Highlights
-
•
Nipah virus (NiV) poses persistent threats to global health due to its high fatality rate.
-
•
A total of 754 human NiV cases with 435 deaths (fatality rate: 58%) were reported globally.
-
•
Bangladesh recorded the highest incidences (341 cases and 241 deaths; fatality rate: 71%).
-
•
Strong global understandings are highly required to minimize future threats of NiV.
Keywords: Nipah outbreaks, Southeast Asia, High fatality, Global understanding, Active surveillance, Vaccine and therapeutics
Abstract
Objectives
Nipah virus (NiV), a bat-borne zoonotic pathogen, poses persistent threats to global public health due to severe clinical manifestation and high case fatality rate (CFR). A critical examination of NiV outbreaks is essential for refining strategies and mitigating the impact of future infections. In this study, we provide a concise update on global NiV outbreaks that occurred during the past 25 years.
Methods
In this geospatial study, we conducted an in-depth examination of the epidemiological characteristics of human NiV cases and deaths from 1998 to 2024 through multiple analyses of public data and official reports.
Results
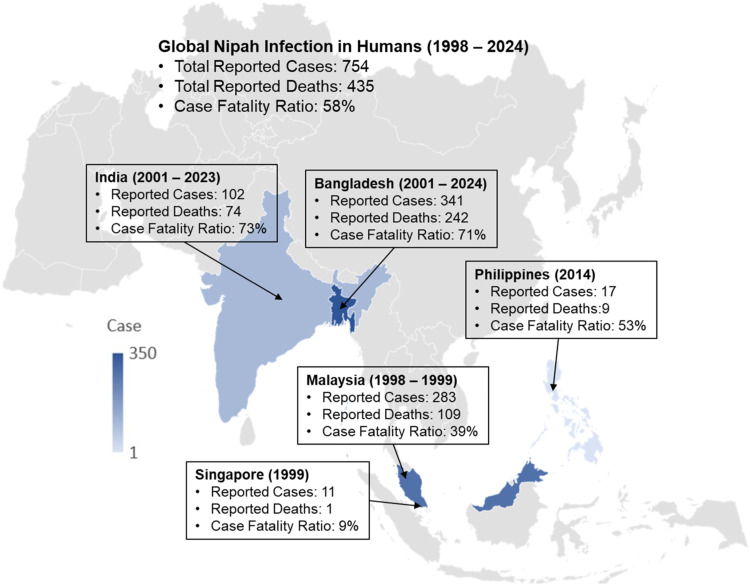
NiV emerged in 1998 in Malaysia during an outbreak among pig farmers. Since then, NiV outbreaks have been documented in five countries of South and Southeast Asia (Bangladesh, India, Malaysia, Philippines, and Singapore). As of May 2024, there have been 754 confirmed human NiV cases with 435 deaths (CFR: 58%) reported in these five countries. Bangladesh records the highest incidence (341 cases and 241 deaths; CFR: 71%) followed by Malaysia (283 cases and 109 deaths; CFR: 39%), India (102 cases and 74 deaths; CFR: 73%), the Philippines (17 cases and nine deaths; CFR: 53%), and Singapore (11 cases and one death; CFR: 9%).
Conclusions
The clinical outcomes of NiV have been underscoring constant global public health threats as no effective therapies and vaccines are available. Strong global understandings, with an eye on developing vaccines and therapeutics, are required to minimize clinical outcomes and future threats of NiV.
Introduction
Nipah virus (NiV), a member of Henipavirus, emerged as a deadly zoonotic pathogen during the past 25 years. Originating from the Pteropodidae family of fruit bats, NiV presents a persistent and alarming health threat due to its severe respiratory and neurological manifestations, potential for human-to-human transmission, and devastatingly high mortality rates [1,2]. The first known outbreak of NiV occurred in Malaysia in 1998 followed by Singapore in 1999. Since then, Southeast and South Asia has witnessed sporadic outbreaks of NiV infection, mainly in Bangladesh, India, and the Philippines. The NiV outbreaks have predominantly involved individuals who came into close contact with infected intermediate hosts or consumed contaminated date palm sap, which could be infected by bat urine or saliva. The case fatality rate (CFR) of NiV infection has ranged from less than 10% to as high as 100%, depending on the specific outbreak and the healthcare infrastructure available for managing cases [[2], [3], [4]]. The epidemiological characteristics of NiV in Bangladesh and India differ from those in Malaysia and Singapore, showing notable variations in CFR and transmission patterns. In Bangladesh and India, NiV infections have a high fatality rate and are not linked to intermediate hosts. In the Philippines, the NiV outbreak is also associated with an intermediate host. In fact, infected people have been initially exposed to horses having acute encephalitis that were butchered and consumed by the people who later confirmed NiV infection [5]. While human NiV cases have been reported to date only in Asia, countries in other regions are at risk wherever there are susceptible animals, the presence of the virus, and a pathway for transmission. With complex transmission dynamics (bats-intermediate hosts-humans) and challenges in prevention and control due to a lack of therapeutics and vaccines, NiV demands increased attention to regularly examine the infection status to improve outbreak predictions and future challenges. This article provides a concise and compelling update on global human NiV outbreaks during the past 25 years. We also highlight the possible threats of NiV and discuss proactive measures to minimize larger and deadlier outbreaks in the future.
Materials and methods
In this geospatial study, we compiled the global human NiV outbreak data. We conducted an in-depth examination of the epidemiological characteristics of NiV infection through a literature review and multiple analyses of public data and official reports. In this regard, we established a multisource database of human NiV outbreaks. This database comprises information obtained through systematic searches of databases: PubMed, Web of Science, and Google Scholar. Additionally, our database included information from gray literature from national and international (World Health Organization, the Institute of Epidemiology, Disease Control and Research [IEDCR] of Bangladesh, and the National Centre for Disease Control of India). We selected the best-matched databases and reported human NiV cases and deaths from 1998 to 2024 were retrieved [1,[4], [5], [6], [7], [8], [9]]. The database contains updated epidemiological information essential for understanding NiV cases and deaths recorded in five affected countries (Bangladesh, India, Malaysia, Philippines, and Singapore). After data extraction, at least two authors cross-checked the data to ensure consistency. Mapping and time-series analyses were conducted using Microsoft® Excel and SAS (version 9.4; Cary, North Carolina, USA). This study did not require ethical clearance as we used only secondary aggregate data that does not allow the identification of individual cases.
Results
NiV emerged in Malaysia during an outbreak among pig farmers in 1998. Since then, NiV outbreaks in humans have been documented in several South and Southeast Asian countries. An updated country-wise global distribution of NiV outbreaks in humans is shown in Figure 1. As of May 2024, there have been 754 confirmed human NiV cases with 435 deaths (global average CFR: 58%) being reported in five countries (Bangladesh, India, Malaysia, Philippines, and Singapore). In the outbreaks from 1998 to 1999 in Malaysia and Singapore, a total of 294 human cases with 110 deaths were reported (average CFR: 39%); most of the cases (n = 283) and deaths (n = 109) accounted for Malaysia (Figure 1), with most of these people having had close contact with NiV-infected pigs. Since then, no other NiV outbreaks have been reported in these two countries, but sporadic outbreaks were reported in Bangladesh, India, and the Philippines. The NiV outbreaks in the Philippines have been recorded in 2014 when nine of the 17 confirmed NiV patients died (CFR: 53%) due to exposure of infected horses (Figure 1).
Figure 1.
Geographical distribution of Nipah virus outbreaks in humans during 1998-2024. Data are as of May 31, 2024.
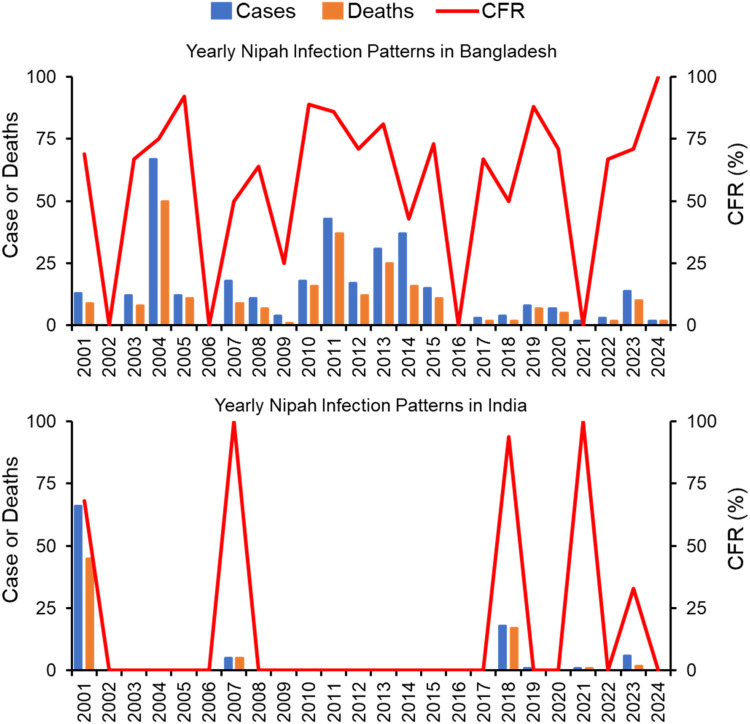
The first human NiV cases were reported in Bangladesh and India in 2001, and the outbreaks continued to flare up in these two countries (Figure 2). Till now consumption of raw date palm sap and half-eaten fruits contaminated with urine or saliva from infected fruit bats are thought to be the primary sources of NiV infection in Bangladesh, where outbreaks are detected almost every year. As of May 2024, Bangladesh recorded the world's highest number of human NiV cases (45% of total global cases) and deaths (56% of total global deaths) (Figure 1). Since 2001, Bangladesh recorded a total of 341 cases with 242 deaths (CFR: 71%) in 34 of the country's 64 districts. During the same period, India recorded 102 cases with 74 deaths (CFR: 73%) in West Bengal and Kerala states.
Figure 2.
Epidemiological patterns of human Nipah virus cases, deaths, and CFR in Bangladesh and India during 2001-2024. Data are as of May 31, 2024. CFR, case fatality rate.
NiV surveillance in both Bangladesh and India is crucial due to the recurring risk of outbreaks in these regions. In Bangladesh, surveillance efforts focus on detecting human cases, especially in areas where raw date palm sap consumption is common, and involve rigorous testing, contact tracing, and public education to prevent transmission. In India, particularly in states like Kerala, surveillance includes early detection, isolation of cases, and extensive monitoring of contacts, along with public awareness campaigns and bolstered healthcare infrastructure to manage potential outbreaks. Despite the increased surveillance and public awareness programs, outbreaks have worsened in recent years in these two countries. India has experienced four out of a total of six outbreaks during 2018-2023. Bangladesh reported the highest NiV cases and deaths in 7 years in 2023 and the country's highest NiV-related mortality rate (CFR: 100%, two cases with two deaths) in 2024. Overall, a total of 311 cases with 119 deaths (CFR: 38%) were reported in Southeast Asia (Malaysia, Singapore, and the Philippines), while 443 cases with 316 deaths (CFR: 71%) were reported in South Asia (Bangladesh and India) during the last 25 years.
Discussion
The epidemiological characteristics of NiV outbreaks in Bangladesh and India differ from those in Malaysia, Singapore, and the Philippines, with notable variations observed in fatality rates and transmission patterns. In Bangladesh and India, NiV infections have high fatality rates and are not associated with known intermediate hosts [1,2,5,9]. The different fatality rates between Southeast and South Asian countries can be attributed to genetic variations of NiV, which may affect their pathogenicity and virulence. Two clades of NiV that have >92% genetic identity and amino acid homology (clade I: NiV-B responsible for outbreaks in Bangladesh and India; clade II: NiV-M responsible for outbreaks in Malaysia, Singapore, and the Philippines) are thought to be one of the causes associated with different fatality rates observed in South and Southeast Asia [3,8]. Additionally, the transmission routes differ between these two regions. In Malaysia, Singapore, and the Philippines, the NiV primarily spreads from pigs or horses to humans, while in Bangladesh, it is often transmitted through the consumption of contaminated date palm sap or half-eaten fruits. Also, human-to-human transmission and vertical transmission have been found in Bangladesh. Differences in healthcare infrastructure and public health responses between these countries may also play a crucial role in the variations in fatality rates. Indeed, environmental and sociocultural factors may also influence the epidemiology and impact of NiV outbreaks in the Southeast and South Asian regions.
Although NiV has demonstrated limited sustained in human-to-human transmission compared to some respiratory pathogens such as influenza or coronaviruses, its potential for adaptation and evolution cannot be underestimated. NiV exhibits genetic diversity and the ability to undergo genetic reassortment which may lead to novel strains with enhanced transmissibility and virulence. One of the primary concerns is the possibility of reassortment between NiV strains and other bat-borne viruses that share genetic and antigenic similarities with NiV [3,8,10]. We could not exclude such possibilities as the COVID-19 pandemic showed that virus strains could emerge that are far more transmissible and virulent than their genetically related predecessors. Along with reassortment ability, some other factors suggest that NiV can potentially cause larger outbreaks or even a pandemic. Firstly, a broad geographical distribution of NiV throughout Asia, the Pacific Islands, Australia, and Africa, which is the home to more than half of the world's population. Anti-NiV or cross-reacting anti-NiV antibodies have been detected in fruit bats from Bangladesh, Cambodia, China, Indonesia, India, Madagascar, Malaysia, New Caledonia, Papua New Guinea, Thailand, and Vietnam [3,8,11]. Also, NiV RNA has been detected in bats in Bangladesh, India, Thailand, and Timor-Leste. Moreover, bat Henipavirus RNA, a species of the Henipavirus genus, is identified in Ghana [8]. Such wide distribution raises concerns about the potential for NiV to disseminate beyond its current endemic region. Also, current trends in climate change and disruption of natural ecosystems due to changes in land use, deforestation, urbanization, and altered agricultural practices in these regions exacerbate the additional risk of increased spillover events due to increased interactions between NiV-carrying bats and humans. As NiV can infect ranges of mammalian species [1,4,5], cross-species transmission events, particularly from bats to intermediate hosts and then to humans, pose an additional threat to larger NiV outbreaks. Indeed, NiV can be transmitted through respiratory droplets [11], enhancing its potential for rapid spread of infection outside its current endemic region. Under such conditions, the lack of specific treatments or vaccines targeting NiV led to additional challenges to control larger outbreaks as asymptomatic and mild cases of NiV can also occur [12,13]. The convergence of these factors provides ample reasons to believe that NiV has the potential to cause larger and deadlier outbreaks in many parts of the world and pose persistent global health threats.
NiV, with its unpredictable emergence in different parts of the world, necessitates comprehensive global approaches. This involves integrating surveillance, public health measures, medical interventions, research, and community engagement. Enhanced surveillance systems in currently endemic and possible risk regions are vital for the early detection and monitoring of NiV in bat populations, intermediate hosts, and humans. In this regard, the international community needs strengthened coordination to implement a “One World, One Health” approach. Applying this approach requires strong national and international collaborations between human health, animal health, and environmental sectors to effectively prevent, detect, and respond to deadly NiV. It is necessary to take evidence-based scientific programs to monitor the genetic changes in NiV regularly and explore the cellular and molecular mechanisms in survivors and fatal cases. Fostering national and international collaborations to develop vaccines and therapeutics and strengthen healthcare systems with high biosafety level laboratories in endemic and possible risk countries are pivotal for preventing and managing NiV outbreaks.
This study has several limitations. We rely on data from national and international NiV surveillance systems. Current approaches for disease surveillance could fail to identify mild or asymptomatic NiV infections in affected areas, particularly in resource-constrained countries due to limited surveillance efforts and inadequate diagnostic methods. Therefore, such data are often limited by under-reporting, misreporting, and delayed reporting, leading to incomplete and inaccurate information. However, the results presented here provide strong evidence that NiV infections occur almost every year in South Asia with very high CFR (>70%). Such high fatality rates, coupled with its complex epidemiology, zoonotic threat, person-to-person transmission, global connectivity, and lack of therapeutics and vaccines, make it a formidable global public health challenge. Addressing this challenge, only a robust global understanding and cumulative efforts can minimize the clinical outcomes from NiV and reduce future threats of larger and more deadly outbreaks.
Declarations of competing interest
The authors have no competing interests to declare.
Acknowledgments
Funding
This work is supported in part by the Japan Society for the Promotion of Science (No. 24K13414).
Acknowledgments
None.
Author contributions
S.K. planned and compiled the manuscript. S.M.F.A., M.A.M., M.N.U, T.S., M.M.R, T.Y., K.K., T.H., and A. N. provided support with data and manuscript editing. All authors discussed and approved the final version of the manuscript.
Data sharing statement
The data that supports this paper is available upon reasonable request from the corresponding author [S.K.].
References
- 1.Chua KB, Bellini WJ, Rota PA, Harcourt BH, Tamin A, Lam SK, et al. Nipah virus: a recently emergent deadly paramyxovirus. Science. 2000;288:1432–1435. doi: 10.1126/science.288.5470.1432. [DOI] [PubMed] [Google Scholar]
- 2.Gurley ES, Montgomery JM, Hossain MJ, Bell M, Azad AK, Islam MR, et al. Person-to-person transmission of Nipah virus in a Bangladeshi community. Emerg Infect Dis. 2007;13:1031–1037. doi: 10.3201/eid1307.061128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cortes-Azuero O, Lefrancq N, Nikolay B, McKee C, Cappelle J, Hul V, et al. The genetic diversity of Nipah virus across spatial scales. J Infect Dis. 2024 doi: 10.1093/infdis/jiae221. [DOI] [PubMed] [Google Scholar]
- 4.Chua KB. Epidemiology, surveillance and control of Nipah virus infections in Malaysia. Malays J Pathol. 2010;32:69–73. [PubMed] [Google Scholar]
- 5.Ching PKG, de los Reyes VC, Sucaldito MN, Tayag E, Columna-Vingno AB, Malbas FF, Jr, et al. Outbreak of henipavirus infection, Philippines, 2014. Emerg Infect Dis. 2015;21:328–331. doi: 10.3201/eid2102.141433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Nipah virus infection. Bangladesh, https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON508; 2024 [accessed 31 May 2024].
- 7.World Health Organization. Nipah virus infection. India, https://www.who.int/emergencies/disease-outbreak-news/item/2023-DON490; 2024 [accessed 31 May 2024].
- 8.European Centre for Disease Prevention and Control. Factsheet on Nipah virus disease, https://www.ecdc.europa.eu/en/infectious-disease-topics/nipah-virus-disease/factsheet-nipah-virus-disease; 2023 [accessed 31 May 2024].
- 9.Satter SM, Aquib WR, Sultana S, Sharif AR, Nazneen A, Alam MR, et al. Tackling a global epidemic threat: Nipah surveillance in Bangladesh, 2006–2021. PLoS Negl Trop Dis. 2023;17 doi: 10.1371/journal.pntd.0011617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halpin K, Young PL, Field HE, Mackenzie JS. Isolation of Hendra virus from pteropid bats: a natural reservoir of Hendra virus. J Gen Virol. 2000;81:1927–1932. doi: 10.1099/0022-1317-81-8-1927. [DOI] [PubMed] [Google Scholar]
- 11.Clayton BA, Middleton D, Bergfeld J, Haining J, Arkinstall R, Wang L, et al. Transmission routes for Nipah virus from Malaysia and Bangladesh. Emerg Infect Dis. 2012;18:1983–1993. doi: 10.3201/eid1812.120875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chadha MS, Comer JA, Lowe L, Rota PA, Rollin PE, Bellini WJ, et al. Nipah virus-associated encephalitis outbreak, Siliguri, India. Emerg Infect Dis. 2006;12:235–240. doi: 10.3201/eid1202.051247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar CPG, Sugunan AP, Yadav P, Kurup KK, Aarathee R, Manickam P, et al. Infections among contacts of patients with Nipah virus. India. Emerg Infect Dis. 2019;25:1007–1010. doi: 10.3201/eid2505.181352. [DOI] [PMC free article] [PubMed] [Google Scholar]