Abstract
Background
Osteoarthritis (OA) and rheumatoid arthritis (RA) are often difficult to distinguish in the early stage of the disease. The purpose of this study was to explore the similarities and differences between the two diseases through Mendelian randomization (MR) and transcriptome analysis.
Methods
We first performed a correlation analysis of phenotypic data from genome-wide association studies (GWAS) of OA and RA. Then, we performed functional and pathway enrichment of differentially expressed genes in OA, RA, and normal patients. The infiltration of immune cells in arthritis was analyzed according to gene expression. Finally, MR analysis was performed with inflammatory cytokines and immune cells as exposures and arthritis as the outcome. The same and different key cytokines and immune cells were obtained by the two analysis methods.
Results
GWAS indicated that there was a genetic correlation between OA and RA. The common function of OA and RA is enriched in their response to cytokines, while the difference is enriched in lymphocyte activation. T cells are the main immune cells that differentiate between OA and RA. MR analysis further revealed that OA is associated with more protective cytokines, and most of the cytokines in RA are pathogenic. In addition, CCR7 on naive CD4 + T cell was positively correlated with OA. SSC-A on CD4 + T cell was negatively correlated with RA, while HLA DR on CD33- HLA DR + was positively correlated with RA.
Conclusion
Our study demonstrated the similarities and differences of immune inflammation between OA and RA, allowing us to better understand these two diseases.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12967-024-05643-4.
Keywords: Osteoarthritis (OA), Rheumatoid arthritis (RA), Inflammatory cytokines, Immune cells, Mendelian randomization
Introduction
Osteoarthritis (OA) and rheumatoid arthritis (RA) are the two most common joint diseases that cause pain and limited mobility in patients. RA and OA have many similarities, both involving joint cartilage degeneration, synovitis, and osteophyte hyperplasia [1]. However, the pathogenesis of these two diseases is different and remains largely unknown. Most inflammatory markers in patients with OA are normal or mildly elevated, while inflammatory markers such as rheumatoid factor and anti-cyclic citrullinated peptide antibodies can be significantly elevated in patients with RA. For a long time, the pathogenesis of OA has focused on the theory of mechanical stress [2]. With the progress of immunological research, the concept of OA has been fundamentally changed [3]. Chronic inflammation caused by various inflammatory factors and immune cells plays a central role in the pathogenesis [4]. The abnormal production of inflammatory factors such as tumor necrosis factor (TNF) and interleukin (IL) not only directly affect articular cartilage, but also regulates the metabolism of other cytokines in articular cartilage [5, 6]. Studies have shown that a variety of immune cells are involved in the occurrence and development of OA. For example, tryptase produced by mast cells can induce chondrocyte inflammation and apoptosis [7]. Some scholars even suggested that OA may be related to innate immune dysfunction [8].
RA is a chronic systemic autoimmune disease characterized by joint synovitis. However, the exact cause of RA is not yet clear, and factors such as environment, infection, genetics are closely related [9]. Among them, it is widely recognized that synovium plays an important role in joint destruction by secreting various immune inflammatory mediators such as proteases and cytokines [10]. The inflammatory factor TNF -α plays a central role in rheumatoid arthritis, mediating synovitis and cartilage destruction, and also accelerating cartilage destruction in osteoarthritis [11]. Cytokines such as IL-1β and IL-6 also accelerate the breakdown of collagen fibers in joints, stimulate the production of inflammatory substances, and promote the occurrence of OA and RA [12, 13]. In addition to cytokines, OA and RA also have a large amount of similar immune cell infiltration. For example, macrophages can secrete a large number of cytokines, chemokines and degrading enzymes, which are positively correlated with the severity of the arthritis [14]. Activated CD4 + T cells can initiate specific immune responses, leading to joint inflammation and bone destruction [15]. Given the correlation between the two diseases and immune inflammation, studying from the perspectives of cytokines and immune cells is of great significance for a deeper understanding of the similarities and differences between the two types of arthritis.
In Mendelian randomization (MR), genetic variation is used as an instrumental variable to represent the causal relationship between exposure and outcomes of interest. Studying causal relationships at the genetic level can reduce bias. Previous MR studies have investigated the relationship between inflammatory cytokines and OA, but we have included more inflammatory cytokines [16]. And combine MR and transcriptome analysis methods to study the relationship between cytokines, immune cells and arthritis. We are the first to use this method to explore the similarities and differences in immune inflammation between OA and RA. This may provide new insights into the role of immune inflammation in OA and RA.
Materials and methods
Transcriptome analysis
Dataset download and identification of DEGs
We used “osteoarthritis” or “rheumatoid arthritis” as keywords to search for related datasets in GEO database (https://www.ncbi.nlm.nih.gov/geo/). The datasets for homo sapiens microarray analysis of OA, RA and normal controls with complete data were selected. Differential analysis was performed by microarray data linear model (limma) software package (version 3.40.6) to obtain differential expression genes (DEGs) between OA and control group, RA and control group, OA and RA. The false discovery rate (FDR) less than 0.01 and | log2-fold change (FC) | greater than 2 were considered to be statistically significant. The results of DEGs are presented by volcano map and heatmap.
Principal component and enrichment analysis
The R software package “stats” (version 3.6.0) was used for principal component analysis (PCA) clustering analysis of gene matrix data. The two-dimensional PCA clustering results were visualized by “prcomp” function. The DEGs were analyzed by Gene Ontology (GO) function and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis. Specifically, we used the annotations in the R package “org.Hs.eg.db” (version 3.1.0) as the background to map genes into the background set. The package “clusterprofiler” (version 3.14.3) was used for enrichment analysis to obtain the results of gene set enrichment. p < 0.05 is considered to be statistically significant. The enrichment pathways and functions were visualized by ggplot2 package.
Evaluation of immune cells
We used two methods to analyze the immune cell composition in two types of arthritis. CIBERSORT infers the relative proportion of immune cell subsets in each sample based on standardized gene expression. Upload data to CIBERSORT (https://cibersort.stanford.edu/). The website defined infiltrating immune cells using LM22 characteristic matrix, and only the data with p value < 0.05 were retained. The microenvironment cell population counter (MCP-counter) method estimates the content of different cell types in samples based on the gene expression characteristics of cells. Violin diagrams are used for visualization. Paired t-test and Mann-Whitney U test were used to assess the significant differences between OA and RA.
MR
Study design and data source
The phenotypic data of OA and RA were obtained from the ATLAS database (https://atlas.ctglab.nl/), and the correlation between the two phenotypes was analyzed using the database [17]. The website uses linkage disequilibrium score regression (LDSC) to first calculate the heritability of two arthritis traits, and then calculate the genetic correlation between them. The significant genetic correlation after Bonferroni correction was visualized in the heatmap (p < 0.05). The summary statistical data of OA was obtained from the IEU OpenGWAS (https://gwas.mrcieu.ac.uk/), including 39,427 European individuals with hip or knee OA (ebi-a-GCST007092), 24,955 knee OA (ebi-a-GCST007090), and 15,704 hip OA (ebi-a-GCST007091). The summary statistical data for RA including 6236 European individuals with RA (finn-b-M13_RHEUMA), 4596 serum positive RA (finn-b-RHEUMA_SEROPOS), and 1937 serum negative RA (finn-b-RHEUMA_SERONEG). Then we conducted MR analysis of immune inflammation on hip, knee OA, and serum positive and negative RA, respectively. The selection of inflammatory cytokines and immune cell instruments was publicly obtained based on previously published MR analysis [18, 19]. Inflammatory cytokines include a total of 91 plasma proteins, such as chemokines, interleukin, fibroblast growth factor, monocyte chemoattractant protein, tumor necrosis factor, etc. (accession numbers GCST90274758 to GCST90274848). Immune cells include 731 phenotypes, including absolute cell count (n = 118), median fluorescence intensity reflecting surface antigen levels (n = 389), morphological parameters (n = 32), and relative cell count (n = 192). Specifically, it contains B cells, T cells, natural killer cells, monocytes, myeloid cells, etc. (accession numbers GCST90001391 to GCST90002121). Finally, we further screened out the same and different inflammatory cytokines and immune cells in OA and RA, and established the connection between cytokines and immune cells.
Defining genetic instruments
The genetic instruments selected by MR needs to meet three important prerequisites, namely, the genetic tool is highly correlated with exposure factors (relevance), but not with confounding factors (independence), and can only affect the results through exposure (exclusion-restriction). Based on the above three assumptions, obtain genetic variations strongly (p < 5 × 10–8) associated with arthritis and immune inflammatory factors. And SNPs in strong linkage disequilibrium (r2 < 0.001) were removed to avoid biased results. The physical distance between genes kb = 10,000. To ensure a strong correlation with the exposure, we removed SNPs with low F statistic < 10. We searched for the relationship between SNPs and phenotypes on the website (http://www.phenoscanner.medschl.cam.ac.uk/), and then removed SNPs related to confounding factors. The included SNPs were statistically analyzed by various methods to infer the causal relationship between arthritis and immune inflammatory factors.
Statistical analysis
All analyses were carried out using the R packages in R version 4.1.3, with a statistically significant p < 0.05. We mainly use the " TwoSampleMR " package to evaluate the causal relationship between immune inflammatory factors and arthritis by IVW, MR Egger regression, and weighted median methods. The IVW method, as the main statistical method, weights each instrumental variable by the reciprocal of variance. If there is no intercept, the weighted average of the effective estimates of all instrumental variables is calculated. To exclude the influence of level pleiotropy, the bias between the intercept term and zero in MR Egger regression was tested. The weighted median is the median of the distribution function obtained by ranking the SNP effect values of all individuals according to their weights. When at least 50% of the information comes from effective instrumental variables, robust estimates can be obtained. The leave-one-out method evaluate the sensitivity of results. By removing SNPs one by one and calculating the effects of merging other units, the effect of a single SNP on outcomes is clarified, and the degree of impact and stability are evaluated. In addition, we also used scatter plots and funnel plots. The scatter plot indicates whether the results are affected by outliers. The funnel plot indicates whether the results are robust or heterogeneous.
Results
Genetic correlation of OA and RA
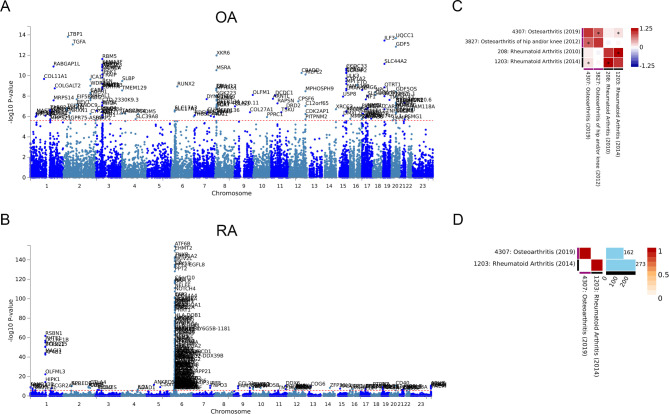
GWAS analysis of the two types of arthritis was carried out in the ATLAS database to identify whether there are shared genetic factors. For OA, 162 independent SNP loci were identified (Fig. 1A). Similarly, 273 independent SNP loci were identified in RA (Fig. 1B). The specific loci are listed in Supplementary Table 1. Comparing the datasets of OA and RA with the multiple GWAS, we found that there was an association between the gene loci of the two diseases (p < 0.05). This indicates that there may be a genetic correlation between OA and RA (Fig. 1C). However, the GWAS results of the two diseases did not find overlapping loci (Fig. 1D).
Fig. 1.
Genetic correlation of OA and RA. (A) Manhattan plot of OA specific 162 SNP loci. (B) Manhattan plot of RA specific 273 SNP loci. (C) Genetic correlation analysis of OA and RA after Bonferroni correction. (D) Overlapping genes between OA and RA
Identification of common and differential genes between OA and RA
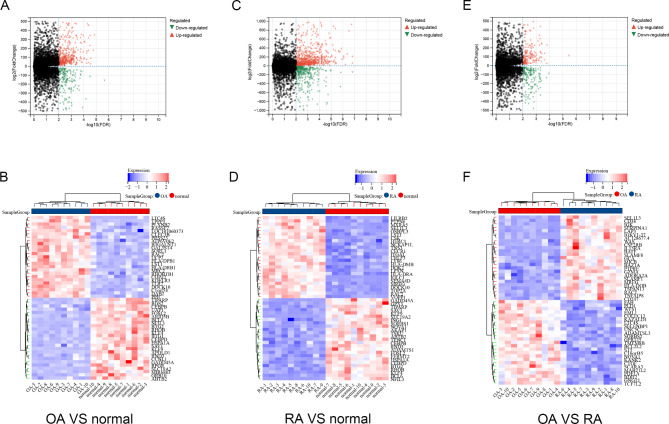
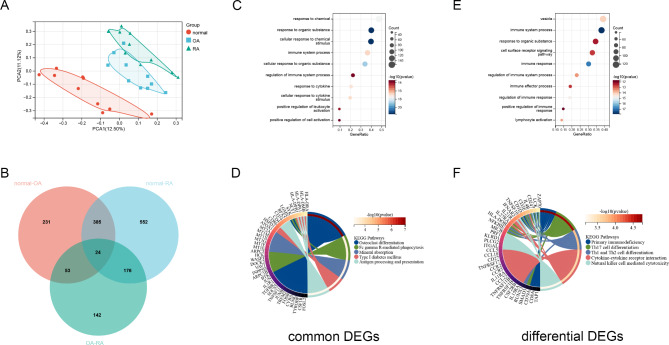
To further investigate the similarities and differences between OA and RA, we used transcriptome data to screen for common and different genes associated with both types of arthritis. The dataset GSE55235 contained 10 normal control samples, 10 OA samples, and 10 RA samples. After screening DEGs with FDR < 0.01 and | log2 FC | > 2, 693 genes were found in OA and normal controls (Fig. 2A-B). 1137 DEGs were found in RA and normal controls, and 395 DEGs were found in OA and RA (Fig. 2C-F). PCA cluster analysis of the dataset showed that there were significant differences between OA, RA and normal controls, while there were similarities between OA and RA (Fig. 3A). The overlap analysis of DEGs showed that 385 DEGs were common DEGs between OA, RA and normal controls. In the Venn diagram, after excluding 24 identical genes, 371 genes were DEGs that differed between OA and RA (Fig. 3B).
Fig. 2.
Differentially expressed genes identification. (A) Volcano map of DEGs between OA and normal controls. (B) Heatmap of top 50 DEGs between OA and normal control. (C) Volcano map of DEGs between RA and normal controls. (D) Heatmap of top 50 DEGs between RA and normal control. (E) Volcano map of DEGs between RA and OA. (F) Heatmap of top 50 DEGs between RA and OA
Fig. 3.
Enrichment analysis between RA and OA. (A) PCA clustering analysis of OA, RA and normal control samples. (B) Venn diagram screening for DEGs that are the common or different between OA and RA. (C) GO functional enrichment analysis of common DEGs between OA and RA. (D) KEGG pathway of analysis of common DEGs between OA and RA. (E) GO functional enrichment analysis of differential DEGs between OA and RA. (F) KEGG pathway of analysis of differential DEGs between OA and RA
Enrichment Analysis of common and differential genes between OA and RA
Enrichment analysis was performed on 385 common DEGs and 371 differential DEGs. GO enrichment analysis showed that the common functions of OA and RA focused on the response to chemical stimuli, the response to cytokine, immune system process. KEGG pathway analysis showed that osteoclast differentiation, mineral absorption and type 1 diabetes mellitus were statistically significant (Fig. 3C-D). The significant differences in functionality between OA and RA are mainly concentrated in the immune system process and lymphocyte activation. In addition, the top enriched pathways include Th17 cell differentiation, Th1 and Th2 cell differentiation and Natural killer cell mediated cytotoxicity (Fig. 3E-F).
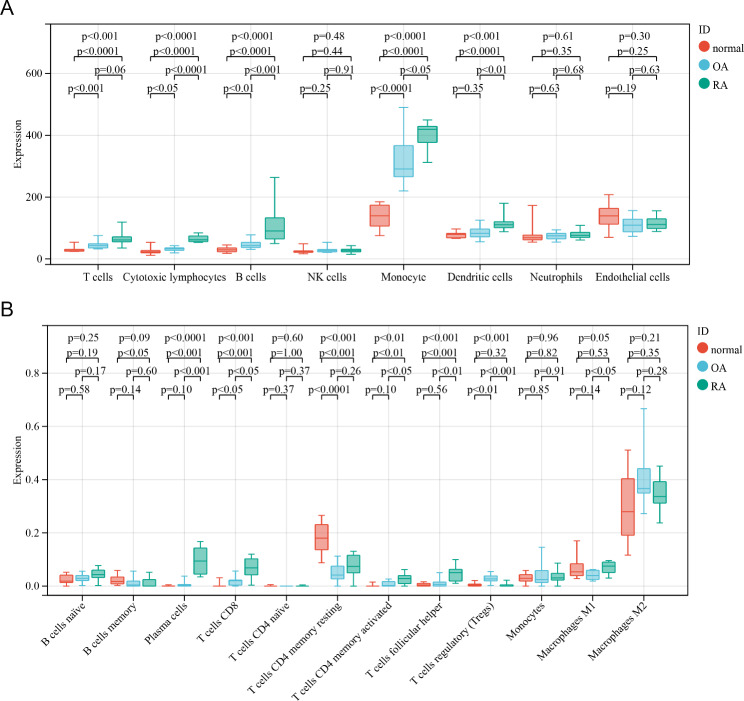
Similar and different immune cell infiltration in OA and RA
In order to further explore the similarities and differences of immune cells between the two diseases, we used two algorithms. The MCP algorithm shows that the number of T cells, cytotoxic lymphocytes, B cells, and monocytes is the lowest in the normal control group, with moderate OA and the highest RA (Fig. 4A). Further validation using the CIBERSORT algorithm revealed that CD8 + T cells and Tregs cells exhibited the same trend. Violin diagram showed that compared with OA, the RA group had more plasma cells, activated CD4 + T cells, and helper T cells. However, compared to the normal control, although the OA group had a higher number of these cells, the difference was not statistically significant (Fig. 4B).
Fig. 4.
Evaluation of immune cells obtained from transcriptome data using different algorithms. (A) The number of immune cells in OA, RA and normal control group obtained by the MCP algorithm. (B) The number of immune cells in OA, RA and normal control group obtained by the CIBERSORT algorithm
Identification of common and differential cytokines between OA and RA using MR analysis
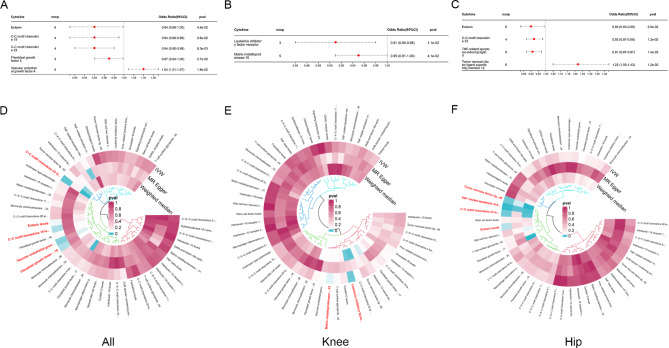
Transcriptome analysis showed that cytokines play a central role in arthritis, and we further analyzed the specific cytokines in arthritis using MR. As shown in Fig. 5, we determined the SNPs of total OA, knee OA, and hip OA, and identified inflammation cytokines associated with three types of OA using the IVW method. Five inflammatory cytokines have a causal relationship with total OA (Eotaxin, C-C motif chemokine 19, C-C motif chemokine 23, Fibroblast growth factor 5 and Vascular endothelial growth factor A), two inflammatory cytokines associated with knee OA (Leukemia inhibitory factor receptor, Matrix metalloproteinase-10), and four inflammatory cytokines associated with hip OA (TNF-related apoptosis-inducing ligand, Eotaxin, C-C motif chemokine 23, Tumor necrosis factor ligand superfamily member 12). Among them, only Vascular endothelial growth factor A and Tumor Necrosis factor ligand superfamily member 12 are risk inflammatory cytokines, while the rest are protective cytokines.
Fig. 5.
Using MR analysis to screen for inflammatory cytokines that have a statistically causal relationship with OA. (A-C) Forest plot of the causal relationships between total OA, knee OA, hip OA and significant inflammatory cytokines (OR < 1 indicates protection, OR > 1 indicates pathogenicity). (D-F) Circular map of causal relationships between total OA, knee OA, hip OA and all inflammatory cytokines (Red represents statistically significant)
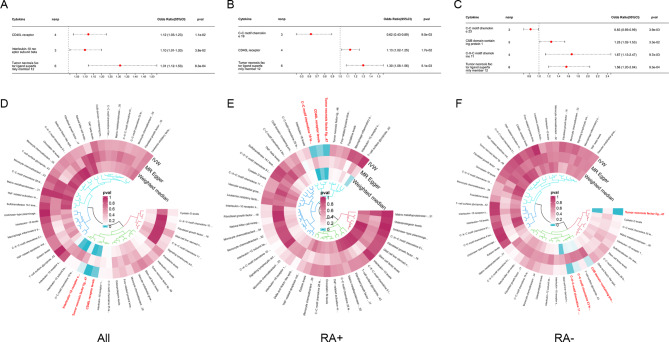
In Fig. 6, we identified inflammatory cytokines associated with total RA, serum positive RA, and serum negative RA. Three inflammatory cytokines increased the risk of total RA (Tumor necrosis factor ligand superfamily member 12, CD40L receptor, Interleukin-10 receptor subunit beta), two increased the risk of serum positive RA (Tumor necrosis factor ligand superfamily member 12, CD40L receptor), and three increased risk of serum negative RA (Tumor necrosis factor ligand superfamily member 12, CUB domain-containing protein 1, C-X-C motif chemokine 11). C-C motif chemokine 19 and C-C motif chemokine 23 are common protective inflammatory cytokines in OA and RA, Tumor Necrosis factor ligand superfamily member 12 is the same pathogenic inflammatory cytokine.
Fig. 6.
Using MR analysis to screen for inflammatory cytokines that have a statistically causal relationship with RA. (A-C) Forest plot of the causal relationships between total RA, serum positive RA, serum negative RA and significant inflammatory cytokines (OR < 1 indicates protection, OR > 1 indicates pathogenicity). (D-F) Circular map of causal relationships between total RA, serum positive RA, serum negative RA and all inflammatory cytokines (Red represents statistically significant)
Identification of common and differential immune cells between OA and RA using MR analysis
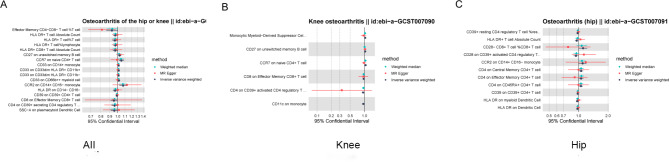
To investigate the causal effects of immune cells on OA and RA, we conducted the same grouping and MR analysis. A total of 28 immune cell phenotypes were identified in OA, including 16 protective phenotypes and 12 pathogenic phenotypes (Fig. 7). Total OA and knee OA have two identical pathogenic immune cells (CD27 on unswitched memory B cell, CCR7 on naive CD4 + T cell), while total OA and hip OA have one identical pathogenic immune cell (CCR2 on CD14 + CD16- monocyte).
Fig. 7.
Using MR analysis to screen for immune cells that have a statistically causal relationship with OA. (A-C) Forest plot of the causal relationships between total OA, knee OA, hip OA and significant immune cells
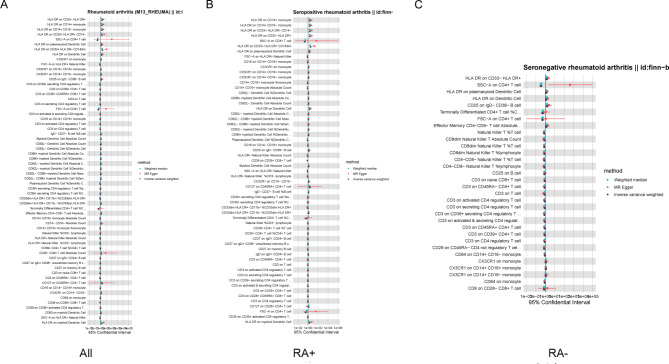
76 immune cell phenotypes were identified in RA, including 45 protective phenotypes and 31 pathogenic phenotypes with significant significance (Fig. 8). After adjusting based on the FDR method (FDR < 0.05), 15 significant immune cell phenotypes were found. Serum positive RA has the most immune cell phenotypes, 5 protective phenotypes, and 9 pathogenic phenotypes. SSC-A on CD4 + T cell is a common protective immune cell phenotype among the three types of RA, while HLA DR on CD33- HLA DR + is a common pathogenic immune cell (Fig. 7). The results of MR Egger regression and weighted median method also demonstrate the robustness of the causal correlation obtained. In addition, HLA DR on Dendritic Cell and HLA DR on myeloid Dendritic Cell are common immune cell phenotypes in OA and RA, but they are protective in OA and pathogenic in RA. All immune cell phenotypes with statistical significance are listed in the Supplementary Tables 2–4.
Fig. 8.
Using MR analysis to screen for immune cells that have a statistically causal relationship with RA. (A-C) Forest plot of the causal relationships between total RA, serum positive RA, serum negative RA and significant immune cells
Discussion
OA has long been believed as a degenerative joint disease caused by aging and biomechanical changes. However, many patients show symptoms of inflammatory arthritis with redness, swelling, heat and pain [20]. In the early MRI examination, sometimes joint effusion and synovial hyperplasia were detected [21]. The increase of C-reactive protein and erythrocyte sedimentation can also be detected in the blood of patients with early OA [22]. Sometimes it is very hard to distinguish from RA. From our research, it can also be seen that OA and RA share some common physiological processes, such as response to cytokines and immune system processes. The difference between the two types of arthritis is that RA has a more severe immune response and lymphocyte activation than OA, with T lymphocytes being the main component. These evidences suggest that OA is not only a degenerative disease, but immune inflammatory mechanisms also play a huge role [23]. Previous studies only focused on a single component of immune inflammation, and studying the mechanisms of arthritis from different perspectives of inflammatory cytokines and immune cells is more comprehensive and meaningful. To our knowledge, this is the first study to investigate GWAS data about OA and RA based on immune inflammation.
Transcriptome analysis shows that not only RA, but also OA is associated with susceptibility genes such as HLA, indicating a correlation between OA and genetics. GWAS data also shows a certain genetic correlation between OA and RA, but OA is associated with more protective cytokines, and the cytokines in RA are mostly pathogenic. This indicates that the inflammatory microenvironment within the joint not only damages the joint, but also has a killing effect on harmful substances. In our study, multiple chemokines were identified to be associated with OA and RA. C-C motif chemokine 19 (CCL19) and C-C motif chemokine 23 (CCL23) are common protective cytokines in OA and RA. Eotaxin, also known as CCL11, has a negative causal relationship with OA (OR = 0.94, 95%CI 0.89–1.00, p = 0.045). C-X-C motif chemokine 11 (CXCL11) has a positive causal relationship with RA(OR = 1.67, 95%CI 1.13–2.47, p = 0.0097). Chemokines are important signaling substances that mediate inflammatory responses, leading to targeted migration and aggregation of immune cells to inflammatory sites, releasing inflammatory factors [24]. Studies have shown that the CCL2/CCR2 signaling axis is involved in mediating monocyte transport, promoting inflammation, and causing tissue damage during the OA process. By inhibiting the synthesis of CCL2 or its binding to CCR2, the accumulation of macrophages in the synovial membrane can be partially reduced to prevent the development of OA in mice [25]. However, chemokines not only have the effect of promoting inflammation, but also have natural antagonistic effects [26]. For example, CCL11 is an antagonist of CCR2, which indicates its protective effect on OA and indirectly verifies the correctness of our research results [27]. The relationship between CXCL11 and RA has not yet been elucidated, but recent studies have shown that pyroptosis and injury of chondrocytes in OA upregulate the expression of CXCL1128. In addition, tumor Necrosis factor ligand superfamily member 12 (TNFSF12) is the same pathogenic inflammatory cytokine in OA and RA. CD40 is also a member of the tumor necrosis factor receptor superfamily (TNFSF5), and CD40L receptors are positively correlated with total RA and serum positive RA. TNF is a pro-inflammatory cytokine produced by cells such as macrophages and monocytes, and is considered a key factor in the occurrence and persistence of arthritis. It promotes the production of enzymes that degrade cartilage and bones, leading to joint damage [29]. It can also induce angiogenesis to further exacerbate the progression of joint inflammation [30]. In another large-scale MR analysis of OA, TNFSF12 was positively correlated with total joint replacement, accelerating the production of various inflammatory cytokines and matrix metalloproteinases in inflammatory joint disease, thereby promoting joint destruction [31]. Meanwhile, inhibiting the excessive activation of the CD40 ligand co-stimulation pathway has been used in clinical trials to treat RA, providing support for the potential involvement of TNFSF in arthritis [32].
As is well known, inflammatory cytokines are synthesized and secreted by immune cells, and inflammatory cytokines also activate immune cells, forming positive feedback regulation [33]. Considering the above factors, we combined transcriptome and MR analysis to explore the relationship between immune cells and arthritis. Our study found that CCR7 on naive CD4 + T cell was positively correlated with total OA and knee OA, while CCR2 on CD14 + CD16- monocyte was positively correlated with total OA and hip OA. Multiple studies have shown that under RA inflammatory conditions, CCL19 attracts T cells to infiltrate the site of autoimmune inflammation [34]. CCR7, as a receptor for CCL19, is expressed on CD4 + T cells in the synovial membrane and binds to CCL19 to stimulate angiogenesis [35]. Therefore, CCL19-CCR7 on naive CD4 + T cell and CCL11-CCR2 on CD14 + CD16- monocyte may be the two inflammatory regulatory axes of OA. It is worth noting that HLA DR on dendritic cell (OR = 0.97, 95%CI 0.95–1.00, p = 0.018) and HLA DR on myeloid dendritic cell (OR = 0.97, 95%CI 0.94–0.99, p = 0.02) are negatively correlated with hip OA. HLA-DRB1 is a known genetic risk factor for RA and has a strong correlation with the onset of RA [36]. We found that multiple HLA DR phenotypes, such as HLA DR on CD14 + CD16 monocyte, HLA DR on CD33 HLA DR+, and HLA DR on Plasmacytoid Dendritic Cell, were pathogenic in serum positive RA. This is consistent with clinical studies, where HLA-DR + patients have a higher positivity rate for rheumatoid factors, earlier joint destruction, and poorer prognosis [37]. In addition, we also found that SSC-A on CD4 + T cell is a common significant immune cell phenotype among the three types of RA. The imbalance of CD4 + T cell subpopulations has always been considered an important factor in regulating the occurrence and development of RA [38]. The CD4 + T cell subpopulation includes helper T cells (Th) and regulatory T cells (Treg), among which Th cells are further divided into Th1 and Th2 cells, playing different roles. Th1 cells secrete cytokines such as TNF, which mediate cytotoxic reactions. Th2 synthesizes cytokines such as IL-10, mediates humoral immunity and antibody generation [39]. The Th1 subgroup in RA exhibits a significant advantage over Th2, and abnormal Th1 cells can reach the joint area through blood circulation, activate their proliferation, and promote the development of RA [40]. Combined transcriptome and MR analysis results indicate that TNF-CD4 + T cells and IL-10-CD4 + T cells may be the two inflammatory regulatory axes of RA.
This study conducted MR analysis of inflammatory cytokines and immune cell phenotypes based on published large GWAS cohort, combined with transcriptome analysis, and the results were highly reliable. However, our results still have certain limitations. The conclusions regarding inflammatory cytokines and immune cells are still speculative and require further experimental verification, which may be our future research direction.
Conclusion
In summary, we verified the correlation between OA and RA from a genetic perspective. And through transcriptome and MR analysis, the same and different cytokines and immune cells were identified, establishing the relationship between cytokines and immune cells. Thereby further establishing the connection between immune inflammation and arthritis. This may provide new insights into the role of immune inflammation in arthritis and the treatment of RA and OA.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Authors’ contributions
Jiaqian Wang: Conceptualization, Project administration, Software, Writing original draft, and Editing. Zhixiang Zhang: Investigation, Visualization, Data curation, Review the draft. Zhiqiang Shao: Methodology, Formal analysis, Supervision, review and editing. Zonghan Xu: Provided ideas for the study, Resources, Funding, review and editing.
Funding
No benefit in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Data availability
The data used to support the findings of this study are included within the article.
Declarations
Ethical approval
This study was approved by the local research ethics committee.
Consent for publication
All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Zhixiang Zhang and Zhiqiang Shao contributed equally to this work.
Contributor Information
Zonghan Xu, Email: xuzonghan@126.com.
Jiaqian Wang, Email: 1580443814@qq.com.
References
- 1.Gessl I, et al. Role of joint damage, malalignment and inflammation in articular tenderness in rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Ann Rheum Dis. 2021;80:884–90. 10.1136/annrheumdis-2020-218744. [DOI] [PubMed] [Google Scholar]
- 2.Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192:230–7. 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- 3.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthritis Cartilage. 2013;21:16–21. 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 4.de Lange-Brokaar BJ, et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012;20:1484–99. 10.1016/j.joca.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 5.Attur M, et al. Increased interleukin-1beta gene expression in peripheral blood leukocytes is associated with increased pain and predicts risk for progression of symptomatic knee osteoarthritis. Arthritis Rheum. 2011;63:1908–17. 10.1002/art.30360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen WC, et al. Resistin enhances IL-1beta and TNF-alpha expression in human osteoarthritis synovial fibroblasts by inhibiting miR-149 expression via the MEK and ERK pathways. FASEB J. 2020;34:13671–84. 10.1096/fj.202001071R. [DOI] [PubMed] [Google Scholar]
- 7.Yoo JM, et al. Inhibitory effects of Viscum Coloratum Extract on IgE/Antigen-Activated mast cells and mast cell-derived inflammatory mediator-activated chondrocytes. Molecules. 2016;22. 10.3390/molecules22010037. [DOI] [PMC free article] [PubMed]
- 8.Kalaitzoglou E, Griffin TM, Humphrey MB. Innate Immune responses and osteoarthritis. Curr Rheumatol Rep. 2017;19. 10.1007/s11926-017-0672-6. [DOI] [PubMed]
- 9.Smolen JS, et al. Rheumatoid arthritis. Nat Rev Dis Primers. 2018;4:18001. 10.1038/nrdp.2018.1. [DOI] [PubMed] [Google Scholar]
- 10.Firestein GS, McInnes IB. Immunopathogenesis of rheumatoid arthritis. Immunity. 2017;46:183–96. 10.1016/j.immuni.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haas S, Straub RH. Disruption of rhythms of molecular clocks in primary synovial fibroblasts of patients with osteoarthritis and rheumatoid arthritis, role of IL-1beta/TNF. Arthritis Res Ther. 2012;14:R122. 10.1186/ar3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y, Wang JY, Zou Y, Ma XW, Meng T. Role of IL-1 family cytokines IL-36, IL-37, IL-38 in Osteoarthritis and Rheumatoid Arthritis: a Comprehensive Review. J Inflamm Res. 2024;17:4001–16. 10.2147/JIR.S474879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou SM, et al. CXCL1 contributes to IL-6 expression in osteoarthritis and rheumatoid arthritis synovial fibroblasts by CXCR2, c-Raf, MAPK, and AP-1 pathway. Arthritis Res Ther. 2020;22:251. 10.1186/s13075-020-02331-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurowska-Stolarska M, Alivernini S. Synovial tissue macrophages in joint homeostasis, rheumatoid arthritis and disease remission. Nat Rev Rheumatol. 2022;18:384–97. 10.1038/s41584-022-00790-8. [DOI] [PubMed] [Google Scholar]
- 15.Argyriou A, et al. Single cell sequencing identifies clonally expanded synovial CD4(+) T(PH) cells expressing GPR56 in rheumatoid arthritis. Nat Commun. 2022;13:4046. 10.1038/s41467-022-31519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang G, et al. Genetic influences of the effect of circulating inflammatory cytokines on osteoarthritis in humans. Osteoarthritis Cartilage. 2023;31:1047–55. 10.1016/j.joca.2022.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Watanabe K, et al. A global overview of pleiotropy and genetic architecture in complex traits. Nat Genet. 2019;51:1339–48. 10.1038/s41588-019-0481-0. [DOI] [PubMed] [Google Scholar]
- 18.Zhao JH, et al. Genetics of circulating inflammatory proteins identifies drivers of immune-mediated disease risk and therapeutic targets. Nat Immunol. 2023;24:1540–51. 10.1038/s41590-023-01588-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orru V, et al. Complex genetic signatures in immune cells underlie autoimmunity and inform therapy. Nat Genet. 2020;52:1036–45. 10.1038/s41588-020-0684-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glyn-Jones S, et al. Osteoarthr Lancet. 2015;386:376–87. 10.1016/S0140-6736(14)60802-3. [Google Scholar]
- 21.Roemer FW, et al. Presence of MRI-detected joint effusion and synovitis increases the risk of cartilage loss in knees without osteoarthritis at 30-month follow-up: the MOST study. Ann Rheum Dis. 2011;70:1804–9. 10.1136/ard.2011.150243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pearle AD, et al. Elevated high-sensitivity C-reactive protein levels are associated with local inflammatory findings in patients with osteoarthritis. Osteoarthritis Cartilage. 2007;15:516–23. 10.1016/j.joca.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 23.van den Bosch MHJ. Inflammation in osteoarthritis: is it time to dampen the alarm(in) in this debilitating disease? Clin Exp Immunol. 2019;195:153–66. 10.1111/cei.13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol. 2004;22:891–928. 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 25.Raghu H, et al. CCL2/CCR2, but not CCL5/CCR5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann Rheum Dis. 2017;76:914–22. 10.1136/annrheumdis-2016-210426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu Rev Immunol. 2007;25:787–820. 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 27.Ogilvie P, et al. Unusual chemokine receptor antagonism involving a mitogen-activated protein kinase pathway. J Immunol. 2004;172:6715–22. 10.4049/jimmunol.172.11.6715. [DOI] [PubMed] [Google Scholar]
- 28.Rozi R, Zhou Y, Rong K, Chen P. Mir-124-3p sabotages lncRNA MALAT1 stability to repress chondrocyte pyroptosis and relieve cartilage injury in osteoarthritis. J Orthop Surg Res. 2022;17:453. 10.1186/s13018-022-03334-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nanus DE, et al. TNFalpha regulates cortisol metabolism in vivo in patients with inflammatory arthritis. Ann Rheum Dis. 2015;74:464–9. 10.1136/annrheumdis-2013-203926. [DOI] [PubMed] [Google Scholar]
- 30.DeBusk LM, et al. Tie2 receptor tyrosine kinase, a major mediator of tumor necrosis factor alpha-induced angiogenesis in rheumatoid arthritis. Arthritis Rheum. 2003;48:2461–71. 10.1002/art.11213. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, et al. Evaluating the causal effect of circulating proteome on the risk of osteoarthritis-related traits. Ann Rheum Dis. 2023. 10.1136/ard-2023-224459. [DOI] [PubMed] [Google Scholar]
- 32.Orozco G, et al. Association of CD40 with rheumatoid arthritis confirmed in a large UK case-control study. Ann Rheum Dis. 2010;69:813–6. 10.1136/ard.2009.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Filali S, Noack M, Geloen A, Pirot F, Miossec P. Effects of pro-inflammatory cytokines and cell interactions on cell area and cytoskeleton of rheumatoid arthritis synoviocytes and immune cells. Eur J Cell Biol. 2023;102:151303. 10.1016/j.ejcb.2023.151303. [DOI] [PubMed] [Google Scholar]
- 34.Weninger W, et al. Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J Immunol. 2003;170:4638–48. 10.4049/jimmunol.170.9.4638. [DOI] [PubMed] [Google Scholar]
- 35.Moschovakis GL, et al. The chemokine receptor CCR7 is a promising target for rheumatoid arthritis therapy. Cell Mol Immunol. 2019;16:791–9. 10.1038/s41423-018-0056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weyand CM, Hicok KC, Conn DL, Goronzy JJ. The influence of HLA-DRB1 genes on disease severity in rheumatoid arthritis. Ann Intern Med. 1992;117:801–6. 10.7326/0003-4819-117-10-801. [DOI] [PubMed] [Google Scholar]
- 37.Listing J, et al. HLA-DRB1 genes, rheumatoid factor, and elevated C-reactive protein: independent risk factors of radiographic progression in early rheumatoid arthritis. Berlin collaborating Rheumatological Study Group. J Rheumatol. 2000;27:2100–9. [PubMed] [Google Scholar]
- 38.Imberti L, Sottini A, Signorini S, Gorla R, Primi D. Oligoclonal CD4 + CD57 + T-cell expansions contribute to the imbalanced T-cell receptor repertoire of rheumatoid arthritis patients. Blood. 1997;89:2822–32. [PubMed] [Google Scholar]
- 39.Cousins DJ, Lee TH, Staynov DZ. Cytokine coexpression during human Th1/Th2 cell differentiation: direct evidence for coordinated expression of Th2 cytokines. J Immunol. 2002;169:2498–506. 10.4049/jimmunol.169.5.2498. [DOI] [PubMed] [Google Scholar]
- 40.Yudoh K, Matsuno H, Nakazawa F, Yonezawa T, Kimura T. Reduced expression of the regulatory CD4 + T cell subset is related to Th1/Th2 balance and disease severity in rheumatoid arthritis. Arthritis Rheum. 2000;43:617–27. 10.1002/1529-0131(200003)43:3%3C617::AID-ANR19%3E3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are included within the article.