1. Introduction
Health system performance improvement has been a policy priority, particularly where healthcare funding comes predominantly from public/government sources. However, measuring and monitoring performance at the health system-level is challenging [[1], [2]], [[1], [2]] partly because attributing healthcare systems' contributions to health outcomes is difficult. Both socioeconomic factors and health system settings influence health outcomes through complex and multi-layered processes [3].
Amenable mortality (AM) is one of the most common measures used to compare healthcare quality and health systems' effectiveness across the countries. AM is defined as the potentially avoidable deaths (aged ≤75 years in general but may vary) that could be averted by the effective interventions and timely utilization of good-quality healthcare services. The definitions acknowledge that all deaths are not avoidable because of the potential effects of co-morbidity, frailty, and patient preference. However, a higher rate of such deaths indicates a poor health system performance [4]. The latest versions of the mortality conditions considered amenable include the diseases preventable by public health interventions [5,6].
Despite the limited and mixed evidence base [7], particularly around the validity of AM as a performance measure in capturing the contributions of timely access and quality of health care (health system performance) at sub-national levels [8], the use of AM has been extended to compare not only the geographic variations within a country [[8], [9], [10], [11], [12]] and that across the sub-population groups including ethnicity [11,13], income quintile [9], socioeconomic status [3,11,14], and occupational group [15], but also to assess the impacts of health policy reforms [16,17]. Between 2016 and 2021, New Zealand (NZ) used AM as one of the six headline health system performance measures [18,19].
The study aims to understand the extent to which the district-level variations in amenable mortality in NZ could be explained in terms of health system factors using a population dataset from 2008 to 2018. We limited our analysis to the pre-COVID 19 era.
2. Methods
We conducted a population-based cross-sectional study for the amenable deaths registered in 20 District Health Boards (DHB) within NZ from 2008 to 2018.
Between 2001 and 2022, the healthcare delivery system in New Zealand was highly decentralized. At the time of this study, 20 DHBs represented the district-level administrative units of the NZ health systems responsible for planning and funding overall services in the country. Under this system, the DHBs directly provided hospital services and local public health monitoring. Primary Health Organizations (PHOs), and other private and non-governmental organizations provided community-based health services, including primary health care [20].
The geography and size/catchment areas of the DHBs varied, and the population (and its composition) has changed over the years. Waitematā DHB featured the largest population (615,100, 12.6 %), with three DHBs - Counties Manukau, Canterbury, and Auckland, each having >10 % of the total estimated resident population in 2018. West Coast DHB had the smallest population (32,400, 0.7 %), and four DHBs each had less than 1.5 % of the total population: Whanganui (1.4 %), South Canterbury (1.2 %), Tairāwhiti(1.0 %) and Wairarapa (1.0 %) [21]. In 2022, the DHBs were merged into a single organisation (Te Whatu Ora - Health New Zealand) [22].
2.1. Data sources
We obtained anonymized, individual-level customized datasets from the National Collections division of the Ministry of Health (MOH). The Mortality Collection database, a specified collection of the mortality data that classifies all deaths registered in NZ with their underlying cause of death (COD), provided the outcome data (AM) [23]. The COD information comes from different sources, including patient records, death certificates, police reports, and hospital discharge summaries. Because of the two-year reporting delays in updating the COD, 2018 was the latest calendar year for which mortality data was available for public use at the time of data collection for this study [24].
Socio-demographic data (age, sex, and self-reported ethnicity) for the study population were collected from the Primary Health Organization (PHO) Enrollment Collection, a nationwide collection of patients enrolled with the primary care providers reported quarterly and available since 2005 [25]. Details of the study variables and data sources are provided in supplementary file 1.
2.2. Measurement
Amenable deaths include all deaths registered in the NZ Mortality Collection dataset for those aged below 75 years at the time of death, where the primary cause of death code is listed in MOH's amenable mortality code lists (supplementary file 2). We followed the ICD-10-Australia Modification classification codes. The age cut-off recommended for NZ is 0–74 years at the death date, although some exceptions apply within this range. The MOH uses the lists of clinical codes developed by Martin Tobias [24] which are updated every five years. We used the 2012 definition for the dataset 2008–2009 years and the 2016 definition for the 2010–2018 years. The changes were minimal with the latter definition incorporating deaths due to hepatitis C virus, uterine cancer, and atrial fibrillation and flutter.
The core criteria for defining amenability of the conditions considered by the MOH's definition comprise the availability of medical or surgical interventions introduced after 1960 and delivered by a doctor or nurse in a healthcare setting, including home. Although screening, diagnosis, and rehabilitation interventions are also included, public health interventions such as food safety and tobacco taxes are excluded. The interventions include only those with evidence of effectiveness (reduced under 75 mortality by >30 %), demonstrated effective by randomized controlled trial, or potential to reduce mortality with a five-year universal coverage period indicated by the observational studies.
The lists also exclude the causes that account for <0.1 % of all under-75 deaths, irrespective of any other inclusion criteria [24]. We also excluded the cases with missing information in the dataset for either age, sex, ethnicity, deprivation, and domicile code, the core variables used in the analysis.
2.3. Data analysis
We identified eligible deaths (AM) from the mortality dataset from 2008 to 2018 using the MOH definition. Descriptive analysis was conducted for amenable deaths over the years and across the DHBs. The raw figure increased from 4,169 in 2008 to 4,746 in 2018 but the proportion of the total all-causes deaths for those aged <75 decreased from 46.9 % to 45.8 % over that period.
The AM records were then merged with a population dataset for all the PHO enrolled population aged 0–74 years, separately for each calendar year.
We merged the numerator and denominator population grouped by six variables: Year (2008–2018), Domicile-codes, Sex (male and female), Age groups (0–74, 5 years interval with 0–4, 5–9, ….,70–74), and Prioritized ethnicity [26] (Māori, Pacific Peoples and Non-Māori Non-Pacific - NMNP) groups as labeled in the respective datasets.
Consistent with previous population-based research in NZ [27], cases with no record for the 'AM' variable in the specific group combination in the merged file were categorized as ‘no amenable deaths’ (they were either alive or may have died from other non-amenable causes) in the respective year. The number of AM cases (numerator) and the denominator population in the merged dataset range from 4,164 and 3,550,611 in 2008 to 4,746 and 4,312,005 in 2018 respectively. Overall, 95.6 % of the amenable mortality cases merged to the denominator population. This means the remaining 4.4 % amenable mortality cases had no record in the PHO Enrolment Collection (denominator) dataset in the respective years. The final dataset allowed us to conduct population-based cross-sectional analyses.
All predictor and grouping variables were checked for their distribution by the outcome variable. With DHB-wise variation as the primary focus of the analysis, we also explored the distribution of the predictor variables across DHBs. Index of Multiple Deprivation (IMD) and Access variables in some of the DHBs had no amenable death data (observations) in deciles 1, 2, and 3 (least deprivation). Because of this, we re-coded the deciles into three broader bands for analysis.
When predictor variables representing the same aspect (e.g., NZDep and IMD as measures of area-level deprivation) were highly correlated, only one predictor with a stronger relationship with the outcome variable was retained, as agreed by the study team. For example, since NZDep2013 and IMD were strongly correlated (correlation coefficient = 0.83, p < 0.001) and both measured relative area-level socioeconomic deprivation, IMD was chosen for further analysis. The New Zealand Index of Deprivation (NZDep) is the most common measure of socio-economic deprivation in NZ. However, it is derived from the census variable and is updated only every five years. Given that the IMD variable is more comprehensive and incorporates the effects of access factors [28], we preferred IMD over NZDep.
The dataset with the General Practitioners (GPs) variable that has data for only up to 2016 was analyzed separately. Auckland DHB, which features a good mix of the population characteristics for most variables, is used as a reference category for the geographic variation analyses. All variables, including the interaction terms in the final model, demonstrated significant effects (p < 0.05) except ‘Rurality’ and ‘GPs’.
The dataset structure in this study was hierarchical, with health outcomes and demographic variables measured at the individual level, socio-economic status (deprivation) measured at the domicile level, and finance and human resource variables measured at the DHB level. Initially, a mixed effects regression analysis (a hierarchical random intercept model) with DHB as a random effect variable and the rest of the predictors as fixed effect variables was conducted. Box 1 below summarizes the three primary models analyzed. The lme4 package in R was used to analysis the data [29]. Technical details of the data analysis is provided in supplementary file 3.
Box 1.
| Models | Structure | Variables | Note |
|---|---|---|---|
| model 1 | multi-level random intercept model (logistic regression) |
Random effect variable: DHB Fixed effect variables: age, ethnicity, sex, deprivation, rurality, finance, and year window (stepwise) |
all model terms had Pr(>Chisq) value < 0.001 |
| model 2 | fixed effect multiple logistic regression model | age, DHB, ethnicity, sex, deprivation, rurality, year window, and finance (orderly) | all model terms had Pr(>Chisq) value < 0.001 |
| model 3 | fixed effect multiple logistic regression model with interaction | model 2 variables plus DHB-year interaction term | All model terms had Pr(>Chisq) value < 0.001, and all Variance Inflation Factors (VIF)s, including those for the interaction terms, were less than five except for the finance variable (VIF = 5.06) |
Alt-text: Box 1
3. Results
The unadjusted mortality ranged from an average of 117.4 per 100,000 population in 2008 to 110.1 in 2018, and 118.9 per 100,000 population for the overall study period (2008–2018). The rates for DHBs ranged from 79.4 per 100,000 population in Waitemata DHB to 197.1 in Whanganui DHB for the same period.
Table 1 shows that DHB as a random effect variable (a cluster variable in the hierarchical random intercept regression model) estimates only 2.1 % (intra-cluster correlation coefficient, ICC = 0.021) of the variability in the chance of having amenable mortality (compared to that of not having it). When adjusted for the effects of the predictor variables (age, sex, ethnicity, deprivation, rurality, years, and annual health expenditure per capita entered as fixed-effect variables), the intra-cluster correlation coefficient of districts as a cluster variable is reduced to less than 1.0 % (ICC = 0.008).
Table 1.
Estimates of the district-level variations in amenable mortality.
| Co-variates (mixed-effect model) | Variance | Std. Dev. | ICC | MOR |
|---|---|---|---|---|
| DHB only | 0.070 | 0.265 | 0.021 | 1.29 |
| Adjusted (individual, area, and DHB level variables) – age, sex, ethnicity, deprivation, rurality, finance, and year | 0.027 | 0.165 | 0.008 | 1.17 |
Note: ICC = intra-cluster correlation coefficient; MOR = median odds ratio.
The median odds ratio estimates with the same model shows that the odds of amenable mortality increases by a factor of 1.3 when comparing a typical lower-risk district to a higher-risk district. This reduces to 1.2 when adjusted for the available predictor variables.
The estimated proportion of variation explained by the mixed-effect adjusted model as contributed by the fixed-effect variables is 61.9 %, calculated as (0.021–0.008)/0.021, the ICC values presented in Table 1. This means, after controlling for the effects of the predictor variables that are considered beyond the control of the DHBs included in the risk-adjustment models in this analysis, district-level health system characteristics (not considered in the model) potentially explain the remaining variations at the district-level health system governance units in NZ.
The fixed-effect regression model shows that the number of districts having significantly different odds compared to the reference DHB reduced to 10 of 19, ranging from an average OR of 0.72, 95 % CI (0.61–0.84) in Waitemata DHB to 2.28, 95 % CI (1.85–2.82) in West Coast DHB. Table 2 shows the adjusted odds of amenable mortality decreased by 16 % in 2016–2018 [OR = 0.84, 95 % CI (0.80–0.88)] compared to that in 2008–2009 (the reference year).
Table 2.
Amenable mortality by other co-variates.
| Variables | Unadjusted – Odds Ratio |
Adjusted – Odds Ratio |
||||
|---|---|---|---|---|---|---|
| OR | LL | UL | OR | LL | UL | |
| Year windows | ||||||
| 2008–09 | Ref | Ref | ||||
| 2010–12 | 0.9919 | 0.9656 | 1.0189 | 0.9501 | 0.9194 | 0.9818 |
| 2013–15 | 0.9761 | 0.9504 | 1.0025 | 0.8887 | 0.8531 | 0.9259 |
| 2016–18 | 0.9615 | 0.9364 | 0.9873 | 0.8395 | 0.7962 | 0.8853 |
| Ethnicity (Prioritized) | ||||||
| Non- Māori Non-Pacific | Ref | Ref | ||||
| Māori | 1.7206 | 1.6849 | 1.7570 | 2.6755 | 2.6148 | 2.7375 |
| Pacific | 1.1542 | 1.1173 | 1.1923 | 1.8608 | 1.7948 | 1.9291 |
| Deprivation (New Zealand Index of Multiple Deprivation) – 3 categories | ||||||
| IMD1 (deciles 1–4) | Ref | Ref | ||||
| IMD2 (deciles 5–6) | 1.4353 | 1.4037 | 1.4675 | 1.3458 | 1.3144 | 1.3778 |
| IMD3 (deciles 7–10) | 1.8820 | 1.8429 | 1.9219 | 1.7618 | 1.7183 | 1.8064 |
| Gender | ||||||
| Female | Ref | Ref | ||||
| Male | 1.4460 | 1.4209 | 1.4716 | 1.4799 | 1.4541 | 1.5061 |
| Age – group | ||||||
| 70–74 | Ref | Ref | ||||
| 00–10 | 0.0239 | 0.0227 | 0.0252 | 0.0173 | 0.0164 | 0.0183 |
| 10–20 | 0.0156 | 0.0146 | 0.0166 | 0.0117 | 0.0110 | 0.0125 |
| 20–30 | 0.0291 | 0.0277 | 0.0307 | 0.0234 | 0.0223 | 0.0247 |
| 30–40 | 0.0361 | 0.0345 | 0.0378 | 0.0312 | 0.0297 | 0.0327 |
| 40–50 | 0.0773 | 0.0748 | 0.0799 | 0.0693 | 0.0671 | 0.0717 |
| 50–60 | 0.1722 | 0.1676 | 0.1768 | 0.1599 | 0.1556 | 0.1642 |
| 60–70 | 0.4201 | 0.4105 | 0.4298 | 0.4104 | 0.4010 | 0.4199 |
| Urban-Rural locality | ||||||
| Non-Urban | Ref | Ref | ||||
| Urban | 0.9164 | 0.8967 | 0.9365 | 1.0176 | 0.9938 | 1.0418 |
| Finance (Annual Health Expenditure per Capita, rescaled 0–1) | ||||||
| AHE_PP* | 1.1989 | 1.1472 | 1.2528 | 1.0176 | 0.9938 | 1.0418 |
| Human Resource (Total Workforce, rescaled 0–1) | ||||||
| TWF_FTE* | 0.8721 | 0.8321 | 0.9142 | 1.3691 | 1.0820 | 1.7324 |
| Human Resource (GP, rescaled 0–1) | ||||||
| GP_FTE** | 0.9996 | 0.9621 | 1.0385 | 1.1198** | 0.9865** | 1.2712** |
OR = Odds Ratio; LL = Lower Limit (95 % confidence interval); UL = Upper Limit (95 % confidence interval).
Note: The estimates are based on the model 2, with covariates: age, DHBs, ethnicity, gender, deprivation, rurality, years, and finance (orderly); NMNP: Non-Māori Non-Pacific; IMD: New Zealand Index of Multiple Deprivation; AHE_PP: Annual Health Expenditure Per Capita rescaled (0–1); HR = Human Resources; TWF_FTEs*: Total DHB Work Force (both clinical and non-clinical) Full-Time Equivalents rescaled (0–1), entered as an alternative to AHE_PP (Variance Inflation Factor = 4.7), AHE_PP with (VIF = 3.7) is preferred in the final model.
GP_FTEs**: General Practice Full Time Equivalent rescaled (0–1), analyzed in a separate dataset (2008–2016), the adjusted OR values are based on the model (model 2 equivalent) without interaction terms. When the DHB*Year interaction term was included (model 3 equivalent), the corresponding OR value was 1.03, 95 % CI (0.85, 1.23). The equivalent Model 2 covariates were: age, DHB, ethnicity, gender, deprivation, rurality, years, and GP_FTE (orderly); The equivalent model 3 covariates: age, ethnicity, gender, deprivation, rurality, GP_FTE, and DHB*Year interaction (orderly); Finance variable not included as it correlates with GP_FTE (human resource) variable, VIF of all terms <5 reported.
Among the variables included in this analysis, ethnicity demonstrated the strongest relationship with the odds of dying from the causes considered amenable to healthcare interventions in NZ after controlling for the effects of the other covariates. The odds of amenable mortality are three times higher for a person identifying as Māori [OR = 2.68, 95 % CI (2.62–2.74)] compared to Non-Māori Non-Pacific. The odds are almost two times higher [OR = 1.86, 95 % CI (1.79–1.93)] for Pacific peoples compared to the reference group. Similarly, people living in the deprivation deciles 5/6 are 35 % more likely to die from amenable causes [OR = 1.35, 95 % CI (1.32–1.38) compared to those living in the least deprived deciles (deciles 1–4) after controlling for the effects of the other covariates.
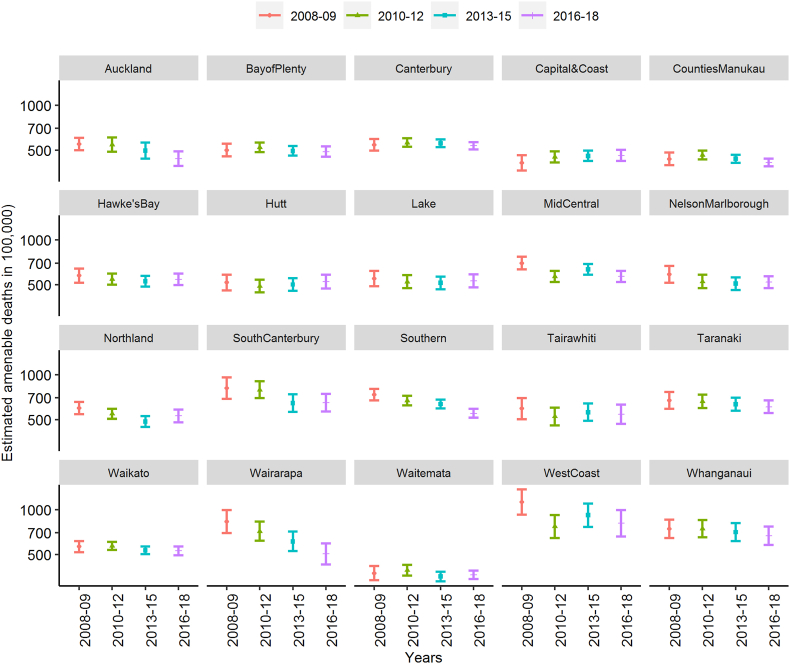
Only two DHBs (Southern DHB and Wairarapa DHB) demonstrate a continued drop each year in the adjusted risk of amenable death over the study period (Fig. 1).
Fig. 1.
Estimated amenable mortality by District Health Boards and Years
Note: The estimations are based on a model with DHB, and Year interaction terms included (model 3). The model terms were age, ethnicity, gender, deprivation, rurality, finance, and DHB-year interaction (orderly). All model terms had a Pr(>Chisq) value < 0.001 except for the rurality, and all Variance Inflation Factors, including those for the interaction terms, were less than 5. Reference group: Non-Māori Non-Pacific females aged 70–74 years living in a non-urban domicile with IMD decile 1–4 and the DHB annual expenditure per capita at its mean.
4. Discussion
In this paper, we demonstrate that only a small proportion of the district-level variations in the risk of amenable mortality could be explained in terms of the health system characteristics. With the assumption that most of the major socio-demographic, geographic, economic, and macro-level health system factors that could influence the district-level health system performance in terms of the risk of amenable mortality are included in the analysis in this study, the model explains 61.9 % variability. It means a maximum of 38.1 % variability across the DHBs could be associated with the district-level health systems' inputs - programs, activities, and initiatives.
We found a significant decline in the odds of amenable mortality in NZ, in recent years (2016–2018) compared to that in 2008–2009, although there is no consistent pattern across the years. The decline in the overall mean estimated amenable death rates (with reference to 2008/2009) is higher among Pacific Peoples and Māori than the non-Māori non-Pacific group, reducing the gaps, particularly after 2010. This could be attributed to the health system policy and program inputs over the years.
However, it is important to note that ethnicity and deprivation exhibit stronger relationships with the odds of amenable deaths, more than the geographic variations demonstrated in this analysis. A person who identifies as being Māori is almost three times more likely to die from a cause that is considered amenable to healthcare interventions in NZ and Pacific People are almost two times more likely to die, when compared to Non-Māori Non-Pacific people. This is significant in terms of health system performance in NZ as it indicates a failure to uphold Māori rights under the Treaty of Waitangi (the founding constitutional document in NZ between Māori and the British Crown) to good governance, self-determination and equity [30,31].
The overall interpretation of the empirical results regarding the variations in population health outcomes in terms of amenable mortality in this study is in line with what Fantini et al. [32] and Allin and Grignon [33] report for the comparable health systems in Italy and Canada respectively. However, it contrasts with Lavergne and McGrail [34] who questioned the appropriateness of amenable mortality for comparing performance at the provincial level health systems in Canada. We acknowledge the concept of ‘performance’ the authors discuss in their work [34] is more comprehensive than measuring only variations in population health outcome that we covered in this paper.
Our estimates essentially confirm and update the evidence reported by Tobias et al. [24] in their study for the data from 1996 to 2006. Some of the parameters included in the analysis, however, are not comparable directly. The results for the deprivation variable we used are not directly comparable to other NZ research that used the conventional social position measures, either individual socioeconomic position or NZDep. Furthermore, the strength of the relationships may also vary, given we adjusted for additional variables. For example, the adjusted odds of amenable deaths among males are 1.48 times than that among females in our analysis compared to an age-adjusted excess risk of 1.75 reported earlier [24]. Similarly, we also confirm that age, sex, ethnicity, and deprivation are the key variables that explain variations across the district in line with the other evidences [5,12].
A population-level analysis of amenable deaths of this scale with the latest available individualized dataset is new to New Zealand. In this analysis, we used two sets of the AM lists – the older version for the dataset from the years 2008–2009 and the updated one that applies to the dataset from 2010 onwards. Similarly, we used the more recent deprivation measure, the Index of Multiple Deprivation (IMD), over the conventional NZDep. The IMD uses 28 indicators grouped into seven domains (income, employment, crime, housing, health, education, and access), allowing us to consider overall deprivation and its drivers (i.e., Domains) separately. Similarly, we incorporated rurality and some of the health system input's proxy measures that may challenge the healthcare services' effective delivery and vary across the NZ districts. By including the DHB-Year interaction effect in the model, we acknowledged the possibilities of the DHBs having differential contributions in reducing amenable deaths over the years.
This analysis also features a few limitations. First, the denominator population comes from the PHO enrolment dataset. The data covered 97.1 % (ranging from 95.5 % to 98.9 %) of the estimated resident population for those aged 0–74 years. There are inherent limitations of primary care enrolment data that apply to our results. These include the differential likelihood of PHO enrolment across different population groups [[35], [36]], [[35], [36]] and limitations inherent in the dataset such as accuracy of address data (e.g., Domicile Code) [35]. Nevertheless, the numerator population (AM) distribution from the mortality dataset, and the denominator population (PHO enrolled) were broadly consistent, with an average of 96.1 % and 97.1 % coverage of the original datasets respectively. These provided a complete set of information available across the study period. Similarly, the share of the total population by the DHBs in our dataset (2008–2018) compares well with that in the estimated resident population for the same period. For example, the highest difference is only three percentage-points (less in our dataset) in Auckland and close to zero in most other DHBs.
We could not capture the population's potential inter-district movements within the study period as the dataset is cross-sectional. The DHB of domicile, rurality, and deprivation of the study population represents the place reflected in the PHO dataset for the particular year regardless of where they died. Therefore, longitudinal studies following a specific population cohort may provide robust estimates of the individuals' risk across the DHBs. We focused on the district-wide variation analysis over the years and could not do further investigations by the cause of deaths (too few cases in some of the districts). Separate studies at the aggregated level may help understand the dynamics within each of the significant cause-categories like Cancers, Cardiovascular Diseases, and Diabetes. Conducting a separate analysis of variations in all-cause deaths across the districts [5] and comparing it with that for the amenable deaths may provide additional insights about the advantages of using amenable deaths to measure health system performance at the district level.
Overall, the key demographic, socioeconomic, and macro-level health system input variables explain most regional variation in amenable mortality. However, some variation remains. It implies that other unobserved local factors influence district performance. National-level performance is more evident for the indigenous population groups, narrowing the gaps, but not consistently across the districts. The district-level financial inputs guided by the population-based funding formula may also have contributed to lowering the inter-district variations in health outcomes, as has also been evident in the English NHS [10]. However, attributing the unexplained variations solely to the health sector-specific performance would be difficult, mainly because the socioeconomic factors and healthcare interventions influence health outcomes through multi-layered and complex mechanisms.
Funding
The author(s) received no specific funding for this work.
Competing interests
We declare no conflicts of interest with respect to the research, authorship, and/or publication of this article.
Declarations of interest
None
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We acknowledge the National Collections team at the Ministry of Health NZ and TAS NZ, who provided the dataset required for this analysis. Similarly, the guidance provided by Associate Professor Barry Milne and Associate Professor Roger Marshall was instrumental during the data processing and analysis. The data processing and analysis work were possible only because of the computing facilities provided by the New Zealand eScience Infrastructure (NeSI). We appreciate the contributions of Dr. Richard Hamblin and Catherine Gerard from the Health Quality and Safety Commission, NZ, who guided us from the beginning of the project and provided feedback on the manuscript. Ethical approval for this study was granted by the University of Auckland Human Participants Ethics Committee on March 12, 2020, Reference 022792.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.puhip.2024.100545.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
References
- 1.Smith P.C. Performance measurement in health care: history, challenges and prospects. Publ. Money Manag. 2005;25(4):213–220. [Google Scholar]
- 2.Smith P.C., Mossialos E., Papanicolas I., Leatherman S. Cambridge University Press; 2009. Performance measurement for health system improvement: experiences, challenges and prospects. [Google Scholar]
- 3.Manderbacka K., Arffman M., Sund R., Karvonen S. Multiple social disadvantage does it have an effect on amenable mortality: a brief report. Int. J. Equity Health. 2014;13(1) doi: 10.1186/s12939-014-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castelli A., Nizalova O. University of York; 2011. Avoidable mortality: what it means and how it is measured. [Google Scholar]
- 5.Tobias M., Yeh L.C. How much does health care contribute to health gain and to health inequality? Trends in amenable mortality in New Zealand 1981–2004. Aust N Z J Public Health. 2009;33(1):70–78. doi: 10.1111/j.1753-6405.2009.00342.x. [DOI] [PubMed] [Google Scholar]
- 6.Nolte E., McKee C.M. Measuring the health of nations: updating an earlier analysis. Health Aff. 2008;27(1):58–71. doi: 10.1377/hlthaff.27.1.58. [DOI] [PubMed] [Google Scholar]
- 7.Lavergne M.R., McGrail K. What, if anything, does amenable mortality tell us about regional health system performance? Healthc. Policy. 2013;8(3):79–90. [PMC free article] [PubMed] [Google Scholar]
- 8.Fantini M.P., Lenzi J., Franchino G., et al. Amenable mortality as a performance indicator of Italian health-care services. BMC Health Serv. Res. 2012;12(1) doi: 10.1186/1472-6963-12-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James P.D., Wilkins R., Detsky A.S., Tugwell P., Manuel D.G. Avoidable mortality by neighbourhood income in Canada: 25 years after the establishment of universal health insurance. J. Epidemiol. Community Health. 2007;61(4):287–296. doi: 10.1136/jech.2006.047092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Currie J., Castillo M.G., Adekanmbi V., Barr B., O'Flaherty M. Evaluating effects of recent changes in NHS resource allocation policy on inequalities in amenable mortality in England, 2007–2014: time-series analysis. J. Epidemiol. Community Health. 2019;73(2):162–167. doi: 10.1136/jech-2018-211141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kunitz S.J., Veazie M., Henderson J.A. Historical trends and regional differences in all-cause and amenable mortality among American Indians and Alaska Natives since 1950. Am J Public Health. 2014;104(SUPPL. 3):S268–S277. doi: 10.2105/AJPH.2013.301684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wróblewska W. Territorial variation in mortality from causes amenable to medical care in Poland. Ann. Agric. Environ. Med. 2017;24(3):489–495. doi: 10.5604/12321966.1233557. [DOI] [PubMed] [Google Scholar]
- 13.Katikireddi S.V., Cezard G., Bhopal R.S., et al. Assessment of health care, hospital admissions, and mortality by ethnicity: population-based cohort study of health-system performance in Scotland. Lancet Public Health. 2018;3(5):e226–e236. doi: 10.1016/S2468-2667(18)30068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wood E., Sallar A.M., Schechter M.T., Hogg R.S. Social inequalities in male mortality amenable to medical intervention in British Columbia. Soc. Sci. Med. 1999;48(12):1751–1758. doi: 10.1016/s0277-9536(99)00081-7. [DOI] [PubMed] [Google Scholar]
- 15.Mustard C.A., Bielecky A., Etches J., et al. Avoidable mortality for causes amenable to medical care, by occupation in Canada, 1991–2001. Can. J. Public Health. 2010;101(6):500–506. doi: 10.1007/BF03403973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng X., Liu Y., Astell-Burt T., et al. Analysis of health service amenable and non-amenable mortality before and since China's expansion of health coverage in 2009. BMJ Open. 2016;6(1) doi: 10.1136/bmjopen-2015-009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gómez-Arias R.D., Bonmati A.N., Pereyra-Zamora P., Rodríguez-Ospina F.L., Agudelo-Londoño S.M. Avoidable mortality and health policies. Colombia. 1985-2002. Colomb. Méd. 2009;40(4):373–386. [Google Scholar]
- 18.Ministry of Health System level measures framework. 2018. https://www.health.govt.nz/new-zealand-health-system/system-level-measures-framework [Available from:
- 19.Ministry of Health Health system indicators framework. 2021. https://www.health.govt.nz/new-zealand-health-system/health-system-indicators-framework [updated 13 December 2021; cited 2021 28 December]. Available from:
- 20.Ministry of Health Overview of the health system. 2017. https://www.health.govt.nz/new-zealand-health-system/overview-health-system [updated 30 March 2017; cited 2021 09 July ]. Available from:
- 21.Statistics N.Z. Subnational population estimates tables: NZ.Stat. 2021. http://nzdotstat.stats.govt.nz/wbos/Index.aspx?DataSetCode=TABLECODE7509 [updated 14 July 2021; cited 2021 14 July]. Available from:
- 22.DPMC NZ. Response to health and disability system review 2021 [updated 21 April 2021; cited 2021 10 July]. Available from: https://dpmc.govt.nz/our-business-units/transition-unit/response-health-and-disability-system-review.
- 23.Ministry of Health . Ministry of Health; 2019. Mortality collection.https://www.health.govt.nz/nz-health-statistics/national-collections-and-surveys/collections/mortality-collection [cited 2021 01 Jan]. Available from: [Google Scholar]
- 24.Ministry of Health . Ministry of Health; Wellington: 2010. Amenable mortality in New Zealand, 1996–2006. [Google Scholar]
- 25.Ministry of Health . Ministry of Health; 2019. Primary health organisation enrolment collection.https://www.health.govt.nz/nz-health-statistics/national-collections-and-surveys/collections/primary-health-organisation-enrolment-collection [cited 2020 15 Nov]. Available from: [Google Scholar]
- 26.Ministry of Health . Ministry of Health; Wellington: 2017. HISO 10001:2017 Ethnicity data protocols. [Google Scholar]
- 27.Milne B.J., Parker K., McLay J., et al. Primary health care access and ambulatory sensitive hospitalizations in New Zealand. J Ambul Care Manage. 2015;38(2):178–187. doi: 10.1097/JAC.0000000000000057. [DOI] [PubMed] [Google Scholar]
- 28.Exeter D.J., Zhao J., Crengle S., Lee A., Browne M. The New Zealand Indices of Multiple Deprivation (IMD): a new suite of indicators for social and health research in Aotearoa, New Zealand. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0181260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hajduk G.K. Introduction to linear mixed models. 2019. https://ourcodingclub.github.io/tutorials/mixed-models/#six [updated 10th September 2019; cited 2021 15 July]. Available from:
- 30.Waitangi Tribunal . Legislation Direct: Lower Hutt; New Zealand: 2019. Hauora: Report on stage one of the health services and outcomes kaupapa inquiry. [Google Scholar]
- 31.Reid P. In: Always speaking’: the Treaty of Waitangi and public policy. Tawhai V., Gray-Sharp K., editors. 2011. Good governance: the case of health equity. Huia: Wellington, New Zealand. [Google Scholar]
- 32.Fantini M.P., Lenzi J., Franchino G., et al. Amenable mortality as a performance indicator of Italian health-care services. BMC Health Serv. Res. 2012;12(1) doi: 10.1186/1472-6963-12-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Allin S., Grignon M. Examining the role of amenable mortality as an indicator of health system effectiveness. Healthc. Policy. 2014;9(3):12–19. [PMC free article] [PubMed] [Google Scholar]
- 34.Lavergne M.R., McGrail K. What, if anything, does amenable mortality tell us about regional health system performance? Healthc. Policy. 2013;8(3):79–90. [PMC free article] [PubMed] [Google Scholar]
- 35.Statistics N.Z. Statistics New Zealand; New Zealand: 2013. Evaluation of administrative data sources for subnational population estimates, Tatauranga Aotearoa, Wellington. [Google Scholar]
- 36.Ministry of Health . Ministry of Health; Wellington: 2021. Enrolment in a primary health organisation.https://www.health.govt.nz/our-work/primary-health-care/about-primary-health-organisations/enrolment-primary-health-organisation [updated 13 Jan 2021; cited 2021 28 Jan]. Available from: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.