Abstract
Glucagon-like peptide-1 (GLP-1) receptor agonists (RAs) are emerging glucose-lowering agents primarily used in managing diabetes and obesity. Recently, GLP-1 RAs have garnered attention for their cardiovascular benefits beyond glycaemic control in patients with type 2 diabetes, exhibiting patterns previously seen in cardiovascular outcomes trials on sodium–glucose cotransporter 2 inhibitors, which now receive a high level of recommendation for the treatment of heart failure (HF). GLP-1 RAs have been increasingly investigated in HF cohorts, but mainly in small-scale studies reporting inconclusive findings regarding clinical outcomes and different safety profiles in HF patients with reduced and preserved ejection fractions. This review discusses the effects of GLP-1 RAs on surrogate HF outcomes, such as cardiac structure and function, exercise capacity and quality of life, in HF patients across the spectrum of left ventricular ejection fraction, to provide insights into the potential of these agents to be investigated in large clinical trials to evaluate clinical outcomes.
Keywords: Glucagon-like peptide-1 receptor agonists, heart failure, quality of life, cardiac function, exercise capacity
Glucagon-like peptide-1 (GLP-1) receptor agonists (RAs) are an emerging class of glucose-lowering drugs that are increasingly used in the treatment of type 2 diabetes (T2D) and obesity. Cardiovascular outcome trials in patients with T2D have demonstrated the efficacy of GLP-1 RAs in reducing major adverse cardiovascular events, including cardiovascular death, MI and stroke, regardless of glycaemic control.[1–8]
Recent European Society of Cardiology guidelines for the management of cardiovascular disease (CVD) in patients with diabetes state that GLP-1 RAs (lixisenatide, liraglutide, semaglutide, exenatide extended release [ER], dulaglutide, efpeglenatide) should be considered for glucose-lowering treatment in patients with T2D at risk of or with heart failure (HF; class IIa A recommendation) alreadytaking sodium–glucose cotransporter 2 inhibitors, without differentiating between HF phenotypes.[9] The consensus statement from the American Association of Clinical Endocrinologists supports the use of GLP-1 RAs as first-line therapy in managing hyperglycaemia in T2D patients with established (or at high risk of) atherosclerotic CVD, as well as in those with chronic kidney disease or a history of stroke or transient ischaemic attack, while recommending the administration of sodium–glucose cotransporter 2 inhibitors to HF patients.[10] The American College of Cardiology/American Heart Association primary prevention of cardiovascular disease guideline supports the use of GLP-1 RAs in patients with T2D and high atherosclerotic CVD risk, but does not mention existing HF.[11]
Current evidence related to the effects of GLP-1 RAs on HF outcomes remains limited. GLP-1 RA cardiovascular outcome trials in patients with T2D reported a neutral impact of the drugs on HF hospitalisation. Of note, the prevalence of HF varied across these trials from 9% to 24%, and HF events were considered a secondary endpoint.[1–8] Most of these studies do not report HF diagnostic criteria or mention HF therapy, and only one trial reported on left ventricular ejection fraction (LVEF) at baseline.[8] Moreover, one should consider the fact that different GLP-1 RAs were used across the studies, with different chemical structures, durations of action and weight-lowering effects, which may impact their efficacy.
An updated meta-analysis of nine randomised controlled trials (RCTs), including 8,920 patients with HF and T2D, reported a 13% reduction in major adverse cardiovascular events in the GLP-1 RA compared with placebo arm.[12] In contrast, no benefit of GLP-1 RAs was observed in terms of all-cause death, HF hospitalisation or cardiovascular death.[12] Of note, that meta-analysis did not differentiate between HF phenotypes.
In the recent STEP HFpEF DM trial, which studied the efficacy of semaglutide among patients with obesity-related HF with preserved ejection fraction (HFpEF) and T2D, a reduction in the time to first HF events was demonstrated in the intervention arm, but this was a secondary endpoint.[13] No RCTs have primarily tested the effects of GLP-1 RAs on HF hard outcomes and/or mortality in patients with HFpEF or HF with reduced ejection fraction (HFrEF).
Generally, surrogate endpoints are expected to predict clinical benefits. In recent years, data have been accumulated suggesting effects of GLP-1 RAs on surrogate HF outcomes in HF patients across LVEF, including cardiac structure and function, exercise capacity and quality of life.[13]
In this review, we summarise the evidence related to the effects of GLP-1 RAs on these outcomes (Figure 1), which may shed light on the potential of these agents to be used in the clinical care of HF patients and investigated in large clinical trials to evaluate clinical outcomes.
Figure 1: Effects of Glucagon-like Peptide-1 Receptor Agonists on Cardiac Function, Exercise Capacity, Quality of Life and Clinical Outcomes in Patients With Heart Failure.
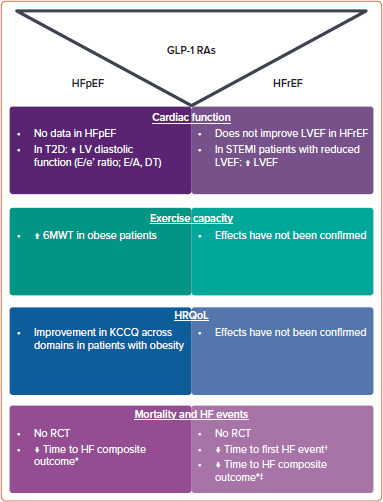
*A pre-specified analysis from the SELECT trial (HF composite outcome = cardiovascular death or HF hospitalisation/emergency visit).† Secondary outcomes of STEP HFpEF trials. ‡ Data from multicentre PRAISE HFpEF DM observational cohort study (HF composite outcome = HF hospitalisation and all-cause mortality). 6MWT = 6-min walking test; DT = deterioration time; GLP-1 RAs = Glucagon-like peptide-1 receptor agonists; HF = heart failure; HFpEF = heart failure with preserved ejection fraction; HFrEF = heart failure with reduced ejection fraction; HRQoL = health-related quality of life; KCCQ = Kansas City Cardiomyopathy Questionnaire; LV = left ventricle; LVEF = left ventricular ejection fraction; T2D = type 2 diabetes; RCT = randomised controlled trials.
Potential Mechanism Underlying the Effects of GLP-1 RAs in Heart Failure
GLP-1 RAs bind to GLP-1 receptors expressed in various tissues, including pancreatic beta cells, the kidneys, heart, brain and gastric mucosa, among other organs. After binding to GLP-1 receptors in pancreatic beta cells, GLP-1 RAs exert their glucose-lowering effect by stimulating glucose-dependent insulin release. Antihyperglycemic effects aside, GLP-1 RAs reduce gastric emptying and suppress appetite centres in the hypothalamus, resulting in weight loss. Systemic effects of GLP-1 RAs, mediated via GLP-1 receptors in other organs and systems, include enhanced endothelial function and myocardial metabolism, natriuresis and anti-inflammatory and blood pressure-lowering effects.[14–17]
All these effects of GLP-1 RAs are crucial for the pathophysiology of HF, with weight loss in obese HF patients being of utmost importance. Obesity is highly prevalent in HF; in HFpEF, the prevalence of obesity reaches up to 80%.[18] Obesity is an important contributor to the pathophysiology of both diabetes and HF, particularly HFpEF.[19] Adipose tissue is a complex endocrine organ that has multiple endocrine and paracrine effects on the heart.[20] Moreover, the high amount of visceral fat and epicardial adipose tissue (EAT), as well as associated plasma volume expansion, in obese individuals results in significant haemodynamic impairment at rest and during exercise, exerting local effects on the heart.[21] Remarkably, even in HF-free patients with morbid obesity, cardiac abnormalities can be seen that are consistent with left ventricular (LV) remodelling and dysfunction, including greater LV mass, LV diastolic dysfunction and elevated LV filling pressure.[22,23]
Obese HFpEF patients have greater LV hypertrophy and pulmonary capillary wedge pressure, increased LV filling pressures and severe right ventricular dysfunction compared with non-obese HFpEF patients.[24,25] Increased EAT also results in a greater pericardial restraint, which affects haemodynamics.[26] Overall, the cardiovascular effects of GLP-1 RAs in HF can be realised through multiple pathways, including reducing visceral fat and EAT in obese HF patients; abolishing obesity-related haemodynamic impairment, pathological LV remodelling and myocardial inflammation; and systemic and glucose-lowering effects.[27]
Effects of GLP-1 RAs on Cardiac Structure and Function
GLP-1 RAs have garnered significant interest due to their potential benefits beyond glycaemic control, including their effects on cardiac function.[28] A landmark pilot study that investigated the efficacy and safety of 72-h GLP-1 RA infusion in patients with acute MI and impaired LVEF (<40%) revealed a notable improvement in LV function, as assessed by echocardiography, compared with control subjects with similar disease characteristics that was independent of diabetes.[29] Furthermore, in a randomised placebo-controlled trial involving patients with ST-segment elevation MI (STEMI) who underwent balloon angioplasty and stent placement, with daily injections of exenatide or placebo for 3 days, revealed exenatide was associated with a smaller infarct size on cardiac MRI conducted 38 days after reperfusion.[30] Echocardiographic assessment at 6 months demonstrated persistent improvements in diastolic function and global longitudinal strain among patients who received exenatide.[30] The reduction in infarct size was confirmed in a larger study of 172 STEMI patients randomised to receive either intravenous exenatide or placebo.[31,32] However, in that study, no significant difference in changes in LVEF were observed between the groups.[31,32] In contrast, the administration of liraglutide to STEMI patients was associated with a small but significant improvement in LVEF assessed at 3 months in both non-diabetic and diabetic subjects.[33]
Although GLP-1 RAs have shown promise in enhancing postischemic LV systolic function in preclinical and clinical settings, studies investigating the effects of GLP-1 RAs on LV diastolic function in patients with T2D reported conflicting findings.[31,34–37] Several small studies reported that liraglutide significantly improved LV diastolic function in T2D patients compared with either placebo or other glucose-lowering agents, which was also associated with improvements in endothelial function and antioxidant and anti-inflammatory activity.[36,37] In contrast, the administration of exenatide was not associated with changes in LV diastolic function.[5] These apparent discrepancies could be explained, in part, by the drug-specific effects of GLP-1 RAs on LV diastolic function, as well as differences in the baseline characteristics of patients, namely less prominent LV diastolic dysfunction in patients in the exenatide cohort compared with patients in the liraglutide studies.[5,36,37]
However, a meta-analysis of 10 placebo-controlled RCTs, including 732 individuals with T2D, found that liraglutide therapy did not influence echocardiographic parameters of diastolic function compared with placebo, including the ratio of early diastolic filling velocity (E) to mitral annular early diastolic velocity (e′; weighted mean difference [WMD] -0.763; 95% CI [-2.157, 0.630]; p=0.283), change in e′ (WMD -0.069; 95% CI [-0.481, 0.343]; p=0.742) and change in E/e′ (WMD -0.683; 95% CI [-1.663, 0.298]; p=0.172).[35] LVEF also remained unchanged with liraglutide therapy compared with placebo (WMD -0.651; 95% CI [-1.649, 0.348]; p=0.202).[35]
A further meta-analysis of 22 RCTs that included a considerably larger cohort (n=61,412) of T2D patients with or without cardiovascular disease and patients with cardiovascular disease alone revealed that treatment with GLP-1 RAs led to improvements in diastolic function (E-wave; standardised mean difference -0.40; 95% CI [-0.60, -0.20]; p<0.001), early diastolic to late diastolic velocities ratio (WMD -0.10; 95% CI [-0.18, -0.02]; p=0.01), E/e′ ratio (WMD -0.97; 95% CI [-1.54, -0.41; p<0.001) and E-wave deceleration time (WMD -9.96 ms; 95% CI [-18.52, -1.41 ms]; p=0.02), although LVEF was not affected.[38]
The effects of GLP-1 RAs on cardiac structure and function were also investigated in HF patients. An early small study of 12 HF patients with New York Heart Association (NYHA) Classes III–IV showed a significant increase in LVEF following 5 weeks of continuous subcutaneous infusion of GLP-1 RAs.[39] However, larger studies have not demonstrated a significant effect of GLP-1 RAs on LV function, including albiglutide, which was administered over a long period (>12 weeks) to non-diabetic, overweight or obese individuals with HF (NYHA Class II–III) and LVEF below 40%.[40] In the FIGHT trial, long-term administration of liraglutide to HF and HFrEF patients with or without diabetes did not significantly improve LVEF after 24 weeks of treatment.[41] However, there was an increase in heart rate and more serious cardiac events, such as arrhythmias and acute coronary syndrome, in patients treated with liraglutide.[41] In a meta-analysis of nine RCTs involving 8,920 patients with HF and coexisting T2D, GLP-1 RAs did not improve LVEF, LV end-diastolic volume or LV end-systolic volume.[12]
Although larger-scale studies are yet to explore the effects of GLP-1 RAs on cardiac structure and function, available evidence suggests that GLP-1 RAs may improve systolic and diastolic function in individuals with T2D who are at high risk of CVD and reduce infarct size after acute MI.[33–37] In individuals with HFrEF, GLP-1 RAs do not affect LV systolic function, whereas the effects of GLP-1 RAs in individuals with HFpEF have not yet been investigated.
Effects of GLP-1 RAs on Exercise Capacity
It may be assumed that GLP-1 RAs could potentially increase exercise capacity primarily by promoting weight loss. Other mechanisms underlying the effects of GLP-1 RAs on exercise capacity include improvements in myocardial energetics, enhanced endothelial function, reductions in systemic inflammation and oxidative stress and modulation of skeletal muscle metabolism.[14–17]
Overall, clinical evidence regarding the effects of GLP-1 RAs on exercise capacity is limited and inconsistent, and varies across the spectrum of HF.[42] The effects of GLP-1 RAs in HFrEF have been studied in several small trials. In FIGHT, there was no significant effect of liraglutide on 6-min walk test (6MWT) distances compared with placebo.[40] In the LIVE trial, which evaluated the effects of liraglutide on LV function in stable chronic HF patients with and without diabetes, at the end of treatment patients from the liraglutide group were able to walk 28 ± 65 m longer during the 6MWT, compared with 3 ± 89 m for patients in the placebo group, with a mean difference of 24 m.[43] However, more patients in the liraglutide than placebo group experienced serious cardiac adverse events, including significant arrhythmia.[43] A similar trend towards a higher risk of unfavourable outcomes was observed in the post hoc analysis of the FIGHT trial.[40]
Another small trial compared the effects of 12 weeks’ treatment with albiglutide (n=27) to placebo (n=30) on cardiac function, cardiac metabolism and exercise capacity in HFrEF.[43] Albiglutide did not improve myocardial glucose use or myocardial oxygen consumption, cardiac efficiency or the 6MWT distance. Surprisingly, a slight improvement in change of peak oxygen consumption (peak VO2) was observed in the albiglutide group compared with placebo (mean 0.9 ± 0.5 ml/kg/min versus -0.6 ± 0.5 ml/kg/min; p=0.02).[40] However, the improvement in peak VO2 was within the margin of measurement error and was not accompanied by a corresponding improvement in the 6MWT distance or quality of life, so this finding needs to be investigated further. Peak VO2 improvement was not supported by the pharmacokinetic/pharmacodynamic modelling, indicating no relationship between exposure to albiglutide and peak VO2.[40] Therefore, current data suggest that GLP-1 RAs do not improve exercise capacity in HFrEF.[44]
More promising evidence is available regarding GLP-1 RAs in obesity-related HFpEF. The benefits of semaglutide in obese HFpEF patients were established in the landmark STEP HFpEF trial.[45] In that trial, patients receiving semaglutide experienced greater reductions in weight (estimated difference -10.7%; p<0.001) and increases in 6MWT distance (estimated difference +20.3 m; p<0.001) compared with those in the control group.[45,46] This result was confirmed by the recent pooled analysis of the STEP HFpEF and STEP HFpEF DM trials, which included 1,145 patients. In that analysis, patients in the semaglutide group showed improvement from baseline to week 52 in both body weight (mean 8.4% reduction; p<0.0001 versus placebo) and 6MWT distance (mean 17.1 m; p<0.001 versus placebo).[47] Because of the high prevalence of frailty and sarcopenic obesity in the HFpEF population, future studies are required to estimate the proportion of lean body mass loss versus fat loss on GLP-1 RA therapy to identify predictors of the disproportionate loss of muscle mass.[45]
Conversely, the relative increase in heart rates secondary to GLP-1 RAs may be potentially beneficial in HFpEF due to the high prevalence of chronotropic incompetence in these patients. Another promising strategy for HFpEF patients may be combining exercise training with GLP-1 RAs, mitigating the risk of sarcopenia and frailty and providing a synergistic effect on physical tolerance and quality of life.[46] Exercise training can also potentially attenuate gastrointestinal side effects related to GLP-1 RAs.[45] Similarly, previous studies showed that caloric restriction with or without aerobic training improved peak VO2 in obese elderly HFpEF patients.[48] Future large studies combining comprehensive cardiac rehabilitation programs and intentional weight loss through GLP-1 RAs, caloric restriction and exercise in obesity-related HFpEF will be highly appreciated. It is hoped that ongoing trials like SUMMIT, which is investigating tirzepatide in participants with HFpEF and obesity, will provide more evidence on incretin-based medications in HFpEF.[49]
Effects of GLP-1 RAs on Quality of Life
In addition to reducing the risk of hospital admission and mortality, improving health-related quality of life (HRQoL) is another treatment target in HF. HRQoL is a broad concept that covers individuals’ views on how their disease and treatment affect their overall wellbeing and physical, psychological and social abilities compared to personal expectations.[50] Reduced HRQoL in HF is multifactorial and related to frequent readmissions and symptom burden, exercise intolerance, emotional distress, loss of independence and social limitations. In addition to HF-associated factors, HRQoL is also attributable to comorbidities, the major ones being T2D and obesity. Patients with HFrEF and concomitant T2D show consistently lower Kansas City Cardiomyopathy Questionnaire (KCCQ) scores than those without T2D.[49] Similarly, higher BMI has detrimental effects on perceived health in HFrEF, which is also highly significant in HFpEF.[51–53] Notably, of the different HF phenotypes, HFpEF is associated with the worst HRQoL, whereas among HFpEF patients, the worst HRQoL had been shown in a subgroup with the highest BMI, a higher proportion of at least Grade 2 obesity and T2D.[53] Moreover, there was a linear relationship between HRQoL and BMI.[53]
Better T2D control and weight reduction with GLP-1 RA therapy may be beneficial in terms of patient-reported health status among the HF cohort. However, in specifically designed placebo-controlled studies of GLP-1 RAs among patients with HFrEF, there were no improvements in HRQoL.[39,40,43] There may be several possible explanations for the failure of GLP-1 RAs in HFrEF studies. A well-known collider bias, termed the ‘obesity paradox’, should be considered.[54] BMI as a metric of obesity has some limitations, such as not being able to differentiate between lean and fat mass, estimate the magnitude of visceral obesity and predict outcomes.[55] Likewise, data on mean BMI among HFrEF participants may not reflect the presence and severity of obesity as a target for the potential effectiveness of GLP-1 RAs.
The FIGHT study showed that more vulnerable HF patients with severely reduced HRQoL and perhaps more advanced stages of the disease may require complex care planning to improve their health status rather than solely increasing glucose and fatty acid metabolism to enhance cardiac metabolism.[40] Indeed, patients at later stages of HF may respond differently to therapy that improves outcomes in less severe disease states.[56] A short duration of treatment may also have prevented significant changes in HRQoL in HFrEF studies.[39,43]
In contrast, among HFpEF cohorts, semaglutide showed clinically meaningful benefits in non-diabetic and diabetic cohorts (Table 1).[13,45] In STEP HFpEF, semaglutide improved in KCCQ clinical summary scores (CSS), one of the study’s primary endpoints, by 7.8 points compared with placebo.[45] In addition, the KCCQ overall summary score improved by 7.5 points compared with placebo.[45] A similar benefit was shown in analyses by baseline KCCQ-CSS tertiles, BMI, and LVEF subgroups.[52,57,58] Moreover, the effect was consistent across all HRQoL domains (e.g. physical limitations score, quality of life score, symptom burden score, symptom frequency score and social limitations score), with estimated treatment differences ranging from 6.7 to 9.6 points.[52] Similarly, the odds of at least 5-, 10-, 15-and 20-point improvements in all KCCQ domains were 1.6-to 2.9-fold higher among semaglutide-than placebo-treated patients.[52] The effect of semaglutide on HRQoL in HFpEF, particularly social and physical function as measured by the KCCQ, is mediated at least in part by the effect on weight reduction.[57]
Table 1: Characteristics of Studies Exploring the Effects of Glucagon-like Peptide-1 Receptor Agonists on Quality of Life in Heart Failure.
| Study, no. Participants | Drug, Follow-up Period | HF Characteristics | Proportion of Patients with T2D/Baseline BMI | QoL Instrument | Baseline QoL Levels | Changes Compared with Placebo [95% CI]; p-value |
|---|---|---|---|---|---|---|
| Lepore et al, 2016[40] n=62 | Albiglutide, 12 weeks | HFrEF on stable GDMT, NYHA II–III (proportions not reported), mean ( ± SD) EF 31 ± 1.6% | Without T2D/mean ± SD BMI 31 ± 7 kg/m2 | MLHFQ | 31 ± 4 | 2.5 ± 4.8*, p=0.61 |
| FIGHT, 2016[41] n=300 | Liraglutide, 180 days | Recently (within 14 days) hospitalised HFrEF, NYHA II–IV (III, 63%; IV, 5%), median EF 25% (IQR 20–33%) | T2D 59%/median BMI 31 kg/m2 (IQR 26–36 kg/m2) | KCCQ-CSS | 46 (32–65) | 1.3 [-4.0, 6.5]†; p=0.64 |
| KCCQ-OSS | 43 (30–61) | 0.6 [-4.5, 5.8]; p=0.81 | ||||
| LIFE, 2017[43] n=241 | Liraglutide, 24 weeks | HF with EF ≤45% on stable GDMT, NYHA I–III (II, 54%; III, 14%), mean ± SD EF 33.7 ± 7.6% | T2D 32%/median BMI 28.0 kg/m2 (IQR 3.8 kg/m2) | MLHFQ | Not reported | -1.6 [-5.3, 2.0]*, p=0.39 |
| StepHFpEF, 2023[45] n=529 | Semaglutide, 52 weeks | HF with EF ≥45% and BMI ≥30 kg/m2, baseline KCCQ-CSS <90 points, NYHA II–IV (III or IV, 33.8%), median EF 57% | Without T2D/median BMI 37.0 kg/m2 (IQR 33.7–41.4 kg/m2) | KCCQ-CSS | 58.9 (41.7–72.9) | 7.8 [4.8–10.9]‡; p<0.001 |
| KCCQ-OSS | Not available | 7.5 [4.4–10.6]‡; p<0.001 | ||||
| STEP HFpEF DM, 2024[13] n=617 | Semaglutide, 52 weeks | HF with EF ≥45% and BMI ≥30 kg/m2, baseline KCCQ-CSS <90 points, NYHA II–IV (III or IV, 29.3%), median EF 56% | Only with T2D (100%)/median BMI 36.9 kg/m2 (IQR 33.6–41.4 kg/m2) | KCCQ-CSS | 59.4 (43.8–72.0) | 7.3 [4.1–10.4]‡; p<0.001 |
| KCCQ-OSS | Not available | 7.3 [4.2–10.4]‡; p<0.001 |
Baseline levels of QoL are presented as mean ± standard deviation or median (IQR); mean values of quantitative variables are presented for the active arm if data for the total cohort were not published. *delta estimates; †between-group difference, adjusted for baseline value; ‡ estimated between-group difference. EF = ejection fraction; GDMT = guideline-directed medical therapy; HF = heart failure; KCCQ-CSS = Kansas City Cardiomyopathy Questionnaire Clinical Summary Scores; KCCQ-OSS = Kansas City Cardiomyopathy Questionnaire Overall Summary Scores; MLHFQ = Minnesota Living With Heart Failure Questionnaire; NYHA = New York Heart Association; QoL = quality of life; T2D = type 2 diabetes.
Mental health is an integral part of the HRQoL construct and requires attention in HF. HF patients frequently experience low mood, depression and cognitive impairment, with worse estimates shown for the HFpEF phenotype.[59] Although some effects of GLP-1 RAs, particularly weight loss, are linked to direct effects of GLP-1 RAs in brain areas responsible for appetite, satiety, food behaviour and other central regulatory mechanisms, data regarding the potential impact of therapy on mental health among HF patients are lacking. Some data suggest neuroprotective properties, and there are ongoing randomised trials among patients with neurological and psychiatric disorders (NCT04466345).[60–62] In addition, published reports suggest efficacy of GLP-1 RAs against weight gain related to the use of antipsychotic medication that may affect a patient’s feelings.[63] Once-weekly GLP-1 RAs may also improve HRQoL through greater treatment satisfaction.[64]
In contrast with prior anti-obesity medications, concerns about suicide intention have not been confirmed for GLP-1 RA therapy.[65,66] Of all adverse events reported, psychiatric side-effects of GLP-1 RAs are not frequent and account for 1.2–4.4% of events.[67,68] Still, the presence of GLP-1 RA-specific psychiatric adverse events that may also be related to drug intolerance requires the development of a strategy to overcome this barrier for the better implementation of GLP-1RA therapy.[67]
Controversy Regarding Effects of GLP-1 RAs in Heart Failure: Direct Drug Effects or Due to Weight Loss?
Previously described cardiovascular effects of GLP-1 RAs are primarily indirect and include enhanced endothelial function and myocardial metabolism, natriuresis and anti-inflammatory and blood pressure-lowering effects. Given that GLP-1 RAs result in weight loss and the pronounced effects of weight loss on haemodynamic disturbance and cardiac reverse remodelling in obese HF patients, the question is, to what extent is the effect of the drugs related to a direct effect on the cardiovascular system in HF?
A study of 5,067 overweight and obese T2D patients failed to demonstrate an association between mild weight reduction and improved cardiac function.[69] In obese HFpEF patients, weight-reduction interventions have significant potential for improving HRQoL.[70] A systematic review of 22 studies investigated the effect of intentional weight loss in overweight and obese patients with HF and demonstrated that all forms of weight loss (lifestyle changes, pharmacotherapy or bariatric surgery) are likely to result in significant improvements of symptoms and HRQoL in HF patients.[70] In the STEP HFpEF trial, higher changes in KCCQ-CSS were linearly associated with weight loss with semaglutide (5.9-point increase in KCCQ-CSS per 10% body weight loss).[45] Remarkably, the recent SELECT trial proved the cardiovascular efficacy of subcutaneous semaglutide in reducing the primary cardiovascular composite endpoint (death from cardiovascular causes, non-fatal MI or non-fatal stroke) in both overweight and obese patients with established atherosclerotic CVD.[71] These data, together with the finding of no effect of prior weight-reduction strategies on hard outcomes in HF, signify very likely beneficial effects of GLP-1RAs that are independent of weight loss. Moreover, a counterintuitive reduction in N-terminal pro B-type natriuretic peptide concentrations on top of weight reduction has been shown in both STEP HFpEF trials, indicating potential direct cardiac effects of the therapies.[72] Still, whether GLP-1RAs in HFpEF exert a disease-modifying effect independent of weight loss remains to be investigated.
The beneficial effects of GLP-1 RAs on HRQoL and other outcomes in HFpEF are likely due to pleiotropic impacts, related or not to weight reduction, such as decongestion (mainly due to the reduction of increased plasma volume associated with obesity and epicardial constrain), reverse cardiac remodelling (mainly due to the decrease in epicardial fat and regression of left atrial and LV myopathy, lowering intracardiac pressure) and anti-inflammatory actions, among others. Additional mechanistic studies are needed to confirm these assumptions.
Future Directions
Recently, Sundaram presented the results of PRAISE HFpEF DM.[73] This was a multicentre observational cohort study from 170 hospitals across the US that investigated the effects of GLP-1 RAs on clinical outcomes, a composite of HF hospitalisation and all-cause mortality, in 1,024 obese HFpEF patients compared with 796 controls receiving dipeptidyl peptidase inhibitor/sulphonylurea therapy. The study showed a 20% risk reduction of the primary outcome in the GLP-1 RA arm (HR 0.80; 95% CI [0.68–0.99]). Furthermore, a prespecified subgroup analysis of HF individuals from the SELECT trial evaluating the effects of semaglutide on cardiovascular outcomes in people with overweight or obesity, which included 2,273 HFpEF and 1,347 HFrEF patients, showed that the therapy was associated with a reduction in the HF composite outcome (cardiovascular death or HF hospitalisation/emergency visit) across LVEF, suggesting that GLP-1 RAs may improve outcomes at least in obese HF patients regardless of diabetes status.[74] However, RCTs are still needed to investigate the effects of GLP-1 RAs on HF outcomes, mortality and clinical outcomes in both HFpEF and HFrEF.
Currently, there is an ongoing randomised double-masked placebo-controlled trial, the SUMMIT trial, studying the efficacy and safety of tirzepatide, a dual agonist of glucose-dependent insulinotropic polypeptide and GLP-1 receptors, versus placebo in HFpEF patients with obesity (NCT04847557). The study’s co-primary endpoints are changes in HRQoL and the composite of cardiovascular death and HF events. That study will provide insights into the potential of GLP-1 RAs to modify the disease course in HFpEF patients.
Conclusion
The effects of GLP-1 RAs on HF surrogate endpoints vary in HF patients across the LVEF spectrum. In HFrEF, the administration of GLP-1 RAs did not improve LV systolic function. In HFpEF, the effects of the drugs on cardiac function and structure have not yet been investigated. GLP-1 RA therapy significantly improved both exercise capacity and HRQoL in individuals with obesity-related HFpEF regardless of diabetes status, but had no effect on these parameters in in HFrEF patients. Data from observational studies and subgroup analyses of RCTs show that GLP-1 RAs reduce HF outcomes and mortality in obese HF patients across the LVEF spectrum. However, given the neutral effect of GLP-1 RAs on surrogate HF outcomes in HFrEF and a potentially increased risk of arrhythmias and HF-related hospitalisations, conducting further large-scale trials in the HFrEF cohort seems complicated. Still, it may be relevant to perform pilot studies to address whether the therapy will benefit selected HFrEF cohorts with obesity.
References
- 1.Pfeffer MA, Claggett B, Diaz R et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 2.Marso SP, Daniels GH, Brown-Frandsen K et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marso SP, Bain SC, Consoli A et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez AF, Green JB, Janmohamed S et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–29. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 5.Holman RR, Bethel MA, Mentz RJ et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2017;377:1228–39. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerstein HC, Colhoun HM, Dagenais GR et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–30. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 7.Husain M, Birkenfeld AL, Donsmark M et al. Oral semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2019;381:841–51. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 8.Fudim M, White J, Pagidipati NJ et al. Effect of once-weekly exenatide in patients with type 2 diabetes mellitus with and without heart failure and heart failure-related outcomes: insights from the EXSCEL trial. Circulation. 2019;140:1613–22. doi: 10.1161/CIRCULATIONAHA.119.041659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marx N, Federici M, Schütt K et al. 2023 ESC guidelines for the management of cardiovascular disease in patients with diabetes. Eur Heart J. 2023;44:4043–140. doi: 10.1093/eurheartj/ehad192. [DOI] [PubMed] [Google Scholar]
- 10.Samson SL, Vellanki P, Blonde L et al. American Association of Clinical Endocrinology consensus statement: comprehensive type 2 diabetes management algorithm – 2023 update. Endocr Pract. 2023;29:305–40. doi: 10.1016/j.eprac.2023.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Arnett DK, Blumenthal RS, Albert MA et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140:e563–95. doi: 10.1161/CIR.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao H, Liu Y, Liu M et al. Clinical outcomes with GLP-1 receptor agonists in patients with heart failure: a systematic review and meta-analysis of randomized controlled trials. Drugs. 2023;83:1293–307. doi: 10.1007/s40265-023-01932-2. [DOI] [PubMed] [Google Scholar]
- 13.Kosiborod MN, Petrie MC, Borlaug BA et al. Semaglutide in patients with obesity-related heart failure and type 2 diabetes. N Engl J Med. 2024;390:1394–1407. doi: 10.1056/NEJMoa2313917. [DOI] [PubMed] [Google Scholar]
- 14.Shah M, Vella A. Effects of GLP-1 on appetite and weight. Rev Endocr Metab Disord. 2014;15:181–7. doi: 10.1007/s11154-014-9289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muller TD, Finan B, Bloom SR et al. Glucagon-like peptide 1 (GLP-1). Mol Metab. 2019;30:72–130. doi: 10.1016/j.molmet.2019.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowlands J, Heng J, Newsholme P, Carlessi R. Pleiotropic effects of GLP-1 and analogs on cell signaling, metabolism, and function. Front Endocrinol (Lausanne) 2018;9:672. doi: 10.3389/fendo.2018.00672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dailey MJ, Moran TH. Glucagon-like peptide 1 and appetite. Trends Endocrinol Metab. 2013;24:85–91. doi: 10.1016/j.tem.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haass M, Kitzman DW, Anand IS et al. Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial. Circ Heart Fail. 2011;4:324–31. doi: 10.1161/CIRCHEARTFAILURE.110.959890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vaduganathan M, Ostrominski JW. Glucagon-like peptide-1 receptor agonists in heart failure: STEPping across the ejection fraction divide. J Am Coll Cardiol. 2023;82:2097–100. doi: 10.1016/j.jacc.2023.09.812. [DOI] [PubMed] [Google Scholar]
- 20.Dzhioeva ON, Timofeev YS, Metelskaya VA et al. Role of epicardial adipose tissue in the pathogenesis of chronic inflammation in heart failure with preserved ejection fraction. Cardiovasc Ther Prevent. 2024;23:3928. doi: 10.15829/1728-8800-2024-3928. [in Russian]. [DOI] [Google Scholar]
- 21.Patel V B, Shah S, Verma S, Oudit GY. Epicardial adipose tissue as a metabolic transducer: role in heart failure and coronary artery disease. Heart Fail Rev. 2017;22:889–902. doi: 10.1007/s10741-017-9644-1. [DOI] [PubMed] [Google Scholar]
- 22.Neeland IJ, Gupta S, Ayers CR et al. Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging. 2013;6:800–7. doi: 10.1161/CIRCIMAGING.113.000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zarich SW, Kowalchuk GJ, McGuire MP et al. Left ventricular filling abnormalities in asymptomatic morbid obesity. Am J Cardiol. 1991;68:377–81. doi: 10.1016/0002-9149(91)90835-9. [DOI] [PubMed] [Google Scholar]
- 24.Obokata M, Reddy YNV, Pislaru SV et al. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136:6–19. doi: 10.1161/circulationaha.116.026807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorimachi H, Omote K, Omar M et al. Sex and central obesity in heart failure with preserved ejection fraction. Eur J Heart Fail. 2022;24:1359–70. doi: 10.1002/ejhf.2563. [DOI] [PubMed] [Google Scholar]
- 26.Koepp K E, Obokata M, Reddy YNV et al. Hemodynamic and functional impact of epicardial adipose tissue in heart failure with preserved ejection fraction. JACC Heart Fail. 2020;8:657–66. doi: 10.1016/j.jchf.2020.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ussher JR, Drucker DJ. Glucagon-like peptide 1 receptor agonists: cardiovascular benefits and mechanisms of action. Nat Rev Cardiol. 2023;20:463–74. doi: 10.1038/s41569-023-00849-3. [DOI] [PubMed] [Google Scholar]
- 28.Tate M, Chong A, Robinson E et al. Selective targeting of glucagon-like peptide-1 signalling as a novel therapeutic approach for cardiovascular disease in diabetes. Br J Pharmacol. 2015;172:721–36. doi: 10.1111/bph.12943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikolaidis LA, Mankad S, Sokos GG et al. Effects of glucagon-like peptide-1 in patients wit h acute myocardial infarction and left ventricular dysfunction after successful reperfusion. Circulation. 2004;109:962–5. doi: 10.1161/01.CIR.0000120505.91348.58. [DOI] [PubMed] [Google Scholar]
- 30.Woo JS, Kim W, Ha SJ et al. Cardioprotective effects of exenatide in patients with ST-segment-elevation myocardial infarction undergoing primary percutaneous coronary intervention: results of exenatide myocardial protection in revascularization study. Arterioscler Thromb Vasc Biol. 2013;33:2252–60. doi: 10.1161/atvbaha.113.301586. [DOI] [PubMed] [Google Scholar]
- 31.Lønborg J, Vejlstrup N, Kelbǣk H et al. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 2012;33:1491–9. doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- 32.Lønborg J, Vejlstrup N, Kelbǣk H et al. Impact of acute hyperglycemia on myocardial infarct size, area at risk, and salvage in patients with STEMI and the association with exenatide treatment: results from a randomized study. Diabetes. 2014;63:2474–85. doi: 10.2337/db13-1849. [DOI] [PubMed] [Google Scholar]
- 33.Chen WR, Hu SY, Chen YD et al. Effects of liraglutide on left ventricular function in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am Heart J. 2015;170:845–54. doi: 10.1016/j.ahj.2015.07.014. [DOI] [PubMed] [Google Scholar]
- 34.Noyan-Ashraf MH, Momen MA, Ban K et al. GLP-1R agonist liraglutide activates cytoprotective pathways and improves outcomes after experimental myocardial infarction in mice. Diabetes. 2009;58:975–83. doi: 10.2337/db08-1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong Z, Chen K, Zhao Y, Xia S. Effects of liraglutide on left ventricular function: a meta-analysis of randomized, placebo-controlled trials. Int J Endocrinol. 2021;2021:9993229. doi: 10.1155/2021/9993229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bizino MB, Jazet IM, Westenberg JJM et al. Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial. Cardiovasc Diabetol. 2019;18:55. doi: 10.1186/s12933-019-0857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiramatsu T, Asano Y, Mabuchi M et al. Liraglutide relieves cardiac dilated function than DPP-4 inhibitors. Eur J Clin Investig. 2018;48:e13007. doi: 10.1111/eci.13007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huixing L, Di F, Daoquan P. Effect of glucagon-like peptide-1 receptor agonists on prognosis of heart failure and cardiac function: a systematic review and meta-analysis of randomized controlled trials. Clin Ther. 2023;45:17–30. doi: 10.1016/j.clinthera.2022.12.006. [DOI] [PubMed] [Google Scholar]
- 39.Sokos GG, Nikolaidis LA, Mankad S et al. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–9. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 40.Lepore JJ, Olson E, Demopoulos L et al. Effects of the novel long-acting GLP-1 agonist, albiglutide, on cardiac function, cardiac metabolism, and exercise capacity in patients with chronic heart failure and reduced ejection fraction. JACC Heart Fail. 2016;4:559–66. doi: 10.1016/j.jchf.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Margulies KB, Hernandez AF, Redfield MM et al. Effects of liraglutide on clinical stability among patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2016;316:500–8. doi: 10.1001/jama.2016.10260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferreira JP, Saraiva F, Sharma A et al. Glucagon-like peptide 1 receptor agonists in patients with type 2 diabetes with and without chronic heart failure: a meta-analysis of randomized placebo-controlled outcome trials. Diabetes Obes Metab. 2023;25:1495–502. doi: 10.1111/dom.14997. [DOI] [PubMed] [Google Scholar]
- 43.Jorsal A, Kistorp C, Holmager P et al. Effect of liraglutide, a glucagon-like peptide-1 analogue, on left ventricular function in stable chronic heart failure patients with and without diabetes (LIVE) – a multicentre, double-blind, randomised, placebo-controlled trial. Eur J Heart Fail. 2017;19:69–77. doi: 10.1002/ejhf.657. [DOI] [PubMed] [Google Scholar]
- 44.Neves JS, Packer M, Ferreira JP. Increased risk of heart failure hospitalization with GLP-1 receptor agonists in patients with reduced ejection fraction: a meta-analysis of the EXSCEL and FIGHT trials. J Card Fail. 2023;29:1107–9. doi: 10.1016/j.cardfail.2023.03.017. [DOI] [PubMed] [Google Scholar]
- 45.Kosiborod MN, Abildstrøm SZ, Borlaug BA et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023;389:1069–84. doi: 10.1056/NEJMoa2306963. [DOI] [PubMed] [Google Scholar]
- 46.Kambic T, Lavie CJ, Eijsvogels TMH. Seeking synergy for novel weight-and glucose-lowering pharmacotherapy and exercise training in heart failure patients with preserved ejection fraction. Eur Heart J. 2024;45:861–3. doi: 10.1093/eurheartj/ehad856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Butler J, Shah SJ, Petrie MC et al. Semaglutide versus placebo in people with obesity-related heart failure with preserved ejection fraction: a pooled analysis of the STEP-HFpEF and STEP-HFpEF DM randomized trials. Lancet. 2024;403:1635–48. doi: 10.1016/S0140-6736(24)00469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cimino G, Vaduganathan M, Lombardi C M et al. Obesity, heart failure with preserved ejection fraction, and the role of glucagon-like peptide-1 receptor agonists. ESC Heart Fail. 2024;11:649–61. doi: 10.1002/ehf2.14560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaduganathan M, Fonarow GC, Greene S J et al. Health-related quality of life in comorbid heart failure with reduced ejection fraction and diabetes mellitus. J Am Coll Cardiol. 2019;74:3176–8. doi: 10.1016/j.jacc.2019.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anker SD, Agewall S, Borggrefe M et al. The importance of patient-reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J. 2014;35:2001–9. doi: 10.1093/eurheartj/ehu205. [DOI] [PubMed] [Google Scholar]
- 51.Butler J, Anker SD, Filippatos G et al. Empagliflozin and health-related quality of life outcomes in patients with heart failure with reduced ejection fraction: the EMPEROR-Reduced trial. Eur Heart J. 2021;42:1203–12. doi: 10.1093/eurheartj/ehaa1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kosiborod MN, Jhund PS, Docherty KF et al. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: results from the DAPA-HF trial. Circulation. 2020;141:90–9. doi: 10.1161/circulationaha.119.044138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reddy YNV, Rikhi A, Obokata M et al. Quality of life in heart failure with preserved ejection fraction: importance of obesity, functional capacity, and physical inactivity. Eur J Heart Fail. 2020;22:1009–18. doi: 10.1002/ejhf.1788. [DOI] [PubMed] [Google Scholar]
- 54.Sato R, von Haehling S. Revisiting t he obesity paradox in heart failure: what is the best anthropometric index to gauge obesity? Eur Heart J. 2023;44:1154–6. doi: 10.1093/eurheartj/ehad079. [DOI] [PubMed] [Google Scholar]
- 55.Yusuf S, Hawken S, Ounpuu S et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366:1640–9. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 56.Mann DL, Givertz MM, Vader JM et al. Effect of treatment with sacubitril/valsartan in patients with advanced heart failure and reduced ejection fraction: a randomized clinical trial. JAMA Cardiol. 2022;7:17–25. doi: 10.1001/jamacardio.2021.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Borlaug BA, Kitzman DW, Davies MJ et al. Semaglutide in HFpEF across obesity class and by body weight reduction: a prespecified analysis of the STEP-HFpEF trial. Nat Med. 2023;29:2358–65. doi: 10.1038/s41591-023-02526-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Butler J, Abildstrøm SZ, Borlaug BA et al. Semaglutide in patients with obesity and heart failure across mildly reduced or preserved ejection fraction. J Am Coll Cardiol. 2023;82:2087–96. doi: 10.1016/j.jacc.2023.09.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bekfani T, Nisser J, Derlien S et al. Psychos ocial factors, mental health, and coordination capacity in patients with heart failure with preserved ejection fraction compared with heart failure with reduced ejection fraction. ESC Heart Fail. 2021;8:3268–78. doi: 10.1002/ehf2.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meissner WG, Remy P, Giordana C et al. Trial of lixisenatide in early Parkinson’s disease. N Engl J Med. 2024;390:1176–85. doi: 10.1056/NEJMoa2312323. [DOI] [PubMed] [Google Scholar]
- 61.Kreiner FF, von Scholten BJ, Kurtzhals P, Gough SCL. Glucagon-like peptide-1 receptor agonists to expand the healthy lifespan: current and future potentials. Aging Cell. 2023;22:e13818. doi: 10.1111/acel.13818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sass MR, Danielsen AA, Köhler-Forsberg O Effect of the GLP-1 receptor agonist semaglutide on metabolic disturbances in clozapine-treated or olanzapine-treated patients with a schizophrenia spectrum disorder: study protocol of a placebo-controlled, randomised clinical trial (SemaPsychiatry). 13. BMJ Open. 2023. p. e068652. [DOI] [PMC free article] [PubMed]
- 63.Prasad F, De R, Korann V et al. Semaglutide for the treatment of antipsychotic-associated weight gain in patients not responding to metformin – a case series. Ther Adv Psychopharmacol. 2023;13:20451253231165169. doi: 10.1177/20451253231165169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Billings LK, Handelsman Y, Heile M et al. Health-related quality of life assessments with once-weekly glucagon-like peptide-1 receptor agonists in type 2 diabetes mellitus. J Manag Care Spec Pharm. 2018;24:S30–41. doi: 10.18553/jmcp.2018.24.9-a.s30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cooper DH, Ramachandra R, Ceban F et al. Glucagon-like peptide 1 (GLP-1) receptor agonists as a protective factor for incident depression in patients with diabetes mellitus: a systematic review. J Psychiatr Res. 2023;164:80–9. doi: 10.1016/j.jpsychires.2023.05.041. [DOI] [PubMed] [Google Scholar]
- 66.McIntyre RS, Mansur RB, Rosenblat JD, Kwan ATH. The association between glucagon-like peptide-1 receptor agonists (GLP-1 RAs) and suicidality: reports to the Food and Drug Administration Adverse Event Reporting System (FAERS). Expert Opin Drug Saf. 2024;23:47–55. doi: 10.1080/14740338.2023.2295397. [DOI] [PubMed] [Google Scholar]
- 67.Tobaiqy M, Elkout H. Psychiatric adverse events associated with semaglutide, liraglutide and tirzepatide: a pharmacovigilance analysis of individual case safety reports submitted to the EudraVigilance database. Int J Clin Pharm. 2024;46:488–95. doi: 10.1007/s11096-023-01694-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen W, Cai P, Zou W, Fu Z. Psychiatric adverse events associated with GLP-1 receptor agonists: a real-world pharmacovigilance study based on the FDA Adverse Event Reporting System database. Front Endocrinol (Lausanne) 2024;15:1330936. doi: 10.3389/fendo.2024.1330936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alonso A, Bahnson JL, Gaussoin SA et al. Effect of an intensive lifestyle intervention on atrial fibrillation risk in individuals with type 2 diabetes: the Look AHEAD randomized trial. Am Heart J. 2015;170:770–7e5. doi: 10.1016/j.ahj.2015.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee VYJ, Houston L, Perkovic A et al. The effect of weight loss through lifestyle interventions in patients with heart failure with preserved ejection fraction – a systematic review and meta-analysis of randomised controlled trials. Heart Lung Circ. 2024;33:197–208. doi: 10.1016/j.hlc.2023.11.022. [DOI] [PubMed] [Google Scholar]
- 71.Peck KH, Dulay MS, Hameed S Intentional weight loss in overweight and obese patients with heart failure: a systematic review. Eur J Heart Fail. 2024. epub ahead of press. [DOI] [PubMed]
- 72.Petrie M. Semaglutide 2.4 mg and NTproBNP in obesity-related HFpEF: insights from the STEP-HFpEF programme. Presented at Heart Failure 2024, Lisbon, Portugal, 11–14 May 2024. https://esc365.escardio.org/presentation/283481 (accessed 22 July 2024)
- 73.Sundaram V. Glucagon-like peptide-1 receptor agonist in obese heart failure with preserved ejection fraction and type 2 diabetes mellitus. Presented at Heart Failure 2024, Lisbon, 11–14 May 2024. https://esc365.escardio.org/presentation/283465 (accessed 22 July 2024)
- 74.Deanfield J. Semaglutide and cardiovascular outcomes in patients with overweight or obesity and heart failure: a pre-specified analysis from the SELECT trial. Presented at Heart Failure 2024, Lisbon, Portugal, 11–14 May 2024. https://esc365.escardio.org/presentation/283493 (accessed 22 July 2024)


