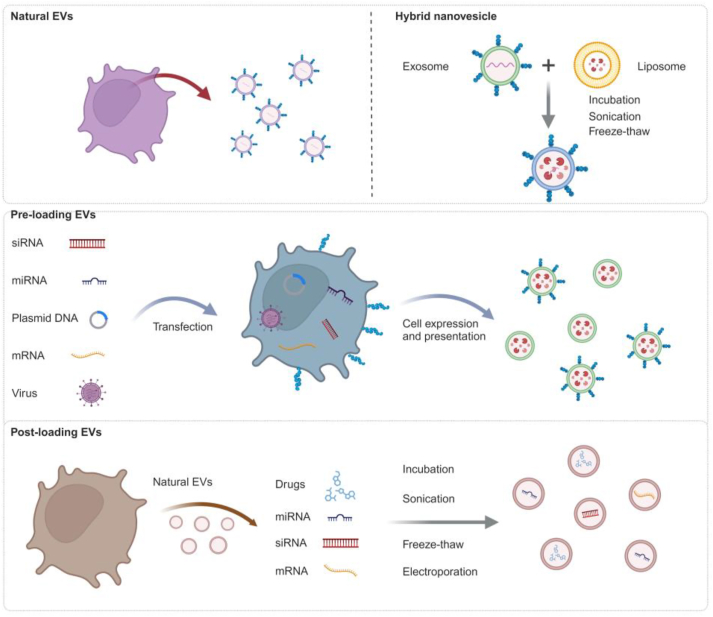
Different technologies have been used to engineer natural EVs to overcome their challenges and limitations150. Chemically engineered EVs, bioengineered EVs, and EV-coated synthetic nanoparticles have been explored for EV-based lung cancer therapeutics (Fig. 5). Natural EVs can be engineered with pre-loading methods (i.e., EV modification takes place during EV formation) or post-loading methods (i.e., EV modification occurs after EV isolation)151,152.
Figure 5.
Diverse strategies, including pre-loading and post-loading methods, to prepare both natural and engineered EVs for downstream applications. Created with Biorender.com.
Chemically engineered EVs are realized by conjugating tumor targeting probes to EVs or by loading them with anticancer reagents via passive or active approaches. Passive loading is accomplished by direct incubation followed by mixing the concentration gradient of antitumor drugs that interact or penetrate membranes of EVs. While this approach is easy to implement and nondestructive (i.e., preserves EV integrity), it's applicability predominantly limited to certain types of drugs since loading is highly dependent on the physiochemical characteristics of the cargo153. To address this issue, active loading techniques have been developed, including electroporation154, 155, 156, sonication157, freeze–thawing158, and extrusion159, which have advantages over passive approaches156. These methods use an external stimulus, such as an electric field, sound energy, mechanical stress, or pressure, to induce transient pores in the EV membrane, enabling enhanced loading efficiency or a broader range of cargoes. But there is a potential concern as to whether these active approaches disrupt EV stability or the unique molecular cargoes of natural EVs. Therefore, additional investigation is required to validate these approaches for diverse EV loading.
Bioengineered EVs can be accomplished by pre-loading methods, such as coincubation and genetic modification in donor cells with targeting properties, encompassing the inclusion of targeted nucleic acids, peptides, or proteins by inducing their expression160, 161, 162. Such approach takes advantage of the inherent cellular machinery to load nucleic acids, peptides or proteins into EVs. Careful consideration should be taken when developing bioengineering methods, overexpression, or direct transfection, emphasizing the potential impact of residual or co-released reagents on EV behavior, false-positive effects, and toxicity.
Bioinspired and biomimetic EVs have also gained popularity in recent years, due to their potential for large-scale production, precise fabrication process control, and reduced cost (compared to the cost of using tissue culture facilities to prepare EVs)163,164. Examples include EV-mimetic nanovesicles, prepared by continuously squeezing donor cells through membrane filters with different pore sizes or by passing donor cells through microchannels165, 166, 167, hybrid EVs produced by the fusion of EVs with conventional biomaterial liposomes168,169, EV membrane-camouflaged nanoparticles created by enveloping synthesize nanoparticles, benefit from both the bio-targeting abilities of EVs and the physiochemical properties of synthetic nanomaterials170, have recently been explored as nanohybrids for improved lung cancer therapeutics.
3.4. EV-based delivery platforms for lung cancers
Natural and engineered EVs have found applications in the realm of lung cancer, enhancing the efficacy of existing approaches. Numerous studies have delved into the integration of these nanotechnological advancements to augment clinically employed therapeutic strategies for lung cancer. These investigations span a spectrum of therapeutic modalities, encompassing traditional methods, such as radio/chemotherapy, and more personalized approaches, such as immunotherapy and targeted therapy. Additionally, nano-based methodologies like photothermal therapy, photodynamic therapy, and sonodynamic therapy have been explored (Fig. 6)171, 172, 173, 174. This collective exploration signifies a comprehensive effort to harness the potential of prepared EVs in advancing diverse therapeutic strategies for lung cancer treatment (Supporting Information Table S4).
Figure 6.
EV-based delivery platforms designed to enhance various therapeutic strategies for lung cancer. (A) EVs enhance chemoimmunotherapy for lung cancers. Hybrid nanovesicles are prepared by fusing CAR T cell-derived EVs carrying perforin and granzyme B with lung-targeting liposomes to deliver paclitaxel (PTX). These hybrid nanovesicles inheriting lung-targeting abilities from liposomes, swiftly accumulate in the lungs after injection. They effectively transported PTX and cytotoxic granules to mesothelin (MSLN)-positive tumor cells via anti-MSLN single-chain variable fragments (scFvs), triggering immunogenic cell death (ICD) and blocking programmed death ligand-1 (PD-L1), thereby enhancing antitumor effect and extending survival in a metastatic lung cancer model. Reprinted with the permission from Ref. 171. Copyright © 2023 American Chemical Society. (B) Utilization of immune cell-derived exosomes to enhance radiotherapy effectiveness by employing engineered M1 macrophages (stimulated from M0 macrophages) using lipopolysaccharide (LPS)-derived exosomes (M1Exos) as potent sensitizers. Engineered M1Exos address key limitations of radiotherapy in three ways: they relieve tumor hypoxia, inhibit DNA damage repair with DNA damage repair inhibitor (DDRi), and remodel the immunosuppressive TME. Expressing catalase (CAT) to alleviate hypoxia and containing DNA repair inhibitors, M1Exos enhance radiotherapy efficacy, while their natural characteristics polarize M2 macrophages to promote antitumor immune responses and inhibit suppressive immune cells. Reprinted with the permission from Ref. 172. Copyright © 2022 The Authors. Advanced Science published by Wiley-VCH GmbH. (C) Encapsulating interleukin-12 messenger RNA within EVs for inhalation presents a targeted strategy for delivery to lung cancer cells. This strategy stimulates an immune response and offers significant protection against primary and subsequent tumor challenges, indicating promising potential for locally delivered cytokine-based immunotherapies. Reprinted with the permission from Ref. 173. Copyright © 2024, The Author(s), under exclusive licence to Springer Nature Limited. (D) EVs enhance PDT, The M2-like tumor-associated macrophages (M2 TAMs)-targeting peptide (CRV), are overexpress the exosomes by fusing the CRV sequence into lysosome-associated membrane glycoprotein 2b (Lamp2b) encoded plasmids. These exosomes, termed CRV-exosomes, were utilized to modify BITT nanoparticles (CEB) (upper panel). CEB, with its dual-targeting capability for both tumor cells and M2 TAMs or M2 macrophages, eliminates these cells via photodynamic and photothermal therapy upon laser irradiation (lower panel). This innovative dual-targeting and phototherapeutic system presents a promising platform for lung cancer therapy, effectively eradicating cancer cells by enhancing PTT, PDT, and reshaping the TME. Reprinted with the permission from Ref. 174. Springer Nature, Copyright © 2023, The Author(s).
3.4.1. EVs deliver traditional chemotherapy drugs
Chemotherapy, a conventional cancer treatment approach, involves administering potent drugs to eliminate rapidly dividing cancer cells, but it often leads to severe side effects due to the high toxicity of these drugs. EVs offer promising delivery vehicles that can enhance the delivery of conventional chemotherapy drugs at reduced dosages, thereby improving the precision and effectiveness of chemotherapy while minimizing adverse side effects. Illustrating this, EVs obtained from A549 cancer cells were used to deliver docetaxel (DTX), a drug that reduces tumor cell proliferation, induces tumor cell apoptosis, and increases reactive oxygen species generation in the TME. Packaging the drug into EVs addressed the limitations of its unsatisfactory solubility, pharmacokinetics, and in vivo performance in the treatment of NSCLC. The EV-DTX complex demonstrated heightened tumor cell cytotoxicity and superior targeting of tumor tissue compared to free DTX175.
3.4.2. EVs enhance radiotherapy for lung cancer
Through targeted delivery, EVs can carry radiation-sensitizing agents directly to cancer cells, minimizing collateral damage to healthy tissues. Additionally, these nanoscale vesicles may enhance radiosensitization, protect healthy tissues, modulate the immune response, and potentially overcome radioresistance, collectively offering a multifaceted strategy to optimize the precision and effectiveness of radiotherapy in lung cancer treatment. Ongoing investigations into EV-based radiotherapeutic approaches hold significant potential for advancing cancer treatment outcomes. M1 macrophage-derived EVs (M1EVs) have been engineered as radiotherapy sensitizers to enhance the efficacy of radiotherapy in three ways: alleviating tumor hypoxia, inhibiting DNA damage repair, and reshaping the immunosuppressive microenvironment172. Similarly, EVs derived from gingival mesenchymal stem cells (GMSCs), characterized by a cup-shaped morphology, exhibit promise when combined with ionizing radiation (IR) for treatment. This combined therapy demonstrates superior efficacy in restraining NSCLC cell proliferation and inducing apoptosis compared to that of IR treatment alone. The effectiveness of this approach is further confirmed in A549 subcutaneous xenograft models, in which the combined therapy effectively suppresses tumor growth176. These radiotherapies, enhanced by EVs, inhibit tumor growth, regulate antitumor immunity, and increase DNA damage to achieve excellent antitumor efficacy.
3.4.3. EVs deliver immunotherapy agents
Immunotherapy represents a revolutionary strategy in lung cancer treatment, harnessing the body's immune system to combat malignant disease. By serving as carriers for immunomodulatory agents and leveraging their intrinsic ability to transport bioactive molecules, EVs facilitate precise and targeted delivery to tumor sites, thereby minimizing off-target effects. Researchers are actively investigating EV-based delivery approaches to amplify the efficacy of immunotherapy, heralding a transformative pathway for improving cancer treatment outcomes. Although CAR T cell–based therapies have shown success in managing various hematopoietic cancers, such methods suffer several limitations, especially the potential development of immunosuppressive and immune rejection mechanisms, and uncontrolled passage, migration, or mutation of engineered cells in vivo, as well as the reliance on autologous cells. EVs have emerged as cell-free immunotherapeutic agents that could address many of these challenges associated with the potential side effects and toxicity of live cell-based therapeutics. For example, CAR T cells engineered to express anti-mesothelin single-chain variable fragment (scFV) transfer EVs containing paclitaxel (PTX), named PTX@CAR-Exo, via an inhalable administration to an orthotopic lung cancer mouse model. Consequently, an accumulation of PTX@CAR-Exo within the tumor area was observed, along with reduced tumor size and metastatic lesions and prolonged survival. Furthermore, there was a reversion of the immunosuppressive TME, which increased IFN-γ and TNF-α levels and CD8+ T cell-mediated clearance of solid tumors177, suggesting a potential opportunity to improve adoptive cell transfer (ACT) therapies by, in part, providing an EV-based platform to promote the costimulation of chemotherapeutic drugs and immune cells. Similarly, a hybrid nanovesicle named Lip-CExo@PTX, incorporating both EVs derived from bispecific CAR T cells which was engineered to target mesothelin (MSLN) and PD-L1, and liposomes targeting lung tissue with their changed ratio of cationic and anionic lipids, sequentially transferred PTX and cytotoxic granules (granzyme B and perforin) to MSLN-positive lung tumors to avoid PD-L1-induced T cell exhaustion and promote PTX-induced chemotherapeutic response. Consistently, hybrid nanovesicles accumulated in lung tissue at a rate of over 95% after intravenous administration, and this attracted more infiltrating lymphocytes to amplify the benefits from PTX-induced immunogenic cell death, further enhancing the antitumor effects171.
3.4.4. EVs deliver genes for cancer therapy
Gene therapy has great potential to revolutionize cancer therapy by regulating the expression level of abnormal genes, such as KRAS, EGFR, and P53 in lung cancer178, 179, 180. As a naturally effective delivery vehicle, EVs have been widely exploited for different biomolecules, including DNA, mRNA, siRNA, miRNA, and antisense oligonucleotides, for abnormal gene knockdown, overcoming the immune rejection which is the biggest obstacle associated with viral vectors for gene therapy181,182. For example, EVs containing adeno-associated virus (AAV) effectively suppressed tumor growth in several preclinical studies from AAV-packaging HEK 293T cells using sequential centrifugation and iodixanol density gradient, show higher gene delivery efficiency and more resistance to antibody neutralization, compared to free AAV lung cancer cell lines and in a xenograft mouse model183. Another study mass-produced engineered EVs by fusing EVs with lipids and loading them with exogenous siRNA via extrusion and electroporation techniques. These EVs were then taken up by lung cancer cells, resulting in highly effective gene silencing showing potential substitute for gene delivery carriers, as they exhibited a 14-fold increase in cellular uptake by lung cancer cells (A549) and achieved a gene silencing effect comparable to that of commercial Lipofectamine RNAiMax184.
3.4.5. EV-based vaccines for lung cancer
Lung cancer vaccines, which evoke an anticancer immune response, are a promising approach to reduce pulmonary malignancy185,186. By enhancing the immune response against cancer while reducing the risk for the formation of embryomas/teratomas and autoimmunity, EVs have been developed as an alternative prophylactic vaccine for lung cancer187. One research group developed a prophylactic vaccine, named the ES-exo/GM-CSF vaccine, which used EVs derived from murine embryonic stem cells that had been engineered to produce granulocyte-macrophage colony stimulating factor (GM-CSF). This vaccine significantly inhibited the growth of metastatic lung tumors through a decrease of tumor-infiltrating-induced the immunosuppressive of T regulatory cells, MDSCs, and TAMs, as well as increased production of effector cytokine production by intra-tumoral CD8+ T cells, which covered several overlapping mechanisms mentioned in section 2188. Similarly, fibroblast activation protein-α (FAP) gene-engineered tumor cell-derived exosome-like nanovesicles (eNVs-FAP), preserving the advantages of natural EVs by using a rapid and high-throughput production approach, can promote the release of IFN-γ from cytotoxic T lymphocyte and the reduction of FAP+ CAFs through inducing lung tumor ferroptosis, indicating eNVs-FAP emerges as a promising candidate vaccine for lung tumor parenchyma and the stroma80.
3.4.6. EVs conjugated with nanoparticles for PTT, SDT, and PDT
Therapeutic strategies that employ nanoparticle-based photothermal therapy (PTT) agents represent a promising alternative to traditional therapy for cancers, attracting significant interest for eliminating primary tumors as well as tumor metastases and recurrence189,190. In one study, platelet exosomes and photothermal sensitive liposomes were combined to create EV-mimetic nanoparticles, which incorporated glucose oxidase and ferric ammonium to enhance chemodynamic therapy and photothermal effect related to damage angiogenesis and oxidize glucose metabolism on tumor-bearing mice191. In another study, tumor-derived EVs encapsulating black phosphorus quantum dots demonstrated strong PTT efficiency for tumor ablation, along with the activated host immunity and increased the number of infiltrating T cells within TME192. Besides PTT, EV-based delivery platforms also show advantages via integrating sonodynamic therapy (SDT), and photodynamic therapy (PDT). One research group utilized EVs to serve as nanocarriers delivering sonosensitizer indocyanine green, resulting in greatly improved sonotoxicity against cancer cells193. Another group loaded EVs with the photodynamic therapy agent 5-aminolevulinic acid, and the EVs were injected into tumor-bearing mice along with doxorubicin, showing the success of a synergistic chemotherapeutic and photodynamic antitumor effect194. These principles, which have already shown potential for other types of tumors, can be easily adapted and explored for lung cancer therapeutic applications.
3.4.7. EVs overcome drug resistance in lung cancer
Accumulating evidence suggests that EV-based carriers represent a new drug delivery platform for overcoming drug resistance, including resistance towards chemotherapy, immunotherapy, and targeted therapy in lung cancer. For instance, regarding chemotherapy resistance, LOC85009 delivered by lung cancer cell-derived EVs suppressed cell proliferation while promoting cell apoptosis through the ATG5-induced autophagy signaling pathway, thus attenuating docetaxel resistance in LUAD42. In addition, the delivery of microRNA-193a by bone marrow mesenchymal stem cell-derived exosomes enhanced the sensitivity of drug-resistant NSCLC cells to cisplatin147. In the context of immunotherapy resistance, EV-mediated circZNF451 delivery restrained anti-PD1 treatment resistance by reshaping the TME characterized by M2 phenotype polarization of macrophages and exhaustion of cytotoxic CD8+ T cells47. And regarding targeted therapy resistance, miR-7 contained in EVs, the levels of which are higher in gefitinib-sensitive PC9 cells than the levels in gefitinib-resistant H1975 cells, can be transferred intercellularly to reverse gefitinib resistance and inhibit tumor growth by targeting the yes-associated protein in NSCLC195. Additionally, the combination of EV-delivered miR-7-5p and everolimus exhibits synergistic anticancer therapeutic efficacy via abrogating the MNK/eIF4E axis and mTOR signaling in NSCLC196.
In addition to potential application as a drug delivery vehicle, targeting EV-mediated resistance has also been recently explored in lung cancer therapy, encompassing mechanisms inhibiting the biogenesis and release of EVs and eliciting anticancer immune response197. Targeting tumor cell-derived EVs containing PD-L1 has shown promise as a therapeutic strategy to overcome cancer immunotherapy resistance, including for lung cancer. Recently, in a lung cancer mouse xenograft model, the FDA-approved drug macitentan amplified the therapeutic efficacy of anti-PD-L1 antibody and antitumor immunity by inhibiting the secretion of EVs containing PD-L1 as well as the subsequent binding of these EVs to CD8+ T cells198.
3.5. Ongoing clinical studies and trials of EV-based lung cancer therapeutics
Given the preference for single-subtype populations of EVs, many obstacles stemming from the isolation, purification, and characterization of clinical-grade EVs remain in addition to obstacles associated with the need for quality control and standardization associated with cell-based therapeutics. Despite this, however, several successful clinical studies and trials of EV-based lung cancer therapeutics have been successfully implemented. The first clinical study of an EV-based therapy was a phase I study of NSCLC patients (human leukocyte antigen A2+, stages III B to IV) expressing Melanoma Antigen Gene family proteins A3 or A4. Engineered to carry MAGE-derived peptides, clinical grade, autologous DC-derived EVs (Dex) were prepared to investigate the safety, feasibility, and treatment efficiency of EV-based immunotherapy199. Despite the study's small sample size, Dex was shown to simultaneously deliver high levels of antigen and costimulatory molecules, prohibiting the activation of immune effectors to maintain the long-term stability of the disease. Moreover, Dex triggered only mild side-effects (grades I and II: injection site reactions, flu-like illness, and peripheral arm pain)200. Given the results from the above phase I study, researchers at the Gustave Roussy and Curie Institutes collaborated to develop an immunotherapeutic approach that incorporated metronomic cyclophosphamide (mCTX) followed by administering vaccinations containing Dex. The mCTX suppressed Treg functions, thereby restoring the effector functions of T and NK cells. Additionally, the study showed that Dex was proficient in activating both innate and adaptive immunity. A phase II clinical trial (NCT01159288) was carried out that used the second generation of Dex (IFN-γ–Dex) loaded with major histocompatibility complex (MHC) class I- and class II-restricted cancer antigens201. The trial aimed to boost NK- and T-cell immune responses as maintenance immunotherapy in unresectable (stage III B to IV) NSCLC patients with response/stabilization to chemotherapeutic induction. It concluded that IFN-γ–Dex is capable of improving immune stimulatory ability and enhancing NK-cell function in patients benefiting from prolonged progression-free survival, although it did not meet the trial's primary endpoint of reaching 50% of patients with progression-free survival by 4-months after chemotherapy149. More recently, a clinical trial in China (ChiCTR-ICR-15006304) evaluated tumor cell-derived EVs packaging methotrexate (TMPs-MTX) combined with pemetrexed-cisplatin chemotherapy in patients with advanced non-squamous NSCLC with malignant pleural effusion. TMPs-MTX demonstrated a manageable safety profile and was an effective treatment, as shown by an elevated rate of objective response, prolonged progression-free survival, and overall survival202. With the boom of this EV field, numerous clinical trials are essential to evaluate the therapeutic and prognostic value of EVs in the context of lung cancer.
4. Conclusions and perspectives
In this review, we have summarized significant advances, which have allowed for unprecedented insight into EV composition and biology and their role in lung cancer metabolism, angiogenesis, progression and metastasis, immunity, and their resistance to lung cancer therapy. We have also highlighted recent technologies, including those for isolating, modifying, and detecting EVs, which underscore their exceptional ability to be exploited as diagnostic, prognostic, and therapeutic agents in lung cancer. Excitingly, with ongoing technological advancements and the establishment of standardized guidelines for EV research, now possible with a better understanding of their biology, we anticipate the future holds further breakthroughs in overcoming the current challenges in the field.
The present understanding of EVs for lung cancer predominantly relies on studies from cell line models. Thus, a remaining challenge is understanding the role of EVs in the complex interplay of normal physiology and homeostasis in vivo, especially considering the challenges in maintaining the phenotypes of ex vivo models. By utilizing in vivo models, researchers can further explore how EVs influence the TME, immune evasion mechanisms, and treatment response in a dynamic and physiologically relevant context. Furthermore, recent advances in imaging techniques allow for real-time visualization of EV dynamics within living organisms, enabling researchers to track EV behavior and distribution in real-time to better mimic what occurs in real patients. Additionally, recently developed organoid models, particularly those employing genome modification technologies beyond cell line studies, recapitulate key features of tumor architecture and microenvironment and offer a platform for studying EV-mediated communication within a more complex three-dimensional context. We believe further studies using these advances will elucidate the multifaceted roles of EVs in lung cancer progression and therapy responses to pave the way for the development of novel diagnostic and therapeutic strategies.
Due to limitations in technology development, most EV-based studies employ crude isolation methods that presumably result in a mixture of heterogeneous EVs for subsequent exploration. As a consequence, the precise cellular origin of EV subgroups is not clear. Advanced approaches that enable individual and pure EV isolation and the tracking of EVs released by distinct cell populations would assist in the clarification of this points. We envision that recent advancements in nanotechnology and single-cell techniques have led to significant progress in separating, analyzing, and tracking the heterogeneity of EVs in vivo. These developments, including the development of single-EV analysis techniques adapted from single-cell techniques, have unveiled previously unknown heterogeneity in EV cargo and facilitated the molecular and functional characterization of specific EV subsets.
While considerable efforts have been dedicated to EV-based applications, numerous difficulties and challenges persist. First, no available standardized procedure for the isolation and storage of EVs exists for all types of EV-based therapeutic applications, including lung cancer. Providing that large-scale clinical-grade EVs are desired, isolation methods adopted for long-term use would essentially ensure reproducibility, purity, minimal impurities, and preservation of the functional properties of EVs. Second, establishing quality release criteria, which should encompass physicochemical quantification, molecular characterization, and functional parameters predicting therapeutic potential is a fundamental requirement for the approval of EV preparations for clinical applications.
The therapeutic potential of EVs in lung cancer is strongly influenced by their half-life and the stability of their cargoes, necessitating strategies to prolong their bioavailability. Despite their role as biomimetic composites, EVs and the cargoes they carry still face degradation and clearance issues in the bloodstream. Another hurdle is minimizing off-target effects and improving tissue-specific targeting. Achieving precise localization of EVs to lung tumors is difficult due to their inherent heterogeneity and interactions with circulating proteins or cellular receptors. Thus, a reliance on innovative targeting strategies is crucial to enhance therapeutic specificity and efficacy. It will be necessary to develop surface modifications and encapsulation techniques that balance EV stability while maintaining functionality.
The choice of administration route significantly influences EV pharmacokinetics and efficacy. While intravenous administration offers systemic delivery, direct inhalation or intratracheal administration targets the lungs more effectively. Each route has advantages and challenges, necessitating careful consideration of factors such as tissue penetration and procedural risks. Determining the optimal dose and dosing strategy is complex and depends on various factors such as disease severity and therapeutic payload. Strategies like dose escalation and fractionated dosing may optimize efficacy while minimizing adverse effects, but further research is needed to establish optimal regimens for different patient populations. Understanding EV pharmacokinetics and pharmacodynamics in vivo is crucial for optimizing therapeutic strategies. Despite progress in elucidating EV biology, their behavior in physiological environments remains poorly understood. Overcoming these challenges requires identifying suitable preclinical in vivo models for evaluating the potential of EV-based therapeutics for lung cancer. Additionally, systematic evaluation in preclinical and clinical settings is essential to predict therapeutic outcomes accurately and accelerate the translation of EV-based therapies for lung cancer management.
Although clinical trials of EV-based therapeutics have shown minimal toxicities careful selection of cell sources for EV production is essential for successful therapeutic outcomes, even unmodified EVs from certain non-malignant cell types are considered to be immunologically inert and can be used as allogenic therapeutics in animal studies and a small set of patients. Comprehensive data from more clinical safety and potency assays are vital for transitioning EV research from the laboratory to clinical practice. Collaborative efforts across disciplines, including by clinicians, analysts, pharmacists, clinical chemists, and engineers will be pivotal in advancing EV research to enhance the management and outcomes of lung cancer patients.
Author contributions
Gaigai Huang: Conceptualization, Writing – original draft, Writing – review & editing. Wenshu Zheng: Conceptualization, Supervision, Visualization, Writing – original draft, Writing – review & editing. Yu Zhou: Conceptualization, Writing – original draft. Meihua Wan: Conceptualization. Tony Hu: Conceptualization, Supervision, Writing – original draft, Writing – review & editing.
Conflicts of interest
The authors have no conflicts of interest to declare.
Acknowledgments
The work was primarily supported by research funding provided by the US Department of Defense (W8IXWH1910926), National Institutes of Health (U01CA252965, R01AI144168, R01AI175618, R01HD090927, R01HD103511, R21NS130542, USA), and Carol Lavin Bernick Faculty Award of Tulane University.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting information to this article can be found online at https://doi.org/10.1016/j.apsb.2024.06.010.
Contributor Information
Wenshu Zheng, Email: wzheng5@tulane.edu.
Meihua Wan, Email: wanmh@scu.edu.cn.
Tony Hu, Email: tonyhu@tulane.edu.
Appendix A. Supporting information
The following are the Supporting information to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 3.Duma N., Santana-Davila R., Molina J.R. Non-small cell lung cancer: epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94:1623–1640. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Feng J., Lian Z., Xia X., Lu Y., Hu K., Zhang Y., et al. Targeting metabolic vulnerability in mitochondria conquers MEK inhibitor resistance in KRAS-mutant lung cancer. Acta Pharm Sin B. 2023;13:1145–1163. doi: 10.1016/j.apsb.2022.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lei X., Li Z., Zhong Y., Li S., Chen J., Ke Y., et al. Gli1 promotes epithelial-mesenchymal transition and metastasis of non-small cell lung carcinoma by regulating snail transcriptional activity and stability. Acta Pharm Sin B. 2022;12:3877–3890. doi: 10.1016/j.apsb.2022.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71:7–33. doi: 10.3322/caac.21654. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman R.M., Sanchez R. Lung cancer screening. Med Clin North Am. 2017;101:769–785. doi: 10.1016/j.mcna.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Latimer K.M. Lung cancer: clinical presentation and diagnosis. FP Essent. 2018;464:23–26. [PubMed] [Google Scholar]
- 9.Aberle D.R., Adams A.M., Berg C.D., Black W.C., Clapp J.D., Fagerstrom R.M., et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395–409. doi: 10.1056/NEJMoa1102873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith H.B., Ward R., Frazier C., Angotti J., Tanner N.T. Guideline-recommended lung cancer screening adherence is superior with a centralized approach. Chest. 2022;161:818–825. doi: 10.1016/j.chest.2021.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Takeshita J., Masago K., Kato R., Hata A., Kaji R., Fujita S., et al. CT-guided fine-needle aspiration and core needle biopsies of pulmonary lesions: a single-center experience with 750 biopsies in Japan. AJR Am J Roentgenol. 2015;204:29–34. doi: 10.2214/AJR.14.13151. [DOI] [PubMed] [Google Scholar]
- 12.Kemp R.A., Reinders D.M., Turic B. Detection of lung cancer by automated sputum cytometry. J Thorac Oncol. 2007;2:993–1000. doi: 10.1097/JTO.0b013e318158d488. [DOI] [PubMed] [Google Scholar]
- 13.Detterbeck F.C., Mazzone P.J., Naidich D.P., Bach P.B. Screening for lung cancer: diagnosis and management of lung cancer, 3rd Ed: American College of Chest Physicians Evidence-based Clinical Practice Guidelines. Chest. 2013;143:e78S–e92S. doi: 10.1378/chest.12-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Remon J., Soria J.C., Peters S. Early and locally advanced non-small-cell lung cancer: an update of the esmo clinical practice guidelines focusing on diagnosis, staging, systemic and local therapy. Ann Oncol. 2021;32:1637–1642. doi: 10.1016/j.annonc.2021.08.1994. [DOI] [PubMed] [Google Scholar]
- 15.Gerlinger M., Rowan A.J., Horswell S., Math M., Larkin J., Endesfelder D., et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Margerie-Mellon C., de Bazelaire C., de Kerviler E. Image-guided biopsy in primary lung cancer: why, when and how. Diagn Interv Imaging. 2016;97:965–972. doi: 10.1016/j.diii.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 17.Su T., Huang S., Zhang Y., Guo Y., Zhang S., Guan J., et al. miR-7/TGF-β2 axis sustains acidic tumor microenvironment-induced lung cancer metastasis. Acta Pharm Sin B. 2022;12:821–837. doi: 10.1016/j.apsb.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaft J.E., Rimner A., Weder W., Azzoli C.G., Kris M.G., Cascone T. Evolution of systemic therapy for stages I–III non-metastatic non-small-cell lung cancer. Nat Rev Clin Oncol. 2021;18:547–557. doi: 10.1038/s41571-021-00501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi C., Zhang C., Fu Z., Liu J., Zhou Y., Cheng B., et al. Antitumor activity of aumolertinib, a third-generation EGFR tyrosine kinase inhibitor, in non-small-cell lung cancer harboring uncommon EGFR mutations. Acta Pharm Sin B. 2023;13:2613–2627. doi: 10.1016/j.apsb.2023.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kemper M., Krekeler C., Menck K., Lenz G., Evers G., Schulze A.B., et al. Liquid biopsies in lung cancer. Cancers (Basel) 2023;15:1430. doi: 10.3390/cancers15051430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pantel K., Alix-Panabières C. Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med. 2010;16:398–406. doi: 10.1016/j.molmed.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Siravegna G., Marsoni S., Siena S., Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531–548. doi: 10.1038/nrclinonc.2017.14. [DOI] [PubMed] [Google Scholar]
- 23.Kalluri R., LeBleu V.S. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katzmann D.J., Babst M., Emr S.D. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell. 2001;106:145–155. doi: 10.1016/s0092-8674(01)00434-2. [DOI] [PubMed] [Google Scholar]
- 25.Stenmark H. Rab GTPases as coordinators of vesicle traffic. Nat Rev Mol Cell Biol. 2009;10:513–525. doi: 10.1038/nrm2728. [DOI] [PubMed] [Google Scholar]
- 26.Gao J., Wei B., de Assuncao T.M., Liu Z., Hu X., Ibrahim S., et al. Hepatic stellate cell autophagy inhibits extracellular vesicle release to attenuate liver fibrosis. J Hepatol. 2020;73:1144–1154. doi: 10.1016/j.jhep.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sinha S., Hoshino D., Hong N.H., Kirkbride K.C., Grega-Larson N.E., Seiki M., et al. Cortactin promotes exosome secretion by controlling branched actin dynamics. J Cell Biol. 2016;214:197–213. doi: 10.1083/jcb.201601025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shurer C.R., Kuo J.C., Roberts L.M., Gandhi J.G., Colville M.J., Enoki T.A., et al. Physical principles of membrane shape regulation by the glycocalyx. Cell. 2019;177 doi: 10.1016/j.cell.2019.04.017. 1757-70.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verweij F.J., Bebelman M.P., George A.E., Couty M., Bécot A., Palmulli R., et al. ER membrane contact sites support endosomal small GTPase conversion for exosome secretion. J Cell Biol. 2022;221:e202112032. doi: 10.1083/jcb.202112032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arya S.B., Chen S., Jordan-Javed F., Parent C.A. Ceramide-rich microdomains facilitate nuclear envelope budding for non-conventional exosome formation. Nat Cell Biol. 2022;24:1019–1028. doi: 10.1038/s41556-022-00934-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 32.Liu N., Hou L., Chen X., Bao J., Chen F., Cai W., et al. Arabidopsis tetraspanin8 mediates exosome secretion and glycosyl inositol phosphoceramide sorting and trafficking. Plant Cell. 2024;36:626–641. doi: 10.1093/plcell/koad285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo B.B., Bellingham S.A., Hill A.F. The neutral sphingomyelinase pathway regulates packaging of the prion protein into exosomes. J Biol Chem. 2015;290:3455–3467. doi: 10.1074/jbc.M114.605253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leidal A.M., Huang H.H., Marsh T., Solvik T., Zhang D., Ye J., et al. The LC3-conjugation machinery specifies the loading of RNA-binding proteins into extracellular vesicles. Nat Cell Biol. 2020;22:187–199. doi: 10.1038/s41556-019-0450-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sriwastva M.K., Teng Y., Mu J., Xu F., Kumar A., Sundaram K., et al. An extracellular vesicular mutant KRAS-associated protein complex promotes lung inflammation and tumor growth. J Extracell Vesicles. 2023;12 doi: 10.1002/jev2.12307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thuya W.L., Kong L.R., Syn N.L., Ding L.W., Cheow E.S.H., Wong R.T.X., et al. FAM3C in circulating tumor-derived extracellular vesicles promotes non-small cell lung cancer growth in secondary sites. Theranostics. 2023;13:621–638. doi: 10.7150/thno.72297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y., Fan J., Xu T., Ahmadinejad N., Hess K., Lin S.H., et al. Extracellular vesicle tetraspanin-8 level predicts distant metastasis in non-small cell lung cancer after concurrent chemoradiation. Sci Adv. 2020;6:eaaz6162. doi: 10.1126/sciadv.aaz6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodrigues G., Hoshino A., Kenific C.M., Matei I.R., Steiner L., Freitas D., et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat Cell Biol. 2019;21:1403–1412. doi: 10.1038/s41556-019-0404-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y., Liu X., Zeng L., Zhao X., Chen Q., Pan Y., et al. Exosomal protein angiopoietin-like 4 mediated radioresistance of lung cancer by inhibiting ferroptosis under hypoxic microenvironment. Br J Cancer. 2022;127:1760–1772. doi: 10.1038/s41416-022-01956-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang D., Zhao C., Xu F., Zhang A., Jin M., Zhang K., et al. Cisplatin-resistant NSCLC cells induced by hypoxia transmit resistance to sensitive cells through exosomal PKM2. Theranostics. 2021;11:2860–2875. doi: 10.7150/thno.51797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng X., Dong K., Ye C., Jiang Y., Zhai S., Cheng R., et al. A breathable, biodegradable, antibacterial, and self-powered electronic skin based on all-nanofiber triboelectric nanogenerators. Sci Adv. 2020;6 doi: 10.1126/sciadv.aba9624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu Z., Tang H., Chen S., Xie Y., Shi L., Xia S., et al. Exosomal LOC85009 inhibits docetaxel resistance in lung adenocarcinoma through regulating ATG5-induced autophagy. Drug Resist Updat. 2023;67 doi: 10.1016/j.drup.2022.100915. [DOI] [PubMed] [Google Scholar]
- 43.Huang J., Ding M., Lu Y., Xu L., Zhang Y., Han S., et al. miR-1246b, a novel miRNA molecule of extracellular vesicles in bronchoalveolar lavage fluid, promotes nodule growth through FGF14 in patients with lung cancer. Cell Death Dis. 2023;14:789. doi: 10.1038/s41419-023-06218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li C., Qin F., Wang W., Ni Y., Gao M., Guo M., et al. hnRNPA2B1-mediated extracellular vesicles sorting of miR-122-5p potentially promotes lung cancer progression. Int J Mol Sci. 2021;22:12866. doi: 10.3390/ijms222312866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu D., Deng S., Li L., Liu T., Zhang T., Li J., et al. TGF-β1-mediated exosomal lnc-MMP2-2 increases blood–brain barrier permeability via the miRNA-1207-5p/EPB41L5 axis to promote non-small cell lung cancer brain metastasis. Cell Death Dis. 2021;12:721. doi: 10.1038/s41419-021-04004-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diao X., Guo C., Zheng H., Zhao K., Luo Y., An M., et al. SUMOylation-triggered ALIX activation modulates extracellular vesicles CIRCTLCD4-RWDD3 to promote lymphatic metastasis of non-small cell lung cancer. Signal Transduct Target Ther. 2023;8:426. doi: 10.1038/s41392-023-01685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao J., Ao Y.Q., Zhang L.X., Deng J., Wang S., Wang H.K., et al. Exosomal circZNF451 restrains anti-PD1 treatment in lung adenocarcinoma via polarizing macrophages by complexing with trim56 and fxr1. J Exp Clin Cancer Res. 2022;41:295. doi: 10.1186/s13046-022-02505-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu W., Ru Z., Zhou Y., Xiao W., Sun R., Zhang S., et al. Lung cancer-derived extracellular vesicles induced myotube atrophy and adipocyte lipolysis via the extracellular IL-6-mediated STAT3 pathway. Biochim Biophys Acta Mol Cell Biol Lipids. 2019;1864:1091–1102. doi: 10.1016/j.bbalip.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 49.Hu W., Xiong H., Ru Z., Zhao Y., Zhou Y., Xie K., et al. Extracellular vesicles-released parathyroid hormone-related protein from lewis lung carcinoma induces lipolysis and adipose tissue browning in cancer cachexia. Cell Death Dis. 2021;12:134. doi: 10.1038/s41419-020-03382-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y.H., Chen Y., Shi L., Han X., Xie J.C., Chen Y., et al. A novel lung cancer stem cell extracellular vesicles lncRNA ROLLCSC modulate non-stemness cancer cell plasticity through miR-5623-3p and miR-217-5p targeting lipid metabolism. Int J Biol Macromol. 2023;256 doi: 10.1016/j.ijbiomac.2023.128412. [DOI] [PubMed] [Google Scholar]
- 51.Tyagi A., Wu S.Y., Sharma S., Wu K., Zhao D., Deshpande R., et al. Exosomal miR-4466 from nicotine-activated neutrophils promotes tumor cell stemness and metabolism in lung cancer metastasis. Oncogene. 2022;41:3079–3092. doi: 10.1038/s41388-022-02322-w. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Yang Q., Luo J., Xu H., Huang L., Zhu X., Li H., et al. Metabolomic investigation of urinary extracellular vesicles for early detection and screening of lung cancer. J Nanobiotechnol. 2023;21:153. doi: 10.1186/s12951-023-01908-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morrissey S.M., Zhang F., Ding C., Montoya-Durango D.E., Hu X., Yang C., et al. Tumor-derived exosomes drive immunosuppressive macrophages in a pre-metastatic niche through glycolytic dominant metabolic reprogramming. Cell Metab. 2021;33 doi: 10.1016/j.cmet.2021.09.002. 2040–58.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Calcinotto A., Kohli J., Zagato E., Pellegrini L., Demaria M., Alimonti A. Cellular senescence: aging, cancer, and injury. Physiol Rev. 2019;99:1047–1078. doi: 10.1152/physrev.00020.2018. [DOI] [PubMed] [Google Scholar]
- 55.Liu Y., Zhan Z., Kang Z., Li M., Lv Y., Li S., et al. Preclinical and early clinical studies of a novel compound SYHA1813 that efficiently crosses the blood–brain barrier and exhibits potent activity against glioblastoma. Acta Pharm Sin B. 2023;13:4748–4764. doi: 10.1016/j.apsb.2023.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fan J., Xu G., Chang Z., Zhu L., Yao J. miR-210 transferred by lung cancer cell-derived exosomes may act as proangiogenic factor in cancer-associated fibroblasts by modulating JAK2/STAT3 pathway. Clin Sci (Lond) 2020;134:807–825. doi: 10.1042/CS20200039. [DOI] [PubMed] [Google Scholar]
- 57.Mao S., Lu Z., Zheng S., Zhang H., Zhang G., Wang F., et al. Exosomal miR-141 promotes tumor angiogenesis via KLF12 in small cell lung cancer. J Exp Clin Cancer Res. 2020;39:193. doi: 10.1186/s13046-020-01680-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hsu Y.L., Hung J.Y., Chang W.A., Lin Y.S., Pan Y.C., Tsai P.H., et al. Hypoxic lung cancer-secreted exosomal miR-23a increased angiogenesis and vascular permeability by targeting prolyl hydroxylase and tight junction protein ZO-1. Oncogene. 2017;36:4929–4942. doi: 10.1038/onc.2017.105. [DOI] [PubMed] [Google Scholar]
- 59.Chang R.M., Fu Y., Zeng J., Zhu X.Y., Gao Y. Cancer-derived exosomal miR-197-3p confers angiogenesis via targeting TIMP2/3 in lung adenocarcinoma metastasis. Cell Death Dis. 2022;13:1032. doi: 10.1038/s41419-022-05420-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu X., Li R., Chen X., Yao J., Wang Q., Zhang J., et al. SYT7 is a key player in increasing exosome secretion and promoting angiogenesis in non-small-cell lung cancer. Cancer Lett. 2023;577 doi: 10.1016/j.canlet.2023.216400. [DOI] [PubMed] [Google Scholar]
- 61.Hoshino D., Kirkbride K.C., Costello K., Clark E.S., Sinha S., Grega-Larson N., et al. Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 2013;5:1159–1168. doi: 10.1016/j.celrep.2013.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hsu Y.L., Huang M.S., Hung J.Y., Chang W.A., Tsai Y.M., Pan Y.C., et al. Bone-marrow-derived cell-released extracellular vesicle miR-92a regulates hepatic pre-metastatic niche in lung cancer. Oncogene. 2020;39:739–753. doi: 10.1038/s41388-019-1024-y. [DOI] [PubMed] [Google Scholar]
- 63.Xu W., Patel N., Deng Y., Ding S., Wang T., Zhang H. Extracellular vesicle-derived LINC00482 induces microglial M2 polarization to facilitate brain metastasis of NSCLC. Cancer Lett. 2023;561 doi: 10.1016/j.canlet.2023.216146. [DOI] [PubMed] [Google Scholar]
- 64.Chen G.Y., Cheng J.C., Chen Y.F., Yang J.C., Hsu F.M. Circulating exosomal integrin β3 is associated with intracranial failure and survival in lung cancer patients receiving cranial irradiation for brain metastases: a prospective observational study. Cancers (Basel) 2021;13:380. doi: 10.3390/cancers13030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ni J., Zhang X., Li J., Zheng Z., Zhang J., Zhao W., et al. Tumour-derived exosomal lncRNA-SOX2OT promotes bone metastasis of non-small cell lung cancer by targeting the miRNA-194-5p/RAC1 signalling axis in osteoclasts. Cell Death Dis. 2021;12:662. doi: 10.1038/s41419-021-03928-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lou F., Huang J., Sima C.S., Dycoco J., Rusch V., Bach P.B. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg. 2013;145:75–81. doi: 10.1016/j.jtcvs.2012.09.030. [DOI] [PubMed] [Google Scholar]
- 67.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 68.Lengel H.B., Mastrogiacomo B., Connolly J.G., Tan K.S., Liu Y., Fick C.N., et al. Genomic mapping of metastatic organotropism in lung adenocarcinoma. Cancer Cell. 2023;41 doi: 10.1016/j.ccell.2023.03.018. 970–85.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Blavier L., Nakata R., Neviani P., Sharma K., Shimada H., Benedicto A., et al. The capture of extracellular vesicles endogenously released by xenotransplanted tumours induces an inflammatory reaction in the premetastatic niche. J Extracell Vesicles. 2023;12 doi: 10.1002/jev2.12326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lahiri A., Maji A., Potdar P.D., Singh N., Parikh P., Bisht B., et al. Lung cancer immunotherapy: progress, pitfalls, and promises. Mol Cancer. 2023;22:40. doi: 10.1186/s12943-023-01740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yao Y., Chen C., Wang J., Xuan H., Chen X., Li Z., et al. Circular RNA cirCATP9A promotes non-small cell lung cancer progression by interacting with HuR and by promoting extracellular vesicles-mediated macrophage M2 polarization. J Exp Clin Cancer Res. 2023;42:330. doi: 10.1186/s13046-023-02916-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rao X., Zhou X., Wang G., Jie X., Xing B., Xu Y., et al. NLRP6 is required for cancer-derived exosome-modified macrophage M2 polarization and promotes metastasis in small cell lung cancer. Cell Death Dis. 2022;13:891. doi: 10.1038/s41419-022-05336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen J., Zhang K., Zhi Y., Wu Y., Chen B., Bai J., et al. Tumor-derived exosomal miR-19b-3p facilitates M2 macrophage polarization and exosomal LINC00273 secretion to promote lung adenocarcinoma metastasis via Hippo pathway. Clin Transl Med. 2021;11:e478. doi: 10.1002/ctm2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hsu Y.L., Hung J.Y., Chang W.A., Jian S.F., Lin Y.S., Pan Y.C., et al. Hypoxic lung-cancer-derived extracellular vesicle microRNA-103a increases the oncogenic effects of macrophages by targeting PTEN. Mol Ther. 2018;26:568–581. doi: 10.1016/j.ymthe.2017.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liang M., Chen X., Wang L., Qin L., Wang H., Sun Z., et al. Cancer-derived exosomal TRIM59 regulates macrophage NLRP3 inflammasome activation to promote lung cancer progression. J Exp Clin Cancer Res. 2020;39:176. doi: 10.1186/s13046-020-01688-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chang W.A., Tsai M.J., Hung J.Y., Wu K.L., Tsai Y.M., Huang Y.C., et al. miR-150-5p-containing extracellular vesicles are a new immunoregulator that favor the progression of lung cancer in hypoxic microenvironments by altering the phenotype of NK cells. Cancers (Basel) 2021;13:6252. doi: 10.3390/cancers13246252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alexander M., Hu R., Runtsch M.C., Kagele D.A., Mosbruger T.L., Tolmachova T., et al. Exosome-delivered microRNAs modulate the inflammatory response to endotoxin. Nat Commun. 2015;6:7321. doi: 10.1038/ncomms8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Than U.T.T., Le H.T., Hoang D.H., Nguyen X.H., Pham C.T., Bui K.T.V., et al. Induction of antitumor immunity by exosomes isolated from cryopreserved cord blood monocyte-derived dendritic cells. Int J Mol Sci. 2020;21:1834. doi: 10.3390/ijms21051834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xu P., Zhang X., Chen K., Zhu M., Jia R., Zhou Q., et al. Tumor cell-derived microparticles induced by methotrexate augment T-cell antitumor responses by downregulating expression of PD-1 in neutrophils. Cancer Immunol Res. 2023;11:501–514. doi: 10.1158/2326-6066.CIR-22-0595. [DOI] [PubMed] [Google Scholar]
- 80.Hu S., Ma J., Su C., Chen Y., Shu Y., Qi Z., et al. Engineered exosome-like nanovesicles suppress tumor growth by reprogramming tumor microenvironment and promoting tumor ferroptosis. Acta Biomater. 2021;135:567–581. doi: 10.1016/j.actbio.2021.09.003. [DOI] [PubMed] [Google Scholar]
- 81.Ma X., Chen Z., Hua D., He D., Wang L., Zhang P., et al. Essential role for Trpc5-containing extracellular vesicles in breast cancer with chemotherapeutic resistance. Proc Natl Acad Sci U S A. 2014;111:6389–6394. doi: 10.1073/pnas.1400272111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Park M., Kim J.W., Kim K.M., Kang S., Kim W., Kim J.K., et al. Circulating small extracellular vesicles activate TYRO3 to drive cancer metastasis and chemoresistance. Cancer Res. 2021;81:3539–3553. doi: 10.1158/0008-5472.CAN-20-3320. [DOI] [PubMed] [Google Scholar]
- 83.Kreger B.T., Johansen E.R., Cerione R.A., Antonyak M.A. The enrichment of survivin in exosomes from breast cancer cells treated with paclitaxel promotes cell survival and chemoresistance. Cancers (Basel) 2016;8:111. doi: 10.3390/cancers8120111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han R., Guo H., Shi J., Wang H., Zhao S., Jia Y., et al. Tumour microenvironment changes after osimertinib treatment resistance in non-small cell lung cancer. Eur J Cancer. 2023;189 doi: 10.1016/j.ejca.2023.05.007. [DOI] [PubMed] [Google Scholar]
- 85.Poggio M., Hu T., Pai C.C., Chu B., Belair C.D., Chang A., et al. Suppression of exosomal PD-L1 induces systemic anti-tumor immunity and memory. Cell. 2019;177 doi: 10.1016/j.cell.2019.02.016. 414–27.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ma S., Mangala L.S., Hu W., Bayaktar E., Yokoi A., Hu W., et al. CD63-mediated cloaking of VEGF in small extracellular vesicles contributes to anti-VEGF therapy resistance. Cell Rep. 2021;36 doi: 10.1016/j.celrep.2021.109549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xiao P., Liu Y.K., Han W., Hu Y., Zhang B.Y., Liu W.L. Exosomal delivery of FTO confers gefitinib resistance to recipient cells through ABCC10 regulation in an m6A-dependent manner. Mol Cancer Res. 2021;19:726–738. doi: 10.1158/1541-7786.MCR-20-0541. [DOI] [PubMed] [Google Scholar]
- 88.Xie H., Yao J., Wang Y., Ni B. Exosome-transmitted circVMP1 facilitates the progression and cisplatin resistance of non-small cell lung cancer by targeting miR-524-5p-METTL3/SOX2 axis. Drug Deliv. 2022;29:1257–1271. doi: 10.1080/10717544.2022.2057617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ma Y., Yuwen D., Chen J., Zheng B., Gao J., Fan M., et al. Exosomal transfer of cisplatin-induced miR-425-3p confers cisplatin resistance in NSCLC through activating autophagy. Int J Nanomedicine. 2019;14:8121–8132. doi: 10.2147/IJN.S221383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang T., Zhang P., Li H.X. CAFS-derived exosomal miRNA-130a confers cisplatin resistance of NSCLC cells through PUM2-dependent packaging. Int J Nanomedicine. 2021;16:561–577. doi: 10.2147/IJN.S271976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gu G., Hu C., Hui K., Zhang H., Chen T., Zhang X., et al. Exosomal miR-136-5p derived from anlotinib-resistant NSCLC cells confers anlotinib resistance in non-small cell lung cancer through targeting PPP2R2A. Int J Nanomedicine. 2021;16:6329–6343. doi: 10.2147/IJN.S321720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang K., Chen J., Li C., Yuan Y., Fang S., Liu W., et al. Exosome-mediated transfer of SNHG7 enhances docetaxel resistance in lung adenocarcinoma. Cancer Lett. 2022;526:142–154. doi: 10.1016/j.canlet.2021.10.029. [DOI] [PubMed] [Google Scholar]
- 93.Wu S., Luo M., To K.K.W., Zhang J., Su C., Zhang H., et al. Intercellular transfer of exosomal wild type EGFR triggers osimertinib resistance in non-small cell lung cancer. Mol Cancer. 2021;20:17. doi: 10.1186/s12943-021-01307-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang X., Qiao D., Chen L., Xu M., Chen S., Huang L., et al. Chemotherapeutic drugs stimulate the release and recycling of extracellular vesicles to assist cancer cells in developing an urgent chemoresistance. Mol Cancer. 2019;18:182. doi: 10.1186/s12943-019-1114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen J., Yang J., Wang W., Guo D., Zhang C., Wang S., et al. Tumor extracellular vesicles mediate anti-PD-L1 therapy resistance by decoying anti-PD-L1. Cell Mol Immunol. 2022;19:1290–1301. doi: 10.1038/s41423-022-00926-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chen G., Huang A.C., Zhang W., Zhang G., Wu M., Xu W., et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560:382–386. doi: 10.1038/s41586-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cohen O., Betzer O., Elmaliach-Pnini N., Motiei M., Sadan T., Cohen-Berkman M., et al. 'Golden' exosomes as delivery vehicles to target tumors and overcome intratumoral barriers: in vivo tracking in a model for head and neck cancer. Biomater Sci. 2021;9:2103–2114. doi: 10.1039/d0bm01735c. [DOI] [PubMed] [Google Scholar]
- 98.Jung K.O., Jo H., Yu J.H., Gambhir S.S., Pratx G. Development and MPI tracking of novel hypoxia-targeted theranostic exosomes. Biomaterials. 2018;177:139–148. doi: 10.1016/j.biomaterials.2018.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Han Z., Liu S., Pei Y., Ding Z., Li Y., Wang X., et al. Highly efficient magnetic labelling allows MRI tracking of the homing of stem cell-derived extracellular vesicles following systemic delivery. J Extracell Vesicles. 2021;10 doi: 10.1002/jev2.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jung K.O., Kim Y.H., Chung S.J., Lee C.H., Rhee S., Pratx G., et al. Identification of lymphatic and hematogenous routes of rapidly labeled radioactive and fluorescent exosomes through highly sensitive multimodal imaging. Int J Mol Sci. 2020;21:7850. doi: 10.3390/ijms21217850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Varga Z., Gyurkó I., Pálóczi K., Buzás E.I., Horváth I., Hegedűs N., et al. Radiolabeling of extracellular vesicles with (99m)Tc for quantitative in vivo imaging studies. Cancer Biother Radiopharm. 2016;31:168–173. doi: 10.1089/cbr.2016.2009. [DOI] [PubMed] [Google Scholar]
- 102.Bhatta R., Han J., Liu Y., Bo Y., Lee D., Zhou J., et al. Metabolic tagging of extracellular vesicles and development of enhanced extracellular vesicle based cancer vaccines. Nat Commun. 2023;14:8047. doi: 10.1038/s41467-023-43914-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lázaro-Ibáñez E., Faruqu F.N., Saleh A.F., Silva A.M., Tzu-Wen Wang J., Rak J., et al. Selection of fluorescent, bioluminescent, and radioactive tracers to accurately reflect extracellular vesicle biodistribution in vivo. ACS Nano. 2021;15:3212–3227. doi: 10.1021/acsnano.0c09873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cho S., Yi J., Kwon Y., Kang H., Han C., Park J. Multifluorescence single extracellular vesicle analysis by time-sequential illumination and tracking. ACS Nano. 2021;15:11753–11761. doi: 10.1021/acsnano.1c02556. [DOI] [PubMed] [Google Scholar]
- 105.Han C., Yang J., Zhang E., Jiang Y., Qiao A., Du Y., et al. Metabolic labeling of cardiomyocyte-derived small extracellular-vesicle (sEV) miRNAs identifies miR-208a in cardiac regulation of lung gene expression. J Extracell Vesicles. 2022;11 doi: 10.1002/jev2.12246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lara P., Palma-Florez S., Salas-Huenuleo E., Polakovicova I., Guerrero S., Lobos-Gonzalez L., et al. Gold nanoparticle based double-labeling of melanoma extracellular vesicles to determine the specificity of uptake by cells and preferential accumulation in small metastatic lung tumors. J Nanobiotechnol. 2020;18:20. doi: 10.1186/s12951-020-0573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pan B.T., Johnstone R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 108.Gandham S., Su X., Wood J., Nocera A.L., Alli S.C., Milane L., et al. Technologies and standardization in research on extracellular vesicles. Trends Biotechnol. 2020;38:1066–1098. doi: 10.1016/j.tibtech.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Seo K., Yoo J.H., Kim J., Min S.J., Heo D.N., Kwon I.K., et al. Ginseng-derived exosome-like nanovesicles extracted by sucrose gradient ultracentrifugation to inhibit osteoclast differentiation. Nanoscale. 2023;15:5798–5808. doi: 10.1039/d2nr07018a. [DOI] [PubMed] [Google Scholar]
- 110.Gámez-Valero A., Monguió-Tortajada M., Carreras-Planella L., Franquesa M., Beyer K., Borràs F.E. Size-exclusion chromatography-based isolation minimally alters extracellular vesicles' characteristics compared to precipitating agents. Sci Rep. 2016;6 doi: 10.1038/srep33641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chattrairat K., Yasui T., Suzuki S., Natsume A., Nagashima K., Iida M., et al. All-in-one nanowire assay system for capture and analysis of extracellular vesicles from an ex vivo brain tumor model. ACS Nano. 2023;17:2235–2244. doi: 10.1021/acsnano.2c08526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yasui T., Paisrisarn P., Yanagida T., Konakade Y., Nakamura Y., Nagashima K., et al. Molecular profiling of extracellular vesicles via charge-based capture using oxide nanowire microfluidics. Biosens Bioelectron. 2021;194 doi: 10.1016/j.bios.2021.113589. [DOI] [PubMed] [Google Scholar]
- 113.Yuan D., Zhao Q., Yan S., Tang S.Y., Alici G., Zhang J., et al. Recent progress of particle migration in viscoelastic fluids. Lab Chip. 2018;18:551–567. doi: 10.1039/c7lc01076a. [DOI] [PubMed] [Google Scholar]
- 114.Tian F., Cai L., Chang J., Li S., Liu C., Li T., et al. Label-free isolation of rare tumor cells from untreated whole blood by interfacial viscoelastic microfluidics. Lab Chip. 2018;18:3436–3445. doi: 10.1039/c8lc00700d. [DOI] [PubMed] [Google Scholar]
- 115.Wang G., Crawford K., Turbyfield C., Lam W., Alexeev A., Sulchek T. Microfluidic cellular enrichment and separation through differences in viscoelastic deformation. Lab Chip. 2015;15:532–540. doi: 10.1039/c4lc01150c. [DOI] [PubMed] [Google Scholar]
- 116.Wang C., Senapati S., Chang H.C. Liquid biopsy technologies based on membrane microfluidics: high-yield purification and selective quantification of biomarkers in nanocarriers. Electrophoresis. 2020;41:1878–1892. doi: 10.1002/elps.202000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Yafouz B., Kadri N.A., Ibrahim F. Dielectrophoretic manipulation and separation of microparticles using microarray dot electrodes. Sensors. 2014;14:6356–6369. doi: 10.3390/s140406356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moore J.H., Varhue W.B., Su Y.H., Linton S.S., Farmehini V., Fox T.E., et al. Conductance-based biophysical distinction and microfluidic enrichment of nanovesicles derived from pancreatic tumor cells of varying invasiveness. Anal Chem. 2019;91:10424–10431. doi: 10.1021/acs.analchem.8b05745. [DOI] [PubMed] [Google Scholar]
- 119.Ibsen S.D., Wright J., Lewis J.M., Kim S., Ko S.Y., Ong J., et al. Rapid isolation and detection of exosomes and associated biomarkers from plasma. ACS Nano. 2017;11:6641–6651. doi: 10.1021/acsnano.7b00549. [DOI] [PubMed] [Google Scholar]
- 120.Davies R.T., Kim J., Jang S.C., Choi E.J., Gho Y.S., Park J. Microfluidic filtration system to isolate extracellular vesicles from blood. Lab Chip. 2012;12:5202–5210. doi: 10.1039/c2lc41006k. [DOI] [PubMed] [Google Scholar]
- 121.Cheung L.S., Sahloul S., Orozaliev A., Song Y.A. Rapid detection and trapping of extracellular vesicles by electrokinetic concentration for liquid biopsy on chip. Micromachines (Basel) 2018;9:306. doi: 10.3390/mi9060306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nam J., Tan J.K., Khoo B.L., Namgung B., Leo H.L., Lim C.T., et al. Hybrid capillary-inserted microfluidic device for sheathless particle focusing and separation in viscoelastic flow. Biomicrofluidics. 2015;9 doi: 10.1063/1.4938389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vaidyanathan R., Naghibosadat M., Rauf S., Korbie D., Carrascosa L.G., Shiddiky M.J., et al. Detecting exosomes specifically: a multiplexed device based on alternating current electrohydrodynamic induced nanoshearing. Anal Chem. 2014;86:11125–11132. doi: 10.1021/ac502082b. [DOI] [PubMed] [Google Scholar]
- 124.Wu M., Ouyang Y., Wang Z., Zhang R., Huang P.H., Chen C., et al. Isolation of exosomes from whole blood by integrating acoustics and microfluidics. Proc Natl Acad Sci U S A. 2017;114:10584–10589. doi: 10.1073/pnas.1709210114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee K., Shao H., Weissleder R., Lee H. Acoustic purification of extracellular microvesicles. ACS Nano. 2015;9:2321–2327. doi: 10.1021/nn506538f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sandfeld-Paulsen B., Jakobsen K.R., Bæk R., Folkersen B.H., Rasmussen T.R., Meldgaard P., et al. Exosomal proteins as diagnostic biomarkers in lung cancer. J Thorac Oncol. 2016;11:1701–1710. doi: 10.1016/j.jtho.2016.05.034. [DOI] [PubMed] [Google Scholar]
- 127.Song Z., Wang S., Liu Y. The diagnostic accuracy of liquid exosomes for lung cancer detection: a meta-analysis. Onco Targets Ther. 2019;12:181–192. doi: 10.2147/OTT.S188832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhu N., Li G., Zhou J., Zhang Y., Kang K., Ying B., et al. A light-up fluorescence resonance energy transfer magnetic aptamer-sensor for ultra-sensitive lung cancer exosome detection. J Mater Chem B. 2021;9:2483–2493. doi: 10.1039/d1tb00046b. [DOI] [PubMed] [Google Scholar]
- 129.Liu C., Zeng X., An Z., Yang Y., Eisenbaum M., Gu X., et al. Sensitive detection of exosomal proteins via a compact surface plasmon resonance biosensor for cancer diagnosis. ACS Sens. 2018;3:1471–1479. doi: 10.1021/acssensors.8b00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sha L., Bo B., Yang F., Li J., Cao Y., Zhao J. Programmable DNA-fueled electrochemical analysis of lung cancer exosomes. Anal Chem. 2022;94:8748–8755. doi: 10.1021/acs.analchem.2c01318. [DOI] [PubMed] [Google Scholar]
- 131.Shin H., Oh S., Hong S., Kang M., Kang D., Ji Y.G., et al. Early-stage lung cancer diagnosis by deep learning-based spectroscopic analysis of circulating exosomes. ACS Nano. 2020;14:5435–5444. doi: 10.1021/acsnano.9b09119. [DOI] [PubMed] [Google Scholar]
- 132.Ding L., Wu Y., Liu L.E., He L., Yu S., Effah C.Y., et al. Universal DNAzyme walkers-triggered CRISPR-Cas12a/Cas13a bioassay for the synchronous detection of two exosomal proteins and its application in intelligent diagnosis of cancer. Biosens Bioelectron. 2023;219 doi: 10.1016/j.bios.2022.114827. [DOI] [PubMed] [Google Scholar]
- 133.Hsu C.C., Yang Y., Kannisto E., Zeng X., Yu G., Patnaik S.K., et al. Simultaneous detection of tumor derived exosomal protein-microRNA pairs with an exo-pros biosensor for cancer diagnosis. ACS Nano. 2023;17:8108–8122. doi: 10.1021/acsnano.2c10970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chang L., Wu H., Chen R., Sun X., Yang Y., Huang C., et al. Microporous PdCuB nanotag-based electrochemical aptasensor with Au@CuCl2 nanowires interface for ultrasensitive detection of PD-L1-positive exosomes in the serum of lung cancer patients. J Nanobiotechnol. 2023;21:86. doi: 10.1186/s12951-023-01845-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tian F., Zhang S., Liu C., Han Z., Liu Y., Deng J., et al. Protein analysis of extracellular vesicles to monitor and predict therapeutic response in metastatic breast cancer. Nat Commun. 2021;12:2536. doi: 10.1038/s41467-021-22913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu D., Yan J., Shen X., Sun Y., Thulin M., Cai Y., et al. Profiling surface proteins on individual exosomes using a proximity barcoding assay. Nat Commun. 2019;10:3854. doi: 10.1038/s41467-019-11486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lee S.E., Park H.Y., Hur J.Y., Kim H.J., Kim I.A., Kim W.S., et al. Genomic profiling of extracellular vesicle-derived DNA from bronchoalveolar lavage fluid of patients with lung adenocarcinoma. Transl Lung Cancer Res. 2021;10:104–116. doi: 10.21037/tlcr-20-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Choi D., Montermini L., Jeong H., Sharma S., Meehan B., Rak J. Mapping subpopulations of cancer cell-derived extracellular vesicles and particles by nano-flow cytometry. ACS Nano. 2019;13:10499–10511. doi: 10.1021/acsnano.9b04480. [DOI] [PubMed] [Google Scholar]
- 139.Jiang C., Fu Y., Liu G., Shu B., Davis J., Tofaris G.K. Multiplexed profiling of extracellular vesicles for biomarker development. Nanomicro Lett. 2021;14:3. doi: 10.1007/s40820-021-00753-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu T., Wang J., Li T., Cui P., Hou B., Zhuang C., et al. Predicting disease progression in advanced non-small cell lung cancer with circulating neutrophil-derived and platelet-derived microparticles. BMC Cancer. 2021;21:939. doi: 10.1186/s12885-021-08628-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Genova C., Tasso R., Rosa A., Rossi G., Reverberi D., Fontana V., et al. Prognostic role of soluble and extracellular vesicle-associated PD-L1, B7-H3 and B7-H4 in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Cells. 2023;12:832. doi: 10.3390/cells12060832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cabeza L., Perazzoli G., Peña M., Cepero A., Luque C., Melguizo C., et al. Cancer therapy based on extracellular vesicles as drug delivery vehicles. J Control Release. 2020;327:296–315. doi: 10.1016/j.jconrel.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 143.Walker S., Busatto S., Pham A., Tian M., Suh A., Carson K., et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics. 2019;9:8001–8017. doi: 10.7150/thno.37097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Nguyen Cao T.G., Kang J.H., Kang S.J., Truong Hoang Q., Kang H.C., Rhee W.J., et al. Brain endothelial cell-derived extracellular vesicles with a mitochondria-targeting photosensitizer effectively treat glioblastoma by hijacking the blood‒brain barrier. Acta Pharm Sin B. 2023;13:3834–3848. doi: 10.1016/j.apsb.2023.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Liu X.N., Zhang C.B., Lin H., Tang X.Y., Zhou R., Wen H.L., et al. microRNA-204 shuttled by mesenchymal stem cell-derived exosomes inhibits the migration and invasion of non-small-cell lung cancer cells via the KLF7/AKT/HIF-1α axis. Neoplasma. 2021;68:719–731. doi: 10.4149/neo_2021_201208N1328. [DOI] [PubMed] [Google Scholar]
- 146.Li X., Wu F. Mesenchymal stem cell-derived extracellular vesicles transfer miR-598 to inhibit the growth and metastasis of non-small-cell lung cancer by targeting THBS2. Cell Death Discov. 2023;9:3. doi: 10.1038/s41420-022-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wu H., Mu X., Liu L., Wu H., Hu X., Chen L., et al. Bone marrow mesenchymal stem cells-derived exosomal microRNA-193a reduces cisplatin resistance of non-small cell lung cancer cells via targeting LRRC1. Cell Death Dis. 2020;11:801. doi: 10.1038/s41419-020-02962-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liang Y., Zhang D., Li L., Xin T., Zhao Y., Ma R., et al. Exosomal microRNA-144 from bone marrow-derived mesenchymal stem cells inhibits the progression of non-small cell lung cancer by targeting CCNE1 and CCNE2. Stem Cell Res Ther. 2020;11:87. doi: 10.1186/s13287-020-1580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Besse B., Charrier M., Lapierre V., Dansin E., Lantz O., Planchard D., et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology. 2016;5 doi: 10.1080/2162402X.2015.1071008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.S E.L.A., Mäger I., Breakefield X.O., Wood M.J. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 151.Johnsen K.B., Gudbergsson J.M., Skov M.N., Pilgaard L., Moos T., Duroux M. A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy. Biochim Biophys Acta. 2014;1846:75–87. doi: 10.1016/j.bbcan.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 152.van der Meel R., Fens M.H., Vader P., van Solinge W.W., Eniola-Adefeso O., Schiffelers R.M. Extracellular vesicles as drug delivery systems: lessons from the liposome field. J Control Release. 2014;195:72–85. doi: 10.1016/j.jconrel.2014.07.049. [DOI] [PubMed] [Google Scholar]
- 153.Bellavia D., Raimondo S., Calabrese G., Forte S., Cristaldi M., Patinella A., et al. Interleukin 3- receptor targeted exosomes inhibit in vitro and in vivo chronic myelogenous leukemia cell growth. Theranostics. 2017;7:1333–1345. doi: 10.7150/thno.17092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liang G., Kan S., Zhu Y., Feng S., Feng W., Gao S. Engineered exosome-mediated delivery of functionally active miR-26a and its enhanced suppression effect in HEPG2 cells. Int J Nanomedicine. 2018;13:585–599. doi: 10.2147/IJN.S154458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wang M., Lv C.Y., Li S.A., Wang J.K., Luo W.Z., Zhao P.C., et al. Near infrared light fluorescence imaging-guided biomimetic nanoparticles of extracellular vesicles deliver indocyanine green and paclitaxel for hyperthermia combined with chemotherapy against glioma. J Nanobiotechnol. 2021;19:210. doi: 10.1186/s12951-021-00907-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhang X., Zhang H., Gu J., Zhang J., Shi H., Qian H., et al. Engineered extracellular vesicles for cancer therapy. Adv Mater. 2021;33 doi: 10.1002/adma.202005709. [DOI] [PubMed] [Google Scholar]
- 157.Kim M.S., Haney M.J., Zhao Y., Mahajan V., Deygen I., Klyachko N.L., et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine. 2016;12:655–664. doi: 10.1016/j.nano.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Haney M.J., Klyachko N.L., Zhao Y., Gupta R., Plotnikova E.G., He Z., et al. Exosomes as drug delivery vehicles for Parkinson's disease therapy. J Control Release. 2015;207:18–30. doi: 10.1016/j.jconrel.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Fuhrmann G., Serio A., Mazo M., Nair R., Stevens M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release. 2015;205:35–44. doi: 10.1016/j.jconrel.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 160.Wang Z., Mo H., He Z., Chen A., Cheng P. Extracellular vesicles as an emerging drug delivery system for cancer treatment: current strategies and recent advances. Biomed Pharmacother. 2022;153 doi: 10.1016/j.biopha.2022.113480. [DOI] [PubMed] [Google Scholar]
- 161.Rädler J., Gupta D., Zickler A., Andaloussi S.E. Exploiting the biogenesis of extracellular vesicles for bioengineering and therapeutic cargo loading. Mol Ther. 2023;31:1231–1250. doi: 10.1016/j.ymthe.2023.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tang L., Yin Y., Cao Y., Fu C., Liu H., Feng J., et al. Extracellular vesicles-derived hybrid nanoplatforms for amplified CD47 blockade-based cancer immunotherapy. Adv Mater. 2023;35 doi: 10.1002/adma.202303835. [DOI] [PubMed] [Google Scholar]
- 163.Jang Y., Park J., Kim P., Park E.J., Sun H., Baek Y., et al. Development of exosome membrane materials-fused microbubbles for enhanced stability and efficient drug delivery of ultrasound contrast agent. Acta Pharm Sin B. 2023;13:4983–4998. doi: 10.1016/j.apsb.2023.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhou X., Miao Y., Wang Y., He S., Guo L., Mao J., et al. Tumour-derived extracellular vesicle membrane hybrid lipid nanovesicles enhance siRNA delivery by tumour-homing and intracellular freeway transportation. J Extracell Vesicles. 2022;11 doi: 10.1002/jev2.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yan Y., Du C., Duan X., Yao X., Wan J., Jiang Z., et al. Inhibiting collagen I production and tumor cell colonization in the lung via miR-29a-3p loading of exosome-/liposome-based nanovesicles. Acta Pharm Sin B. 2022;12:939–951. doi: 10.1016/j.apsb.2021.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wen Y., Fu Q., Soliwoda A., Zhang S., Zheng M., Mao W., et al. Cell-derived nanovesicles prepared by membrane extrusion are good substitutes for natural extracellular vesicles. Extracellular vesicle. 2022;1:100004. doi: 10.1016/j.vesic.2022.100004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Neupane Y.R., Handral H.K., Alkaff S.A., Chng W.H., Venkatesan G., Huang C., et al. Cell-derived nanovesicles from mesenchymal stem cells as extracellular vesicle-mimetics in wound healing. Acta Pharm Sin B. 2023;13:1887–1902. doi: 10.1016/j.apsb.2022.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Evers M.J.W., van de Wakker S.I., de Groot E.M., de Jong O.G., Gitz-François J.J.J., Seinen C.S., et al. Functional siRNA delivery by extracellular vesicle-liposome hybrid nanoparticles. Adv Healthc Mater. 2022;11 doi: 10.1002/adhm.202101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Piffoux M., Silva A.K.A., Wilhelm C., Gazeau F., Tareste D. Modification of extracellular vesicles by fusion with liposomes for the design of personalized biogenic drug delivery systems. ACS Nano. 2018;12:6830–6842. doi: 10.1021/acsnano.8b02053. [DOI] [PubMed] [Google Scholar]
- 170.Lopes D., Lopes J., Pereira-Silva M., Peixoto D., Rabiee N., Veiga F., et al. Bioengineered exosomal-membrane-camouflaged abiotic nanocarriers: neurodegenerative diseases, tissue engineering and regenerative medicine. Mil Med Res. 2023;10:19. doi: 10.1186/s40779-023-00453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhu T., Chen Z., Jiang G., Huang X. Sequential targeting hybrid nanovesicles composed of chimeric antigen receptor T-cell-derived exosomes and liposomes for enhanced cancer immunochemotherapy. ACS Nano. 2023;17:16770–16786. doi: 10.1021/acsnano.3c03456. [DOI] [PubMed] [Google Scholar]
- 172.Ma X., Yao M., Gao Y., Yue Y., Li Y., Zhang T., et al. Functional immune cell-derived exosomes engineered for the trilogy of radiotherapy sensitization. Adv Sci. 2022;9 doi: 10.1002/advs.202106031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Liu M., Hu S., Yan N., Popowski K.D., Cheng K. Inhalable extracellular vesicle delivery of IL-12 mRNA to treat lung cancer and promote systemic immunity. Nat Nanotechnol. 2024;19:565–575. doi: 10.1038/s41565-023-01580-3. [DOI] [PubMed] [Google Scholar]
- 174.Lin Y., Yi M., Guan X., Chen E., Yang L., Li S., et al. "Two birds with one stone" strategy for the lung cancer therapy with bioinspired AIE aggregates. J Nanobiotechnol. 2023;21:49. doi: 10.1186/s12951-023-01799-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang Y., Guo M., Lin D., Liang D., Zhao L., Zhao R., et al. Docetaxel-loaded exosomes for targeting non-small cell lung cancer: preparation and evaluation in vitro and in vivo. Drug Deliv. 2021;28:1510–1523. doi: 10.1080/10717544.2021.1951894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Liang X., Cai H., Sun H., Li N., Zhao W., Liu Q., et al. Nanoscale exosomes derived from gingiva mesenchymal stem cells for radiotherapy-induced apoptosis in non-small cell lung cancer cells. ACS Appl Nano Mater. 2023;6:13533–13542. [Google Scholar]
- 177.Zheng W., Zhu T., Tang L., Li Z., Jiang G., Huang X. Inhalable CAR-T cell-derived exosomes as paclitaxel carriers for treating lung cancer. J Transl Med. 2023;21:383. doi: 10.1186/s12967-023-04206-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lu J., Yu D. Adenoviral-vector based siRNA for mutant K-ras as a promising tool for lung cancer gene therapy: a license to kill. Cancer Biol Ther. 2006;5:1724–1725. doi: 10.4161/cbt.5.12.3761. [DOI] [PubMed] [Google Scholar]
- 179.Huang H., Yi X., Wei Q., Li M., Cai X., Lv Y., et al. Edible and cation-free kiwi fruit derived vesicles mediated EGFR-targeted siRNA delivery to inhibit multidrug resistant lung cancer. J Nanobiotechnology. 2023;21:41. doi: 10.1186/s12951-023-01766-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Swisher S.G., Roth J.A., Komaki R., Gu J., Lee J.J., Hicks M., et al. Induction of p53-regulated genes and tumor regression in lung cancer patients after intratumoral delivery of adenoviral p53 (INGN 201) and radiation therapy. Clin Cancer Res. 2003;9:93–101. [PubMed] [Google Scholar]
- 181.Munagala R., Aqil F., Jeyabalan J., Kandimalla R., Wallen M., Tyagi N., et al. Exosome-mediated delivery of RNA and DNA for gene therapy. Cancer Lett. 2021;505:58–72. doi: 10.1016/j.canlet.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Uddin N., Binzel D.W., Shu D., Fu T.M., Guo P. Targeted delivery of RNAi to cancer cells using RNA-ligand displaying exosome. Acta Pharm Sin B. 2023;13:1383–1399. doi: 10.1016/j.apsb.2022.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Liu B., Li Z., Huang S., Yan B., He S., Chen F., et al. AAV-containing exosomes as a novel vector for improved gene delivery to lung cancer cells. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.707607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Jhan Y.Y., Prasca-Chamorro D., Palou Zuniga G., Moore D.M., Arun Kumar S., Gaharwar A.K., et al. Engineered extracellular vesicles with synthetic lipids via membrane fusion to establish efficient gene delivery. Int J Pharm. 2020;573 doi: 10.1016/j.ijpharm.2019.118802. [DOI] [PubMed] [Google Scholar]
- 185.Yaddanapudi K., Mitchell R.A., Putty K., Willer S., Sharma R.K., Yan J., et al. Vaccination with embryonic stem cells protects against lung cancer: is a broad-spectrum prophylactic vaccine against cancer possible? PLoS One. 2012;7 doi: 10.1371/journal.pone.0042289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lin F.K., Chui Y.L. Generation of induced pluripotent stem cells from mouse cancer cells. Cancer Biother Radiopharm. 2012;27:694–700. doi: 10.1089/cbr.2012.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lv F., Liu H., Zhao G., Zhao E., Yan H., Che R., et al. Therapeutic exosomal vaccine for enhanced cancer immunotherapy by mediating tumor microenvironment. iScience. 2022;25 doi: 10.1016/j.isci.2021.103639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Meng S., Whitt A.G., Stamp B.F., Eaton J.W., Li C., Yaddanapudi K. Exosome-based cancer vaccine for prevention of lung cancer. Stem Cell Investig. 2023;10:2. doi: 10.21037/sci-2022-030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Li Y., Liu Z., Hou Y., Yang G., Fei X., Zhao H., et al. Multifunctional nanoplatform based on black phosphorus quantum dots for bioimaging and photodynamic/photothermal synergistic cancer therapy. ACS Appl Mater Inter. 2017;9:25098–25106. doi: 10.1021/acsami.7b05824. [DOI] [PubMed] [Google Scholar]
- 190.Yang Y., Hu D., Lu Y., Chu B., He X., Chen Y., et al. Tumor-targeted/reduction-triggered composite multifunctional nanoparticles for breast cancer chemo-photothermal combinational therapy. Acta Pharm Sin B. 2022;12:2710–2730. doi: 10.1016/j.apsb.2021.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ma Y., Zhang Y., Han R., Li Y., Zhai Y., Qian Z., et al. A cascade synergetic strategy induced by photothermal effect based on platelet exosome nanoparticles for tumor therapy. Biomaterials. 2022;282 doi: 10.1016/j.biomaterials.2022.121384. [DOI] [PubMed] [Google Scholar]
- 192.Liu Q., Fan T., Zheng Y., Yang S.L., Yu Z., Duo Y., et al. Immunogenic exosome-encapsulated black phosphorus nanoparticles as an effective anticancer photo-nanovaccine. Nanoscale. 2020;12:19939–19952. doi: 10.1039/d0nr05953f. [DOI] [PubMed] [Google Scholar]
- 193.Nguyen Cao T.G., Kang J.H., You J.Y., Kang H.C., Rhee W.J., Ko Y.T., et al. Safe and targeted sonodynamic cancer therapy using biocompatible exosome-based nanosonosensitizers. ACS Appl Mater Inter. 2021;13:25575–25588. doi: 10.1021/acsami.0c22883. [DOI] [PubMed] [Google Scholar]
- 194.Qian R., Jing B., Jiang D., Gai Y., Zhu Z., Huang X., et al. Multi-antitumor therapy and synchronous imaging monitoring based on exosome. Eur J Nucl Med Mol Imaging. 2022;49:2668–2681. doi: 10.1007/s00259-022-05696-x. [DOI] [PubMed] [Google Scholar]
- 195.Chen R., Qian Z., Xu X., Zhang C., Niu Y., Wang Z., et al. Exosomes-transmitted miR-7 reverses gefitinib resistance by targeting YAP in non-small-cell lung cancer. Pharmacol Res. 2021;165 doi: 10.1016/j.phrs.2021.105442. [DOI] [PubMed] [Google Scholar]
- 196.Liu S., Wang W., Ning Y., Zheng H., Zhan Y., Wang H., et al. Exosome-mediated miR-7-5p delivery enhances the anticancer effect of everolimus via blocking MNK/eIF4E axis in non-small cell lung cancer. Cell Death Dis. 2022;13:129. doi: 10.1038/s41419-022-04565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cheng H.Y., Su G.L., Wu Y.X., Chen G., Yu Z.L. Extracellular vesicles in anti-tumor drug resistance: mechanisms and therapeutic prospects. J Pharm Anal. 2023 doi: 10.1016/j.jpha.2023.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lee C.H., Bae J.H., Choe E.J., Park J.M., Park S.S., Cho H.J., et al. Macitentan improves antitumor immune responses by inhibiting the secretion of tumor-derived extracellular vesicle PD-L1. Theranostics. 2022;12:1971–1987. doi: 10.7150/thno.68864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lamparski H.G., Metha-Damani A., Yao J.Y., Patel S., Hsu D.H., Ruegg C., et al. Production and characterization of clinical grade exosomes derived from dendritic cells. J Immunol Methods. 2002;270:211–226. doi: 10.1016/s0022-1759(02)00330-7. [DOI] [PubMed] [Google Scholar]
- 200.Morse M.A., Garst J., Osada T., Khan S., Hobeika A., Clay T.M., et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. NCT01159288 . 2017. Clinicaltrials Gov.https://clinicaltrials.Gov Available from: [Google Scholar]
- 202.Dong X., Huang Y., Yi T., Hu C., Gao Q., Chen Y., et al. Intrapleural infusion of tumor cell-derived microparticles packaging methotrexate or saline combined with pemetrexed-cisplatin chemotherapy for the treatment of malignant pleural effusion in advanced non-squamous non-small cell lung cancer: a double-blind, randomized, placebo-controlled study. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.1002938. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.