Key Points
Question
What are the effectiveness and safety of long-term use of muscle relaxant medications for the treatment of chronic pain?
Findings
In this systematic review of 44 studies including 2482 participants, 9 unique muscle relaxant medications were assessed. Muscle relaxants may be more beneficial than placebo for treating trigeminal neuralgia, painful cramps, and neck pain, but for fibromyalgia, low back pain, and other syndromes, they did not appear to be beneficial.
Meaning
These findings suggest that long-term use of muscle relaxants may only be beneficial for certain syndromes; clinicians should consider deprescribing if pain-related goals are not met.
This systematic review evaluates the efficacy and safety of long-term use of skeletal muscle relaxants for low back pain, fibromyalgia, headaches, painful cramps or spasticity, and other syndromes.
Abstract
Importance
Stricter opioid prescribing guidelines have increased prescriptions of skeletal muscle relaxants (SMRs) for chronic pain, but the efficacy of long-term use of SMRs for chronic pain is unknown.
Objective
To systematically review the effectiveness or efficacy of long-term use of SMRs for chronic pain.
Evidence Review
Two reviewers systematically searched Ovid MEDLINE, Embase (Ovid), Web of Science, CINAHL, and Cochrane through December 4, 2023. They included articles published in English, Spanish, or Italian. Only randomized clinical trials (RCTs) and cohort studies with comparator groups evaluating at least 1-month duration of SMRs for chronic pain were included. The reviewers dually reviewed data abstraction, risk-of-bias, and quality. They characterized studies by chronic pain syndrome: low back pain, fibromyalgia, headaches, painful cramps or spasticity, and other syndromes.
Findings
A total of 30 RCTs with 1314 participants and 14 cohort studies with 1168 participants assessed SMRs for chronic pain. Studies were primarily short-term (4-6 weeks). Nine unique SMRs were represented by the studies identified. Eleven studies (25%) examined baclofen, 8 (18%) examined tizanidine, and 7 (16%) examined cyclobenzaprine. Evidence for effectiveness was strongest for SMRs used for trigeminal neuralgia, neck pain, and painful cramps; evidence suggested SMRs for fibromyalgia, low back pain, and other syndromes were not more beneficial than placebo. The most common adverse effects were sedation and dry mouth. RCTs had a low to moderate risk of bias, and the quality of cohort studies was fair to good.
Conclusions and Relevance
In this systematic review of long-term use of SMRs for chronic pain, findings suggest that their long-term use may benefit patients with painful spasms or cramps and neck pain; their long-term use for low back pain, fibromyalgia, and headaches did not appear to be beneficial. Clinicians should be vigilant for adverse effects and consider deprescribing if pain-related goals are not met.
Introduction
Chronic pain, commonly defined as pain that lasts beyond 3 months and/or extends past normal tissue healing time,1 affects millions of US residents, with a 2021 prevalence of 21%.2 More than 50 million US adults experience pain most days or every day,1 making chronic pain one of the most significant public health problems in the United States.3 The Centers for Disease Control and Prevention’s Clinical Practice Guideline for Prescribing Opioids for Pain4,5 and the Department of Health and Human Services’ National Pain Strategy6 call for a multimodal approach to pain management, incorporating nonpharmacologic and nonopioid pharmacologic treatment options, leaving considerable latitude for shared decision-making between patients and clinicians. Guidelines specific to certain pain syndromes, such as the American College of Physicians’ clinical practice guideline on treatments for low back pain, emphasize nonopioid medications including nonsteroidal anti-inflammatories (NSAIDs) and muscle relaxant medications.7
Centrally acting skeletal muscle relaxants (SMRs) are a pharmacologically diverse category of medications that include antispasticity and antispasmodic medications, such as baclofen, carisoprodol, chlorzoxazone, cyclobenzaprine, metaxalone, methocarbamol, orphenadrine, and tizanidine. They are indicated for acute musculoskeletal conditions including spasms and low back pain; they are also used off-label for numerous other pain and nonpain conditions.8 SMRs are to be used with caution because of central nervous system adverse effects, including drowsiness and dizziness, particularly when used in combination with other centrally acting medications.9 Because of these adverse effects and a lack of evidence regarding the long-term efficacy of SMRs, recommendations generally limit their use to a maximum duration of 2 to 3 weeks.9 However, SMR prescribing doubled between 2005 and 2016, and physician visits for continuing SMR prescriptions tripled during the same period, indicating a shift toward longer duration of use and for nonacute (including chronic) pain syndromes.10
Prior systematic reviews9,11 on the effectiveness or efficacy of longer-term use of SMRs were conducted before this growth of use, focused on specific conditions such as low back pain, and were limited to clinical trials. Given the increase in use of this medication class, and because approximately one-third of patients being prescribed SMRs do not have a preceding musculoskeletal disorder diagnosis,12 a broader examination of their long-term use for multiple chronic conditions is needed. The aim of this systematic review was to evaluate the effectiveness or efficacy of long-term (≥4 weeks) use of SMRs for chronic (≥3 months) pain.
Methods
The reporting of this systematic review was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) standards of quality.13 We developed a protocol for study eligibility a priori and registered it in the PROSPERO database of systematic reviews (CRD42019128973).14 This study was not considered human participant research by the Yale School of Medicine Human Investigation Committee.
Analysis Team
We crafted a research team to draw from multiple sources of expertise.15 Our team included clinician-investigators who provide primary care (B.J.O. and K.L.M.), specialized addiction treatment (B.J.O., K.L.M., J.S.M., and W.C.B.), specialized pain management (W.C.B. and J.S.M.), and specialized palliative care (J.S.M.) as well as a medical librarian with experience in systematic reviews (M.C.F.) and medical trainees (B.G. and Z.A.).
Data Sources and Searches
Our search was structured around the following domains: (1) population of interest (painful syndrome lasting ≥3 months); (2) intervention of interest (use of a nonbenzodiazepine SMR for ≥4 weeks); (3) an adequate comparator; (4) outcomes pertaining to pain severity, pain interference, or quality of life; and (5) study type (randomized clinical trial or cohort study involving at least 10 participants). These inclusion criteria are summarized in Table 1.
Table 1. Inclusion Criteria Following the Population, Intervention, Comparison, Outcome, and Study Design Framework.
| Element | Inclusion criteria |
|---|---|
| Population | Adults ages ≥18 y with chronic pain or painful muscle spasms (experiencing pain on most days for >3 mo) |
| Intervention | Daily use of an oral nonbenzodiazepine antispasmotic drug(s) for 4 weeks or longer |
| Comparison | Placebo, other pain medication, nonpharmacologic pain treatment, nonexposed cohort, baseline (historical) data |
| Outcome | Any outcome(s) that pertains to pain severity, pain interference, or quality of life |
| Study design | Randomized clinical trials, observational studies with a nonexposed cohort, observational studies with baseline evaluation, with at least 10 patients in the intervention arm |
We performed a comprehensive search of the following databases: Ovid MEDLINE, Embase (Ovid), Web of Science, CINAHL, and Cochrane. We performed all searches on December 4, 2023. Search results were pooled in EndNote version 21 (Clarivate) and duplicates removed before uploading to Covidence, a systematic review software. We identified additional studies by scanning other systematic reviews and bibliographies. We also searched the websites of the following preidentified organizations for appropriate references to studies that may not have been indexed in the databases above (ie, grey literature): Society for General Internal Medicine, Substance Abuse and Mental Health Services Administration, and the American Academy of Pain Medicine. We limited our search to studies with human participants and those published in English, Spanish, or Italian. We did not impose a date-of-publication restriction on study inclusion.
To produce relevant controlled vocabulary and keyword terms, we analyzed 5 previously identified key articles using the Yale MeSH Analyzer.16 In each database, we ran scoping searches and used an iterative process to translate and refine the search strategies. We used the previously identified articles to validate the success of our searches (for exact search terminology and syntax, see the eAppendix in Supplement 1).
Study Selection
Two authors independently screened titles and abstracts using a screening algorithm developed a priori. All disagreements were resolved by consensus with the input from the first author. We used Covidence, a systematic review software, to facilitate screeners’ independent organization, retrieval, and assessment of articles.17
Data Extraction and Quality Assessment
For each screened article, 2 authors independently abstracted information about the context, participants, intervention, and outcomes into a standardized form. If desired information was not published, we contacted the first author of the article by email to inquire. To pool the varied expertise on our team, at least 2 team members read all screened studies. As we anticipated considerable heterogeneity of settings in which included studies may have taken place, our data synthesis process drew from realist synthesis, an analytic approach driven by realist theory that considers the interaction between context, mechanism, and outcome in evaluating an intervention.18,19,20 In the realist synthesis strategy, reviewers delineated the contextual influences (C) that were hypothesized to have contributed to the relevant mechanisms (R) to generate the outcomes (O) of interest.19,20 Contextual influences, in this case, were defined by the type of pain syndrome identified. We arrived at consensus for C-R-O sequences during iterative consultations among members of the research team.
Two reviewers independently completed the quality assessment of each study using the Cochrane Risk of Bias Tool for randomized clinical trials21 and the Newcastle-Ottawa Scales for observational studies.22 Disagreements were resolved by consensus with the input of the first author.
Data Analysis
Like previous systematic reviews of the effectiveness of interventions on chronic pain conditions9,11,23 and further informed by the variety of studies identified, we classified interventions by pain syndrome groupings. The groupings were low back pain, fibromyalgia, headaches, painful cramps or spasticity, and other syndromes.
Results
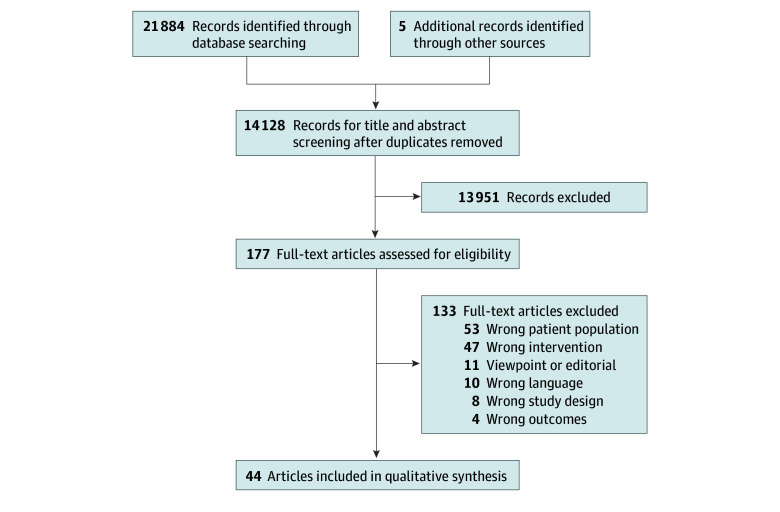
Our search yielded 21 889 articles, and 14 128 remained after the removal of duplicates. Following title and abstract screens, we screened 177 full-text articles for eligibility and identified 44 articles that met criteria for inclusion, each representing a unique study (Figure).24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67 These included 30 randomized clinical trials24,25,26,27,28,29,30,31,32,33,34,35,36,37,39,41,45,46,48,50,51,53,54,58,59,60,61,64,65,67 with 1314 participants and 14 cohort studies38,40,42,43,44,47,49,55,56,57,62,63,66 with 1168 participants.
Figure. Flowchart of Study Selection.
Description of Studies
We identified 5 studies that addressed low back pain (eTable 1 in Supplement 1),53,54,60,64,66 11 studies that addressed fibromyalgia or related disorders (eTable 2 in Supplement 1),28,31,32,41,42,43,48,51,52,59,62 10 studies that addressed headaches or trigeminal neuralgia (eTable 3 in Supplement 1),27,37,40,45,46,47,50,56,57,67 10 studies that addressed painful muscle cramps or spasticity (eTable 4 in Supplement 1),24,25,26,34,36,38,49,58,61 and 8 studies that addressed other pain syndromes, including osteoarthritis,29,39 cervical spondylosis,30 neuropathy,35,55 cancer pain,63 gastric reflux–related pain,65 and orchialgia44 (eTable 5 in Supplement 1).
Nine muscle relaxant medications were represented by the studies identified. The most common were as follows: 11 studies (25%) examined baclofen,26,36,38,44,47,50,57,63,64,65,67 8 (18%) examined tizanidine,37,40,42,43,46,55,56,62 and 7 (16%) examined cyclobenzaprine.28,31,32,35,41,45,51 Other studies examined eperisone,30,39,52,53,54 quinine,34,60,61 carisoprodol,33,58,59 orphenadrine,25,27,49 chlormezanone,29,48 and methocarbamol.24,66
While a plurality of studies took place in the United States or Canada, 12 took place in Europe,29,31,37,46,48,52,53,57,59,60,65,66 12 took place in Asia,26,30,35,39,44,47,50,54,56,63,64,67 3 in Africa,24,25,36 and 1 in Australia/New Zealand.61 Other characteristics of the included studies are found in Table 2.
Table 2. Characteristics of 44 Included Studies .
| Characteristic | Studies, No. (%) (N = 44) |
|---|---|
| Study type | |
| Randomized clinical trial | 30 (68) |
| Cohort study | 14 (32) |
| Study region | |
| United States or Canada | 16 (36) |
| Europe | 12 (27) |
| Asia | 12 (27) |
| Africa | 3 (6) |
| Australia/New Zealand | 1 (2) |
| Year of publication | |
| 2020-2023 | 4 (9) |
| 2010-2019 | 11 (25) |
| 2000-2009 | 13 (30) |
| 1990-1999 | 6 (14) |
| 1980-1989 | 7 (16) |
| 1970-1979 | 1 (2) |
| 1960-1969 | 2 (4) |
| Pain syndrome | |
| Low back pain | 5 (11) |
| Fibromyalgia | 11 (25) |
| Headache or trigeminal neuralgia | 10 (23) |
| Cramps or painful spasticity | 10 (23) |
| Other | 8 (18) |
| Muscle relaxant | |
| Baclofen | 11 (25) |
| Tizanidine | 8 (18) |
| Cyclobenzaprine | 7 (16) |
| Eperisone | 5 (11) |
| Quinine | 3 (7) |
| Carisoprodol | 3 (7) |
| Orphenadrine | 3 (7) |
| Chlormezanone | 2 (4) |
| Methocarbamol | 2 (4) |
| Intervention duration | |
| 4 wk | 13 (30) |
| 4-12 wk | 24 (56) |
| >12 wk | 7 (16) |
Quality Assessment
The risk of bias among randomized clinical studies was low to moderate (eTable 6 in Supplement 1). Risk of bias most commonly manifested as lack of blinding of participants and personnel as well as lack of blinding of outcomes assessments. Cohort studies were of fair to good quality (eTable 7 in Supplement 1). The most common reasons for low quality assessment were low comparability of cohorts based on design or analysis (13 of 14) and low-quality selection of the nonexposed cohort (12 of 14).
Interventions for Back Pain
Among the 5 studies of interventions for back pain, 4 were RCTs53,54,60,64 and included a total of 98 patients in the intervention arms; 1 cohort study66 included 374 individuals in the intervention arm (eTable 1 in Supplement 1). Two of the 5 studies involved eperisone,53,54 1 involved baclofen,64 1 involved quinine,60 and 1 involved methocarbamol.66 Eperisone with tramadol was not associated with improvements in pain severity more than tizanidine with tramadol.53 In a study comparing eperisone with physical therapy and McKenzie therapy (a form of physical therapy that approaches the type of symptomatic complaint more than the anatomic location of the pain), pain scores were most improved in the McKenzie group.54 When quinine was compared with placebo for back pain related to ankylosing spondylitis, mean scores for pain and function did not differ between groups.60 In a study comparing baclofen with placebo and with baclofen and acupuncture, pain scores decreased in all groups but returned to baseline in the baclofen-only group 5 weeks after discontinuation, whereas scores remained improved in the baclofen with acupuncture group.64 In a propensity score–matched (cohort) study comparing methocarbamol with long-term oral opioid analgesics,66 both arms showed clinical improvement, with superior improvement and fewer adverse events in the methocarbamol group. Prevalence of adverse effects in eperisone groups ranged from none to 17% and included somnolence. Adverse effects in the methocarbamol group occurred in 10% of patients and included somnolence and dizziness. No adverse effects were documented for quinine nor baclofen.
Interventions for Fibromyalgia and Similar Disorders
Among 11 studies of intervention for fibromyalgia and similar disorders, 7 were RCTs28,31,32,41,48,51,59 and 4 were cohort studies42,43,52,62 and included a total of 391 patients (eTable 2 in Supplement 1). Five studies involved cyclobenzaprine,28,31,32,41,51 3 involved tizanidine,42,43,62 and 1 study each involved chlormezanone,48 eperisone,52 and carisoprodol.59 Among those involving cyclobenzaprine, all were RCTs. In 3 studies,28,41,51 cyclobenzaprine was associated with improvement in sleep disturbance but with no difference from placebo in other outcomes. In an RCT comparing cyclobenzaprine with amitriptyline,32 both groups improved clinically over 6 months with no difference between groups. Prevalence of adverse effects ranged from none to 98% and included somnolence, dry mouth, and, for those taking a sublingual formulation, tongue and sublingual numbness. Among studies examining tizanidine for fibromyalgia,42,43,62 all were cohort studies, and documented improvements in pain intensity for participants beyond baseline; 1 study43 examined outcomes 1 week after tizanidine was stopped and noted that pain intensity worsened again. Prevalence of adverse effects were none to 66% and included somnolence, headaches, and dizziness. In RCTs examining chlormezanone and carisoprodol, pain was not improved in the intervention groups compared with placebo. A cohort study involving eperisone52 showed improvements in pain scales compared with celecoxib at 2, 4, and 6 weeks of treatment. Chlormezanone was associated with nausea (prevalence 48%); no adverse effects were noted for carisoprodol or eperisone.
Interventions for Headaches
Among 10 studies of interventions for headaches, including trigeminal neuralgia, 6 were RCTs27,37,45,46,50,67 and 4 were cohort studies,40,47,56,57 including a total of 558 patients in the intervention arms (eTable 3 in the Supplement). Four studies focused on tizanidine,37,40,46,56 3 on baclofen,47,50,57 and 1 each on cyclobenzaprine45 and orphenadrine.27 Studies involving tizanidine demonstrated improvement from baseline in pain severity; 1 six-week RCT37 demonstrated improvement vs placebo, and another, also a 6-week RCT,46 did not. Drowsiness, dry mouth, vivid dreams, and hallucinations were reported in tizanidine groups; in 1 study,40 25% of participants in the tizanidine group dropped out due to adverse effects. Studies involving baclofen demonstrated improvement from baseline; 1 study50 comparing baclofen and carbamazepine vs carbamazepine alone found improved reduction of pain in the combination group. Adverse effect incidence ranged from none to 35% and included sedation, vomiting, diarrhea, nausea, weakness, and constipation. Orphenadrine compared with diazepam27 and cyclobenzaprine compared with placebo45 did not confer improved reductions in symptoms.
Interventions for Painful Cramps or Spasticity
Among 10 studies of interventions for painful cramps or spasticity, 8 were RCTs24,25,26,34,36,58,61 and 2 were cohort studies38,49; they included 330 patients in total in the intervention arms (eTable 4 in Supplement 1). Six addressed nocturnal leg cramps,24,25,34,36,38,49 with 4 of these24,25,36,38 among patients with cirrhosis of the liver. Overall, 3 focused on baclofen,26,36,38 2 focused on orphenadrine,25,49 2 on carisoprodol,33,58 2 on quinine,34,61 and 1 on methocarbamol.24 Baclofen was associated with significant improvements in cramp frequency, duration, and severity compared with placebo, but not when compared with transcutaneous electrical nerve stimulation. Orphenadrine, carisoprodol, and methocarbamol were associated with improved cramp frequency beyond placebo. Adverse effects were documented for the baclofen, orphenadrine, and carisoprodol groups.
Interventions for Other Syndromes
The 8 studies of intervention for other syndromes addressed osteoarthritis, cervical pain, orchialgia, pain associated with cancer, neuropathic pain, gastric reflux–related pain, and osteroarthritis of multiple joints (eTable 5 in Supplement 1). Chlormezanone was associated with reduced number of breaks in sleep among those with neck osteoarthritis, but not osteoarthritis of the hip, knee, lumbar spine, or shoulder.29 Eperisone was also associated with improved neck pain over placebo at 6 weeks.30
Discussion
This systematic review identified 44 studies that investigated the long-term use of SMRs, including 9 specific medications, for a range of chronic pain conditions. Evidence for effectiveness was strongest for SMRs used for muscle spasms, painful cramps, and neck pain; in studies of SMRs for fibromyalgia, low back pain, headaches, and other syndromes, some showed small benefits and some did not, and on balance studies did not suggest a benefit. The most common adverse effects were sedation (and other central nervous system–related effects, including dizziness) and dry mouth. No studies measured the misuse of SMRs. Most studies lasted only a month or slightly longer, and this short duration may bias toward higher efficacy (many pharmacologic treatments for pain show declining efficacy over time) and toward lower adverse effects (which may develop over time, including misuse of SMRs).
This summary of the evidence raises concerns given the growth in SMR prescriptions over the last decade,10 including for more than 1 in 6 patients seeking care for chronic back pain in a national study of Medicare beneficiaries.68 Furthermore, previous studies suggest that as many as 30% of individuals using opioids are also prescribed SMRs,69,70 which increase risks of opioid-related overdose, particularly in those taking SMRs for longer durations.71 Therefore, despite increasing prevalence and increasing risks of their use, our systematic review suggests only limited evidence of efficacy for long-term use of SMRs for a small subset of pain syndromes.
This review broadens the work of prior reviews that have focused on individual medications and specific pain syndromes. Two recent systematic reviews23,72 examining the effectiveness and safety of multiple medication classes in the management of acute and chronic nonspecific lower back pain found no evidence of difference between SMRs and placebo in the management of chronic nonspecific low-back pain. Another review73 focused on the efficacy and safety of cyclobenzaprine for myofascial pain and found insufficient evidence to support the use of cyclobenzaprine. While nonspecific back pain tends to be the most common reason for SMR prescriptions in the United States, approximately one-third of office visits during which SMRs were prescribed addressed non–back pain syndromes, suggesting that in practice SMRs are used for a variety of indications for which evidence is limited.10
Most studies included in this review examined the efficacy of SMRs in comparison with placebo or, in the case of cohort studies, in comparison with a historical control. However, the painful syndromes studied in this review have effective therapies available, against whose efficacy SMRs should be measured. For example, in the case of chronic nonspecific low-back pain, moderate-quality evidence supports the effectiveness of exercise, multidisciplinary rehabilitation, acupuncture, and mindfulness-based stress reduction.7 In the case of fibromyalgia, graded exercise is the mainstay of therapy, and among pharmacotherapy, tricyclic antidepressants and serotonin-norepinephrine reuptake inhibitors have meta-analytic evidence of efficacy.74,75,76 For patients already prescribed long-term SMRs, interventions are needed to assist clinicians to engage in shared decision-making with patients about deprescribing SMRs. This may be particularly true for older patients, for whom risks of adverse events may be greater. Academic detailing and tapering guidelines, which have shown some success in deprescribing of opioids and other medications, may inform these interventions.77,78
Limitations
This systematic review was limited to only English-, Spanish-, and Italian-language publications, so studies from countries where these languages are not spoken, including low- and middle-income countries, may not have been included. The varying nature of the clinical sites, pain syndrome definitions, qualifying medications, and durations of therapy precluded meta-analyses, so we used a narrative synthesis based on a realist framework to identify the evidence for different clinical contexts.20 Finally, because we included only quantitative studies to facilitate comparison across studies, we did not include qualitative studies that may offer valuable insights into complex care processes and patient experiences,79 which are particularly important in pain management.80
Conclusions
This systematic review identified 44 studies examining the effectiveness or efficacy of long-term use of SMRs to treat chronic painful conditions, a clinical practice that has expanded considerably in recent decades. Long-term use of SMRs for chronic pain may be beneficial for patients with painful spasms or cramps and neck pain; evidence was equivocal for their long-term use for low back pain, fibromyalgia, and headaches. Clinicians should be vigilant for adverse effects and consider deprescribing if pain-related goals are not met.
eAppendix. Embase Search Strategy Syntax
eTable 1. Characteristics of Interventions for Low Back Pain
eTable 2. Characteristics of Interventions for Fibromyalgia and Similar Disorders
eTable 3. Characteristics of Interventions for Headaches or Trigeminal Neuralgia
eTable 4. Characteristics of Interventions for Painful Muscle Cramps or Spasticity
eTable 5. Characteristics of Interventions for Other Pain Syndromes
eTable 6. Risk of Bias Assessment for Randomized Trials (Cochrane Risk of Bias Tool)
eTable 7. Quality Assessment for Cohort Studies (Newcastle-Ottawa Scale)
Data Sharing Statement
References
- 1.Yong RJ, Mullins PM, Bhattacharyya N. Prevalence of chronic pain among adults in the United States. Pain. 2022;163(2):e328-e332. doi: 10.1097/j.pain.0000000000002291 [DOI] [PubMed] [Google Scholar]
- 2.Rikard SM, Strahan AE, Schmit KM, Guy GP Jr. Chronic pain among adults—United States, 2019-2021. MMWR Morb Mortal Wkly Rep. 2023;72(15):379-385. doi: 10.15585/mmwr.mm7215a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Institute of Medicine . Relieving Pain in America: A Blueprint for Transforming Prevention. Care, Education, and Research; 2011. [PubMed] [Google Scholar]
- 4.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15):1624-1645. doi: 10.1001/jama.2016.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. 2022;71(3):1-95. doi: 10.15585/mmwr.rr7103a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Department of Health and Human Services’ Interagency Pain Research Coordinating Committee . National pain strategy: a comprehensive population health-level strategy for pain. Accessed August 13, 2024. https://www.iprcc.nih.gov/national-pain-strategy-overview
- 7.Qaseem A, Wilt TJ, McLean RM, et al. ; Clinical Guidelines Committee of the American College of Physicians . Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2017;166(7):514-530. doi: 10.7326/M16-2367 [DOI] [PubMed] [Google Scholar]
- 8.See S, Ginzburg R. Skeletal muscle relaxants. Pharmacotherapy. 2008;28(2):207-213. doi: 10.1592/phco.28.2.207 [DOI] [PubMed] [Google Scholar]
- 9.van Tulder MW, Touray T, Furlan AD, Solway S, Bouter LM; Cochrane Back Review Group . Muscle relaxants for nonspecific low back pain: a systematic review within the framework of the Cochrane Collaboration. Spine (Phila Pa 1976). 2003;28(17):1978-1992. doi: 10.1097/01.BRS.0000090503.38830.AD [DOI] [PubMed] [Google Scholar]
- 10.Soprano SE, Hennessy S, Bilker WB, Leonard CE. Assessment of physician prescribing of muscle relaxants in the United States, 2005-2016. JAMA Netw Open. 2020;3(6):e207664. doi: 10.1001/jamanetworkopen.2020.7664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdel Shaheed C, Maher CG, Williams KA, McLachlan AJ. Efficacy and tolerability of muscle relaxants for low back pain: systematic review and meta-analysis. Eur J Pain. 2017;21(2):228-237. doi: 10.1002/ejp.907 [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Delcher C, Reisfield GM, Wei YJ, Brown JD, Winterstein AG. Utilization patterns of skeletal muscle relaxants among commercially insured adults in the United States from 2006 to 2018. Pain Med. 2021;22(10):2153-2161. doi: 10.1093/pm/pnab088 [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269. [DOI] [PubMed] [Google Scholar]
- 14.National Institute for Health Research . PROSPERO: International Prospective Register of Systematic Reviews. Accessed June 16, 2018. https://www.crd.york.ac.uk/PROSPERO/
- 15.Harris J, Croot L, Thompson J, Springett J. How stakeholder participation can contribute to systematic reviews of complex interventions. J Epidemiol Community Health. 2016;70(2):207-214. doi: 10.1136/jech-2015-205701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cushing/Whitney Medical Library of Yale University . Yale MeSH Analyzer. Accessed July 25, 2024. https://mesh.med.yale.edu/
- 17.Veritas Health Information . Covidence systematic review software. Accessed June 15, 2018. http://www.covidence.com
- 18.Pawson R. The Science of Evaluation: A Realist Manifesto. SAGE Publications; 2013. doi: 10.4135/9781473913820 [DOI] [Google Scholar]
- 19.Wong G, Greenhalgh T, Westhorp G, Buckingham J, Pawson R. RAMESES publication standards: realist syntheses. BMC Med. 2013;11:21. doi: 10.1186/1741-7015-11-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenhalgh T, Wong G, Westhorp G, Pawson R. Protocol—realist and meta-narrative evidence synthesis: evolving standards (RAMESES). BMC Med Res Methodol. 2011;11:115. doi: 10.1186/1471-2288-11-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JP, Altman DG, Gøtzsche PC, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group . The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessment the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa Hospital Research Institute; 2008. [Google Scholar]
- 23.Cashin AG, Wand BM, O’Connell NE, et al. Pharmacological treatments for low back pain in adults: an overview of Cochrane Reviews. Cochrane Database Syst Rev. 2023;4(4):CD013815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abd-Elsalam S, Arafa M, Elkadeem M, et al. Randomized-controlled trial of methocarbamol as a novel treatment for muscle cramps in cirrhotic patients. Eur J Gastroenterol Hepatol. 2019;31(4):499-502. doi: 10.1097/MEG.0000000000001310 [DOI] [PubMed] [Google Scholar]
- 25.Abd-Elsalam S, Ebrahim S, Soliman S, et al. Orphenadrine in treatment of muscle cramps in cirrhotic patients: a randomized study. Eur J Gastroenterol Hepatol. 2020;32(8):1042-1045. doi: 10.1097/MEG.0000000000001622 [DOI] [PubMed] [Google Scholar]
- 26.Aydin G, Tomruk S, Keleş I, Demir SO, Orkun S. Transcutaneous electrical nerve stimulation versus baclofen in spasticity: clinical and electrophysiologic comparison. Am J Phys Med Rehabil. 2005;84(8):584-592. doi: 10.1097/01.phm.0000171173.86312.69 [DOI] [PubMed] [Google Scholar]
- 27.Bakris GL, Mulopulos GP, Tiwari S, Franklin C. An effective alternative for muscle contraction headaches: orphenadrine citrate. IMJ Ill Med J. 1982;161(2):106-108. [PubMed] [Google Scholar]
- 28.Bennett RM, Gatter RA, Campbell SM, Andrews RP, Clark SR, Scarola JA. A comparison of cyclobenzaprine and placebo in the management of fibrositis: a double-blind controlled study. Arthritis Rheum. 1988;31(12):1535-1542. doi: 10.1002/art.1780311210 [DOI] [PubMed] [Google Scholar]
- 29.Berry H, Liyanage SP, Durance RA, Goode JD, Swannell AJ. A double-blind study of benorylate and chlormezanone in musculoskeletal disease. Rheumatol Rehabil. 1981;20(1):46-49. doi: 10.1093/rheumatology/20.1.46 [DOI] [PubMed] [Google Scholar]
- 30.Bose K. The efficacy and safety of eperisone in patients with cervical spondylosis: results of a randomized, double-blind, placebo-controlled trial. Methods Find Exp Clin Pharmacol. 1999;21(3):209-213. doi: 10.1358/mf.1999.21.3.534831 [DOI] [PubMed] [Google Scholar]
- 31.Cantini F, Bellandi F, Niccoli L, Di Munno O. Fluoxetin combined with cyclobenzaprine in the treatment of fibromyalgia. Minerva Med. 1994;85(3):97-100. [PubMed] [Google Scholar]
- 32.Carette S, Bell MJ, Reynolds WJ, et al. Comparison of amitriptyline, cyclobenzaprine, and placebo in the treatment of fibromyalgia: a randomized, double-blind clinical trial. Arthritis Rheum. 1994;37(1):32-40. doi: 10.1002/art.1780370106 [DOI] [PubMed] [Google Scholar]
- 33.Chesrow EJ, Kaplitz SE, Breme JT, Vetra H. Use of carisoprodol (soma) for treatment of leg cramps associated with vascular, neurologic or arthritic disease. J Am Geriatr Soc. 1963;11:1014-1016. doi: 10.1111/j.1532-5415.1963.tb00340.x [DOI] [PubMed] [Google Scholar]
- 34.Connolly PS, Shirley EA, Wasson JH, Nierenberg DW. Treatment of nocturnal leg cramps: a crossover trial of quinine vs vitamin E. Arch Intern Med. 1992;152(9):1877-1880. doi: 10.1001/archinte.1992.00400210099016 [DOI] [PubMed] [Google Scholar]
- 35.Elchami Z, Asali O, Issa MB, Akiki J. The efficacy of the combined therapy of pregabalin and cyclobenzaprine in the treatment of neuropathic pain associated with chronic radiculopathy. Eur J Pain Suppl. 2011;5(1):275. doi: 10.1016/S1754-3207(11)70950-6 [DOI] [Google Scholar]
- 36.Elfert AA, Abo Ali L, Soliman S, et al. Randomized placebo-controlled study of baclofen in the treatment of muscle cramps in patients with liver cirrhosis. Eur J Gastroenterol Hepatol. 2016;28(11):1280-1284. doi: 10.1097/MEG.0000000000000714 [DOI] [PubMed] [Google Scholar]
- 37.Fogelholm R, Murros K. Tizanidine in chronic tension-type headache: a placebo controlled double-blind cross-over study. Headache. 1992;32(10):509-513. doi: 10.1111/j.1526-4610.1992.hed3210509.x [DOI] [PubMed] [Google Scholar]
- 38.Henry Z, Northup PG. Baclofen is safe and efficacious for treatment of muscle cramps in patients with cirrhosis: a pilot study. Gastroenterology. 2014;(suppl 1):S-988. doi: 10.1016/S0016-5085(14)63593-9 [DOI] [Google Scholar]
- 39.Kaur N, Singh H, Gupta AC. Randomized controlled trial of etodolac versus combination of etodolac and eperisone in patients of knee osteoarthritis. Pain Res Treat. 2013;2013:273695. doi: 10.1155/2013/273695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krusz JC, Belanger J, Mills C. Tizanidine: a novel effective agent for the treatment of chronic headaches. Headache Q. 2000;11(1):41-45. [Google Scholar]
- 41.Lederman S, Arnold LM, Vaughn B, Kelley M, Sullivan GM. Efficacy and safety of sublingual cyclobenzaprine for the treatment of fibromyalgia: results from a randomized, double-blind, placebo-controlled trial. Arthritis Care Res (Hoboken). 2023;75(11):2359-2368. doi: 10.1002/acr.25142 [DOI] [PubMed] [Google Scholar]
- 42.Malanga GA, Gwynn MW, Smith R, Miller D. Tizanidine is effective in the treatment of myofascial pain syndrome. Pain Physician. 2002;5(4):422-432. doi: 10.36076/ppj.2002/5/422 [DOI] [PubMed] [Google Scholar]
- 43.McClain D. An open label dose finding trial of tizanidine [Zanaflex] for treatment of fibromyalgia. J Musculoskeletal Pain. 2002;10(4):7-18. doi: 10.1300/J094v10n04_02 [DOI] [Google Scholar]
- 44.Mohseni-Rad H, Razzaghdoust A, Mishan MA, Gholamrezaie HR, Hosseinkhani A. Terazosin or baclofen in young men with chronic orchialgia: a cohort study of 499 patients. Urologia. 2020;87(1):35-40. doi: 10.1177/0391560319873531 [DOI] [PubMed] [Google Scholar]
- 45.Mueller L. Efficacy and safety of cyclobenzaprine hydrochloride extended-release for the treatment of chronic migraine: a randomized, double-blind, placebo-controlled trial. AHS Scientific Meeting, Los Angeles, California; 2014. [Google Scholar]
- 46.Murros K, Kataja M, Hedman C, et al. Modified-release formulation of tizanidine in chronic tension-type headache. Headache. 2000;40(8):633-637. doi: 10.1046/j.1526-4610.2000.040008633.x [DOI] [PubMed] [Google Scholar]
- 47.Parmar BS, Shah KH, Gandhi IC. Baclofen in trigeminal neuralgia—a clinical trial. Indian J Dent Res. 1989;1(4):109-113. [PubMed] [Google Scholar]
- 48.Pattrick M, Swannell A, Doherty M. Chlormezanone in primary fibromyalgia syndrome: a double blind placebo controlled study. Br J Rheumatol. 1993;32(1):55-58. doi: 10.1093/rheumatology/32.1.55 [DOI] [PubMed] [Google Scholar]
- 49.Popkin RJ. Orphenadrine citrate (Norflex) for the treatment of “restless legs” and related syndromes. J Am Geriatr Soc. 1971;19(1):76-79. doi: 10.1111/j.1532-5415.1971.tb01557.x [DOI] [PubMed] [Google Scholar]
- 50.Puri N, Rathore A, Dharmdeep G, et al. A clinical study on comparative evaluation of the effectiveness of carbamazepine and combination of carbamazepine with baclofen or capsaicin in the management of trigeminal neuralgia. Niger J Surg. 2018;24(2):95-99. doi: 10.4103/njs.NJS_8_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quimby LG, Gratwick GM, Whitney CD, Block SR. A randomized trial of cyclobenzaprine for the treatment of fibromyalgia. J Rheumatol Suppl. 1989;19:140-143. [PubMed] [Google Scholar]
- 52.Reale C, Spinoglio A, Di Marco P, et al. Eperisone hydrochloride versus celecoxib in fibromyalgia management. Pain Pract. 2012;(suppl 1):60. [Google Scholar]
- 53.Rossi M, Ianigro G, Liberatoscioli G, et al. Eperisone versus tizanidine for treatment of chronic low back pain. Minerva Med. 2012;103(3):143-149. [PubMed] [Google Scholar]
- 54.Sakai Y, Matsuyama Y, Nakamura H, et al. The effect of muscle relaxant on the paraspinal muscle blood flow: a randomized controlled trial in patients with chronic low back pain. Spine (Phila Pa 1976). 2008;33(6):581-587. doi: 10.1097/BRS.0b013e318166e051 [DOI] [PubMed] [Google Scholar]
- 55.Semenchuk MR, Sherman S. Effectiveness of tizanidine in neuropathic pain: an open-label study. J Pain. 2000;1(4):285-292. doi: 10.1054/jpai.2000.9435 [DOI] [PubMed] [Google Scholar]
- 56.Shimomura T, Awaki E, Kowa H, Takahashi K. Treatment of tension-type headache with tizanidine hydrochloride: its efficacy and relationship to the plasma MHPG concentration. Headache. 1991;31(9):601-604. doi: 10.1111/j.1526-4610.1991.hed3109601.x [DOI] [PubMed] [Google Scholar]
- 57.Steardo L, Leo A, Marano E. Efficacy of baclofen in trigeminal neuralgia and some other painful conditions: a clinical trial. Eur Neurol. 1984;23(1):51-55. doi: 10.1159/000115677 [DOI] [PubMed] [Google Scholar]
- 58.Stern FH. Value of carisoprodol (Soma) in relieving leg cramps. J Am Geriatr Soc. 1963;11:1008-1013. doi: 10.1111/j.1532-5415.1963.tb00339.x [DOI] [PubMed] [Google Scholar]
- 59.Vaerøy H, Abrahamsen A, Førre O, Kåss E. Treatment of fibromyalgia (fibrositis syndrome): a parallel double blind trial with carisoprodol, paracetamol and caffeine (Somadril comp) versus placebo. Clin Rheumatol. 1989;8(2):245-250. doi: 10.1007/BF02030081 [DOI] [PubMed] [Google Scholar]
- 60.Williamson L, Illingworth H, Smith D, Mowat A. Oral quinine in ankylosing spondylitis: a randomized placebo controlled double blind crossover trial. J Rheumatol. 2000;27(8):2054-2055. [PubMed] [Google Scholar]
- 61.Woodfield R, Goodyear-Smith F, Arroll B. N-of-1 trials of quinine efficacy in skeletal muscle cramps of the leg. Br J Gen Pract. 2005;55(512):181-185. [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao Y, Michalek JE, Russell IJ. Effects of tizanidine on cerebrospinal fluid substance P in patients with fibromyalgia. Round Table Series: Royal Society of Medicine. 2002;75:23-28. [Google Scholar]
- 63.Yomiya K, Matsuo N, Tomiyasu S, et al. Baclofen as an adjuvant analgesic for cancer pain. Am J Hosp Palliat Care. 2009;26(2):112-118. doi: 10.1177/1049909108327968 [DOI] [PubMed] [Google Scholar]
- 64.Zaringhalam J, Manaheji H, Rastqar A, Zaringhalam M. Reduction of chronic non-specific low back pain: a randomised controlled clinical trial on acupuncture and baclofen. Chin Med. 2010;5:15. doi: 10.1186/1749-8546-5-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pauwels A, Raymenants K, Geeraerts A, et al. Clinical trial: a controlled trial of baclofen add-on therapy in PPI-refractory gastro-oesophageal reflux symptoms. Aliment Pharmacol Ther. 2022;56(2):231-239. doi: 10.1111/apt.17068 [DOI] [PubMed] [Google Scholar]
- 66.Ueberall MA, Mueller-Schwefe G. COMET—effectiveness and tolerability of methocarbamol versus oral opioid-analgesics as add-on measure in patients with non-specific low back pain refractory to recommended 1st line treatments: a retrospective analysis of depersonalized propensity score matched open-label real-world 4-week data from the German Pain e-Registry. Curr Med Res Opin. 2022;38(2):237-253. doi: 10.1080/03007995.2021.2003105 [DOI] [PubMed] [Google Scholar]
- 67.Kookna JC, Acharya J. Assessment of effectiveness of carbamazepine and combination of carbamazepine with baclofen or capsaicin in the management of trigeminal neuralgia. Neuroquantology. 2022;20(15):6207-6210. [Google Scholar]
- 68.Ly DP. Evaluation and treatment patterns of new low back pain episodes for elderly adults in the United States, 2011-2014. Med Care. 2020;58(2):108-113. doi: 10.1097/MLR.0000000000001244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garg RK, Fulton-Kehoe D, Franklin GM. Patterns of opioid use and risk of opioid overdose death among Medicaid patients. Med Care. 2017;55(7):661-668. doi: 10.1097/MLR.0000000000000738 [DOI] [PubMed] [Google Scholar]
- 70.Larochelle MR, Zhang F, Ross-Degnan D, Wharam JF. Trends in opioid prescribing and co-prescribing of sedative hypnotics for acute and chronic musculoskeletal pain: 2001-2010. Pharmacoepidemiol Drug Saf. 2015;24(8):885-892. doi: 10.1002/pds.3776 [DOI] [PubMed] [Google Scholar]
- 71.Li Y, Delcher C, Wei YJ, et al. Risk of opioid overdose associated with concomitant use of opioids and skeletal muscle relaxants: a population-based cohort study. Clin Pharmacol Ther. 2020;108(1):81-89. doi: 10.1002/cpt.1807 [DOI] [PubMed] [Google Scholar]
- 72.Cashin AG, Folly T, Bagg MK, et al. Efficacy, acceptability, and safety of muscle relaxants for adults with non-specific low back pain: systematic review and meta-analysis. BMJ. 2021;374(1446):n1446. doi: 10.1136/bmj.n1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Leite FM, Atallah AN, El Dib R, et al. Cyclobenzaprine for the treatment of myofascial pain in adults. Cochrane Database Syst Rev. 2009;2009(3):CD006830. doi: 10.1002/14651858.CD006830.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;2014(1):CD007115. doi: 10.1002/14651858.CD007115.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.O’Malley PG, Balden E, Tomkins G, Santoro J, Kroenke K, Jackson JL. Treatment of fibromyalgia with antidepressants: a meta-analysis. J Gen Intern Med. 2000;15(9):659-666. doi: 10.1046/j.1525-1497.2000.06279.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Macfarlane GJ, Kronisch C, Dean LE, et al. EULAR revised recommendations for the management of fibromyalgia. Ann Rheum Dis. 2017;76(2):318-328. doi: 10.1136/annrheumdis-2016-209724 [DOI] [PubMed] [Google Scholar]
- 77.Soumerai SB, Avorn J. Principles of educational outreach (‘academic detailing’) to improve clinical decision making. JAMA. 1990;263(4):549-556. doi: 10.1001/jama.1990.03440040088034 [DOI] [PubMed] [Google Scholar]
- 78.Trotter Davis M, Bateman B, Avorn J. Educational outreach to opioid prescribers: the case for academic detailing. Pain Physician. 2017;20(2S):S147-S151. doi: 10.36076/ppj.2017.s151 [DOI] [PubMed] [Google Scholar]
- 79.Pope C, Mays N. Reaching the parts other methods cannot reach: an introduction to qualitative methods in health and health services research. BMJ. 1995;311(6996):42-45. doi: 10.1136/bmj.311.6996.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lennox Thompson B, Gage J, Kirk R. Living well with chronic pain: a classical grounded theory. Disabil Rehabil. 2020;42(8):1141-1152. doi: 10.1080/09638288.2018.1517195 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Embase Search Strategy Syntax
eTable 1. Characteristics of Interventions for Low Back Pain
eTable 2. Characteristics of Interventions for Fibromyalgia and Similar Disorders
eTable 3. Characteristics of Interventions for Headaches or Trigeminal Neuralgia
eTable 4. Characteristics of Interventions for Painful Muscle Cramps or Spasticity
eTable 5. Characteristics of Interventions for Other Pain Syndromes
eTable 6. Risk of Bias Assessment for Randomized Trials (Cochrane Risk of Bias Tool)
eTable 7. Quality Assessment for Cohort Studies (Newcastle-Ottawa Scale)
Data Sharing Statement