Abstract
Background
Remnant cholesterol (RC), a potent atherogenic lipid, has been shown to be strongly correlated with insulin resistance and the pathogenesis of diabetes mellitus. However, the relationship between RC and normoglycemia reversal in individuals with impaired fasting glucose (IFG) is crucial and remains unclear. This investigation, which aimed to clarify this association, is important for understanding and potentially improving the management of diabetes.
Method
This study, which included 15,019 IFG participants from 11 Chinese cities between 2010 and 2016, was conducted with a rigorous research process. Cox regression analysis revealed intriguing findings regarding the relationship between RC and normoglycemia reversal in individuals with IFG. Potential nonlinear associations were further explored via smooth curve-fitting techniques and 4-knot restricted cubic spline functions, ensuring a comprehensive analysis. To examine the validity of the results, an array of subgroup and sensitivity analyses were conducted, further bolstering the robustness of the findings.
Results
By the end of the 2.89-year median follow-up period, 6,483 of the 15,019 IFG participants (43.17%) had reverted to normoglycemia. The findings, which reveal that increased RC levels are inversely associated with the likelihood of normoglycemia reversal, are novel and significant. According to the fully adjusted Cox proportional hazards model analysis, an increase of one standard deviation in RC was associated with a 20% decrease in the likelihood of normoglycemia reversal among IFG participants (HR: 0.80, 95% CI: 0.77–0.82). A nonlinear association between RC and normoglycemia reversal was observed, with an inflection point at 41.37 mg/dL. This suggests that the growth rate of the likelihood of reversion decreased and stabilized after the inflection point was reached. Moreover, significant interactions were observed between the age groups, providing a more nuanced understanding of this complex relationship.
Conclusion
Among Chinese adults with IFG, RC exhibited a negative nonlinear relationship with the probability of normoglycemia reversal. When RC levels reached or exceeded 41.38 mg/dL, the probability of achieving normoglycemia progressively diminished and subsequently stabilized. Maintaining RC levels below 41.38 mg/dL can significantly improve the probability of normoglycemia reversal among individuals with IFG, especially those aged 60 years or older.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-024-02286-8.
Keywords: Impaired fasting glucose, Remnant cholesterol, Reversion to normoglycemia, Nonlinear association, Competitive risk model
Introduction
Prediabetes, characterized by impaired glucose tolerance (IGT) and impaired fasting glucose (IFG), is a critical stage preceding the onset of type 2 diabetes and plays a crucial role in diabetes prevention and management [1]. Studies have indicated that approximately 374 million adults worldwide are affected by prediabetes [2]. In 2021, individuals with IFG constitute approximately 5.8% of the global population, and estimates suggest that this percentage may increase to 6.5% by 2045. Similarly, the prevalence of IGT was predicted to be 9.1% in 2021, with predictions indicating a growth rate of 10.0% by 2045 [3]. Prediabetes not only puts individuals at a greater risk of developing diabetes but also correlates with an increased likelihood of cardiovascular disease, stroke, and other long-term health issues [4–7]. However, approximately 20–50% of individuals with prediabetes revert to normal blood glucose levels instead of developing type 2 diabetes, which is contingent upon various factors [1, 8]. Studies have shown that patients who revert to normoglycemia exhibit a significantly reduced risk of diabetes onset [9]. Moreover, research has shown that reversion to normoglycemia can mitigate the risk of cardiovascular issues and other persistent health conditions [10–13]. Consequently, it is imperative to implement effective interventions to facilitate the return of patients with IFG to normoglycemia.
Remnant cholesterol (RC) is a potent inducer of atherosclerosis. It predominantly comprises intermediate-density lipoproteins (IDLs) and very low-density lipoproteins (VLDLs) during fasting and chylomicron remnants after meals. It constitutes a triglyceride-rich lipoprotein particle (TRL) [14]. In contrast to traditional high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C), RC is an independent risk factor for atherosclerotic cardiovascular disease [15–17]. It has a robust link with adverse cardiovascular outcomes [18]. Lipoprotein particles characterized by an elevated RC typically include increased triglyceride (TG) levels. Pioneering studies among Taiwanese Chinese individuals diagnosed with diabetes have demonstrated that hypertriglyceridemia is associated with various pathological conditions, including cardiovascular diseases, albuminuria, and diabetic retinopathy [19–23]. Furthermore, elevated RC levels increase the likelihood of developing insulin resistance (IR), obesity, and metabolic syndrome [24–26]. Recent studies tracking specific groups of individuals have shown that RC can predict the onset of diabetes more effectively than can conventional lipids [27–30]. However, the extant literature provides no concrete evidence of an association between RC and normoglycemia reversal in individuals with IFG. In light of the extant literature on RC and diabetes, this study investigated the potential association between RC and normoglycemia reversal in individuals with IFG. It has been hypothesized that a higher RC may have an inverse and nonlinear relationship with the possibility of returning to normoglycemia. Thus, this study employed a large Chinese cohort to explore the association between RC and normoglycemia reversal in individuals with IFG. The findings from the secondary data analysis aimed to inform early intervention strategies for promoting normoglycemia in this population.
Methods
Data and study population
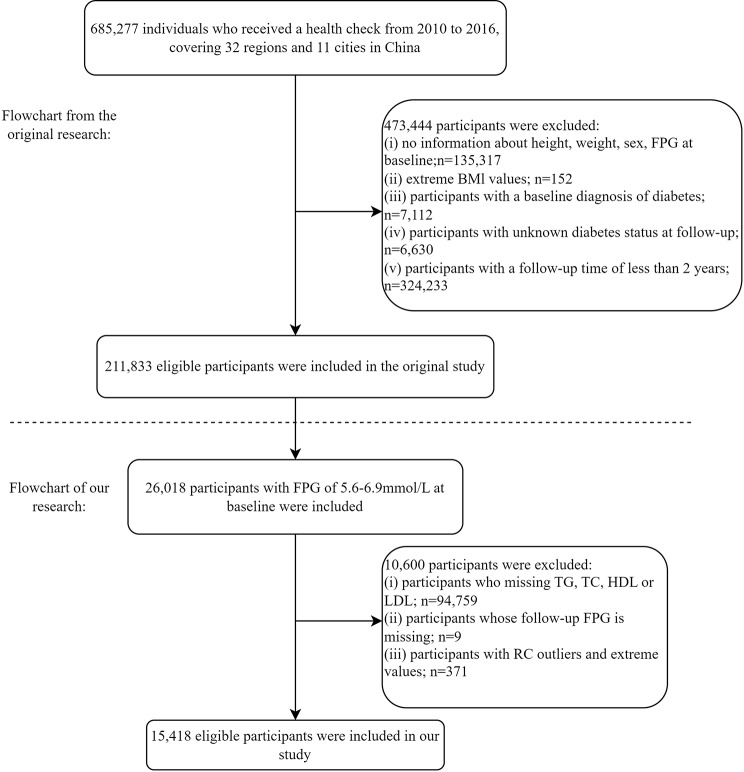
This retrospective cohort study analyzed historical data from a computerized database established by a Rich Healthcare Group in China, encompassing the medical records of health examination participants from 32 regions across 11 cities in China from 2010 to 2016. The initial cohort comprised individuals who had at least two visits during this period and were at least 20 years old, resulting in a total sample size of 685,277. Following the methodology of Chen et al.., who examined the association between body mass index (BMI) and diabetes [31], several exclusions were made: (1) participants who were missing baseline data on weight, height, sex, or fasting glucose (n = 135,317); (2) participants who were diagnosed with diabetes at baseline (n = 7,112); and (3) participants whose diabetes status was unclear throughout the entire follow-up period (n = 6,630). These participants either did not have their diabetes status evaluated or had ambiguous results during follow-up, which could compromise the reliability of the analysis. (4) Extreme BMI values (n = 152). These extreme values could be due to data entry errors or very rare outliers, which could unduly influence the statistical analysis, and (5) participants with follow-up durations < 2 years (n = 324,233). After these exclusions, the final sample consisted of 211,833 participants.
This study used a dataset from Chen et al.. to identify participants with IFG at baseline [31]. The main aim was to explore the association between RC and normoglycemia reversal among IFG participants, as well as the role of RC in this process. To address this objective, the analysis further excluded (1) participants who did not fulfill the initial diagnostic standards for IFG; (2) participants missing baseline values for HDL-C, LDL-C, total cholesterol (TC), and TG; (3) participants lacking fasting plasma glucose (FPG) data; and (4) participants with abnormal RC values, including extreme values and those that deviated more than three standard deviations (SDs) from the average. Figure 1 presents a detailed flowchart of the study procedure.
Fig. 1.
Flow chart of the study participants
Data source
The raw materials utilized in this research stemmed from the collection of information that was initially distributed by Chen et al.. in 2018, which is openly available on Dryad at www.datadryad.org. The dataset, titled “Data from: Association of body mass index and age with incident diabetes in Chinese adults: a population-based cohort study,” is available for open access at 10.5061/dryad.ft8750v [32]. This publication allows free access to CC BY-NC 4.0, enabling unrestricted sharing, copying, and adaptation for noncommercial use.
Measurement of baseline clinical indicators
At each scheduled assessment, participants were required to complete an extensive questionnaire evaluating their demographics, lifestyle, medical records, and family background regarding chronic conditions, including information on sex, age, diabetes history, family history of diabetes, drinking, and smoking status. Height and weight were measured by proficient personnel, and blood pressure was measured via a conventional mercury sphygmomanometer.
After fasting for at least 10 h, venous blood samples were collected. These samples were analyzed via an automated analyzer (Beckman 5800) to quantify the serum HDL-C, LDL-C, TC, TG, FPG, blood urea nitrogen (BUN), creatinine (Cr), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) levels. The entire data collection and analysis process was conducted meticulously and rigorously adhered to ethical guidelines and data privacy protection principles [31].
Independent variables and outcome measures
To determine RC levels, the following equation is employed: RC = TC − HDL-C − LDL-C [33]. In line with the American Diabetes Association’s (ADA) updated recommendations for 2022 [34], IFG in this investigation is characterized by an FPG concentration between 100 mg/dL (5.6 mmol/L) and 125 mg/dL (6.9 mmol/L), with no self-reported diabetes diagnosis. Normoglycemia is defined as an FPG ≤ 100 mg/dL (5.6 mmol/L).
Handling of missing values
This dataset included certain instances of missing values, as follows: diastolic blood pressure (DBP) in 5 cases (0.03%), systolic blood pressure (SBP) in 5 cases (0.03%), ALT in 34 cases (0.23%), AST in 8,062 cases (53.7%), BUN in 364 cases (2.36%), Cr in 114 cases (0.76%), smoking status in 10,407 cases (69.3%), and drinking status in 10,407 cases (69.3%). The pattern of missing data, corroborated by the correlation matrix, aligns with the idea of the missing at random (MAR) assumption [35].
Multivariate imputation was implemented via the chained equation (MICE) technique for multiple imputations to address the potential bias introduced by these missing entries. Predictive mean matching was used to manage missing continuous variables, and random forest imputation was used to address missing categorical variables. Ten iterations are performed to generate the final imputation model. This algorithm uses the interdependencies among all variables to impute missing values by considering the covariance structure among different variables, thereby augmenting the precision and reliability of the imputation process [35].
Statistical analyses
RC was stratified into quartiles. In terms of variable type, different statistical measures were used for description: the mean ± SD for normally distributed continuous variables, the median (interquartile range, IQR) for skewed continuous variables, and frequencies and percentages for categorical variables. The chi-square test was used to analyze categorical variables, whereas one-way ANOVA was used to assess differences in continuous variables across different RC quartiles. The H test was used to examine the differences between groups for continuous variables that exhibited nonnormal distributions. Kaplan‒Meier survival curves were used to estimate the cumulative rate of reversion from IFG to normoglycemia within different quartiles of RC. Statistical disparities among the groups were compared via the log-rank test.
Univariate and multivariate Cox proportional hazards regression models were used primarily to elucidate the link between RC and the restoration of normoglycemia among individuals with IFG. Before modeling, the variance inflation factor (VIF) was calculated to evaluate multicollinearity between variables. Cox regression models were adjusted by excluding variables with considerable multicollinearity (VIF values > 5), such as TC and body weight (Additional file 1: Table S1). In alignment with the STROBE guidelines and extant literature [36], nonsignificant variables identified in the univariable Cox regression analysis, such as height, were excluded. Following this initial analysis, three Cox regression models were developed: Model I without covariate adjustment; Model II was adjusted for key sociodemographic variables, including age, sex, systolic and diastolic blood pressure, alcohol consumption, smoking status, family history of diabetes, and BMI; and Model III was further adjusted for additional variables, such as FPG, LDL-C, HDL-C, TG, BUN, Cr, AST, and ALT levels, providing a comprehensive evaluation of metabolic and renal function. Additionally, a Schoenfeld residual test was performed to assess whether the influence of RC remained constant over time and to determine if the proportional hazards assumption of the Cox model was true. If this assumption is violated, the model’s results may be biased.
Considering that some patients with IFG may progress to diabetes during the follow-up period, potentially influencing the reversion to normoglycemia, a competing risk model was used to analyze the cumulative incidence. Specifically, the Fine and Gray method [37, 38], which accounts for multiple mutually exclusive events, was applied to provide a more robust and comprehensive risk assessment. Diabetes development was a competing risk that could hinder normoglycemia reversal in this study.
Additionally, a fully adjusted Cox regression model incorporating smooth curve fitting techniques and 4-knot restricted cubic splines was employed to elucidate the complex nonlinear relationship between RC and normoglycemia reversal in individuals with IFG. Previous studies and statistical guidelines have suggested that the use of four knots effectively captures the most nonlinear relationships in biomedical data. This methodological approach also enabled us to plot dose‒response curves depicting the probability of reversion at varying levels of RC. Upon identifying a nonlinear relationship in the dose‒response curve, a recursive algorithm was employed to pinpoint critical inflection points where the influence of RC on reversion to normoglycemia significantly changed. These inflection points elucidate the RC thresholds at which the relative risk of reversion begins to accelerate or decelerate.
Several sensitivity analyses were performed to confirm that the findings were as solid and dependable as possible. First, to evaluate the consistency of the effect of RC across different stratifications, the data were reanalyzed via a Cox regression model with RCs categorized into quartiles. Second, additional sensitivity factors, such as drinking, smoking status, and family history of diabetes [39–42], which were previously shown to be strongly associated with the likelihood of diabetes, were excluded, and Cox regression analysis was conducted to confirm the independent impact of RC on IFG reversal in RCs. Third, the data were reanalyzed, excluding variables with a high proportion of missing values, to determine their potential impact on the results. Finally, to capture potential nonlinear relationships between continuous covariates and the outcome, a generalized additive model (GAM) was implemented in the fully adjusted model, and the potential impact of unmeasured confounding variables on the results was estimated by computing the E value [43].
Subgroup analyses were performed to explore further the relationship between RC and normoglycemia reversal among IFG individuals with different demographic and clinical characteristics. Stratifications included sex, age (< 30, 30–45, 45–60, ≥ 60 years) [44], BMI (< 24, 24–28, ≥ 28 kg/m2) [45], TG levels (< 1.7, ≥ 1.7 mmol/L) [46], drinking, smoking status, and family history of diabetes. Each stratum was fully adjusted for the appropriate factors. To assess how RCs and different stratification variables interact, researchers have used the likelihood ratio test to identify significant differences among groups.
A two-sided P value ≤ 0.05 was considered statistically significant. All the statistical analyses were performed via R software (version 4.4.0; R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
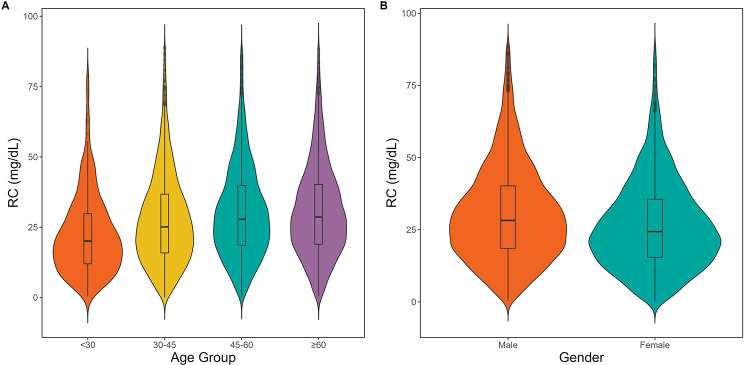
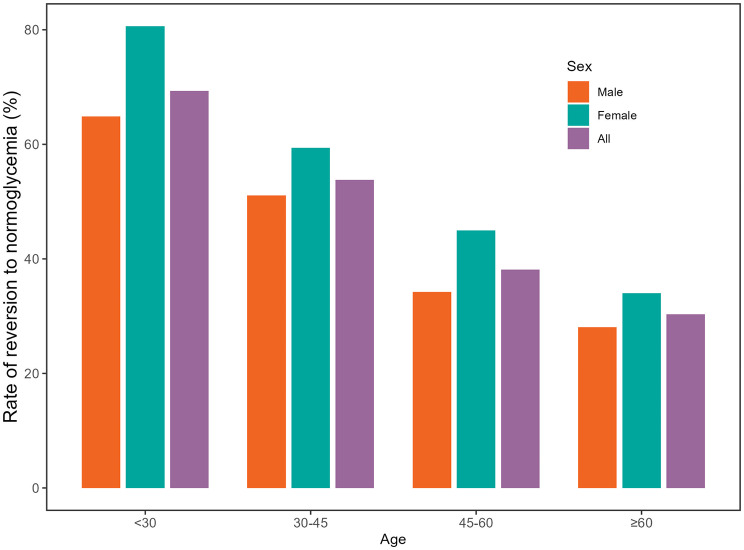
This study included 15,019 eligible participants (Fig. 1). The average age of the cohort was 50.95 ± 13.47 years, comprising 9,713 males (64.67%) and 5,306 females (35.33%). Baseline characteristics stratified by RC quartiles (Q1: <17.40, Q2:17.40–27.07, Q3:27.07–38.23, and Q4: ≥38.23) are detailed in Table 1. Significant positive trends were observed in age; weight; height; FPG, TG, TC, AST, ALT, and Cr levels; SBP; DBP; and BMI with increasing RC levels, whereas HDL-C levels tended to decrease. Additionally, higher RC levels were associated with greater proportions of males, current and past drinkers, and smokers, whereas lower proportions of females and never-smokers were found (all P < 0.001). Notably, RC levels were significantly positively associated with different age groups, and males presented markedly higher RC levels than females did (Fig. 2). Figure 3 shows that males tended to transition from IFG to normoglycemia across various age groups, and this reversion rate significantly decreased with advancing age.
Table 1.
Baseline characteristics of participants according to RC quartiles
| RC quartiles, mg/dL | P value | ||||
|---|---|---|---|---|---|
| Q1 (< 17.40) | Q2 (17.40-27.07) | Q3 (27.07–38.23) | Q4 (≥ 38.23) | ||
| N | 3787 | 3733 | 3745 | 3754 | |
| Age, years | 48.37 ± 13.80 | 50.88 ± 13.70 | 51.76 ± 13.26 | 52.80 ± 12.71 | < 0.001 |
| Gender | < 0.001 | ||||
| Male | 2174 (57.4) | 2324 (62.3) | 2541 (67.9) | 2674 (71.2) | |
| Female | 1613 (42.6) | 1409 (37.7) | 1204 (32.1) | 1080 (28.8) | |
| Height, cm | 165.97 ± 8.36 | 166.16 ± 8.27 | 167.01 ± 8.45 | 167.41 ± 8.36 | < 0.001 |
| Weight, kg | 65.66 ± 11.88 | 68.22 ± 11.57 | 70.64 ± 12.07 | 72.45 ± 12.08 | < 0.001 |
| BMI, kg/m2 | 23.73 ± 3.29 | 24.62 ± 3.23 | 25.23 ± 3.23 | 25.75 ± 3.18 | < 0.001 |
| SBP, mmHg | 125.25 ± 17.57 | 126.94 ± 17.94 | 127.92 ± 17.34 | 129.89 ± 17.67 | < 0.001 |
| DBP, mmHg | 76.78 ± 11.06 | 78.08 ± 11.19 | 78.97 ± 10.90 | 80.09 ± 11.34 | < 0.001 |
| FPG, mmol/L | 5.92 ± 0.31 | 5.93 ± 0.31 | 5.96 ± 0.32 | 6.00 ± 0.33 | < 0.001 |
| TC, mmol/L | 4.49 ± 0.78 | 4.84 ± 0.79 | 5.14 ± 0.81 | 5.64 ± 0.91 | < 0.001 |
| TG, mmol/L | 1.12 ± 0.68 | 1.43 ± 0.81 | 1.79 ± 0.95 | 2.59 ± 1.45 | < 0.001 |
| HDL-C, mmol/L | 1.45 ± 0.29 | 1.39 ± 0.27 | 1.30 ± 0.27 | 1.20 ± 0.27 | < 0.001 |
| LDL-C, mmol/L | 2.76 ± 0.65 | 2.89 ± 0.67 | 3.01 ± 0.69 | 3.10 ± 0.78 | < 0.001 |
| ALT, U/L | 23.91 ± 24.26 | 25.80 ± 20.49 | 29.13 ± 21.11 | 33.29 ± 24.57 | < 0.001 |
| AST, U/L | 24.10 ± 9.04 | 25.40 ± 11.99 | 26.53 ± 10.90 | 28.53 ± 12.71 | < 0.001 |
| BUN, mmol/L | 4.95 ± 1.22 | 5.01 ± 1.25 | 5.02 ± 1.25 | 5.04 ± 1.25 | 0.015 |
| Cr, umol/L | 71.87 ± 16.26 | 72.68 ± 15.77 | 73.46 ± 16.45 | 74.15 ± 16.14 | < 0.001 |
| Family history of diabetes | 86 (2.3) | 96 (2.6) | 98 (2.6) | 112 (3.0) | 0.285 |
| Smoking status | < 0.001 | ||||
| Current | 472 (12.5) | 601 (16.1) | 800 (21.4) | 999 (26.6) | |
| Past | 91 (2.4) | 94 (2.5) | 109 (2.9) | 114 (3.0) | |
| Never | 3224 (85.1) | 3038 (81.4) | 2836 (75.7) | 2641 (70.4) | |
| Drinking status | < 0.001 | ||||
| Current | 75 (2.0) | 93 (2.5) | 84 (2.2) | 138 (3.7) | |
| Past | 453 (12.0) | 486 (13.0) | 572 (15.3) | 516 (13.7) | |
| Never | 3259 (86.1) | 3154 (84.5) | 3089 (82.5) | 3100 (82.6) | |
Abbreviations BMI body mass index, SBP systolic blood pressure, DBP diastolic blood pressure, FPG fasting plasma glucose, TG triglyceride, TC total cholesterol, HDL-C high-density lipoprotein cholesterol, LDL-C low-density lipoprotein cholesterol, ALT alanine aminotransferase, AST aspartate aminotransferase, BUN blood urea nitrogen, Cr creatinine, RC remnant cholesterol
Fig. 2.
Distribution of RCs by age and sex. (A) Violin plots display RC distributions across age groups: under 30, 30–45, 45–60, and above 60 years, with embedded boxplots showing medians and interquartile ranges. (B) Plots comparing RC levels between males and females, highlighting differences in distribution and central tendencies
Fig. 3.
Incidence rates of reversion from IFG to normoglycemia stratified by sex and age
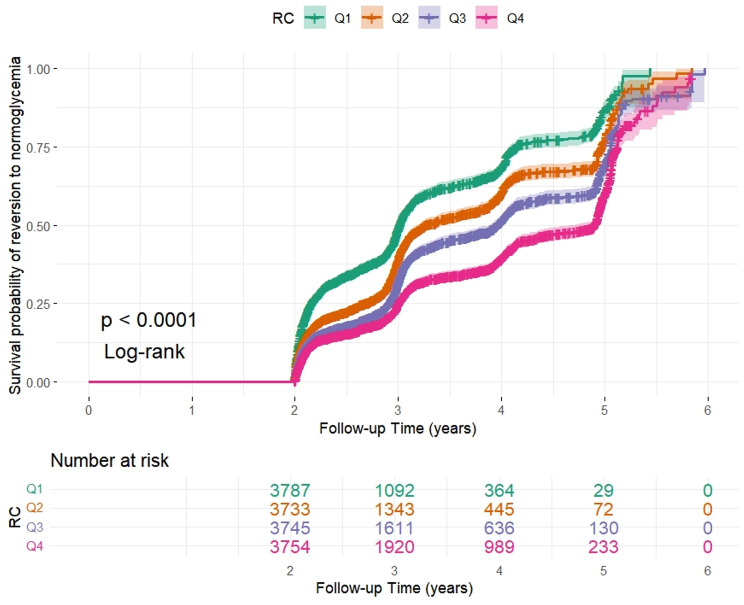
Over 2.89 years, 6,483 participants (43.17%) reverted to normoglycemia, with an incidence rate of 145 cases per 1,000 person-years. The incidence rates were 185, 152, 135, and 114 cases per 1,000 person-years for each RC quartile. The reversion rates for the total population and each RC quartile were as follows: 42.8% (42.0–43.6%), 49.9% (48.3–51.5%), 43.5% (41.9–45.1%), 41.0% (39.4–42.6%), and 36.8% (35.3–38.4%) (Table 2). The Kaplan‒Meier curves in Fig. 4 show the likelihood of shifting back to normoglycemia in IFG individuals according to RC quartiles throughout the follow-up period. The cumulative reversion risk was significantly different between the groups (P < 0.001), indicating that the proportional hazards assumption remained intact. The overall trend showed that the cumulative reversion risk increased over time across all RC quartiles.
Table 2.
Incidence and reversion rates of IFG to normoglycemia by RC quartiles
| RC | Participants(n) | Reversion events(n) | Reversal rate (95% CI) (%) | Per 1000 person-year |
|---|---|---|---|---|
| Total | 15,019 | 6432 | 42.83 (42.03, 43.62) | 144.83 |
| Q1 (< 17.40) | 3787 | 1889 | 49.88 (48.28, 51.49) | 185.05 |
| Q2 (17.40-27.07) | 3733 | 1625 | 43.53 (41.93, 45.14) | 152.26 |
| Q3 (27.07–38.23) | 3745 | 1535 | 40.99 (39.41, 42.59) | 135.25 |
| Q4 (≥ 38.23) | 3754 | 1383 | 36.84 (35.30, 38.41) | 113.52 |
| P for trend | < 0.001 |
Abbreviations RC, remnant cholesterol; CI, confidence interval
Fig. 4.
Kaplan‒Meier analysis of reversion from IFG to normoglycemia according to RC
Association between RC and normoglycemia reversal in IFG individuals
Univariate Cox regression analysis of baseline variables and the transition from IFG to normoglycemia (Additional file 1: Table S2) revealed that all variables, except height, were strongly associated with the possibility of normoglycemia reversal (P < 0.001). Variables exhibiting high collinearity (TC and weight, variance inflation factor (VIF) > 5) were removed from subsequent analyses (Additional file 1: Table S1).
Table 3 presents three multivariate Cox regression models to further evaluate the association between RC and normoglycemia reversal among individuals with IFG. The findings indicated a strong inverse association between RC and normoglycemia reversal (all P < 0.001). The unadjusted model (Model I) revealed a 27% decrease in the reversion rate (HR = 0.73, 95% CI: 0.71–0.75) for each SD increase in RC. Models II and III revealed that the hazard ratios for reversion to normoglycemia were 0.79 (95% CI: 0.77–0.81) and 0.80 (95% CI: 0.77–0.82), respectively, after adjusting for potential covariates. This suggests a 21% and 20% decrease in the probability of normoglycemia reversal, respectively, compared with those of the Q1 group. Additionally, the Schoenfeld residual plots showed no significant deviation from zero, supporting the Cox proportional hazards assumption (P = 0.5294) (Additional file 2: Figure S1).
Table 3.
Multivariable Cox regression analyses for the association between RC and reversion from IFG to normoglycemia
| HR (95%CI) | E value | ||||
|---|---|---|---|---|---|
| Model I | Model II | Model III | Model IV | ||
| Reversion to normoglycemia | |||||
| RC (per SD increase) | 0.73 (0.71, 0.75) | 0.79 (0.77, 0.81) | 0.80 (0.77, 0.82) | 0.78(0.75,0.81) | 1.66 |
| P value | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
| RC quartile | |||||
| IQ1 | Ref | Ref | Ref | Ref | |
| Q2 | 0.72 (0.68, 0.77) | 0.80 (0.75, 0.86) | 0.81 (0.75, 0.86) | 0.79(0.74, 0.84) | |
| Q3 | 0.57 (0.53, 0.61) | 0.67 (0.62, 0.71) | 0.68 (0.64, 0.74) | 0.66(0.62, 0.71) | |
| Q4 | 0.43 (0.40, 0.46) | 0.53 (0.49, 0.57) | 0.55 (0.51, 0.60) | 0.53(0.49, 0.58) | |
| P for trend | < 0.001 | < 0.001 | < 0.001 | < 0.001 | |
Abbreviations HR, hazard ratio; CI, confidence interval; Ref, reference
Model I was unadjusted
Model II adjusted for age, sex, family history of diabetes, smoking status, drinking status, SBP, DBP, and BMI
Model III adjusted for age, sex, family history of diabetes, smoking status, drinking status, SBP, DBP, BMI, FPG, TG, ALT, AST, HDL-C, LDL-C, BUN, and Cr
Model IV was adjusted for age (smooth), sex, family history of diabetes, smoking status, drinking status, SBP (smooth), DBP (smooth), BMI (smooth), FPG (smooth), TG (smooth), ALT (smooth), AST (smooth), HDL-C (smooth), LDL-C (smooth), BUN (smooth), and Cr (smooth)
Competing risks multivariable Cox proportional hazards regression analysis
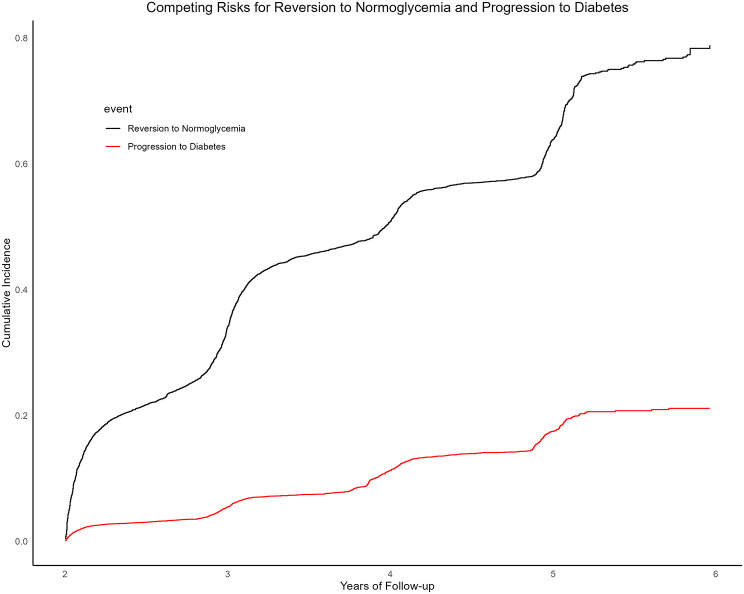
Patients with IFG may experience two primary outcomes during the follow-up period: reversion to normoglycemia or progression to diabetes. Neglecting either outcome can result in a misestimation of risk, potentially leading to either underestimation or overestimation. In this study, progression to diabetes posed a competing risk that could hinder normoglycemia reversal. Competing risk curves demonstrated distinct cumulative incidence rates for these two mutually exclusive events (Fig. 5). Table 4 shows the results of the competing hazard analysis. In unadjusted Model I, a noticeable reverse correlation was observed between RC and normoglycemia reversal in individuals with IFG (SHR = 0.93, 95% CI: 0.93–0.94). After adjusting for age, sex, BMI, alcohol consumption, smoking status, and a family history of diabetes, the inverse association remained significant (Model II: SHR = 0.81, 95% CI: 0.80–0.84). Further adjustments for FPG, TG, AST, ALT, HDL, LDL, BUN, and Cr in Model III revealed a significant inverse relationship between RC and normoglycemia reversal in individuals with IFG (SHR = 0.82, 95% CI: 0.80–0.85). When RCs were categorized into quartiles, the competing risk analysis yielded consistent results.
Fig. 5.
Cumulative incidence curve of reversion from IFG to normoglycemia and progression to diabetes
Table 4.
Competing risk analysis for reversion to normoglycemia considering the competing risk of progression to diabetes
| SHR (95%CI) | |||
|---|---|---|---|
| Model I | Model II | Model III | |
| Reversion to normoglycemia | |||
| RC (per SD increase) | 0.75 (0.73, 0.77) | 0.81 (0.80, 0.84) | 0.82 (0.80, 0.85) |
| P value | < 0.001 | < 0.001 | < 0.001 |
| RC quartile | |||
| Q1 | Ref | Ref | Ref |
| Q2 | 0.74 (0.69, 0.79) | 0.83 (0.78, 0.89) | 0.83 (0.77, 0.88) |
| Q3 | 0.59 (0.56, 0.64) | 0.71 (0.66, 0.76) | 0.72 (0.67, 0.77) |
| Q4 | 0.45 (0.43, 0.49) | 0.58 (0.54, 0.63) | 0.61 (0.56, 0.66) |
| P for trend | < 0.001 | < 0.001 | < 0.001 |
Abbreviations SHR subdistribution hazard ratios, CI confidence interval, Ref, reference
Model I was unadjusted
Model II adjusted for age, sex, family history of diabetes, smoking status, drinking status, SBP, DBP, and BMI
Model III adjusted for age, sex, family history of diabetes, smoking status, drinking status, SBP, DBP, BMI, FPG, TG, ALT, AST, HDL-C, LDL-C, BUN, and Cr
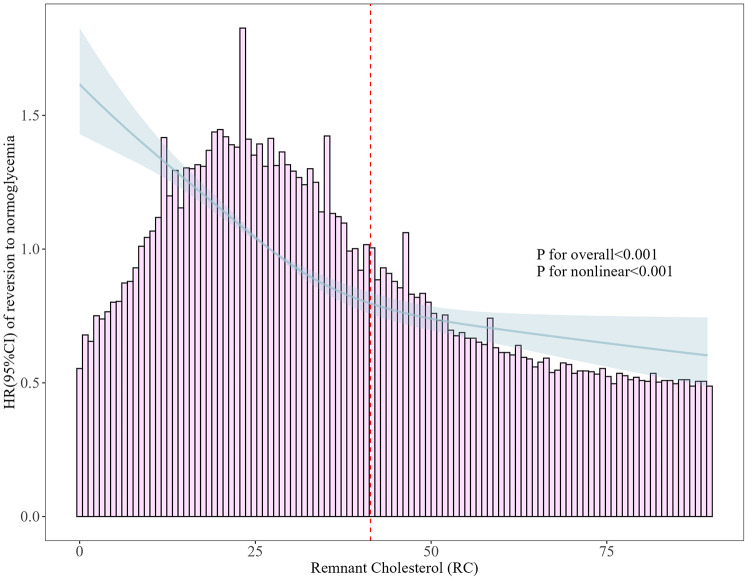
Nonlinear association between RC and normoglycemia reversal among IFG individuals
To assess the nonlinear association between RC and normoglycemia reversal among individuals with IFG, we implemented a fully adjusted Cox regression model incorporating 4-knot restricted cubic splines. The dose‒response curve is shown in Fig. 6. A significant nonlinear relationship was observed (P for nonlinearity < 0.001). The inflection point at RC = 41.38 mg/dL was then determined via a recursive algorithm, where the effect of the RC changed. A two-piecewise Cox proportional hazard model was fitted on the basis of this inflection point. Before the inflection point, hazard ratios (HRs) were calculated at 0.66 per SD increase (95% CI: 0.63–0.68) and 0.93 (95% CI: 0.87–0.93 per SD increase) after the inflection point (Table 5).
Fig. 6.
Nonlinear association between RC and reversion from the IFG to normoglycemia
Table 5.
Results of the two-piecewise Cox regression model
| Reversion to normoglycemia | HR(95%CI) | P value |
|---|---|---|
| Fitting model by standard Cox regression | 0.73 (0.71, 0.75) | < 0.001 |
| Fitting model by two-piecewise cox regression | ||
| Inflection point of RC (mg/dL) | 41.38 | |
| < 41.38 mg/dL (per SD increase) | 0.66 (0.63, 0.68) | < 0.001 |
| ≥ 41.38 mg/dL(per SD increase) | 0.93 (0.87, 0.99) | 0.031 |
Abbreviations HR, hazard ratio; CI, confidence interval
Adjusted for age, sex, family history of diabetes, smoking status, drinking status, SBP, DBP, BMI, FPG, TG, ALT, AST, HDL-C, LDL-C, BUN, and Cr
Sensitivity analyses
Several sensitivity analyses were performed to confirm that the findings were as solid and dependable as possible. (1) After stratifying RCs into quartiles and incorporating them as categorical variables in the Cox regression model (Model III), a significant inverse association was observed between RC and normoglycemia reversal in IFG individuals across all quartiles, with trend strengthening (Q1: reference; Q2:0.81; Q3:0.68; Q4:0.55), which is consistent with the findings of the analyses in Table 2 when RC was considered a continuous variable. (2) After additional sensitivity factors (alcohol consumption, smoking status, and family history of diabetes) were excluded, the impact of RC on reversion to normoglycemia among IFG individuals was still significant (HR = 0.79, 95% CI: 0.76–0.82). Additional file 1: Table S3 shows that the reanalysis results validate the main analysis. (3) Variables with more than 10% missing values were excluded (such as AST, with 8,062 cases (53.7%) missing; smoking status, with 10,407 cases (69.3%) missing; and drinking status, with 10,407 cases (69.3%) missing). The reanalysis results were the original analysis results (HR = 0.79, 95% CI: 0.77–0.82) (Additional file 1: Table S4). (4) The findings obtained from the GAM analyses (Model IV) (HR = 0.78, 95% CI: 0.75–0.81) were broadly similar to those of the fully adjusted Model III (HR = 0.80, 95% CI: 0.77–0.82) (Table 3). Additionally, the E value was 1.66, indicating that an unmeasured confounder would need a relative risk of at least 1.66 with both RC and reversion to normoglycemia to fully explain the observed association. Overall, sensitivity analyses indicated that the findings were robust and unaffected by various factors.
Subgroup analyses
Subgroup analyses were performed to assess the varying effects of RC on the reversal of IFG to normoglycemia in different subgroups. The results, detailed in Table 6 (P for interaction < 0.05), indicated significant interactions between RC and age but not with sex, BMI, family history of diabetes, TG, SBP, DBP, alcohol consumption, or smoking status. Notably, RC had a greater influence on the probability of normoglycemia reversal in IFG individuals aged ≥ 60 years.
Table 6.
Exploratory subgroup analysis of the association between RC (per SD increase) and reversion to normoglycemia
| Characteristic | No. of participants | HR (95% CI) | P value | P for interaction |
|---|---|---|---|---|
| Gender | 0.213 | |||
| Male | 9173 | 0.80 (0.77, 0.83) | < 0.001 | |
| Female | 5306 | 0.77 (0.73, 0.81) | < 0.001 | |
| Age, years | < 0.001 | |||
| < 30 | 799 | 0.78 (0.68, 0.89) | < 0.001 | |
| 30–45 | 4785 | 0.82 (0.78, 0.87) | < 0.001 | |
| 45–60 | 5647 | 0.80 (0.76, 0.84) | < 0.001 | |
| ≥ 60 | 3788 | 0.70 (0.65, 0.76) | < 0.001 | |
| BMI, kg/m2 | 0.158 | |||
| < 24 | 6115 | 0.73 (0.70, 0.77) | < 0.001 | |
| 24–28 | 2411 | 0.82 (0.78, 0.86) | < 0.001 | |
| ≥ 28 | 6493 | 0.87 (0.80, 0.95) | < 0.001 | |
| TG, mmol/L | 0.441 | |||
| < 1.7 | 9147 | 0.75 (0.71, 0.78) | < 0.001 | |
| ≥ 1.7 | 5871 | 0.88 (0.84, 0.92) | < 0.001 | |
| Family history of diabetes | 0.329 | |||
| Yes | 392 | 0.66 (0.52, 0.84) | < 0.001 | |
| No | 14,627 | 0.80 (0.77, 0.82) | < 0.001 | |
| Smoking status | 0.149 | |||
| Current | 2872 | 0.80 (0.74, 0.86) | < 0.001 | |
| Past | 408 | 0.78 (0.62, 0.98) | < 0.001 | |
| Never | 11,739 | 0.80 (0.76, 0.80) | < 0.001 | |
| Drinking status | 0.170 | |||
| Current | 390 | 0.92 (0.78, 1.20) | < 0.001 | |
| Past | 2027 | 0.82 (0.72, 0.89) | < 0.001 | |
| Never | 12,602 | 0.78 (0.75, 0.81) | < 0.001 | |
| SBP, mmHg | 0.451 | |||
| < 140 | 11,653 | 0.79 (0.76, 0.82) | < 0.001 | |
| ≥ 140 | 3366 | 0.78 (0.73, 0.84) | < 0.001 | |
| DBP, mmHg | 0.068 | |||
| < 90 | 12,798 | 0.79 (0.76, 0.81) | < 0.001 | |
| ≥ 90 | 2221 | 0.82 (0.75, 0.90) | < 0.001 |
Adjusted for age, sex, family history of diabetes, smoking status, drinking status, SBP, DBP, BMI, FPG, TG, ALT, AST, HDL-C, LDL-C, BUN, and Cr
In each case, the model is not adjusted for the stratification variable
Discussion
This study used a large cohort to retrospectively explore the relationship between RC and normoglycemia reversal in individuals with IFG. After adjusting for potential confounders, the findings revealed a significant inverse relationship between RC and normoglycemia reversal in individuals with IFG. Moreover, the dose‒response curve revealed a significant nonlinear relationship between RC and normoglycemia, indicating a potential threshold effect of RC on this reversion.
Numerous investigations have shown that reversion from IFG to normoglycemia exhibits substantial variability across global populations, influenced by a combination of geographical disparity, ethnic diversity, genetic predispositions, and lifestyle. For example, a longitudinal cohort analysis conducted over a decade in Tehran revealed that approximately 40% of 1,329 subjects achieved normoglycemia [47]. Concurrently, a study focusing on Indian adolescents reported a reversion to normal glucose tolerance in 70.6% of participants during a median observational period of seven years [48]. Similarly, a prospective analysis involving 9,637 Mexicans reported a reversion incidence of 22.6% [49], whereas an extensive cohort examination among Chinese adults indicated that 44.92% (n = 6,393) of 14,231 participants reverted to normoglycemia within two years [10]. Moreover, a notable reversion rate following pharmacological and lifestyle modifications has been reported in diverse studies [12, 50–52]. During a nearly 3-year follow-up, this study revealed that approximately 43.7% of individuals with IFG successfully transitioned to normoglycemia. Notably, the aforementioned research verified that a considerable number of prediabetic patients with different backgrounds returned to normoglycemia. Therefore, conducting more in-depth epidemiological studies and actively identifying controllable factors influencing the transition from IFG to normoglycemia are necessary for preventing diabetes and its complications.
In recent years, research has demonstrated that RC is not only significantly linked to cardiovascular disease but also may affect the occurrence and progression of diabetes. A cross-sectional study utilizing the CNHS database revealed that individuals with high RCs and low LDL-C levels presented a greater diabetes risk within the broader population (4.04 times greater than those with low RCs and LDL-C levels) and that this effect was more sensitive in women [53]. Another population-based study in rural China revealed that each 1 SD increase in RC was associated with a 34% greater likelihood of developing type 2 diabetes [54]. Furthermore, cross-sectional insights from the NHANES cohort revealed a robust positive relationship between RC and the incidence of diabetes (OR = 2.259, 95% CI, 1.797–2.838) [55]. Longitudinal studies within general and cardiovascular-compromised cohorts have demonstrated that RC not only significantly correlates with diabetes incidence but also surpasses other traditional lipid metrics as a prognosticator of emergent diabetes [27–29]. Concurrently, empirical evidence from studies on gestational diabetes corroborates these findings [56, 57]. Nonetheless, existing research has primarily examined the link between RC and diabetes, neglecting to explore the connection between RC and normoglycemia reversal in individuals with IFG. Hence, it is postulated that augmentation of RC may inversely affect the likelihood of IFG reversion to a normoglycemic state. Through meticulous statistical scrutiny and a suite of sensitivity analyses, it was found that an elevated RC diminished the probability of IFG patients reverting to normoglycemia. As a readily quantifiable lipid marker, fluctuations in the RC could represent a critical, controllable predictor of IFG reversal to normoglycemic conditions. A salient conclusion from the Diabetes Prevention Program Outcomes Study (DPPOS) indicated that individuals who achieved normoglycemia experienced a significant 56% decrease in the risk of developing subsequent diabetes. In contrast, those who remained prediabetic did not experience such a decrease [9]. Thus, these findings provide novel insights for clinical practice, bearing significant implications not only in preventing diabetes progression but also in facilitating proactive lipid management strategies to enhance glucose normalization.
The level of remaining cholesterol is negatively correlated with the reversion of IFG to normoglycemia; although the precise mechanisms involved remain elusive, they are likely related to β-cell dysfunction. Unlike LDL-C particles, RC particles are more prevalent, larger, and have a higher cholesterol content [58]. Research posits that a cholesterol-rich milieu may undermine the viability of pancreatic β-cells, thereby attenuating insulin production [59, 60]. Research has shown that elevated RC levels catalyze atherogenesis, exacerbating IR [61, 62]. Clinically, the atherogenic ramifications of lipid parameters and the resulting IR are pivotal factors in diabetes pathophysiology, with the renin‒angiotensin‒aldosterone system (RAAS) serving a crucial function [63, 64]. An imbalance in renin and angiotensin II levels can trigger various pathological conditions, particularly because of the salient role of angiotensin II in incipient atherosclerotic plaque formation [65, 66]. RAAS dysregulation also augments the production of proinflammatory cytokines and oxidative stress, further aggravating atherogenesis and IR and attenuating insulin secretion [67]. Moreover, increased RC levels may precipitate chronic low-grade inflammation, increasing the levels of proinflammatory cytokines such as IL-6 and TNF-α, impacting insulin signaling and promoting IR [68, 69]. Additionally, considering the inherent nature of RC as a cholesterol variant, elevated RC levels facilitate lipid deposition in tissues, such as the liver, muscles, and pancreatic islets, culminating in lipotoxicity. This lipotoxicity impairs insulin receptor functionality and instigates disturbances in glucose metabolism [70, 71]. Consequently, reducing RC levels could enhance pancreatic β-cell functionality and thereby aid in normalizing glucose metrics.
Furthermore, in the present investigation, a nonlinear relationship and saturation effect between RC and normoglycemia reversal in individuals with IFG were ascertained for the first time. Using a two-piecewise Cox proportional hazards regression model, a pivotal inflection point for RC was discerned at 41.38 mg/dL. Below this threshold, each SD decrease in the RC led to a 34% greater chance of reverting to normoglycemia. Beyond this threshold, each increase in SD resulted in only a 7% increase, indicating that the growth trend of the likelihood of reversion decreased and stabilized after the inflection point was reached. Baseline analyses stratified by this inflection point (Additional file 1: Table S5) demonstrated that participants with RC levels below 41.38 mg/dL were predominantly younger and, barring HDL-C and BUN, had lower values for other physical and laboratory markers, such as BMI, FPG, ALT, AST, Cr, SBP, DBP, LDL-C, and TG, albeit with a greater propensity for smoking and drinking. Previous studies postulated that certainse covariates may impede the reversion from IFG to normoglycemia [45, 72, 73], implying that the more significant impact of RC on reversion within the < 41.38 mg/dL group could be attributed to reduced levels of these risk factors. Once the RC surpasses the inflection point, the heightened presence of risk factors attenuates the influence of RC on reversion, offering a plausible explanation for the observed statistical trends. The identification of different nonlinear associations in studies investigating the relationships between other lipid metabolism markers and metabolic diseases further corroborates these findings [29, 74–77]. These results underscore the significance of RC levels in the glucose recovery of patients with IFG, suggesting that RC, as an early biomarker, could assist clinicians in the early identification of patients with an elevated potential for recovery and the formulation of more efficacious intervention strategies, thereby offering novel insights and methodologies for diabetes prevention and management. Further research is needed to determine whether this association can be used to predict long-term vascular outcomes and diabetic complications. Such studies would be invaluable for establishing RC as a comprehensive risk factor and guiding more targeted interventions.
Subsequent subgroup analysis revealed interaction effects among age groups concerning the influence of RC on the reversal of glucose levels from the IFG to normoglycemia. Interestingly, these findings indicate that, compared with their younger counterparts, elderly IFG patients aged > 60 years are more susceptible to the effects of RC on normoglycemia. This observation is consistent with a Korean cohort study that reported a lower risk of diabetes in the upper RC quartile with increasing age [27]. The etiology of the observed age-related differences in the influence of RC on normoglycemia in individuals with IFG remains unclear. Further research is needed to understand the nonlinear association between RC and normoglycemia reversal among individuals with IFG, emphasizing variations across age groups and thereby facilitating the formulation of more efficacious intervention strategies.
Study strengths and limitations
The primary strengths of this study are as follows: (1) This is the first study to focus exclusively on the IFG cohort to assess the relationship between RC and normoglycemia reversal. (2) This study used multicenter cohort data from 11 cities across China to generate a large and representative Chinese cohort. (3) This study identified a crucial inflection point for the nonlinear association between RC and normoglycemia reversal, offering valuable insights for future interventions. (4) A series of sensitivity analyses were performed, including stratifying RCs into quartiles, integrating continuous covariates as curves within the model via the GAM, computing the E value, and omitting variables characterized by substantial missing data or those linked with diabetes-related factors such as drinking, smoking status, and family history of diabetes. (5) Potential confounders were probed through subgroup analyses, and the interaction effects between RC and variables such as age group were discerned.
However, this research has several limitations: (1) The external validity of the findings to populations beyond China may be constrained due to the single-source nature of the data, which were exclusively from the Rich Healthcare Group. Although the dataset encompasses a substantial cohort of 11 cities across China and possesses a degree of representativeness, it was sourced primarily from a health examination database. Consequently, it may not comprehensively capture the heterogeneity of the entire Chinese population or extrapolate it to populations in other nations. Hence, caution is needed when these results are generalized to other regions or populations. Future research should incorporate multiple data sources to validate the findings, increasing the robustness of the conclusions and providing more universally applicable evidence. (2) The operational definition of diabetes employed in this study excluded diagnoses on the basis of a 2-hour oral glucose tolerance test or glycated hemoglobin levels, instead of relying exclusively on FPG and self-reported measures. (3) This research constitutes a secondary analysis of extant data. It does not account for factors such as waist circumference, fasting insulin levels, sleep patterns, physical activity, or dietary habits that could regulate glucose. Nevertheless, potential confounding effects were quantitatively assessed by computing the E value (1.66). (4) While this study employed a retrospective cohort design, it is crucial to emphasize that the findings offer evidence of a relationship between RC and normoglycemia reversal among individuals with IFG rather than a causal relationship. Further research should utilize methodologies such as randomized controlled trials to provide additional evidence. (5) For clinicians, the use of a composite index that requires calculations can be inconvenient. The incorporation of computer algorithms to calculate and display key lipid composite indices and routine lipid measurements is recommended. (6) This study did not assess the influence of other atherogenic lipid biomarkers, such as lipoprotein(a) and apolipoprotein(B), which have been linked to vascular complications in Chinese populations [78, 79].
Conclusion
This study demonstrated a significant nonlinear inverse relationship between RC and normoglycemia reversal in Chinese adults with IFG. The identified inflection point of 41.38 mg/dL suggests a critical window for intervention. Clinicians should consider incorporating RC monitoring into routine care for IFG patients, particularly those aged 60 years or older. Implementing personalized RC-lowering strategies, including lifestyle modifications and lipid-lowering therapies, before the RC levels reach this threshold may significantly improve the likelihood of IFG reversal. Further research is warranted to validate these findings in diverse populations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We express our gratitude to Chen, Ying et al. for their role in data collection and sharing, which greatly facilitated our research.
Abbreviations
- RC
Remnant cholesterol
- HR
Hazard ratio
- IFG
Impaired fasting glucose
- BMI
Body mass index
- TG
Triglyceride
- TC
Total cholesterol
- IR
Insulin resistance
- VLDLs
Very low-density lipoproteins
- IDLs
Intermediate-density lipoproteins
- TRL
Triglyceride-rich lipoprotein particle
- HDL-C
High-density lipoprotein cholesterol
- LDL-C
Low-density lipoprotein cholesterol
- SBP
Systolic blood pressure
- DBP
Diastolic blood pressure
- ALT
Alanine aminotransferase
- AST
Aspartate transaminase
- Cr
Creatinine
- BUN
Blood urea nitrogen
- CCR
Creatinine
- MICE
Multivariate imputation by chained equations
- SD
Standard deviation
- IQR
Interquartile range
- VIF
Variance inflation factor
- GAM
Generalized additive model
- DPPOS
Diabetes prevention program outcome study
- RAAS
Renin-angiotensin-aldosterone system
Author contributions
LX-K and YQ-W conceptualized and designed the study, and were primarily responsible for drafting the initial manuscript. HQ-Y and MT-G were primarily responsible for analyzing the patient data and revising the manuscript critically for important intellectual content. YX-Z and JR-L participated in the literature review and the search for pertinent data. SW and ZZ-X also reviewed and edited the final manuscript to ensure accuracy and compliance with journal standards.
Funding
This work was supported by the Chengdu Medical College - Nanbu County People’s Hospital Clinical Science Research Fund, grant number 23LHNBSYB02; 2022 Open Topic Fund of Clinical Medical Research Center for Elderly Diseases (Jointly Funded by Chengdu Medical College and Nanbu County People’s Hospital), grant number 2022LHNBSYB05; 2023 Clinical Science Research Fund Project of Chengdu Medical College, the Third Affiliated Hospital of Chengdu Medical College and Chengdu Pidu District People’s Hospital and the Open Project of the Sichuan Collaborative Innovation Center for Aging and Elderly Health, grant number 23LHPDZYB24.
Data availability
The datasets supporting the conclusions of this article are available in the Dryad database, which is publicly accessible. (https://datadryad.org/stash/data/set/doi:10.5061/dryad.8q0p192).
Declarations
Ethics approval and consent to participate
The Rich Healthcare Group Review Board authorized the original study, which adhered to the Helsinki Declaration’s requirements. Furthermore, for the current retrospective investigation, the Rich Healthcare Group Review Board waived informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Laixi Kong and Yuqing Wu contributed equally to this work.
Contributor Information
Shuang Wu, Email: ws910902@163.com.
Zhenzhen Xiong, Email: xzz62308631@163.com.
References
- 1.Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012;379(9833):2279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. IDF Diabetes Atlas 10th edition. [Accessed 2023 Aug 28]. https://diabetesatlas.org/en/resources/…
- 3.Rooney MR, Fang M, Ogurtsova K, Ozkan B, Echouffo-Tcheugui JB, Boyko EJ, Magliano DJ, Selvin E. Global prevalence of Prediabetes. Diabetes Care. 2023;46(7):1388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Porrini E, Diaz JM, Moreso F, Lauzurrica R, Ibernon M, Torres IS, Ruiz RB, Rodriguez RA, Mallen PD, Bayes-Genis B, et al. Prediabetes is a risk factor for cardiovascular disease following renal transplantation. KIDNEY INT. 2019;96(6):1374–80. [DOI] [PubMed] [Google Scholar]
- 5.Huang Y, Cai X, Mai W, Li M, Hu Y. Association between prediabetes and risk of cardiovascular disease and all cause mortality: systematic review and meta-analysis. BMJ-BRIT MED J. 2016;355:i5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang A, Zhang J, Zuo Y, Tian X, Chen S, Wu S, Zhao X, Wang Y. Prediabetes and risk of stroke and its subtypes by hypertension status. DIABETES-METAB RES. 2022;38(4):e3521. [DOI] [PubMed] [Google Scholar]
- 7.Hubbard D, Colantonio LD, Tanner RM, Carson AP, Sakhuja S, Jaeger BC, Carey RM, Cohen LP, Shimbo D, Butler M, et al. Prediabetes and Risk for Cardiovascular Disease by Hypertension Status in black adults: the Jackson Heart Study. Diabetes Care. 2019;42(12):2322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busquets-Cortes C, Bennasar-Veny M, Lopez-Gonzalez AA, Fresneda S, Abbate M, Yanez AM. Utility of fatty liver index to predict reversion to normoglycemia in people with prediabetes. PLoS ONE. 2021;16(4):e249221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE. Effect of regression from prediabetes to normal glucose regulation on long-term reduction in diabetes risk: results from the diabetes Prevention Program outcomes Study. Lancet. 2012;379(9833):2243–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Wu S, Song Q, Wang X. Reversion from Pre-diabetes Mellitus to Normoglycemia and Risk of Cardiovascular Disease and all-cause mortality in a Chinese Population: a prospective cohort study. J AM HEART ASSOC. 2021;10(3):e19045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Zhang P, Wang J, Gong Q, An Y, Qian X, Zhang B, Li H, Gregg EW, Bennett PH et al. Associations of progression to diabetes and regression to normal glucose tolerance with development of cardiovascular and microvascular disease among people with impaired glucose tolerance: a secondary analysis of the 30 year Da Qing Diabetes Prevention Outcome Study. DIABETOLOGIA 2021, 64(6):1279–1287. [DOI] [PubMed]
- 12.Amer OE, Sabico S, Alfawaz HA, Aljohani N, Hussain SD, Alnaami AM, Wani K, Al-Daghri NM. Reversal of Prediabetes in Saudi Adults: Results from an 18 Month Lifestyle Intervention. NUTRIENTS 2020, 12(3):804. [DOI] [PMC free article] [PubMed]
- 13.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. NEW ENGL J MED. 2002;346(6):393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginsberg HN, Packard CJ, Chapman MJ, Boren J, Aguilar-Salinas CA, Averna M, Ference BA, Gaudet D, Hegele RA, Kersten S, et al. Triglyceride-rich lipoproteins and their remnants: metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. EUR HEART J. 2021;42(47):4791–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nordestgaard BG: Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights From Epidemiology, Genetics, and, Biology. CIRC RES 2016, 118(4):547–563. [DOI] [PubMed]
- 16.Wang K, Wang R, Yang J, Liu X, Shen H, Sun Y, Zhou Y, Fang Z, Ge H. Remnant cholesterol and atherosclerotic cardiovascular disease: metabolism, mechanism, evidence, and treatment. FRONT CARDIOVASC MED. 2022;9:913869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnett JR, Hooper AJ, Hegele RA. Remnant cholesterol and atherosclerotic Cardiovascular Disease Risk. J AM COLL CARDIOL. 2020;76(23):2736–9. [DOI] [PubMed] [Google Scholar]
- 18.Quispe R, Martin SS, Michos ED, Lamba I, Blumenthal RS, Saeed A, Lima J, Puri R, Nomura S, Tsai M, et al. Remnant cholesterol predicts cardiovascular disease beyond LDL and ApoB: a primary prevention study. EUR HEART J. 2021;42(42):4324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen AH, Tseng CH. The role of triglyceride in cardiovascular disease in Asian patients with type 2 diabetes–a systematic review. Rev Diabet Stud. 2013;10(2–3):101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tseng CH, Tseng CP, Chong CK, Cheng JC, Tai TY. Independent association between triglycerides and coronary artery disease in Taiwanese type 2 diabetic patients. INT J CARDIOL. 2006;111(1):80–5. [DOI] [PubMed] [Google Scholar]
- 21.Tai TY, Tseng CH, Sung SM, Huang RF, Chen CZ, Tsai SH. Retinopathy, neuropathy and nephropathy in non-insulin-dependent diabetic patients. J FORMOS MED ASSOC. 1991;90(10):936–40. [PubMed] [Google Scholar]
- 22.Tseng CH. Lipid abnormalities associated with urinary albumin excretion rate in Taiwanese type 2 diabetic patients. KIDNEY INT. 2005;67(4):1547–53. [DOI] [PubMed] [Google Scholar]
- 23.Tseng CH, Tseng CP, Chong CK. Joint effects of hypertension, smoking, dyslipidemia and obesity and angiotensin-converting enzyme DD genotype on albuminuria in Taiwanese patients with type 2 diabetes mellitus. CLIN BIOCHEM. 2010;43(7–8):629–34. [DOI] [PubMed] [Google Scholar]
- 24.Zou Y, Kuang M, Zhong Y, Jiang C. Remnant cholesterol can identify individuals at higher risk of metabolic syndrome in the general population. SCI REP-UK. 2023;13(1):5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohnishi H, Saitoh S, Takagi S, Ohata J, Isobe T, Kikuchi Y, Takeuchi H, Shimamoto K. Relationship between insulin-resistance and remnant-like particle cholesterol. ATHEROSCLEROSIS. 2002;164(1):167–70. [DOI] [PubMed] [Google Scholar]
- 26.Klop B, Elte JW, Cabezas MC. Dyslipidemia in obesity: mechanisms and potential targets. NUTRIENTS. 2013;5(4):1218–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huh JH, Roh E, Lee SJ, Ihm SH, Han KD, Kang JG. Remnant cholesterol is an independent predictor of type 2 diabetes: a Nationwide Population-based Cohort Study. Diabetes Care. 2023;46(2):305–12. [DOI] [PubMed] [Google Scholar]
- 28.Xie G, Zhong Y, Yang S, Zou Y. Remnant cholesterol is an independent predictor of New-Onset diabetes: a single-Center Cohort Study. DIABET METAB SYND OB. 2021;14:4735–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng X, Jiang M, Ren X, Han L. The longitudinal association of remnant cholesterol with diabetes in middle-aged and elderly Chinese: a nationwide population-based cohort study. J DIABETES COMPLICAT. 2023;37(1):108360. [DOI] [PubMed] [Google Scholar]
- 30.Carvalho L, Bensenor IM, Nogueira A, Duncan BB, Schmidt MI, Blaha MJ, Toth PP, Jones SR, Santos RD, Lotufo PA, et al. Increased particle size of triacylglycerol-enriched remnant lipoproteins, but not their plasma concentration or lipid content, augments risk prediction of incident type 2 diabetes. Diabetologia. 2021;64(2):385–96. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Zhang XP, Yuan J, Cai B, Wang XL, Wu XL, Zhang YH, Zhang XY, Yin T, Zhu XH, et al. Association of body mass index and age with incident diabetes in Chinese adults: a population-based cohort study. BMJ OPEN. 2018;8(9):e21768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Y, et al. Data from: Association of body mass index and age withincident diabetes in Chinese adults: a population-based cohort study Dryad. Dataset. 2018. 10.5061/dryad.ft8750v. [Google Scholar]
- 33.Nordestgaard BG, Varbo A. Triglycerides and cardiovascular disease. Lancet. 2014;384(9943):626–35. [DOI] [PubMed] [Google Scholar]
- 34.Classification and Diagnosis of Diabetes. Standards of Medical Care in Diabetes-2022. Diabetes Care. 2022;45(Suppl 1):S17–38. [DOI] [PubMed] [Google Scholar]
- 35.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. STAT MED. 2011;30(4):377–99. [DOI] [PubMed] [Google Scholar]
- 36.Fitchett E, Seale AC, Vergnano S, Sharland M, Heath PT, Saha SK, Agarwal R, Ayede AI, Bhutta ZA, Black R, et al. Strengthening the reporting of Observational studies in Epidemiology for Newborn infection (STROBE-NI): an extension of the STROBE statement for neonatal infection research. LANCET INFECT DIS. 2016;16(10):e202–13. [DOI] [PubMed] [Google Scholar]
- 37.Cooper H, Wells S, Mehta S. Are competing-risk models superior to standard Cox models for predicting cardiovascular risk in older adults? Analysis of a whole-of-country primary prevention cohort aged >/=65 years. INT J EPIDEMIOL. 2022;51(2):604–14. [DOI] [PubMed] [Google Scholar]
- 38.Basak R, Mistry H, Chen RC. Understanding competing risks. INT J RADIAT ONCOL. 2021;110(3):636–40. [DOI] [PubMed] [Google Scholar]
- 39.Ustulin M, Rhee SY, Chon S, Ahn KK, Lim JE, Oh B, Kim SH, Baik SH, Park Y, Nam MS, et al. Importance of family history of diabetes in computing a diabetes risk score in Korean prediabetic population. SCI REP-UK. 2018;8(1):15958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu X, Fang X, Wu M. Prevalence, awareness, treatment and control of type 2 diabetes in southeast China: a population-based study. J DIABETES INVEST. 2024;15(8):1034–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cai X, Zhu Q, Cao Y, Liu S, Wang M, Wu T, Hong J, Ahmat A, Aierken X, Li N. A Prediction Model Based on Noninvasive Indicators to Predict the 8-Year Incidence of Type 2 Diabetes in Patients with Nonalcoholic Fatty Liver Disease: A Population-Based Retrospective Cohort Study. BIOMED RES INT 2021, 2021:5527460. [DOI] [PMC free article] [PubMed]
- 42.Cai X, Zhu Q, Wu T, Zhu B, Aierken X, Ahmat A, Li N. Development and Validation of a Novel Model for Predicting the 5-Year Risk of Type 2 Diabetes in Patients with Hypertension: A Retrospective Cohort Study. BIOMED RES INT 2020, 2020:9108216. [DOI] [PMC free article] [PubMed]
- 43.Haneuse S, VanderWeele TJ, Arterburn D. Using the E-Value to assess the potential effect of unmeasured confounding in Observational studies. JAMA-J AM MED ASSOC. 2019;321(6):602–3. [DOI] [PubMed] [Google Scholar]
- 44.Bek T. Systemic risk factors contribute differently to the development of proliferative diabetic retinopathy and clinically significant macular oedema. Diabetologia. 2020;63(11):2462–70. [DOI] [PubMed] [Google Scholar]
- 45.Yang H, Kuang M, Yang R, Xie G, Sheng G, Zou Y. Evaluation of the role of atherogenic index of plasma in the reversion from Prediabetes to normoglycemia or progression to diabetes: a multi-center retrospective cohort study. CARDIOVASC DIABETOL. 2024;23(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Karpov Y, Khomitskaya Y. PROMETHEUS: an observational, cross-sectional, retrospective study of hypertriglyceridemia in Russia. CARDIOVASC DIABETOL. 2015;14:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alizadeh Z, Baradaran HR, Kohansal K, Hadaegh F, Azizi F, Khalili D. Are the determinants of the progression to type 2 diabetes and regression to normoglycemia in the populations with pre-diabetes the same? FRONT ENDOCRINOL. 2022;13:1041808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mehreen TS, Kamalesh R, Pandiyan D, Kumar DS, Anjana RM, Mohan V, Ranjani H. Incidence and predictors of Dysglycemia and Regression to Normoglycemia in Indian adolescents and young adults: 10-Year Follow-Up of the ORANGE Study. DIABETES TECHNOL THE. 2020;22(12):875–82. [DOI] [PubMed] [Google Scholar]
- 49.Sevilla-Gonzalez M, Merino J, Moreno-Macias H, Rojas-Martinez R, Gomez-Velasco DV, Manning AK. Clinical and metabolomic predictors of regression to normoglycemia in a population at intermediate cardiometabolic risk. CARDIOVASC DIABETOL. 2021;20(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. NEW ENGL J MED. 2001;344(18):1343–50. [DOI] [PubMed] [Google Scholar]
- 51.Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359(9323):2072–7. [DOI] [PubMed] [Google Scholar]
- 52.Ramachandran A, Snehalatha C, Mary S, Selvam S, Kumar CK, Seeli AC, Shetty AS. Pioglitazone does not enhance the effectiveness of lifestyle modification in preventing conversion of impaired glucose tolerance to diabetes in Asian Indians: results of the Indian Diabetes Prevention Programme-2 (IDPP-2). DIABETOLOGIA 2009, 52(6):1019–1026. [DOI] [PubMed]
- 53.Hu X, Liu Q, Guo X, Wang W, Yu B, Liang B, Zhou Y, Dong H, Lin J. The role of remnant cholesterol beyond low-density lipoprotein cholesterol in diabetes mellitus. CARDIOVASC DIABETOL. 2022;21(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yuan L, Liu J, Huang Z, Zhao Y, Feng Y, Yang X, Hu H, Zhang J, Li T, Li Y, et al. Elevated remnant cholesterol increase 6-year type 2 diabetes mellitus onset risk. CLIN CHIM ACTA. 2023;541:117253. [DOI] [PubMed] [Google Scholar]
- 55.Wu Y, Wei Q, Li H, Yang H, Wu Y, Yu Y, Chen Q, He B, Chen F. Association of remnant cholesterol with hypertension, type 2 diabetes, and their coexistence: the mediating role of inflammation-related indicators. LIPIDS HEALTH DIS. 2023;22(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gao Y, Hu Y, Xiang L. Remnant cholesterol, but not other cholesterol parameters, is associated with gestational diabetes mellitus in pregnant women: a prospective cohort study. J TRANSL MED. 2023;21(1):531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang W, Li N, Wang X, Zhang X, Tu M, Lin L, Li Q, Zhang H, Liu J, Yang X, et al. Remnant cholesterol is Associated with Gestational Diabetes Mellitus: a Cohort Study. J CLIN ENDOCR METAB. 2023;108(11):2924–30. [DOI] [PubMed] [Google Scholar]
- 58.Sokooti S, Flores-Guerrero JL, Heerspink H, Connelly MA, Bakker S, Dullaart R. Triglyceride-rich lipoprotein and LDL particle subfractions and their association with incident type 2 diabetes: the PREVEND study. CARDIOVASC DIABETOL. 2021;20(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hao M, Head WS, Gunawardana SC, Hasty AH, Piston DW. Direct effect of cholesterol on insulin secretion: a novel mechanism for pancreatic beta-cell dysfunction. Diabetes. 2007;56(9):2328–38. [DOI] [PubMed] [Google Scholar]
- 60.Lu X, Liu J, Hou F, Liu Z, Cao X, Seo H, Gao B. Cholesterol induces pancreatic beta cell apoptosis through oxidative stress pathway. CELL STRESS CHAPERON. 2011;16(5):539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Varbo A, Benn M, Tybjaerg-Hansen A, Jorgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J AM COLL CARDIOL. 2013;61(4):427–36. [DOI] [PubMed] [Google Scholar]
- 62.2019 ESC/EAS guidelines for the. Management of dyslipidaemias: lipid modification to reduce cardiovascular risk. ATHEROSCLEROSIS. 2019;290:140–205. [DOI] [PubMed] [Google Scholar]
- 63.Kane JP, Pullinger CR, Goldfine ID, Malloy MJ. Dyslipidemia and Diabetes Mellitus: role of lipoprotein species and interrelated pathways of lipid metabolism in diabetes mellitus. CURR OPIN PHARMACOL. 2021;61:21–7. [DOI] [PubMed] [Google Scholar]
- 64.Jandeleit-Dahm K, Cooper ME. Hypertension and diabetes: role of the renin-angiotensin system. ENDOCRIN METAB CLIN. 2006;35(3):469–90. [DOI] [PubMed] [Google Scholar]
- 65.Poznyak AV, Bharadwaj D, Prasad G, Grechko AV, Sazonova MA, Orekhov AN. Renin-angiotensin system in Pathogenesis of atherosclerosis and treatment of CVD. INT J MOL SCI. 2021;22(13):6702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Durante A, Peretto G, Laricchia A, Ancona F, Spartera M, Mangieri A, Cianflone D. Role of the renin-angiotensin-aldosterone system in the pathogenesis of atherosclerosis. CURR PHARM Des. 2012;18(7):981–1004. [DOI] [PubMed] [Google Scholar]
- 67.Favre GA, Esnault VL, Van Obberghen E. Modulation of glucose metabolism by the renin-angiotensin-aldosterone system. AM J PHYSIOL-ENDOC M. 2015;308(6):E435–49. [DOI] [PubMed] [Google Scholar]
- 68.Rehman K, Akash MS. Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J BIOMED SCI. 2016;23(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wagner R, Jaghutriz BA, Gerst F, Barroso OM, Machann J, Schick F, Loffler MW, Nadalin S, Fend F, Konigsrainer A, et al. Pancreatic steatosis associates with impaired insulin secretion in genetically predisposed individuals. J CLIN ENDOCR METAB. 2020;105(11):3518–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sandesara PB, Virani SS, Fazio S, Shapiro MD. The forgotten lipids: triglycerides, remnant cholesterol, and atherosclerotic Cardiovascular Disease Risk. ENDOCR REV. 2019;40(2):537–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saponaro C, Gaggini M, Carli F, Gastaldelli A. The subtle balance between Lipolysis and Lipogenesis: a critical point in metabolic homeostasis. NUTRIENTS. 2015;7(11):9453–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Siegel LC, Sesso HD, Bowman TS, Lee IM, Manson JE, Gaziano JM. Physical activity, body mass index, and diabetes risk in men: a prospective study. AM J MED. 2009;122(12):1115–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sun Y, Wang Z, Huang Z, Hu H, Han Y. The Association between the Triglyceride-to-High-Density Lipoprotein Cholesterol Ratio and the risk of Progression to Diabetes from prediabetes: a 5-year Cohort Study in Chinese adults. FRONT ENDOCRINOL. 2022;13:947157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li M, Zhang W, Zhang M, Li L, Wang D, Yan G, Qiao Y, Tang C. Nonlinear relationship between untraditional lipid parameters and the risk of prediabetes: a large retrospective study based on Chinese adults. CARDIOVASC DIABETOL. 2024;23(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zheng X, Zhang X, Han Y, Hu H, Cao C. Nonlinear relationship between atherogenic index of plasma and the risk of prediabetes: a retrospective study based on Chinese adults. CARDIOVASC DIABETOL. 2023;22(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mo Z, Han Y, Cao C, Huang Q, Hu Y, Yu Z, Hu H. Association between non-high-density lipoprotein to high-density lipoprotein ratio and reversion to normoglycemia in people with impaired fasting glucose: a 5-year retrospective cohort study. DIABETOL METAB SYNDR. 2023;15(1):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zha F, Cao C, Hong M, Hou H, Zhang Q, Tang B, Hu H, Han Y, Zan Y, Wang Y, et al. The nonlinear correlation between the cardiometabolic index and the risk of diabetes: a retrospective Japanese cohort study. FRONT ENDOCRINOL. 2023;14:1120277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tseng CH. Lipoprotein(a) is an independent risk factor for peripheral arterial disease in Chinese type 2 diabetic patients in Taiwan. Diabetes Care. 2004;27(2):517–21. [DOI] [PubMed] [Google Scholar]
- 79.Tseng CH. Apolipoprotein B is an independent risk factor for microalbuminuria in Taiwanese patients with type 2 diabetes. Diabetes Care. 2003;26(10):2965–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets supporting the conclusions of this article are available in the Dryad database, which is publicly accessible. (https://datadryad.org/stash/data/set/doi:10.5061/dryad.8q0p192).