Abstract
A 20-year-old man with metastatic large cell neuroendocrine carcinoma of the lung was treated with the delta-like ligand 3–targeting bispecific T cell engager, tarlatamab. Treatment was complicated by transient cytokine release syndrome but resulted in a partial response. Bispecific T cell engagers may offer a novel treatment approach for large cell neuroendocrine carcinoma of the lung.
Keywords: Tarlatamab, Large cell neuroendocrine carcinoma, DLL3, Bispecific T-cell engager, Cytokine release syndrome, Case report
Introduction
Large cell neuroendocrine carcinoma (LCNEC) is a rare subtype of lung cancer with limited response to chemotherapy. The advent of bispecific T cell engagers (BiTEs) has offered the opportunity to use native T cells to engage cell surface cancer targets with an off-the-shelf therapeutic. Tarlatamab is a BiTE against delta-like ligand 3 (DLL3) with promising activity against SCLC.1 DLL3 expression is also found in LCNEC.2, 3, 4 Here, we present the case of a young man with metastatic LCNEC of the lung with response to tarlatamab.
Case Presentation
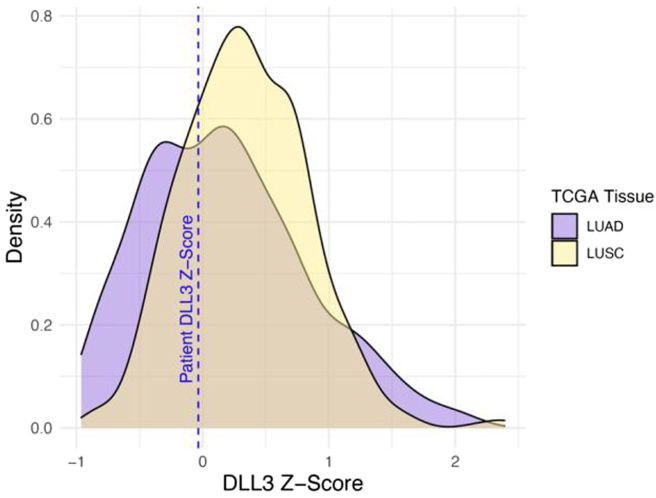
A 20-year-old man without smoking history or other toxic exposures presented to the emergency department with 1 month of progressive and severe back spasms and abdominal pain. Computed tomography imaging (Fig. 1A) revealed large right hilar mass (5.2 × 4.7 × 7.7 cm) with rim-enhancing hypodensities in the liver and lytic lesions in T2, T6, and L1. Brain imaging and scrotal ultrasound results were within normal limits. A core liver biopsy evaluation revealed positive results for transcription termination factor 1, synaptophysin, and chromogranin, negative results for p53 and retinoblastoma, and LCNEC with a Ki-67 of 95%. The case was reviewed by a second experienced lung pathologist who concurred with the diagnosis. Targeted DNA (Tempus xT 648 gene panel) and whole transcriptomic RNA next-generation sequencing (Tempus xR) did not reveal actionable genomic alterations. MDM2 and MYCL copy number gain and RB1 and TBL1XR1 copy number loss were noted on genomic profiling with a tumor mutational burden of 1.6 mutations per megabase. Transcriptomic analysis revealed DLL3 expression (Fig. 2). Furthermore, z-scores are used to compare expression levels between samples from RNA sequencing data. They represent the number of SDs that a gene's RNA expression level for a given sample differs from the mean of all other genes within that sample. In this case, the z-score for DLL3 expression in the patient's sample was 0, indicating DLL3 RNA expression was close to the average RNA expression of all the sample's genes. The patient’s DLL3 z-score was similar to that of patients with lung adenocarcinoma and squamous cell carcinoma in the Cancer Genome Atlas. SCLC is not included in the Cancer Genome Atlas.
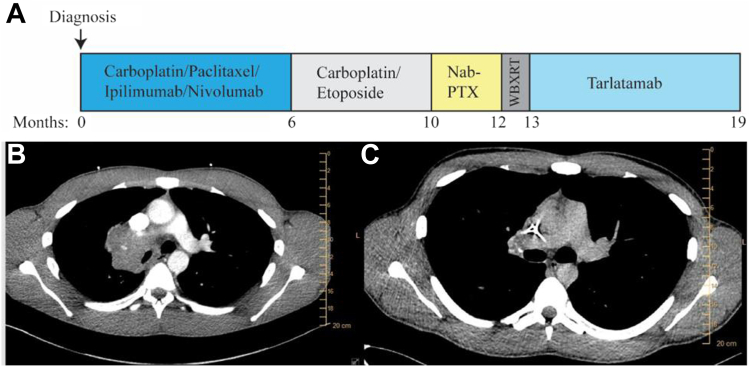
Figure 1.
(A) Timeline of treatment. (B) Baseline diagnostic imaging and (C) imaging after 2 cycles of carboplatin, paclitaxel, ipilimumab, and nivolumab.
Figure 2.
Transcriptomic analysis of DLL3 expression. DLL3, delta-like ligand 3; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; TCGA, The Cancer Genome Atlas; PTX, paclitaxel.
After discussion of multiple reasonable regimens and the patient’s values, the CheckMate 9LA regimen of carboplatin, paclitaxel, ipilimumab, and nivolumab was chosen through shared decision-making. Treatment resulted in dramatic efficacy (Fig. 1B and C), without any immune-related adverse events, but progression was found after 6 months. He was subsequently treated with carboplatin and etoposide. As this was second-line therapy, etoposide was continued after 4 cycles of doublet therapy, but only two “maintenance” cycles were given due to treatment interruption for nonmedical reasons, followed by progression, providing a total of 4 months of disease control. Subsequently, nab-paclitaxel was given with best response of progression after two cycles. This progression included numerous brain metastases, treated with whole-brain radiotherapy. The patient requested next-line therapy.
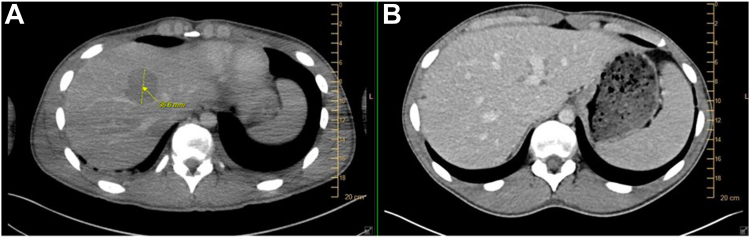
Given his cancer biologically and clinically behaved much like SCLC, and because it was known (at least at an RNA level) to express DLL3 (Fig. 2), compassionate use on a single-patient protocol was pursued, and he was the first patient to have one granted. Cycle 1, day 1 was administered inpatient and resulted in grade 3 cytokine release syndrome (CRS) requiring medical intensive care unit admission, vasopressors, dexamethasone, and tocilizumab. Day 8 was administered inpatient with preceding prophylactic tocilizumab and resulted in grade 2 CRS, which quickly resolved after the administration of tocilizumab and dexamethasone. It is important to note that this degree of CRS is uncommon with tarlatamab. In a phase 2 study in extensive-stage SCLC, grade 3 CRS occurred in only 1% of patients.1 Of note, our patient was far younger than those represented in that study. Day 15 and subsequent infusions were administered outpatient without complications. Tarlatamab resulted in partial response (Fig. 3A and B), which lasted 6 months before both systemic and intracranial progression. The patient was subsequently treated with temozolomide (2 mo) and carboplatin/irinotecan (2 mo) without response and is actively pursuing early-phase clinical trials.
Figure 3.
Imaging (A) before and (B) after tarlatamab.
Discussion
LCNEC of the lung is a rare subtype with poor prognosis and limited response to chemotherapy. Recent genomic profiling studies of LCNEC revealed SCLC-like subtypes with DLL3 expression in a large fraction of tumors.2, 3, 4 Here, we present the first case of a patient with metastatic LCNEC treated with the DLL3 T cell engager, tarlatamab, having treatment response. Treatment with tarlatamab was well tolerated, with longer duration of disease control than the preceding two lines of chemotherapy indicating this could be a promising treatment strategy for LCNEC. Tarlatamab was recently approved for relapsed extensive-stage SCLC. In addition to several SCLC studies, it is being evaluated for neuroendocrine prostate cancer (NCT04702737), and further study for neuroendocrine non-SCLC is indicated. Additional DLL3/CD3 BiTE compounds are being investigated in neuroendocrine cancers, and early reports have revealed activity in LCNEC of the lung (NCT04429087), although these data were not available at the time of our request for compassionate use of tarlatamab.5
The optimal biomarker for selecting patients for treatment with tarlatamab is not yet known. In extensive-stage SCLC, responses were found regardless of DLL3 expression.1 Our patient did not have residual tissue to evaluate DLL3 expression by immunohistochemistry and declined additional tissue biopsies at progression. As no standard options existed, transcriptomic analysis from standard-of-care sequencing was used to hypothesize which clinical trial or compassionate use treatment to pursue. Larger studies evaluating DLL3 RNA expression would be needed to establish its utility as a biomarker for selecting patients for BiTE therapy, but it could be a promising strategy as RNA sequencing is more broadly performed clinically. This patient also experienced grade 3 CRS, which is uncommon with tarlatamab. Prophylactic use of tocilizumab decreased the severity and duration of CRS with day 8 step-dosing. This approach has been explored with BiTEs for multiple myeloma with high rates of CRS and could provide a strategy to shorten hospitalization times or safely administer these agents in the outpatient setting.6
Conclusion
Here, we report the first case of LCNEC of the lung treated with tarlatamab. The patient achieved partial response, with longer disease control on tarlatamab than previous or subsequent lines of chemotherapy. Targeting DLL3 in NSCLC with neuroendocrine differentiation and optimal biomarkers for patient selection should be studied further.
CRediT Authorship Contribution Statement
Shetal A. Patel: Conceptualization, Writing-Original draft.
Young Whang: Writing-review and editing.
Chaely Medley: Writing-review and editing.
Kevin Chen: Writing-review and editing.
Jasmine Jordan: Investigation, Writing-review and editing.
Dante Bortone: Investigation, Writing-review and editing.
Benjamin Vincent: Investigation, Writing-review and editing.
Jared Weiss: Conceptualization, Investigation, Writing-Original draft, Writing-review and editing.
Disclosure
Dr. Patel has received research funding (to institution) from Amgen. Dr. Weiss has received consulting fees from Amgen. Amgen provided drug-only support for the compassionate-use protocol described. The remaining authors declare no conflict of interest.
Acknowledgments
The authors thank the patient for providing consent for this case report.
Footnotes
Cite this article as: Patel SA, Whang Y, Medley C, et al. Tarlatamab for large cell neuroendocrine carcinoma in a young adult: a case report. JTO Clin Res Rep. 2024;5:100712.
References
- 1.Ahn M.J., Cho B.C., Felip E., et al. Tarlatamab for patients with previously treated small-cell lung cancer. N Engl J Med. 2023;389:2063–2075. doi: 10.1056/NEJMoa2307980. [DOI] [PubMed] [Google Scholar]
- 2.Alì G., Di Stefano I., Poma A.M., et al. Prevalence of delta-like protein 3 in a consecutive series of surgically resected lung neuroendocrine neoplasms. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.729765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hermans B.C.M., Derks J.L., Thunnissen E., et al. DLL3 expression in large cell neuroendocrine carcinoma (LCNEC) and association with molecular subtypes and neuroendocrine profile. Lung Cancer. 2019;138:102–108. doi: 10.1016/j.lungcan.2019.10.010. [DOI] [PubMed] [Google Scholar]
- 4.George J., Walter V., Peifer M., et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nat Commun. 2018;9:1048. doi: 10.1038/s41467-018-03099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wermke M., Felip E., Gambardella V., et al. Phase I trial of the DLL3/CD3 bispecific T-cell engager BI 764532 in DLL3-positive small-cell lung cancer and neuroendocrine carcinomas. Future Oncol. 2022;18:2639–2649. doi: 10.2217/fon-2022-0196. [DOI] [PubMed] [Google Scholar]
- 6.Scott S.A., Marin E.M., Maples K.T., et al. Prophylactic tocilizumab to prevent cytokine release syndrome (CRS) with teclistamab: a single-center experience. Blood Cancer J. 2023;13:191. doi: 10.1038/s41408-023-00963-y. [DOI] [PMC free article] [PubMed] [Google Scholar]