Abstract
Background
The diagnosis of peripheral isolated nodular lesions that are suspected as pulmonary tuberculosis (PTB) is challenging, which are not easily accessible via conventional bronchoscopy. This study evaluated the combined use of Xpert MTB/RIF assay and endobronchial ultrasonography with a guide sheath (EBUS-GS) for detecting MTB infection in peripheral lung bands, for early detection of PTB.
Methods
The clinical data of 232 patients with suspected peripheral nodular PTB who underwent EBUS-GS between June 2020 and October 2023 were retrospectively reviewed. The sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and area under the curve (AUC) of acid-fast bacilli smear, culture, Xpert MTB/RIF assay, and pathological examination were calculated. To assess diagnostic accuracy, the results of the four methods were directly compared with the final clinical diagnosis.
Results
In total, 146 and 86 patients were clinically diagnosed with peripheral nodular PTB and non-PTB, respectively. The sensitivity, specificity, PPV, NPV, and AUC values of combined Xpert MTB/RIF assay and EBUS-GS were 47.26%, 100.0%, 100.0%, 52.76%, and 0.74; those of acid-fast bacilli smear were 8.22%, 97.67%, 85.71%, 38.53%, and 0.53; those of culture were 31.51%, 100.0%, 100.0%, 46.24%, and 0.66; and those of pathological examination were 23.97%, 97.67%, 94.59%, 43.08%, and 0.61, respectively.
Conclusion
The diagnostic accuracy of the combined Xpert MTB/RIF assay and EBUS-GS was significantly better than that of other conventional tests. Hence, this novel technique can be routinely applied for diagnosing and managing peripheral nodular PTB.
Keywords: Peripheral nodular pulmonary tuberculosis, Xpert MTB/RIF, Endobronchial ultrasound with guide sheath, Mycobacterium tuberculosis infection, Pulmonary tuberculosis
Background
Tuberculosis (TB), an infectious disease of the respiratory system caused by Mycobacterium tuberculosis (MTB), is a chronic disease that significantly affects human health [1]. An epidemiological survey by the World Health Organization revealed that 10.6 million new TB cases were recorded in 2021 [2]. According to the site of MTB infection, it is classified as pulmonary TB (PTB) and extrapulmonary TB (EPTB). PTB accounts for approximately 80% of all TB cases [3, 4]. As the respiratory tract is connected to the outside world, patients with PTB, particularly those who are not diagnosed and managed timely, are considered the main source of TB infection. Strict screening and standardized treatment are required for control the TB epidemic more effectively [5]. As high-resolution computed tomography (CT) of the chest is frequently used during health checkups, lung abnormalities can be initially detected, and the lesion type can be further classified. If a diagnosis of TB is made, anti-TB treatment is administered as early as possible. If a malignant tumor is suspected, early surgery is required. Currently, for lesions located in the inner or middle lung bands, conventional bronchoscopy can be employed to reach the lesion site. Routine lavage or biopsy combined with macrogene sequencing and histopathological examination can facilitate a relatively easy diagnosis [6, 7]. However, for peripheral lung lesions (i.e., those located in the branches below the bronchial tubes in the lung segments), the lesion site cannot be reached via conventional bronchoscopy. Moreover, the positivity rate of conventional lavage is low. Thus, the early diagnostic rate remains unsatisfactory [8].
Ultrasound bronchoscopy-guided transbronchial lung biopsy, a novel diagnostic technique, can effectively reach the grade 8–9 bronchus, probe peripheral lesions of the airways, and access surrounding blood vessels [9, 10]. It has superior diagnostic performance, is associated with a lower rate of complications, and is extensively used in the diagnosis of peripheral lung lesions, particularly in oncology [11, 12]. However, the application of this technique in the field of PTB remains limited. If this technique is combined with a guide sheath (GS), the GS can be locally fixed in the lumen, thereby allowing multiple samplings of the lesion and further reducing the risk of bleeding due to physical compression of the GS during surgery.
PTB with isolated nodular lesions is a special type of TB that commonly lacks the characteristic clinical manifestations of TB and is easily confused with other lung diseases such as peripheral lung cancer, lung sarcoma, benign tumors of the lungs, and granulomatous lesions [13, 14]. Diagnosing nodular PTB at an early stage is challenging, particularly if the lesion is located in the peripheral lung bands. In such cases, conventional bronchoscopy fails to adequately approach the lesion. Additionally, CT-guided percutaneous lung aspiration biopsy, which is used to collect samples, is associated with an increased risk of surgical complications such as pneumothorax and bleeding. Hence, the diagnostic accuracy is reduced [15]. Recent research has shown that endobronchial ultrasonography (EBUS) with a GS (EBUS-GS) can be used to reach the bronchial lumen of the peripheral lungs and collect samples via precise positioning for lavage or puncture biopsy [16]. At this stage, this technique has been widely used in clinical practice, particularly for diagnosing early-stage lung cancer [17, 18]. However, its application in the field of TB is still relatively rare. Owing to the limited sampling via EBUS-GS, diagnosis based on histopathological biopsy alone is challenging, and diagnostic errors can easily occur. Therefore, the current study aimed to evaluate the use of molecular biology-based diagnostic techniques (e.g., Xpert MTB/RIF assay) to facilitate a comprehensive clinical diagnosis of TB. Furthermore, the diagnostic efficacy of the Xpert MTB/RIF assay combined with EBUS-GS for peripheral nodular PTB was examined.
Methods
Study design
This single-center retrospective study was conducted at the Tuberculosis Diagnosis and Treatment Center of Zhejiang Chinese and Western Medicine Integrated Hospital from June 2020 to October 2023. The digital medical records of patients suspected of having peripheral nodular PTB who were admitted to our facility were examined. All patients who underwent comprehensive laboratory tests, including routine blood test, coagulation function test, blood biochemical indicator test, tumor marker examination, and T-SPOT.TB assay, were enrolled. After screening for bronchoscopy contraindications, patients underwent lung puncture biopsy using EBUS-GS. Next, they immediately underwent bronchial flushing. EBUS-GS biopsy samples were sent for pathological examination, and bronchial flushing fluid samples were sent for acid-fast bacilli (AFB) smear, culture, and Xpert MTB/RIF assay.
PTB was diagnosed by clinicians based on the diagnostic standard of the People’s Republic of China for Tuberculosis (WS 288–2017). The final clinical diagnostic standard was a composite reference standard (CRS). For PTB diagnosis, patients must meet at least one of the following criteria: (1) positive results in bronchial flushing fluid microbiological tests (including culture or Xpert MTB/RIF assay); (2) histopathological findings on lung puncture consistent with TB pathology, with typical lesions of caseous necrotic granuloma and Langerhans’ giant cells observed around the lesion; and (3) reduction or disappearance of lung nodules after 1 month of anti-TB treatment. Otherwise, patients were diagnosed with non-PTB conditions.
The inclusion criteria were as follows: (1) patients with solid or subsolid solitary lung nodules with a diameter of 8–30 mm on chest CT, located in the outer area of the lung; (2) those without significant abnormalities of the heart or lung, blood clotting disorders, or any other contraindications for standard bronchoscopy; and (3) those not taking oral anticoagulant medications. The exclusion criteria were as follows: (1) patients with different lung lesions located in the inner and middle bands of the lungs; (2) patients with multiple areas of patchy, solid, or cavitated lesions on lung CT; (3) those with lesions located in a subsegment of the bronchus that could be accessed via plain bronchoscopy; and (4) those with an unclear final diagnosis. Due to the retrospective nature of the study, the need to obtain informed consent was waived. This research was evaluated and approved by the Ethics Committee of Zhejiang Chinese and Western Medicine Integrated Hospital (2023–09–025). Moreover, it was performed in accordance with the principles of the Declaration of Helsinki.
Experimental methods
Equipment and instruments
A bronchoscope (Olympus, BF-P260F) with an external diameter of 4.0 mm and an internal diameter of 2.0 mm, ultrasound imaging equipment (Olympus, EndoEchoEU-M2000), ultrasound probe driver (Olympus; MAJ-1720), ultrasound probe (Olympus; UM-S20-17 S) with an external diameter of 1.4 mm, GS (Olympus, K-201) with an external diameter of 1.95 mm, and disposable biopsy forceps were used.
Procedure
Before EBUS-GS examination, all patients underwent thin-layer chest CT (0.75-mm slice thickness; AS 64-slice CT system, PHILIPS, Netherlands). Chest CT images were reviewed by two experienced pulmonologists before the procedure, who then used the bronchial pathway to target the lesion. Before surgery, the anesthesiologist performed transoral laryngeal mask mechanical ventilation under intravenous anesthesia after 6 h of food and water fasting. After the administration of anesthesia, the scope was introduced into healthy and affected bronchi, and their branches were routinely explored sequentially. Then, according to the virtual path established on CT, the operator entered the subsegmental bronchus where the lesion was located, and the ultrasound probe was used to reach the bronchial lumen of the target lesion via the biopsy hole. After their detection, the abnormal echoes were localized. After fixing the bronchoscope at the same location, the ultrasound probe was removed and the biopsy forceps were fed into the biopsy tube via the GS to obtain biopsy samples from a region close to the lesion site. The biopsy forceps were withdrawn after repeated clamping of 3–5 pieces of tissue. Then, the punctured tissues were sent for routine pathological examination. Subsequently, approximately 60 mL of saline was injected into the bronchial lumen of the lesion via the GS for flushing. The bronchial flushing fluid was absorbed back into a sterile container, uniformly aliquoted, and subjected to AFB smear, culture, and Xpert MTB/RIF assay.
Diagnostic specimen handling
AFB smear
About 1 mL of centrifuged lavage fluid samples was placed on a dry and clean glass slide, left to dry, and subsequently heat fixed and stained using Ziehl–Neelsen stain. After placing the stained sample under a microscope, at least one antacid bacillus was observed in 300 different fields of view. Thus, the result was considered positive.
Culture
An equivalent amount of 4% sodium hydroxide solution was combined with the lavage sample, and the mixture was left to stand for 15 min. Subsequently, sterile phosphate buffer was added until the total volume reached 50 mL. After centrifugation, the precipitate was inoculated into the liquid medium. For liquid culture, the BACTEC MGIT 960 Mycobacterium culture system (BD Diagnostic Systems, Sparks, MD) was used.
Xpert MTB/RIF assay
Fresh bronchoalveolar lavage fluid (BALF) samples were centrifuged, yielding a 1-mL sediment. This sediment was then combined with 2 mL of the sample treatment solution. The mixture was left at room temperature for 15 min and then vigorously vortexed for 8 min. Next, 2 mL of the mixture was added to the Xpert MTB/RIF cartridge (Cepheid, USA), which was then loaded onto the testing module. Finally, the system automatically reported results within 2 h.
Pathological examination
A neutral formalin fixative was used to fully soak the specimen, after which pathological specimens were obtained. Next, a series of processes such as dehydration, embedding, sectioning, staining, and filming of the sampled tissues were performed. The pathological sections were then analyzed under a microscope. Results showed the presence of caseous necrosis in the lesion. Hence, a pathological diagnosis of the disease was made based on the patient’s clinical information and pathological and morphological characteristics.
Statistical analysis
SPSS software version 22.0 (SPSS Inc., Chicago, USA) was used for statistical analysis. MedCalc Statistical v15.2.2 software (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org) was used to calculate the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), area under the receiver operating characteristic curve (AUC), and 95% confidence interval (CI) of the four tests. CRS was selected as the final diagnostic standard to assess the diagnostic accuracy of the tests. Additionally, paired data were compared using the McNemar’s test. AUCs were compared using the Z-test. To compare internal variations among the four tests, a Venn diagram (https://www.xiantaozi.com/literatures) was constructed. A P-value of < 0.05 was considered to indicate statistical significance.
Results
Baseline clinical characteristics of the participants
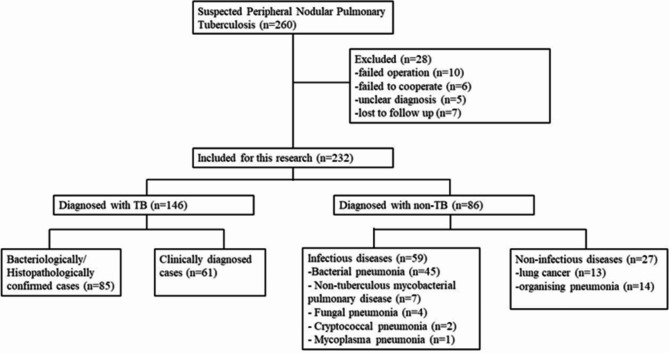
The medical records of the patients (n = 260) clinically suspected of having peripheral nodular PTB and admitted to our institution between June 2020 and October 2023 were examined. However, 28 patients with insufficient data were excluded from the study. Finally, 232 patients were included in this retrospective analysis. The average age of the patients was 51.41 ± 16.99 years, and 151 (65.09%) of them were male. In total, 175 (75.43%) patients showed positive results in the T-SPOT.TB assay, and 79 (34.05%) were previously diagnosed with PTB and were receiving anti-TB treatment. Meanwhile, all patients tested negative for the human immunodeficiency virus. Based on CRS, which is the ultimate diagnostic criteria, 146 (62.93%) patients were diagnosed with peripheral nodular PTB and 86 (37.07%) had no PTB. In the non-PTB group, 13 patients were diagnosed with lung cancer, 14 with organizing pneumonia, 45 with bacterial pneumonia, 7 with nontuberculous mycobacterial pulmonary disease, 4 with fungal pneumonia, 2 with cryptococcal pneumonia, and 1 with mycoplasma pneumonia. Figure 1 shows the status of 260 patients who were admitted due to suspicion of peripheral nodular PTB.
Fig. 1.
Status of 260 patients with suspected peripheral nodular PTB
Diagnostic performance of AFB smear, culture, Xpert MTB/RIF assay, and pathological examination for peripheral nodular PTB
The overall sensitivity, specificity, PPV, NPV, and AUC of AFB smear using EBUS-GS for detecting peripheral nodular PTB were 8.22% (95% CI: 4.32–13.92%), 97.67% (91.85–99.72%), 85.71% (57.19–98.22%), 38.53% (32.04–45.34%), and 0.53 (0.46–0.60), respectively.
The overall sensitivity, specificity, PPV, NPV, and AUC of culture using EBUS-GS for detecting peripheral nodular PTB were 31.51% (95% CI: 24.08–39.71%), 100.00% (95.80–100.00%), 100.00% (92.29–100.00%), 46.24% (38.91–53.68%), and 0.66 (0.59–0.72), respectively.
The overall sensitivity, specificity, PPV, NPV, and AUC of the Xpert MTB/RIF assay using EBUS-GS for detecting peripheral nodular PTB were 47.26% (95% CI: 38.95–55.68%), 100.00% (95.80–100.00%), 100.00% (94.79–100.00%), 52.76% (44.80–60.62%), and 0.74 (0.67–0.79), respectively.
The overall sensitivity, specificity, PPV, NPV, and AUC of pathological examination using EBUS-GS for detecting peripheral nodular PTB were 23.97% (95% CI: 17.30–31.73%), 97.67% (91.85–99.72%), 94.59% (81.81–99.34%), 43.08% (36.02–50.34%), and 0.61 (0.54–0.67), respectively.
The results of the four tests are displayed in Table 1.
Table 1.
The diagnostic accuracy of the four tests for peripheral nodular pulmonary tuberculosis
| Test | Sensitivity (95% CI) |
Specificity (95% CI) |
PPV (95% CI) |
NPV (95% CI) |
AUC (95% CI) |
|---|---|---|---|---|---|
| AFB smear |
8.22 (4.32–13.92) |
97.67 (91.85–99.72) |
85.71 (57.19–98.22) |
38.53 (32.04–45.34) |
0.53 (0.46–0.60) |
| Culture |
31.51 (24.08–39.71) |
100.00 (95.80–100.00) |
100.00 (92.29–100.00) |
46.24 (38.91–53.68) |
0.66 (0.59–0.72) |
| Xpert MTB/RIF |
47.26 (38.95–55.68) |
100.00 (95.80–100.00) |
100.00 (94.79–100.00) |
52.76 (44.80–60.62) |
0.74 (0.69–0.79) |
| Pathological examination |
23.97 (17.30–31.73) |
97.67 (91.85–99.72) |
94.59 (81.81–99.34) |
43.08 (36.02–50.34) |
0.61 (0.54–0.67) |
Comparison of the diagnostic accuracies of the four tests during EBUS-GS
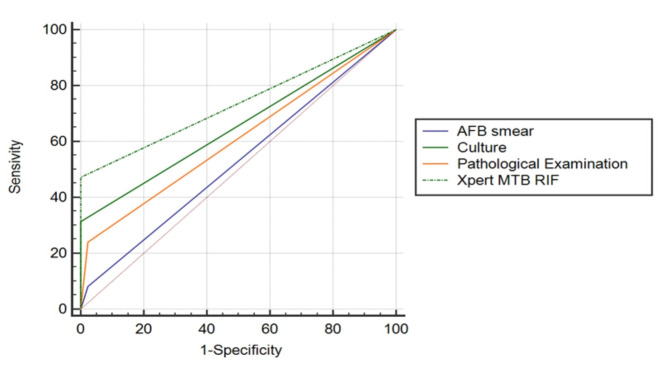
The diagnostic accuracies of AFB smear, culture, Xpert MTB/RIF assay, and pathological examination combined with EBUS-GS were compared in patients suspected of having peripheral nodular PTB, with CRS being used as the ultimate diagnostic criterion. AFB smear had the lowest diagnostic accuracy among the four diagnostic methods, and the difference between each of the two tests was statistically significant (P < 0.01). Both culture and pathological examination had moderate diagnostic accuracy without a statistically significant difference (P > 0.05). Moreover, the Xpert MTB/RIF assay showed the greatest diagnostic accuracy (P < 0.01). Table 2 shows the comparison of the tests. Figure 2 depicts the receiver operating characteristic curves of the four tests.
Table 2.
The diagnostic accuracy of MTB culture, Xpert MTB/RIF assay, and pathological examination for peripheral nodular PTB
| Test | Sensitivity (P-value) |
Specificity (P-value) |
AUC (P-value) |
|---|---|---|---|
|
Culture vs. Xpert MTB/RIF |
< 0.001 | 1.000 | < 0.001 |
|
Culture vs. pathological examination |
0.843 | < 0.001 | 0.068 |
|
Xpert MTB/RIF vs. pathological examination |
< 0.001 | < 0.001 | < 0.001 |
AUC: area under the curve
Fig. 2.
shows the ROC curves of the four tests
Complications
Among the 232 patients who underwent EBUS-GS, 7 (3.02%) presented with pneumothorax. Furthermore, five patients recovered with oxygen therapy, and two required thoracic drainage. In total, two (0.87%) patients had intra-airway hemorrhage, which was treated with topical epinephrine spray.
Discussion
The diagnosis of isolated nodular lesions that are suspected as PTB is challenging [19], particularly if the nodular foci are located in the peripheral bands of the lung, which are not easily accessible via conventional bronchoscopy. This leads to a decreased detection rate, making it difficult to obtain a definitive diagnosis. It is difficult to distinguish PTB from primary lung cancer in the peripheral lungs. Even in regions where TB is endemic, it is still misdiagnosed as primary lung cancer [20, 21]. Therefore, the EBUS-GS technique should be adopted, which is a more accurate, minimally invasive, and safer method of obtaining specimens when combined with TB molecular biology testing, thereby improving the diagnostic yield of peripheral nodular PTB [22].
Results showed that AFB smear with EBUS-GS had the least diagnostic accuracy for peripheral nodular PTB (with a sensitivity of 8.22%, specificity of 97.67%, and AUC of 0.53). These values were similar to those reported by Yao et al. [23]. In our previous study, the detection efficacy of AFB smear was higher than that in the present study, probably due to the fact that the patients in the previous study had a wider range of lung lesions, which made it easier to obtain positive results [24]. However, AFB smear is not a reliable method for MTB detection, and a positive outcome does not conclusively confirm PTB. In our study, two patients with positive AFB smear were diagnosed with nontuberculous mycobacterial infection, and the bacterial strains were identified via culture. Thus, AFB smear, which is the conventional diagnostic approach for TB, has the least diagnostic precision.
Culture, which is another traditional diagnostic technique, showed a fairly high level of accuracy. The current study revealed that the sensitivity, specificity, and AUC of culture were 31.51%, 100.00%, and 0.66, respectively. These results were significantly better than those of AFB smear (P < 0.001). However, culture has a disadvantage that cannot be ignored, i.e., the need for several weeks to obtain the results. Thus, it cannot be used as an early diagnostic technique [25]. The delayed intermediate time affects the identification and management of PTB, which may lead to incorrect diagnosis and transmission [26].
Using EBUS-GS, we successfully acquired biopsy samples from solitary nodular TB. The sensitivity, specificity, and AUC of pathological examination were 23.97%, 97.67%, and 0.61, respectively. Its diagnostic efficacy was similar to that of culture (P > 0.05), and both tests had significantly higher diagnostic efficacy than AFB smear (P < 0.001). However, pathological examination also requires few days to obtain results. The diagnostic sensitivity of pathological examination during EBUS-GS was significantly lower than that of CT-guided lung puncture biopsy, owing to the narrow inner diameter of the GS and limited extent of operation using the biopsy needle [27]. Inserting the needle vertically to obtain tissue samples from deep within the lesion is challenging, leading to reduced sensitivity of pathological examination. Occasionally, TB is pathologically challenging to distinguish from nontuberculous mycobacterial lung disease [28]. In this study, for a patient with a pathological diagnosis of granulomatous inflammation who showed a positive culture for nontuberculous mycobacteria, the final diagnosis was nontuberculous mycobacterial lung disease. Furthermore, in one case, the pathological examination results were indicative of chronic suppurative inflammation with suspected granuloma formation, which was eventually diagnosed as bacterial pneumonia after 2 weeks of anti-infective treatment with cefoperazone sodium and sulbactam sodium. In the non-PTB group, 13 patients were newly diagnosed with lung cancer, which facilitated early surgical treatment and improved the prognosis.
The current study showed that the Xpert MTB/RIF assay in conjunction with EBUS-GS had the highest efficacy in diagnosing peripheral nodular PTB. Additionally, the results were obtained within 2 h, and the method confirmed rifampicin resistance, thereby enhancing its clinical utility. However, the sensitivity was 47.26%, lower than that reported in a previous study, which revealed that the sensitivity of the Xpert MTB/RIF assay using BALF samples was 58.9% in patients with smear-negative or sputum-sparse PTB [29]. This could be attributed to the fact that our study involved lesion sizes of 8–30 mm, which were significantly smaller than the lesions in other studies. Moreover, the lesion sites in our study were located in the peripheral lung bands away from the central bronchiole, and conventional bronchoscopy cannot easily reach the lesions. Cao et al. found that when using EBUS-GS for the same lesion type, the sensitivity of the Xpert MTB/RIF assay was 34.78%, with an AUC of 0.674, which was slightly lower than that in our study. However, when using puncture tissue for Xpert MTB/RIF assay, the sensitivity and AUC were significantly higher, but with more complications [30]. In general, EBUS-GS causes less harm and is associated with fewer issues than conventional invasive examinations [31]. Meanwhile, the Xpert MTB/RIF assay can provide a high and relatively rapid diagnostic output [32]. The use of both techniques yields exceptional diagnostic outcomes for isolated pulmonary nodules caused by MTB and is beneficial in distinguishing nontuberculous respiratory diseases. In our study, we found that the Xpert MTB/RIF assay could diagnose 18 more patients with PTB, whereas the other tests yielded negative results. Controlling TB transmission is facilitated by a considerably increased rate of detection, which provides timely access to anti-TB treatment and decreases the spread of bacteria.
This study had some limitations. It was conducted retrospectively at a single center and had a limited number of participants. Therefore, the diagnostic significance of the Xpert MTB/RIF assay combined with EBUS-GS for solitary pulmonary nodules caused by MTB requires validation through a clinical multicenter study. Additionally, the operators of EBUS-GS need to have a high level of operating skills. Some lesions may not be accessible, or there could be failure in puncturing due to the angle of the bronchoscope.
Conclusion
The combination of Xpert MTB/RIF and EBUS-GS is clinically valuable in the diagnosis of peripheral isolated PTB, as it has good sensitivity and high specificity. Therefore, implementation of this technique in hospitals in TB endemic areas can help in the early screening of PTB and control of TB epidemics.
Acknowledgements
We would like to express our feelings to the patients and colleagues in our department.
Abbreviations
- AFB smear
Acid-fast bacilli smear
- AUC
Area under the curve
- BALF
Bronchoalveolar lavage fluid
- CI
Confidence interval
- CRS
Composite reference standard
- CT
Computed tomography
- EBUS-GS
Endobronchial ultrasonography with a guide sheath
- EBUS
Endobronchial ultrasonography
- EPTB
Extrapulmonary tuberculosis
- GS
Guide sheath
- MTB
Mycobacterium tuberculosis
- NPV
Negative predictive value
- PPV
Positive predictive value
- PTB
Pulmonary tuberculosis
- TB
Tuberculosis
Author contributions
L.Z. and Q.H. designed the study protocol and reviewed the literature. Y.Y. and X.R. collected the clinical data. Q.H. and H.L. performed the statistical analysis of the data. L.Z. and Y.Y. completed the writing of the first draft. Q.H. reviewed and revised the manuscript. All authors read and approved the final manuscript.
Funding
Funding for this research was provided by the HangZhou Municipal Health Commission (Grant number: A20200673) and the Health Commission of Zhejiang Province (Grant number: 2022KY984).
Data availability
Data is provided within the manuscript, and is available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Due to the retrospective nature of the study, the need to obtain informed consent was waived. This research was evaluated and approved by the Ethics Committee of Zhejiang Chinese and Western Medicine Integrated Hospital (2023–09–025). Moreover, it was performed in accordance with the principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pathakumari B, Devasundaram S, Raja A. Altered expression of antigen-specific memory and regulatory T-cell subsets differentiate latent and active tuberculosis. Immunology. 2018;153:325–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bagcchi S. WHO’s global tuberculosis report 2022. Lancet Microbe. 2023;4:e20. [DOI] [PubMed] [Google Scholar]
- 3.Antonangelo L, Faria CS, Sales RK. Tuberculous pleural effusion: diagnosis & management. Expert Rev Respir Med. 2019;13:747–59. [DOI] [PubMed] [Google Scholar]
- 4.Toujani S, Ben Salah N, Cherif J, Mjid M, Ouahchy Y, Zakhama H, et al. Primary infection and pulmonary tuberculosis. Rev Pneumol Clin. 2015;71:73–82. [DOI] [PubMed] [Google Scholar]
- 5.Suárez I, Fünger SM, Kröger S, Rademacher J, Fätkenheuer G, Rybniker J. The diagnosis and treatment of tuberculosis. Dtsch Arztebl Int. 2019;116:729–35. [DOI] [PubMed] [Google Scholar]
- 6.Liu HC, Gao YL, Li DF, Zhao XY, Pan YQ, Zhu CT. Value of Xpert MTB/RIF using bronchoalveolar lavage fluid for the diagnosis of pulmonary tuberculosis: a systematic review and meta-analysis. J Clin Microbiol. 2021;59:e02170–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu X, Chen Y, Ouyang H, Liu J, Luo X, Huang Y, et al. Tuberculosis diagnosis by metagenomic next-generation sequencing on bronchoalveolar lavage fluid: a cross-sectional analysis. Int J Infect Dis. 2021;104:50–7. [DOI] [PubMed] [Google Scholar]
- 8.Lam S, Shah PL. Bronchoscopic diagnosis of peripheral lung lesions. Respiration. 2021;100:764–6. [DOI] [PubMed] [Google Scholar]
- 9.Livi V, Barisione E, Zuccatosta L, Romagnoli M, Praticò A, Michieletto L, et al. Competence in navigation and guided transbronchial biopsy for peripheral pulmonary lesions. Panminerva Med. 2019;61:280–9. [DOI] [PubMed] [Google Scholar]
- 10.Biswas A, Mehta HJ, Sriram PS. Diagnostic yield of the virtual bronchoscopic navigation system guided sampling of peripheral lung lesions using ultrathin bronchoscope and protected bronchial brush. Turk Thorac J. 2019;20:6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris K, Puchalski J, Sterman D. Recent advances in bronchoscopic treatment of peripheral lung cancers. Chest. 2017;151:674–85. [DOI] [PubMed] [Google Scholar]
- 12.Kurihara Y, Tashiro H, Takahashi K, Tajiri R, Kuwahara Y, Kajiwara K, et al. Factors related to the diagnosis of lung cancer by transbronchial biopsy with endobronchial ultrasonography and a guide sheath. Thorac Cancer. 2022;13:3459–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martini K, Loubet A, Bankier A, Bouam S, Morand P, Cassagnes L, et al. Nodular reverse halo sign in active pulmonary tuberculosis: a rare CT feature? Diagn Interv Imaging. 2020;101:281–7. [DOI] [PubMed] [Google Scholar]
- 14.Moreira-Neto C, Moreira C Jr., Tolentino D, Duker JS. Nodular posterior scleritis associated with presumed ocular tuberculosis: a multimodal imaging case report. Am J Ophthalmol Case Rep. 2019;16:100558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim J, Lee KH, Cho JY, Kim J, Shin YJ, Lee KW. Usefulness of CT-guided percutaneous transthoracic needle lung biopsies in patients with suspected pulmonary infection. Korean J Radiol. 2020;21:526–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang QC, Xuan WX, Li HL, Sun GN, Cheng DJ, Wang Z, et al. Diagnostic value of LungPoint navigation combined with EBUS-GS & ROSE in peripheral pulmonary nodules. Indian J Med Res. 2022;156:529–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu J, Tang F, Gu Y. A prospective study on the diagnosis of peripheral lung cancer using endobronchial ultrasonography with a guide sheath and computed tomography-guided transthoracic needle aspiration. Ther Adv Med Oncol. 2018;10:1758834017752269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka A, Akamatsu H, Kawabata H, Ariyasu H, Nakamura Y, Yamamoto N. Peripheral pulmonary carcinoid tumor diagnosed by endobronchial-ultrasound-guided bronchoscopy. Respirol Case Rep. 2016;4:10–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim CH, Lim JK, Lee SY, Won DI, Cha SI, Park JY, et al. Predictive factors for tuberculosis in patients with a TB-PCR-negative bronchial aspirate. Infection. 2013;41:187–94. [DOI] [PubMed] [Google Scholar]
- 20.Sun W, Zhang L, Liang J, Li X, Yang Y, Sun W, et al. Comparison of clinical and imaging features between pulmonary tuberculosis complicated with lung cancer and simple pulmonary tuberculosis: a systematic review and meta-analysis. Epidemiol Infect. 2022;150:e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang XL, Shan W. Application of dynamic CT to identify lung cancer, pulmonary tuberculosis, and pulmonary inflammatory pseudotumor. Eur Rev Med Pharmacol Sci. 2017;21:4804–9. [PubMed] [Google Scholar]
- 22.Zhu J, Gu Y. Diagnosis of peripheral pulmonary lesions using endobronchial ultrasonography with a guide sheath and computed tomography guided transthoracic needle aspiration. Clin Respir J. 2019;13:765–72. [DOI] [PubMed] [Google Scholar]
- 23.Yao L, Chen S, Sha W, Gu Y. The diagnostic performance of endobronchial ultrasound with Xpert MTB/RIF ultra in smear-negative pulmonary tuberculosis. BMC Infect Dis. 2023;23:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou L, Zou X, Hu Q, Hua H, Qi Q. Determination of the diagnostic accuracy of nanopore sequencing using bronchoalveolar lavage fluid samples from patients with sputum-scarce pulmonary tuberculosis. J Infect Chemother. 2023;30:98–103. [DOI] [PubMed] [Google Scholar]
- 25.Noussair L, Bert F, Leflon-Guibout V, Métivier R, Chauvet C, Napol C, et al. Evaluation of the culture-enhanced Xpert MTB/RIF assay for the diagnosis of smear-negative tuberculosis. Med Mal Infect. 2019;49:467–70. [DOI] [PubMed] [Google Scholar]
- 26.Said B, Charlie L, Getachew E, Wanjiru CL, Abebe M, Manyazewal T. Molecular bacterial load assay versus culture for monitoring treatment response in adults with tuberculosis. SAGE Open Med. 2021;9:20503121211033470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brioulet J, David A, Sagan C, Cellerin L, Frampas E, Morla O. Percutaneous CT-guided lung biopsy for the diagnosis of persistent pulmonary consolidation. Diagn Interv Imaging. 2020;101:727–32. [DOI] [PubMed] [Google Scholar]
- 28.Jain D, Ghosh S, Teixeira L, Mukhopadhyay S. Pathology of pulmonary tuberculosis and non-tuberculous mycobacterial lung disease: facts, misconceptions, and practical tips for pathologists. Semin Diagn Pathol. 2017;34:518–29. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Shen Y, Wang L, Ju L, Wu X, Wang P, et al. Efficacy of the Xpert Mycobacterium tuberculosis/rifampicin assay for diagnosing sputum-smear negative or sputum-scarce pulmonary tuberculosis in bronchoalveolar lavage fluid. Int J Infect Dis. 2021;107:121–6. [DOI] [PubMed] [Google Scholar]
- 30.Cao J, Gu Y, Wu XC, Cheng LP, Wang L, Qu QR, et al. EBUS-GS with the GeneXpert MTB/RIF assay for diagnosis of Mycobacterium tuberculosis infection of isolated pulmonary nodules. Eur J Med Res. 2023;28:370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu CH, Yuan Q, Yu LK, Wang W, Lin Y. Endobronchial ultrasound transbronchial biopsy with guide-sheath for the diagnosis of solitary pulmonary nodules. Oncotarget. 2017;8:58272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chhajed PN, Vaidya PJ, Mandovra NP, Chavhan VB, Lele TT, Nair R, et al. EBUS-TBNA in the rapid microbiological diagnosis of drug-resistant mediastinal tuberculous lymphadenopathy. ERJ Open Res. 2019;5:00008–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is provided within the manuscript, and is available from the corresponding author on reasonable request.