Abstract
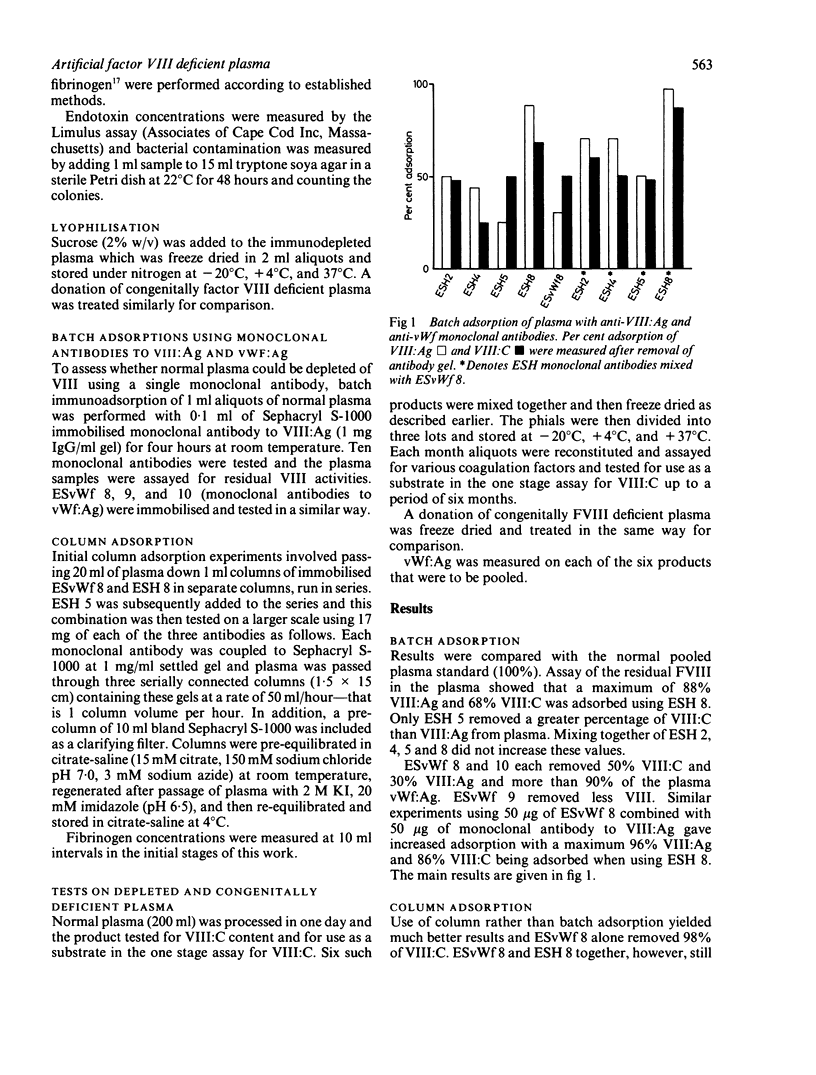
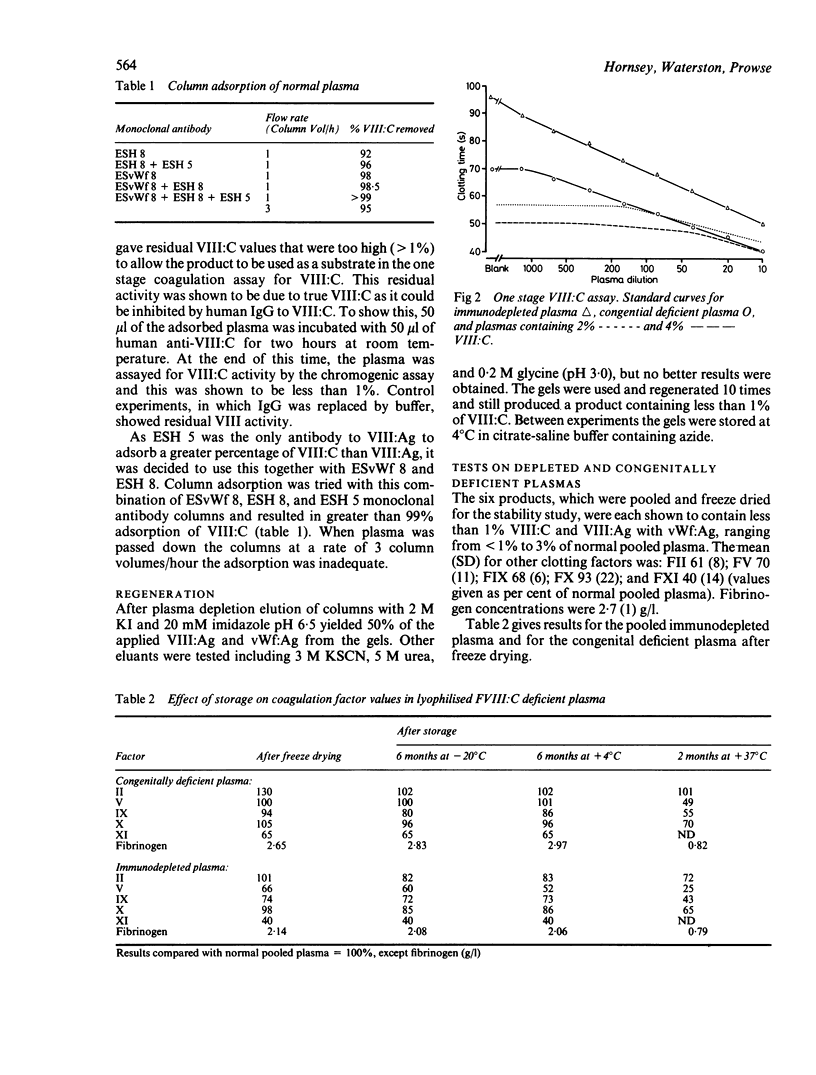
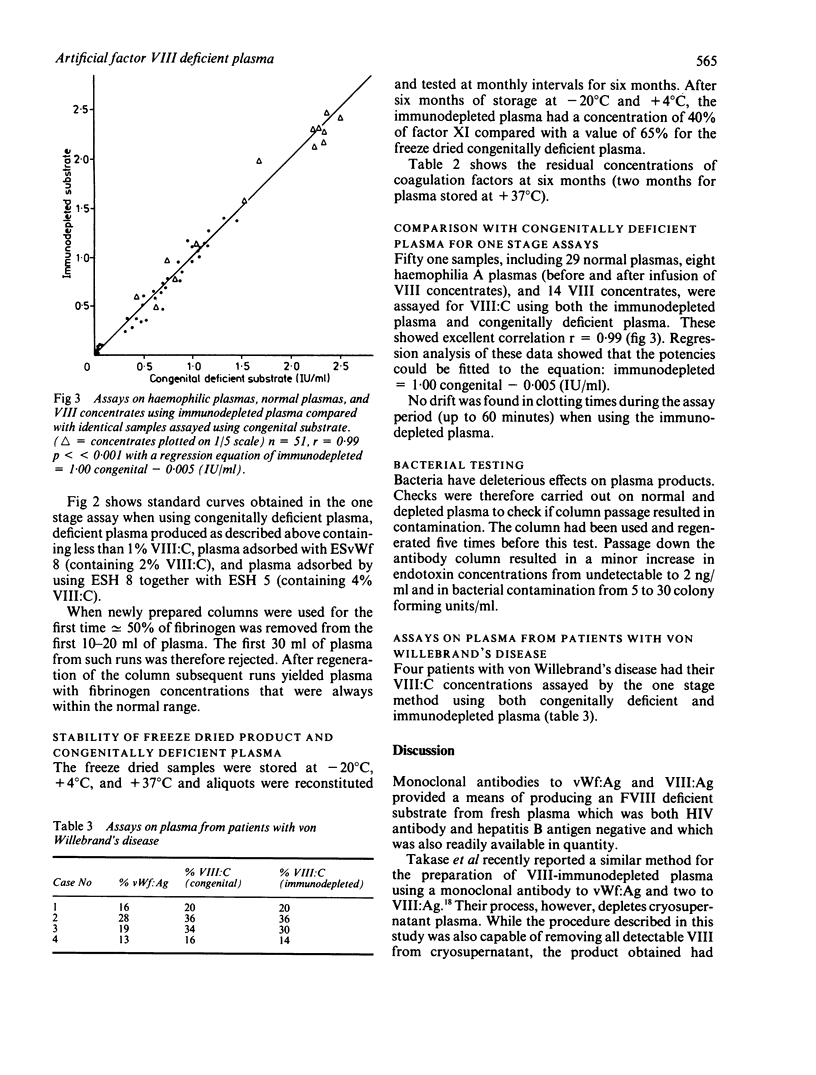
Monoclonal antibodies to factor VIII antigen (VIII:Ag) and von Willebrand factor (vWf:Ag) were immobilised on Sephacryl S-1000 and tested for their ability to deplete normal human citrated plasma of factor VIII. A combination of two antibodies to VIII:Ag and one antibody to vWf:Ag was required to produce plasma containing less than 0.01 IU/ml. Its performance in the one stage coagulation assay of VIII:C was equivalent to that of congenital VIII deficient plasma for the assay of normal and haemophilic plasma and factor VIII concentrates. Storage of freeze dried aliquots of this product at -20 degrees C, +4 degrees C, and 37 degrees C showed that it could be used as a substrate for at least six months when stored at temperatures +4 degrees C and below.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BIGGS R., EVELING J., RICHARDS G. The assay of antihaemophilic-globulin activity. Br J Haematol. 1955 Jan;1(1):20–34. doi: 10.1111/j.1365-2141.1955.tb05484.x. [DOI] [PubMed] [Google Scholar]
- Chantarangkul V., Ingram G. I., Thorn M. B., Darby S. C. An artificial 'haemophilic' plasma for one-stage factor-VIII assay. Br J Haematol. 1978 Nov;40(3):471–488. doi: 10.1111/j.1365-2141.1978.tb05818.x. [DOI] [PubMed] [Google Scholar]
- ELLIS B. C., STRANSKY A. A quick and accurate method for the determination of fibronogen in plasma. J Lab Clin Med. 1961 Sep;58:477–488. [PubMed] [Google Scholar]
- Exner T., Rickard K. A., Speers S. Factor VIII deficient plasma for laboratory tests prepared from normal plasma and a human antibody. Haemostasis. 1977;6(3):157–162. doi: 10.1159/000214175. [DOI] [PubMed] [Google Scholar]
- Furlan M., Felix R., Beck E. A. Preparation of factor VIII-deficient plasma by immunoadsorption. Vox Sang. 1979;36(6):342–346. doi: 10.1111/j.1423-0410.1979.tb04508.x. [DOI] [PubMed] [Google Scholar]
- Griffin B. D., Micklem L. R., McCann M. C., James K., Pepper D. S. The production and characterisation of a panel of ten murine monoclonal antibodies to human procoagulant factor VIII. Thromb Haemost. 1986 Feb 28;55(1):40–46. [PubMed] [Google Scholar]
- HARDISTY R. M., MACPHERSON J. C. A one-stage factor VIII (antihaemophilic globulin) assay and its use on venous and capillary plasma. Thromb Diath Haemorrh. 1962 May 15;7:215–228. [PubMed] [Google Scholar]
- Hornsey V. S., Griffin B. D., Pepper D. S., Micklem L. R., Prowse C. V. Immunoaffinity purification of factor VIII complex. Thromb Haemost. 1987 Feb 3;57(1):102–105. [PubMed] [Google Scholar]
- Hornsey V., Micklem L. R., McCann M. C., James K., Dawes J., McClelland D. B., Prowse C. V. Enhancement of factor VIII-von Willebrand factor ristocetin cofactor activity by monoclonal antibodies. Thromb Haemost. 1985 Aug 30;54(2):510–514. [PubMed] [Google Scholar]
- Kohn J., Wilchek M. A new approach (cyano-transfer) for cyanogen bromide activation of Sepharose at neutral pH, which yields activated resins, free of interfering nitrogen derivatives. Biochem Biophys Res Commun. 1982 Aug;107(3):878–884. doi: 10.1016/0006-291x(82)90604-0. [DOI] [PubMed] [Google Scholar]
- McPherson J., Soberano M. E., Macdonald C., Zucker M. B. Evidence that von Willebrand factor is not required for the clotting of plasma in the presence of platelets and kaolin (Hardisty-Hutton test). Thromb Haemost. 1984 Apr 30;51(2):272–274. [PubMed] [Google Scholar]
- Mertens K., van Wijngaarden A., Bertina R. M. The role of factor VIII in the activation of human blood coagulation factor X by activated factor IX. Thromb Haemost. 1985 Oct 30;54(3):654–660. [PubMed] [Google Scholar]
- Muntean W., Hathaway W. E., Montgomery R. R. Influence of high molecular weight factor VIII on the measurement of low molecular weight factor VIII procoagulant in different assay systems. Br J Haematol. 1982 Aug;51(4):649–658. doi: 10.1111/j.1365-2141.1982.tb02829.x. [DOI] [PubMed] [Google Scholar]
- Ofosu F., Cassidy K., Blajchman M. A., Hirsh J. Immunodepletion of human plasma factor VIII. Blood. 1980 Oct;56(4):604–607. [PubMed] [Google Scholar]
- Prowse C., Hornsey V., McKay G., Waterston Y. Room temperature, microtray chromogenic assay of factor VIII:C. Vox Sang. 1986;50(1):21–25. doi: 10.1111/j.1423-0410.1986.tb04840.x. [DOI] [PubMed] [Google Scholar]
- Rosén S. Assay of factor VIII:C with a chromogenic substrate. Scand J Haematol Suppl. 1984;40:139–145. doi: 10.1111/j.1600-0609.1984.tb02556.x. [DOI] [PubMed] [Google Scholar]
- Takase T., Rotblat F., Goodall A. H., Kernoff P. B., Middleton S., Chand S., Denson K. W., Austen D. E., Tuddenham E. G. Production of factor VIII deficient plasma by immunodepletion using three monoclonal antibodies. Br J Haematol. 1987 Aug;66(4):497–502. doi: 10.1111/j.1365-2141.1987.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Wright J. F., Hunter W. M. A convenient replacement for cyanogen bromide-activated solid phases in immunoradiometric assays. J Immunol Methods. 1982;48(3):311–325. doi: 10.1016/0022-1759(82)90332-5. [DOI] [PubMed] [Google Scholar]


