Abbreviations
AF, Atrial fibrillation
BPVT, Bioprosthetic valve thrombosis
CAG, Coronary angiography
CPK, Creatine-phospho-kinase
CT, Computed tomography
EKG, Electrocardiogram
LV, Left ventricle
LVEF, LV ejection fraction
MPG, Mean trans-valvular pressure gradient
MRI, Magnetic resonance imaging
MVA, Mitral valve area
NT-ProBNP, N terminal pro B type natriuretic peptide
PHT, Pressure half-time
TEE, Transesophageal echocardiogram
TTE, Transthoracic echocardiogram
INTRODUCTION
Bioprosthetic valve replacement is widely performed nowadays in valvular diseases caused by degeneration, infection, injury, or congenital defect due to the claims of extended durability of the newer generation and less thrombogenic risk without the need for long-term anticoagulation as compared to mechanical valves. However, recent studies demonstrated a significant prevalence of bioprosthetic valve thrombosis (BPVT) due to more frequent access to echocardiogram, cardiac computed tomography (CT), and magnetic resonance imaging (MRI) for post-operative evaluation. We herein describe a case of subacute bioprosthetic mitral valve thrombosis complicating with severe mitral stenosis, occurring two weeks after surgical porcine mitral valve replacement despite post-operative oral anticoagulation therapy.
CASE PRESENTATION
A 75-year-old woman with history of mitral valve prolapse, hypertension and diabetes experienced a sudden onset of dyspnea for four days as her initial presentation to our emergency department. Her vital signs were stable at presentation other than desaturation with SpO2 around 94% in ambient air. On physical examination, bilateral lung basal rales and grade IV/VI holosystolic murmur over the mitral area were noted. Initial laboratory results yielded elevated cardiac enzymes (creatine-phospho-kinase (CPK) 257 U/L, creatine kinase myocardial band 43 U/L, troponin T 0.025 ng/ml and N terminal pro B type natriuretic peptide (NT-ProBNP) level (2532 pg/ml). The electrocardiogram (EKG) showed sinus tachycardia with ST-segment depression over the anterolateral leads. Chest X-ray revealed acute pulmonary edema. Transthoracic echocardiogram (TTE) disclosed hyperdynamic left ventricle (LV) with the LV ejection fraction (LVEF) of 60% and acute severe mitral regurgitation. Further transesophageal echocardiogram (TEE) demonstrated the posterior mitral leaflet aneurysm and the severe mitral regurgitation due to the posterior scallop of posterior mitral leaflet chordal rupture.
A presumptive diagnosis of acute severe mitral regurgitation due to chordal rupture was made. Coronary angiography (CAG) revealed patent coronary arteries. However, rapid atrial fibrillation (AF) occurred during CAG and subcutaneous enoxaparin 1 mg/kg twice daily and oral amiodarone 200 mg twice daily were prescribed. Enoxaparin was omitted one day before the operation and the patient underwent surgical porcine mitral valve replacement (27-mm St. Jude Epic; E100-27M-00, Brazil) smoothly. A routine postoperative TTE on day 6 demonstrated normal functioning of an implanted bioprosthetic mitral valve with preserved LVEF. On the same day, due to persisting AF and adequate control of postoperative bleeding, anticoagulation therapy with oral warfarin 1 mg once daily was prescribed with an INR goal of greater than 1.5.
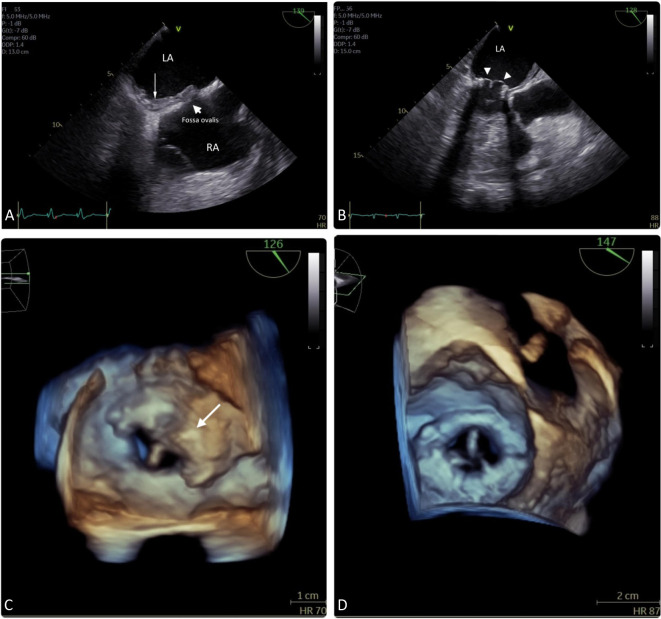
Unfortunately, the patient exhibited sudden dyspnea on day 16. EKG showed rapid AF and chest X-ray revealed acute pulmonary congestion. Subacute bioprosthetic valve thrombosis with severe mitral stenosis [mitral valve area (MVA) of 1.01 cm2 by pressure half-time (PHT) and mean trans-valvular pressure gradient (MPG) of 10.5 mmHg] and pericardial effusion were detected on a TTE with GE VividTM E95, a 4D cardiovascular ultrasound. The subsequent TEE disclosed a mural thrombus over the left atrium around the ring of mitral valve scaffold extending to the inter-atrial septum and restricted mobility of one leaflet of the porcine mitral valve with MVA of 1.01 cm2 by PHT and trans-mitral valve MPG of 9.2 mmHg, indicative of severe mitral stenosis (Figures 1A and 1C). Blood cultures, autoimmune and coagulation profiles yielded negative findings (Table 1). Subacute bioprosthetic valve thrombosis was impressed, and after shared decision-making with the patient and family, subcutaneous enoxaparin 1 mg/kg twice daily was provided rather than a surgical option. Oral warfarin was also adjusted to 2.5 mg daily due to inadequate INR goal. The follow-up TEE one week after treatment disclosed gradually diminishing mural thrombus over the inter-atrial septum and para mitral annulus ring with recovering mobility of bioprosthetic mitral valve (Figures 1B and 1D). Then, the patient was discharged from the hospital under stable hemodynamic status with oral warfarin 2.5 mg once daily with INR of 2.18. Transthoracic echocardiogram followed up 2 months later revealed a complete resolution of the thrombus with an MVA of 1.8 cm2 by PHT and MPG of 6 mmHg. Transthoracic echocardiogram followed up after one year demonstrated a well-functioning bioprosthetic mitral valve with MVA of 1.8 cm2 by PHT and MPG of 7 mmHg and no obvious thrombus was detected so far.
Figure 1.
(A) Mural thrombus (arrow) over left atrium, extending to the inter-atrial septum. (B) Diminishing inter-atrial septum thrombus (arrow) with thin mitral leaflets (arrowhead). (C) Thrombus (arrow) over mitral bioprosthesis. (D) Resolution of thrombus with recovery of bioprosthetic mitral valve mobility.
Table 1. Laboratory results.
| Result | Reference | |
| WBC | 7.73 K/mcl | 4.0-11.0 K/mcl |
| Hemoglobin | 14.6 g/dL | 12.0-16.0 g/dL |
| Hemotocrit | 42.8% | 37.0%-50.0% |
| Platelet | 181 K/mcl | 130-400 K/mcl |
| Glucose | 175 mg/dL | 120-200 mg/dL |
| BUN | 25.0 mg/dL | 6.0-20.0 mg/dL |
| Creatinine | 0.9 mg/dL | 0.5-0.9 mg/dL |
| GOT | 54.0 U/L | < 40 U/L |
| GPT | 28.0 U/L | < 40 U/L |
| Na | 142 mEq/L | 136-145 mEq/L |
| K | 4.2 mEq/L | 3.5-5.1 mEq/L |
| Ca | 8.6 mg/dl | 8.6-10.2 mg/dl |
| Mg | 2.7 mg/dl | 1.6-2.6 mg/dl |
| P | 2.3 mg/dl | 2.7-4.5 mg/dl |
| Albumin | 3.6 g/dl | 3.5-5.2 mEq/L |
| NT-proBNP | 2532 pg/ml | < 125 pg/ml |
| CPK | 257 U/L | 20-200 U/L |
| CK-MB | 43.0 U/L | < 25.0 U/L |
| Troponin T | 0.025 ng/ml | 0.000-0.014 ng/ml |
| PT (INR) | 17.4 sec (1.29) | 11-15 sec (0.78-1.12) |
| APTT | 35.9 sec | 32-45.1 sec |
| TSH | 3.34 uIU/ml | 0.27-4.20 uIU/ml |
| Free T4 | 1.26 ng/dl | 0.93-1.70 ng/dl |
| ANA | Negative | Negative |
| c-ANCA | Negative | Negative |
| p-ANCA | Negative | Negative |
| Protein C | 73.5% | 70-140% |
| Protein S | 103.3% | 63.5-149.0% |
ANA, antinuclear antibody; APTT, activated partial thromboplastin time; BUN, blood urea nitrogen; Ca, calcium; c-ANCA, cytoplasmic-antineutrophil cytoplasmic antibody; CK-MB, creatine kinase myocardial band; CPK, creatine phosphokinase; GOT, glutamyl oxaloacetic transaminase (aspartate aminotransferase); GPT, glutamyl pyruvic transaminase (alanine aminotransferase); K, potassium; Mg, magnesium; Na, sodium; NT-proBNP, N terminal pro B type natriuretic peptide; P, phosphorus; p-ANCA, perinuclear-antineutrophil cytoplasmic antibody; PT (INR), prothrombin time (international normalized ratio); TSH, thyroid stimulating hormone; WBC, white blood cells.
DISCUSSION
Due to the increasing prevalence of degenerative and infective valvular diseases, increasing the lifespan and maturation of surgical procedures, prosthetic valve replacement, either mechanical or bioprosthetic, is performed worldwide. The choice of valve usually depends on the patient’s age, preference and compliance with lifelong anticoagulation therapy.1,2 Bioprosthetic porcine and bovine valves are most widely used for being less thrombogenic without the need of long-term anti coagulation as compared to their mechanical counterparts and the extended durability of new generation bioprosthetic valves. However, recent studies demonstrated a significant incidence of BPVT due to more convenient access to TTE, cardiac CT and MRI for postoperative evaluation.3 The clinical spectrum of BPVT can range from incidental findings in asymptomatic patients to acute heart failure and cardiogenic shock in symptomatic ones. According to the Mayo Clinic pathology database between 1997 and 2013, the prevalence of histologically proven BPVT after explantation occurs at rates of 10.9%, 12.7%, 12.1%, and 11.6% within the aortic, mitral, tricuspid, and pulmonary positions, respectively.3 Another report showed that the freedom from valve thrombosis at 5 years was 98.3% with St Jude Epic porcine mitral valve.4 Symptomatic BPVT occurs in less than 1% of patients undergoing surgical valve implantation.5 Factors associated with higher thrombotic rate include bioprosthetic porcine valve, implantation in mitral position, recipients with hypercoagulable status, anticardiolipin syndrome, previous thromboembolic events, recent withdrawal of anticoagulation, subtherapeutic INR, atrial fibrillation and low left ventricular ejection fraction.6-8 Sixty-five percent of BPVT occurred > 12 months after implantation where peak incidence was at 13 to 24 months.3,9 Recipients of bioprosthetic mitral valve replacement who were anticoagulated have a lower thromboembolic risk than those who were not.10 Current ESC and ACC/AHA guidelines recommend three to six months of oral anticoagulation with vitamin-K antagonists after surgical bioprosthetic mitral valve replacement to maintain the INR at a level of 2.5 in low bleeding risk patients even with no other indications for anticoagulation.1,2 In patients with confirmed BPVT, vitamin K antagonist or unfractionated heparin is recommended in hemodynamically stable conditions, and otherwise, surgery or fibrinolysis should be considered. Naser et al. showed that recovery from BPVT was significantly faster in mitral than aortic (median 2.5 vs. 4.8 months, p = 0.038) and tricuspid (median 5.9 months, p = 0.025 vs. mitral) positions.11
In our case, even with early initiation of anticoagulants after surgery, subacute BPVT still occurred 16 days after surgery. After adequate anticoagulants with INR greater than 2.0, the valvular and LA mural thrombus diminished gradually, accompanied by recovery of her mitral bioprosthetic valve function. Although current guidelines and consensus suggest resuming anticoagulants soon after operation if bleeding is manageable, the optimal timing to initiate anticoagulation after mitral valve operation remains at the discretion of the surgeon and ICU doctors. In our case, the underlying atrial fibrillation, being mitral valve replacement, recent withdrawal of anticoagulant due to surgery and inadequate anticoagulant dosing might play important roles in resulting bioprosthetic mitral valve thrombosis. Reducing the anticoagulation-free window and timely achieving the INR goal at around 2.5 may prevent further events in the future.
LEARNING POINT
Timing and dosage of anticoagulation after surgical bioprosthetic valve replacement.
DECLARATION OF CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Otto C. ACC/AHA guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Joint Committee on clinical practice guidelines. Circulation. 2020;2021:143. doi: 10.1161/CIR.0000000000000923. [DOI] [PubMed] [Google Scholar]
- 2.Vahanian A. 2021 ESC/EACTS guidelines for the management of valvular heart disease: developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2022;43:2022. doi: 10.1093/eurheartj/ehac051. [DOI] [PubMed] [Google Scholar]
- 3.Puri R, Auffret V, Rodés-Cabau J. Bioprosthetic valve thrombosis. J Am Coll Cardiol. 2017;69:2193–2211. doi: 10.1016/j.jacc.2017.02.051. [DOI] [PubMed] [Google Scholar]
- 4.Nakazato T, et al. Midterm clinical outcomes of the St Jude Medical epic porcine bioprosthesis in the mitral position. Circulation. 2018;83:110–116. doi: 10.1253/circj.CJ-18-0483. [DOI] [PubMed] [Google Scholar]
- 5.Bouwmeester S, El Farissi M, Houthuizen P. Bioprosthetic mitral valve thrombosis. Netherlands Heart Journal. 2022;30:239–240. doi: 10.1007/s12471-022-01659-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrescu I, et al. Long-term outcomes of anticoagulation for bioprosthetic valve thrombosis. J Am Coll Cardiol. 2020;75:857–866. doi: 10.1016/j.jacc.2019.12.037. [DOI] [PubMed] [Google Scholar]
- 7.Rajappan R, Vydianathan P, Sundaram RS. A case of bioprosthetic valve thrombosis: is it time to revise our thinking? JIAE. 2022;6:59–62. [Google Scholar]
- 8.Scantlebury DC, Nkomo VT, Enriquez-Sarano M. Antiphospholipid syndrome and recurrent thrombotic valve disease. J Am Coll Cardiol. 2013;61:e177. doi: 10.1016/j.jacc.2012.12.058. [DOI] [PubMed] [Google Scholar]
- 9.Egbe AC, et al. Bioprosthetic valve thrombosis versus structural failure: clinical and echocardiographic predictors. J Am Coll Cardiol. 2015;66:2285–2294. doi: 10.1016/j.jacc.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 10.Heras M, et al. High risk of thromboemboli early after bioprosthetic cardiac valve replacement. J Am Coll Cardiol. 1995;25:1111–1119. doi: 10.1016/0735-1097(94)00563-6. [DOI] [PubMed] [Google Scholar]
- 11.Naser JA, et al. Gradient changes in bioprosthetic valve thrombosis: duration of anticoagulation and strategies to improve detection. Open Heart. 2021;8 doi: 10.1136/openhrt-2021-001608. [DOI] [PMC free article] [PubMed] [Google Scholar]