Abstract
Chimeric antigen receptor (CAR) T cell therapy has come of age, offering a potentially curative option for patients who are refractory to standard anti-cancer treatments. The success of CAR T cell therapy in the setting of acute lymphoblastic leukemia (ALL) and specific types of B cell lymphoma led to rapid regulatory approvals of CD19-directed CAR T cells, first in the United States and subsequently across the globe. Despite these major milestones in the field of immuno-oncology, growing experience with CAR T cells has also highlighted the major limitations of this strategy, namely challenges associated with manufacturing a bespoke patient–specific product, intrinsic immune cell defects leading to poor CAR T cell function as well as persistence, and/or tumor cell resistance resulting from loss or modulation of the targeted antigen. In addition, both on- and off-tumor immunotoxicities and the financial burden inherent in conventional cellular biomanufacturing often hamper the success of CAR T cell-based treatment approaches. Herein, we provide an overview of the opportunities and challenges related to first form of gene transfer therapy to gain commercial approval in the United States. Ongoing advances in the areas of genetic engineering, precision genome editing, toxicity mitigation methods and cell manufacturing will improve the efficacy and safety of CAR T cells for hematologic malignancies and expand the use of this novel class of therapeutics to reach solid tumors.
Keywords: Chimeric Antigen Receptor, CAR T cell, Cancer, Immunotherapy
Introduction
The field of cancer immunotherapy has undergone transformative changes over the last several years and is currently progressing at an unprecedented pace to further advance recent therapeutic successes. Much enthusiasm related to harnessing the power of the immune system to reduce unmet medical needs in hematologic malignancies and solid tumors has been attributed to the remarkable clinical results of checkpoint inhibitors and chimeric antigen receptor (CAR) T cells. The United States Food and Drug Administration (FDA) approval of two CAR T cell therapies in 2017 for the treatment of advanced B cell cancers in pediatric and adult patients represents a milestone in cellular immunotherapy. These treatments were subsequently approved by the European Union, the United Kingdom and Canada in 2018, marking a global paradigm shift from conventional management strategies to a potentially curative approaches based on living and self-replicating CAR T cell products.
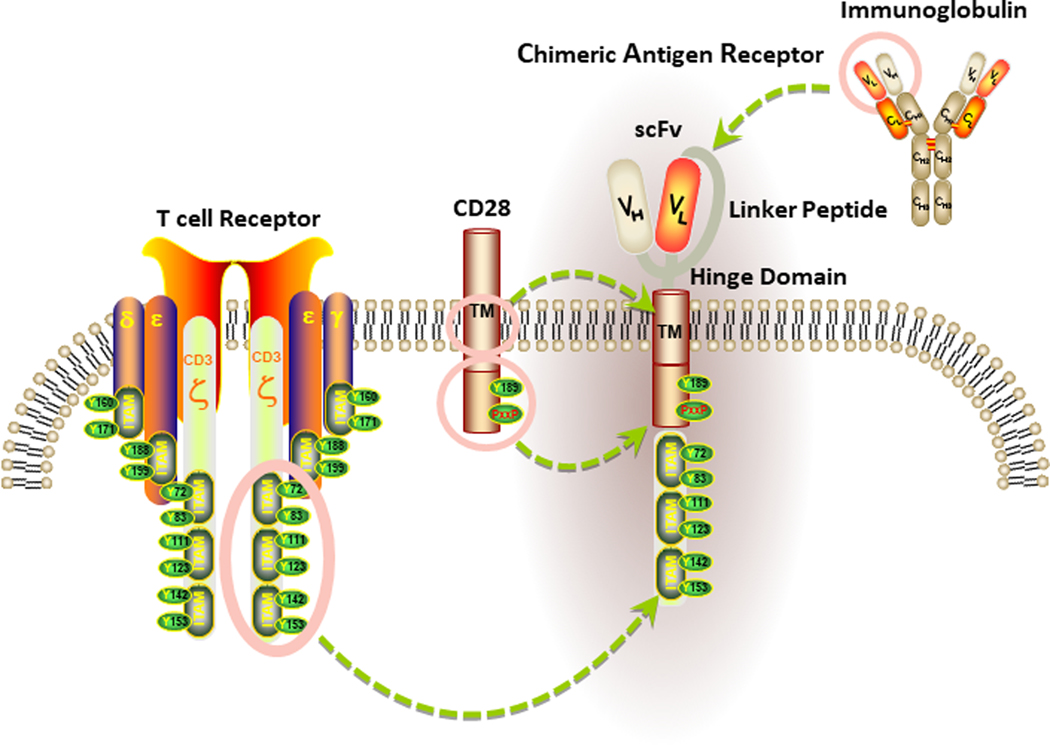
Genetic engineering can be used to create CARs, which are synthetic hybrid receptors that combine an extracellular binding domain (typically derived from a single-chain variable fragment (scFv) fusion protein of heavy (VH) and light chain (VL) immunoglobulin variable regions), with intracellular signaling modules to activate T cell effector functions. The signaling components of a CAR are often derived from endogenous T cell receptors as well as co-stimulatory molecules that are required for optimal T lymphocyte activation. Because recognition by CARs is based on scFv binding to native intact surface antigens, recognition of cancer cells does not require major histocompatability complex restriction nor effective processing and presentation of epitopes[1–5]. However, CAR recognition requires surface expression of the targeted antigen (Figure 1).
Figure 1. Basic Structure of a chimeric antigen receptor (CAR).
The antigen recognition domain of a CAR is typically a single-chain variable fragment (scFv) comprised of the variable light (VL) and heavy (VH) chains of an immunoglobulin, connected by a short linker peptide. This binding moiety is fused to a hinge region that is anchored to the plasma membrane by a transmembrane (TM) domain. In the diagram above, the TM domain of the CAR is derived from the CD28 costimulatory receptor. Signaling components of a CAR are localized within the receptor endodomain. Because endogenous T cell activation requires the phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs), the cytoplasmic portion of CD3ζ is commonly used as the main endodomain component of a CAR to drive signal 1. Signal 2, which is provided in the form of costimulation and is required for optimal T cell activation, is triggered by activation of an intracellular costimulatory receptor endodomain fused to CD3ζ (e.g., CD28).
Biomanufacturing of a CAR T cell product typically involves cell collection from a patient by leukapheresis, followed by elutriation to remove myeloid cells, bulk T cell enrichment, activation and CAR transgene delivery. The latter production step is usually achieved by integration of viral vectors or transposons encoding the synthetic receptors that direct tumor cell recognition. The gene-engineered T cells are then expanded ex vivo to clinical scale and infused back into the patient’s body to attack and destroy chemotherapy-resistant cancer (Figure 2)[6, 7]. One of the current critical constraints in CAR T cell therapy is its highly patient-specific nature, which often results in variable efficacy across autologous T cell infusion products. Several different strategies are currently used for the generation and administration of CAR T cells, but each approach possesses drawbacks ranging from limited availability of reagents, cost of goods, lack of efficiency in production, issues involving scalability and inconsistent product potency. Development of new strategies to generate reproducible, broadly effective and durably persistent CAR T cells that are more potent at lower doses and have enhanced availability due to automated manufacturing and lower costs will undoubtedly result in the next-generation of “best-in-class” adoptive cellular therapies for cancer.
Figure 2. Autologous CAR T cell production schema.
The generation of autologous CAR T cells begins with leukapheresis of a patient, followed by T cell enrichment and activation. Activated T lymphocytes are transduced (e.g., using a lentiviral vector) to facilitate introduction and sometimes permanent integration of the CAR transgene. Gene-modified T cells are then expanded in either static or dynamic culture, cryopreserved and infused back into the patient.
Success and Limitations of CAR T cell Therapy for Hematologic Malignancies
In aggregate, hematologic cancers have a high prevalence, and with few exceptions, most are not cured with currently available therapies. Striking results from several centers have demonstrated that the adoptive transfer of genetically engineered T cells can mediate durable complete remissions in individuals with a variety of refractory hematologic malignancies. Notably, CAR T cells have exhibited powerful anti-tumor effects in leukemia and lymphoma, leading to the first FDA approval of this treatment strategy over two years ago.
Several groups, including our own, have reported complete response (CR) rates of >80% in relapsed/refractory B-ALL patients treated with anti-CD19 CAR T cells[8–10]. Additional clinical studies confirmed the anti-tumor efficacy of CD19-directed CAR T cells for the treatment of refractory B cell lymphoma, with overall response rates ranging from 50–80%[11–13]. Other trials have demonstrated that potentially targeting rare CD19-positive multiple myeloma stem cells may also be a viable treatment option, with disease eradication evident 12 months post-CAR T cell infusion[14, 15]. Furthermore, results from multiple centers indicate that treatment of advanced myeloma patients with CAR T cells directed against the B-cell maturation antigen (BCMA) hold promise as well[16, 17]. In several of these trials, CRs were typically associated with robust proliferation of transferred lymphocytes, with a clear advantage of long-term persistence of the CAR T cells[18, 9, 19–23]. Longitudinal studies of CAR T cell engraftment have demonstrated that these cells remain functional, and have the ability to persist for several years to over a decade in patients, suggesting that they are capable of establishing immunological memory[24, 25]. Thus, a single treatment with CAR T cells can induce clearance of tumor burdens that far exceed the number of infused T cells, and these lymphocytes can persist to mediate long-term durable remission.
Despite a >80% CR rate with CD19-directed CAR T cell therapy in pediatric ALL, relapse-free survival 12 months post-infusion is 59%[8]. The major route to CAR T cell failure in these cases is through loss of the CD19 antigen or epitope, and this is observed regardless of otherwise adequate persistence of transferred T cells. Antigen loss is likely due to genetic deletion or selection of a CD19 variant encoding an isoform that lacks the transmembrane domain or a portion of the protein targeted by the anti-CD19 CAR scFv[26–28]. Fry et al. also demonstrated antigen escape as a mechanism of resistance to anti-CD22 CAR T cell therapy. In this study, relapse was mediated by proliferation of tumor cells with diminished antigen site density that permitted CD22-positive cell escape, rather than antigen-negative disease[29]. Results from a phase II trial in diffuse large B cell lymphoma (DLBCL) with a CD19-targeting CAR showed CR rates of 51%, and relapse rates at 14% (median follow-up of 15.4 months), of which 27% were due to antigen loss[12]. Relapse by antigen escape has also been observed in CAR T cell therapy of multiple myeloma[30]. These findings highlight the prevalence of antigen escape as a major relapse mechanism in CAR T cell treatment of B cell malignancies and suggest the need for improving tumor cell targeting (e.g., combination strategies directed against multiple antigens)[31].
Autologous T cells engineered to express a CD19-targeted CAR may also be dramatically effective for some patients with relapsed chronic lymphocytic leukemia (CLL), with an overall response rate of 57%[19], but sustained CRs occur in only ~27% of patients[19, 32]. In CLL, response to anti-CD19 CAR T cells correlates closely with in vivo T cell proliferative capacity. Responding patients display a profound CAR T cell expansion early after infusion, while many non-responding (NR) patients lack detectable transferred cells at any time point post-infusion, indicating a failure of proliferation and/or engraftment of the CAR T cells[20]. In both CLL and ALL, lack of CAR T cell engraftment and proliferation may be attributed, at least in part, to activation of naturally occurring negative immune checkpoint molecules (e.g. PD-1 and CTLA-4)[33, 34, 20, 35], a reduction in stem cell memory/central memory functions[36, 23, 37, 20, 24], metabolic dysfunction[38] and senescent proliferative arrest (reviewed in [34]). We and others have demonstrated that some of these intrinsic defects can be detected at the time of T cell collection and following CAR T cell manufacturing[20, 35, 38]. The development of a comprehensive understanding of the baseline determinants of response and resistance to CAR T cell therapy will offer prospects for improving cell manufacturing and potentially managing patients treated with this approach.
Together with remarkable anti-tumor efficacy, adoptive transfer of CAR T cells has resulted in significant and unique toxicities. Indeed, the success of CAR T cell therapy for hematologic malignancies has been compromised by serious side effects arising from cytokine release syndrome (CRS) and neurotoxicity, both which may result in death of patients. Upon encountering a tumor antigen, CAR T cells are engineered to kill the targeted tumor cell and expand (both mediated in part by release of cytokines), leading to a positive feedback-mediated proliferation of the transferred cells. Because this elevation in CAR T cell numbers results in further increases in tumor cell engagement, cytokine levels surge and eventually become toxic. In the context of CD19-directed CAR T cell therapy, CRS is observed in the majority of patients with B-ALL and in subsets of individuals with B-CLL and B-NHL[39, 40]. This syndrome is characterized by increased levels of cytokines/chemokines (IL-6, TNFα, IL-2, IL-1, IL-2Rα, IFNγ, GM-CSF, MIP-1α, etc.) and additional inflammatory markers (ferritin, C-reactive protein), together with fever, hypotension, myalgia and other systemic symptoms. Current management of CRS involves blockade of pro-inflammatory cytokine signaling, treatment with corticosteroids or activation of engineered suicide genes that trigger CAR T cell death. Because all of these strategies require CAR T cell suppression, clinicians must often decide between mitigating toxicity and potentially inhibiting the expansion and anti-tumor effector activity of the transferred T cells. A newly described attractive approach to reduce CRS without impairing therapeutic responses elicited by CAR T cells involves inhibition of catecholamine synthesis[41]. In practical terms, this self-amplifying feed-forward catecholamine loop can be pharmacologically interrupted either prior to or concurrent with adoptive T cell transfer to modulate the inflammatory response.
CAR T cells may also cause certain neurological effects, collectively referred to as neurotoxicities. These toxicities caused by CAR T cells are diverse, and not localized to any specific region of the central nervous system (CNS). In this regard, patients may experience delirium, hallucinations, cognitive defects, tremors, ataxia, seizures, and focal motor or sensory deficits[42–44]. Cerebral edema has led to deaths in a small number of patients[45–47]. Neurotoxicities may occur simultaneously with signs of CRS such as hypotension, but they may also occur independently, indicating that the pathobiology of CRS and neurotoxicity is distinct[44]. Due to the variability in the onset and severity of neurotoxicity, close monitoring is required throughout the CAR T cell treatment course.
One of the most striking toxicities associated with genetically-directed T cells is organ damage that occurs when transferred T cells target healthy tissues. In the case of “on-target, off-tumor” related toxicity, CAR T cells may react against normal tissues that have shared expression of the targeted antigen. With CD19-directed CAR T cell strategies, transferred T cells are designed to kill malignant B cells, but in the process, they can also destroy healthy B cells. The resulting B cell aplasia could lead to hypogammaglobulinemia because activated B cells differentiate into antibody-secreting plasma cells. As another example of off-tumor toxicity, in the earliest trials of CAR T cell therapy, cholestasis was observed in renal cell carcinoma patients infused with engineered lymphocytes targeted against carbonic anhydrase IX, which is also expressed on normal bile duct epithelial cells[48, 49]. In another study, a patient with metastatic colorectal cancer treated with anti-ERBB2 (Her-2/neu) CAR T cells experienced pulmonary toxicity and subsequently died. This was attributed, at least in part, to expression of ERBB2 on normal lung tissue[50]. Although not documented in any clinical trial, “off-target, off-tumor” aberrant reactivity may also arise when CAR T cells cross-react against an antigen expressed on normal tissue that is similar to the targeted antigen present on the tumor. This type of toxicity has been reported in trials of T cells engineered to express transgenic T cell receptors. In this regard, two different studies have revealed severe toxicity, including lethal events, after treatment with T cells redirected to the testis antigen, MAGE-A3[51, 52]. These cases emphasize the need for careful target antigen selection in the context of adoptive T cell therapy.
The Promise of CAR T cell Immunotherapy for Solid Tumors
The success exhibited by CAR T cells in hematologic malignancies provides rationale for translation of this technology to much more common and challenging solid tumor indications. These diseases are responsible for greater than three quarters of cancer-related deaths in humans, and therefore represent a large unmet medical need. Early clinical studies in solid tumors demonstrated poor CAR T cell anti-tumor efficacy and varying levels of toxicity[53, 48, 50, 54]. However, more current reports of patients with glioblastoma, pancreatic cancer, mesotheliomas and sarcomas treated with CAR T cells have supported the feasibility of this approach through demonstration of transient anti-tumor activity and the absence of serious adverse events[55–58]. Notably, in a recent study, CAR T cells directed against IL-13Rα2 induced a complete regression of metastatic glioblastoma in a single patient[59]. Some of the following valuable lessons learned from the above trials will undoubtedly drive the design and improvement of future CAR T cell therapies for non-hematopoietic malignancies: i) despite the trafficking of CAR T cells to the tumor site, initial proliferation and elicitation of some degree of effector activity, clinically meaningful responses are rarely observed; ii) anti-tumor potency is frequently limited by lack of substantial expansion and/or survival of CAR T cells in the tumor microenvironment (TME); iii) significant decreases in targeted antigen expression have been documented following CAR T cell infusion[57], suggesting transient on-target, on-tumor activity and highlighting antigen loss/heterogeneity as a critical barrier to the success of this approach, and iv) on-target, off-tumor toxicity reported in some trials, irrespective of very low antigen levels[60].
The precise causes of the limited success of CAR T cell therapy in solid tumors remain elusive, but are likely multifactorial. In a number of different cancers, identifying specific tumor antigens that are highly and uniformly expressed has been challenging. Unlike the situation in hematologic malignancies, CAR T cells must traffic to solid tumor sites and surmount stromal elements to infiltrate into the tumor bed and elicit antigen-specific cytotoxicity, regardless of antigen heterogeneity or loss. Even if trafficking and infiltration are achieved, T cells can become hypofunctional due to a hostile TME. Accordingly, rapidly dividing malignant cells exhibit aerobic glycolysis, which creates a hypoxic TME devoid of glucose and other nutrients that can render infiltrating CAR T cells susceptible to oxidative stress[61]. Due to the TME-associated pro-inflammatory milieu, tumor cells also upregulate inhibitory ligands for T cells such as Programmed death-ligand 1 (PD-L1) and Galectin 9. Accessory cells in the TME, namely cancer associated fibroblasts (CAFs), tumor-associated macrophages (TAMs)/neutrophils (TANs), regulatory T cells (TREGS) and myeloid derived suppressor cells (MDSCs) may further potentiate CAR T cell dysfunction and reduce the survival of these engineered lymphocytes[57]. Furthermore, secretion of transforming growth factor beta (TGFβ) and vascular endothelial growth factor (VEGF) by TME cells leads to the formation of abnormal tumor vasculature and promotes an opposing anti-inflammatory polarization of TAMs. M2-polarized TAMs inhibit T cell-mediated immune responses to tumor antigens by secreting other soluble immunosuppressive factors, such as IL-10, arginase-1 (Arg-1) and nitric oxide (NO)[62–66]. Indoleamine-2,3-dioxygenase (IDO) and prostaglandin E2 (PGE2) are also abundantly produced in the TME, and these molecules may hamper T cell activation and effector activity [67–71]. Additional barriers operative in the TME that lead to suppression of CAR T cell-mediated anti-tumor immunity are reviewed by Martinez and Moon[72]. Thus, numerous obstacles unique to solid tumors compared to hematologic malignancies likely contribute to the lack of CAR T cell efficacy in non-hematopoietic cancers to date.
Important advancements in CAR T cell engineering to induce multiple costimulatory factors[73, 74], drive generation of cytokines[75, 76, 69] as well as secretion of soluble immune checkpoint inhibitors[77–79] or bispecific T cell engagers[80] have shown promise in pre-clinical models, and some of these approaches are currently being tested in early-phase human trials. To overcome the issue of identifying target antigens that are selective for solid tumors, CAR T cells directed against aberrant protein products of RNA splice variants or cancer-specific glycans have been created[81, 82]. In a more recent study, single-domain antibody (nanobody) CAR T cells were successfully targeted to the TME via PD-L1 or to the tumor stroma and vasculature through the EIIIB+ fibronectin splice variant, which are conserved across multiple solid tumor types[83]. Other sensing and switching strategies have also been incorporated into CAR T cell design to generate engineered lymphocytes conditionally specific for the TME[84–86]. Finally, repeated infusions of freshly expanded CAR T cells systemically[79], regionally[87, 59] or intratumorally[88] may enhance the persistence and function of the cells in toxic TMEs. Developments in CAR T cell therapy over the coming years in the areas of safety, reliability and efficacy against solid tumors will ultimately determine how revolutionary this new platform will be in the broader battle against cancer.
Increasing Access to CAR T cell Therapies and Reducing Financial Toxicity
The great potential of CAR T cell therapy has been demonstrated, particularly in the setting of hematologic malignancies. However, there are major limitations associated with accessing this technology. Currently, it is a highly specialized product, and therefore, the time required for autologous cell culture can limit the number of individuals who can be treated. Unfortunately, conventional manufacturing strategies are unable to meet the demand due to problems with scaling out, and a second major issue is the high cost of production. The current manufacturing of commercially available CAR T cells involves a patient-specific platform requiring numerous manual processing steps. One of the most expensive aspects of the cell culture process is the cost of human labor. Tisangenlecleucel, the first FDA approved CAR T cell product from Novartis, is marketed for treatment of pediatric B-ALL and costs $475,000[89]. Axicabtagene, the anti-CD19 CAR T cell product from Kite/Gilead Pharma approved for treatment of DLBCL, is priced at $373,000[90]. The expense of this drug is for product manufacturing alone, and does not include additional costs incurred by treatment, such as admission to intensive care units following infusion. Furthermore, the T cells collected from patients and used as starting material for cellular manufacturing are likely to have developed cancer-related T cell dysfunction, which may not be reversible[91] and these baseline defects often result in generation of poor quality infusion products[20, 35].
Disease progression prior to or during CAR T cell manufacturing also remains a significant barrier to the broader implementation of adoptive cellular therapies for cancer. In the JULIET study, where an anti-CD19 CAR T cell therapy was used to treat DLBCL and follicular lymphoma (FL), 13% of patients never received their autologous product due to disease progression and/or death[13]. With the same autologous product used to treat B-ALL, 7.6% of enrolled and apheresed patients died before infusion[8]. For these reasons, development of “universal” CAR T cell therapy in a safe and effective manner would rapidly expand application of this technology to many more patients than only those who can receive autologous cellular products. Healthy donor CAR T cells can be produced from a patient’s previously human leukocyte antigen (HLA)-matched hematopoietic stem cell transplant donor or from an unrelated donor. In the latter approach, genome editing (e.g., CRISPR/Cas9-mediated knock-out of the T cell receptor and HLA class I via ablation of β2 microglobulin[92]) can permit the administration of modified cells to non-HLA matched recipients. There is increasing enthusiasm for development of universal, off-the-shelf allogeneic CAR T cell products. T cells collected from healthy individuals could be used to create large quantities of allogeneic tumor-specific CAR T cells that could be administered to virtually any patient. As proof-of-concept, CAR T cells derived from healthy unrelated donors have exhibited anti-leukemic efficacy in children with relapsed B-ALL[93]. If this approach can be scaled-out, it would accelerate the pace of drug delivery and make CAR T cell therapy a viable option for lymphopenic and critically ill cancer patients who often do not possess sufficient numbers of functional T cells for treatment.
In developed economies, there is an ongoing debate about increasing prices for cancer therapies[94, 95]. These diseases contribute disproportionally to the national burden of healthcare costs. In the United States, the average cost of most orally administered cancer drugs by 2014 exceeded $135,000 per year, which is six times the price of similar therapies approved in the early 2000s[96]. Although the vast majority of newly diagnosed cancer patients are expected to respond to initial treatment regimens that incorporate continuously administered targeted drugs, these strategies are typically not curative. Furthermore, despite these advances, and even in individuals who achieve remission, nearly all patients relapse with disease that becomes progressively more refractory to successive lines of therapy. Thus, prolonged treatment with targeted agents has significant medical, social and economic costs, and patients who become resistant have a very poor prognosis[97].
Checkpoint inhibitors are a new class of cancer drugs that induce responses in a subset of patients with previously incurable malignancies. The best-documented example of this therapy is in metastatic melanoma, where about 20% of patients have long-term survival after treatment with CTLA-4 blocking antibodies[98, 99]. Reproducible clinical benefits of checkpoint inhibitors are observed in 15–30% of individuals with a range of different malignancies. However, this is an expensive treatment, costing as much as $150,000 per year in the United States when administered as a single agent. Annual costs of combinations of checkpoint inhibitors may exceed $250,000 for each patient[100]. In addition, because it is not currently possible to predict which patients will respond, the price tag of checkpoint therapies surpasses $1,000,000 per life saved[101]. Not unlike targeted drugs, another major limitation of this approach is the need for recurrent administration. In contrast, we believe that one-time infusions of CAR T cells can potentially effect long-term durable remissions in many cancers, thereby conserving considerable financial resources over time.
Due to its highly personalized nature, the custom CAR T cell manufacturing process is accompanied by high development and production costs, stringent regulatory requirements associated with gene transfer, and reimbursement challenges. As described above, there is a critical need to control the ever-increasing prices of cancer therapy. In the case of CAR T cells, improved cell manufacturing is the most obvious strategy to lower the cost of treatment and improve access to this emerging technology. For example, we recently developed a culture system that yields sufficient numbers of highly functional CAR T cells in 3-days, compared to the standard 9- to 12-day procedure currently used in industry[102]. This abridged culture process should be considerably less expensive, and together with a dose reduction achieved due to increased product potency, could greatly reduce manufacturing costs. As a separate strategy, if the aforementioned universal allogeneic CAR T cell products prove to be clinically effective, we anticipate that the cost of goods for production will drastically decrease. Thus, these proposed innovative manufacturing approaches will help to further facilitate integration of CAR T cell therapy into standard medical management of cancer.
Site Level Considerations and Prospects for Bringing CAR T cell Therapies to Global Patient Populations
More than 500 clinical trials around the world are investigating CAR T cells for the treatment of advanced cancer, most of which are in the U.S. and China [103]. Adoption of CAR T cell therapy by treatment centers is a significantly involved process requiring close collaboration between academic or commercial institutions, hospitals and regulatory agencies. This presents a unique challenge for current healthcare systems, as it represents a novel treatment paradigm in which patients are infused with a “living drug.” For safe and successful implementation of a bespoke cellular therapy, several variables must be considered including regulatory framework, hospital or treatment center infrastructure, specialized staff training, and logistical coordination for the shipping of leukapheresis and/or cellular products (i.e., applicable to centralized manufacturing models). If manufacturing is decentralized, centers must also consider product comparability across sites and practice strategies to minimize variability related to the production process [6].
Hospitals or cancer centers that aim to administer CAR T cells must guarantee the safety and traceability of the process as well as the engineered cellular product from start to finish. The requirements to become a CAR T cell center should be thorough and exhaustive due to the nature of this treatment. Some of these requisites include but are not limited to a leukapheresis unit, onsite medical laboratories, various clinical facilities such as hematology and neurology departments, transfusion services, and an intensive care unit with specialized training for treating adverse events related to CAR T cell product administration. Ideally, such centers should also have a functional clinical CAR T cell unit that can integrate multidisciplinary teams with experience in collaborating to care for patients. In addition, hospital or center managers should be able to provide necessary development resources to all staff. Centers should have the accreditation of national authorities and quality improvement programs such as the Foundation for the Accreditation of Cellular Therapy (FACT) and the Joint Accreditation Committee of the International Society for Cell & Gene Therapy (ISCT) and the European Society for Blood and Marrow Transplantation (EBMT; JACIE). The JACIE has established international standards/best practices for hematopoietic cellular therapy product collection, processing and administration [104].
Choosing the patients who will benefit most from therapy is another crucial consideration. Centers must have a multidisciplinary committee that can evaluate patients who meet the criteria for inclusion. This entails reviewing the diagnosis as well as indication for treatment, and evaluation of the risk of experimental therapy. Following patient enrollment and the scheduling of collections and infusions, adherence to Good Manufacturing Practice (GMP) standards is crucial to maintain the quality and safety of the cellular product. Transportation and reception of the leukapheresis material and CAR T cell infusion product must be carefully monitored by pharmacists to evaluate temperature conditions, shipping time periods (if applicable) and storage conditions upon receipt. These healthcare specialists must inspect the traceability of the cells from the time of collection to CAR T cells infusion and ensure the availability of drugs used for the treatment of adverse events related to therapy. Finally, the aforementioned integrated clinical groups trained in all stages of CAR T cell use/monitoring and care protocols must be available 24 hours per day and 7 days a week for patient management.
Despite the demanding requirements of running CAR T cell clinical trials, successful implementation of this approach across different institutions around the globe has been demonstrated by the multi-center ELIANA trial for treatment of relapsed or refractory B cell ALL using CD19-directed CAR T cells [105]. During this trial, use of the global supply chain to distribute a U.S. manufactured cellular product was demonstrated to be feasible. Furthermore, administration and clinical monitoring by outside centers resulted in similar efficacy and safety compared to a previous single-center study where manufacturing and treatment were completed on-site [106]. The experience gained from implementation of the ELIANA trial across 25 sites in 11 countries can be used as a roadmap for institutions wishing to implement their own cellular therapy programs. The currently active JULIET trial is another global, multi-center investigation of the efficacy and safety of anti-CD19 CAR T cells in adult patients with relapsed or refractory DLBCL [107]. A key difference in this study compared to ELIANA is the use of two different manufacturing facilities, with one in Europe and the other in the U.S. to generate a CAR T cell product for the treatment of separate patient cohorts. A careful comparison of product potency between these two facilities will provide insight into the feasibility of transitioning flexible cellular engineering processes from a single academic or commercial facility to highly controlled procedures that can be universally implemented. Given the success of CAR T cells in treating patients with B cell cancers in the U.S. and other parts of the globe, scaling out cell manufacturing capacity will permit assessment of the safety and efficacy of CAR T cell therapies in larger cohorts of patients around the world.
Conclusion and Future Vision
Clinical trials of CAR T cell therapy for a number of incurable malignancies are now global, and commercialization of many of these strategies is expected in the near future. FDA approval of CD19-directed CAR T cells for the treatment of relapsed/refractory ALL and DLBCL has heralded an emerging industry of potentially curative cell-based immunotherapies, now valued at many billions of dollars[108, 109]. Despite these successes, the efficacy of CAR T cells in the setting of both hematopoietic and non-hematopoietic cancers is often hampered by poor therapeutic levels of CAR T cell expansion, the lack of durable persistence of these cells, failure to achieve deep molecular remissions (i.e., defined as incomplete elimination of minimum residual disease), diminished anti-tumor function/survival in the immunosuppressive TME, antigen escape, cytokine-associated immunotoxicity and/or off-tumor related tissue damage. The application of several new technologies aimed at improving CAR T cell development and biomanufacturing that succeed in increasing anti-tumor potency, preventing resistance, mitigating severe adverse events and reducing financial toxicity will undoubtedly produce safer, more clinically efficacious CAR T cells, which will be more affordable and therefore more widely available. Finally, diligent site-level management of CAR T cell centers, anticipation of regulatory concerns and harmonization of manufacturing practices will serve to further streamline integration of these therapies into standard medical management of cancer.
Acknowledgements
This work was supported by the Bob Levis Funding Group (JAF). Additional funding comes from P01 CA214278 (JJM and JAF), R01 CA241762 (JJM and JAF) and U54 CA244711 (JAF) grants from the National Cancer Institute, a U01 AG066100 (JAF) grant from the National Institute on Aging, the Gabrielle’s Angel Foundation (JAF) and the Alliance for Cancer Gene Therapy (JAF). The design of figures was aided by licensed materials from ScienceSlides (http://www.visiscience.com) and the Parker Institute for Cancer Immunotherapy.
Footnotes
Compliance with Ethical Standards:
Conflict of Interest
JJM and JAF hold patents related to CAR T cell therapy and receive associated royalties. The remaining authors have no competing interests.
References
- 1.Kuwana Y, Asakura Y, Utsunomiya N et al. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochemical and biophysical research communications. 1987;149(3):960–8. doi: 10.1016/0006-291x(87)90502-x. [DOI] [PubMed] [Google Scholar]
- 2.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(24):10024–8. doi: 10.1073/pnas.86.24.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell. 1991;64(5):891–901. doi: 10.1016/0092-8674(91)90314-o. [DOI] [PubMed] [Google Scholar]
- 4.Krause A, Guo HF, Latouche JB et al. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. The Journal of experimental medicine. 1998;188(4):619–26. doi: 10.1084/jem.188.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milone MC, Fish JD, Carpenito C et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17(8):1453–64. doi: 10.1038/mt.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levine BL, Miskin J, Wonnacott K, Keir C. Global Manufacturing of CAR T Cell Therapy. Molecular therapy Methods & clinical development. 2017;4:92–101. doi: 10.1016/j.omtm.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vormittag P, Gunn R, Ghorashian S, Veraitch FS. A guide to manufacturing CAR T cell therapies. Current opinion in biotechnology. 2018;53:164–81. doi: 10.1016/j.copbio.2018.01.025. [DOI] [PubMed] [Google Scholar]
- 8.Maude SL, Laetsch TW, Buechner J et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–48. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JH, Riviere I, Gonen M et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):449–59. doi: 10.1056/NEJMoa1709919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghorashian S, Kramer AM, Onuoha S et al. Enhanced CAR T cell expansion and prolonged persistence in pediatric patients with ALL treated with a low-affinity CD19 CAR. Nat Med. 2019. doi: 10.1038/s41591-019-0549-5. [DOI] [PubMed] [Google Scholar]
- 11.Schuster SJ, Svoboda J, Chong EA et al. Chimeric Antigen Receptor T Cells in Refractory B-Cell Lymphomas. N Engl J Med. 2017;377(26):2545–54. doi: 10.1056/NEJMoa1708566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neelapu SS, Locke FL, Bartlett NL et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med. 2017;377(26):2531–44. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuster SJ, Bishop MR, Tam CS et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 14.Garfall AL, Maus MV, Hwang WT et al. Chimeric Antigen Receptor T Cells against CD19 for Multiple Myeloma. N Engl J Med. 2015;373(11):1040–7. doi: 10.1056/NEJMoa1504542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan Z, Cao J, Cheng H et al. A combination of humanised anti-CD19 and anti-BCMA CAR T cells in patients with relapsed or refractory multiple myeloma: a single-arm, phase 2 trial. Lancet Haematol. 2019. doi: 10.1016/S2352-3026(19)30115-2. [DOI] [PubMed] [Google Scholar]
- 16.Raje N, Berdeja J, Lin Y et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2019;380(18):1726–37. doi: 10.1056/NEJMoa1817226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen AD, Garfall AL, Stadtmauer EA et al. B cell maturation antigen-specific CAR T cells are clinically active in multiple myeloma. J Clin Invest. 2019;129(6):2210–21. doi: 10.1172/JCI126397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maude SL, Frey N, Shaw PA et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Porter DL, Hwang WT, Frey NV et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med. 2015;7(303):303ra139. doi: 10.1126/scitranslmed.aac5415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fraietta JA, Lacey SF, Orlando EJ et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24(5):563–71. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Turtle CJ, Hanafi LA, Berger C et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest. 2016;126(6):2123–38. doi: 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee DW, Kochenderfer JN, Stetler-Stevenson M et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–28. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hurton LV, Singh H, Najjar AM et al. Tethered IL-15 augments antitumor activity and promotes a stem-cell memory subset in tumor-specific T cells. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(48):E7788–E97. doi: 10.1073/pnas.1610544113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fraietta JA, Nobles CL, Sammons MA et al. Disruption of TET2 promotes the therapeutic efficacy of CD19-targeted T cells. Nature. 2018;558(7709):307–12. doi: 10.1038/s41586-018-0178-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scholler J, Brady TL, Binder-Scholl G et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med. 2012;4(132):132ra53. doi: 10.1126/scitranslmed.3003761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sotillo E, Barrett DM, Black KL et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov. 2015;5(12):1282–95. doi: 10.1158/2159-8290.Cd-15-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bagashev A, Sotillo E, Tang CHA et al. CD19 Alterations Emerging after CD19-Directed Immunotherapy Cause Retention of the Misfolded Protein in the Endoplasmic Reticulum. Mol Cell Biol. 2018;38(21). doi:UNSP e00383–18 10.1128/MCB.00383-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orlando EJ, Han X, Tribouley C et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nature Medicine. 2018;24(10):1504-+. doi: 10.1038/s41591-018-0146-z. [DOI] [PubMed] [Google Scholar]
- 29.Fry TJ, Shah NN, Orentas RJ et al. CD22-targeted CAR T cells induce remission in B-ALL that is naive or resistant to CD19-targeted CAR immunotherapy. Nat Med. 2018;24(1):20–8. doi: 10.1038/nm.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ali SA, Shi V, Maric I et al. T cells expressing an anti-B-cell maturation antigen chimeric antigen receptor cause remissions of multiple myeloma. Blood. 2016;128(13):1688–700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh Z, Ross S, Fry TJ. Multi-Specific CAR Targeting to Prevent Antigen Escape. Curr Hematol Malig Rep. 2019. doi: 10.1007/s11899-019-00537-5. [DOI] [PubMed] [Google Scholar]
- 32.Geyer MB, Riviere I, Senechal B et al. Safety and tolerability of conditioning chemotherapy followed by CD19-targeted CAR T cells for relapsed/refractory CLL. JCI Insight. 2019;5. doi: 10.1172/jci.insight.122627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fedorov VD, Themeli M, Sadelain M. PD-1- and CTLA-4-based inhibitory chimeric antigen receptors (iCARs) divert off-target immunotherapy responses. Sci Transl Med. 2013;5(215):215ra172. doi: 10.1126/scitranslmed.3006597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasakovski D, Xu L, Li Y. T cell senescence and CAR-T cell exhaustion in hematological malignancies. Journal of hematology & oncology. 2018;11(1):91. doi: 10.1186/s13045-018-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finney OC, Brakke HM, Rawlings-Rhea S et al. CD19 CAR T cell product and disease attributes predict leukemia remission durability. J Clin Invest. 2019;129(5):2123–32. doi: 10.1172/JCI125423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu Y, Zhang M, Ramos CA et al. Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood. 2014;123(24):3750–9. doi: 10.1182/blood-2014-01-552174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blaeschke F, Stenger D, Kaeuferle T et al. Induction of a central memory and stem cell memory phenotype in functionally active CD4(+) and CD8(+) CAR T cells produced in an automated good manufacturing practice system for the treatment of CD19(+) acute lymphoblastic leukemia. Cancer immunology, immunotherapy : CII. 2018;67(7):1053–66. doi: 10.1007/s00262-018-2155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Bruggen JAC, Martens AWJ, Fraietta JA et al. Chronic lymphocytic leukemia cells impair mitochondrial fitness in CD8(+) T cells and impede CAR T-cell efficacy. Blood. 2019;134(1):44–58. doi: 10.1182/blood.2018885863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teachey DT, Lacey SF, Shaw PA et al. Identification of Predictive Biomarkers for Cytokine Release Syndrome after Chimeric Antigen Receptor T-cell Therapy for Acute Lymphoblastic Leukemia. Cancer Discov. 2016;6(6):664–79. doi: 10.1158/2159-8290.CD-16-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood. 2016;127(26):3321–30. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staedtke V, Bai RY, Kim K et al. Disruption of a self-amplifying catecholamine loop reduces cytokine release syndrome. Nature. 2018;564(7735):273–7. doi: 10.1038/s41586-018-0774-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karschnia P, Jordan JT, Forst DA et al. Clinical presentation, management, and biomarkers of neurotoxicity after adoptive immunotherapy with CAR T cells. Blood. 2019;133(20):2212–21. doi: 10.1182/blood-2018-12-893396. [DOI] [PubMed] [Google Scholar]
- 43.Gust J, Taraseviciute A, Turtle CJ. Neurotoxicity Associated with CD19-Targeted CAR-T Cell Therapies. CNS Drugs. 2018;32(12):1091–101. doi: 10.1007/s40263-018-0582-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hunter BD, Jacobson CA. CAR T-cell associated neurotoxicity: Mechanisms, clinicopathologic correlates, and future directions. J Natl Cancer Inst. 2019. doi: 10.1093/jnci/djz017. [DOI] [PubMed] [Google Scholar]
- 45.Torre M, Solomon IH, Sutherland CL et al. Neuropathology of a Case With Fatal CAR T-CellAssociated Cerebral Edema. J Neuropathol Exp Neurol. 2018;77(10):877–82. doi: 10.1093/jnen/nly064. [DOI] [PubMed] [Google Scholar]
- 46.Guha-Thakurta N, Wierda WG. Cerebral edema secondary to chimeric antigen receptor T-cell immunotherapy. Neurology. 2018;91(18):843. doi: 10.1212/WNL.0000000000006436. [DOI] [PubMed] [Google Scholar]
- 47.Gust J, Hay KA, Hanafi LA et al. Endothelial Activation and Blood-Brain Barrier Disruption in Neurotoxicity after Adoptive Immunotherapy with CD19 CAR-T Cells. Cancer Discov. 2017;7(12):1404–19. doi: 10.1158/2159-8290.CD-17-0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lamers CH, Sleijfer S, Vulto AG et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J Clin Oncol. 2006;24(13):e20–2. doi: 10.1200/JCO.2006.05.9964. [DOI] [PubMed] [Google Scholar]
- 49.Lamers CH, Sleijfer S, van Steenbergen S et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Molecular therapy : the journal of the American Society of Gene Therapy. 2013;21(4):904–12. doi: 10.1038/mt.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morgan RA, Yang JC, Kitano M et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Molecular therapy : the journal of the American Society of Gene Therapy. 2010;18(4):843–51. doi: 10.1038/mt.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Linette GP, Stadtmauer EA, Maus MV et al. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood. 2013;122(6):863–71. doi: 10.1182/blood2013-03-490565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morgan RA, Chinnasamy N, Abate-Daga D et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J Immunother. 2013;36(2):133–51. doi: 10.1097/CJI.0b013e3182829903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kershaw MH, Westwood JA, Parker LL et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12(20 Pt 1):6106–15. doi: 10.1158/1078-0432.CCR-06-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park JR, Digiusto DL, Slovak M et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Molecular therapy : the journal of the American Society of Gene Therapy. 2007;15(4):825–33. doi: 10.1038/sj.mt.6300104. [DOI] [PubMed] [Google Scholar]
- 55.Louis CU, Savoldo B, Dotti G et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood. 2011;118(23):6050–6. doi: 10.1182/blood-2011-05-354449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahmed N, Brawley VS, Hegde M et al. Human Epidermal Growth Factor Receptor 2 (HER2) -Specific Chimeric Antigen Receptor-Modified T Cells for the Immunotherapy of HER2-Positive Sarcoma. J Clin Oncol. 2015;33(15):1688–96. doi: 10.1200/JCO.2014.58.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Rourke DM, Nasrallah MP, Desai A et al. A single dose of peripherally infused EGFRvIII-directed CAR T cells mediates antigen loss and induces adaptive resistance in patients with recurrent glioblastoma. Sci Transl Med. 2017;9(399). doi: 10.1126/scitranslmed.aaa0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beatty GL, O’Hara MH, Lacey SF et al. Activity of Mesothelin-Specific Chimeric Antigen Receptor T Cells Against Pancreatic Carcinoma Metastases in a Phase 1 Trial. Gastroenterology. 2018;155(1):29–32. doi: 10.1053/j.gastro.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown CE, Alizadeh D, Starr R et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N Engl J Med. 2016;375(26):2561–9. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thistlethwaite FC, Gilham DE, Guest RD et al. The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity. Cancer immunology, immunotherapy : CII. 2017;66(11):1425–36. doi: 10.1007/s00262-017-2034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oliver AJ, Lau PKH, Unsworth AS et al. Tissue-Dependent Tumor Microenvironments and Their Impact on Immunotherapy Responses. Frontiers in immunology. 2018;9:70. doi: 10.3389/fimmu.2018.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Terabe M, Matsui S, Park JM et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. The Journal of experimental medicine. 2003;198(11):1741–52. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zea AH, Rodriguez PC, Atkins MB et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer research. 2005;65(8):3044–8. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 64.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117(5):1155–66. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Munder M, Eichmann K, Modolell M. Alternative metabolic states in murine macrophages reflected by the nitric oxide synthase/arginase balance: competitive regulation by CD4+ T cells correlates with Th1/Th2 phenotype. Journal of immunology. 1998;160(11):5347–54. [PubMed] [Google Scholar]
- 66.Brown JM, Recht L, Strober S. The Promise of Targeting Macrophages in Cancer Therapy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23(13):3241–50. doi: 10.1158/1078-0432.CCR-16-3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Munn DH, Mellor AL. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends in immunology. 2016;37(3):193–207. doi: 10.1016/j.it.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hornyak L, Dobos N, Koncz G et al. The Role of Indoleamine-2,3-Dioxygenase in Cancer Development, Diagnostics, and Therapy. Frontiers in immunology. 2018;9:151. doi: 10.3389/fimmu.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mellor AL, Lemos H, Huang L. Indoleamine 2,3-Dioxygenase and Tolerance: Where Are We Now? Frontiers in immunology. 2017;8:1360. doi: 10.3389/fimmu.2017.01360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miao J, Lu X, Hu Y et al. Prostaglandin E2 and PD-1 mediated inhibition of antitumor CTL responses in the human tumor microenvironment. Oncotarget. 2017;8(52):89802–10. doi: 10.18632/oncotarget.21155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang D, DuBois RN. The Role of Prostaglandin E(2) in Tumor-Associated Immunosuppression. Trends in molecular medicine. 2016;22(1):1–3. doi: 10.1016/j.molmed.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Martinez M, Moon EK. CAR T Cells for Solid Tumors: New Strategies for Finding, Infiltrating, and Surviving in the Tumor Microenvironment. Frontiers in immunology. 2019;10:128. doi: 10.3389/fimmu.2019.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weinkove R, George P, Dasyam N, McLellan AD. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clinical & translational immunology. 2019;8(5):e1049. doi: 10.1002/cti2.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert opinion on biological therapy. 2015;15(8):1145–54. doi: 10.1517/14712598.2015.1046430. [DOI] [PubMed] [Google Scholar]
- 75.Yeku OO, Brentjens RJ. Armored CAR T-cells: utilizing cytokines and pro-inflammatory ligands to enhance CAR T-cell anti-tumour efficacy. Biochemical Society transactions. 2016;44(2):412–8. doi: 10.1042/BST20150291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Koneru M, Purdon TJ, Spriggs D, Koneru S, Brentjens RJ. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology. 2015;4(3):e994446. doi: 10.4161/2162402X.2014.994446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Suarez ER, Chang de K, Sun J et al. Chimeric antigen receptor T cells secreting anti-PD-L1 antibodies more effectively regress renal cell carcinoma in a humanized mouse model. Oncotarget. 2016;7(23):34341–55. doi: 10.18632/oncotarget.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li S, Siriwon N, Zhang X et al. Enhanced Cancer Immunotherapy by Chimeric Antigen Receptor-Modified T Cells Engineered to Secrete Checkpoint Inhibitors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23(22):6982–92. doi: 10.1158/10780432.CCR-17-0867. [DOI] [PubMed] [Google Scholar]
- 79.Rafiq S, Yeku OO, Jackson HJ et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nature biotechnology. 2018;36(9):847–56. doi: 10.1038/nbt.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi BD, Yu X, Castano AP et al. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nature biotechnology. 2019;37(9):1049–58. doi: 10.1038/s41587-019-0192-1. [DOI] [PubMed] [Google Scholar]
- 81.Wilkie S, Picco G, Foster J et al. Retargeting of human T cells to tumor-associated MUC1: the evolution of a chimeric antigen receptor. Journal of immunology. 2008;180(7):4901–9. doi: 10.4049/jimmunol.180.7.4901. [DOI] [PubMed] [Google Scholar]
- 82.Posey AD Jr., Schwab RD, Boesteanu AC et al. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity. 2016;44(6):1444–54. doi: 10.1016/j.immuni.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xie YJ, Dougan M, Jailkhani N et al. Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(16):7624–31. doi: 10.1073/pnas.1817147116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nature biotechnology. 2013;31(1):71–5. doi: 10.1038/nbt.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu X, Ranganathan R, Jiang S et al. A Chimeric Switch-Receptor Targeting PD1 Augments the Efficacy of Second-Generation CAR T Cells in Advanced Solid Tumors. Cancer research. 2016;76(6):1578–90. doi: 10.1158/0008-5472.CAN-15-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roybal KT, Rupp LJ, Morsut L et al. Precision Tumor Recognition by T Cells With Combinatorial Antigen-Sensing Circuits. Cell. 2016;164(4):770–9. doi: 10.1016/j.cell.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Adusumilli PS, Cherkassky L, Villena-Vargas J et al. Regional delivery of mesothelin-targeted CAR T cell therapy generates potent and long-lasting CD4-dependent tumor immunity. Sci Transl Med. 2014;6(261):261ra151. doi: 10.1126/scitranslmed.3010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tchou J, Zhao Y, Levine BL et al. Safety and Efficacy of Intratumoral Injections of Chimeric Antigen Receptor (CAR) T Cells in Metastatic Breast Cancer. Cancer Immunol Res. 2017;5(12):115261. doi: 10.1158/2326-6066.CIR-17-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bach PB, Giralt SA, Saltz LB. FDA Approval of Tisagenlecleucel: Promise and Complexities of a $475000 Cancer Drug. Jama. 2017;318(19):1861–2. doi: 10.1001/jama.2017.15218. [DOI] [PubMed] [Google Scholar]
- 90.Lin JK, Muffly LS, Spinner MA et al. Cost Effectiveness of Chimeric Antigen Receptor T-Cell Therapy in Multiply Relapsed or Refractory Adult Large B-Cell Lymphoma. J Clin Oncol. 2019;37(24):2105–19. doi: 10.1200/JCO.18.02079. [DOI] [PubMed] [Google Scholar]
- 91.Schietinger A, Philip M, Krisnawan VE et al. Tumor-Specific T Cell Dysfunction Is a Dynamic Antigen-Driven Differentiation Program Initiated Early during Tumorigenesis. Immunity. 2016;45(2):389–401. doi: 10.1016/j.immuni.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ren J, Liu X, Fang C et al. Multiplex Genome Editing to Generate Universal CAR T Cells Resistant to PD1 Inhibition. Clinical cancer research : an official journal of the American Association for Cancer Research. 2017;23(9):2255–66. doi: 10.1158/1078-0432.CCR-16-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qasim W, Zhan H, Samarasinghe S et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci Transl Med. 2017;9(374). doi: 10.1126/scitranslmed.aaj2013. [DOI] [PubMed] [Google Scholar]
- 94.Lentz R, Benson AB 3rd, Kircher S. Financial toxicity in cancer care: Prevalence, causes, consequences, and reduction strategies. Journal of surgical oncology. 2019;120(1):85–92. doi: 10.1002/jso.25374. [DOI] [PubMed] [Google Scholar]
- 95.Witte J, Mehlis K, Surmann B et al. Methods for measuring financial toxicity after cancer diagnosis and treatment: a systematic review and its implications. Annals of oncology : official journal of the European Society for Medical Oncology. 2019;30(7):1061–70. doi: 10.1093/annonc/mdz140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dusetzina SB. Drug Pricing Trends for Orally Administered Anticancer Medications Reimbursed by Commercial Health Plans, 2000–2014. JAMA oncology. 2016;2(7):960–1. doi: 10.1001/jamaoncol.2016.0648. [DOI] [PubMed] [Google Scholar]
- 97.Byrd JC, Jones JJ, Woyach JA, Johnson AJ, Flynn JM. Entering the era of targeted therapy for chronic lymphocytic leukemia: impact on the practicing clinician. J Clin Oncol. 2014;32(27):3039–47. doi: 10.1200/JCO.2014.55.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hodi FS, O’Day SJ, McDermott DF et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wolchok JD, Kluger H, Callahan MK et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pabla S, Conroy JM, Nesline MK et al. Proliferative potential and resistance to immune checkpoint blockade in lung cancer patients. Journal for immunotherapy of cancer. 2019;7(1):27. doi: 10.1186/s40425-019-0506-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Andrews A. Treating with Checkpoint Inhibitors-Figure $1 Million per Patient. American health & drug benefits. 2015;8(Spec Issue):9. [PMC free article] [PubMed] [Google Scholar]
- 102.Ghassemi S, Nunez-Cruz S, O’Connor RS et al. Reducing Ex Vivo Culture Improves the Antileukemic Activity of Chimeric Antigen Receptor (CAR) T Cells. Cancer Immunol Res. 2018;6(9):1100–9. doi: 10.1158/2326-6066.CIR-17-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.ClinicalTrials.Gov. CAR therapy-. 2019 https://clinicaltrials.gov/ct2/results?term=CAR+therapy-&recrs=abdf. Accessed 12/7 2019. [Google Scholar]
- 104.Yakoub-Agha I, Chabannon C, Bader P et al. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE). Haematologica. 2020;105(2):297–316. doi: 10.3324/haematol.2019.229781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maude SL, Laetsch TW, Buechner J et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. New England Journal of Medicine. 2018;378(5):439–48. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maude SL, Teachey DT, Rheingold SR et al. Sustained remissions with CD19-specific chimeric antigen receptor (CAR)-modified T cells in children with relapsed/refractory ALL. Journal of Clinical Oncology. 2016;34(15_suppl):3011-. doi: 10.1200/JCO.2016.34.15_suppl.3011. [DOI] [Google Scholar]
- 107.Schuster SJ, Bishop MR, Tam CS et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. New England Journal of Medicine. 2018;380(1):45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 108.Brindley DA, Davie NL, Sahlman WA et al. Promising growth and investment in the cell therapy industry during the first quarter of 2012. Cell stem cell. 2012;10(5):492–6. doi: 10.1016/j.stem.2012.04.018. [DOI] [PubMed] [Google Scholar]
- 109.Santomasso B, Bachier C, Westin J, Rezvani K, Shpall EJ. The Other Side of CAR T-Cell Therapy: Cytokine Release Syndrome, Neurologic Toxicity, and Financial Burden. American Society of Clinical Oncology educational book American Society of Clinical Oncology Annual Meeting. 2019;39:433–44. doi: 10.1200/EDBK_238691. [DOI] [PubMed] [Google Scholar]