Abstract
A middle-aged woman presented with hypertensive emergency after corticosteroid treatment for Sjögren syndrome-associated multiple mononeuropathy with suspected systemic sclerosis. Hypertensive heart failure with hyperreninemia improved with antihypertensives, including aliskiren; however, she became hemodialysis-dependent. Clinical findings and biopsy-proven thrombotic microangiopathy indicated conditions resembling scleroderma renal crisis (SRC). Severe hypertension and heart failure with hyperreninemia occurred after stopping aliskiren for hypotension due to diverticular bleeding, which improved after the reintroduction of aliskiren. Aliskiren appears to be effective in managing hypertensive heart failure in patients with SRC. Nevertheless, hemodialysis remained necessary in our case, and whether or not aliskiren can restore the renal function is unclear.
Keywords: aliskiren, hyperreninemia, hypertension, multiple mononeuropathy, scleroderma renal crisis, thrombotic microangiopathy
Introduction
The renin-angiotensin system (RAS) is involved in hypertensive emergencies due to scleroderma renal crisis (SRC) (1). Injury to the renal arterial endothelium is believed to initiate progressive renal vascular injury, which triggers hyperreninemia and accelerated hypertension (2). Inhibition of RAS, mainly by angiotensin-converting enzyme inhibitors (ACEIs), improves severe hypertension and acute hypertension-mediated damage to various organs, such as the kidneys, heart, retina, brain, and large arteries (3). However, a recent systemic review showed that 54.9% of patients with SRC treated with ACEIs required either temporal or permanent dialysis (4), and this condition is associated with high mortality.
We encountered a middle-aged woman who presented with an SRC-like condition, acute heart failure, and renal failure accompanied by hyperreninemia. This presentation occurred after corticosteroid treatment for Sjögren syndrome-associated multiple mononeuropathy with systemic sclerosis (SSc). Despite effective control of her blood pressure and heart failure using antihypertensive medications including aliskiren fumarate (a direct renin inhibitor), she ultimately required ongoing hemodialysis. Interestingly, we observed that aliskiren effectively managed severe hypertension and acute heart failure with hyperreninemia, even after the initiation of hemodialysis in our patient.
Although whether or not aliskiren can aid in the recovery of the renal function in patients with SRC is unclear, it may be worthwhile to further investigate its efficacy in improving the renal function in individuals with SRC.
Case Report
A 48-year-old woman presented with severe numbness in the left thumb and left index finger, left toe, and ulnar side of the back of the left hand over a 10-month period. More recently, she experienced painful numbness in both soles of the feet and in the left fourth and fifth fingers. In addition, she exhibited swelling of the left thumb and left index finger and had an ulcer on the tip of her right fingers. The skin of her face, extensor side of the arms, back of the hands, and back showed a poikioderma-like appearance. Both palms showed erythema punctatum and chest thickening. She had a history of atopic dermatitis since 20 years old and had dry mouth. She believed that her skin lesions were due to atopic dermatitis.
A neurological examination and neuromuscular electrodiagnosis revealed multiple mononeuropathies. The patient did not show any signs or symptoms related to infectious diseases or malignancy. Laboratory test results for urine, blood count, and blood chemistry, including creatinine kinase, were almost normal. Immunologic tests showed a C-reactive protein level of 0.38 mg/dL, anti-nuclear antibody titer of 1:2,560 with discrete-speckled pattern, and positive anti-centromere antibody presence. However, anti-Scl 70, anti-RNA polymerase III, anti-Jo-1, and anti-RNP antibodies were negative. Chest computed tomography revealed no abnormalities. Although systemic scleroderma (SSc) was suspected, a dermatologist could not confirm skin thickening of the fingers, resulting in it not being classified as SSc (5).
Despite the absence of anti-SS-A/B antibodies and no significant lymphocytic sialadenitis on a lip biopsy, the patient had dry mouth, positive findings on the Saxon test, a decreased function on salivary gland scintigraphy, and a positive finding combination of Schirmer's test and the fluorescein staining test. These findings confirmed the diagnosis of Sjögren syndrome (6). Therefore, she was diagnosed with Sjögren syndrome-associated multiple mononeuropathy (7) and treated upon admission to our neurology department with methylprednisolone pulse therapy (1,000 mg/day intravenously [i.v.] for 3 consecutive days). Oral prednisolone (PSL) was then administered at 40 mg/day for 2 weeks and tapered, resulting in considerable improvement in her neurological symptoms. The patient was treated at a neurology outpatient clinic. Her blood pressure at the time of discharge was 132/64 mmHg, and her serum creatinine level was 0.65 mg/dL.
Four months after corticosteroid treatment with 20 mg/day PSL, she presented to our emergency department with a 2-week history of fatigue and dyspnea and was admitted to our hospital because of a hypertensive emergency. She had no pre-existing renal disease or hypertension four months prior to presentation. Upon presentation, the patient had a blood pressure of 226/172 mmHg, heart rate of 144 beats/min, and SpO2 of 77% (room air). Oxygen was supplied immediately using a reservoir mask. The results of blood tests showed a hemoglobin level of 14.3 g/dL, white blood cell count of 14,300 /μL, platelet count of 19.8×104/μL, urea nitrogen level of 47.1 mg/dL, serum creatinine level of 1.95 mg/dL, aspartate aminotransferase level of 105 U/L, alanine aminotransferase level of 80 U/L, alkaline phosphatase level of 422 U/L, lactate dehydrogenase level of 713 U/L, sodium level of 138 mEq/L, potassium level of 3.5 mEq/L, chloride level of 93 mEq/L, bicarbonate level of 16.9 mEq/L, and C-reactive protein level of 3.69 mg/dL. The urine test data were as follows: protein 2+, occult blood ±, red blood cells: 5-9/high-power field, proteinuria of 0.8 g/g creatinine, urinary N-acetyl-β-D-glucosaminidase of 4.9 U/L, and alpha1-microglobulin of 65.92 mg/L. There were no signs of uveitis during a dilated eye examination and no hypertensive changes on a fundosated eye examination. Chest radiography revealed butterfly shadow and severe cardiomegaly. She was diagnosed with a hypertensive emergency with acute heart and renal failure.
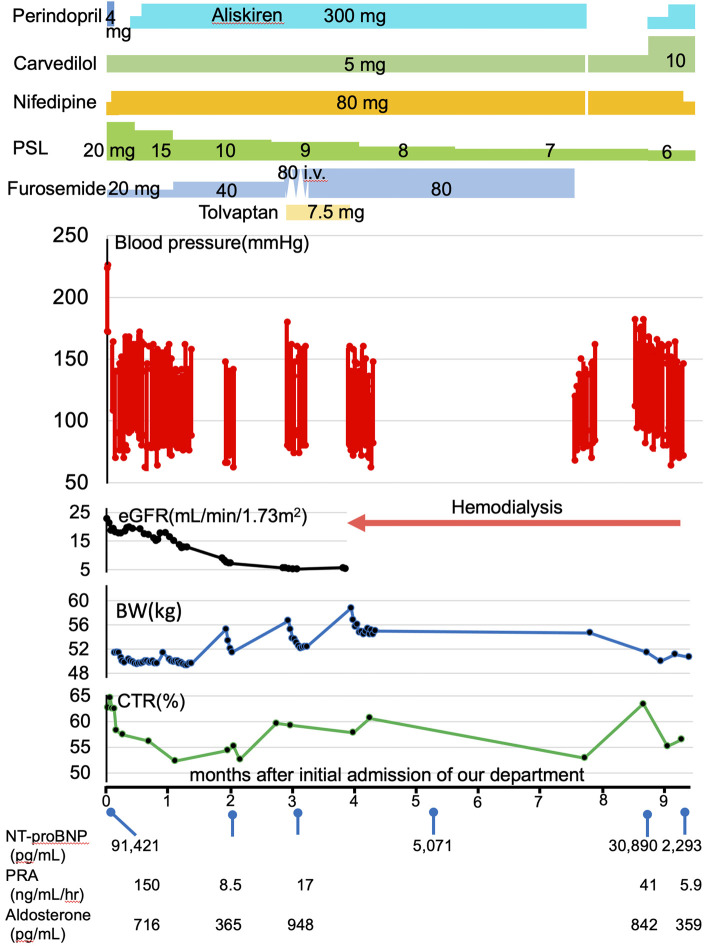
Treatment with perindopril at 4 mg/day resulted in an abrupt drop in systolic blood pressure to 140 mmHg along with a 35% reduction in the estimated glomerular filtration rate (eGFR). Consequently, the perindopril treatment was discontinued. Since there was no improvement in the eGFR, an alternative approach was adopted, starting with the use of nifedipine CR at 80 mg/day and subsequently adding carvedilol at 5 mg/day. An N-terminal prohormone of brain natriuretic peptide level of 91,421 pg/mL, renin activity of 150 ng/mL/h, and aldosterone concentration of 716 pg/mL were also detected (Fig. 1). Echocardiography showed diffuse hypokinesis, a reduced ejection fraction (EF) of 44%, E/e' of 11.79, left ventricular hypertrophy, and mild pericardial effusion without pulmonary artery hypertension. Abdominal computed tomography (CT) revealed normal kidneys.
Figure 1.
The time sequence of clinical findings. PSL: prednisolone, eGFR: estimated glomerular filtration rate, BW: body weight, CTR: costophrenic angle, NT-proBNP: N-terminal prohormone of brain natriuretic peptide, PRA: plasma renin activity, Ald: aldosterone concentration
After ruling out renal arterial stenosis by Doppler ultrasound and magnetic resonance angiography, which caused renovascular hypertension, aliskiren fumarate was added and increased to 300 mg/day. Her blood pressure stabilized at approximately 140-150/80 mmHg. Her heart failure improved, and her EF increased to 54%; however, her renal function continued to decline (Fig. 1).
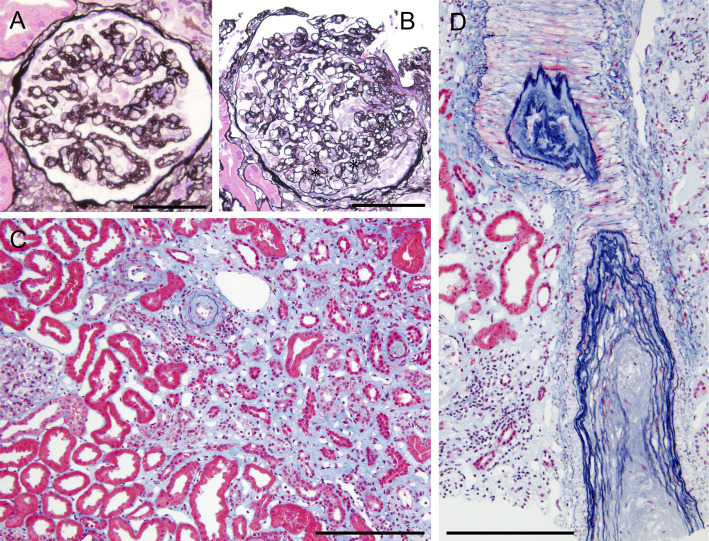
To confirm a definite diagnosis, a left kidney biopsy was performed, which was complicated by bleeding and hematoma around the left kidney and left retroperitoneum. At the time of interventional radiology, non-active bleeding was confirmed, and potential intrarenal small arterial narrowing suggested possible vasospasm. A kidney biopsy showed 1 glomerulus with global sclerosis out of 54 glomeruli obtained. Most of the remaining glomeruli showed capillary wrinkling, irregular double contours, and collapse of the capillary tufts (Fig. 2A). Some glomeruli showed mesangiolysis (Fig. 2B). Mild tubular atrophy, interstitial fibrosis with edema involving 60% of the tubulointerstitial areas, and no notable infiltration of inflammatory cells into the tubulointerstitial area were observed. The distribution of tubulointerstitial damage was zonal, indicating ischemic injury following vascular compromise (Fig. 2C). Many of the small arteries and arterioles showed marked intimal thickening with hyalinosis and luminal narrowing or obstruction (Fig. 2C, D). Immunofluorescence showed negative staining for IgA, IgG, IgM, C1q, and C3. Electron microscopy revealed swollen glomerular endothelial cells with loss of fenestration, subendothelial widening, and mild microvillous transformation of podocytes (Fig. 3). No electron-dense deposits were observed. Collectively, these histological findings suggest malignant nephrosclerosis and tubulointerstitial damage represented by subacute thrombotic microangiopathy (TMA).
Figure 2.
Light micrographs of a kidney biopsy. A: A glomerulus shows capillary wrinkling, irregular double contours and collapse of capillary tufts. Periodic acid-methenamine silver staining. Original magnification ×400. Bar=100 μm. B: A glomerulus shows mesangiolysis (asterisks). Periodic acid-methenamine silver staining. Original magnification ×200. Bar=100 μm. C: There are mild tubular atrophy and interstitial fibrosis with edema, and distribution of the tubulointerstitial damage is zonal. Elastica-Masson staining. Original magnification ×200. Bar=100 μm. C and D: The small arteries and arterioles show marked intimal thickening with hyalinosis, indicating luminal narrowing or obstruction. Elastica-Masson staining. Original magnification ×200. Bar=100 μm.
Figure 3.
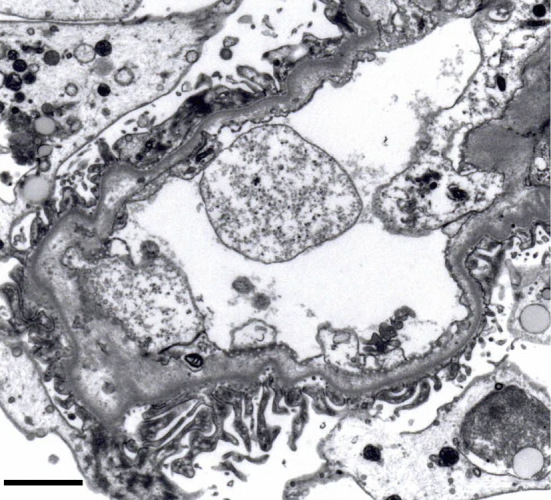
Electron microscopy. A glomerulus shows swollen glomerular endothelial cells with loss of fenestrations, subendothelial widening, and mild microvillous transformation of podocytes.
Her ADAMTS13 activity level was 99%, and ADAMTS13 inhibitor concentration was <0.5 U/mL, features which rendered thrombotic thrombocytopenic purpura-like status relatively unlikely. Our patient did not meet the American College of Rheumatology (ACR) or European League Against Rheumatism (EULAR) classification criteria for SSc, with a total score of 7 (puffy fingers: 2; fingertip ulcer: 2; and anti-centromer antibody: 3) and ≥9 points being considered to indicate SSc (5). However, based on her clinicopathological findings, the clinical judgment was an SRC-like condition that induced malignant hypertension and subsequent renal TMA. She was taking PSL at a dose of 15 mg/day, which was considered necessary to suppress her symptoms of multiple mononeuropathies. However, there is no significant evidence regarding whether stopping or reducing steroid treatment improves renal outcomes in patients with SRC (3). We planned to taper PSL treatment. Her serum creatinine levels continued to rise despite good blood pressure control and suppression of hyperreninemia (Fig. 1), and she required hemodialysis four months after antihypertensive treatment.
Approximately three months after hemodialysis introduction, the patient developed diverticular bleeding and hypotension. All antihypertensive drugs were stopped and then restarted, except for aliskiren. She had a two-week history of slight exertional dyspnea and presented to our hospital with dyspnea one month after stopping aliskiren. She was receiving nifedipine CR at 80 mg/day, carbedilol at 5 mg/day, and PSL at 7 mg/day. Her blood pressure was 182/112 mmHg, her heart rate was 110 beats/min, and SpO2 was 100% under a 2 L/min oxygen supply via a nasal cannula. Chest radiography revealed butterfly shadow and severe cardiomegaly. She was diagnosed with a hypertensive emergency with hypertensive heart failure. A total of 2.6 kg of dry weight reduction was performed for four hemodialysis sessions. However, echocardiography showed diffuse hypokinesis, a reduced EF of 45%, and E/e' of 11.37 with left ventricular hypertrophy without pulmonary artery hypertension. A renin activity of 41 ng/mL/h and aldosterone concentration of 842 pg/mL have been reported (Fig. 1). She underwent maintenance hemodialysis with the same dry weight. The carbedilol dose was increased to 10 mg/day, and aliskiren was reintroduced and increased to 300 mg/day. Her blood pressure stabilized at <140/<80 mmHg, EF returned to 60% with reduced serum renin activity, and she was discharged.
Discussion
The clinical manifestations and prognosis of SSc are variable, with the majority of patients exhibiting skin thickening and variable involvement of the internal organs. Although our patient did not fulfill the classification criteria for SSc, she had no history of hypertension or renal diseases. However, the use of a high dose of corticosteroids, which is considered a high risk factor for SRC (8), sudden onset of hypertension, severe high-renin hypertension with progressive renal failure, and renal pathology of TMA with a normal ADAMTS13 activity level and without ADAMTS13 inhibitor suggested an SRC-like condition.
The patient was also diagnosed with SS. Three SS patients with anti-centromere antibodies and high titers of anti-nuclear antibody (1:320) and negative anti-SS A/B antibodies among 402 patients diagnosed with primary SS developed clinical features suggestive of limited cutaneous SSc (9). Interestingly, the presence of anti-centromere antibodies among 402 patients with SS is reported to allow the identification of a subset of patients with “SS overlap syndrome”. The subset of patient show a wide diversity of autoimmune diseases, including SSc (10). We noticed possible widespread constriction of the small arteries in the present patient's kidneys. SRC is associated with reversible vasospasm and vasculopathy of the arcuate and interlobular renal arterial circulation (1,11). However, kidney bleeding may trigger intrarenal vasospasm, although it generally remains localized. Therefore, it is likely that the clinicopathological and potential radiological findings in our patient indicated that her hypertensive emergency was caused by a condition resembling SRC.
Despite the lack of randomized controlled trials, several cohort studies have shown a survival benefit with the use of ACEIs in patients with SRC (8). However, even if patients with SRC are treated with ACEIs, their prognosis remains poor. The current patient survival likelihood is 70-82% at 1 year and 50-60% at 5 years, despite renal replacement therapy support (12,13). Perindopril was immediately introduced to our patient after the initial hypertensive emergency; however, we stopped it and used nifedipine CR because of an abrupt drop in the systolic blood pressure along with a 35% reduction in the eGFR by perindopril. Carbedilol and aliskiren were introduced to control her blood pressure. Of note, however: we could have safely reintroduced a low dose of perindopril or used a low-dose angiotensin II receptor blocker (ARB) as an alternative.
It has been reported that aliskiren was employed in two cases of SRC (14,15). In one case, aliskiren was added to the ARB candesartan cilexetil, while in the other case, aliskiren was added to the selective endothelin-A receptor antagonist sitaxsentan. As a result, the renal function and blood pressure were effectively stabilized. Consequently, we chose to use aliskiren instead of ACEIs or ARB to treat our patient. However, it is important to note that in practical use, PRA measurements show persistent and artificial suppression for hours following a single intravenous or oral administration of the direct renin inhibitor (16). Although the acute heart failure improved in our patient, her kidney function subacutely deteriorated, and hemodialysis was required. The direct renin inhibitor aliskiren has blood pressure-lowering effects similar to those of ACEIs and angiotensin II receptor blockers (17). Aliskiren has been reported to reduce renin activity and modestly lower blood pressure in patients undergoing chronic hemodialysis (18). However, no strong evidence has confirmed the benefits of aliskiren in SRC (3). Furthermore, there was a case of SRC that did not respond to corticosteroids, plasmapheresis, or renin-angiotensin pathway inhibitors, including enalapril maleate and ariskiren (19).
Increased renin production is the main cause of severe hypertension in patients with SRC. Hyperplasia of the juxtaglomerular apparatus due to reduced intrarenal blood flow may continuously produce renin in patients after SRC, even in those on hemodialysis for a long period. It has been reported that the serum renin level will rise again if ACEIs are stopped in patients with SRC, which can cause complications, such as congestive heart failure (20). Our patient experienced a hypertensive emergency with acute heart failure and hyperreninemia after stopping aliskiren while on hemodialysis. This supports the notion that ACEIs should be continued if dialysis has been initiated with the goal of dialysis independence (12). Renal recovery can occur up to two years after the onset of SRC in patients undergoing dialysis and using ACEIs (13). Our patient had not discontinued hemodialysis for more than two years following the introduction of hemodialysis. Whether we should have attempted ACEI treatment additively or in substitution for aliskiren while expecting the discontinuation of hemodialysis is unclear.
In summary, aliskiren effectively reduced blood pressure and ameliorated heart failure in the presence of hyperreninemia. Nevertheless, whether or not aliskiren can facilitate the recovery of the renal function in patients with SRC remains unclear. Therefore, further research is needed to explore the potential of aliskiren to enhance the renal function, particularly in individuals in the early phases of SRC. This investigation provides valuable insights into the management and treatment options for this challenging condition.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Steen VD. Kidney involvement in systemic sclerosis. Presse Med 43: e305-314, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Batal I, Domsic RT, Medsger TA, Bastacky S. Scleroderma renal crisis: a pathology perspective. Int J Rheumatol 2010: 543704, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foocharoen C, Tonsawan P, Pongkulkiat P, Anutrakulchai S, Mahakkanukrauh A, Suwannaroj S. Management review of scleroderma renal crisis: an update with practical pointers. Mod Rheumatol 33: 12-20, 2022. [DOI] [PubMed] [Google Scholar]
- 4.de Zubiría-Maria A, Florez-Suarez JB, Mendez-Patarroyo P, Quintana-López G. Pharmacological treatment of scleroderma renalcrisis: a systematic literature review. Rev Colomb Reumatol 27: 111-125, 2020. [Google Scholar]
- 5.van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum 65: 2737-2747, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujibayashi T, Sugai S, Miyasaka N, Hayashi Y, Tsubota K. Revised Japanese criteria for Sjögren's syndrome (1999): availability and validity. Mod Rheumatol 14: 425-434, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Perzyńska-Mazan J, Maślińska M, Gasik R. Neurological manifestations of primary Sjögren's syndrome. Reumatologia 56: 99-105, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kowal-Bielecka O, Fransen J, Avouac J, et al. Update of EULAR recommendations for the treatment of systemic sclerosis. Ann Rheum Dis 76: 1327-1339, 2017. [DOI] [PubMed] [Google Scholar]
- 9.Ramos-Casals M, Nardi N, Brito-Zerón P, et al. Atypical autoantibodies in patients with primary Sjögren syndrome: clinical characteristics and follow-up of 82 cases. Semin Arthritis Rheum 35: 312-321, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Salliot C, Gottenberg JE, Bengoufa D, Desmoulins F, Miceli-Richard C, Mariette X. Anticentromere antibodies identify patients with Sjögren's syndrome and autoimmune overlap syndrome. J Rheumatol 34: 2253-2258, 2007. [PubMed] [Google Scholar]
- 11.Scorza R, Rivolta R, Mascagni B, Berruti V, Bazzi S, Castagnone D, Quarto di Palo F. Effect of iloprost infusion on the resistance index of renal vessels of patients with systemic sclerosis. J Rheumatol 24: 1944-1948, 1997. [PubMed] [Google Scholar]
- 12.Steen VD, Medsger TA Jr. Long-term outcomes of scleroderma renal crisis. Ann Intern Med 133: 600-603, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Penn H, Howie AJ, Kingdon EJ, et al. Scleroderma renal crisis: patient characteristics and long-term outcomes. QJM 100: 485-494, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Fukasawa H, Furuya R, Ishigaki S, Kinoshita N, Isobe S, Fujigaki Y. Hyponatremia in a patient with scleroderma renal crisis: a potential role of activated renin-angiotensin system. BMC Nephrol 13: 47, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dhaun N, MacIntyre IM, Bellamy CO, Kluth DC. Endothelin receptor antagonism and renin inhibition as treatment options for scleroderma kidney. Am J Kidney Dis 54: 726-731, 2009. [DOI] [PubMed] [Google Scholar]
- 16.Azizi M, Webb R, Nussberger J, Hollenberg NK. Renin inhibition with aliskiren: where are we now, and where are we going? J Hypertens 24: 243-256, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Gradman AH, Pinto R, Kad R. Current concepts: renin inhibition in the treatment of hypertension. Curr Opin Pharmacol 8: 120-126, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Ishimitsu T, Ohta S, Ohno E, et al. Long-term antihypertensive effects of aliskiren, a direct renin inhibitor, in chronic hemodialysis patients. Ther Apher Dial 17: 524-531, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Thomas CP, Nester CM, Phan AC, Sharma M, Steele AL, Lenert PS. Eculizumab for rescue of thrombotic microangiopathy in PM-Scl antibody-positive autoimmune overlap syndrome. Clin Kidney J 8: 698-670, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steen VD, Mayes MD, Merkel PA. Assessment of kidney involvement. Clin Exp Rheumatol 21: S29-31, 2003. [PubMed] [Google Scholar]