Summary
Once considered a metabolic waste product, today it is considered an important signaling molecule continuously forming under aerobic conditions. Lactate, a molecule primarily known as a byproduct of glycolysis, has gained importance in recent years due to its multifaceted role in various biological processes. Misconceptions surrounding lactate have persisted for centuries, especially the belief that elevated lactate levels were solely a result of low oxygen levels shaped early understanding. However, current research challenges this view and expands our comprehension of lactate’s various roles. Unfortunately, despite all of the mentioned above lactate is rooted in modern society as a deterrent word and many people do not know its value in the human body, let alone clinical implementations or physical performance. The main goal of this review is to refresh current knowledge regarding lactate research and spread the overall information among a professional society.
Keywords: Lactate, Lactate metabolism, Lactic acid, Disease metabolism, Lactate shuttle
What is lactate?
Since its discovery in 1780 by Carl Wilhelm Scheele, lactate has often been mistaken for a mere hypoxic waste product as a consequence of oxygen deprivation during muscle contractions with many harmful effects. The “low O2 induces high lactate” paradigm has led to the simplistic conclusion that “high lactate means low O2”, a position that was developed based on, that time breakthrough research on amphibians, by Nobel Prize laureates [1] and on humans [2]. It was not until the 1980s, when the shuttle transfer of lactate between cells was introduced, that a paradigm shift in the understanding of the role of lactate in metabolism occurred [3]. The physiology and biochemistry of lactate’s role have been comprehensively reviewed, since it has been decades, new insights have emerged, especially regarding the role of lactate and the lactate shuttle in fundamental physiology, metabolism, and clinical applications. Since then, evidence for lactate as a major player in the coordination of whole-cell metabolism has spread rapidly. It now serves as a link between glycolytic and aerobic pathways, and according to the lactate shuttle theory, this connection extends across cells, tissues, and organs [4–9].
Lactate is a metabolite generated through glycolysis, a fundamental metabolic process occurring within the cytoplasm of cells. This process commences with glucose, sourced from the bloodstream, undergoing glycolysis, ultimately splitting into two pyruvate molecules. This enzymatic transformation simultaneously brings a small but essential amount of adenosine triphosphate (ATP), which serves as a primary energy source for cellular functions. In specific physiological circumstances, such as during intense exercise or when the body is exposed to a stressor, pyruvate is converted into lactate in a much higher ratio than when resting. This conversion process is facilitated by the activity of lactate dehydrogenase (LDH) enzymes, which play a crucial role in regenerating nicotinamide adenine dinucleotide (NAD+). This regeneration of NAD+ is vital for sustaining the continuation of glycolysis, ensuring the cell’s energy demands are met. Importantly, lactate does not stay only within cells but is efficiently transported out into the bloodstream. In this extracellular environment, lactate primarily exists in the form of lactate anions. This phenomenon is a fundamental aspect of the body’s metabolic regulation, allowing for the efficient exchange of lactate between different tissues and organs, ensuring that energy demands are met even under challenging conditions [8–10]. Both L- and D-stereoisomers of lactate are produced and metabolized to pyruvate through the enzyme LDH. However, LDH has stereoselectivity, so D-lactate production and metabolism require D-LDH, and L-lactate requires L-LDH. The resting values of lactate in the human body approximates 1mM [11], physiological serum concentration of lactate is 1 to 2 mM12, with L-lactate being the predominant physiological enantiomer. Plasma ratio of D-lactate versus L-lactate is estimated to be 1: 100 under normal conditions [12]. Unless stated otherwise, ‘lactate’ always refers to the L-lactate.
In summary, lactate is a dynamic and multifunctional metabolite that appears to be a critical component of cellular energy metabolism, especially during strenuous activities or stress increasing situations. Its generation through glycolysis, facilitated by LDH enzymes, and subsequent transport via the bloodstream exemplify its role as a vital player in maintaining cellular energy homeostasis and overall metabolic balance. It’s noteworthy that lactate isn’t solely a product of anaerobic metabolism; it’s continually produced and distributed within cells, tissues, and organs under aerobic conditions, playing multifunctional roles in metabolism and signaling [7,11,13,14].
Lactate as a metabolic link
“Lactate is the fulcrum of metabolic regulation in vivo” [13]. Lactate plays a pivotal role in metabolic regulation, bridging glycolysis and mitochondrial respiration. It is no longer viewed as a waste product but as a chief messenger in a complex feedback loop. Short-term challenges to ATP supply stimulate lactate production, leading to immediate, short-term, and long-term cellular adaptations to support ATP homeostasis [5,6]. Lactate metabolism at the whole-body level is crucial for three primary reasons: 1. It serves as a significant energy source 2. It acts as the principal precursor for gluconeogenesis and 3. It functions as a universal signaling molecule, referred to as a ‘lactormone,’ with autocrine, paracrine, and endocrine-like effects [12,14–18]. Important to mention is that lactate suppresses concentrations of circulating free fatty acids (FFA) by inhibiting adipose lipolysis through receptor binding, specifically via Hydroxycarboxylic acid receptor 1, previously known as GPR81. Short-term lactate inhibits both lipolysis and mitochondrial free fatty acid oxidation, but long-term exposure upregulates mitochondrial biogenesis, glucose tolerance, and lipid oxidation in humans. Mentioned effects have been observed in rodent models [6,20], and later confirmed in humans [21,23]. These findings only underlining lactate’s role in regulating FFA and metabolism.
Blood lactate concentration and fat oxidation measurements offer an indirect means to evaluate metabolic flexibility, mitochondrial function, and oxidative capacity during exercise. Given lactate’s significant influence on fat and carbohydrate (CHO) metabolism, a compromised ability to clear lactate due to reduced mitochondrial function may substantially impact fat oxidation and CHO oxidation, potentially triggering metabolic dysregulation and, consequently, various metabolic disorders like type 2 diabetes [24].
The latest findings highlight the diverse clinical applications and potential therapeutic roles of lactate in various medical conditions, ranging from anti-inflammatory treatments to heart failure management and brain injury care. Additionally, further research discovers its potential implications in cancer development, progression and eventual treatment. The main opportunities of clinical applications will be discussed in the further chapters.
Is fatigue induced by lactate?
Lactate serves as a crucial metabolic link during exercise, with a dual role in supporting muscle function and optimizing overall performance. To understand its contribution, it’s essential to place lactate within the broader context of exercise-induced fatigue. As exercise intensity escalates, muscles increase lactate production as a protective mechanism. This process helps remove excess protons (H+) from active muscle fibers, stabilizing intracellular pH and sustaining muscle contraction. Contrary to the misconception that lactate induces fatigue, it is an essential element of the organism’s adaptive response to strenuous exercise [7]. Lactate’s versatility extends beyond pH regulation. It swiftly traverses the bloodstream to distant tissues, where it becomes a valuable energy source. The presence of lactate during fatigue is inevitable, but it more seems like a self-defense mechanism of a human body to survive high stress, than the main cause of fatigue.
Lactate shuttle theory
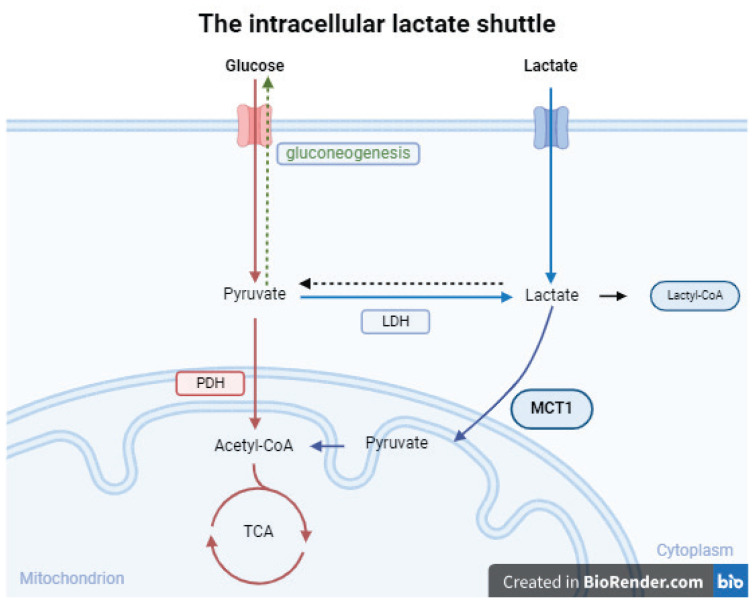
The concept of lactate shuttles describes its roles in delivering oxidative and gluconeogenic substrates, as well as its involvement in cell signaling. This linkage, proposed by the lactate shuttle hypothesis, operates across various cells, tissues, and organs, transcending compartmental barriers. Lactate is considered a messenger in a complex feedback loop, responding to short-term challenges in ATP supply by stimulating lactate production. This phenomenon is vital for immediate and long-term cellular adaptations to maintain ATP homeostasis. The “cell-cell lactate shuttle” and “intracellular lactate shuttle” (Fig. 1) concepts describe how lactate contributes to the delivery of oxidative and gluconeogenic substrates and participates in cell signaling, both within and between cells and tissues. Most if not all cell-cell and intracellular lactate shuttles are driven by a concentration or pH gradient or by redox state [11]. Lactate can cross the cell membrane by free diffusion of undissociated acid or transport via astereospecific pH-sensitive transport protein - monocarboxylate transporter (MCT) [12], which are bidirectional, allowing tissues to switch between lactate release and uptake based on concentration and pH changes. Lactate metabolism is ubiquitous and plays a role in energy supply, glycemia maintenance, cerebral metabolism, and signaling, with implications for various clinical conditions, such as glucose control, traumatic brain injury (TBI), heart failure, and inflammation [5–7,11,12].
Fig. 1.
Lactate is produced from glycolysis or glutamine decomposition and is transported to mitochondria via the MCT’s through the membrane. In cytoplasm Lactate is oxidized to pyruvate, which enters mitochondria and is metabolized through the tricarboxylic acid cycle. In the other pathway, lactate is converted to glucose through gluconeogenesis. Lactate can be converted into Lactyl-CoA and is involved in the lactylation of histones and nonhistone proteins. Lactate is entering the mitochondria on its own through the MCT 1, even under aerobic conditions [11]. LDH – lactate dehydrogenase, PDH – pyruvate dehydrogenase, MCT1 - monocarboxylate transporter 1, TCA-tricarboxylic acid cycle (Figure was created in Biorender.com and partly adapted from [27]).
Essential and non-negligible part of lactate shuttling is mitochondrial lactate oxidation complex (mLOC) [25]. The mLOC includes some essential components of lactate oxidation such as MCTs, its membrane chaperone basigin (BSG or CD147), LDH, and cytochrome oxidase [11]. This theory highlights the significance of mitochondria in lactate oxidation. The mLOC likely establishes gradients of protons and lactate, essential for the function of lactate shuttle systems. The significance of the intracellular lactate shuttle lies in its role as a link between glycolysis and oxidative metabolism, with glycolytic products serving as crucial substrates for oxidative processes [26,27].
Lactate shuttling involves the export of lactate by one cell type and its import by another within the tumor microenvironment. This phenomenon, observed in various physiological and pathological conditions, is crucial due to lactate’s role as an energy-rich metabolite for gluconeogenesis and ATP synthesis. The Cori cycle exemplifies lactate shuttling between skeletal muscle and the liver. In the tumor microenvironment, which comprises hypoxic and normoxic cell populations, glycolysis depends on oxygen. Cancer cells, unable to oxidize lactate, rely on glycolysis for ATP synthesis, and glucose’s impact on oxygen uptake further emphasizes the importance of lactate shuttling for mitochondrial respiration [27].
Astrocyte-neuron lactate shuttle
The astrocyte-neuron lactate shuttle (ANLS), is a metabolic concept that describes the exchange of lactate between astrocytes and neurons in the brain. This shuttle is crucial for brain energy metabolism and function. In the ANLS, glucose is taken up by astrocytes and metabolized through glycolysis to produce lactate, which serves as an essential energy source for nearby neurons [28,29]. Neurons can efficiently utilize lactate to meet their energy demands, especially during periods of increased brain activity or when glucose availability is limited. This shuttle system helps maintain brain function and supports processes like memory, cognition, and neurotransmitter production. It allows for the efficient distribution of energy substrates within the brain and ensures that neurons have a readily available energy source, even under challenging conditions [30,31]. The ANLS highlights the dynamic and collaborative nature of brain metabolism, where different cell types work together to ensure the brain’s energy needs are met, contributing to overall brain health and function.
Contrary, there are some studies challenging the ANLS paradigm [32]. Either that the lactate flows in the opposite direction (neuron to astrocytes) [33] or that neurons primarily rely on glucose uptake and glycolysis instead of astrocyte-produced lactate [34]. Although there is evidence in both approaches, it is yet not possible to state which approach is correct and future research is needed.
Monocarboxylate Transporters (MCTs)
Critical to lactate shuttling are monocarboxylate transporters (MCTs), proteins that facilitate lactate transport across membranes. MCTs are bidirectional, allowing tissues to switch between lactate release and uptake based on concentration and pH changes. Expression patterns of MCTs vary with muscle fiber type and in different tissues [35]. MCTs are ubiquitous, expressed in most tissues [36], including cancers [37] where they support lactate shuttling and are currently targets for cancer therapy [13]. Highly glycolytic cells utilize MCT transporters to export lactate and protons produced by LDH, therefore, MCTs are critical regulators of intracellular lactate and pH. However, MCT functions are also necessary for lactate import into cells that use lactate as an oxidative metabolite (heart and skeletal muscle) or as a substrate for gluconeogenesis (liver and kidney) [38]. MCT1 is expressed in most tissues at low levels, whereas MCT4 is expressed at high levels in type 2 muscle fibers and at lower levels in other tissues, such as testis, lung, and placenta, and in some cell types such as chondrocytes, leukocytes, and astrocytes. MCT2 is primarily expressed in liver, kidney, and neurons in the brain, while MCT3 expression is restricted to retinal pigment epithelium and the choroid plexus [14,39].
Physical performance
Lactate measurements are nowadays widely used performance tests. Whether it is an endurance, sprint or fast paced game, each discipline will benefit from a specifically designed test. There are specific protocols of different length distinguished by the desired interest of performance. Coming from 30 seconds maximal bout (Wingate test) up to marathon like endurance testing which could last for hours. Following the right approach, researchers are able to set the lactate threshold (LT) or the Vmax values [24,40], which are crucial parts of a training program design. Therefore, lactate can be an important signaling molecule, not only in clinical applications but even in the professional performance sport. Additionally, San Millán & Brooks [24] found very strong correlation between lactate raise and CHO uptake during continuous elevated workload, which comes all the way back to the aforementioned metabolic flexibility.
When is lactate elevated?
Now that we know what exactly is lactate and what it does in the human body, comes the important question, when is lactate elevated or lowered? Since in introduction lactate is referred to as a signaling molecule, the signaling has to come from the elevation or drop from the basal value. Drop of lactate is not a very common phenomenon and will be briefly discussed at the end of this section. Therefore, the main signal from the lactate measurement comes from the elevated value.
Lactate exhibits ubiquitous systemic distribution, effectively preventing localized lactate accumulation. When lactate does accumulate, it often signifies underlying health issues such as acute inflammation or insufficient perfusion [41]. This accumulation can trigger appropriate physiological responses, including the promotion of regulatory T cell development, which aids in resolving inflammation. However, in the context of cancer, this propensity can have adverse consequences, as it may suppress anticancer immune responses [8]. Resting value of lactate in healthy humans in blood is around 1 mM [11] (values might differ across the literature, 1–2mM in serum according to Manosalva et al. [12]. Though, it can rise up to ~10 mM in inflammatory pathologies and even 30–40 mM in cancerous tissues [42–44].
Clinical causes of elevated lactate
Cancer metabolism
Cancer cells are known for their upregulated glucose uptake and excessive lactate production, even in oxygen-rich environments, a phenomenon recognized as the ‘Warburg effect’ [45]. Recent research has revealed intriguing parallels between cancer and exercise-related metabolic patterns, suggesting that gene mutations driving increased lactate production, termed ‘lactagenesis,’ are central to both; the cause and the purpose of the Warburg effect. Dysregulated lactate metabolism and signaling are thus pivotal factors in cancer development, aligning with the evolving understanding of lactate shuttling in cancer metabolism [46–48]. Cancer cells exploit this mechanism to utilize lactate not only as an energy source but also as an export molecule, effectively acidifying the tumor microenvironment and promoting tumor invasion into surrounding tissues [49]. Moreover, this process creates an inappropriate positive feedback loop due to continually high lactate levels, inducing confused cell signaling in carcinogenesis. This loop intensifies glucose uptake, glycolysis, lactate production, accumulation, and release, while simultaneously diminishing mitochondrial function. Additionally, it upregulates MCT1 and MCT4 expression, further supporting angiogenesis, immune evasion, cell migration, and metastasis. This collective cascade of events actively encourages carcinogenesis and the progression to cancer [14,48,50,51].
Warburg effect
The Warburg effect is a phenomenon often observed in cancer cells. It involves a shift in the way cancer cells generate energy. Normally, healthy cells primarily produce energy through a process of oxidative phosphorylation, which occurs in the mitochondria and is highly efficient. However, cancer cells often rely more on glycolysis, which is a less efficient process that occurs in the cytoplasm. This increased glycolytic activity is a sign of many cancer cells, because as a result, they consume large amounts of glucose and produce lactate [43]. This metabolic shift towards glycolysis is thought to provide cancer cells with the building blocks they need for rapid growth and division. The Warburg effect has become an important focus in cancer research, as understanding the metabolic changes in cancer cells may offer new opportunities for cancer diagnosis and treatment [48]. Important to mention, this is not a dead end of a cancer metabolism, more like the beginning of the entire process.
The reverse Warburg effect
The concept of reverse Warburg effect suggests that tumor cells can induce nearby stromal cells to adopt glycolysis, producing and releasing lactate, ketones, and potentially pyruvate. Cancer cells then take up these metabolites for oxidative metabolism. Cancer cells create a “pseudo-hypoxic” environment in stromal fibroblasts by secreting hydrogen peroxide. This triggers the activation of Hypoxia-inducible factor 1-alpha (HIF-1), leading to increased glycolysis and the expression of MCT4 [14].
Inflammation
Nowadays, lactate can be a very useful indicator of acute disease (Table 1). Since we know that elevated basal level of lactate can indicate disrupted homeostasis in the human body, it should be included in basal blood tests after receiving patients to the hospital admission or ICU. Diagnostics of lactate are valid in both acute and chronic states [27]. For the past two decades, researchers have observed the buildup of lactate in the synovial fluid of rheumatoid arthritis patients [52]. This phenomenon arises due to the increased metabolic requirements of synovial cells. Notably, it has been proposed that assessing synovial lactate levels could serve as a dependable marker for distinguishing between various forms of inflammatory arthritis [42,44]. Lactate levels can also increase according to the grade of inflammation. Considering the range of lactate in healthy humans ca 1 mM, with the inflammation process, the buildup can increase up to 10mM [44]. Inflammation triggers metabolic shifts leading to increased tissue acidity, mainly due to elevated lactate production from enhanced glycolysis. Lactate can modulate various signaling pathways, utilizing mechanisms such as post-translational modifications, G-protein coupled receptors, and transcription factors, thus regulating the expression of immune-related molecules and enzymes, it acts as an immunomodulatory molecule able to control T-cell effector functions during inflammation [42]. The role of lactate in chronic inflammatory diseases (CIDs) seems to inhibit key glycolytic enzymes, reducing T cell migration. Different lactate transporter patterns in CD4+ and CD8+ T cells influence their responses within inflamed tissues. This option underlines the complexity of immune responses in CIDs and lactate’s contribution to the acidity in inflammatory sites.
Table 1.
| Cause of lactate increase | Signaling mechanism | Clinical importance |
|---|---|---|
| Cancer | Increased glycolytic activity of tumor, tumor tissue hypoxia, decreased clearance of lactate with severe metastases | Potential therapeutic targets, acidic microenvironment is critical for tumorigenesis, angiogenesis, and metastasis |
| Cardiogenic or hypovolemic shock, advanced heart failure, or severe trauma | Decreased O2 delivery to tissues | The majority of lactic acidosis cases |
| TBI | Energy metabolism | Neuroprotective effects, biomarker of systemic physiology |
| Sepsis | Energy metabolism, reduced clearance of lactate even in hemodynamically stable patients | Biomarker of severity of disease, predictors of prognosis and mortality rate |
| Cardiovascular disease | Energy metabolism, increases sympathetic nerve activity and arterial blood pressure | Predictors of prognosis and mortality rate, reduce myocardial reperfusion injury |
| Respiratory disease | Energy metabolism | Biomarker of severity of disease, indicators of diagnosis and therapeutic effect |
| Kidney and liver disease | Decreased lactate clearance | Predictors of prognosis and mortality rate |
| Arthritis | Energy metabolism | Indicators of diagnosis |
| Vigorous exercise, seizures, or shivering | Increased O2 requirements | The decrease in pH and hyperlactatemia is transient |
Traumatic injury
Elevation of basal lactate is often considered an indicator after traumatic injuries, but does not necessarily indicate hypoxia. Persistently elevated lactate is already a valuable predictor of post-surgery complications, rehabilitation, or even mortality, especially in the first 48 hours [53]. Hip fracture patients with lactate higher than 3.0 mM on arrival to the hospital have four times higher chance of mortality within a month compared to those with lactate below 3.0 mM. Prolonged elevated lactate may be the outcome from the stress response in trauma or critical care, involving catecholamines liberating skeletal muscle glycogen as lactate. Although the understanding of the entire process is still not fully described, persistently high lactate remains a useful clinical marker [54].
Injury and Illness
Emerging insights into lactate’s metabolic role have initiated investigations into its potential applications in improving outcomes for individuals with injuries and illnesses [55,56]. Elevated blood lactate levels, often observed during high-intensity exercise, are nowadays increasingly recommended to mitigate risk factors for conditions like diabetes, coronary artery disease, and cardiometabolic disease [57]. Hyperlactatemia is also common in severe injuries and critical illnesses like sepsis [54,55,58], where it serves as a predictor of mortality. Contrary to traditional notions linking hyperlactatemia to ischemia and hypoxia, recent theories involving the lactate shuttle mechanism suggest that lactate elevation is a protective response to injury and illness [17]. There has been found consistent evidence that decreasing lactate concentrations are associated with improved patient prognosis across various clinical situations. These observations extend beyond septic patients and are applicable to a diverse range of patients with hyperlactatemia. While the changes in lactate levels tend to occur gradually, making it challenging to provide specific recommendations for the rate of decrease, regular monitoring, possibly as frequently as every 1–2 hours, can help identify those likely to recover from those at higher risk of adverse outcomes. Furthermore, studying lactate kinetics appears to be valuable not only in cases of severe hyperlactatemia but also in patients with different initial lactate levels and it underscores the importance of monitoring and managing lactate levels as a prognostic indicator in various clinical contexts, highlighting its relevance in critical care and clinical practice [53]. Additionally, there is an emerging evidence that deliberately evoked hyperlactatemia may have therapeutic benefits in specific conditions, like improving myocardial function in septic shock [59], post-cardiac surgery, and acute heart failure [60], as well as potential applications in TBI [56,61] and tumor treatment strategies targeting the Warburg effect.
Exercise-induced lactate increase
Elevated lactate during physical activity is a well-known phenomenon. Lactate is mostly connected to the exhaustion and fatigue, and is commonly mistaken for their precursor, due to its occurrence during high intensity exercise and the following exhaustion. Lactate is mostly elevated due to increased energetic and metabolic demands during high intensity exercise and muscle contraction glycolysis is accelerated, leading to upregulation in lactate and pyruvate concentrations and increasing the lactate/pyruvate ratio (L/P). At rest, this ratio in muscle is around 10. However, during moderate-intensity exercise, the ratio increases significantly [62]. This rise in the L/P ratio results in the flooding of the monocarboxylate pair into the mitochondrial reticulum [15,63,64]. This influx gives rise to acetyl-CoA production and subsequently leads to the formation of malonyl-CoA. Malonyl-CoA, in turn, inhibits the entry of activated FFA into the mitochondrial matrix by blocking carnitine-palmitoyl transferase-1 (CPT1) [13,64]. Additionally, the accumulation of acetyl-CoA may downregulate β-ketothiolase, which is the terminal and rate-limiting enzyme in the mitochondrial β-oxidation pathway.
Why is lactate research still relevant in the future?
According to the latest research, lactate appears to be a helpful molecule that is ubiquitous and supports the affected tissue with energy, instead of being the main producer of acidosis. Regarding the aforementioned information, about the abilities of lactate as a signaling molecule, we should come to the main question: Why is lactate produced in the human body? What is its main purpose? And when we find out, how do we use it?
Lactate is maybe a more important energy source than we thought, because lactate is preferred over glucose, when infused in the human body [65], also in working human skeletal muscles and heart [15,66–68], similarly in the brain [39,56,69,70]. Since we know that lactate is the preferred energy source over glucose, it opens the whole new chapter of research of lactate usage in treatment of illness and injuries. Only the latest reviews found that strains of lactic acid bacteria exert health-promoting functions such as immunomodulatory improvement of intestinal integrity, resistance to pathogens, prevention of lactose intolerance, anticancer effects, reduction of depression and anxiety symptoms, anti-obesity and anti-diabetic activities, and decrease serum cholesterol levels [12,71].
Cancer research
The role of lactate in cancer, tumorigenesis, and tumor metabolism has gained renewed interest, since lactate is no longer viewed as a metabolic dead-end or a fatigue-causing waste product. Release of lactate may support carcinogenesis through a series of steps, including increased glucose uptake, upregulation of glycolytic enzyme expression and activity, decreased mitochondrial function, enhanced lactate production, accumulation, and release, and the upregulation of transporters MCT1 and MCT4 for lactate exchange. Throughout these processes, lactate emerges as a central metabolic compound that is both involved and necessary at every stage of the tumor sequence [11,46,48,72].
Lactate holds significant importance in cancer research due to its multifaceted roles within the tumor microenvironment. Sonveaux et al. [37] discovered the crucial concept of lactate shuttling in tumors, where cancer cells utilize lactate from the microenvironment for respiration, presenting a challenging aspect. Efforts to hinder this process are actively underway, with researchers focusing on developing inhibitors for monocarboxylate transporters MCT1 and MCT4 [14,50,51]. However, challenges concerning MCT specificity persist, exemplified by instances like AstraZeneca’s AR-C155858, which initially inhibited MCT1 and MCT2 but led to MCT4 upregulation and increased carcinogenesis in rodent models both in vivo and in vitro [73]. While newer MCT-1 inhibitors show promise in specific cancers [50], the pursuit of cancer-specific MCT blockers remains a significant area of research interest. Moreover, quantitative imaging techniques for lactate measurements have emerged as a valuable tool for assessing target specificity, tumor response, and clinical outcomes during cancer treatment, highlighting the pivotal role of lactate measurements in current clinical research and practice [43]. Additionally, lactate’s antioxidant properties may contribute to cancer therapy resistance, as it can counteract the oxidative stress induced by anticancer treatments like radiation and chemotherapy, potentially leading to enhanced chemo-resistance and tumor survival [43].
Recent discoveries are revealing that histone lactylation, mediated by lactyl-CoA, influencing gene expression and physiological states like cancer, and inflammation [74,75]. Lactate’s involvement in histone lactylation underscores its role in tumor progression. Additionally, histone lactylation emerges as a regulator of immune responses and homeostasis. This finding suggests its significance in diverse pathophysiological conditions beyond cancer. Understanding histone lactylation mechanisms offers insights into lactate’s functions and potential therapeutic paths across various diseases [76].
This multifaceted nature of lactate underscores its significance in advancing our understanding of cancer biology and treatment strategies. Targeting lactate metabolism and MCTs in the future cancer research seems like the right move towards cancer treatment.
Traumatic brain injuries (TBI)
In resting individuals, glucose primarily fuels the brain 14. However, lactate becomes pivotal in scenarios like TBI and exercise. Comatose TBI patients utilize a substantial portion of circulating blood glucose through gluconeogenesis from lactate, supplying up to 57 % of brain fuel both directly and indirectly [11,61]. During exercise, healthy individuals experience significant increases in plasma lactate levels, contributing up to 25 % of the brain’s total energy requirement. Lactate sometimes supplants glucose as the preferred brain fuel, and its provision to a healthy brain reduces glucose uptake, emphasizing cerebral lactate shuttling [77,78]. Furthermore, lactate acts as a signaling molecule for the secretion of cerebral brain-derived neurotrophic factor (BDNF), facilitating neuroprotective and cognitive functions [56,70,77]. Elevated circulating lactate levels, induced by exercise or infusion, correlate with increased BDNF levels, potentially influencing cognitive processes, memory, and synaptic plasticity. Recent studies reinforce lactate’s positive impact on cognitive function [77,78], demonstrating correlations between executive function, decision-making, blood lactate levels, and cerebral lactate uptake. Moreover, lactate is implicated in long-term memory consolidation in the hippocampus [11], highlighting its significance in cognitive processes and brain function.
Lactate therapy has emerged as a promising approach for brain injury resuscitation, with ongoing experimental and clinical trials worldwide. Initial brain injuries trigger hyperlactatemia as part of the immediate stress response, but prolonged hospitalization and inadequate nutrition result in low blood lactate levels, affecting cerebral glucose uptake. Intravenous lactate infusion is explored as a means to provide an alternative energy substrate for the brain. Lactate not only serves as a brain fuel source following injury but also exhibits anti-inflammatory properties by binding to the lactate receptor GPR81, inhibiting inflammatory pathways [11,51]. This approach is particularly advantageous when oral or gastric tube feeding is not feasible due to polytrauma or complications. Lactate infusion involves specific target blood lactate concentrations, typically ranging from 2 to 5 mM. Brain injury conditions under investigation for lactate therapy include TBI and subarachnoid bleeding [70,79,80]. Experimental evidence from animal studies confirms lactate’s ability to sustain neuronal activity during glucose deprivation [81], and patients with traumatic brain injuries utilize peripheral lactate as a brain energy substrate [56]. Lactate transport across cell membranes via MCTs further underscores its relevance in brain metabolism [81].
Intravenous L-lactate infusion has demonstrated the potential to minimize cerebral swelling and intracranial pressure following brain injury. It also holds promise in reducing inflammation in other injured tissues as well [11]. Peripherally produced lactate serves as an energy source for the injured brain, both directly through cerebral uptake and indirectly as a precursor for gluconeogenesis. This finding underscores the use of arterial lactate supplementation to compensate for reduced cerebral glucose uptake in TBI cases [56]. The brain takes up and oxidizes lactate from the systemic circulation during injury, just as it does during physical exercise [77,78].
In summary, lactate’s multifaceted roles in brain metabolism, cognitive function, and potential therapeutic applications in brain injury resuscitation underscore its growing importance in neuroscience research and clinical practice.
Sepsis
Emerging evidence challenges the conventional wisdom that elevated lactate levels primarily signify oxygen debt or tissue hypoxia in sepsis. Instead, research suggests that lactate production in sepsis is closely tied to increased aerobic glycolysis, often triggered by the activation of the stress response, particularly adrenergic stimulation. This change in perspective indicates that lactate may serve as an essential intermediate metabolite and even a potential energy source in the septic condition. Moreover, experimental and human studies consistently support the notion that sepsis-associated hyperlactatemia (SAHL) might be an adaptive survival response. Recent findings hint at lactate oxidation as a means to enhance bioenergetic efficiency, emphasizing the potential benefits of elevated lactate levels during sepsis. It becomes apparent that lactate in sepsis may not solely reflect oxygen debt or hypoxia but is an intricate aspect of metabolic alterations that could contribute to the body’s adaptive mechanisms in times of stress and illness. These insights may offer a promising direction for developing reliable predictive models for sepsis patients, potentially reshaping the understanding and management of this life-threatening condition [54,55]. Additionally, evaluating isotonic or hypertonic sodium-L-lactate as resuscitation solutions for sepsis merits further consideration within this evolving paradigm [17,66,82].
Anti-inflammation abilities
Lactate-containing compounds have shown promise in limiting inflammation following injury and are being explored as a potential anti-inflammatory resuscitation fluid in various therapies, including acute pancreatitis, hepatitis, and dengue fever [11,82]. Lactate could be also used as an inflammation predictor. Therefore, lactate measurements could help us indicate possible inflammation in the body, and with given information about anti-inflammatory abilities, it seems that the lactate excess in the affected tissue is due to the body’s immune response. Targeting lactate transporters could deliver new ways of the inflammatory disorder treatment [42].
Gut health and the microbiome
Recent research highlights lactate’s potential in promoting gut health and its association with the gut microbiome [83]. Lactate has been found to downregulate inflammation in intestinal cells and exhibit anti-inflammatory effects in colitis models, suggesting that lactate-containing foods could benefit gut microbiota and protect against pathogens [84–86]. It is a natural fermentation product produced by various gut bacteria, particularly Lactobacillus and Bifidobacterium genera [87]. While often overshadowed by other substrates like butyrate and acetate [88], lactate’s high turnover rate and potential impact on gut health through supporting mucosal integrity and diverse microbiota composition make it an area of growing interest, suggesting that practices promoting gut lactate presence may have significant implications for health and disease.
Clinical applications
Lactate serves as a major fuel source for a healthy heart, preferred over glucose and free fatty acids. Therefore, the treatment of heart failure is moving towards utilizing exogenous lactate to enhance cardiac function [13,66,89]. Lactate monitoring may play a crucial role in identifying signs of severe sepsis [9], but slight increases in blood lactate levels, even within the normal range, could signal an underlying infection or impending metabolic challenge. Recognizing the significance of these fluctuations can aid in early intervention and improved patient care, potentially extending to the use of lactate-based fluids in sepsis, inflammation, uremia, and cardiac conditions management [59,90]. Sodium lactate infusion has not been integrated into standard care for any patient group, as revealed in a recent review of 34 studies [91], despite the suggested potential benefits of such treatment.
Strains of lactic acid bacteria exert health-promoting functions such as immunomodulatory improvement of intestinal integrity, resistance to pathogens, prevention of lactose intolerance, anti-cancer effects, reduction of depression and anxiety symptoms, anti-obesity and anti-diabetic activities, and decrease serum cholesterol levels [12]. Not to forget to mention the role of lactate in control of proinflammatory T cell motility and effector functions. The latest research provides a potential molecular mechanism for T cell entrapment and functional changes, and offers targeted therapeutic interventions for the treatment of chronic inflammatory disorders [42].
Metabolic flexibility
Studies show that the lactate metabolism is very flexible and trainable. According to San Millan & Brooks [24] and our unpublished results, professional athletes are showing superior capabilities to oxidize lactate, as well as CHO and lipid-derived fuel energy sources, and also keep capacity for lipid oxidation at different exercise intensities, compared to moderately active people who subsequently show superior results to patients with metabolic syndrome. Most of the patients with metabolic syndrome and some of the moderately active people are mostly relying on CHO metabolism in low intensity activities, let alone resting. This means blood lactate increase even with the slightest movement activity, and because lactate has profound effects on fat and CHO metabolism, a poor lactate clearance capacity due to lactate oxidation complex limitations greatly affect fat oxidation and CHO oxidation, which could result in metabolic dysregulation, and which, in turn, may give rise to insulin resistance, diabetes, and possibly other chronic diseases, including cardiometabolic diseases [24,92]. Significantly lower fat oxidation, and higher levels of lactate, at low intensity exercise in patients with metabolic syndrome suggest poor mLOC protein expression and mitochondrial dysfunction. Therefore, correlating both seems to represent a valid indirect method to evaluate mitochondrial power and function during a long bout of exercise in individuals ranging from patients to professional athletes.
Exerkines are signaling molecules released in response to exercise, including: myokines, cardiokines, adipokines, etc. Exerkines have potential importance for improving cardiovascular, metabolic, immune and neurological health. Thus, exerkines holds the potential in treatment of cardiovascular disease, type 2 diabetes mellitus and obesity, and possibly to facilitate healthy aging [93,94].
Skeletal muscles produce myokines in response to exercise, which allow for crosstalk between the muscle and other organs. Myokines have been allocated to have specific functions in humans including effects on cognition, lipid and glucose metabolism, bone formation, endothelial cell function, hypertrophy, skin structure, and tumor growth [95]. This suggests that myokines may be useful biomarkers for monitoring exercise in patients with cancer, diabetes, or neurodegenerative diseases [23,95].
TGF-β2 is an exercise-induced adipokine that improves glucose tolerance and insulin sensitivity. This effect is seen in mice and human fat tissue following exercise. Lactate stimulates the production of TGF-β2 in human fat cells. When lactate levels are reduced during exercise in mice, it blocks the positive effects on glucose tolerance. Research shows that exercise enhances overall metabolism by facilitating communication between different organs, particularly muscle and fat tissue, through a signaling process involving lactate and TGF-β2 [96].
Conclusion
Lactate is a long-debated topic, which has been accompanied by many misunderstandings. Compared to the past decades we are moving by leaps and bounds ahead of understanding such a complicated molecule, but still there is a long and rough path ahead. Once feared, lactate deserves redemption within professional society. It is no longer a waste product of glycolysis, but an energy source for the entire body in critical conditions. Taken sepsis, inflammation, injuries or any other special condition where hyperlactatemia occurs, lactate is more like a signaling molecule of upregulated glycolytic metabolism and the savior that is providing energy, instead of a waste which is the main source of acidosis.
The future of lactate research has many paths in many fields. Resuscitation and supplementation of lactate is ongoing yet not fully discovered practice, where L-lactate supplementation shows very promising results not only in critically ill sepsis patients, but also in TBI patients, lactate preserves the brain function, and is the first taken energy source. Another field of interest is predictive models of patient’s recovery, not only time, but even success rate, and mortality after severe injuries. And last but not least, accessing metabolism flexibility, whether we are talking about athletes, the general population or patients. Lactate measurements during rest, or several intensities can give us really helpful insights into one’s metabolic health and performance capabilities. These data could be helpful predictors for diabetes, pre-diabetes or patients with metabolic syndrome as a prevention or even the cure, considering exercise is medicine.
Acknowledgements
This work was supported by the Cooperatio Program, research area Immunity and Infection.
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Fletcher WM, Hopkins FG. Lactic acid in amphibian muscle1. J Physiol. 1907;35(4):247–309. doi: 10.1113/jphysiol.1907.sp001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hill AV, Lupton H. Muscular Exercise, Lactic Acid, and the Supply and Utilization of Oxygen. QJM. 1923;os-16(62):135–171. doi: 10.1093/qjmed/os-16.62.135. [DOI] [Google Scholar]
- 3.Gladden LB. Lactate metabolism: a new paradigm for the third millennium. J Physiol. 2004;558(1):5–30. doi: 10.1113/jphysiol.2003.058701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benninga H. A history of lactic acid making: a chapter in the history of biotechnology. Br J Hist Sci. 1991;24(4):474–475. doi: 10.1017/S0007087400027692. [DOI] [Google Scholar]
- 5.Brooks GA. Lactate shuttles in Nature. Biochem Soc Trans. 2002;30(2):258–264. doi: 10.1042/bst0300258. [DOI] [PubMed] [Google Scholar]
- 6.Brooks GA. Cell-cell and intracellular lactate shuttles: Lactate: Darth Vader or Jedi Knight of exercise physiology? J Physiol. 2009;587(23):5591–5600. doi: 10.1113/jphysiol.2009.178350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferguson BS, Rogatzki MJ, Goodwin ML, Kane DA, Rightmire Z, Gladden LB. Lactate metabolism: historical context, prior misinterpretations, and current understanding. Eur J Appl Physiol. 2018;118(4):691–728. doi: 10.1007/s00421-017-3795-6. [DOI] [PubMed] [Google Scholar]
- 8.Rabinowitz JD, Enerbäck S. Lactate: the ugly duckling of energy metabolism. Nat Metab. 2020;2(7):566–571. doi: 10.1038/s42255-020-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraut JA, Madias NE. Lactic Acidosis. N Engl J Med. 2014;371(24):2309–2319. doi: 10.1056/NEJMra1309483. [DOI] [PubMed] [Google Scholar]
- 10.Rogatzki MJ, Ferguson BS, Goodwin ML, Gladden LB. Lactate is always the end product of glycolysis. Front Neurosci. 2015:9. doi: 10.3389/fnins.2015.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brooks GA. The science and translation of lactate shuttle theory. Cell Metab. 2018;27(4):757–785. doi: 10.1016/j.cmet.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 12.Manosalva C, Quiroga J, Hidalgo AI, et al. Role of lactate in inflammatory processes: friend or foe. Front Immunol. 2022;12:808799. doi: 10.3389/fimmu.2021.808799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks GA. Lactate as a fulcrum of metabolism. Redox Biol. 2020;35:101454. doi: 10.1016/j.redox.2020.101454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty JR, Cleveland JL. Targeting lactate metabolism for cancer therapeutics. J Clin Invest. 2013;123(9):3685–3692. doi: 10.1172/JCI69741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bergman BC, Wolfel EE, Butterfield GE, et al. Active muscle and whole body lactate kinetics after endurance training in men. J Appl Physiol. 1999;87(5):1684–1696. doi: 10.1152/jappl.1999.87.5.1684. [DOI] [PubMed] [Google Scholar]
- 16.Bergman BC, Horning MA, Casazza GA, Wolfel EE, Butterfield GE, Brooks GA. Endurance training increases gluconeogenesis during rest and exercise in men. Am J Physiol - Endocrinol Metab. 2000;278(241–2):E244–E251. doi: 10.1152/ajpendo.2000.278.2.E244. [DOI] [PubMed] [Google Scholar]
- 17.Brooks GA, Arevalo JA, Osmond AD, Leija RG, Curl CC, Tovar AP. Lactate in contemporary biology: a phoenix risen. J Physiol. 2022;600(5):1229–1251. doi: 10.1113/JP280955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emhoff CAW, Messonnier LA, Horning MA, Fattor JA, Carlson TJ, Brooks GA. Gluconeogenesis and hepatic glycogenolysis during exercise at the lactate threshold. J Appl Physiol. 2013;114(3):297–306. doi: 10.1152/japplphysiol.01202.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hashimoto T, Hussien R, Oommen S, Gohil K, Brooks GA. Lactate sensitive transcription factor network in L6 cells: activation of MCT1 and mitochondrial biogenesis. FASEB J. 2007;21(10):2602–2612. doi: 10.1096/fj.07-8174com. [DOI] [PubMed] [Google Scholar]
- 20.Liu C, Wu J, Zhu J, et al. Lactate inhibits lipolysis in fat cells through activation of an orphan g-protein-coupled receptor GPR81. J Biol Chem. 2009;284(5):2811–2822. doi: 10.1074/jbc.M806409200. [DOI] [PubMed] [Google Scholar]
- 21.Pedersen MGB, Rittig N, Søndergaard E, Gormsen LC, Møller N. 1660-P: Lactate Inhibits Lipolysis in Healthy Males-A Randomized Crossover Trial. Diabetes. 2023;72(Supplement_1):1660-P. doi: 10.2337/db23-1660-P. [DOI] [Google Scholar]
- 22.Takeuchi N, Higashida K, Nakai N. Inhibition of the mitochondrial respiratory chain reduces catecholamine-stimulated lipolysis via increasing lactate production in 3T3-L1 adipocytes. Mol Med Rep. 2023;28(6):1–6. doi: 10.3892/mmr.2023.13116. [DOI] [PubMed] [Google Scholar]
- 23.Brooks GA, Osmond AD, Arevalo JA, et al. Lactate as a myokine and exerkine: drivers and signals of physiology and metabolism. J Appl Physiol Bethesda Md 1985. 2023;134(3):529–548. doi: 10.1152/japplphysiol.00497.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.San-Millán I, Brooks GA. Assessment of metabolic flexibility by means of measuring blood lactate, fat, and carbohydrate oxidation responses to exercise in professional endurance athletes and less-fit individuals. Sports Med. 2018;48(2):467–479. doi: 10.1007/s40279-017-0751-x. [DOI] [PubMed] [Google Scholar]
- 25.Hashimoto T, Hussien R, Brooks GA. Colocalization of MCT1, CD147, and LDH in mitochondrial inner membrane of L6 muscle cells: evidence of a mitochondrial lactate oxidation complex. Am J Physiol-Endocrinol Metab. 2006;290(6):E1237–E1244. doi: 10.1152/ajpendo.00594.2005. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto T, Brooks GA. Mitochondrial lactate oxidation complex and an adaptive role for lactate production. Med Sci Sports Exerc. 2008;40(3):486. doi: 10.1249/MSS.0b013e31815fcb04. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Yang Y, Zhang B, et al. Lactate metabolism in human health and disease. Signal Transduct Target Ther. 2022;7(1):1–22. doi: 10.1038/s41392-022-01151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bélanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14(6):724–738. doi: 10.1016/j.cmet.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 29.Pellerin L, Pellegri G, Bittar PG, et al. Evidence Supporting the Existence of an Activity-Dependent Astrocyte-Neuron Lactate Shuttle. Dev Neurosci. 1998;20(4–5):291–299. doi: 10.1159/000017324. [DOI] [PubMed] [Google Scholar]
- 30.Bouzier-Sore AK, Voisin P, Bouchaud V, Bezancon E, Franconi JM, Pellerin L. Competition between glucose and lactate as oxidative energy substrates in both neurons and astrocytes: a comparative NMR study. Eur J Neurosci. 2006;24(6):1687–1694. doi: 10.1111/j.1460-9568.2006.05056.x. [DOI] [PubMed] [Google Scholar]
- 31.Wyss MT, Jolivet R, Buck A, Magistretti PJ, Weber B. In Vivo evidence for lactate as a neuronal energy source. J Neurosci. 2011;31(20):7477–7485. doi: 10.1523/JNEUROSCI.0415-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab. 2012;32(7):1107–1138. doi: 10.1038/jcbfm.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simpson IA, Carruthers A, Vannucci SJ. Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab. 2007;27(11):1766–1791. doi: 10.1038/sj.jcbfm.9600521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang BL. Brain activity-induced neuronal glucose uptake/glycolysis: Is the lactate shuttle not required? Brain Res Bull. 2018;137:225–228. doi: 10.1016/j.brainresbull.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 35.Brown MA, Brooks GA. Trans-stimulation of lactate transport from rat sarcolemmal membrane vesicles. Arch Biochem Biophys. 1994;313(1):22–28. doi: 10.1006/abbi.1994.1353. [DOI] [PubMed] [Google Scholar]
- 36.Garcia CK, Goldstein JL, Pathak RK, Anderson RGW, Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: Implications for the Cori cycle. Cell. 1994;76(5):865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 37.Sonveaux P, Végran F, Schroeder T, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118(12):3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halestrap AP, Wilson MC. The monocarboxylate transporter family--role and regulation. IUBMB Life. 2012;64(2):109–119. doi: 10.1002/iub.572. [DOI] [PubMed] [Google Scholar]
- 39.Bergersen LH. Lactate transport and signaling in the brain: potential therapeutic targets and roles in body-brain interaction. J Cereb Blood Flow Metab. 2015;35(2):176–185. doi: 10.1038/jcbfm.2014.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hering GO, Hennig EM, Riehle HJ, Stepan J. A lactate kinetics method for assessing the maximal lactate steady state workload. Front Physiol. 2018;9:310. doi: 10.3389/fphys.2018.00310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pucino V, Certo M, Bulusu V, et al. Lactate buildup at the site of chronic inflammation promotes disease by inducing CD4+ T Cell metabolic rewiring. Cell Metab. 2019;30(6):1055–1074.e8. doi: 10.1016/j.cmet.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Haas R, Smith J, Rocher-Ros V, et al. Lactate regulates metabolic and pro-inflammatory circuits in control of T cell migration and effector functions. PLOS Biol. 2015;13(7):e1002202. doi: 10.1371/journal.pbio.1002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hirschhaeuser F, Sattler UGA, Mueller-Klieser W. Lactate: A metabolic key player in cancer. Cancer Res. 2011;71(22):6921–6925. doi: 10.1158/0008-5472.CAN-11-1457. [DOI] [PubMed] [Google Scholar]
- 44.Pucino V, Bombardieri M, Pitzalis C, Mauro C. Lactate at the crossroads of metabolism, inflammation, and autoimmunity. Eur J Immunol. 2017;47(1):14–21. doi: 10.1002/eji.201646477. [DOI] [PubMed] [Google Scholar]
- 45.Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol. 1927;8(6):519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goodwin ML, Gladden LB, Nijsten MWN, Jones KB. Lactate and Cancer: Revisiting the Warburg effect in an era of lactate shuttling. Front Nutr. 2015:1. doi: 10.3389/fnut.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hui S, Ghergurovich JM, Morscher RJ, et al. Glucose feeds the TCA cycle via circulating lactate. Nature. 2017;551(7678):115–118. doi: 10.1038/nature24057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.San-Millán I, Brooks GA. Reexamining cancer metabolism: Lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis. 2017;38(2):119–133. doi: 10.1093/carcin/bgw127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Glancy B, Kane DA, Kavazis AN, Goodwin ML, Willis WT, Gladden LB. Mitochondrial lactate metabolism: history and implications for exercise and disease. J Physiol. 2021;599(3):863–888. doi: 10.1113/JP278930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Doherty JR, Yang C, Scott KEN, et al. Blocking lactate export by inhibiting the myc target MCT1 disables glycolysis and glutathione synthesis. Cancer Res. 2014;74(3):908–920. doi: 10.1158/0008-5472.CAN-13-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Draoui N, Feron O. Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis Model Mech. 2011;4(6):727–732. doi: 10.1242/dmm.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gobelet C, Gerster JC. Synovial fluid lactate levels in septic and non-septic arthritides. Ann Rheum Dis. 1984;43(5):742–745. doi: 10.1136/ard.43.5.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vincent JL, Quintairos e Silva A, Couto L, Taccone FS. The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care. 2016;20:257. doi: 10.1186/s13054-016-1403-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baysan M, Baroni GD, van Boekel AM, Steyerberg EW, Arbous MS, van der Bom JG. The added value of lactate and lactate clearance in prediction of in-hospital mortality in critically ill patients with sepsis. Crit Care Explor. 2020;2(3):e0087. doi: 10.1097/CCE.0000000000000087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garcia-Alvarez M, Marik P, Bellomo R. Sepsis-associated hyperlactatemia. Crit Care. 2014;18(5):503. doi: 10.1186/s13054-014-0503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Glenn TC, Martin NA, Horning MA, et al. Lactate: Brain fuel in human traumatic brain injury: a comparison with normal healthy control subjects. J Neurotrauma. 2015;32(11):820–832. doi: 10.1089/neu.2014.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Robinson MM, Dasari S, Konopka AR, et al. Enhanced protein translation underlies improved metabolic and physical adaptations to different exercise training modes in young and old humans. Cell Metab. 2017;25(3):581–592. doi: 10.1016/j.cmet.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Marik P, Bellomo R. Lactate clearance as a target of therapy in sepsis: A flawed paradigm. OA Crit Care. 2013;1(1) doi: 10.13172/2052-9309-1-1-431. [DOI] [Google Scholar]
- 59.Marik P, Bellomo R. A rational approach to fluid therapy in sepsis. Br J Anaesth. 2016;116(3):339–349. doi: 10.1093/bja/aev349. [DOI] [PubMed] [Google Scholar]
- 60.Nalos M, Leverve XM, Huang SJ, et al. Half-molar sodium lactate infusion improves cardiac performance in acute heart failure: a pilot randomised controlled clinical trial. Crit Care. 2014;18(2):R48. doi: 10.1186/cc13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brooks GA, Martin NA. Cerebral metabolism following traumatic brain injury: new discoveries with implications for treatment. Front Neurosci. 2015:8. doi: 10.3389/fnins.2014.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henderson GC, Horning MA, Wallis GA, Brooks GA. Letter to the editor. Am J Physiol-Endocrinol Metab. 2007;292(1):E366–E366. doi: 10.1152/ajpendo.00363.2006. [DOI] [PubMed] [Google Scholar]
- 63.Passarella S, De Bari L, Valenti D, Pizzuto R, Paventi G, Atlante A. Mitochondria and l -lactate metabolism. FEBS Lett. 2008;582(25–26):3569–3576. doi: 10.1016/j.febslet.2008.09.042. [DOI] [PubMed] [Google Scholar]
- 64.Saddik M, Gamble J, Witters LA, Lopaschuk GD. Acetyl-CoA carboxylase regulation of fatty acid oxidation in the heart. J Biol Chem. 1993;268(34):25836–25845. doi: 10.1016/S0021-9258(19)74465-2. [DOI] [PubMed] [Google Scholar]
- 65.Miller BF, Fattor JA, Jacobs KA, et al. Lactate and glucose interactions during rest and exercise in men: Effect of exogenous lactate infusion. J Physiol. 2002;544(3):963–975. doi: 10.1113/jphysiol.2002.027128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bergman BC, Tsvetkova T, Lowes B, Wolfel EE. Myocardial glucose and lactate metabolism during rest and atrial pacing in humans. J Physiol. 2009;587(9):2087–2099. doi: 10.1113/jphysiol.2008.168286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gertz EW, Wisneski JA, Neese R, Bristow JD, Searle GL, Hanlon JT. Myocardial lactate metabolism: Evidence of lactate release during net chemical extraction in man. Circulation. 1981;63(6 I):1273–1279. doi: 10.1161/01.CIR.63.6.1273. [DOI] [PubMed] [Google Scholar]
- 68.Stanley WC, Gertz EW, Wisneski JA, Neese RA, Morris DL, Brooks GA. Lactate extraction during net lactate release in legs of humans during exercise. J Appl Physiol. 1986;60(4):1116–1120. doi: 10.1152/jappl.1986.60.4.1116. [DOI] [PubMed] [Google Scholar]
- 69.Wolahan SM, Hirt D, Braas D, Glenn TC. Role of metabolomics in traumatic brain injury research. Neurosurg Clin N Am. 2016;27(4):465–472. doi: 10.1016/j.nec.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolahan SM, Mao HC, Real C, Vespa PM, Glenn TC. Lactate supplementation in severe traumatic brain injured adults by primed constant infusion of sodium L-lactate. J Neurosci Res. 2018;96(4):688–695. doi: 10.1002/jnr.24085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.de Melo Pereira GV, de Oliveira Coelho B, Magalhães AI, Júnior, Thomaz-Soccol V, Soccol CR. How to select a probiotic? A review and update of methods and criteria. Biotechnol Adv. 2018;36(8):2060–2076. doi: 10.1016/j.biotechadv.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 72.Hussien R, Brooks GA. Mitochondrial and plasma membrane lactate transporter and lactate dehydrogenase isoform expression in breast cancer cell lines. Physiol Genomics. 2011;43(5):255–264. doi: 10.1152/physiolgenomics.00177.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Le Floch R, Chiche J, Marchiq I, et al. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc Natl Acad Sci U S A. 2011;108(40):16663–16668. doi: 10.1073/pnas.1106123108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xie Y, Hu H, Liu M, et al. The role and mechanism of histone lactylation in health and diseases. Front Genet. 2022;13:949252. doi: 10.3389/fgene.2022.949252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jiang J, Huang D, Jiang Y, et al. Lactate modulates cellular metabolism through histone lactylation-mediated gene expression in non-small cell lung cancer. Front Oncol. 2021;11:647559. doi: 10.3389/fonc.2021.647559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang D, Tang Z, Huang H, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574(7779):575–580. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hashimoto T, Tsukamoto H, Takenaka S, et al. Maintained exercise-enhanced brain executive function related to cerebral lactate metabolism in men. FASEB J. 2018;32(3):1417–1427. doi: 10.1096/fj.201700381RR. [DOI] [PubMed] [Google Scholar]
- 78.van Hall G, Stømstad M, Rasmussen P, et al. Blood lactate is an important energy source for the human brain. J Cereb Blood Flow Metab. 2009;29(6):1121–1129. doi: 10.1038/jcbfm.2009.35. [DOI] [PubMed] [Google Scholar]
- 79.Oddo M, Levine JM, Frangos S, et al. Brain lactate metabolism in humans with subarachnoid hemorrhage. Stroke. 2012;43(5):1418–1421. doi: 10.1161/STROKEAHA.111.648568. [DOI] [PubMed] [Google Scholar]
- 80.Patet C, Suys T, Carteron L, Oddo M. Cerebral lactate metabolism after traumatic brain injury. Curr Neurol Neurosci Rep. 2016;16(4):31. doi: 10.1007/s11910-016-0638-5. [DOI] [PubMed] [Google Scholar]
- 81.Mächler P, Wyss MT, Elsayed M, et al. In Vivo Evidence for a Lactate Gradient from Astrocytes to Neurons. Cell Metab. 2016;23(1):94–102. doi: 10.1016/j.cmet.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 82.Hoque R, Farooq A, Ghani A, Gorelick F, Mehal WZ. Lactate reduces liver and pancreatic injury in toll-like receptor- and inflammasome-mediated inflammation via GPR81-mediated suppression of innate immunity. Gastroenterology. 2014;146(7):1763–1774. doi: 10.1053/j.gastro.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Turnbaugh PJ, Ley RE, Hamady M, Fraser-Liggett CM, Knight R, Gordon JI. The human microbiome project. Nature. 2007;449(7164):804–810. doi: 10.1038/nature06244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Iraporda C, Romanin DE, Rumbo M, Garrote GL, Abraham AG. The role of lactate on the immunemodulatory properties of the nonbacterial fraction of kefir. Food Res Int. 2014;62:247–253. doi: 10.1016/j.foodres.2014.03.003. [DOI] [Google Scholar]
- 85.Iraporda C, Errea A, Romanin DE, et al. Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology. 2015;220(10):1161–1169. doi: 10.1016/j.imbio.2015.06.004. [DOI] [PubMed] [Google Scholar]
- 86.Iraporda C, Romanin DE, Bengoa AA, et al. Local treatment with lactate prevents intestinal inflammation in the TNBS-induced colitis model. Front Immunol. 2016;7(DEC) doi: 10.3389/fimmu.2016.00651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hill C, Guarner F, Reid G, et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 88.Galland L. The gut microbiome and the brain. J Med Food. 2014;17(12):1261–1272. doi: 10.1089/jmf.2014.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matejovic M, Radermacher P, Fontaine E. Lactate in shock: a high-octane fuel for the heart? Intensive Care Med. 2007;33(3):406–408. doi: 10.1007/s00134-006-0524-8. [DOI] [PubMed] [Google Scholar]
- 90.Leverve X, Mustafa I, Novak I, et al. Lactate metabolism in acute uremia. J Ren Nutr. 2005;15(1):58–62. doi: 10.1053/j.jrn.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 91.van Gemert LA, de Galan BE, Wevers RA, ter Heine R, Willemsen MA. Lactate infusion as therapeutical intervention: a scoping review. Eur J Pediatr. 2022;181:2227–2235. doi: 10.1007/s00431-022-04446-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Koňaříková E, Marković A, Korandová Z, Houštěk J, Mráček T. Current progress in the therapeutic options for mitochondrial disorders. Physiol Res. 2020;69(6):967–994. doi: 10.33549/physiolres.934529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chow LS, Gerszten RE, Taylor JM, et al. Exerkines in health, resilience and disease. Nat Rev Endocrinol. 2022;18(5):273–289. doi: 10.1038/s41574-022-00641-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heo J, Noble EE, Call JA. The role of exerkines on brain mitochondria: a mini-review. J Appl Physiol. 2023;134(1):28–35. doi: 10.1152/japplphysiol.00565.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Severinsen MCK, Pedersen BK. Muscle-organ crosstalk: the emerging roles of myokines. Endocr Rev. 2020;41(4):594–609. doi: 10.1210/endrev/bnaa016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takahashi H, Alves CRR, Stanford KI, et al. TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat Metab. 2019;1(2):291–303. doi: 10.1038/s42255-018-0030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]