Abstract
Background
SARS-CoV-2 infection during pregnancy is known to be associated with poor pregnancy outcomes, including pre-eclampsia (PE), prematurity, perinatal and maternal mortality. Data on the burden of SARS-CoV-2 infection among pregnant women and their offspring in Sub-Saharan Africa is limited. We aimed to estimate SARS-CoV-2 seroprevalence and determine PE biomarkers in Mozambican pregnant women with perinatal loss.
Methods
A cross-sectional study was conducted among women who had a fetal or an early neonatal death at the Maputo Central Hospital (MCH), Mozambique. Anti-SARS-CoV-2 IgG/IgM were determined in maternal and umbilical cord blood and PE biomarkers (sFlt-1 and PIGF) in maternal blood. SARS-CoV-2 RT-PCR was performed in placenta and fetal lung biopsies from participants found to be SARS-CoV-2 seropositive.
Results
A total of 100 COVID-19 unvaccinated women were included in the study from March 2021 to April 2022. Total SARS-CoV-2 antibodies were detected in 68 [68%; 95CI (58 – 76)] maternal and 55 [55%; 95CI (54 – 74)] cord blood samples. SARS-CoV-2 IgM was detected in 18 cord blood samples and a positive placental RT-PCR in three of these participants. The proportion of women with moderate to high sFlt-1/PIGF ratio was higher in SARS-CoV-2 seropositive women than in those seronegative (71.2% vs 28.8%, p = 0.339), although the difference was not statistically significant.
Conclusions
SARS-CoV-2 seroprevalence among Mozambican women with perinatal loss was high during the second pandemic year, and there was evidence of vertical transmission in stillbirths. Findings also suggest that maternal SARS-CoV-2 infection may increase the risk of developing PE.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12884-024-06800-9.
Keywords: SARS-CoV-2 seroprevalence, Pregnancy, Stillbirths, Preeclampsia, SFlt-1/PlGF
Background
The Coronavirus 2019 (COVID-19) pandemic negatively affected maternal and neonatal health globally, largely due to the disruption of prenatal follow-up of pregnancies, limited services and diagnostics– especially in countries with fragile health systems [1, 2]. SARS-CoV-2 infection in pregnancy is associated with adverse outcomes such as pre-eclampsia (PE), preterm birth and perinatal and maternal mortality [3, 4]. Despite evidence of increased morbidity and mortality, data on the burden of SARS-CoV-2 infection among pregnant women and their offspring in Sub-Saharan Africa (SSA) remain limited.
Maternal SARS-CoV-2 infection and pregnancy complications such as PE have overlapping clinical features (hypertension, proteinuria, endothelial and multi-organ complications), which may have led to misdiagnosis and mismanagement of patients during the pandemic [3]. PE is one of the leading causes of maternal and perinatal mortality in SSA [5]. Although the cause remains uncertain, it is suggested that abnormal placentation and excessive production of anti-angiogenic factors such as the soluble fms-like tyrosine kinase 1 (sFlt-1) play a key role in the development and progression of PE [3, 6]. A PE-like syndrome in pregnant women with SARS-CoV-2 can be differentiated using PE biomarkers and SARS-CoV-2 diagnostic tests [3, 6, 7]. A high ratio of sFlt-1 to placental growth factor (PIGF) is predictive of PE and/or fetal growth restriction (FGR) [3, 6], and can be used in distinguishing SARS-CoV-2 cases and guiding clinical intervention [1, 3].
Like many African countries, Mozambique has experienced four waves of the COVID-19 pandemic and reported 233,842 confirmed COVID-19 cases as of August 11, 2024 [8]. However, the extent of SARS-CoV-2 exposure in pregnant women and their babies during the subsequent waves of the pandemic is poorly known. Pregnant women have been an effective sentinel population for serological surveillance in assessing the burden of several infectious diseases in the general population [9, 10]. Serological analysis of infectious diseases serves to strengthen case surveillance and generate information on disease circulation and immunity [10, 11]. Therefore, the detection and monitoring of anti-SARS-CoV-2 immunoglobulins (Ig) in high-risk populations is of paramount importance. In this study, we estimated the seroprevalence of SARS-CoV-2 in Mozambican pregnant women with perinatal loss and their offspring, during the second year of the COVID-19 pandemic.
Methods
Study design and population
This cross-sectional study aimed to determine the prevalence of SARS-CoV-2 antibodies (total Ig, IgM and IgG) in maternal and cord blood of women who experienced perinatal deaths and their offspring; and to assess the prevalence of clinical PE and angiogenic biomarkers (sFlt-1, PIGF) and its association with maternal SARS-CoV-2 serostatus. In addition, we analyzed post-mortem biopsies of fetal tissue of participants with SARS-CoV-2 positivity and ascertained the causes of death. The study included pregnant women who had a fetal or an early neonatal death at the Maputo Central Hospital (MCH). MCH is a 1500-bed government-funded quaternary hospital located in Maputo city, and has a yearly record of approximately 8000 births and 400–500 perinatal deaths. This study was conducted before the roll-out of COVID-19 vaccine for pregnant women in the country.
Study procedures
All pregnant women who had experienced a fetal or early neonatal death (defined as newborn dying within the first 12 h of life in the study) at the MCH were invited to participate in the study. All study procedures were explained and if in agreement, participants signed informed consent forms. Demographic, clinical, and obstetric information were recorded accordingly. Peripheral venous blood was then collected from the mother and umbilical cord blood from their offspring for serological analysis (SARS-CoV-2 antibodies and PE biomarkers). Fetal tissues and placental biopsies were collected by minimally invasive tissue sampling (MITS) technique from all participants, to further analyze the causes of death [12].
Laboratory methods
The Atellica® IM SARS-CoV-2 Total chemiluminescent immunoassay analyzer (COV2T, Siemens Healthcare Diagnostics Inc.) was used for semi-qualitative determination of total SARS-CoV-2 Ig to the spike protein of SARS-CoV-2, with a reported specificity of 99.8% and sensitivity of 100% [13]. A total SARS-CoV-2 antibody titer level of < 1 U/mL was considered non-reactive (negative). In all reactive (positive) samples, anti SARS-CoV2 IgG and IgM were also determined using the Siemens Atellica® IM SARS-CoV-2 IgG (sCOVG, Siemens Healthcare Diagnostics Inc.) and the quantitative Suspension Array Technology (qSAT) assays based on xMAP Luminex platform, respectively. sCOVG was used for the qualitative and quantitative detection of IgG antibodies The manufacturer reported a specificity of 99.9% and sensitivities of 96.41% at least 21 days post-PCR testing [14]. qSAT assays have been reported to have a specificity of 100% and sensitivity of 95.78% or 95.65% (≥ 14—21 days since symptom onset) [15]. In women with positive total Ig, the levels of IgM and IgG of 0.99 U/mL and below were considered negative.
Before fixation, samples from the maternal side, fetal side, umbilical cord and membranes of the placenta were collected for microbiological analysis. The placenta was then examined according to the Amsterdam placental workshop consensus [16]. After undergoing fixation in formalin for 24 h, the three full-thickness samples from the central two-thirds of the placental disk, umbilical cord, amniotic membranes and of any grossly visible lesions were collected for histological analysis. All post-mortem tissue specimens obtained by MITS underwent microbiological and histological analyses by microbiologists and pathologists at the Barcelona Hospital Clinic laboratory (Spain), using routine histopathology, immunohistochemistry and microbiology methods described elsewhere [17]. Additionally, confirmatory SARS-CoV-2 RT-PCR tests were performed on fetal lung and placental (fetal side) tissue of participants and offsprings with positive SARS-CoV-2 antibodies (total Ig and IgM) as per the World Health Organization (WHO) guidelines [18]. rRT-PCR was performed using LightMix ModularDx Kit SARS-CoV (COVID19) E-gene (Tib Molbiol, Roche Diagnostics) with Roche LightCycler® 480 II according to manufacturer’s instructions.
Maternal serum levels of PE biomarkers (sFlt-1 and PIGF) were measured in serum with Elecsys® Immunoassays using the Cobas 6000 analyzer system (Roche Diagnostics, Penzberg, Germany). The intra- and inter-assay coefficients of variation for both biomarkers were lower than 4 and 8%, respectively.
Determination of the cause of death
The fetal cause of death was established by a panel of specialists comprising pathologists, obstetricians, and pediatricians (with expertise in neonatology) that reviewed all clinical and laboratory information of study participants. Using the WHO application of International Classification of diseases- 10 (ICD-10) to deaths during the perinatal period (ICD-PM), immediate and underlying causes in the causal chain of events leading to death were assigned. Maternal conditions were included in the sequence of events that led to fetal or neonatal death [17]. In stillbirths, the causes of death were classified as: (1) congenital malformations, (2) congenital infection, (3) intrauterine hypoxia, (4) disorders related with pregnancy and fetal development. For neonates, the causes of death were classified into: (1) perinatal asphyxia or hypoxia 2) infectious diseases, (2) intrapartum complications, (3) preterm complications, (4) congenital malformations, (5) other conditions.
Statistical analysis and definitions
SARS-CoV-2 seropositivity was defined as the yielding a positive result in any antibody tests against SARS-CoV-2 (total Ig, IgG and/or IgM). Fetal SARS-CoV-2 infection was classified as confirmed, possible, unlikely, or not infected using the Shah’s Classification System for Maternal–Fetal-Neonatal SARS-CoV-2 Infections [19]. For cases of congenital infection in fetal death defining a ‘confirmed’ case requires the detection of SARS-CoV-2 by PCR (positive PCR) from either fetal or placental tissue [19]. The sFtl-1/PIGF ratio cut-offs were categorized as normal/low risk (< 38) and moderate to high risk (≥ 38) [6]. Gestational hypertension was defined as systolic blood pressure ≥ 140 mm Hg or diastolic blood pressure of ≥ 90 mm Hg or more, or both, on two occasions at least 4 h apart after 20 weeks of gestation [20]. Clinical PE was defined as new onset hypertension (systolic blood pressure ≥ 140 mmHg and/or diastolic pressure ≥ 90 mmHg) with new onset proteinuria and/or evidence of multi-organ dysfunction; and eclampsia as generalized seizures together with PE criteria [20].
Participant´s demographic, clinical and obstetric variables included age, area of residence (Maputo city and outside Maputo city), history of gestational hypertension during current pregnancy, infectious diseases (HIV, tuberculosis, syphilis) and antenatal care history. Gravidity was categorized as primigravida (first pregnancy) and multigravida (≥ 2 pregnancies). Regarding the offspring, the gestational age at birth was reported as completed weeks and grouped into < 28 weeks (extremely preterm); 28–31 weeks (very preterm); 32–36 weeks (moderate to late preterm); and ≥ 37 weeks (term); type of death (fetal and neonatal) and birth weight were included. Birth weight was categorized as extremely low (< 1000 g), very low (1000 g – 1499 g), low (1500 g – 2499 g) and normal birth weight (≥ 2500 g). Unless specified otherwise, the term offspring is used to refer to both fetuses and neonates.
Quantitative variables were expressed as mean ± standard deviation (SD) for data that follows the normal distribution and other cases were described using the median and interquartile range (IQR). Categorical variables were expressed as frequencies and percentages. Seroprevalence was reported as a ratio of seropositive participants to the total number of study participants or samples tested, with a 95% confidence interval (95CI). The Chi-square and Fisher´s exact test were used to compare the prevalence of clinical and biomarkers of PE between pregnant women according to their SARS-CoV-2 serostatus. For non-parametric data, Wilcoxon rank sum test were used. Odds ratios were calculated to assess the strength of associations. All statistical analyses were performed using Stata v 16.0 software, with a two-sided p-value of < 0.05 considered statistically significant.
Results
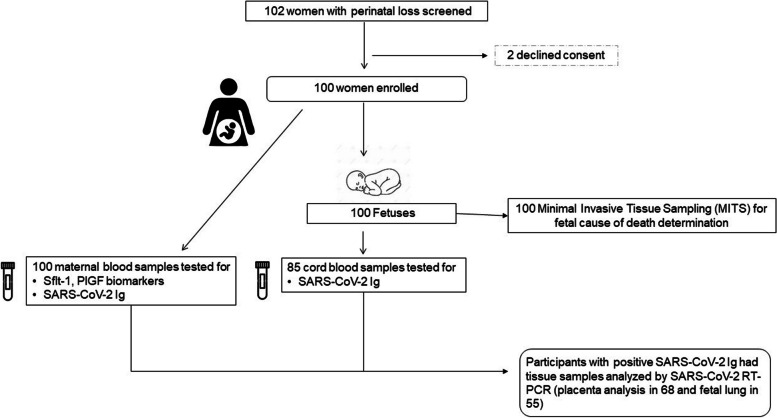
Between March 2021 and April 2022, a total of 102 eligible women were invited to participate in the study; two of them declined consent and 100 women were enrolled in the study. The study flow chart is shown in Fig. 1. A total of 100 maternal and 85 cord blood samples were analyzed for SARS-CoV-2 total Ig and RT-PCR was performed on fetal-side placental and fetal lung tissues of participants who tested positive for total Ig and IgM.
Fig. 1.
Study profile
Table 1 presents baseline characteristics of participants; the mean maternal age was 29 years (SD ± 6.74), 16% were HIV-infected, 40% had a history of hypertension, 58 had PE and nine had eclampsia. Among their offspring, 98 were stillbirths and two were early neonatal deaths.
Table 1.
Demographic and clinical characteristics of study participants
| Women | N = 100 |
| Age, mean (SD) | 29.29 (6.74) |
| Gestational age, mean (SD) | 34.20 (0.392) |
| Area of residence | |
| Maputo city | 64 (64.0) |
| Outside Maputo city | 36 (36.0) |
| Gravidity | |
| Primigravida | 22 (22.2) |
| Multigravida | 62 (77.8) |
| Pregnancy type | |
| Singleton | 99 (99.0) |
| Multiplea | 1 (1.0) |
| Hemoglobin (g/dL), mean(SD) [n] | 11.4 (0.28) [49] |
| History of severe anemia (< 7 g/dL) | 4 (4.0) |
| HIV infection | 16 (16.0) |
| Tuberculosis | 1 (1.0) |
| Syphilis | 1 (1.0) |
| History of gestational hypertension during current pregnancy | 40 (40.0) |
| Systolic arterial blood pressure at admission(mmHg), mean (SD) [n] | 146.8 (31.17) [100] |
| Diastolic arterial blood pressure at admission (mmHg), mean (SD) [n] | 97.8 (20.30) [100] |
| Preeclampsia | 58 (58.0) |
| Eclampsia | 9 (9.0) |
| Mode of delivery | |
| Vaginal | 89 (89.0) |
| Caesarean section | 11 (11.0) |
| Preterm delivery | 72 (72.0) |
| Offspring, N | N = 100 |
| Male | 67 (67.0) |
| Gestagional age at birth, weeks | |
| Extremely preterm (< 28) | 3 (3.0) |
| Very preterm (28–31) | 27 (27.0) |
| Moderate to late preterm (32–36) | 42 (42.0) |
| Term (≥ 37) | 28 (28.0) |
| Birth weight (grams), mean(SD) [n] | 1887.59 (940.1) [100] |
| Extremely low birth weight (< 1000) | 11 (11.0) |
| Very low birth weight (1000—1499) | 28 (28.0) |
| Low birth weight (1500—2499) | 27 (27.0) |
| Normal birth weight ≥ 2500 | 34 (34.0) |
| Type of death | |
| Fetal | 98 (98) |
| Neonatal | 2 (2) |
aOnly one twin was included in the study
Values are number n (%) unless indicated otherwise
The prevalence of SARS-CoV-2 antibodies in maternal and cord blood is presented in Table 2. SARS-CoV-2 total Ig was detected in 68 out of 100 maternal blood samples [68%; 95% CI: 57.9–77] and in 55 out of 85 cord blood samples analysed [64.7%; 95% CI: 53.6–74.8] (with 15 cord blood samples having insufficient volume for testing). For specific Ig isotypes, 60 out of 66 maternal samples were positive for IgM [90.9%; 95% CI: 81.2–96.6], and 53 out of 66 samples were positive for IgG [90.9%; 95% CI: (81.2–96.6)]. Notably, 49 of these samples [49/66; 74.2%; 95%CI: (62 – 84.2)] had both IgM and IgG antibodies. Among the 55 SARS-CoV-2 seropositive cord blood samples, IgM was detected in 18 out of 35 samples [51.4%; 95% CI: (34 – 68.6)], and IgG was detected in 19 out of 27 samples [70.4%; 95% CI: (49.8 – 86.2)]. Eleven cord blood samples [52.3%; 95%CI: (29.8 – 74.9)] tested positive for both IgM and IgG, while the remaining samples had insufficient volume for comprehensive anti-SARS-CoV IgM and/or IgG analyses.
Table 2.
Seroprevalence of anti SARS-CoV-2 immunoglobulins in maternal and cord blood samples
| Maternal blooda | Cord bloodb | |||||
|---|---|---|---|---|---|---|
| Positive | Positive | |||||
| n | N | % (95CI) | n | N | % (95CI) | |
| Total Ig | 68 | 100 | 68 (57.9—77) | 55 | 85 | 64.7 (53.6 – 74.8) |
| By Isotopes | ||||||
| IgM | 60 | 66 | 90.9 (81.2–96.6) | 18 | 35 | 51.4 (34 – 68.6) |
| IgG | 53 | 66 | 80.3 (68.7 – 89.1) | 19 | 27 | 70.4 (49.8 – 86.2) |
95CI 95% Confidence Interval
aTwo maternal blood samples out of the 68 that tested total Ig positive had insufficient volume for anlysing the IgM and IgG serotypes
bTotal Ig were analyzed in 85 available cord blood samples; the IgM and IgG were analyzed in remaining 35 and 27 samples, respectively, out of the 55 with positive Ig total ¡
Among the 68 placental biopsies tested, 3 (4.4%) were positive for SARS-CoV-2 by RT-PCR, while all 55 fetal lung biopsies were negative (see Supplementary Material, Table S1). These three positive biopsies corresponded to three out of the 18 stillborn fetuses with positive IgM in cord blood. The immediate cause of death of these three cases was intrauterine hypoxia in two and pneumonia in the third. Overall, for the 100 offspring included in the study, the most common causes of death were intrauterine hypoxia (68%) and pneumonia (24%) (Table 3).
Table 3.
Identified causes of perinatal death in study participants by MITS
| Immediate cause n [% (95 CI)] | Comorbid or antecedent cause n (% [95CI]) | |
|---|---|---|
| Perinatal deaths, N = 100 | ||
| Stillbirths (n = 98) | ||
| Congenital birth defectsa | 2 (2% [0.5 – 7.8]) | 1 (5.2% [0.6 – 32.5]) |
| Congenital Infection | 25 (25% [17.4 – 34.5]) | 8 (42.1% [21.5 – 65.9]) |
| Pneumoniab | 24 (24% [16.6 – 33.4]) | 4 (21.1% [7.5 – 46.5]) |
| Cytomegalovirus | 1 (1% [0.1 – 6.9]) | 4 (21.1% [7.5 – 46.5]) |
| Disorders related to length of gestation and fetal growthc | 5 (5% [2.1 – 11.6]) | 0 |
| Umbilical cord complicationsd | 0 (0) | 2 (10.5% [2.3 – 36.1]) |
| Intrauterine hypoxia | 66 (66% [56.1 – 74.7]) | 3 (15.8% [4.7 – 41.3]) |
| Early neonatal deaths (n = 2) | ||
| Intrauterine hypoxia and asphyxia | 2 (2% [0.5 – 7.8]) | |
aOne congenital malformation of limbs, one gastrochisis as main fetal cause of death and one congenital CNS malformation as contributing condition to cause of death
b22 pneumonia cases had maternal chorioamnionitis and evidence of inflammation in fetal lung tissue and two cases fetal lung inflammation only; in nine pneumonia cases, the following pathogens were isolated: Mycoplasma (one case), Streptococcus agalactiae (seven cases) and Staphylococcus aureus (one case)
cFour fetal meconium aspirations and one macrosomia
dCord compression
Table 4 shows the prevalence of clinical PE and sFlt-1/PIGF ratio in pregnant women by SARS-CoV-2 serostatus. Although the difference was not statistically significant, the proportion of women with clinical PE was higher in women who were SARS-CoV-2 seropositive (49/68; 72.1%) than in those who were seronegative (17/32; 27.9%). Angiogenic biomarkers showed a similar pattern with a higher proportion of SARS-CoV-2 seropositive women having a moderately to significantly elevated sFlt-1/PIGF ratio (≥ 38) than those who were seronegative [71.2% (47/68) versus 28.8% (19/32), p = 0.339].
Table 4.
Prevalence of preeclampsia and sFlt-1/PIGF ratio by maternal SARS-CoV-2 serostatus in study participants
|
SARS-CoV-2 seronegative n (%) N = 32 |
SARS-CoV-2 seropositive n (%) N = 68 |
p-value | OR [95%CI] | p-valuec | |
|---|---|---|---|---|---|
| Clinical Preeclampsia, (N = 100) | 0.209a | 1.8 [0.73 – 4.26] | 0.207 | ||
| No | 15 (41.7) | 19 (59.4) | |||
| Yes | 17 (27.9) | 49 (72.1) | |||
| sFlt-1/PIGF ratio, (N = 100) | |||||
| Median (IQR) | 58.2 (2.19–1163.7) | 131.8 (0.4–2439) | 0.205b | 1.01 [0.99- 1.002] | 0.199 |
| Normal/Low (< 38) | 13 (38.2) | 21 (61.8) | 0.337a | 1.5 [0.64 – 3.67] | 0.339 |
| Moderate to high (≥ 38) | 19 (28.8) | 47 (71.2) | |||
aChi-square
bWilcoxon rank sum test
cLogistic regression
sFlt-1 soluble Fms-like tyrosine kinase 1; PIGF- Placenta growth factor
Discussion
To our knowledge, this is the first study to report SARS-CoV-2 vertical transmission in stillbirths in the African region. We observed a high seroprevalence of SARS-CoV-2 among unvaccinated Mozambican pregnant women with perinatal loss during the second year of the COVID-19 pandemic. The study findings also support the evidence of increased sFlt-1/PIGF ratio in SARS-CoV-2 seropositive pregnant women.
SARS-CoV-2 seroprevalence among the study women was 68%, much higher than the rates reported during the first year of the pandemic in pregnant women of the same province which ranged from 9.2% to 11.3% [21, 22]. Similar increasing trends in SARS-CoV-2 seroprevalence were reported in neighboring countries during subsequent COVID-19 epidemic waves [23–25]. During the second year of the pandemic, seroprevalence estimates among pregnant women in South Africa was 64%, and 82.1% in unvaccinated pregnant women in Kenya [24, 25]. In Malawi, a multicenter study found a prevalence of COVID-19 infection of 60%, 70%, and 53% among expectant mothers during the second, third, and fourth waves, respectively [23]. This is also consistent with the high seropositivity rate (72.7%) reported among Indian pregnant women during the same period [26].
The study findings show the presence of SARS-CoV-2 IgM antibodies in the cord blood of 18 stillborn fetuses with confirmed SARS-CoV-2 infection of the fetal side placenta tissue in three of these cases. Given that maternal IgM antibodies do not cross the placental barrier, these findings suggest in utero transmission [18]. It could be hypothesized that placental exosomes may have triggered an adaptive immune response to SARS-CoV-2 in the fetuses [27]. However, maternal SARS-CoV-2 infection with placental involvement has also been associated with intrauterine transmission of the virus [28, 29]. For instance, two cases of vertical transmission were reported in neonates born to mothers with COVID-19 where viral RNA was detected on placental samples [28]. Similar findings, with syncytiotrophoblast infection were reported in South Africa [29]. Although placental infection with SARS-CoV-2 does not always result in fetal infection, it is a likely risk factor for maternal–fetal transmission [30]. A recent study conducted in Italy in 25 expectant mothers with SARS-CoV-2 infection and 24 controls found significantly lower levels of Interferon-γ (IFN-γ) in both peripheral and cord blood of SARS-CoV-2-positive mothers with mild symptoms, suggesting that infection can affect the fetal microenvironment in the absence of severe maternal symptoms and can increase susceptibility to infection [31]. While vertical transmission of SARS-CoV-2 is reported to range from 1 to 4%, [18, 30], positivity rates and timing of exposure have been described to vary between regions [32].
Although the mechanisms of maternal–fetal transmission are not well understood, severe SARS-CoV-2 infection and anti-SARS-CoV-2 IgM positivity in pregnant women have been associated with increased placenta abnormalities or insufficiency [30, 32, 33]. Evidence shows that placental trophoblast necrosis together with chronic histiocytic intervillositis are common findings in placentas associated with intrauterine SARS-CoV-2 transmission [34]. This placental inflammation can trigger a severe fetal inflammatory response syndrome, leading to fetal hypoxia, blood vessel damage, and blood–brain barrier compromise [34, 35]. In our study population, intrauterine hypoxia accounted for most of the perinatal deaths. Additionally, emerging variants of SARS-CoV-2 and their impact on immunomodulatory responses in pregnancy may also increase transmissibility from mother to fetus, emphasizing the need to better understand fetal and neonatal vulnerability to SARS-CoV-2 infection [31, 36].
Apart from the adverse maternal and fetal outcomes that are associated with SARS-CoV-2 infection in pregnancy, this research found a marked increase of sFlt-1/PIGF ratio in SARS-CoV-2 seropositive women compared to those who were seronegative. Consistent with other studies, high angiogenic imbalance increases the likelihood of developing PE during pregnancy [37, 38]. A meta-analysis of 28 studies involving 790,954 pregnant women, found that women with SARS-CoV-2 infection were more likely to develop PE with severe features (OR 1.76; 95CI [1.18 to 2.63]), eclampsia (OR 1.97; 95CI [1.01 to 3.84]), and HELLP syndrome (OR 2.10; 95CI [1.48 to 2.97]) than uninfected women [39]. Furthermore, SARS-CoV-2 infection in the first or second trimester may increase the risk of having PE later in pregnancy [37]. However, some studies observed a higher angiogenic imbalance in pregnant women with hypertensive disorders (HDP) and no SARS-CoV-2 infection compared to those with infection. This can be attributed to the severity of the underlying hypertensive disorder and placental insufficiency that this group of women had and not necessarily with SARS-CoV-2 infection [40]. To date, sFlt-1/PIGF ratio is considered not to be a good predictor of severity of SARS-CoV-2 infection, but it is a reliable predictor of PE [37, 38].
A potential study limitation to consider, is the fact that some serum samples had insufficient volume, resulting in incomplete testing of positive blood samples for SARS-CoV-2 specific IgM and IgG antibodies—especially for cord blood. Despite this limitation, the study findings provide unique data on congenital SARS-CoV-2 infection in stillborns and significantly contributes to the scarce body of literature on SARS-CoV-2 epidemiology in the SSA region.
Conclusions
SARS-CoV-2 seroprevalence was high among unvaccinated pregnant women during the second year of the pandemic in this area of southern SSA, with increased PE biomarkers in women with SARS-CoV-2 antibodies. Further research is required to fully understand the impact of SARS-CoV-2 exposure on fetal and neonatal health.
Supplementary Information
Acknowledgements
The authors acknowledge and extend their gratitude to the study participants. We would also like to thank all the MIBio study personnel, Laura Garcia Otero and Mireia Piqueras for contributing to project coordination and Alba Morató for statistical analysis support. ISGlobal acknowledges support from the grant CEX2023-0001290-S funded by MCIN/AEI/ 10.13039/501100011033, and support from the Generalitat de Catalunya through the CERCA Program. We also acknowledge support from the Department of Research and Universities of the Government of Catalonia (2021 SGR 01573) for the present publication. CISM is supported by the Government of Mozambique and the Spanish Agency for International Development (AECID).
Abbreviations
- Ig
Immunoglobulin
- PE
Pre-eclampsia
- SSA
Sub-Saharan Africa
- sFlt-1
Fsms-like tyrosine kinase 1
- PlGF
Placental Growth Factor
Authors’ contributions
Conceived and designed the study: CM and RG. Gave inputs to study design: TN, CC, AM, EL, JS, MM-R, MMa, MJM, JO, NR, CM, RG. TN, CC, AM, EL, JS, JNC, MM-R, MMa, MJM, JO, NR, CM, and RG contributed to data acquisition. MC did the statistical analysis. MC and RG wrote the first draft of the manuscript. All authors interpreted the data and critically reviewed the manuscript. All authors had access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding
This study was funded by the Bill and Melinda Gates Foundation (BMGF), grant number INV-023750. None of the funders or supporting institutions had any role on study design, data collection, analysis, report writing or publication plan.
Availability of data and materials
De-identified individual data and the data dictionary are not openly available due to reasons of sensitivity and are available to others upon reasonable request to the corresponding author. A data transfer agreement will be signed between the project consortium and the requesting institution before data sharing. Data are located in controlled access data storage at the Barcelona Institute for Global Health.
Declarations
Ethics approval and consent to participate
The study protocol and informed consent form were reviewed and approved by the National Committee on Health Bioethics of Mozambique (Ref: 392/CNBS/20) and the Hospital Clinic of Barcelona Ethics Committee (Spain). Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sheikh J, Lawson H, Allotey J, Yap M, Balaji R, Kew T, et al. Global variations in the burden of SARS-CoV-2 infection and its outcomes in pregnant women by geographical region and country’s income status: a meta-analysis. BMJ Glob Heal. 2022;7:e010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carbone L, Raffone A, Sarno L, Travaglino A, Saccone G, Gabrielli O, et al. Invasive prenatal diagnosis during COVID-19 pandemic. Arch Gynecol Obstet. 2022;305:797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sathiya R, Rajendran J, Sumathi S. COVID-19 and preeclampsia: overlapping features in pregnancy. Rambam Maimonides Med J. 2022;13:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nachega JB, Sam-Agudu NA, Machekano RN, Rosenthal PJ, Schell S, de Waard L, et al. Severe acute respiratory syndrome coronavirus 2 infection and pregnancy in Sub-Saharan Africa: A 6-country retrospective cohort analysis. Clin Infect Dis an Off Publ Infect Dis Soc Am. 2022;75:1950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Musarandega R, Nyakura M, Machekano R, Pattinson R, Munjanja SP. Causes of maternal mortality in Sub-Saharan Africa: a systematic review of studies published from, to 2020. J Glob Health. 2015;2021:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stepan H, Herraiz I, Schlembach D, Verlohren S, Brennecke S, Chantraine F, et al. Implementation of the sFlt-1/PlGF ratio for prediction and diagnosis of pre-eclampsia in singleton pregnancy: implications for clinical practice. Ultrasound Obstet Gynecol Off J Int Soc Ultrasound Obstet Gynecol. 2015;45:241–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papageorghiou AT, Deruelle P, Gunier RB, Rauch S, García-May PK, Mhatre M, et al. Preeclampsia and COVID-19: results from the INTERCOVID prospective longitudinal study. Am J Obstet Gynecol. 2021;225:289.e1–289.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Geneva WHO. WHO COVID-19 dashboard - Mozambique. 2024. https://data.who.int/dashboards/covid19/cases?m49=508&n=c.
- 9.Mayor A, Menéndez C, Walker PGT. Targeting pregnant women for malaria surveillance. Trends Parasitol. 2019;35:677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Global Influenza Programme. WHO Consultation to Adapt Influenza Sentinel Surveillance Systems for Including COVID-19 (Virtual meeting): Meeting Report. Geneva: World Health Organization; 2020.
- 11.Hajissa K, Islam MA, Hassan SA, Zaidah AR, Ismail N, Mohamed Z. Seroprevalence of SARS-CoV-2 Antibodies in Africa: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health. 2022;19(12):7257. 10.3390/ijerph19127257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rakislova N, Fernandes F, Lovane L, Jamisse L, Castillo P, Sanz A, et al. Standardization of minimally invasive tissue sampling specimen collection and pathology training for the child health and mortality prevention surveillance network. Clin Infect Dis. 2019;69(Suppl 4):S302–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duggan J, Andrews N, Brooks T, Migchelsen S, Bown A. Evaluation of Siemens Atellica-IM Total (COV2T) SARS-CoV-2 serology assay for the detection of anti-SARS-CoV-2 total antibodies. Public Health England; 2020.
- 14.Duggan J, Andrews N, Otter A, Brooks T. Evaluation of Siemens Atellica-IM Total (COV2T) SARS-CoV-2 serology assay for the detection of anti-SARS-CoV-2 total antibodies. Public Health England; 2021.
- 15.Dobaño C, Vidal M, Santano R, Jiménez A, Chi J, Barrios D, et al. Highly sensitive and specific multiplex antibody assays to quantify immunoglobulins M, A, and G against SARS-CoV-2 Antigens. J Clin Microbiol. 2021;59:e01731–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khong TY, Mooney EE, Ariel I, Balmus NCM, Boyd TK, Brundler M-A, et al. Sampling and definitions of placental lesions: amsterdam placental workshop group consensus statement. Arch Pathol Lab Med. 2016;140:698–713. [DOI] [PubMed] [Google Scholar]
- 17.Menendez C, Castillo P, Martínez MJ, Jordao D, Lovane L, Ismail MR, et al. Validity of a minimally invasive autopsy for cause of death determination in stillborn babies and neonates in Mozambique: n observational study. PLOS Med. 2017;14: e1002318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization. Definition and categorization of the timing of mother-to-child transmission of SARS-CoV-2. Geneva: World Health Organization; 2021.
- 19.Shah PS, Diambomba Y, Acharya G, Morris SK, Bitnun A. Classification system and case definition for SARS-CoV-2 infection in pregnant women, fetuses, and neonates. Acta Obstet Gynecol Scand. 2020;99:565–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.ACOG. The American College of Obstetricians and Gynecologists, 2020.Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin Summary, Number 222. Obstet Gynecol. 2020;2020(135):1492–5. [DOI] [PubMed] [Google Scholar]
- 21.Charles CM, Osman NB, Arijama D, Matingane B, Sitoé T, Kenga D, et al. Clinical and epidemiological aspects of SARS-CoV-2 infection among pregnant and postpartum women in Mozambique: a prospective cohort study. Reprod Health. 2022;19:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.González R, Nhampossa T, Figueroa-Romero A, Mendes A, Mazuze M, García-Otero L, et al. SARS-CoV-2 seropositivity and hiv viral load among mozambican pregnant women. JAIDS J Acquir Immune Defic Syndr. 2023;92:115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mndala L, Monk EJM, Phiri D, Riches J, Makuluni R, Gadama L, et al. Comparison of maternal and neonatal outcomes of COVID-19 before and after SARS-CoV-2 omicron emergence in maternity facilities in Malawi ( MATSurvey ): data from a national maternal surveillance platform. Lancet Glob Heal. 2022;10:e1623–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawry S, Le Roux J, Wolter N, Mbatha P, Bhiman J, Balkus J, et al. High prevalence of SARS-CoV-2 antibodies in pregnant women after the second wave of infections in the inner-city of Johannesburg, Gauteng Province, South Africa. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2022;125:241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucinde RK, Mugo D, Bottomley C, Karani A, Gardiner E, Aziza R, et al. Sero-surveillance for IgG to SARS-CoV-2 at antenatal care clinics in three Kenyan referral hospitals: epeated cross-sectional surveys 2020–21. PLoS ONE. 2022;17: e0265478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma KA, Singh N, Hillman S, Mathur P, Yadav K, Garg A, et al. Seroprevalence of SARS-CoV-2 antibodies among first-trimester pregnant women during the second wave of the pandemic in India. Int J Gynecol Obstet. 2023;160:74–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dogra N, Ledesma-Feliciano C, Sen R. Developmental aspects of SARS-CoV-2, potential role of exosomes and their impact on the human transcriptome. J Dev Biol. 2021;9:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patanè L, Morotti D, Giunta MR, Sigismondi C, Piccoli MG, Frigerio L, et al. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019–positive mothers and neonates at birth. Am J Obstet Gynecol MFM. 2020;2:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Birkhead M, Glass AJ, Allan-Gould H, Goossens C, Wright CA. Ultrastructural evidence for vertical transmission of SARS-CoV-2. Int J Infect Dis Off Publ Int Soc Infect Dis. 2021;111:10–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Çelik E, Vatansever C, Ozcan G, Kapucuoglu N, Alatas C, Beşli Y, et al. Placental deficiency during maternal SARS-CoV-2 infection. Placenta. 2021;117:47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cennamo M, La Civita E, Sarno L, Carbone G, Di Somma S, Cabaro S, et al. Low interferon-γ levels in cord and peripheral blood of pregnant women infected with SARS-CoV-2. Microorganisms. 2023;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allotey J, Chatterjee S, Kew T, Gaetano A, Stallings E, Fernández-García S, et al. SARS-CoV-2 positivity in offspring and timing of mother-to-child transmission: living systematic review and meta-analysis. BMJ. 2022;376: e067696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwartz DA, Morotti D. Placental Pathology of COVID-19 with and without fetal and neonatal infection: trophoblast necrosis and chronic histiocytic intervillositis as risk factors for transplacental transmission of SARS-CoV-2. Viruses. 2020;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alcover N, Regiroli G, Benachi A, Vauloup-Fellous C, Vivanti AJ, De Luca D. Systematic review and synthesis of stillbirths and late miscarriages following SARS-CoV-2 infections. Am J Obstet Gynecol. 2023. 10.1016/j.ajog.2023.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernandez-Lopez JM, Hernandez-Medina C, Medina-Corvalan C, Rodenas M, Francisca A, Perez-Garcia C, et al. Neuronal progenitors of the dentate gyrus express the SARS-CoV-2 cell receptor during migration in the developing human hippocampus. Cell Mol Life Sci. 2023;80:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ismael N, van Wyk S, Tegally H, Giandhari J, San JE, Moir M, et al. Genomic epidemiology of SARS-CoV-2 during the first four waves in Mozambique. PLOS Glob Public Heal. 2023;3: e0001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nobrega GM, Guida JP, Novaes JM, Solda LM, Pietro L, Luz AG, et al. Role of biomarkers (sFlt-1/PlGF) in cases of COVID-19 for distinguishing preeclampsia and guiding clinical management. Pregnancy Hypertens. 2023;31:32–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Malicka E, Szymusik I, Rebizant B, Dąbrowski F, Brawura-Biskupski-Samaha R, Kosińska-Kaczyńska K. SFlt-1/PlGF ratio is not a good predictor of severe COVID-19 nor of adverse outcome in pregnant women with SARS-CoV-2 infection-a case-control study. Int J Environ Res Public Health. 2022;19:15054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Conde-Agudelo A, Romero R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol. 2022;226:68–89.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soldavini CM, Di Martino D, Sabattini E, Ornaghi S, Sterpi V, Erra R, et al. sFlt-1/PlGF ratio in hypertensive disorders of pregnancy in patients affected by COVID-19. Pregnancy Hypertens. 2022;27:103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified individual data and the data dictionary are not openly available due to reasons of sensitivity and are available to others upon reasonable request to the corresponding author. A data transfer agreement will be signed between the project consortium and the requesting institution before data sharing. Data are located in controlled access data storage at the Barcelona Institute for Global Health.