Abstract
Progressive multifocal leukoencephalopathy (PML) is a demyelinating disease of the central nervous system caused by a reactivation of the polyomavirus JC (JCV) within a setting of immunosuppression. The nature of the immune response that contains replication of this virus is unknown. We have explored JCV-specific cellular immune responses in patients with PML and control subjects. JCV antigen-stimulated peripheral blood mononuclear cells (PBMC) of four human immunodeficiency virus (HIV)-infected patients who were survivors of PML and one HIV-uninfected patient recently diagnosed with PML lysed autologous B-lymphoblastoid cell lines expressing either the JCV T regulatory protein or the VP1 major capsid protein. This lysis was mediated by CD8+ T lymphocytes and was major histocompatibility complex class I restricted. These cells were therefore cytotoxic T lymphocytes (CTL). JCV-specific CTL could not be detected in PBMC of three HIV-infected PML patients who had progressive neurologic disease and an eventual fatal outcome. These data suggest that the JCV-specific cellular immune response may play a crucial role in the containment of PML. This finding may also prove useful as a favorable prognostic marker in the clinical management of these patients.
JC virus (JCV) is the etiologic agent of progressive multifocal leukoencephalopathy (PML), a central nervous system (CNS) disease that occurs in patients with underlying immunosuppression (20). JCV infects most adults but does not cause disease in healthy individuals. Viral reactivation occurs in 4% of patients with AIDS and occasionally other individuals who are profoundly immunosuppressed. This reactivation leads to a lytic infection of oligodendrocytes and an associated demyelination of the CNS. As there is no treatment for PML, this disease constitutes a growing medical problem, since its incidence continues to increase in human immunodeficiency virus (HIV)-infected patients despite the introduction of potent antiretroviral medications (2, 22). PML is also a threat to increasing numbers of patients who are immunosuppressed in association with organ transplantation.
Our understanding of the immune response to JCV is limited. Seroconversion occurs in childhood (24), and immunoglobulin G (IgG) antibodies specific for JCV can be detected in approximately 90% of the normal adult population by a hemagglutination inhibition assay or enzyme-linked immunosorbent assay (25). Pregnant women in the third trimester have a measurable rise of serum JCV antibodies (5). The humoral immune response appears to be incapable of preventing PML. Indeed, most individuals with PML have preexisting anti-JCV IgG antibodies in their serum and do not demonstrate a rise in titer of these antibodies at the onset of neurologic disease (11).
Intrathecal synthesis of anti-JCV VP1 protein IgG antibodies has been detected in 76% of PML patients. However, no clinical or biologic differences were noted in these patients compared to those without detectable JCV-specific antibodies in the cerebrospinal fluid (CSF) (25). A rise in CSF JCV-specific antibodies did not prevent a fatal outcome in two patients with PML (3, 9). In addition, IgM antibodies have not been detected in the serum or CSF of individuals with PML. Finally, JCV-specific antibodies do not prevent virus excretion in the urine in immunocompetent individuals (4).
Since JCV reactivation occurs as a consequence of immune suppression and humoral immunity does not appear to be critical in controlling JCV spread, cell-mediated immunity may play a role in the containment of JCV. However, studies of this immune response have been limited. A generalized impairment of cell-mediated immunity in PML patients has been documented, with anergy in these individuals to skin tests with common antigens (6, 10, 15, 16) and impaired lymphocyte proliferation responses to the mitogen phytohemagglutinin (12, 15) and to allogeneic cells (12). In seven patients with PML, in vitro lymphocyte proliferation to mitogens was blunted, corresponding to an expected generalized depression of cell-mediated immunity (26). In these patients, the production of leukocyte migration inhibitory factor by lymphocytes in response to JCV antigens was nondetectable, suggesting a selective deficiency in the cellular immune response to JCV. More recently, the major histocompatibility complex (MHC) class I and II molecules were found to be expressed at high levels within PML lesions, indicating that an absence of antigen presentation due to decreased MHC expression does not explain the uncontrolled replication of JCV in the CNS (1).
To begin exploring the role of the JCV-specific cellular immune response in containing JCV replication, we sought to determine whether JCV-specific cytotoxic T lymphocytes (CTL) could be demonstrated in peripheral blood mononuclear cells (PBMC) of individuals with PML. CTL responses specific for virus-infected cells have traditionally been measured by expanding effector T-cell populations in vitro by antigen-specific or nonspecific approaches and then assessing the ability of these cells to induce lysis and the release of radioactive label from 51Cr-labeled virus-expressing target cells (18).
In developing an assay for JCV-specific CTL, we generated recombinant vaccinia virus-JCV constructs to express JCV gene products in both stimulator and target cells. Two JCV genes were selected for expression by recombinant vaccinia viruses. The VP1 gene was chosen since it encodes the major capsid protein of JCV, and the T gene was chosen because it encodes a multifunctional protein that regulates transcription of the early gene sequences and defines the switch to transcription of the structural genes. Together, these two genes account for two-thirds of the viral genome. To facilitate the generation of these recombinant vaccinia viruses, the T and VP1 JCV genes were individually subcloned into the plasmid pAbt 4587 (a generous gift of Therion Biologics, Cambridge, Mass.) from the pM1TC plasmid, which contains the entire JCV genome. The presence of the nucleotide sequence T5NT in the DNA of the recombinant genes causes early termination of protein synthesis in vaccinia virus. Using PCR-based conservative mutagenesis, one T5NT sequence in the VP1 gene and three T5NT sequences in the T gene were modified without causing a change in the amino acid composition of the expressed protein. Nucleotide sequences of the mutated recombinant clones of the T and VP1 JCV genes were verified by automated sequencing of both strands and were found to be equivalent to the coding potential of the pM1TC plasmid.
Molecular clones containing the T and VP1 JCV genes were inserted into vaccinia virus (NYCBH strain A) at a nonessential site in the HindIII M region by in vivo recombination (19). The vaccinia virus 40K transcriptional promoter was used to direct expression of the JCV genes. The presence in the recombinant vaccinia virus of a full-length DNA insert of the desired JCV genes was demonstrated by DNA PCR amplification from infected BSC-40 cells. Expression of the JCV genes by the recombinant viruses was initially demonstrated by an in situ enzyme-linked immunosorbent assay (black plaque assay) performed directly on viral plaques in BSC-40 cells (21). Briefly, cell monolayers infected with the JCV T recombinant vaccinia virus were incubated with the mouse monoclonal antibody SV40 T antigen (T Ag; Ab-2) specific for SV40 T Ag (Oncogene Research Products, Cambridge, Mass.), which cross-reacts with JCV T Ag. The NCL-JC mouse monoclonal antibody (Novo Laboratories), which recognizes JCV VP1 Ag, was used on cells infected with the JCV VP1 recombinant vaccinia virus. The presence of the desired proteins in the infected cells was revealed by incubation of cells with a horseradish peroxidase-conjugated goat anti-mouse antibody (Dako) and addition of dianisidine. The expression of JCV proteins of the correct predicted size was determined by radioimmunoprecipitation on the lysates of a B-lymphoblastoid cell line (B-LCL) from an HIV-negative patient with PML; the B-LCL was infected with the recombinant vaccinia viruses using the monoclonal antibodies mentioned above (Fig. 1). JCV recombinant protein synthesis was also assessed in the B-LCL of an HIV-positive patient with PML and in two healthy controls using the same method, and it was found to be identical (data not shown).
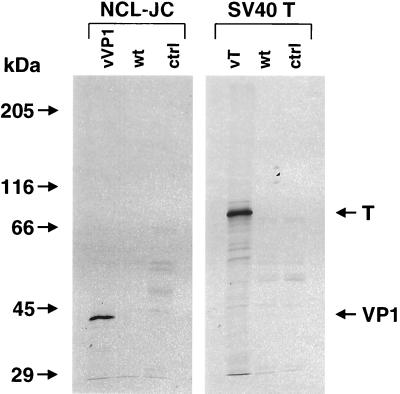
FIG. 1.
Radioimmunoprecipitation of the JCV VP1 and T proteins expressed by recombinant vaccinia viruses. B-LCL from an HIV-negative patient with PML were infected at an MOI of 10 PFU/cell for 16 h with either the recombinant vaccinia virus containing the VP1 (vVP1) or T (vT) genes, or the wild-type vaccinia virus (wt). Uninfected cells served as controls (ctrl). The monoclonal antibodies NCL-JC, specific for the VP1 protein, and SV40 T, which cross-reacts with the T protein of JCV, were used for the immunoprecipitations. Protein products of approximately 43 and 86 kDa were identified, corresponding to the expected size of the JCV VP1 and T proteins, respectively.
Autologous B-LCLs infected with these recombinant vaccinia viruses were made to serve as both stimulator and target cells. These B-LCLs were generated by incubating 107 Ficoll-diatrizoate-isolated PBMC with supernatant of an Epstein-Barr virus (EBV)-producing cell line. Stimulator cells were prepared by infecting autologous B-LCLs with the recombinant vaccinia viruses expressing either the T or VP1 JCV genes at a multiplicity of infection (MOI) of 10 PFU/cell for 16 h, followed by Ficoll-diatrizoate gradient centrifugation, fixation for 15 min in 1.5% paraformaldehyde, and incubation for 15 min in 0.2 M glycine.
For preparation of target cells, the same recombinant vaccinia viruses were used to express the JCV T or VP1 genes in autologous EBV-transformed B-LCL. B-LCLs similarly transformed with wild-type vaccinia virus were used as control target cells. Aliquots of 106 B-LCL were incubated overnight with the respective vaccinia viruses at an MOI of 10 PFU/cell. The next morning, target cells were counted and labeled with 100 μCi of 51Cr for 90 min. These cells were washed, and 104 cells were added as targets to the effector cells in 96-well U-bottom plates in a final volume of 200 μl/well.
Effector cells were generated by mixing Ficoll-diatrizoate gradient centrifugation-isolated PBMC with stimulator cells at a ratio of 1:1. Cells were maintained in culture in the presence of interleukin-2 for 11 to 14 days. The stimulated cells were then analyzed in a 51Cr-release assay (18) performed in U-bottom microtiter plates (Costar, Cambridge, Mass.). A total of 104 51Cr-labeled target cells were plated per well, and effector cells were added at various effector-to-target (E:T) ratios. These cell mixtures were incubated in culture medium in a volume of 0.2 ml/well for 4 h at 37°C. Supernatants were then harvested and their radioactivity was measured. Samples were assayed in duplicate.
To measure the immune response against JCV T and VP1 proteins, functional CTL assays were performed on PBMC of a total of 21 study subjects, including 7 HIV-positive PML patients, 3 HIV-negative PML patients, 7 HIV-positive patients with other neurologic diseases, and 4 healthy HIV-negative control subjects. The diagnosis of PML was ascertained by clinical and neuroradiological criteria and confirmed by brain biopsy or by positive JCV PCR in the CSF. Of the seven HIV-positive PML patients, four were survivors whose disease had improved or remained stable for 15 months to 4½ years after their initial diagnosis of PML while on highly active antiretroviral therapy (HAART). Two of the three HIV-negative PML patients were also stable 3 to 8 years after their initial diagnosis. One of them had a history of non-Hodgkin's lymphoma and had received cytosine arabinoside for treatment of PML. The other patient had a history of a thymoma and was treated for PML with alpha interferon. The third HIV-negative PML patient had a history of B-cell lymphoma and was improving neurologically without any treatment 6 months after being diagnosed with PML. Of the seven HIV-positive patients with other neurological diseases, one had cytomegalovirus polyradiculopathy, three had HIV encephalopathy, and three had leukoencephalopathy of unknown origin. All of these patients had negative JCV PCR in CSF samples, and two of them had a brain biopsy which showed no evidence of PML (Table 1).
TABLE 1.
Detection of JCV T- and VP1-specific effector cells in PBMC of PML patients and control subjects
| Diagnosisa | JCV T (no. pos/no. tested) | JCV VP1 (no. pos/no. tested) |
|---|---|---|
| HIV+ PML survivor | 3/4 | 4/4 |
| HIV+ PML progressor | 0/3 | 0/3 |
| HIV− PML early | 0/1 | 1/1 |
| HIV− PML survivor | 0/2 | 0/2 |
| HIV+ OND | 0/7 | 1/7 |
| HIV− control | 0/4 | 0/4 |
HIV+, HIV positive; HIV−, HIV negative; PML early, patient diagnosed 6 months prior to testing and showing clinical improvement; OND, other neurological diseases. See text for details.
Cytolytic activity specific for the JCV T or VP1 protein could be demonstrated in PBMC of the four HIV-positive PML survivors and the HIV-negative patient recently diagnosed with PML (Fig. 2). Of the seven other HIV-positive patients evaluated, one had detectable CTL specific for the VP1 protein only. This patient had focal leukoencephalopathy with clinical and radiological findings consistent with PML but had undetectable JCV DNA in the CSF by PCR. He was already on HAART when the diagnostic test was performed. None of the healthy control subjects' PBMC displayed cytolytic activity specific for the T or VP1 proteins.
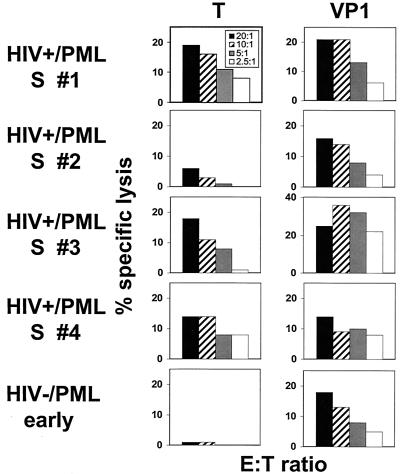
FIG. 2.
PBMC from HIV type 1 (HIV-1)-infected survivors of PML (HIV+/PML S # 1 to 4) have cytolytic activity specific for the JCV T and VP1 proteins. T- and VP1-specific effector cells could be detected in three of four and four of four HIV-positive PML survivors, respectively. PBMC from one recently diagnosed HIV-negative PML patient who showed clinical improvement had cytolytic activity against the VP1 protein. PBMC were stimulated with autologous, fixed B-LCL expressing the T or VP1 protein in interleukin-2-containing medium and assessed for effector function using autologous target cells infected with recombinant vaccinia virus-T, -VP1, or the wild-type vaccinia virus. The percent specific lysis indicates the difference in specific 51Cr release between cells infected with the T- or VP1-expressing vaccinia virus and those infected with the wild-type vaccinia virus. E:T ratios are shown in the box in the upper left panel.
To rule out the possibility that stimulation by EBV or vaccinia virus proteins expressed on stimulator cells was responsible for the in vitro expansion of JCV-specific effector cells, further experiments were performed in which effector cells were incubated with autologous B-LCL infected with wild-type vaccinia virus. Such stimulated lymphocytes did not mediate any specific lysis of JCV-expressing target cells (Fig. 3).
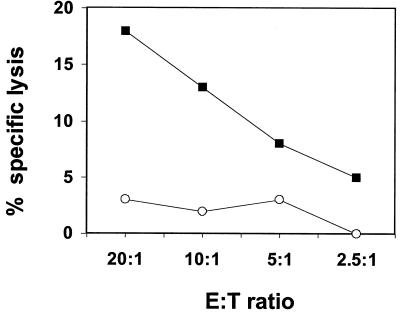
FIG. 3.
PBMC of an HIV-negative PML patient stimulated with autologous fixed B-LCL infected with a recombinant vaccinia virus expressing the JCV VP1 protein (filled squares), but not with the wild-type vaccinia virus (open circles), mediated cytolytic activity specific for the JCV VP1 protein.
To assess the reproducibility of this assay, as well as the persistence of these cell populations in individuals, PBMC of an HIV-positive PML survivor were assayed serially for cytolytic activity specific for the JCV T and VP1 proteins. Persistent JCV T- and VP1-specific cell lysis was demonstrable over a 5-month period (Fig. 4).
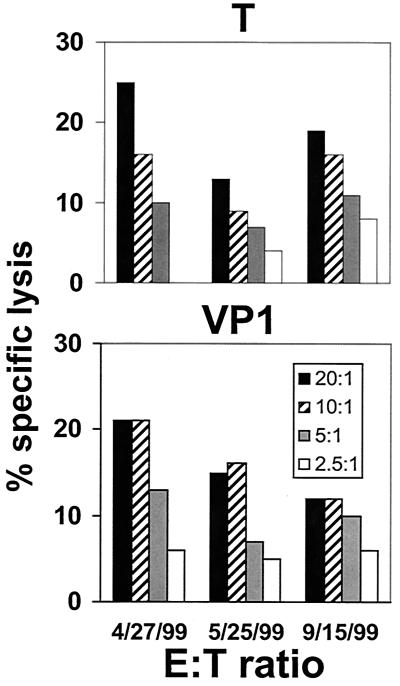
FIG. 4.
PBMC from an HIV-1-infected survivor of PML mediated cytolytic activity specific for the JCV T and VP1 proteins detectable in three successive assays. The average percentage of specific lysis and standard deviation at an E:T ratio of 20:1 was 19% ± 6% for JCV T protein and 16% ± 4.6% for the VP1 protein.
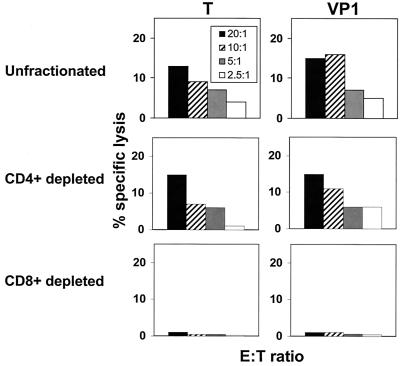
The T-lymphocyte subset containing these JCV-specific effector cells was then determined. PBMC were stimulated for 11 to 14 days with JCV antigen and then incubated with CD4- or CD8-specific immunomagnetic beads (Dynal) at a cell:bead ratio of 1:3. The cells were separated in a magnetic field, and their purity was assessed by flow cytometry. The CD8-depleted cells contained less than 2.3% CD8+ cells and greater than 55% CD4+ cells; the CD4-depleted cells contained less than 1.2% CD4+ cells and more than 83% CD8+ cells. JCV-specific effector cells were demonstrable in the CD4+ cell-depleted but not the CD8+ cell-depleted lymphocyte populations (Fig. 5).
FIG. 5.
Unfractionated and CD4+ T lymphocyte-depleted but not CD8+ T lymphocyte-depleted PBMC of an HIV-1-infected survivor of PML mediated cytolytic activity specific for the JCV T and VP1 proteins.
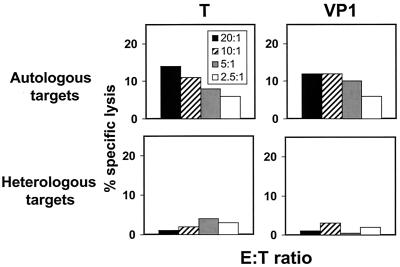
Finally, we pursued studies to determine the MHC restriction of the JCV-specific effector cell-target cell interactions. The MHC class I alleles expressed by the PBMC evaluated in these studies were determined using standard serologic tissue-typing procedures. Fully MHC class I-mismatched target cells were selected from a panel of previously characterized B-LCLs. Lysis of recombinant vaccinia virus-JCV-infected autologous and fully allogeneic target cells was assessed in a standard 51Cr-release assay. These effector cells lysed MHC class I-matched but not mismatched target cells (Fig. 6). Therefore, these experiments demonstrated that the JCV-specific effector cells were MHC class I-restricted CD8+CTL.
FIG. 6.
PBMC of an HIV-1-infected survivor of PML lysed autologous target cells but not fully MHC class I-mismatched target cells infected with recombinant vaccinia viruses expressing the JCV protein T or VP1.
This study, which included 10 patients with PML with variable clinical outcome, is the first demonstration of JCV-specific CTL in individuals with PML. The four evaluated HIV-positive PML survivors had detectable JCV-specific CTL that recognized the T and/or the VP1 protein, whereas neither of the three evaluated HIV-positive PML patients with progressive neurologic disease and a fatal outcome had detectable JCV-specific CTL. As expected, the PML survivors had a higher CD4+ cell count (140 to 771 cells/μl; mean, 358 cells/μl) than those patients with fatal disease (9 to 104 cells/μl; mean, 53 cells/μl), and the PML survivors also had a lower plasma HIV viral load (<50 copies/ml in three of four PML survivors, versus 12,000 to 75,000 copies/ml in those with fatal disease). HIV-positive patients with other neurological diseases had CD4+ cell counts ranging from 7 to 446 cells/μl (mean, 230 cells/μl), and three of six patients had undetectable plasma HIV viral loads (<50 to 60,000 copies/ml). These results suggest that the persistence of a JCV-specific CD8+ cellular immune response is associated with long-term survival in HIV-positive PML patients.
The HIV-negative patient recently diagnosed with PML who showed clinical improvement without treatment had a positive CTL response specific for the VP1 protein. It is somewhat surprising that the two HIV-negative PML survivors had no detectable CTL responses specific for the JCV T or VP1 protein. These patients had been treated with cytosine arabinoside or alpha interferon for PML and had been neurologically stable for 3 to 8 years at the time of testing. It is therefore possible that the absence of T- or VP1-specific CTL in these patients reflects a burnt-out disease with low levels of JCV antigen production. In addition, these two patients were also older than the HIV-positive PML survivors (56 to 62 years versus 32 to 45 years, respectively). Loss of specific CTL as a function of age has been reported in both the experimental and clinical setting (7, 17). Alternatively, their cellular immune responses may be directed against other JCV proteins.
One HIV-positive patient had detectable CTL responses specific for the VP1 protein. He had focal leukoencephalopathy with clinical and radiological findings consistent with PML but had undetectable JCV DNA in the CSF by PCR. This patient was already on HAART when the diagnostic CSF PCR was performed. It is possible that he indeed had PML but that the JC viral load in the CSF was below the limit of detection at the time he was receiving HAART (8). Alternatively, this cytolytic response might reflect a cross-reactive immune response directed against another polyomavirus, such as a virus that is closely related to JC virus but whose DNA cannot be amplified with the JCV-specific PCR primers.
None of the healthy control subjects' PBMC displayed cytolytic activity specific for the T or VP1 proteins. JC viremia is usually not detectable in immunocompetent individuals (13, 14, 23). JCV remains confined to the kidney tubular epithelial cells, and 30% of healthy people shed JCV in the urine (13). It is possible that JCV antigenic stimulation persists at low levels in healthy people, and that JCV-specific CTL prevent viral dissemination in the bloodstream. This JCV-specific CTL response could, however, be restricted to the kidney. JCV-specific CTL may prevent JCV spread in the bloodstream, but their intraparenchymal localization may preclude their detection in the peripheral blood. Alternatively, the CTL responses might be directed against other JCV proteins.
The detection of JCV-specific CTL in HIV-positive PML survivors and HIV-negative PML patients with improving clinical status suggests that this cellular immune response may be important in the containment of PML. This finding may also have direct clinical relevance as a favorable prognostic marker in the clinical management of these patients.
Acknowledgments
We thank Therion Biologics for providing the plasmid pAbt 4587 and the vaccinia virus strain used in this study.
This work was supported by Public Health Service grant no. NS-01919, NS-41198, and AI-28691 to I.J.K. and AI-20729 to N.L.L. R.D.P. is the recipient of grants from the Geneva University Hospital, Switzerland, and from the Carlos and Elsie de Reuter Foundation.
REFERENCES
- 1.Achim C L, Wiley C A. Expression of major histocompatibility complex antigens in the brains of patients with progressive multifocal leukoencephalopathy. J Neuropathol Exp Neurol. 1992;51:257–263. doi: 10.1097/00005072-199205000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Ammassari A, Cingolani A, Pezzotti P, De Luca D A, Murri R, Giancola M L, Larocca L M, Antinori A. AIDS-related focal brain lesions in the era of highly active antiretroviral therapy. Neurology. 2000;55:1194–1200. doi: 10.1212/wnl.55.8.1194. [DOI] [PubMed] [Google Scholar]
- 3.Berner B, Krieter D H, Rumpf K W, Grunewald R W, Beuche W, Weber T, Muller G A. Progressive multifocal leukoencephalopathy in a renal transplant patient diagnosed by JCV-specific DNA amplification and an intrathecal humoral immune response to recombinant virus protein 1. Nephrol Dial Transplant. 1999;14:462–465. doi: 10.1093/ndt/14.2.462. [DOI] [PubMed] [Google Scholar]
- 4.Coleman D V, Gardner S D, Mulholland C, Fridiksdottir V, Porter A A, Lilford R, Valdimarsson H. Human polyomavirus in pregnancy. A model for the study of defence mechanisms to virus reactivation. Clin Exp Immunol. 1983;53:289–296. [PMC free article] [PubMed] [Google Scholar]
- 5.Daniel R, Shah K, Madden D, Stagno S. Serological investigation of the possibility of congenital transmission of papovavirus JC. Infect Immun. 1981;33:319–321. doi: 10.1128/iai.33.1.319-321.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellison G W. Progressive multifocal leukoencephalopathy (PML). I. Investigation of the immunologic status of a patient with lymphosarcoma and PML. J Neuropathol Exp Neurol. 1969;28:501–506. doi: 10.1097/00005072-196907000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Fagiolo U, Amadori A, Cozzi E, Bendo R, Lama M, Douglas A, Palu G. Humoral and cellular immune response to influenza virus vaccination in aged humans. Aging (Milano) 1993;5:451–458. doi: 10.1007/BF03324202. [DOI] [PubMed] [Google Scholar]
- 8.Giudici B, Vaz B, Bossolasco S, Casari S, Brambilla A M, Luke W, Lazzarin A, Weber T, Cinque P. Highly active antiretroviral therapy and progressive multifocal leukoencephalopathy: effects on cerebrospinal fluid markers of JC virus replication and immune response. Clin Infect Dis. 2000;30:95–99. doi: 10.1086/313598. [DOI] [PubMed] [Google Scholar]
- 9.Guillaume B, Sindic C J, Weber T. Progressive multifocal leukoencephalopathy: simultaneous detection of JCV DNA and anti-JCV antibodies in the cerebrospinal fluid. Eur J Neurol. 2000;7:101–106. doi: 10.1046/j.1468-1331.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- 10.Horn G V, Bastian F O, Moake J L. Progressive multifocal leukoencephalopathy: failure of response to transfer factor and cytarabine. Neurology. 1978;28:794–797. doi: 10.1212/wnl.28.8.794. [DOI] [PubMed] [Google Scholar]
- 11.Johnson R T. Viral infections of the nervous system. New York, N.Y: Lippincott-Raven; 1998. pp. 241–248. [Google Scholar]
- 12.Knight A, O'Brien P, Osoba D. Progressive multifocal leukoencephalopathy. Immunologic aspects. Ann Intern Med. 1972;77:229–233. doi: 10.7326/0003-4819-77-2-229. [DOI] [PubMed] [Google Scholar]
- 13.Koralnik I J, Boden D, Mai V X, Lord C I, Letvin N L. JC virus DNA load in patients with and without progressive multifocal leukoencephalopathy. Neurology. 1999;52:253–260. doi: 10.1212/wnl.52.2.253. [DOI] [PubMed] [Google Scholar]
- 14.Koralnik I J, Schmitz J E, Lifton M A, Forman M A, Letvin N L. Detection of JC virus DNA in peripheral blood cell subpopulations of HIV-1-infected individuals. J Neurovirol. 1999;5:430–435. doi: 10.3109/13550289909029484. [DOI] [PubMed] [Google Scholar]
- 15.Marriott P J, O'Brien M D, Mackenzie I C, Janota I. Progressive multifocal leucoencephalopathy: remission with cytarabine. J Neurol Neurosurg Psychiatry. 1975;38:205–209. doi: 10.1136/jnnp.38.3.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathews T, Wisotzkey H, Moossy J. Multiple central nervous system infections in progressive multifocal leukoencephalopathy. Neurology. 1976;26:9–14. doi: 10.1212/wnl.26.1.9. [DOI] [PubMed] [Google Scholar]
- 17.Mbawuike I N, Acuna A, Caballero D, Pham-Nguyen K, Gilbert B, Potrillon P, Harmon M. Reversal of age-related deficient influenza virus-specific CTL responses and IFN-gamma production by monophosphoryl lipid A. Cell Immunol. 1996;173:64–78. doi: 10.1006/cimm.1996.0252. [DOI] [PubMed] [Google Scholar]
- 18.Miller M D, Lord C I, Stallard V, Mazzara G P, Letvin N L. The gag-specific cytotoxic T lymphocytes in rhesus monkeys infected with the simian immunodeficiency virus of macaques. J Immunol. 1990;144:122–128. [PubMed] [Google Scholar]
- 19.Nakano E, Panicali D, Paoletti E. Molecular genetics of vaccinia virus: demonstration of marker rescue. Proc Natl Acad Sci USA. 1982;79:1593–1596. doi: 10.1073/pnas.79.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padgett B L, Walker D L, ZuRhein G M, Eckroade R J, Dessel B H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971;i:1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- 21.Smith G L, Murphy B R, Moss B. Construction and characterization of an infectious vaccinia virus recombinant that expresses the influenza hemagglutinin gene and induces resistance to influenza virus infection in hamsters. Proc Natl Acad Sci USA. 1983;80:7155–7159. doi: 10.1073/pnas.80.23.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tantisiriwat W, Tebas P, Clifford D B, Powderly W G, Fichtenbaum C J. Progressive multifocal leukoencephalopathy in patients with AIDS receiving highly active antiretroviral therapy. Clin Infect Dis. 1999;28:1152–1154. doi: 10.1086/514762. [DOI] [PubMed] [Google Scholar]
- 23.Tornatore C, Berger J R, Houff S A, Curfman B, Meyers K, Winfield D, Major E O. Detection of JC virus DNA in peripheral lymphocytes from patients with and without progressive multifocal leukoencephalopathy. Ann Neurol. 1992;31:454–462. doi: 10.1002/ana.410310426. [DOI] [PubMed] [Google Scholar]
- 24.Walker D L, Padgett B L. The epidemiology of human polyomaviruses. Prog Clin Biol Res. 1983;105:99–106. [PubMed] [Google Scholar]
- 25.Weber T, Trebst C, Frye S, Cinque P, Vago L, Sindic C J, Schulz-Schaeffer W J, Kretzschmar H A, Enzensberger W, Hunsmann G, Luke W. Analysis of the systemic and intrathecal humoral immune response in progressive multifocal leukoencephalopathy. J Infect Dis. 1997;176:250–254. doi: 10.1086/514032. [DOI] [PubMed] [Google Scholar]
- 26.Willoughby E, Price R W, Padgett B L, Walker D L, Dupont B. Progressive multifocal leukoencephalopathy (PML): in vitro cell-mediated immune responses to mitogens and JC virus. Neurology. 1980;30:256–262. doi: 10.1212/wnl.30.3.256. [DOI] [PubMed] [Google Scholar]