Summary
There are no cures for neurodegenerative protein conformational diseases (PCDs), such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and Huntington’s disease (HD). Emerging evidence suggests the gut microbiota plays a role in their pathogenesis, though the influences of specific bacteria on disease-associated proteins remain elusive. Here, we reveal the effects of 229 human bacterial isolates on the aggregation and toxicity of Aβ1-42, α-synuclein, and polyglutamine tracts in Caenorhabditis elegans expressing these culprit proteins. Our findings demonstrate that bacterial effects on host protein aggregation are consistent across different culprit proteins, suggesting that microbes affect protein stability by modulating host proteostasis rather than selectively targeting disease-associated proteins. Furthermore, we found that feeding C. elegans proteoprotective Prevotella corporis activates the heat shock response, revealing an unexpected discovery of a microbial influence on host proteostasis. Insight into how individual bacteria affect PCD proteins could open new strategies for prevention and treatment by altering the abundance of microbes.
Subject areas: Bacteriology, Biological sciences, Molecular neuroscience, Neuroscience
Graphical abstract
Highlights
-
•
Characterization of 229 bacterial isolates on host protein stability
-
•
Bacteria affect the stability of disease-associated proteins
-
•
Proteoprotective Prevotella corporis activates the host heat shock response
-
•
Gram-negative aerobes induce broad proteotoxicity in the host
Bacteriology; Biological sciences; Molecular neuroscience; Neuroscience
Introduction
Neurodegenerative protein conformational diseases (PCDs) are characterized by disturbances in proteostasis that result in the aggregation of disease-associated proteins, ultimately leading to tissue death.1 Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS) are among the most prevalent neurodegenerative diseases, with AD recognized by the World Health Organization (WHO) as one of the leading causes of death worldwide.2 However, despite the increasing prevalence of PCDs, their complex etiologies continue to obscure potential therapeutic targets. The sporadic onset and variable severity of neurodegenerative diseases, along with their idiopathic nature, suggest the role of a triggering factor in their onset and progression. Multiple factors have been associated with the pathogenicity of PCDs, including an expanding body of evidence that suggests the involvement of microbes, but primarily those within the human gut microbiota. The human gut microbiota is considered an “organ” due to its production of essential proteins and metabolites, including vitamins, hormones, and neurotransmitters.3,4 Hence, dysbiosis of the gut microbiota has been linked to various ailments, including neurodegenerative diseases.5
The complexity of the microbiome has made it challenging to determine the precise role of bacteria in the pathogenesis of neurodegenerative diseases. In addition, most of the evidence that associates bacteria with the occurrence of neurodegenerative diseases is based on correlation.6 Interestingly, correlational evidence does not demonstrate any selectivity between different neurodegenerative diseases, despite each disease featuring a unique, specific culprit protein species. For example, a lower abundance of Prevotella spp. has been observed in patients with different PCDs, including PD and ALS.7,8,9,10,11,12,13,14,15 Due to this lack of specificity, we hypothesized that bacteria could be affecting these diseases through the host proteostasis network—upstream of protein aggregate formation. This hypothesis is further supported by our previous study in which we identified gram-negative enteropathogens that significantly disrupt proteostasis across tissues in Caenorhabditis elegans.16 The utility of C. elegans as a model to study host-microbe interaction is strengthened by its unique ability to be colonized by a single bacterial strain. Such a feature simplifies the complexity of the microbiome, allowing us to study the effect of individual species on host proteostasis.
Here, we characterized the effect of 229 unique bacterial isolates from the Human Microbiome Project on the aggregation of disease-associated proteins in C. elegans. We used transgenic nematodes expressing Aβ1-42, α-synuclein, and polyglutamine (polyQ), proteins that adopt amyloidal conformation and are associated with AD, PD, and HD, respectively, in which their toxic aggregation culminates in neurodegeneration. Surprisingly, our results suggest that bacteria-mediated enhancement or suppression of host protein aggregation is not specific to any particular culprit protein. Instead, our data demonstrate that bacteria broadly affect the aggregation of metastable proteins present within the host proteome. Furthermore, we also observed that the proteostasis-modulating effects of intestinal bacteria reach distal tissues. Thus, our results indicate that bacteria influence host proteostasis, ultimately affecting the ability of both proximal and distal tissues to buffer protein folding. To date, the present study provides the most comprehensive characterization of the effect of individual constituents of the human microbiome on host proteostasis. Our findings reveal the bacterial contribution to the stability of not only proteins associated with AD, PD, and HD, but also endogenous host proteins, and may extrapolate to a broader range of metastable proteins implicated in various proteinopathies. Collectively, our results provide a framework for the development of microbiome-based risk factor assessments and disease management strategies.
Results
Characterization of the human microbiome on host proteostasis
We obtained a comprehensive Human Microbiome Project collection of 229 unique bacterial isolates from Biodefense and Emerging Infections Research Resources Repository (BEI Resources) and assessed their effect on C. elegans proteostasis. The method used to conduct this experiment is illustrated in Figure 1. The collection encompasses isolates from a range of diverse phyla and a variety of anatomical sites (Figure 2). In our previous study, we used aggregation-prone polyQ tracts as a sensor of the protein folding environment to demonstrate that gram-negative pathogens disrupt host proteostasis.16 Among animals expressing intestine-, muscle-, and neuron-specific polyQ::YFP (yellow fluorescent protein), we previously found that proteostasis in the intestine was most robustly affected by the colonizing bacteria.16 As such, we performed our initial screen using the intestinal polyQ model (Figure 2). To eliminate the possibility that bacteria will affect C. elegans development, worms were grown to adulthood prior to being transferred to the bacterial strains of interest. PolyQ aggregation was assessed by fluorescent microscopy. To ensure the validity of our results, we also assessed polyQ aggregation by western blotting. Both quantitative methods yielded similar results, validating our approach.
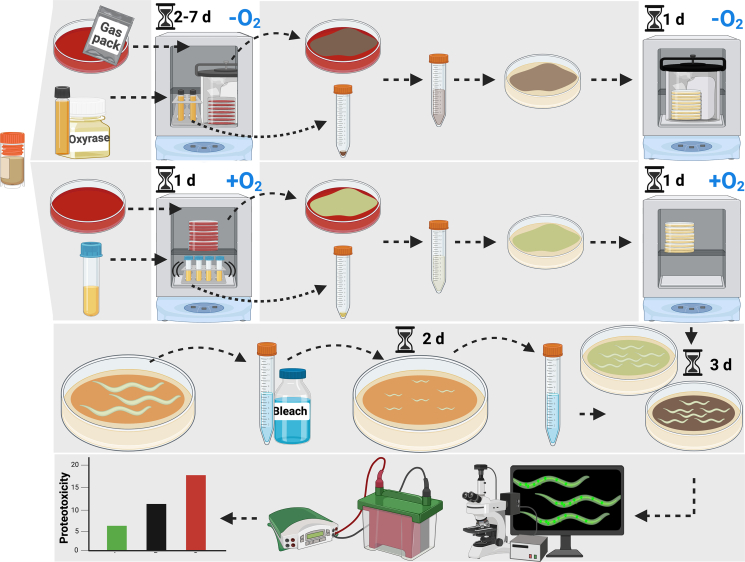
Figure 1.
Schematic illustrating the method used to assess the effect of bacterial isolates on C. elegans proteostasis
Details can be found in STAR methods. In brief, anaerobic bacteria were cultured on TSA-blood plates using anaerobic gas packs or in liquid broth supplemented with oxyrase for 2–7 days, while aerobic bacteria were cultured on TSA-blood plates or in liquid broth for one day. Cultured bacteria were transferred to nematode growth media (NGM) plates and incubated overnight with (for anaerobic bacteria) or without (aerobic bacteria) anaerobic gas packs. Worms were synchronized by the bleaching method and cultured on control E. coli OP50 until young adults (2 days), washed and transferred to NGM plates containing anaerobically or aerobically grown test bacteria, and cultured for an additional three days. Intestinal polyQ aggregates were quantified by manual counting and western blotting the insoluble fractions.
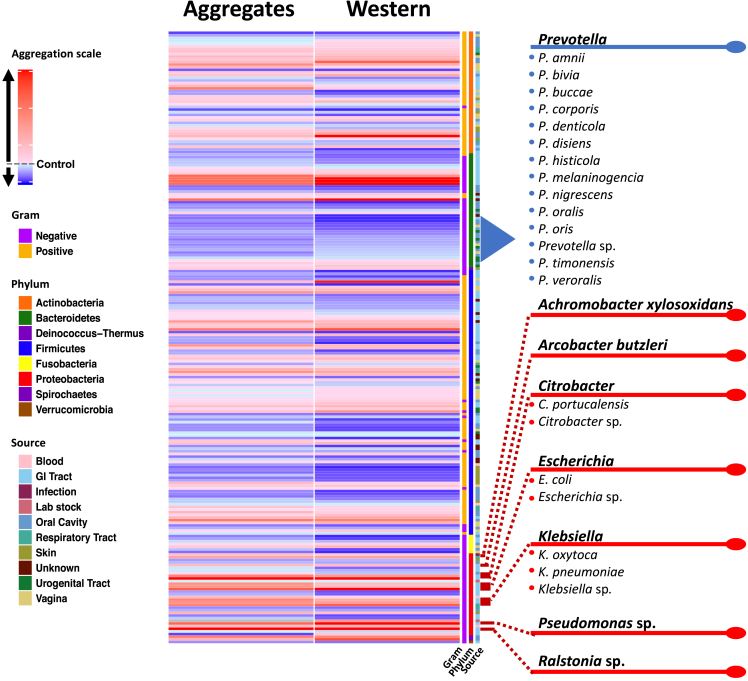
Figure 2.
Heatmap summarizing the results of the screen that assessed the effect of human bacterial isolates on C. elegans intestinal polyQ aggregation
PolyQ aggregation was quantified by microscopy (Aggregates column) and western blotting (Western column). Each of the 229 rows represents a single bacterial strain. Aggregation data are normalized to worms fed control E. coli OP50. The aggregation scale is color-coded from blue to red, where red indicates increasing polyQ aggregation relative to control. The characteristics (Gram, phylum, and source) of each bacterial isolate are included in the last three columns.
Bacteria from the isolate collection exhibited a differential effect on C. elegans proteostasis as indicated by increases and decreases in polyQ aggregation compared to worms fed control Escherichia coli OP50 (Figure 2). Figure 2 summarizes the screen, revealing bacteria that most robustly affected host proteostasis. Prevotella was the only genus that consistently resulted in low polyQ aggregation across all 229 strains tested (Figure 2). Conversely, significant host proteotoxicity was observed in nematodes that were fed Achromobacter xylosoxidans and Arcobacter butzleri, as well as Citrobacter, Escherichia, Klebsiella, Pseudomonas, and Ralstonia spp. (Figure 2). A detailed list of all bacterial strains and their effect on polyQ aggregation is summarized in Table S1. Intriguingly, our data align with previous research linking the depletion or enrichment of these bacteria in patients with PCDs, which is further described in the discussion section of the present study. To our knowledge, this is the first-ever comprehensive screen that assessed the effect of human bacterial isolates on host proteostasis.
Prevotella spp. mitigate the proteotoxic aggregation of diverse culprit proteins
Out of the 229 strains tested, the Prevotella genus had the highest number of members that suppressed polyQ aggregation. We further retested 17 Prevotella spp. using the intestinal polyQ44 model. To increase the robustness of the response, we started feeding animals test bacteria immediately after hatching. Although we observed an enhanced suppression of aggregation for most retested strains, there were few isolates that did not significantly inhibit polyQ aggregation, likely due to the timing of feeding. Furthermore, as expected, feeding worms test bacteria beginning at the L1 larval stage affected development (Figure 3A, “Aggregates”). Out of all strains, Prevotella buccae, Prevotella oris, and Prevotella corporis significantly suppressed polyQ aggregation without causing any detectable developmental delay (Figure 3A, “Aggregates”). Therefore, we followed up with these three strains. Motility defects are associated with neurodegenerative PCDs. As such, we assessed whether the three Prevotella spp. alleviate aggregate-dependent motility defects caused by culprit proteins expressed in the intestine and distal tissues. We used our well-established and validated time-off-pick (TOP) assay that relies on C. elegans motility as a readout of proteotoxicity.16,17 Consistent with their suppression of polyQ aggregation, all three strains alleviated aggregate-dependent motility defects, with P. corporis having the strongest effect (Figure 3A, “Motility”). To determine whether the observed suppression is dependent on polyQ, we used N2 wild-type worms and a model expressing a shorter polyQ tract, polyQ33. The results revealed no effect on the N2 worms and a less robust suppression of the motility defect for polyQ33, indicating that Prevotella suppressed proteotoxicity. Given that dietary restriction enhances proteostasis and suppresses polyQ aggregation,18 we assessed pharyngeal pumping to determine whether the proteoprotective effect mediated by P. corporis could be the result of caloric restriction. Our results showed no significant difference in pharyngeal pumping between worms fed E. coli OP50 and those fed P. corporis (Figure S1), indicating that the observed proteoprotection is not caused by caloric restriction.
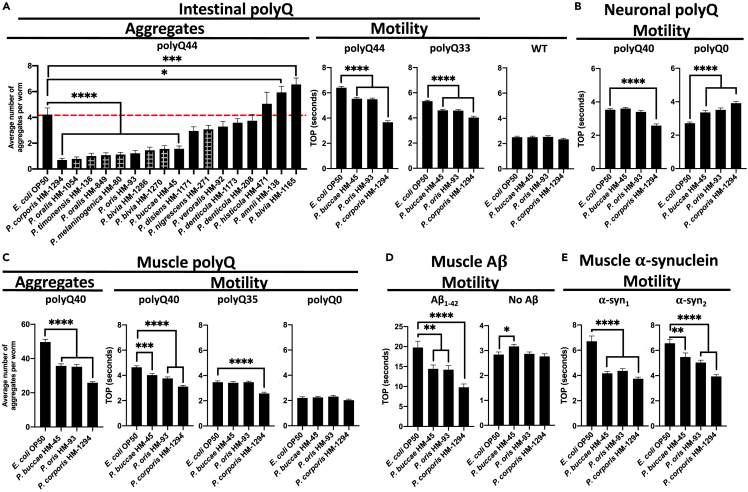
Figure 3.
The effect of Prevotella spp. on proteins associated with neurodegenerative diseases
(A) The effect of Prevotella spp. on C. elegans intestinal polyQ aggregation (“Aggregates”) and the associated toxicity (“Motility”). Checkered bars represent bacteria-associated developmental delay. The three strongest suppressors of polyQ aggregation that did not affect development were tested using intestinal polyQ, (B) neuronal polyQ, (C) muscle polyQ, (D) muscle Aβ1-42, and (E) muscle α-synuclein. Data are represented as the average number of aggregates or TOP (seconds) per worm obtained from at least two independent experiments for a total of 30–60 worms. Error bars represent standard error of the mean (SEM). Statistical significance was calculated using one-way ANOVA followed by multiple comparison Dunnett’s post-hoc test (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
Because Prevotella spp. are anaerobic and may not thrive well in the ambient conditions in which C. elegans are cultured, we investigated whether dead bacteria retain their proteoprotective properties. To accomplish this, we fed worms paraformaldehyde (PFA)-killed bacteria and found that P. corporis still significantly suppressed polyQ aggregation relative to E. coli OP50 control (Figure S2), suggesting that live bacteria are not necessary to elicit protection.
To determine whether Prevotella also affects polyQ in distal tissues, we employed the neuronal and muscle models. Because worms expressing the neuronal polyQ do not exhibit quantifiable aggregates, we used the TOP assay as a proteotoxicity readout. Our results show that of the three strains, only P. corporis showed significant suppression of the motility defect in worms expressing neuronal polyQ40 (Figure 3B). Unexpectedly, P. buccae, P. oris, and P. corporis increased motility defects in worms expressing the neuronal empty vector control (polyQ0); the reason for this is unknown, but importantly does not diminish the observed beneficial effects of Prevotella on neuronal polyQ. In a manner similar to the intestinal polyQ, we observed Prevotella-mediated suppression of aggregation and proteotoxicity in worms expressing muscle-specific polyQ (Figure 3C).
The association between the low abundance of gut Prevotella and diverse neurodegenerative PCDs suggests that the bacterial influence on these diseases is independent of the culprit protein species.8,9,10,11,12,13,14 As such, we hypothesized that bacteria might affect protein aggregation by modulating host proteostasis, and if this is the case, Prevotella spp. should enhance proteostasis in worms expressing various aggregation-prone proteins. To test the effect of Prevotella on other disease-associated proteins, we chose three muscle-specific models: one expressing Aβ1-42, and two expressing α-synuclein.19,20,21 These culprit proteins are associated with Alzheimer’s and Parkinson’s diseases, respectively. Additionally, these models exhibit age-dependent proteotoxicity, which is consistent with the age-dependent progression of neurodegenerative diseases.16,22,23 To investigate progressive proteotoxicity in the Aβ1-42 and α-synuclein models,19,20,21 we assessed their motility between days three to five post-hatching (Figure S3). Nematodes expressing Aβ1-42 and α-synuclein had age-dependent motility defects that were markedly greater than those of the controls (Figure S3). We examined the effect of P. buccae, P. oris, and P. corporis on proteotoxicity associated with each of these three models. In a manner similar to the polyQ models, P. buccae, P. oris, and P. corporis reduced aggregate-dependent toxicity compared to wild-type control (Figure 3A), particularly with P. corporis having the strongest effect (Figures 3D and 3E). Bacteria that strongly enhance or suppress host proteostasis induce notable differences in the aggregation of Aβ1-42, which parallels the extent of the motility defect (Figure S4). Both Aβ1-42 aggregation and the associated toxicity were suppressed by P. corporis and enhanced by Pseudomonas aeruginosa (Figure S4), a bacterium that strongly disrupted host proteostasis in our previous studies.16,24 Since all of our transgenic worms express exogenous proteins, we wanted to determine whether Prevotella can also provide protection against the misfolding of endogenous proteins. To accomplish this, we employed two strains that carry temperature-sensitive mutations in endogenous proteins: myosin heavy chain (UNC-54) that misfolds and leads to paralysis at the restrictive temperature (25°C),25,26 and DYN-1, featuring a modified GTPase domain that is highly expressed in motor neurons, resulting in loss of coordination at the restrictive temperature (25°C).27 In agreement with the proteoprotective effect against misfolding and aggregation of exogenous proteins, Prevotella suppressed the temperature-dependent motility defect of both worm strains at the restrictive temperature, further supporting its proteoprotective role (Figure S5). Collectively, our findings suggest the broader potential of Prevotella in mitigating the pathogenesis of neurodegenerative diseases.
Proteotoxic bacteria enhance the aggregation and toxicity of PCD-associated proteins
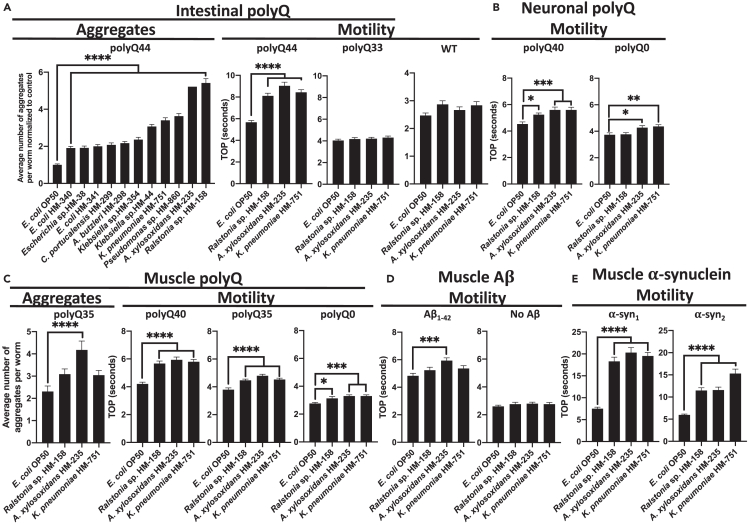
To confirm the effect of the most robust proteotoxic strains identified in our original screen (Figure 2; Table S1), we assessed polyQ44 aggregation in animals fed gram-negative aerobes (Figure 4A, “Aggregates”). We concentrated on these specific bacteria because they did not affect worm development, which is known to influence proteostasis.28 We found that Ralstonia sp., A. xylosoxidans, Pseudomonas sp., and Klebsiella pneumoniae were the strongest inducers of polyQ aggregation (Figure 4A). As we have already demonstrated the proteotoxic potential of P. aeruginosa in our previous work,16,24 we focused on the three remaining species in follow-up experiments. To assess the proteotoxic effect of these bacteria, we employed the TOP assay to measure the motility of worms expressing aggregating polyQ44 and non-aggregating polyQ33. While we detected a significant enhancement in the motility defect in animals expressing polyQ44, no significant changes were observed in wild-type or animals expressing polyQ33 (Figure 4A). These results indicate that the impairment of motility induced by bacteria is contingent on polyQ aggregation rather than general bacterial pathogenicity. The aggregate-dependent motility defects were also observed in worms expressing neuronal and muscle-specific polyQ; however, the phenotype was less robust and we did detect some decrease in motility in control animals (Figures 4B and 4C). A. xylosoxidans also elevated muscle-specific polyQ aggregation (Figure 4C). To test the microbial influence on other disease-associated proteins, we employed worms expressing muscle-specific Aβ1-42 and α-synuclein. A. xylosoxidans induced proteotoxicity associated with both proteins (Figure 4D), and all three strains induced proteotoxicity associated with α-synuclein (Figure 4E). To determine whether the observed proteotoxicity is attributed to increased bacterial colonization, we quantified intestinal bacteria. Our results revealed that K. pneumoniae, Ralstonia sp., and A. xylosoxidans colonized less than E. coli OP50 (Figure S6), indicating that the proteotoxicity mediated by these strains is not explained by their colonization efficiencies. To delve deeper into the mechanism that underlies the bacterial proteotoxicity, we built upon our previous study that implicated secreted bacterial products in host proteotoxicity,24 and exposed worms to spent culture supernatants from K. pneumoniae and A. xylosoxidans, two most robust inducers of polyQ aggregation. Our results revealed that supernatants from these bacteria significantly increased polyQ aggregation, whereas supernatants from P. corporis and E. coli OP50 had no effect (Figure S7). These results are intriguing as they suggest that the mechanism by which bacteria disrupt protein stability may involve secreted factors that can induce protein aggregation in proximal and distal tissues. Additionally, we demonstrate that bacteria can enhance the proteotoxicity of diverse disease-associated proteins, supporting their role in the pathogenesis of neurodegenerative PCDs.
Figure 4.
The effect of proteotoxic bacteria on proteins associated with neurodegenerative diseases
(A) The effect of gram-negative, aerobic bacteria on C. elegans intestinal polyQ aggregation (“Aggregates”) and the associated toxicity (“Motility”). Three robust enhancers of polyQ aggregation were tested using intestinal polyQ, (B) neuronal polyQ, (C) muscle polyQ, (D) muscle Aβ1-42, and (E) muscle α-synuclein. Data are represented as the average number of aggregates or TOP (seconds) per worm obtained from at least two independent experiments for a total of 30–60 worms. Data in (A) (Aggregates) are normalized to worms fed the control E. coli OP50. Error bars represent SEM. Statistical significance was calculated using one-way ANOVA followed by multiple comparison Dunnett’s post-hoc test (∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001).
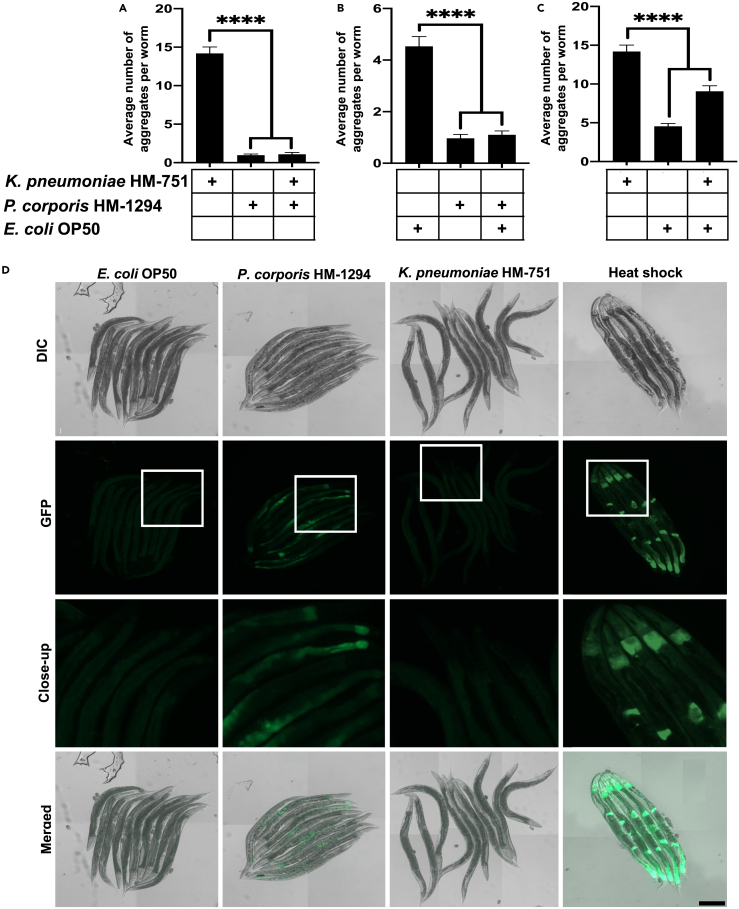
The human microbiota is composed of a diverse array of bacterial species that coexist and interact within a complex polymicrobial community. As such, we investigated the potential of a proteoprotective species to mitigate bacterial proteotoxicity by co-colonizing worms with P. corporis and either K. pneumoniae or E. coli OP50. Our findings revealed that P. corporis suppressed the polyQ aggregation associated with K. pneumoniae and E. coli OP50 (Figures 5A and 5B). We tested whether the same suppressive effect would result from co-colonizing worms with K. pneumoniae and E. coli OP50 and found that the resulting aggregation profile was an integrative reflection of the individual effects observed when each strain was introduced in isolation (Figure 5C). Collectively, these results indicate that, in addition to suppressing proteotoxicity from intrinsic factors like aging and host-encoded metastable proteins, P. corporis also suppresses host proteotoxicity mediated by extrinsic factors such as proteotoxic bacteria.
Figure 5.
The effect of P. corporis on bacteria-induced polyQ aggregation and activation of the heat-shock response
(A–C) The effect of co-colonizing intestinal polyQ44 worms with (A) K. pneumoniae HM-751 and P. corporis HM-1294, (B) E. coli OP50 and P. corporis HM-12924, (C) K. pneumoniae HM-751 and E. coli OP50. Data are represented as the average number of aggregates per intestinal polyQ44 worm obtained from three independent experiments for a total of 60 worms. Statistical significance was calculated using one-way analysis of variants (ANOVA) followed by multiple comparison Dunnett’s post-hoc test (∗∗∗∗p < 0.0001).
(D) Nomarski and fluorescent (EGFP) images of transgenic worms expressing transcriptional fusion reporter hsp70p::GFP, fed E. coli OP50, P. corporis HM-1294, K. pneumoniae HM-751, and as a positive control, heat-shocked worms fed E. coli OP50. Scale bar is 200 μm.
To investigate the mechanistic basis behind the ability of P. corporis to safeguard against host proteotoxicity, we explored its potential to activate the heat shock response (HSR), a critical and evolutionary conserved cellular defense mechanism that responds to protein misfolding and proteotoxic stress. Using C. elegans expressing a transcriptional fluorescent reporter, hsp70p::GFP, we monitored HSR activation in worms fed P. corporis. While control E. coli OP50 and proteotoxic K. pneumoniae did not elicit any detectable response, we found that P. corporis induced the reporter, indicating robust HSR activation (Figure 5D). This result highlights a potential mechanism by which bacteria can alleviate proteotoxicity in the host by activating protective stress responses. To our knowledge, this is the first documentation of bacterial induction of a protective HSR in the host, which opens new opportunities for bacteria-mediated therapeutic strategies against PCDs.
Discussion
In the present study, we screened a comprehensive collection of 229 unique bacterial isolates from the Human Microbiome Project for their ability to affect host proteostasis from within the intestinal milieu. Our follow-up experiments on the most robust beneficial and detrimental bacteria confirmed their influence on proteostasis across host tissues, affecting the stability of proteins associated with Alzheimer’s, Parkinson’s, and Huntington’s diseases. The phylogenetic analysis revealed clustering of select proteotoxic and proteoprotective bacteria, indicating that genetically related bacteria affect host proteostasis in a similar manner, but with a different magnitude (Figure S8). To our knowledge, this is the first comprehensive characterization of bacteria from human microbiomes on host proteostasis. Surprisingly, our data suggest that bacteria do not selectively target any specific host proteins associated with PCDs, but rather affect proteostasis in general, leading to the aggregation and proteotoxicity of any destabilized proteins present within the proteome, such as exogenous polyQ, Aβ1-42, and α-synuclein, tested in our experiments. While this is a generalized mechanism, there could be microbes that exclusively affect a specific disease.
Numerous studies that employed genomic analyses of microbial compositions in affected patients revealed a connection between comparable gut dysbioses and diverse neurodegenerative PCDs.6 These correlational studies from human subjects support our conclusion that bacteria impact host protein stability by modulating host proteostasis. This mechanism is likely mediated by the interplay between host proteostasis and immune responses to bacteria.29,30
Many of the studies aiming to identify neurodegenerative PCD etiology or treat the disease have concentrated on host-targeted therapeutics, primarily focused on targeting the aggregating proteins or the affected cell types. However, this approach has not been successful in pre-clinical or clinical trials.31,32 Perhaps the focal point of the host-targeted approach occurs too late in the aggregation cascade given that the changes in the gut microbiota can happen prior to any clinical manifestation.33,34 Indeed, individuals with neurodegenerative PCDs have insufficient proteostatic capacity.35 While some studies have attempted to treat neurodegenerative PCDs by enhancing components of the host proteostasis network, these have not been successful.31 In support of our results indicating that bacteria affect host proteostasis, a shift toward microbial-centered therapeutic approaches has shown more promise in lessening disease symptoms. For example, eradication of Helicobacter pylori in AD patients was associated with improved disease presentation in a clinical trial and a population-based study.36,37 Interestingly, the beneficial effect of antibiotics on the progression of PCDs, when administered post-onset, is absent when antibiotics were used prior to disease onset; notably, general antibiotic use has been associated with increased risk for PCDs as well as gut dysbiosis.38,39,40 While H. pylori is a good clinical example of microbial contribution to neurodegenerative diseases, we did not expect to detect proteotoxicity associated with this bacterium in our C. elegans model due to a limitation of our approach that includes transferring and incubating bacteria at sub-optimal temperature—a factor that facilitates H. pylori virulence.41 Additional reports suggest that H. pylori can indirectly affect the composition of the human gut microbiota.42 Microbe-targeted therapeutic approaches hold promise and are further bolstered by a recent study that demonstrated that gut microbiota and serum derived from AD patients accelerate disease symptoms and affect neurogenesis in healthy rats and tissue culture, respectively.43
In our screen (Figure 2), Prevotella stood out as the only genus devoid of any species that exhibit proteotoxicity toward the host. Furthermore, our findings indicate that Prevotella spp. have a broad and suppressive effect on host protein aggregation, regardless of the specific disease-associated protein (Figure 3). Sequencing data suggest that a low abundance of Prevotella in the guts of patients with PCDs enhances disease pathogenesis.7,8,9,10,11,12,13,14 However, despite the fact that the Prevotella genus contains over 50 species, many studies report their results at the genus level.44 Our data indicate that various Prevotella species exhibit a differential effect on host proteostasis (Figure 3A). Such various effects of individual species could be explained by large variability in their genomes.44 The species, P. intermedia, P. nigrescense, and P. melanigencia have been consistently associated with infection,45,46,47 inflammation,48,49,50,51 and have also been linked to AD and associated mortality.52 Conversely, it has been shown that P. buccae and P. corporis, two strains of Prevotella that exhibited strong suppression of protein aggregation and the associated toxicity in the present study, were found not to induce inflammation.53 Instead, P. buccae and P. corporis were shown to induce the expression of mucin-associated membrane proteins MUC3 and MUC4.53 Interestingly, induction of MUC3 expression by a probiotic cocktail prevented adherence of enteropathogenic E. coli.54 MUC4 has been shown to be essential for maintaining mucus barrier function and intestinal homeostasis in mice, as its absence resulted in severe large intestinal bleeding and significant down-regulation of antimicrobial peptides.55 Though preliminary, the evidence suggesting the protective role of Prevotella in maintaining intestinal integrity is interesting, as intestinal integrity is often compromised in people with neurodegenerative PCDs.56 Damage to the intestinal epithelium can lead to translocation of bacteria and bacterial products, resulting in systemic inflammation and the breakdown of the blood-brain barrier, contributing to a variety of diseases, including PCDs.57,58 The translocation of bacterial products becomes even more relevant given our results showing the proteotoxic effect of secreted bacterial factors (Figure S7). The contrasting associations of different Prevotella species in host health and disease highlight the importance of studying bacteria at the species level, as demonstrated in the present study, to unravel the precise roles of bacteria in disease pathogenesis. The Bacillus genus is another example that supports the need for species-level studies, as this genus encompasses diverse species that exhibit opposing effects on the host; Bacillus anthracis is a controlled bioweapon,59 whereas Bacillus subtilis is a probiotic that has been shown to play a protective role against the aggregation of α-synuclein in C. elegans, partly through its metabolites.60
The physiological relevance of our findings is supported by studies that focus on pathogenic bacteria and subsequently establish a connection with PCDs. For example, the robust induction of proteotoxicity by K. pneumoniae in C. elegans models expressing different aggregation-prone proteins (Figure 4) is in agreement with a previous study that identified an overabundance of K. pneumoniae in the gut microbiota of individuals with AD.61 Furthermore, a positive correlation has been demonstrated between the presence of K. pneumoniae in the gut and elevated levels of the AD-associated biomarker, C-reactive protein, in blood samples of AD patients.62 Another study found an elevated abundance of Ralstonia in mucosal samples from individuals with PD,63 which is another bacterium that was robustly proteotoxic to our C. elegans models (Figure 4). Interestingly, this bacterium was also found to be more prevalent in individuals with autism,64 which is another disease that has been associated with the presence of misfolded proteins.65,66,67,68 Autism has also been associated with both Achromobacter and Pseudomonas genera.69,70 Both Achromobacter and Pseudomonas are linked to cystic fibrosis (CF), which is another PCD that does not feature neurodegeneration;71,72 Achromobacter was reported to exacerbate the disease,73 and P. aeruginosa is a predominant pathogenic species that colonizes the lungs of CF patients.74 A. xylosoxidans induced proteotoxicity in all our disease models (Figure 4), and Pseudomonas notably increased polyQ aggregation (Figure 4A, “Aggregates”). Furthermore, we previously demonstrated that P. aeruginosa exerts robust proteotoxic effects on C. elegans.16,24 The presence of Pseudomonas, in conjunction with another bacterial genus, was successfully used to differentiate individuals with AD from control subjects.75 Collectively, the aforementioned studies are in agreement with our results and further support the wide-ranging impact of bacteria on protein-folding diseases. It is remarkable that the abovementioned bacteria induce proteotoxicity consistently across our C. elegans models expressing distinct disease proteins. The convergence of our results with existing literature linking these bacteria to PCDs strongly reinforces the notion that bacteria affect the host proteostasis network, broadly affecting protein stability and, consequently, disease pathogenesis.
While our results support a wider-reaching impact of bacteria on the stability of host proteins, it is worth noting that bacteria can selectively target components of the host proteostasis network. This notion is supported by our data showing that P. corporis acts as a potent protector against host proteotoxicity (Figures 3, 5A, 5B, S4, and S5) and an activator of the HSR (Figure 5D). Reports demonstrating that activation of the HSR leads to a reduction of protein aggregation support our findings,76 suggesting that activation of the HSR is a mechanism through which P. corporis confers proteoprotection to the host. Another example of bacterial influence on host proteostasis is illustrated by P. aeruginosa, which produces toxins that target the mitochondrial unfolded protein response (UPRMT),77 a transcriptional pathway that ensures proper protein folding and clearance.78 As such, when the UPRMT is overwhelmed or disrupted, proteotoxicity can occur, leading to accelerated protein aggregation.79 Our previous work demonstrated that P. aeruginosa and E. coli can also disrupt host proteostasis through the production of bacteria-derived protein aggregates.16,24 Additionally, P. aeruginosa FapC amyloids were shown to cross-seed with Aβ1-42.80 Interestingly, the fap operon is also present in the genomes of Burkholderiales, which include Ralstonia and Achromobacter.81,82 The E. coli amyloid, curli, enhanced the aggregation of α-synuclein in vivo.83 K. pneumoniae is also capable of producing amyloids,84 and was proteotoxic to all C. elegans lines (Figure 4). The underlying mechanisms of the bacteria-induced proteotoxicity remain elusive; however, it is likely that metastable proteins of bacterial origin sequester host chaperones. Such sequestration would diminish the capacity of the proteostasis network to buffer the folding of endogenous proteins. A similar mechanism is supported by the organismal ability to buffer protein polymorphisms present within the host proteome, which is hindered by the introduction of misfolded proteins.85
Another way bacteria can cause proteotoxicity in their host is through inflammation, which initiates a cascade where disrupted proteostasis amplifies inflammation and perpetuates a continuous cycle.30 Bacteria have the capacity to stimulate the generation and release of reactive oxygen and nitrogen species, which trigger inflammation and have the potential to disrupt host proteostasis.30 Moreover, the endotoxin hypothesis of neurodegeneration postulates that endotoxin from the gut can breach the blood-brain barrier and trigger neuroinflammation and neurodegeneration.86,87 This process is thought to be initiated by bacterial dysbiosis, leading to an increase in gram-negative bacterial species that contribute to systemic inflammation.
Maintaining a balanced microbiome is critical for human health and longevity. However, the microbial equilibrium can be disrupted by many factors, including the overgrowth of pathogenic bacteria and antimicrobial use, leading to a state of gut dysbiosis. Notably, Prevotella spp., which we demonstrated to have a proteoprotective effect (Figure 3), can be depleted by antimicrobial use.30 Conversely, the proteotoxic bacteria identified in our study, A. xylosoxidans, K. pneumoniae, and Ralstonia sp. (Figure 4), are resistant to many antibiotics.82,88,89,90,91 This raises a new concern in the current era of increasing antimicrobial resistance, as poor antimicrobial stewardship might enrich not only for multidrug-resistant bacteria, but also for multidrug-resistant bacteria that are proteotoxic and pose potential risk factors for neurodegenerative disease. The implications of antibiotic-induced gut dysbiosis on PCD pathogenesis become particularly intriguing when corroborated with our data showing that P. corporis completely inhibited the proteotoxicity of K. pneumoniae (Figure 5A). However, P. corporis alone also protected the host against proteotoxicity (Figure 3), suggesting that beneficial bacteria may serve an additional role by counteracting the detrimental effects on host proteins induced by other bacterial species. While our data indicate the protection is likely due to P. corporis enhancing the host’s capacity to effectively manage bacteria-induced proteotoxicity through activation of the HSR (Figure 5D), it is possible that the presence of P. corporis also directly hinders the detrimental potential of proteotoxic bacteria. Ongoing research is aimed at elucidating the precise signals and mechanisms by which P. corporis exerts its protective effects.
The clinical relevance of our findings, which suggest a protective role of P. corporis against protein misfolding and aggregation by suppressing bacteria-induced proteotoxicity and activation of the host HSR (Figure 5D), is bolstered by previous literature that links decreased Prevotella abundance with exacerbated PCD.7,8,9,10,11,12,13,14 This is further supported by studies that implicate the HSR as a promising therapeutic target for neurodegenerative disease. For example, several studies observed a reduction in AD risk with moderate to frequent sauna bathing, suggesting that intermittent, temperature-induced activation of the HSR protects against proteotoxicity,92,93 a mechanism that has been demonstrated in C. elegans.94 Intranasal administration of human chaperone HSP70 improved AD-like symptoms in two mouse models of AD.95 Treatment of ALS mice with arimoclomol, a drug that increases the levels of HSP70 by interacting with the HSR transcription factor, heat shock factor-1 (HSF-1),96 delayed disease progression.97 Additionally, a study demonstrated that the cytoprotective effect of the FDA-approved ALS drug, riluzole, is dependent on its ability to increase cytosolic HSF-1.98 Collectively, the abovementioned research implicating activation of the HSR as an intervention strategy for neurodegenerative diseases reinforces the clinical importance of our findings linking the ability of P. corporis to suppress proteotoxicity and activate the HSR in its host. To our knowledge, this is the first-ever report of bacteria inducing a protective stress response in the host. Such a finding bolsters the feasibility of a microbial management strategy to treat neurodegenerative disease.
A comprehensive understanding of individual bacterial residents of the human microbiota in the pathogenesis of neurodegenerative PCDs is crucial in developing effective interventions. Our study challenges the traditional approach of solely focusing on host-targeted therapeutics for neurodegenerative PCDs. Instead, modulating the gut microbiota may offer an effective strategy for preventing and managing these devastating diseases. Gut-targeted interventions will likely have to be implemented early in the disease or even prior to its onset. While our results indicate that bacteria generally affect host proteostasis, ultimately influencing the stability of host proteins, further studies are needed to decipher the role of proteoprotective and proteotoxic species in humans and devise approaches to control their levels.
Limitations of the study
Our study has several limitations that need to be acknowledged. First, using C. elegans as a model organism may not fully recapitulate the complexity of human neurodegenerative diseases. A major limitation of our study is the temperature at which nematodes are cultured. While worms grow at the optimal temperatures of 15°C–25°C, bacteria that colonize the human gut are exposed to human body temperature. As such, it is possible that specific factors, either beneficial or detrimental, are not synthesized by bacteria at temperatures lower than 37°C. Our single bacterial strain approach also has limitations in that it does not capture the complexity of the microbial interactions within the human gut microbiota. Furthermore, our study addresses the short-term effect of bacterial colonization, but the long-term effects will have to be explored using other models with longer lifespans. Despite these limitations, our approach and model are best suited to our specific research objectives, and the results are supported by correlational studies observed in patients with neurodegenerative diseases.
Resource availability
Lead contact
Additional information and request for resources should be directed to and will be fulfilled by lead contact, Daniel M. Czyż (dczyz@ufl.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
All data reported in this paper will be shared by the lead author upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in the paper is available from the lead contact upon request.
Acknowledgments
We thank Dr. Richard Morimoto and Sue Fox for providing C. elegans polyQ strains (Northwestern University), and Dr. Janine Kirstein (Leibniz Institute on Aging − Fritz Lipmann Institute) for providing the C. elegans Aβ1-42 strain and the "No Aβ" control. All other C. elegans strains were provided by the Caenorhabditis Genetics Center, which is funded by the NIH Office of Research Infrastructure Programs (P40 OD010440). Human bacterial isolates (listed in Table S1) were obtained through BEI Resources, NIAID, and NIH as part of the Human Microbiome Project. We thank Dr. Mark Gorelik for training RB and for assistance with computational analysis. We would like to thank the funders who have supported our work on the bacterial contribution to protein conformational diseases, namely the National Institute on Aging (R03AG070580), the Infectious Diseases Society of America – Microbial Pathogenesis in Alzheimer’s Disease, the Glenn Foundation for Medical Research, and AFAR Grant for Junior Faculty. Furthermore, we acknowledge and thank the Department of Microbiology and Cell Science for start-up funding. The graphical abstract and Figure 1 were created using BioRender.com.
Author contributions
Conceptualization: D.M.C.; methodology: A.C.W. and D.M.C.; software: R.B. and M.J.B.; validation: A.C.W., R.B., Y.M.A., and A.S.B.; formal analysis: A.C.W. and D.M.C.; investigation: D.M.C., A.C.W., R.B., Y.M.A., and A.S.B.; resources: D.M.C.; writing − original draft: A.C.W. and D.M.C.; writing − reviewing & editing: A.C.W. and D.M.C.; visualization: A.C.W., R.B., and M.J.B.; supervision: D.M.C. and A.C.W.; project administration: D.M.C.; funding acquisition: D.M.C.
Declaration of interests
The authors declare no competing interest.
STAR★Methods
Key resources table
C. elegans strains, bacterial strains, and reagents.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Living Colors JL-8 primary monoclonal antibody | Takara Bio | Cat#632381; RRID: AB_2313808 |
| Goat anti-mouse HRP secondary antibody | Thermo Scientific | Prod#31430; RRID: AB_2548904 |
| Bacterial and virus strains | ||
| Escherichia coli OP50 | Caenorhabditis Genetics Center | WB OP50: RRID: WB-STRAIN: OP50; NCBI TaxID: 637912; DC199 |
| Pseudomonas aeruginosa PAO1 | Shuman Lab (University of Chicago) | PAO1; DC3 |
| Acetobacteraceae sp. | BEI Resources | HM-648 |
| Achromobacter xylosoxidans | BEI Resources | HM-235 |
| Acidaminococcus sp. | BEI Resources | HM-81 |
| Acidaminococcus sp. | BEI Resources | HM-853 |
| Acinetobacter radioresistens | BEI Resources | HM-107 |
| Actinomyces cardiffensis | BEI Resources | HM-147 |
| Actinomyces gerencseriae | BEI Resources | HM-97 |
| Actinomyces graevenitzii | BEI Resources | HM-236 |
| Actinomyces israelii | BEI Resources | HM-98 |
| Actinomyces johnsonii | BEI Resources | HM-1070 |
| Actinomyces massiliensis | BEI Resources | HM-814 |
| Actinomyces neuii | BEI Resources | HM-1266 |
| Actinomyces odontolyticus | BEI Resources | HM-94 |
| Actinomyces sp. | BEI Resources | HM-1090 |
| Actinomyces sp. | BEI Resources | HM-146 |
| Actinomyces urogenitalis | BEI Resources | HM-1089 |
| Actinomyces viscosus | BEI Resources | HM-238 |
| Aggregatibacter aphrophilus | BEI Resources | HM-206 |
| Akkermansia sp. | BEI Resources | HM-844 |
| Alloscardovia omnicolens | BEI Resources | HM-1282 |
| Anaerococcus hydrogenalis | BEI Resources | HM-1292 |
| Anaerococcus lactolyticus | BEI Resources | HM-1034 |
| Anaerostipes sp. | BEI Resources | HM-220 |
| Arcobacter butzleri | BEI Resources | HM-298 |
| Arthrobacter albus | BEI Resources | HM-1152 |
| Atopobium parvulum | BEI Resources | HM-1035 |
| Atopobium parvulum | BEI Resources | HM-1084 |
| Atopobium sp. | BEI Resources | HM-839 |
| Bacteroides caccae | BEI Resources | HM-728 |
| Bacteroides cellulosilyticus | BEI Resources | HM-726 |
| Bacteroides dorei | BEI Resources | HM-717 |
| Bacteroides eggerthii | BEI Resources | HM-210 |
| Bacteroides finegoldii | BEI Resources | HM-727 |
| Bacteroides fragilis | BEI Resources | HM-20 |
| Bacteroides fragilis | BEI Resources | HM-709 |
| Bacteroides ovatus | BEI Resources | HM-222 |
| Bacteroides salyersiae | BEI Resources | HM-725 |
| Bacteroides sp. | BEI Resources | HM-18 |
| Bacteroides stercoris | BEI Resources | HM-1036 |
| Bacteroides vulgatus | BEI Resources | HM-720 |
| Bacteroidetes | BEI Resources | HM-4 |
| Bifidobacterium adolescentis | BEI Resources | HM-633 |
| Bifidobacterium angulatum | BEI Resources | HM-1189 |
| Bifidobacterium breve | BEI Resources | HM-856 |
| Bifidobacterium longum | BEI Resources | HM-846 |
| Bifidobacterium sp. | BEI Resources | HM-30 |
| Campylobacter coli | BEI Resources | HM-296 |
| Campylobacter upsaliensis | BEI Resources | HM-297 |
| Capnocytophaga ochracea | BEI Resources | HM-15 |
| Capnocytophaga sp. | BEI Resources | HM-840 |
| Capnocytophaga sputigena | BEI Resources | HM-1037 |
| Cardiobacterium valvarum | BEI Resources | HM-477 |
| Catabacter hongkongensis | BEI Resources | HM-1192 |
| Citrobacter portucalensis | BEI Resources | HM-299 |
| Citrobacter sp. | BEI Resources | HM-34 |
| Clostridiales bacterium | BEI Resources | HM-1098 |
| Clostridiales bacterium | BEI Resources | HM-793 |
| Clostridiales sp. | BEI Resources | HM-1182 |
| Clostridium bolteae | BEI Resources | HM-318 |
| Clostridium cadaveris | BEI Resources | HM-1039 |
| Clostridium cadaveris | BEI Resources | HM-1041 |
| Clostridium citroniae | BEI Resources | HM-315 |
| Clostridium clostridioforme | BEI Resources | HM-306 |
| Clostridium difficile | BEI Resources | HM-745 |
| Clostridium difficile | BEI Resources | HM-746 |
| Clostridium difficile | BEI Resources | HM-88 |
| Clostridium innocuum | BEI Resources | HM-173 |
| Clostridium orbiscindens | BEI Resources | HM-1044 |
| Clostridium orbiscindens | BEI Resources | HM-303 |
| Clostridium sp. | BEI Resources | HM-287 |
| Clostridium sp. | BEI Resources | HM-36 |
| Clostridium symbiosum | BEI Resources | HM-309 |
| Collinsella sp. | BEI Resources | HM-304 |
| Coprobacillus sp. | BEI Resources | HM-85 |
| Coprococcus sp. | BEI Resources | HM-794 |
| Corynebacterium amycolatum | BEI Resources | HM-109 |
| Corynebacterium sp. | BEI Resources | HM-1295 |
| Corynebacterium sp. | BEI Resources | HM-784 |
| Corynebacterium tuscaniense | BEI Resources | HM-1153 |
| Deinococcus grandis | BEI Resources | HM-111 |
| Dermabacter sp. | BEI Resources | HM-857 |
| Dorea formicigenerans | BEI Resources | HM-300 |
| Eggerthella sp. | BEI Resources | HM-1099 |
| Enterococcus faecalis | BEI Resources | HM-432 |
| Enterococcus faecium | BEI Resources | HM-968 |
| Erysipelotrichaceae sp. | BEI Resources | HM-180 |
| Escherichia coli | BEI Resources | HM-340 |
| Escherichia coli | BEI Resources | HM-341 |
| Escherichia sp. | BEI Resources | HM-38 |
| Eubacterium infirmum | BEI Resources | HM-369 |
| Eubacterium sp. | BEI Resources | HM-766 |
| Facklamia sp. | BEI Resources | HM-289 |
| Faecalibacterium prausnitzii | BEI Resources | HM-473 |
| Finegoldia magna | BEI Resources | HM-1285 |
| Fusobacterium gonidiaformans | BEI Resources | HM-1274 |
| Fusobacterium nucleatum | BEI Resources | HM-75 |
| Fusobacterium nucleatum | BEI Resources | HM-260 |
| Fusobacterium sp. | BEI Resources | HM-871 |
| Fusobacterium ulcerans | BEI Resources | HM-57 |
| Gardnerella vaginalis | BEI Resources | HM-1105 |
| Gemella asaccharolytica | BEI Resources | HM-1242 |
| Gemella haemolysans | BEI Resources | HM-239 |
| Gemella morbillorum | BEI Resources | HM-240 |
| Gemella sanguinis | BEI Resources | HM-241 |
| Granulicatella adiacens | BEI Resources | HM-1047 |
| Helicobacter pullorum | BEI Resources | HM-124 |
| Helicobacter pylori | BEI Resources | HM-273 |
| Hungatella hathewayi | BEI Resources | HM-308 |
| Klebsiella oxytoca | BEI Resources | HM-624 |
| Klebsiella pneumoniae | BEI Resources | HM-751 |
| Klebsiella sp. | BEI Resources | HM-354 |
| Klebsiella sp. | BEI Resources | HM-44 |
| Lachnoanaerobaculum sp. | BEI Resources | HM-780 |
| Lactobacillis oris | BEI Resources | HM-560 |
| Lactobacillus crispatus | BEI Resources | HM-375 |
| Lactobacillus gasseri | BEI Resources | HM-399 |
| Lactobacillus gasseri | BEI Resources | HM-647 |
| Lactobacillus iners | BEI Resources | HM-702 |
| Lactobacillus jensenii | BEI Resources | HM-646 |
| Lactobacillus johnsonii | BEI Resources | HM-643 |
| Lactobacillus parafarraginis | BEI Resources | HM-478 |
| Lactobacillus rhamnosus | BEI Resources | HM-106 |
| Leptotrichia goodfellowii | BEI Resources | HM-12 |
| Listeria monocytogenes | BEI Resources | HM-1048 |
| Mageeibacillus indolicus | BEI Resources | HM-1095 |
| Megasphaera micronuciformis | BEI Resources | HM-1172 |
| Microbacterium sp. | BEI Resources | HM-841 |
| Micrococcus luteus | BEI Resources | HM-114 |
| Mobiluncus mulieris | BEI Resources | HM-125 |
| Neisseria flavescens | BEI Resources | HM-115 |
| Neisseria mucosa | BEI Resources | HM-242 |
| Neisseria sp. | BEI Resources | HM-91 |
| Olsenella sp. | BEI Resources | HM-1239 |
| Olsenella uli | BEI Resources | HM-877 |
| Oribacterium sinus | BEI Resources | HM-13 |
| Oscillibacter sp. | BEI Resources | HM-1030 |
| Oxalobacter formigenes | BEI Resources | HM-1 |
| Paenibacillus barengoltzii | BEI Resources | HM-1049 |
| Paenisporosarcina sp. | BEI Resources | HM-788 |
| Parabacteroides goldsteinii | BEI Resources | HM-1050 |
| Parabacteroides merdae | BEI Resources | HM-729 |
| Parvimonas micra | BEI Resources | HM-1052 |
| Parvimonas sp. | BEI Resources | HM-1253 |
| Parvimonas sp. | BEI Resources | HM-207 |
| Peptoniphilus lacrimalis | BEI Resources | HM-1161 |
| Peptoniphilus sp. | BEI Resources | HM-825 |
| Peptoniphilus sp. | BEI Resources | HM-263 |
| Peptostreptococcaceae bacterium | BEI Resources | HM-483 |
| Peptostreptococcus sp. | BEI Resources | HM-1051 |
| Phascolarctobacterium sp. | BEI Resources | HM-179 |
| Plesiomonas sp. | BEI Resources | HM-791 |
| Porphyromonas gingivalis | BEI Resources | HM-1071 |
| Porphyromonas gingivalis | BEI Resources | HM-1073 |
| Porphyromonas sp. | BEI Resources | HM-1064 |
| Porphyromonas sp. | BEI Resources | HM-781 |
| Porphyromonas uenonis | BEI Resources | HM-130 |
| Prevotella amnii | BEI Resources | HM-138 |
| Prevotella bivia | BEI Resources | HM-1165 |
| Prevotella bivia | BEI Resources | HM-1270 |
| Prevotella bivia | BEI Resources | HM-1286 |
| Prevotella buccae | BEI Resources | HM-45 |
| Prevotella corporis | BEI Resources | HM-1294 |
| Prevotella denticola | BEI Resources | HM-1173 |
| Prevotella denticola | BEI Resources | HM-208 |
| Prevotella disiens | BEI Resources | HM-1171 |
| Prevotella histicola | BEI Resources | HM-471 |
| Prevotella melaninogenica | BEI Resources | HM-80 |
| Prevotella nigrescens | BEI Resources | HM-271 |
| Prevotella oralis | BEI Resources | HM-1054 |
| Prevotella oralis | BEI Resources | HM-849 |
| Prevotella oris | BEI Resources | HM-93 |
| Prevotella sp. | BEI Resources | HM-1103 |
| Prevotella sp. | BEI Resources | HM-16 |
| Prevotella sp. | BEI Resources | HM-5 |
| Prevotella timonensis | BEI Resources | HM-136 |
| Prevotella veroralis | BEI Resources | HM-92 |
| Propionibacterium acidifaciens | BEI Resources | HM-8 |
| Propionibacterium acnes | BEI Resources | HM-491 |
| Propionibacterium propionicum | BEI Resources | HM-209 |
| Propionibacterium sp. | BEI Resources | HM-843 |
| Pseudomonas sp. | BEI Resources | HM-860 |
| Psychrobacter sp. | BEI Resources | HM-332 |
| Ralstonia sp. | BEI Resources | HM-158 |
| Rhodococcus erythropolis | BEI Resources | HM-116 |
| Rothia aeria | BEI Resources | HM-818 |
| Rothia dentocariosa | BEI Resources | HM-245 |
| Rothia mucilaginosa | BEI Resources | HM-1055 |
| Ruminococcaceae sp. | BEI Resources | HM-79 |
| Ruminococcus gnavus | BEI Resources | HM-1056 |
| Ruminococcus lactaris | BEI Resources | HM-1057 |
| Scardovia wiggsiae | BEI Resources | HM-470 |
| Selenomonas noxia | BEI Resources | HM-270 |
| Selenomonas sp. | BEI Resources | HM-564 |
| Shigella sp. | BEI Resources | HM-87 |
| Shuttleworthia sp. | BEI Resources | HM-882 |
| Sneathia amnii | BEI Resources | NR-50515 |
| Solobacterium moorei | BEI Resources | HM-1058 |
| Solobacterium moorei | BEI Resources | HM-1059 |
| Sporosarcina sp. | BEI Resources | HM-331 |
| Staphylococcus aureus | BEI Resources | HM-466 |
| Staphylococcus capitis | BEI Resources | HM-117 |
| Staphylococcus caprae | BEI Resources | HM-143 |
| Staphylococcus epidermidis | BEI Resources | HM-140 |
| Staphylococcus haemolyticus | BEI Resources | HM-1164 |
| Staphylococcus hominis | BEI Resources | HM-119 |
| Staphylococcus lugdunensis | BEI Resources | HM-141 |
| Staphylococcus warneri | BEI Resources | HM-120 |
| Stomatobaculum longum | BEI Resources | HM-480 |
| Streptococcus anginosus | BEI Resources | HM-282 |
| Streptococcus cristatus | BEI Resources | HM-163 |
| Streptococcus downei | BEI Resources | HM-475 |
| Streptococcus gallolyticus | BEI Resources | HM-272 |
| Streptococcus intermedius | BEI Resources | HM-368 |
| Streptococcus mitis | BEI Resources | HM-262 |
| Streptococcus parasanguinis | BEI Resources | HM-1060 |
| Streptococcus pneumoniae | BEI Resources | HM-145 |
| Streptococcus salivarius | BEI Resources | HM-121 |
| Streptococcus sobrinus | BEI Resources | HM-1063 |
| Streptococcus sp. | BEI Resources | HM-885 |
| Streptococcus vestibularis | BEI Resources | HM-561 |
| Sutterella wadsworthensis | BEI Resources | HM-852 |
| Tissierellia bacterium | BEI Resources | HM-1096 |
| Treponema denticola | BEI Resources | HM-569 |
| Treponema denticola | BEI Resources | HM-575 |
| Varibaculum cambriense | BEI Resources | HM-1190 |
| Veillonella atypica | BEI Resources | HM-1301 |
| Veillonella montpellierensis | BEI Resources | HM-1157 |
| Veillonella sp. | BEI Resources | HM-778 |
| Weissella cibaria | BEI Resources | HM-1200 |
| Chemicals, peptides, and recombinant proteins | ||
| Levamisole | Fisher Scientific | Cat#ICN15522805 |
| Cholesterol | MP Biomedicals | Cat#101382 |
| Powdered nonfat milk | Research Products International | M17200-1000 |
| Tween 20 | Fisher BioReagents | Cat#BP337-100 |
| Trans-Blot Turbo Midi-size Transfer Stacks | BioRad | Cat#1704273 |
| Trans-Blot Turbo Midi-size PDVF membrane | BioRad | Cat#10026933 |
| Trans-Blot Turbo 5× transfer buffer | BioRad | Cat#10026938 |
| Criterion XT Precast Gel | BioRad | Cat#3450124 |
| XT 4× Sample Buffer | BioRad | Cat#1610791 |
| 20× Reducing Agent | BioRad | Cat#1610792 |
| XT MOPS | BioRad | Cat#1610788 |
| Clarity Western ECL | BioRad | Cat#1705061 |
| Oxyrase | OXYRASE | Cat#OB-0100 |
| Experimental models: organisms/strains | ||
| C. elegans: Strain AM738: rmIs297[vha-6p::q44::yfp; rol-6(su1006)] | Morimoto Lab (Northwestern University) | AM738; intestinal polyQ44 |
| C. elegans: Strain 712: rmIs281[vha-6p::q33::yfp; rol-6(su1006 | Morimoto Lab (Northwestern University) | AM712; intestinal polyQ33 |
| C. elegans: Strain AM141: rmIs133[unc-54p::q40::yfp] | Morimoto Lab (Northwestern University) | AM141; muscle polyQ40 |
| C. elegans: Strain AM140: rmIs132[unc-54p::q35::yfp] | Morimoto Lab (Northwestern University) | AM140; muscle polyQ35 |
| C. elegans: Strain AM134: rmIs126[unc-54p::q0::yfp] | Morimoto Lab (Northwestern University) | AM134; muscle polyQ0 |
| C. elegans: Strain AM101: rmIs110[F25B3.3p::q40::yfp] | Morimoto Lab (Northwestern University) | AM101; neuronal polyQ40 |
| C. elegans: Strain AM52: rmIs182[F25B3.3p::q0::yfp] | Morimoto Lab (Northwestern University) | AM52; neuronal polyQ0 |
| C. elegans: Strain JKM7: Is[myo-3p::Signalpeptide-Abeta(1–42)::hsp-3(IRES)::wrmScarlet-Abeta(1–42)::unc-54(3′UTR)+rps-0p::HygroR] | Kirstein Lab (University of Bremen, Germany) | JKM7; muscle Aβ1-42 |
| C. elegans: Strain JKM8: Ex[myo-3p::wrmScarlet-Abeta::unc-54(3′UTR)+rps-0p::HygroR] | Kirstein Lab (University of Bremen, Germany) | JKM8, no Aβ1-42 |
| C. elegans: Strain DDP1: uonEx1[unc-54::alpha-synuclein::CFP+unc-54::alpha-synuclein::YFP(Venus)] | Caenorhabditis Genetics Center | WB Strain: DDP1 WormBaseID: WBStrain00005628 This paper: α-syn1 |
| C. elegans: Strain NL5901: pkIs2386[unc-54p::alphasynuclein::YFP+unc-119(+)] | Caenorhabditis Genetics Center | WB Strain: NL5901 WormBase ID: WBStrain00029035 This paper: α-syn2 |
| C. elegans: Strain N2 | Caenorhabditis Genetics Center | WB Strain: N2 WormBase ID: WBStrain00000001 This paper: WT |
| C. elegans: Strain CB1301: unc-54(e1301) | Caenorhabditis Genetics Center | WB Strain: CB1301 WormBase ID: WBStrain00004294 This paper: unc-54(ts) |
| C. elegans: Strain CX51: dyn-1(ky51) X. | Caenorhabditis Genetics Center | WB Strain: CX51 WormBase ID: WBStrain00005217 This paper: dyn-1(ts) |
| C. elegans: Strain AM446: rmIs223[C12C8.1p::gfp; rol-6(su1006)] | Morimoto Lab (Northwestern University) | AM446; hsp70p::GFP |
| Software and algorithms | ||
| GraphPad Prism v8.4.0 | GraphPad Software, Inc | http://www.graphpad.com |
| BioRender | BioRender | www.biorender.com |
| RStudio | RStudio Integrated Development for R | http://www.rstudio.com/ |
| BV-BCR | Bacterial and Viral Bioinformatics Resources Center | https://www.bv-brc.org/ |
| Unicycler | N/A | https://doi.org/10.1371/JOURNAL.PCBI.1005595 |
| Prokka | N/A | https://doi.org/10.1093/bioinformatics/btu153 |
| MAFFT | N/A | https://doi.org/10.1093/nar/gkf436 |
| RAxML | N/A | https://doi.org/10.1093/bioinformatics/btu033 |
| Other | ||
| Thermo Scientific- Anaeropack | Fisher Scientific | Cat#23-246-376 |
| ProSignal Blotting Film | Prometheus | Cat#30-810L |
Experimental model and study participant details
C. elegans maintenance
C. elegans strains were maintained as previously described.99 For experiments that generated the data represented in the heatmap (Figure 2), nematodes were kept on E. coli OP50 for two days at 20°C, washed three times and transferred to indicated bacteria, where they were kept at 22.5°C for three days. For all other experiments, age-synchronized nematodes were plated on indicated bacteria as L1s at 22.5°C, where they remained until the time of assay, except for the experiment in Figure S5 ("Muscle"), in which worms were cultured in temperatures indicated in the figure, and S5 ("Neuronal") in which worms were cultured at 15°C, assayed, then shifted to 25°C for 1 h prior to the motility assay. All C. elegans strains used in this study can be found in the key resources table.
Method details
Bacterial culture conditions
All bacteria were cultured at 37°C. Table S1 lists the growth condition for each bacteria represented in Figure 2 (1–5): 1) anaerobically in reinforced clostridial broth with Oxyrase, 2) facultative conditions (no shaking, tube filled to top) in brain heart infusion broth (BHI), 3) aerobic conditions, shaking at 220 revolutions per minute (RPM) in BHI, 4) anaerobic conditions on tryptic soy agar (TSA) supplemented with 5% defibrinated sheep’s blood, 5) aerobic conditions on TSA supplemented with 5% defibrinated sheep’s blood. E. coli OP50 controls were grown in a manner consistent with which experiment was performed. Bacteria were washed and resuspended in M9 before being seeded on NGM. All follow-up experiments were performed with bacteria grown on TSA supplemented with 5% defibrinated sheep’s blood at 37°C except for the intestinal confirmation experiment of the proteotoxic bacteria (Figure 4A, "Aggregates"), in which bacteria were grown according to growth condition "3" described above. Except for Prevotella spp. which were grown under anaerobic conditions, bacteria were grown aerobically in follow-up experiments.
Aggregate quantification
Fluorescent aggregates were quantified using Leica MZ10F Modular Stereo Microscope equipped with CoolLED pE300lite 365 dir mount STEREO with filter set ET GFP-MZ10F. Fluorescent aggregates in worms used to generate data for the heatmap (Figure 2; Table S1) were manually quantified after having been cultured as indicated in "C. elegans maintenance." For all other experiments, worms were plated on indicated bacteria as L1s and fluorescent aggregates were manually quantified after four days (intestinal polyQ44) or three days (muscle polyQ35, 40) of life. Aggregates of worms that were quantified to generate the data used in the heatmap were quantified blind.
Motility assays
All motility assays were performed at room temperature, as previously described.16,17 In brief, the TOP assay entails sliding a worm pick made with an eyebrow hair under the mid-section of the worm and using a ticking second timer to count the number of seconds it takes for the worm to crawl off entirely. Longer TOP (seconds) indicates a more severe motility defect. TOP (seconds) of transgenic nematodes harboring tissue-specific aggregating protein was assessed on day three (muscle polyQ, muscle Aβ1-42, unc-54(ts), dyn-1(ts), and respective controls) or four (intestine polyQ, neuron polyQ, muscle α-synuclein and respective controls) after plating on indicated bacteria as L1s. Worms carrying the temperature-sensitive mutation in dyn-1(ts) were assayed before and after a 1-h shift to the restrictive temperature (see “C. elegans maintenance” for details).
Feeding worms PFA-killed bacteria
Bacteria were cultured on TSA-blood plates under aerobic (E. coli OP50) or anaerobic (P. corporis) conditions, as described in “bacterial culture conditions.” Bacterial lawns were washed and resuspended in M9 and adjusted to OD600 = 2. PFA was added at a final concentration of 0.5% and incubated at 37°C, shaking at 220 RPM for 1 h. After incubation, samples were washed four times by centrifugation at 5,000 × g for 10 min and resuspension in M9. Bacterial killing was confirmed by spotting 20 μL of each sample onto TSA-blood plates and incubating under the above-specified conditions. Dead bacterial samples were seeded onto NGM and allowed to dry overnight. Once dried, age-synchronized L1 worms expressing intestinal polyQ44 were plated onto them and maintained for four days in a 22.5°C incubator. PolyQ aggregates were quantified as described in “aggregate quantification.”
Pharyngeal pumping
Pharyngeal pumping was assessed using a modified protocol from Raizen et al., 2012.100 Bacteria were cultured on TSA-blood plates in anaerobic conditions and seeded as described in “bacterial culture conditions.” Age-synchronized L1 N2s were plated onto NGM containing indicated bacteria and kept at 22.5°C for four days until the assay. After four days, each worm was picked onto a fresh plate of NGM seeded with the indicated bacteria and was given 10-15 min to acclimate. The number of pharyngeal pumps was counted over a 30-s period. A single pump was scored as a complete backward motion of the terminal bulb grinder.101 This was repeated on the same worm for a total of 10 times per worm.
Exposing worms to bacterial supernatants
Bacteria were grown on TSA-blood plates under aerobic (E. coli OP50, K. pneumoniae, A. xylosoxidans) or anaerobic (P. corporis) conditions. E. coli OP50 pellets and bacterial supernatants were obtained as follows: bacterial lawns were resuspended in M9, adjusted to OD600 = 2, centrifuged at 5,000 × g for 15 min and sterile filtered. E. coli OP50 pellets were washed and resuspended in M9 (No SN) or supernatants from indicated bacteria. Suspensions were seeded onto NGM and allowed to dry overnight prior to having worms (intestinal polyQ44) plated on them. Details regarding worm handling and aggregate quantification can be found in “aggregate quantification.”
Co-colonization assays
Bacteria were grown on TSA-blood plates under aerobic (E. coli OP50, K. pneumoniae) or anaerobic (P. corporis) conditions. Bacterial lawns were resuspended in M9 and indicated bacteria were mixed 1:1 (CFU/mL) and seeded on NGM. The seeded plates were allowed to dry for 6–8 h in ambient conditions prior to having worms (intestinal polyQ44) plated on them. Details regarding worm handling and aggregate quantification can be found in “aggregate quantification.”
Quantification of intestinal bacteria
Bacterial loads in the C. elegans intestine (N2, WT) were quantified as we have done previously. C. elegans were lysed using the BeadBug.102 Bacteria were cultured on TSA-blood plates in aerobic conditions as described in “bacterial culture conditions.” Age-synchronized L1 N2s were plated onto NGM containing indicated bacteria and kept at 22.5°C for four days until the assay.
Live imaging
Nematodes expressing muscle-specific Aβ1-42 were plated as L1s on NGM containing E. coli OP50, P. corporis HM-1294, and P. aeruginosa PAO1 for three days were mounted on a 3% agarose pad, frozen to reduce background fluorescence, and imaged. Nematodes expressing hsp70p::GFP were plated as L1s onto NGM containing E. coli OP50 for two days, washed with M9 and transferred onto NGM containing indicated bacteria for 24 h, and were paralyzed with 2 mM levamisole, mounted on a 3% agarose pad, and imaged. Fluorescent and Nomarski images were taken using Zeiss Axio Observer 7 microscope equipped with Axiocam 503 mono camera, Solid-State Light Source Colibri 7, and a 10× EC Plan-Neofluar objective (0.3 NA) and were processed using ZEN Tiles & Positions Module in Zeiss ZenPro Software.
Gel electrophoresis and western blotting
Worms were prepared for gel electrophoresis and western blotting as previously described with a few modifications.16 In brief, M9 was used to lift worms from NGM which were washed until superficial bacteria was removed. Worms were plated on unseeded NGM, were allowed to dry briefly and 50 worms were picked into 10 μL M9 with 1 mM phenylmethylsulfonyl fluoride (PMSF) in non-skirted screw-cap microcentrifuge tubes and frozen overnight or longer at −80°C. Tubes were pulse-centrifuged and three, 1.5 mm zirconium beads were added to each tube. The following two steps were repeated a minimum of two times; if large worm particulates were observed, these steps were repeated again with the entire sample set until no tubes contained worm pieces that were visible under a dissecting microscope: Samples were homogenized by placing tubes in a Bead Bug homogenizer at 280 × 10 rates per minute (RPM) for 90 s. Tubes were cooled on ice and centrifuged for 1–2 min at a low speed to re-settle worms and worm particulates. Samples were transferred to new tubes with XT loading buffer and reducing agent. Samples were heated at 98°C for 7 min, cooled,16 and entire samples were loaded on 4–12% gradient sodium dodecyl sulfate-polyacrylamide gels (SDS-PAGE) to separate proteins through electrophoresis. Proteins were transferred onto polyvinyl difluoride (PDVF) membrane, blocked with 5% nonfat milk powder in PBS-Tween-20 (PBST) and probed with Living Colors JL-8 monoclonal primary antibody (1:1000) for 48 h at 4°C on orbital shaker and underwent three, 5-min washes with 0.1% PBST followed by incubation 1:10,000 with goat-anti-mouse horseradish peroxidase conjugated secondary antibody for at least 2 h followed by three, 10-min washes with 0.1% PBST. Chemiluminescence was achieved by incubation with Clarity Western ECL substrate. Insoluble fractions were analyzed using ImageJ (v1.52) or ChemiDox XRS+System with Image Lab Image Capture and Analysis Software. Western blots to obtain the insoluble fractions from worms that were used to generate the data used in the heatmap were conducted blind.
Heatmap generation
Heatmap was constructed in R-studio using the ComplexHeatmap package from the Bioconductor project. The experimental data were fed into R-studio as an organized.CSV file. The heatmap is the graphical representation of this data, clustering the bacteria by phylum.
Phylogenetic tree generation
Whole genome sequences were assembled using the Bacterial and Viral Bioinformatics Resources Center (BV-BCR) via Unicycler.103 All genomes were annotated in BV-BRC using Prokka.104 Phylogenetic analysis was conducted in BV-BRC based on concatenated alignments of 100 single-copy genes using mafft,105 and the results were assessed for maximum likelihood using RAxML.106 Sequences that did not have 100 complete single-copy genes due to poor sequencing quality were excluded.
Quantification and statistical analysis
Quantification of aggregate counts per worm in Figure 2 was performed on a cohort of ten worms to corroborate the findings from the western blot analysis. In the rest of the figures, data are represented as the average number of aggregates, TOP (seconds), or otherwise specified per worm obtained from two independent experiments for a total of 60 worms (Figures S2A–S2C and 3A “Aggregates”), 45–60 worms normalized to the control (Figure 4A, “Aggregates”); three independent experiments for a total of 98 worms (Figure S7) or 60 worms (Figure 5) or 45 worms (Figure 3A “Motility” (polyQ44, polyQ33), Figures 3B, 3C, and 4A “Motility,” Figure 4C “Aggregates”) or 30 worms (Figure 3A “Motility” (N2), Figures 3D, 3E, 4A, 4B, and 4C “Motility,” Figures 4D, 4E, S4B, S5, and S6), or 20 worms (Figure S1); two independent experiments for a total of 20 worms (Figure S3). Data are described as statistically significant when p < 0.05 as determined by Student’s t-test or one-way ANOVA followed by multiple comparison Dunnett’s post-hoc test (statistical tests used are indicated in figure legends) performed using Graphpad Prism 8.4.3 or later. Degrees of significance are denoted by asterisks such that ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. Error bars represent standard error of the mean. The heatmap (Figure 2) is represented as data normalized to the control E. coli OP50.
Published: August 27, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.110828.
Supplemental information
“Western” indicates the band intensity of the polyQ insoluble fraction of worms fed indicated bacteria normalized to those fed E. coli OP50 control. “Count” indicates the average number of aggregates per worm of worms fed indicated bacteria normalized to those fed E. coli OP50 control. Parenthesized numbers (1–5) reflect the remaining conditions in which bacteria were grown:(1) anaerobically in reinforced clostridial broth (RCB) with Oxyrase, (2) facultative conditions (no shaking, tube filled to top) in brain heart infusion broth (BHI), (3) aerobic conditions, shaking at 220 revolutions per minute (RPM) in BHI, (4) anaerobic conditions on tryptic soy agar (TSA) supplemented with 5% defibrinated sheep’s blood, (5) aerobic conditions on TSA supplemented with 5% defibrinated sheep’s blood.
References
- 1.Soto C. Unfolding the role of protein misfolding in neurodegenerative diseases. Nat. Rev. Neurosci. 2003;4:49–60. doi: 10.1038/nrn1007. [DOI] [PubMed] [Google Scholar]
- 2.Alzheimers Dement Alzheimer’s disease facts and figures. J. Alzheimers Assoc. 2022;18:700–789. doi: 10.1002/alz.12638. [DOI] [PubMed] [Google Scholar]
- 3.Oliphant K., Allen-Vercoe E. Macronutrient metabolism by the human gut microbiome: major fermentation by-products and their impact on host health. Microbiome. 2019;7:91. doi: 10.1186/s40168-019-0704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke G., Stilling R.M., Kennedy P.J., Stanton C., Cryan J.F., Dinan T.G. Minireview: Gut Microbiota: The Neglected Endocrine Organ. Mol. Endocrinol. 2014;28:1221–1238. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Intili G., Paladino L., Rappa F., Alberti G., Plicato A., Calabrò F., Fucarino A., Cappello F., Bucchieri F., Tomasello G., et al. From Dysbiosis to Neurodegenerative Diseases through Different Communication Pathways: An Overview. Biology. 2023;12:195. doi: 10.3390/biology12020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fang P., Kazmi S.A., Jameson K.G., Hsiao E.Y. The Microbiome as a Modifier of Neurodegenerative Disease Risk. Cell Host Microbe. 2020;28:201–222. doi: 10.1016/j.chom.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jin M., Li J., Liu F., Lyu N., Wang K., Wang L., Liang S., Tao H., Zhu B., Alkasir R. Analysis of the Gut Microflora in Patients With Parkinson’s Disease. Front. Neurosci. 2019;13 doi: 10.3389/fnins.2019.01184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scheperjans F., Aho V., Pereira P.A.B., Koskinen K., Paulin L., Pekkonen E., Haapaniemi E., Kaakkola S., Eerola-Rautio J., Pohja M., et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov. Disord. 2015;30:350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 9.Hertzberg V.S., Singh H., Fournier C.N., Moustafa A., Polak M., Kuelbs C.A., Torralba M.G., Tansey M.G., Nelson K.E., Glass J.D. Gut microbiome differences between amyotrophic lateral sclerosis patients and spouse controls. Amyotroph. Lateral Scler. Front. Degener. 2022;23:91–99. doi: 10.1080/21678421.2021.1904994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vidal-Martinez G., Chin B., Camarillo C., Herrera G.V., Yang B., Sarosiek I., Perez R.G. A Pilot Microbiota Study in Parkinson’s Disease Patients versus Control Subjects, and Effects of FTY720 and FTY720-Mitoxy Therapies in Parkinsonian and Multiple System Atrophy Mouse Models. J. Park. Dis. 2020;10:185–192. doi: 10.3233/JPD-191693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unger M.M., Spiegel J., Dillmann K.-U., Grundmann D., Philippeit H., Bürmann J., Faßbender K., Schwiertz A., Schäfer K.-H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 2016;32:66–72. doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 12.Li F., Wang P., Chen Z., Sui X., Xie X., Zhang J. Alteration of the fecal microbiota in North-Eastern Han Chinese population with sporadic Parkinson’s disease. Neurosci. Lett. 2019;707 doi: 10.1016/j.neulet.2019.134297. [DOI] [PubMed] [Google Scholar]
- 13.Bedarf J.R., Hildebrand F., Coelho L.P., Sunagawa S., Bahram M., Goeser F., Bork P., Wüllner U. Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients. Genome Med. 2017;9:39. doi: 10.1186/s13073-017-0428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aho V.T.E., Pereira P.A.B., Voutilainen S., Paulin L., Pekkonen E., Auvinen P., Scheperjans F. Gut microbiota in Parkinson’s disease: Temporal stability and relations to disease progression. EBioMedicine. 2019;44:691–707. doi: 10.1016/j.ebiom.2019.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallen Z.D., Demirkan A., Twa G., Cohen G., Dean M.N., Standaert D.G., Sampson T.R., Payami H. Metagenomics of Parkinson’s disease implicates the gut microbiome in multiple disease mechanisms. Nat. Commun. 2022;13:6958. doi: 10.1038/s41467-022-34667-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walker A.C., Bhargava R., Vaziriyan-Sani A.S., Pourciau C., Donahue E.T., Dove A.S., Gebhardt M.J., Ellward G.L., Romeo T., Czyż D.M. Colonization of the Caenorhabditis elegans gut with human enteric bacterial pathogens leads to proteostasis disruption that is rescued by butyrate. PLoS Pathog. 2021;17 doi: 10.1371/journal.ppat.1009510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker A.C., Bhargava R., Brust A.S., Owji A.A., Czyż D.M. Time-off-pick Assay to Measure Caenorhabditis elegans Motility. Bio. Protoc. 2022;12 doi: 10.21769/BioProtoc.4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steinkraus K.A., Smith E.D., Davis C., Carr D., Pendergrass W.R., Sutphin G.L., Kennedy B.K., Kaeberlein M. Dietary restriction suppresses proteotoxicity and enhances longevity by an hsf-1-dependent mechanism in Caenorhabditis elegans. Aging Cell. 2008;7:394–404. doi: 10.1111/j.1474-9726.2008.00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodhicharla R., Nagarajan A., Winter J., Adenle A., Nazir A., Brady D., Vere K., Richens J., O’Shea P., Bell D.R., de Pomerai D. Effects of α-Synuclein Overexpression in Transgenic Caenorhabditis elegans Strains. CNS Neurol. Disord.: Drug Targets. 2012;11:965–975. doi: 10.2174/1871527311211080005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Ham T.J., Thijssen K.L., Breitling R., Hofstra R.M.W., Plasterk R.H.A., Nollen E.A.A. C. elegans Model Identifies Genetic Modifiers of α-Synuclein Inclusion Formation During Aging. PLoS Genet. 2008;4 doi: 10.1371/journal.pgen.1000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gallrein C., Iburg M., Michelberger T., Koçak A., Puchkov D., Liu F., Ayala Mariscal S.M., Nayak T., Kaminski Schierle G.S., Kirstein J. Novel amyloid-beta pathology C. elegans model reveals distinct neurons as seeds of pathogenicity. Prog. Neurobiol. 2021;198 doi: 10.1016/j.pneurobio.2020.101907. [DOI] [PubMed] [Google Scholar]
- 22.Morley J.F., Brignull H.R., Weyers J.J., Morimoto R.I. The threshold for polyglutamine-expansion protein aggregation and cellular toxicity is dynamic and influenced by aging in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA. 2002;99:10417–10422. doi: 10.1073/pnas.152161099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor R.C., Dillin A. Aging as an event of proteostasis collapse. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walker A.C., Bhargava R., Dove A.S., Brust A.S., Owji A.A., Czyż D.M. Bacteria-Derived Protein Aggregates Contribute to the Disruption of Host Proteostasis. Int. J. Mol. Sci. 2022;23:4807. doi: 10.3390/ijms23094807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ben-Zvi A., Miller E.A., Morimoto R.I. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc. Natl. Acad. Sci. USA. 2009;106:14914–14919. doi: 10.1073/pnas.0902882106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macleod A.R., Waterston R.H., Fishpool R.M., Brenner S. Identification of the structural gene for a myosin heavy-chain in Caenorhabditis elegans. J. Mol. Biol. 1977;114:133–140. doi: 10.1016/0022-2836(77)90287-X. [DOI] [PubMed] [Google Scholar]
- 27.Clark S.G., Shurland D.-L., Meyerowitz E.M., Bargmann C.I., van der Bliek A.M. A dynamin GTPase mutation causes a rapid and reversible temperature-inducible locomotion defect in C. elegans. Proc. Natl. Acad. Sci. USA. 1997;94:10438–10443. doi: 10.1073/pnas.94.19.10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Labbadia J., Morimoto R.I. Proteostasis and longevity: when does aging really begin? F1000Prime Rep. 2014;6:7. doi: 10.12703/P6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tansey M.G., Wallings R.L., Houser M.C., Herrick M.K., Keating C.E., Joers V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022;22:657–673. doi: 10.1038/s41577-022-00684-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker A., Czyz D.M. Oh my gut! Is the microbial origin of neurodegenerative diseases real? Infect. Immun. 2023;91 doi: 10.1128/iai.00437-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sweeney P., Park H., Baumann M., Dunlop J., Frydman J., Kopito R., McCampbell A., Leblanc G., Venkateswaran A., Nurmi A., Hodgson R. Protein misfolding in neurodegenerative diseases: implications and strategies. Transl. Neurodegener. 2017;6 doi: 10.1186/s40035-017-0077-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mullane K., Williams M. Preclinical Models of Alzheimer’s Disease: Relevance and Translational Validity. Curr. Protoc. Pharmacol. 2019;84 doi: 10.1002/cpph.57. [DOI] [PubMed] [Google Scholar]
- 33.Savica R., Carlin J.M., Grossardt B.R., Bower J.H., Ahlskog J.E., Maraganore D.M., Bharucha A.E., Rocca W.A. Medical records documentation of constipation preceding Parkinson disease. Neurology. 2009;73:1752–1758. doi: 10.1212/WNL.0b013e3181c34af5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferreiro A.L., Choi J., Ryou J., Newcomer E.P., Thompson R., Bollinger R.M., Hall-Moore C., Ndao I.M., Sax L., Benzinger T.L.S., et al. Gut microbiome composition may be an indicator of preclinical Alzheimer’s disease. Sci. Transl. Med. 2023;15 doi: 10.1126/scitranslmed.abo2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tseng C.-S., Chao Y.-W., Liu Y.-H., Huang Y.-S., Chao H.-W. Dysregulated proteostasis network in neuronal diseases. Front. Cell Dev. Biol. 2023;11 doi: 10.3389/fcell.2023.1075215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang Y.-P., Chiu G.-F., Kuo F.-C., Lai C.-L., Yang Y.-H., Hu H.-M., Chang P.-Y., Chen C.-Y., Wu D.-C., Yu F.-J. Eradication of Helicobacter pylori Is Associated with the Progression of Dementia: A Population-Based Study. Gastroenterol. Res. Pract. 2013;2013 doi: 10.1155/2013/175729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kountouras J., Boziki M., Gavalas E., Zavos C., Grigoriadis N., Deretzi G., Tzilves D., Katsinelos P., Tsolaki M., Chatzopoulos D., Venizelos I. Eradication of Helicobacter pylori may be beneficial in the management of Alzheimer’s disease. J. Neurol. 2009;256:758–767. doi: 10.1007/s00415-009-5011-z. [DOI] [PubMed] [Google Scholar]
- 38.Elvers K.T., Wilson V.J., Hammond A., Duncan L., Huntley A.L., Hay A.D., van der Werf E.T. Antibiotic-induced changes in the human gut microbiota for the most commonly prescribed antibiotics in primary care in the UK: a systematic review. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2019-035677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun J., Zhan Y., Mariosa D., Larsson H., Almqvist C., Ingre C., Zagai U., Pawitan Y., Fang F. Antibiotics use and risk of amyotrophic lateral sclerosis in Sweden. Eur. J. Neurol. 2019;26:1355–1361. doi: 10.1111/ene.13986. [DOI] [PubMed] [Google Scholar]
- 40.Mertsalmi T.H., Pekkonen E., Scheperjans F. Antibiotic exposure and risk of Parkinson’s disease in Finland: A nationwide case-control study. Mov. Disord. 2020;35:431–442. doi: 10.1002/mds.27924. [DOI] [PubMed] [Google Scholar]
- 41.Goodwin C.S., Armstrong J.A. Microbiological aspects of Helicobacter pylori (Campylobacter pylori) Eur. J. Clin. Microbiol. Infect. Dis. 1990;9:1–13. doi: 10.1007/BF01969526. [DOI] [PubMed] [Google Scholar]
- 42.Iino C., Shimoyama T. Impact of Helicobacter pylori infection on gut microbiota. World J. Gastroenterol. 2021;27:6224–6230. doi: 10.3748/wjg.v27.i37.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grabrucker S., Marizzoni M., Silajdžić E., Lopizzo N., Mombelli E., Nicolas S., Dohm-Hansen S., Scassellati C., Moretti D.V., Rosa M., et al. Microbiota from Alzheimer’s patients induce deficits in cognition and hippocampal neurogenesis. Brain. 2023;146 doi: 10.1093/brain/awad303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tett A., Pasolli E., Masetti G., Ercolini D., Segata N. Prevotella diversity, niches and interactions with the human host. Nat. Rev. Microbiol. 2021;19:585–599. doi: 10.1038/s41579-021-00559-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Summanen P.H., Talan D.A., Strong C., McTeague M., Bennion R., Thompson J.E., Jr., Väisänen M.-L., Moran G., Winer M., Finegold S.M. Bacteriology of Skin and Soft-Tissue Infections: Comparison of Infections in Intravenous Drug Users and Individuals with No History of Intravenous Drug Use. Clin. Infect. Dis. 1995;20:S279–S282. doi: 10.1093/clinids/20.Supplement_2.S279. [DOI] [PubMed] [Google Scholar]
- 46.Civen R., Jousimies-Somer H., Marina M., Borenstein L., Shah H., Finegold S.M. A Retrospective Review of Cases of Anaerobic Empyema and Update of Bacteriology. Clin. Infect. Dis. 1995;20:S224–S229. doi: 10.1093/clinids/20.supplement_2.s224. [DOI] [PubMed] [Google Scholar]
- 47.Socransky S.s., Haffajee A.d., Cugini M.a., Smith C., Kent R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051X.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 48.Kim S.-J., Choi E.-Y., Kim E.G., Shin S.-H., Lee J.-Y., Choi J.-I., Choi I.-S. Prevotella intermedia lipopolysaccharide stimulates release of tumor necrosis factor-α through mitogen-activated protein kinase signaling pathways in monocyte-derived macrophages. FEMS Immunol. Med. Microbiol. 2007;51:407–413. doi: 10.1111/j.1574-695X.2007.00318.x. [DOI] [PubMed] [Google Scholar]
- 49.Xu P., Shao R.R., Zhang S., Tan Z.W., Guo Y.T., He Y. The mechanism on Prevotella melaninogenica promoting the inflammatory progression of oral lichen planus. Clin. Exp. Immunol. 2022;209:215–224. doi: 10.1093/cei/uxac054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marietta E.V., Murray J.A., Luckey D.H., Jeraldo P.R., Lamba A., Patel R., Luthra H.S., Mangalam A., Taneja V. Human Gut-Derived Prevotella histicola Suppresses Inflammatory Arthritis in Humanized Mice. Arthritis Rheumatol. 2016;68:2878–2888. doi: 10.1002/art.39785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larsen J.M. The immune response to Prevotella bacteria in chronic inflammatory disease. Immunology. 2017;151:363–374. doi: 10.1111/imm.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beydoun M.A., Beydoun H.A., Hossain S., El-Hajj Z.W., Weiss J., Zonderman A.B. Clinical and Bacterial Markers of Periodontitis and Their Association with Incident All-Cause and Alzheimer’s Disease Dementia in a Large National Survey. J. Alzheimers Dis. 2020;75:157–172. doi: 10.3233/JAD-200064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ilhan Z.E., Łaniewski P., Tonachio A., Herbst-Kralovetz M.M. Members of Prevotella Genus Distinctively Modulate Innate Immune and Barrier Functions in a Human Three-Dimensional Endometrial Epithelial Cell Model. J. Infect. Dis. 2020;222:2082–2092. doi: 10.1093/infdis/jiaa324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mack D.R., Michail S., Wei S., McDougall L., Hollingsworth M.A. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 1999;276:G941–G950. doi: 10.1152/ajpgi.1999.276.4.G941. [DOI] [PubMed] [Google Scholar]
- 55.Pothuraju R., Pai P., Chaudhary S., Siddiqui J.A., Cox J.L., Kaur S., Rachagani S., Roy H.K., Bouvet M., Batra S.K. Depletion of transmembrane mucin 4 (Muc4) alters intestinal homeostasis in a genetically engineered mouse model of colorectal cancer. Aging. 2022;14:2025–2046. doi: 10.18632/aging.203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seguella L., Sarnelli G., Esposito G. Leaky gut, dysbiosis, and enteric glia activation: the trilogy behind the intestinal origin of Parkinson’s disease. Neural Regen. Res. 2020;15:1037–1038. doi: 10.4103/1673-5374.270308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Obrenovich M.E.M. Leaky Gut, Leaky Brain? Microorganisms. 2018;6 doi: 10.3390/microorganisms6040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mu Q., Kirby J., Reilly C.M., Luo X.M. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front. Immunol. 2017;8:598. doi: 10.3389/fimmu.2017.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.CDC Bioterrorism and Anthrax: The Threat. Anthrax. 2024. https://www.cdc.gov/anthrax/bioterrorism/index.html
- 60.Goya M.E., Xue F., Sampedro-Torres-Quevedo C., Arnaouteli S., Riquelme-Dominguez L., Romanowski A., Brydon J., Ball K.L., Stanley-Wall N.R., Doitsidou M. Probiotic Bacillus subtilis Protects against α-Synuclein Aggregation in C. elegans. Cell Rep. 2020;30:367–380.e7. doi: 10.1016/j.celrep.2019.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haran J.P., Bhattarai S.K., Foley S.E., Dutta P., Ward D.V., Bucci V., McCormick B.A. Alzheimer’s Disease Microbiome Is Associated with Dysregulation of the Anti-Inflammatory P-Glycoprotein Pathway. mBio. 2019;10 doi: 10.1128/mBio.00632-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kaiyrlykyzy A., Kozhakhmetov S., Babenko D., Zholdasbekova G., Alzhanova D., Olzhayev F., Baibulatova A., Kushugulova A.R., Askarova S. Study of gut microbiota alterations in Alzheimer’s dementia patients from Kazakhstan. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-19393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keshavarzian A., Green S.J., Engen P.A., Voigt R.M., Naqib A., Forsyth C.B., Mutlu E., Shannon K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015;30:1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- 64.Ragusa M., Santagati M., Mirabella F., Lauretta G., Cirnigliaro M., Brex D., Barbagallo C., Domini C.N., Gulisano M., Barone R., et al. Potential Associations Among Alteration of Salivary miRNAs, Saliva Microbiome Structure, and Cognitive Impairments in Autistic Children. Int. J. Mol. Sci. 2020;21:6203. doi: 10.3390/ijms21176203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Atkin T.A., Brandon N.J., Kittler J.T. Disrupted in Schizophrenia 1 forms pathological aggresomes that disrupt its function in intracellular transport. Hum. Mol. Genet. 2012;21:2017–2028. doi: 10.1093/hmg/dds018. [DOI] [PubMed] [Google Scholar]
- 66.Fujita E., Dai H., Tanabe Y., Zhiling Y., Yamagata T., Miyakawa T., Tanokura M., Momoi M.Y., Momoi T. Autism spectrum disorder is related to endoplasmic reticulum stress induced by mutations in the synaptic cell adhesion molecule, CADM1. Cell Death Dis. 2010;1:e47. doi: 10.1038/cddis.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jaco A.D., Lin M.Z., Dubi N., Comoletti D., Miller M.T., Camp S., Ellisman M., Butko M.T., Tsien R.Y., Taylor P. Neuroligin Trafficking Deficiencies Arising from Mutations in the α/β-Hydrolase Fold Protein Family. J. Biol. Chem. 2010;285:28674–28682. doi: 10.1074/jbc.M110.139519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jęśko H., Cieślik M., Gromadzka G., Adamczyk A. Dysfunctional proteins in neuropsychiatric disorders: From neurodegeneration to autism spectrum disorders. Neurochem. Int. 2020;141 doi: 10.1016/j.neuint.2020.104853. [DOI] [PubMed] [Google Scholar]
- 69.De Angelis M., Piccolo M., Vannini L., Siragusa S., De Giacomo A., Serrazzanetti D.I., Cristofori F., Guerzoni M.E., Gobbetti M., Francavilla R. Fecal Microbiota and Metabolome of Children with Autism and Pervasive Developmental Disorder Not Otherwise Specified. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Taniya M.A., Chung H.-J., Al Mamun A., Alam S., Aziz M.A., Emon N.U., Islam M.M., Hong S.T.S., Podder B.R., Ara Mimi A., et al. Role of Gut Microbiome in Autism Spectrum Disorder and Its Therapeutic Regulation. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.915701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gabrielaite M., Bartell J.A., Nørskov-Lauritsen N., Pressler T., Nielsen F.C., Johansen H.K., Marvig R.L. Transmission and Antibiotic Resistance of Achromobacter in Cystic Fibrosis. J. Clin. Microbiol. 2021;59:10. doi: 10.1128/jcm.02911-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Veschetti L., Boaretti M., Saitta G.M., Passarelli Mantovani R., Lleò M.M., Sandri A., Malerba G. Achromobacter spp. prevalence and adaptation in cystic fibrosis lung infection. Microbiol. Res. 2022;263 doi: 10.1016/j.micres.2022.127140. [DOI] [PubMed] [Google Scholar]
- 73.Marsac C., Berdah L., Thouvenin G., Sermet-Gaudelus I., Corvol H. Achromobacter xylosoxidans airway infection is associated with lung disease severity in children with cystic fibrosis. ERJ Open Res. 2021;7 doi: 10.1183/23120541.00076-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhagirath A.Y., Li Y., Somayajula D., Dadashi M., Badr S., Duan K. Cystic fibrosis lung environment and Pseudomonas aeruginosa infection. BMC Pulm. Med. 2016;16:174. doi: 10.1186/s12890-016-0339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xi J., Ding D., Zhu H., Wang R., Su F., Wu W., Xiao Z., Liang X., Zhao Q., Hong Z., et al. Disturbed microbial ecology in Alzheimer’s disease: evidence from the gut microbiota and fecal metabolome. BMC Microbiol. 2021;21:226. doi: 10.1186/s12866-021-02286-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brunquell J., Bowers P., Westerheide S.D. Fluorodeoxyuridine enhances the heat shock response and decreases polyglutamine aggregation in an HSF-1-dependent manner in Caenorhabditis elegans. Mech. Ageing Dev. 2014;141–142:1–4. doi: 10.1016/j.mad.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 77.Deng P., Uma Naresh N., Du Y., Lamech L.T., Yu J., Zhu L.J., Pukkila-Worley R., Haynes C.M. Mitochondrial UPR repression during Pseudomonas aeruginosa infection requires the bZIP protein ZIP-3. Proc. Natl. Acad. Sci. USA. 2019;116:6146–6151. doi: 10.1073/pnas.1817259116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haeussler S., Conradt B. Methods to Study the Mitochondrial Unfolded Protein Response (UPRmt) in Caenorhabditis elegans. Methods Mol. Biol. 2022;2378:249–259. doi: 10.1007/978-1-0716-1732-8_16. [DOI] [PubMed] [Google Scholar]
- 79.Haynes C.M., Ron D. The mitochondrial UPR – protecting organelle protein homeostasis. J. Cell Sci. 2010;123:3849–3855. doi: 10.1242/jcs.075119. [DOI] [PubMed] [Google Scholar]
- 80.Javed I., Zhang Z., Adamcik J., Andrikopoulos N., Li Y., Otzen D.E., Lin S., Mezzenga R., Davis T.P., Ding F., Ke P.C. Accelerated Amyloid Beta Pathogenesis by Bacterial Amyloid FapC. Adv. Sci. 2020;7 doi: 10.1002/advs.202001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dueholm M.S., Otzen D., Nielsen P.H. Evolutionary Insight into the Functional Amyloids of the Pseudomonads. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Isler B., Kidd T.J., Stewart A.G., Harris P., Paterson D.L. Achromobacter Infections and Treatment Options. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.01025-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen S.G., Stribinskis V., Rane M.J., Demuth D.R., Gozal E., Roberts A.M., Jagadapillai R., Liu R., Choe K., Shivakumar B., et al. Exposure to the Functional Bacterial Amyloid Protein Curli Enhances Alpha-Synuclein Aggregation in Aged Fischer 344 Rats and Caenorhabditis elegans. Sci. Rep. 2016;6 doi: 10.1038/srep34477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bieler S., Estrada L., Lagos R., Baeza M., Castilla J., Soto C. Amyloid formation modulates the biological activity of a bacterial protein. J. Biol. Chem. 2005;280:26880–26885. doi: 10.1074/jbc.M502031200. [DOI] [PubMed] [Google Scholar]
- 85.Gidalevitz T., Ben-Zvi A., Ho K.H., Brignull H.R., Morimoto R.I. Progressive disruption of cellular protein folding in models of polyglutamine diseases. Science. 2006;311:1471–1474. doi: 10.1126/science.1124514. [DOI] [PubMed] [Google Scholar]
- 86.Brown G.C., Camacho M., Williams-Gray C.H. The Endotoxin Hypothesis of Parkinson’s Disease. Mov. Disord. 2023;38:1143–1155. doi: 10.1002/mds.29432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brown G.C. The endotoxin hypothesis of neurodegeneration. J. Neuroinflammation. 2019;16:180. doi: 10.1186/s12974-019-1564-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amoureux L., Bador J., Fardeheb S., Mabille C., Couchot C., Massip C., Salignon A.-L., Berlie G., Varin V., Neuwirth C. Detection of Achromobacter xylosoxidans in Hospital, Domestic, and Outdoor Environmental Samples and Comparison with Human Clinical Isolates. Appl. Environ. Microbiol. 2013;79:7142–7149. doi: 10.1128/AEM.02293-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chalhoub H., Kampmeier S., Kahl B.C., Van Bambeke F. Role of Efflux in Antibiotic Resistance of Achromobacter xylosoxidans and Achromobacter insuavis Isolates From Patients With Cystic Fibrosis. Front. Microbiol. 2022;13 doi: 10.3389/fmicb.2022.762307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ryan M.P., Adley C.C. Ralstonia spp.: emerging global opportunistic pathogens. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:291–304. doi: 10.1007/s10096-013-1975-9. [DOI] [PubMed] [Google Scholar]
- 91.Li Y., Kumar S., Zhang L., Wu H., Wu H. Characteristics of antibiotic resistance mechanisms and genes of Klebsiella pneumoniae. Open Med. 2023;18 doi: 10.1515/med-2023-0707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Laukkanen T., Kunutsor S., Kauhanen J., Laukkanen J.A. Sauna bathing is inversely associated with dementia and Alzheimer’s disease in middle-aged Finnish men. Age Ageing. 2017;46:245–249. doi: 10.1093/ageing/afw212. [DOI] [PubMed] [Google Scholar]
- 93.Patrick R.P., Johnson T.L. Sauna use as a lifestyle practice to extend healthspan. Exp. Gerontol. 2021;154 doi: 10.1016/j.exger.2021.111509. [DOI] [PubMed] [Google Scholar]
- 94.Kumsta C., Chang J.T., Schmalz J., Hansen M. Hormetic heat stress and HSF-1 induce autophagy to improve survival and proteostasis in C. elegans. Nat. Commun. 2017;8 doi: 10.1038/ncomms14337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bobkova N.V., Garbuz D.G., Nesterova I., Medvinskaya N., Samokhin A., Alexandrova I., Yashin V., Karpov V., Kukharsky M.S., Ninkina N.N., et al. Therapeutic Effect of Exogenous Hsp70 in Mouse Models of Alzheimer’s Disease. J. Alzheimers Dis. 2014;38:425–435. doi: 10.3233/JAD-130779. [DOI] [PubMed] [Google Scholar]
- 96.Kirkegaard T., Gray J., Priestman D.A., Wallom K.-L., Atkins J., Olsen O.D., Klein A., Drndarski S., Petersen N.H.T., Ingemann L., et al. Heat Shock Protein-based therapy as a potential candidate for treating sphingolipidoses. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aad9823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kieran D., Kalmar B., Dick J.R.T., Riddoch-Contreras J., Burnstock G., Greensmith L. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat. Med. 2004;10:402–405. doi: 10.1038/nm1021. [DOI] [PubMed] [Google Scholar]
- 98.Yang J., Bridges K., Chen K.Y., Liu A.Y.-C. Riluzole increases the amount of latent HSF1 for an amplified heat shock response and cytoprotection. PLoS One. 2008;3 doi: 10.1371/journal.pone.0002864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raizen D., Song B.-M., Trojanowski N., Young-Jai Y. WormBook, The C. elegans Research Community. WormBook; 2012. Methods for Measuring Pharyngeal Behaviors. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Keane J., Avery L. Mechanosensory inputs influence Caenorhabditis elegans pharyngeal activity via ivermectin sensitivity genes. Genetics. 2003;164:153–162. doi: 10.1093/genetics/164.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Walker A.C., Bhargava R., Vaziriyan-Sani A.S., Brust A.S., Czyz D.M. Quantification of Bacterial Loads in Caenorhabditis elegans. Bioprotocol. 2022;12 doi: 10.21769/BioProtoc.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wick R.R., Judd L.M., Gorrie C.L., Holt K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017;13 doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics. 2014;30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 105.Katoh K., Misawa K., Kuma K.i., Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30:3059–3066. doi: 10.1093/nar/gkf436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
“Western” indicates the band intensity of the polyQ insoluble fraction of worms fed indicated bacteria normalized to those fed E. coli OP50 control. “Count” indicates the average number of aggregates per worm of worms fed indicated bacteria normalized to those fed E. coli OP50 control. Parenthesized numbers (1–5) reflect the remaining conditions in which bacteria were grown:(1) anaerobically in reinforced clostridial broth (RCB) with Oxyrase, (2) facultative conditions (no shaking, tube filled to top) in brain heart infusion broth (BHI), (3) aerobic conditions, shaking at 220 revolutions per minute (RPM) in BHI, (4) anaerobic conditions on tryptic soy agar (TSA) supplemented with 5% defibrinated sheep’s blood, (5) aerobic conditions on TSA supplemented with 5% defibrinated sheep’s blood.
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead author upon request.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyze the data reported in the paper is available from the lead contact upon request.