Abstract
Hippocampal inhibitory interneurons exert a powerful influence on learning and memory. Inhibitory interneurons are known to play a major role in many diseases that affect memory, and to strongly influence brain functions required for memory-related tasks. While previous studies involving genetic, optogenetic, and pharmacological manipulations have shown that hippocampal interneurons play essential roles in spatial and episodic learning and memory, exactly how interneurons affect local circuit computations during spatial navigation is not well understood. Given the significant anatomical, morphological, and functional heterogeneity in hippocampal interneurons, one may suspect cell-type specific roles in circuit computations. Here we review emerging evidence of CA1 hippocampal interneurons’ role in local circuit computations that support spatial learning and memory and discuss open questions about CA1 interneurons in spatial learning.
Learning is the process by which an animal acquires knowledge or skills through experience. This process includes forming neural representations of novel information which are initially stored for short-term access and then stabilized into long-term memory through the process of consolidation [1]. The hippocampus is essential for rapid learning and consolidation of spatial and episodic memory, or memories of events [2–4]. Hippocampal excitatory pyramidal cell activity is thought to represent the internal perception of the external environment such as one’s position in space-time dimensions and associations between external stimuli relevant for task performance [5–13]. Inhibitory interneurons strongly influence brain functions required for memory-related tasks and are known to play a major role in many diseases with common symptoms of memory impairment [14–20]. While genetic, optogenetic, and pharmacological interventions have shown that hippocampal interneurons play essential roles in spatial and episodic learning and memory [14,20–22], exactly how interneurons affect local circuit computations during behavior is an active area of investigation and debate. Recent studies have revealed significant functional heterogeneity in spatial and contextual selectivity, as well as heterogeneity in temporal dynamics of various interneurons, suggesting cell-type specific roles in circuit computations [23–25]. Indeed, interneuron subclasses in CA1 not only exhibit transcriptomic heterogeneity, but also target different cell-types and cellular compartments in CA1 (Fig. 1A) [26–28].
Figure 1. Inhibitory roles in place field formation and maintenance.

A. Simplified diagram of CA1 interneuron subclasses and their inhibitory synaptic targets on a pyramidal cell (Pyr.) with circles and triangle illustrating the location of the cell body of the interneuron and pyramidal cell, respectively. Calretinin (CR)- and vasoactive intestinal polypeptide (VIP)-expressing interneurons inhibit dendritic-targeting O-LM cells that primarily express somatostatin (SST), as well as soma-targeting parvalbumin-expressing basket cells (PVBC), thereby disinhibiting pyramidal cells. Basket cells co-expressing cholecystokinin (CCK) and VIP target the soma of pyramidal cells, whereas CCK and calbindin (CB)-expressing interneurons typically target the dendrites. Axo-axonic Chandelier (AAC) cells primarily target the axon initial segment. Note that the diagram focuses on interneurons described in this review and is not a comprehensive overview of hippocampal inhibitory circuitry. B. Prior work shows that spatially selective reduction in inhibition is not required for new field formation but may be important for stabilization and maintenance of new fields. Top, several types of inhibitory interneurons that synapsed onto a pyramidal cell were identified and targeted by their expression of vesicular GABA transporter (VGAT). The inhibitory neurons included multiple cell-types with multiple possible synaptic target locations. Bottom, no change in presynaptic inhibition was found prior to the lap where a new place field formed, but as the place field stabilized, the presynaptic interneuron developed “inverse” spatial selectivity such that it fired less than baseline levels at the postsynaptic pyramidal cell’s place field location [37]. C. Spatial selectivity in place cells is inducible with cell-type-specific reduction in inhibition. Optogenetic activation or silencing of axo-axonic cells targeting the axon initial segment of the pyramidal cell led to place field remapping in vivo during behavior [51]. Specifically, optogenetic silencing led to induction of new, persistent place fields at the photostimulated location. In contrast optogenetic activation led to no significance change in the number of place fields at the stimulation site although rare pre-existing place fields were suppressed.
Here we review the emerging role of hippocampal interneurons in circuit computations for spatial learning and memory and open questions about interneurons in learning. In this review, we focus on recent papers investigating the main hippocampal output region CA1 using recording and manipulation of select neuronal populations during hippocampus-dependent spatial navigation tasks. While one of the primary established roles of interneurons is the generation of neural oscillations, this has been reviewed elsewhere and therefore will not be the focus here [29–36]. Recent studies have shown that CA1 interneurons exhibit previously underappreciated feature selectivity that is relevant for spatial learning and memory [37,38]. Growing evidence suggests that this selectivity for features—whether it be a specific location, type of information, or context—may be attributed to specific subclasses of interneurons [23]. Thus, we review how interneuron roles in computations for learning and memory are actively being rewritten by recent findings investigating cell-type-specific inhibitory populations during behavior.
Formation and Maintenance of Place Codes
When an animal navigates an environment, hippocampal pyramidal cells develop location-specific receptive fields, or place fields, where they preferentially fire action potentials when the animal is in a specific part of the environment [13]. Place cells have been studied extensively as an elegant example of how neural firing patterns develop to form internal representations of the external world [39,40]. Some studies have shown that place fields tend to cluster around reward zones [6,10,41–43], consistent with the idea that they prioritize behaviorally relevant locations over less important ones. Recent work has shown that place cells are causally involved in goal-directed behaviors [44]. For example, optogenetically stimulating groups of place cells that typically fired when animals were at a rewarded location led mice to lick at an unrewarded location, behaving as if they were at the reward location [44]. Thus, it is important to understand how interneurons affect place field formation and maintenance because place fields are relevant for memory-guided navigation.
The role of inhibitory interneurons in place field formation and maintenance is currently debated in the field. On one hand, one study showed near uniform inhibitory firing across spatial positions in CA1 of head-fixed navigating mice [45]. Under uniform inhibitory firing, spatial modulation of excitatory cells is thought to arise primarily from spatially specific excitatory inputs or strengthening of excitatory synapses, requiring little spatially-specific inhibitory input [46,47]. On the other hand, spatial selectivity in CA1 interneurons has been previously reported in varying degrees [38,48–50], and some have speculated that location-specific release of inhibition at least partly contributes to the spatial tuning of place cells [48]. Other studies that have examined CA1 excitatory and inhibitory responses to spontaneously developed or optogenetically induced place fields in head-fixed behaving mice have reported seemingly conflicting findings [23,37,51–53]. Below we highlight recent empirical data investigating the role of interneurons in place field formation and maintenance.
A recent study reports a role for CA1 interneurons in place field maintenance [37]. This study by Geiller et al. (2021) elucidated interneuron effects on place fields in mice by using in vivo single-cell electroporation combined with monosynaptic retrograde tracing and optogenetics to identify synaptically connected cells in vivo [37]. The authors retrogradely labeled neurons presynaptic to an electroporated starter pyramidal cell and found that over 90% of the presynaptic inputs were from local inhibitory interneurons. Given this observation, the authors then expressed a genetically encoded Ca2+ indicator in all inhibitory interneurons using the VGAT-Cre mouse line, followed by electroporation of a starter pyramidal cell in CA1. This approach allowed for calcium imaging of functionally coupled pyramidal cells and interneurons in head-fixed mice running on a belt decorated with different tactile cues and licking for randomly delivered water rewards. Strikingly, while there was no detectable reduction in the activity of presynaptic interneurons during spontaneous place field formation, presynaptic interneurons showed “inverse” spatial selectivity once newly formed place fields stabilized, with strong reduction in firing at field locations of the starter pyramidal cell (Fig. 1B). These findings contrast with prior work that showed inhibitory inputs are spatially uniform and contrast to theories that reduced inhibition is required for place field formation [45]. The discrepancy in spatial selectivity of interneurons between different studies may be due to the cells included. Geiller et al. specifically examined interneurons that were presynaptic to cells with place fields in the current environment and these cells may have higher spatial selectivity than other interneurons, e.g. those that synapse onto cells without a current place field. Additionally, differences in the behavior paradigm (like randomly delivered reward versus reward delivered in specific parts of the track), may affect the animal’s use of position-specific cues leading to differences in spatial selectivity of interneurons.
In contrast, another study suggests CA1 interneurons do play a key role in place field formation, but these effects may be interneuron subtype specific. Dudok et al. (2021) show that disinhibition by axo-axonic Chandelier cells, which target the axon initial segment of pyramidal cells, can induce place field formation [51]. In this study, the authors created a new genetic mouse line to specifically target and optogenetically manipulate CA1 axo-axonic Chandelier cells [51]. This work found axo-axonic cells inhibit firing activity of pyramidal cells in awake behaving mice. Both optogenetic activation and silencing of axo-axonic cells led to remapping of place fields. Interestingly, optogenetic silencing induced new place fields near the photostimulation site that persisted during post-photostimulation laps while optogenetic activation did not change the total number of place fields (Fig. 1C). Thus, location-specific reduction in axo-axonic cells’ firing may be sufficient to generate place fields at that location. Together, the findings of Dubok et al. and Geiller et al. suggest that spatially organized activity of interneurons plays a key role in the development and maintenance of hippocampal representations of spatial experience with the exact role dependent on the interneuron subclass.
Indeed, spatial selectivity in CA1 interneurons differs between interneuron subclasses, or interneurons that have the same molecular markers, like parvalbumin, but differ in their morphological classification, like basket cells and bistratified cells [23]. Geiller et al. (2020) used three-dimensional 2-photon calcium imaging and molecular verification by immunohistochemistry to survey several subclasses of CA1 interneurons simultaneously recorded in behaving head-fixed mice. This study found that many interneuron subclasses showed spatial selectivity, although the degree or stability of modulation varied by subclass. For example, spatial selectivity was more stable across sessions for parvalbumin-positive basket cells than for somatostatin-positive interneurons, although they both had similar spatial modulation. Given the diversity in interneuron responses, inhibitory populations likely contribute significantly to spatial representations that are typically thought to be represented by excitatory populations with significant spatial modulation. Indeed, Geiller et al. (2020) found that the animal’s current position was decoded above chance levels from CA1 interneuron activity alone although decoding performance was better with CA1 place cells than with interneurons only.
Beyond the development of individual place fields, Rolotti et al. (2022) suggest that local feedback inhibition may control the size of the excitatory population to represent a specific location [52]. This is analogous to the previously reported role of dentate somatostatin interneurons in controlling the size of fear memory ensembles [54]. In this study, Rolotti et al. used a tamoxifen-dependent Cre virus and Cre-dependent excitatory opsin ChRmine to achieve sparse opsin expression across CA1 subpopulations in head-fixed mice running on a treadmill for randomly delivered water rewards. By varying the dose of tamoxifen injected in each mouse, the authors titrated the fraction of opsin-expressing CA1 pyramidal cells, while co-injection of GCaMP6f allowed for imaging calcium dynamics of the entire CA1 excitatory population. Rolotti et al. found that most single neurons could be optogenetically induced to develop place fields at the stimulation location that lasted at least 24 hours post-stimulation. Thus, new place fields may require strong enough excitatory drive above a certain threshold. Indeed, they showed that the percentage of pyramidal cells that were stimulated to fire together affected the efficacy of place field induction at the stimulation location [52]. Stimulation of fewer cells together due to lower opsin expression density led to a higher induction of place fields at the stimulation location compared to mice with higher opsin expression density. The authors then reasoned that a larger stimulated excitatory subpopulation might be more likely to recruit local interneurons, providing lateral inhibition onto nearby CA1 pyramidal cells. As a result, place fields could be more difficult to induce due to this lateral inhibition. Consistent with this hypothesis, the number of activated excitatory cells in a subpopulation was increased by chemogenetically suppressing local inhibition with inhibitory DREADDs specific to interneurons. Similarly, McKenzie et al. (2021), which used relatively low-power stimulation of CA1 pyramidal cells in head-fixed behaving mice, observed induction failures at the stimulation site perhaps due to larger recruitment of interneurons than place field induction via intracellular recordings [53]. These experiments suggest that interneurons play a key role in a competitive mechanism by which some pyramidal cells are selected to be part of a neuronal assembly while other pyramidal cells are suppressed and excluded [55,56]. Such groups of neurons firing together during an experience would then strengthen their connections which is thought to be the basis of memory formation [57,58].
Interneurons have been hypothesized to play a key role in developing and refining spatial codes thus improving the signal-to-noise of such coding [59]. These recent studies support this hypothesis and refine it by showing that specific functions vary by interneuron subclass. Together, these studies point to a model in which interneurons refine spatial codes in multiple ways. First, the activity of some spatially modulated interneurons can decode animal location [23]. Second, presynaptic interneuron activity exhibits an inverse relationship with new place field activity to maintain sharp and stable place fields [37]. Third, while interneurons disinhibit high firing cells at their place field locations, interneurons also suppress low firing pyramidal cells such that cells with inadequate excitatory drive do not participate in an assembly [52]. Thus, these studies suggest that interneurons increase spatial coding signal-to-noise to stabilize new memories and suppress low or unstable excitatory activity with their exact roles differing by cell-type. The notion of cell-type-specific spatial selectivity in interneurons is further supported by cell-type-specific plasticity mechanisms discovered in CA1, including at glutamatergic synapses onto interneurons and at inhibitory synapses onto excitatory pyramidal cells [60–63]. Spatially-selective interneurons may also play a role in behavior timescale synaptic plasticity (BTSP) in which excitatory cells exhibit extended periods of subthreshold depolarizations, or dendritic plateau potentials, over behavioral (seconds) timescales, although this has traditionally been attributed to excitatory inputs [47,64]. Furthermore, spatial selectivity that seems to be driven by excitatory inputs may have an inhibitory component. Previous in vitro work has shown that the efficacy of inhibition depends on the distance to adjacent excitatory inputs on the same hippocampal dendritic branch, while excitatory and inhibitory drives are also balanced across dendritic branches [65]. This within-dendrite inhibitory influence over excitatory inputs may contribute to spatial selectivity of excitatory inputs. Future work is necessary to determine which plasticity mechanisms contribute to different phases of spatial learning.
Learning Goal-Directed Navigation
The hippocampus is essential for rapid learning, whether after a single experience or a few minutes [2–4]. While recent papers described above examined place field formation and maintenance as a model of memory formation, place field formation occurs even when animals explore open fields and does not necessarily require animals to express their learning via behavioral changes [39]. To study learning-related behaviors explicitly, a few recent studies have shed light on the role of interneurons in circuit function as animals learn to find new goal locations [66,67]. These studies have examined neural inputs and connections as well as place fields that tend to cluster around reward location (Fig. 2A).
Figure 2. Inhibitory roles in learning goal-directed navigation.
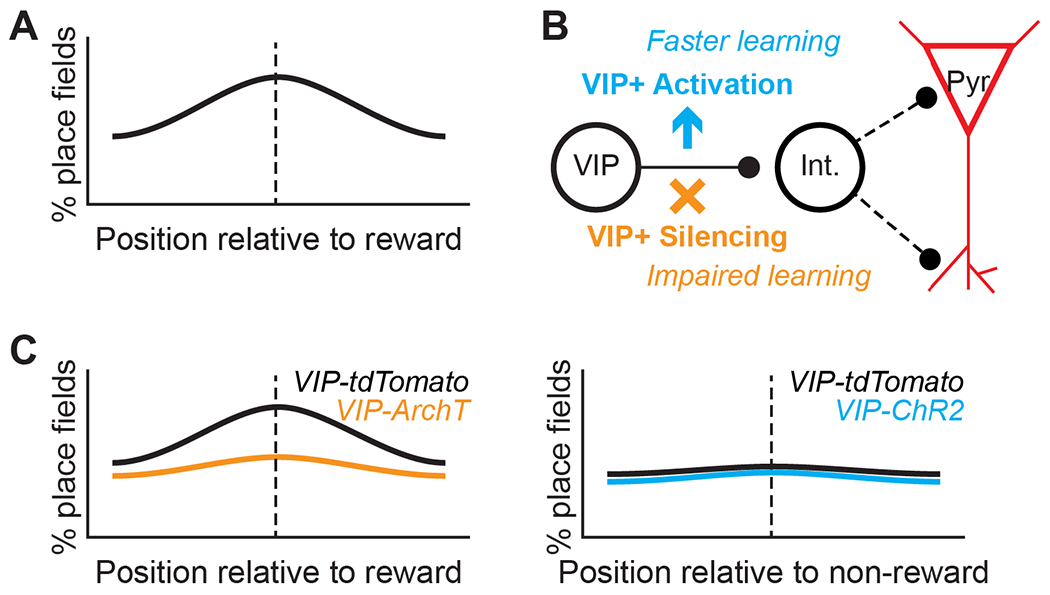
A. Place cells overrepresent behaviorally relevant locations such as reward zones and, while place fields tile the entire environment, more place fields cluster around these important locations. B. Vasoactive intestinal polypeptide (VIP)-expressing interneurons target other interneurons (Int.) that provides either perisomatic or dendritic inhibition onto pyramidal cells (Pyr.). In a recent study investigating a local CA1 disinhibitory circuit in goal-directed learning, optogenetically activating VIP interneurons (light blue) was found to induce faster learning of a new reward zone, as demonstrated by increased licking near the reward location [66]. Optogenetic silencing (yellow) led to impaired learning in the same goal-directed navigation task. These findings suggest that new goal learning (and its behavioral expression) is mediated by local inhibition and disinhibition of pyramidal cells. C. Left, consistent with these behavioral changes in B, optogenetic silencing of VIP neurons led to a reduced proportion of place cells with fields near the goal (yellow, inhibitory opsin ArchT in VIP interneurons). Right, in contrast to the observed behavioral effects, VIP optogenetic activation (light blue, excitatory opsin ChR2 in VIP interneurons) did not lead to observable changes in the proportion of goal-representing place cells. These results suggest that transient release of pyramidal cells from local inhibition is necessary, but not sufficient, to induce learning-dependent neuronal reorganization.
Interneuron-targeting interneurons, which ultimately disinhibit pyramidal cells, were recently discovered to play a key role in goal-directed spatial learning in the work by Turi et al. (2019) [66]. This study focused on CA1 vasoactive intestinal polypeptide (VIP) positive interneurons, a subset of interneurons that primarily target parvalbumin- or somatostatin-expressing interneurons [68,69], and ultimately release pyramidal cells from perisomatic or dendritic inhibition. After verifying that these VIP interneurons were indeed disinhibitory on CA1 pyramidal cells in vitro and in vivo, the authors virally expressed GCaMP6f in the dorsal CA1 region of VIP-Cre mice to observe chronic Ca2+ activity in head-fixed mice running on a treadmill. The authors first found that VIP interneurons could be separated into two groups based on whether they were positively or negatively modulated by speed. Upon training in a behavioral task that required the mice to learn to lick at a specific location, the firing of both VIP interneuron groups was modulated by proximity to reward, but in opposite directions. Interestingly, this reward modulation was not observed if the mice did not have to learn the task as in random foraging conditions. The authors then tested the hypothesis that disinhibition of CA1 pyramidal cells is necessary for overrepresentation of goal location by place cells during learning and subsequent improved performance. To do this, Turi et al. virally expressed either the opsin channelrhodopsin or the opsin archaerhodopsinT in CA1 of VIP-Cre mice to optogenetically activate or silence, respectively, CA1 VIP interneurons in mice learning a new reward location. In support of their hypothesis, the authors found that optogenetic silencing of VIP interneurons reduced the number of place cells near goal locations as well as the rate of learning measured by licking earlier as animals approached the reward location (Fig. 2 B,C). Mice learned significantly faster than controls when CA1 VIP interneurons were optogenetically stimulated near the reward location, even if it did not affect CA1 pyramidal cell reorganization. Based on these results, the authors concluded that disinhibition mediated by VIP interneurons is necessary, but not sufficient, for learning-dependent reorganization of CA1 pyramidal cells.
Prior work has shown that the strength of connections between pyramidal cells and interneurons rapidly reorganize during spatial learning [67]. Dupret et al. (2013) found that rats rapidly learning new goal zones in an otherwise familiar environment had learning-dependent changes in the strengths of putative monosynaptic connections between pre-synaptic excitatory place cells and post-synaptic inhibitory cells in CA1 [67]. This study showed that putative pyramidal-to-interneuron connections alter input weight distributions following learning, with the direction of change dependent on whether the assembly represented new learning. Pyramidal assemblies that represented newly learned goal locations strengthened their connections to postsynaptic interneurons that preferentially fired when the new map was expressed. Conversely, these same pyramidal assemblies weakened their coupling to interneurons whose firing was more correlated with the old map. Furthermore, the changes in connection strength were more likely to occur around rewarded locations compared to unrewarded locations, even though pairing events were observed in all locations [67]. These findings indicate that local pyramidal-to-inhibitory circuits reconfigure dynamically based on both learned information and their affiliation to the map of relevance, and thus may contribute to outputting learned behavior. While this study was unable to differentiate between different subclasses of interneurons, it would be important for future work to determine whether and how cell-type-specific changes in connection strengths drive new learning [23].
Together these studies show that CA1 interneurons develop learning-dependent changes important for goal-directed behavior. First, interneurons alter firing responses to specific spatial cues that predict reward, with the magnitude and direction of response modulation varied by subclass. Second, interneurons rapidly reorganize over learning by redistributing their synaptic weights onto nearby neurons. Ultimately, interneuron changes in firing activity and synaptic strength lead to stable representation of newly learned locations directly relevant for task performance.
Functional Outcomes of Inhibitory Deficits in Disease
Interneurons are sensitive to disease states and deficits in inhibitory activity are likely to lead to network dysfunction due to their powerful influences on neural circuits [70–72]. Multiple studies have shown abnormal inhibitory activity of multiple subclasses in several animal models of Alzheimer’s disease (AD), revealing common pathophysiological mechanisms [19,73–78]. Furthermore, abnormal GABAergic inhibition is observed in depression, autism spectrum disorder, and Down syndrome, suggesting the sensitivity of inhibitory neurons to disease and potential common inhibitory mechanisms across multiple pathological conditions [79–81]. Recent studies using in vivo electrophysiology in animal models of disease have demonstrated the need for subclass-specific investigation of interneurons that may affect different aspects of spatial learning and memory. Paterno et al. (2021) using the Cntnap2 KO mouse model of autism showed reduced inhibitory transmission in vitro by parvalbumin-expressing interneurons onto CA1 pyramidal cells in KO mice, which also had impaired performance in spatial object recognition and object congruence tasks [82]. This model also had layer- and frequency-specific deficits in gamma phase-amplitude coupling, which could be directly related to deficits in subclass-specific transmission at inhibitory synapses. In another study, Chung et al. (2020) found that in mice injected with toxic amyloid beta oligomers, optogenetically activating CA1 parvalbumin- or somatostatin-expressing interneurons restored peak gamma or theta power, respectively, to normal levels in vivo, albeit during anesthesia [83]. The optogenetic manipulation also led to resynchronization of pyramidal cell spiking relative to gamma or theta oscillation, respectively. While this study did not examine mice during task performance, it would be of interest to consider interneuron subclass-specific rescue of frequency-specific oscillations during spatial memory-guided behavior. Similar to these observations, our study using the 5XFAD genetic mouse model of AD and a virtual reality spatial navigation task found fewer and weaker sharp-wave ripples in AD mice, which correlated with a significant reduction in the strength of putative interneuron-onto-pyramidal neuron monosynaptic connections in CA1 [84]. These studies suggest that interneurons are involved in memory impairment in multiple models of disease either by disrupting oscillations or driving network imbalances and hyperactivity. Considering recent studies revealing the diversity and specialization of inhibitory populations and their roles for spatial coding and memory formation described above, interneuron dysfunction likely plays more direct roles in memory impairment, however empirical data for the specific roles of interneurons in circuit dysfunction in disease models is lacking.
Current Gaps in Knowledge
Together these recent studies reveal previously unappreciated spatial selectivity in some interneuron types which in turn increases the signal-to-noise ratio of spatial codes and enables learning [23,37,51,52]. We must also keep important experimental limitations in mind when considering these findings. First, several studies have made clever use of induced place fields [37,52,53]. However, these place fields are generated artificially so may differ in their inputs or structure than naturally occurring place fields. Second, many of these studies were performed in head-fixed mice. This approach enables complex imaging and electrophysiology, but limits vestibular inputs to animals among other limitations [85]. Third, optogenetic stimulation can have many unintended side effects [86]. Fourth, imaging of hippocampus often includes extensive damage to overlying cortex which may affect hippocampal functions or animal behavior since interactions between hippocampus and cortex are involved in cognition [87–89]. Even with these limitations, these studies have provided key insights into interneuron roles in spatial coding.
These findings raise several key questions. First, it remains to be examined whether inhibitory interneurons are crucial in selecting specific assemblies over others to represent or respond to particular experiences. Second, the specific plasticity mechanisms at play in the development of interneuron spatial selectivity and in changing connection strength between inhibitory and excitatory cells are not well understood. Third, while inhibitory deficits have been well-documented in diseases with learning and memory impairments, the diverse circuit mechanisms by which interneurons influence learning and memory have not been elucidated. Finally, multiple brain regions may have unifying inhibitory mechanisms for learning and disease susceptibility, which opens avenues for future research. Studies that bridge multiple scales, from single neurons to networks to behavior, will shine light on the inhibitory mechanisms of learning and memory in health and disease.
Acknowledgements:
A.C. Singer acknowledges 1RF1AG078736, the Packard Foundation, NIH-NINDS R01 NS109226, McCamish Foundation, Friends and Alumni of Georgia Tech, and the Lane Family. N. Jeong acknowledges the Michael Kuhar Neuroscience Research Fellowship. We would like to thank members of the Singer lab and Lary Walker for feedback on the manuscript.
Footnotes
Declaration of interest: none
References
Papers of special interest (•) or outstanding interest (••)
- 1.Carr MF, Jadhav SP, Frank LM: Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Publ Gr 2011, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim SM, Frank LM: Hippocampal Lesions Impair Rapid Learning of a Continuous Spatial Alternation Task. PLoS One 2009, 4:e5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moser E, Moser MB, Andersen P: Spatial learning impairment parallels the magnitude of dorsal hippocampal lesions, but is hardly present following ventral lesions. J Neurosci 1993, 13:3916–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S: Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron 2003, 38:305–315. [DOI] [PubMed] [Google Scholar]
- 5.Wang CH, Monaco JD, Knierim JJ: Hippocampal Place Cells Encode Local Surface-Texture Boundaries. Curr Biol 2020, 30:1397–1409.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gauthier JL, Tank DW: A Dedicated Population for Reward Coding in the Hippocampus. Neuron 2018, 99:179–193.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacDonald CJ, Lepage KQ, Eden UT, Eichenbaum H: Hippocampal “time cells” bridge the gap in memory for discontiguous events. Neuron 2011, 71:737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Góis ZHTD, Tort ABL: Characterizing Speed Cells in the Rat Hippocampus. Cell Rep 2018, 25:1872–1884.e4. [DOI] [PubMed] [Google Scholar]
- 9.Lee H, Ghim JW, Kim H, Lee D, Jung MW: Hippocampal neural correlates for values of experienced events. J Neurosci 2012, 32:15053–15065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer AC, Frank LM: Rewarded Outcomes Enhance Reactivation of Experience in the Hippocampus. Neuron 2009, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knierim JJ: Dynamic interactions between local surface cues, distal landmarks, and intrinsic circuitry in hippocampal place cells. J Neurosci 2002, 22:6254–6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka KZ, He H, Tomar A, Niisato K, Huang AJY, McHugh TJ: The hippocampal engram maps experience but not place. Science (80-) 2018, 361:392–397. [DOI] [PubMed] [Google Scholar]
- 13.O’Keefe J, Dostrovsky J: The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res 1971, 34:171–175. [DOI] [PubMed] [Google Scholar]
- 14.Artinian J, Jordan A, Khlaifia A, Honore E, Fontaine A La, Racine AS, Laplante I, Lacaille JC: Regulation of Hippocampal Memory by mTORC1 in Somatostatin Interneurons. J Neurosci 2019, 39:8439–8456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lovett-Barron M, Kaifosh P, Kheirbek MA, Danielson N, Zaremba JD, Reardon TR, Turi GF, Hen R, Zemelman BV., Losonczy A: Dendritic inhibition in the hippocampus supports fear learning. Science (80-) 2014, 343:857–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bissonette GB, Schoenbaum G, Roesch MR, Powell EM: Interneurons are necessary for coordinated activity during reversal learning in orbitofrontal cortex. Biol Psychiatry 2015, 77:454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delorme J, Wang L, Kuhn FR, Kodoth V, Ma J, Martinez JD, Raven F, Toth BA, Balendran V, Medina AV, et al. Sleep loss drives acetylcholine- And somatostatin interneuron-mediated gating of hippocampal activity to inhibit memory consolidation. Proc Natl Acad Sci U S A 2021, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen K, Fuchs EC, Jaschonek H, Bannerman DM, Monyer H: Gap Junctions between Interneurons Are Required for Normal Spatial Coding in the Hippocampus and Short-Term Spatial Memory. J Neurosci 2011, 31:6542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Losa M, Tracy TE, Ma K, Verret L, Clemente-Perez A, Khan AS, Cobos I, Ho K, Gan L, Mucke L, et al. Nav1.1-Overexpressing Interneuron Transplants Restore Brain Rhythms and Cognition in a Mouse Model of Alzheimer’s Disease. Neuron 2018, doi: 10.1016/j.neuron.2018.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews-Zwilling Y, Gillespie AK, Kravitz AV., Nelson AB, Devidze N, Lo I, Yoon SY, Bien-Ly N, Ring K, Zwilling D, et al. Hilar GABAergic Interneuron Activity Controls Spatial Learning and Memory Retrieval. PLoS One 2012, 7:e40555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira da Cruz JF, Busquets-Garcia A, Zhao Z, Varilh M, Lavanco G, Bellocchio L, Robin L, Cannich A, Julio-Kalajzić F, Lesté-Lasserre T, et al. Specific Hippocampal Interneurons Shape Consolidation of Recognition Memory. Cell Rep 2020, 32:108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Salas-Quiroga A, García-Rincón D, Gómez-Domínguez D, Valero M, Simón-Sánchez S, Paraiso-Luna J, Aguareles J, Pujadas M, Muguruza C, Callado LF, et al. Long-term hippocampal interneuronopathy drives sex-dimorphic spatial memory impairment induced by prenatal THC exposure. Neuropsychopharmacology 2020, 45:877–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.•.Geiller T, Vancura B, Terada S, Troullinou E, Chavlis S, Tsagkatakis G, Tsakalides P, Ócsai K, Poirazi P, Rózsa BJ, et al. Large-Scale 3D Two-Photon Imaging of Molecularly Identified CA1 Interneuron Dynamics in Behaving Mice. Neuron 2020, 0. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper is one of the first comprehensive characterizations of activity profiles of major subclasses of CA1 interneurons in navigating mice. The subclasses included parvalbumin-expressing basket cells, somatostatin-positive cells, bistratified cells, cholecystokinin-positive cells, axo-axonic cells, and Ivy/Neugliaform cells.
- 24.Pelkey KA, Chittajallu R, Craig MT, Tricoire L, Wester JC, McBain CJ: Hippocampal gabaergic inhibitory interneurons. Physiol Rev 2017, 97:1619–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klausberger T, Somogyi P: Neuronal diversity and temporal dynamics: The unity of hippocampal circuit operations. Science (80-) 2008, 321:53–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francavilla R, Villette V, Martel O, Topolnik L: Calcium dynamics in dendrites of hippocampal CA1 interneurons in awake mice. Front Cell Neurosci 2019, 13:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sik A, Penttonen M, Ylinen A, Buzsáki G: Hippocampal CA1 interneurons: an in vivo intracellular labeling study. J Neurosci 1995, 15:6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris KD, Hochgerner H, Skene NG, Magno L, Katona L, Bengtsson Gonzales C, Somogyi P, Kessaris N, Linnarsson S, Hjerling-Leffler J: Classes and continua of hippocampal CA1 inhibitory neurons revealed by single-cell transcriptomics. PLOS Biol 2018, 16:e2006387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen K, Monyer H: Interneuron control of hippocampal oscillations. Curr Opin Neurobiol 2015, 31:81–87. [DOI] [PubMed] [Google Scholar]
- 30.Mann EO, Radcliffe CA, Paulsen O: Hippocampal gamma-frequency oscillations: from interneurones to pyramidal cells, and back. J Physiol 2005, 562:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strüber M, Sauer J-F, Bartos M: Parvalbumin expressing interneurons control spike-phase coupling of hippocampal cells to theta oscillations. Sci Rep 2022, 12:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Butler JL, Paulsen O: Hippocampal network oscillations — recent insights from in vitro experiments. Curr Opin Neurobiol 2015, 31:40–44. [DOI] [PubMed] [Google Scholar]
- 33.Antonoudiou P, Tan YL, Kontou G, Louise Upton A, Mann EO: Parvalbumin and Somatostatin Interneurons Contribute to the Generation of Hippocampal Gamma Oscillations. J Neurosci 2020, 40:7668–7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buzsáki G: Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 2015, 25:1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colgin LL: Rhythms of the hippocampal network. Nat Rev Neurosci 2016, 17:239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dvorak D, Radwan B, Sparks FT, Talbot ZN, Fenton AA: Control of recollection by slow gamma dominating mid-frequency gamma in hippocampus CA1. 2018, doi: 10.1371/journal.pbio.2003354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.••.Geiller T, Sadeh S, Rolotti SV., Blockus H, Vancura B, Negrean A, Murray AJ, Rózsa B, Polleux F, Clopath C, et al. Local circuit amplification of spatial selectivity in the hippocampus. Nature 2021, doi: 10.1038/s41586-021-04169-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work shows evidence for a role of spatially nonuniform disinhibition in maintaining stable place fields but argues against a role for such disinhibition in place field formation.
- 38.Ego-Stengel V, Wilson MA: Spatial selectivity and theta phase precession in CA1 interneurons. Hippocampus 2007, 17:161–174. [DOI] [PubMed] [Google Scholar]
- 39.Wilson MA, McNaughton BL, Filipkowski RK, Silva AJ, Eichenbaum H: Dynamics of the hippocampal ensemble code for space. Science 1993, 261:1055–1058. [DOI] [PubMed] [Google Scholar]
- 40.Best PJ, White AM: Hippocampal cellular activity: A brief history of space. Proc Natl Acad Sci U S A 1998, 95:2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JS, Briguglio JJ, Cohen JD, Romani S, Lee Correspondence AK, Lee AK: The Statistical Structure of the Hippocampal Code for Space as a Function of Time, Context, and Value. Cell 2020, 183:1–16. [DOI] [PubMed] [Google Scholar]
- 42.Dupret D, O’Neill J, Pleydell-Bouverie B, Csicsvari J: The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat Neurosci 2010, 13:995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hollup SA, Molden S, Donnett JG, Moser MB, Moser EI: Accumulation of Hippocampal Place Fields at the Goal Location in an Annular Watermaze Task. J Neurosci 2001, 21:1635–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Robinson NTM, Descamps LAL, Russell LE, Nutbrown R, Schmidt-Hieber C, Hä Usser Correspondence M, Buchholz MO, Bicknell BA, Antonov GK, Lau JYN, et al. Targeted Activation of Hippocampal Place Cells Drives Memory-Guided Spatial Behavior. Cell 2020, 183:1586–1599.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grienberger C, Milstein AD, Bittner KC, Romani S, Magee JC: Inhibitory suppression of heterogeneously tuned excitation enhances spatial coding in CA1 place cells. Nat Neurosci 2017, 20:417. [DOI] [PubMed] [Google Scholar]
- 46.Bittner KC, Grienberger C, Vaidya SP, Milstein AD, Macklin JJ, Suh J, Tonegawa S, Magee JC: Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nat Neurosci 2015 188 2015, 18:1133–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bittner KC, Milstein AD, Grienberger C, Romani S, Magee JC: Behavioral time scale synaptic plasticity underlies CA1 place fields. Science (80-) 2017, 357:1033–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hangya B, Li Y, Muller RU, Czurkó A: Complementary spatial firing in place cell-interneuron pairs. J Physiol 2010, doi: 10.1113/jphysiol.2010.194274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilent WB, Nitz DA: Discrete Place Fields of Hippocampal Formation Interneurons. J Neurophysiol 2007, 97:4152–4161. [DOI] [PubMed] [Google Scholar]
- 50.Nitz D, McNaughton B: Differential Modulation of CA1 and Dentate Gyrus Interneurons During Exploration of Novel Environments. J Neurophysiol 2004, 91:863–872. [DOI] [PubMed] [Google Scholar]
- 51.••.Dudok B, Szoboszlay M, Paul A, Klein PM, Liao Z, Hwaun E, Szabo GG, Geiller T, Vancura B, Wang BS, et al. Recruitment and inhibitory action of hippocampal axo-axonic cells during behavior. Neuron 2021, 109:3838–3850.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that hippocampal axo-axonic cells inhibit CA1 principal cells and silencing their activity induces novel place fields in behaving mice.
- 52.•.Rolotti SV, Ahmed MS, Szoboszlay M, Geiller T, Negrean A, Blockus H, Gonzalez KC, Sparks FT, Solis Canales AS, Tuttman AL, et al. Local feedback inhibition tightly controls rapid formation of hippocampal place fields. Neuron 2022, 110:783–794.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work shows that lcoal inhibition controls the density limit of co-active pyramidal cells, and the size of the active excitatory subpopulation inversely correlates with spatially selective induction efficacy for optogenetically induced place fields.
- 53.McKenzie S, Huszár R, English DF, Kim K, Christensen F, Yoon E, Buzsáki G: Preexisting hippocampal network dynamics constrain optogenetically induced place fields. Neuron 2021, 109:1040–1054.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stefanelli T, Bertollini C, Lüscher C, Muller D, Mendez P: Hippocampal Somatostatin Interneurons Control the Size of Neuronal Memory Ensembles. Neuron 2016, 89:1074–1085. [DOI] [PubMed] [Google Scholar]
- 55.Rao-Ruiz P, Yu J, Kushner SA, Josselyn SA: Neuronal competition: microcircuit mechanisms define the sparsity of the engram. Curr Opin Neurobiol 2019, 54:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roux L, Buzsaki G: Tasks for inhibitory interneurons in intact brain circuits. Neuropharmacology 2015, 88:10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Buzsáki G: Neural Syntax: Cell Assemblies, Synapsembles, and Readers. Neuron 2010, 68:362–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wallace DJ, Kerr JND: Chasing the cell assembly. Curr Opin Neurobiol 2010, 20:296–305. [PubMed] [Google Scholar]
- 59.McNaughton BL, Morris RGM: Hippocampal synaptic enhancement and information storage within a distributed memory system. Trends Neurosci 1987, 10:408–415. [Google Scholar]
- 60.Nissen W, Szabo A, Somogyi J, Somogyi P, Lamsa KP: Cell Type-Specific Long-Term Plasticity at Glutamatergic Synapses onto Hippocampal Interneurons Expressing either Parvalbumin or CB1 Cannabinoid Receptor. J Neurosci 2010, 30:1337–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szabo A, Somogyi J, Cauli B, Lambolez B, Somogyi P, Lamsa KP: Calcium-Permeable AMPA Receptors Provide a Common Mechanism for LTP in Glutamatergic Synapses of Distinct Hippocampal Interneuron Types. J Neurosci 2012, 32:6511–6516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Udakis M, Pedrosa V, Chamberlain SEL, Clopath C, Mellor JR: Interneuron-specific plasticity at parvalbumin and somatostatin inhibitory synapses onto CA1 pyramidal neurons shapes hippocampal output. 2020, 11:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bannon NM, Chistiakova M, Volgushev M: Synaptic Plasticity in Cortical Inhibitory Neurons: What Mechanisms May Help to Balance Synaptic Weight Changes? Front Cell Neurosci 2020, 14:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Milstein AD, Li Y, Bittner KC, Grienberger C, Soltesz I, Magee JC, Romani S: Bidirectional synaptic plasticity rapidly modifies hippocampal representations. Elife 2021, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu G: Local structural balance and functional interaction of excitatory and inhibitory synapses in hippocampal dendrites. Nat Neurosci 2004 74 2004, 7:373–379. [DOI] [PubMed] [Google Scholar]
- 66.Turi GF, Li W-K, Chavlis S, Pandi I, O’Hare J, Priestley JB, Grosmark AD, Liao Z, Ladow M, Zhang JF, et al. Vasoactive Intestinal Polypeptide-Expressing Interneurons in the Hippocampus Support Goal-Oriented Spatial Learning. Neuron 2019, 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dupret D, O’Neill J, Csicsvari J, O’Neill J, Csicsvari J: Dynamic reconfiguration of hippocampal interneuron circuits during spatial learning. Neuron 2013, 78:166–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tyan L, Chamberland S, Magnin E, Camiré O, Francavilla R, Suzanne David L, Deisseroth K, Topolnik L: Dendritic Inhibition Provided by Interneuron-Specific Cells Controls the Firing Rate and Timing of the Hippocampal Feedback Inhibitory Circuitry. J Neurosci 2014, 34:4534–4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Francavilla R, Villette V, Luo X, Chamberland S, Muñoz-Pino E, Camiré O, Wagner K, Kis V, Somogyi P, Topolnik L: Connectivity and network state-dependent recruitment of long-range VIP-GABAergic neurons in the mouse hippocampus. Nat Commun 2018 91 2018, 9:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marín O: Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci 2012 132 2012, 13:107–120. [DOI] [PubMed] [Google Scholar]
- 71.Ruden JB, Dugan LL, Konradi C: Parvalbumin interneuron vulnerability and brain disorders. Neuropsychopharmacol 2020 462 2020, 46:279–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Paterno R, Casalia M, Baraban SC: Interneuron deficits in neurodevelopmental disorders: Implications for disease pathology and interneuron-based therapies. Eur J Paediatr Neurol 2020, 24:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reid HMO, Chen-Mack N, Snowden T, Christie BR: Understanding Changes in Hippocampal Interneurons Subtypes in the Pathogenesis of Alzheimer’s Disease: A Systematic Review. Brain Connect 2021, 11:159–179. [DOI] [PubMed] [Google Scholar]
- 74.Palop JJ, Chin J, Roberson ED, Wang J, Thwin MT, Bien-Ly N, Yoo J, Ho KO, Yu G-QQ, Kreitzer A, et al. Aberrant Excitatory Neuronal Activity and Compensatory Remodeling of Inhibitory Hippocampal Circuits in Mouse Models of Alzheimer’s Disease. Neuron 2007, doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verret L, Mann EO, Hang GB, Barth AMI, Cobos I, Ho K, Devidze N, Masliah E, Kreitzer AC, Mody I, et al. Inhibitory Interneuron Deficit Links Altered Network Activity and Cognitive Dysfunction in Alzheimer Model. Cell 2012, 149:708–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leung L, Andrews-Zwilling Y, Yoon SY, Jain S, Ring K, Dai J, Wang MM, Tong L, Walker D, Huang Y: Apolipoprotein E4 Causes Age- and Sex-Dependent Impairments of Hilar GABAergic Interneurons and Learning and Memory Deficits in Mice. PLoS One 2012, doi: 10.1371/journal.pone.0053569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li G, Bien-Ly N, Andrews-Zwilling Y, Xu Q, Bernardo A, Ring K, Halabisky B, Deng C, Mahley RW, Huang Y: GABAergic Interneuron Dysfunction Impairs Hippocampal Neurogenesis in Adult Apolipoprotein E4 Knockin Mice. Cell Stem Cell 2009, doi: 10.1016/j.stem.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Busche MA, Eichhoff G, Adelsberger H, Abramowski D, Wiederhold KH, Haass C, Staufenbiel M, Konnerth A, Garaschuk O: Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science (80-) 2008, doi: 10.1126/science.1162844. [DOI] [PubMed] [Google Scholar]
- 79.Filice F, Janickova L, Henzi T, Bilella A, Schwaller B: The Parvalbumin Hypothesis of Autism Spectrum Disorder. Front Cell Neurosci 2020, 14:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Contestabile A, Magara S, Cancedda L: The GABAergic hypothesis for cognitive disabilities in down syndrome. Front Cell Neurosci 2017, 11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Umschweif G, Medrihan L, McCabe KA, Sagi Y, Greengard P: Activation of the p11/SMARCA3/Neurensin-2 pathway in parvalbumin interneurons mediates the response to chronic antidepressants. Mol Psychiatry 2021 267 2021, 26:3350–3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Paterno R, Marafiga JR, Ramsay H, Li T, Salvati KA, Baraban SC: Hippocampal gamma and sharp-wave ripple oscillations are altered in a Cntnap2 mouse model of autism spectrum disorder. Cell Rep 2021, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chung H, Park K, Jang HJ, Kohl MM, Kwag J: Dissociation of somatostatin and parvalbumin interneurons circuit dysfunctions underlying hippocampal theta and gamma oscillations impaired by amyloid β oligomers in vivo. Brain Struct Funct 2020, 225:935–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.•.Prince SM, Paulson AL, Jeong N, Zhang L, Amigues S, Singer AC: Alzheimer’s pathology causes impaired inhibitory connections and reactivation of spatial codes during spatial navigation. Cell Rep 2021, 35:109008. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper links synaptic weakening between interneurons and principal cells to reduced number and power of sharp-wave ripple activity in vivo in navigating mice with amyloid patholoy.
- 85.Minderer M, Harvey CD, Donato F, Moser EI: Virtual reality explored. Nat 2016 5337603 2016, 533:324–325. [DOI] [PubMed] [Google Scholar]
- 86.Allen BD, Singer AC, Boyden ES: Principles of designing interpretable optogenetic behavior experiments. Learn Mem 2015, 22:232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Sigurdsson T, Duvarci S: Hippocampal-Prefrontal Interactions in Cognition, Behavior and Psychiatric Disease. Front Syst Neurosci 2015, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang F, Schoenbaum G, Kahnt T: Interactions between human orbitofrontal cortex and hippocampus support model-based inference. PLOS Biol 2020, 18:e3000578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Anderson MC, Floresco SB: Prefrontal-hippocampal interactions supporting the extinction of emotional memories: the retrieval stopping model. Neuropsychopharmacol 2021 471 2021, 47:180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]


